Isinglass as an Alternative Biopolymer Membrane for Green Electrochemical Devices: Initial Studies of Application in Electric Double-Layer Capacitors and Future Perspectives
Abstract
:1. Introduction
2. Materials and Methods
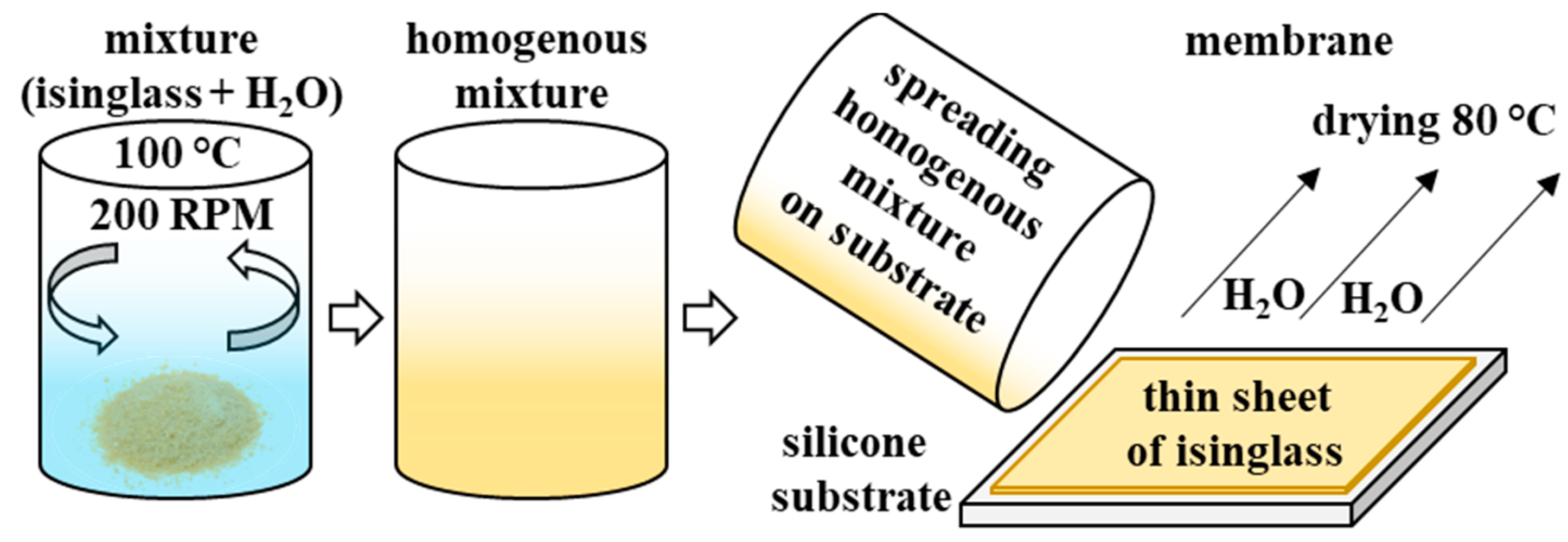
2.1. Preparation of Biopolymer Membrane
2.2. Electrochemical Cell Preparation
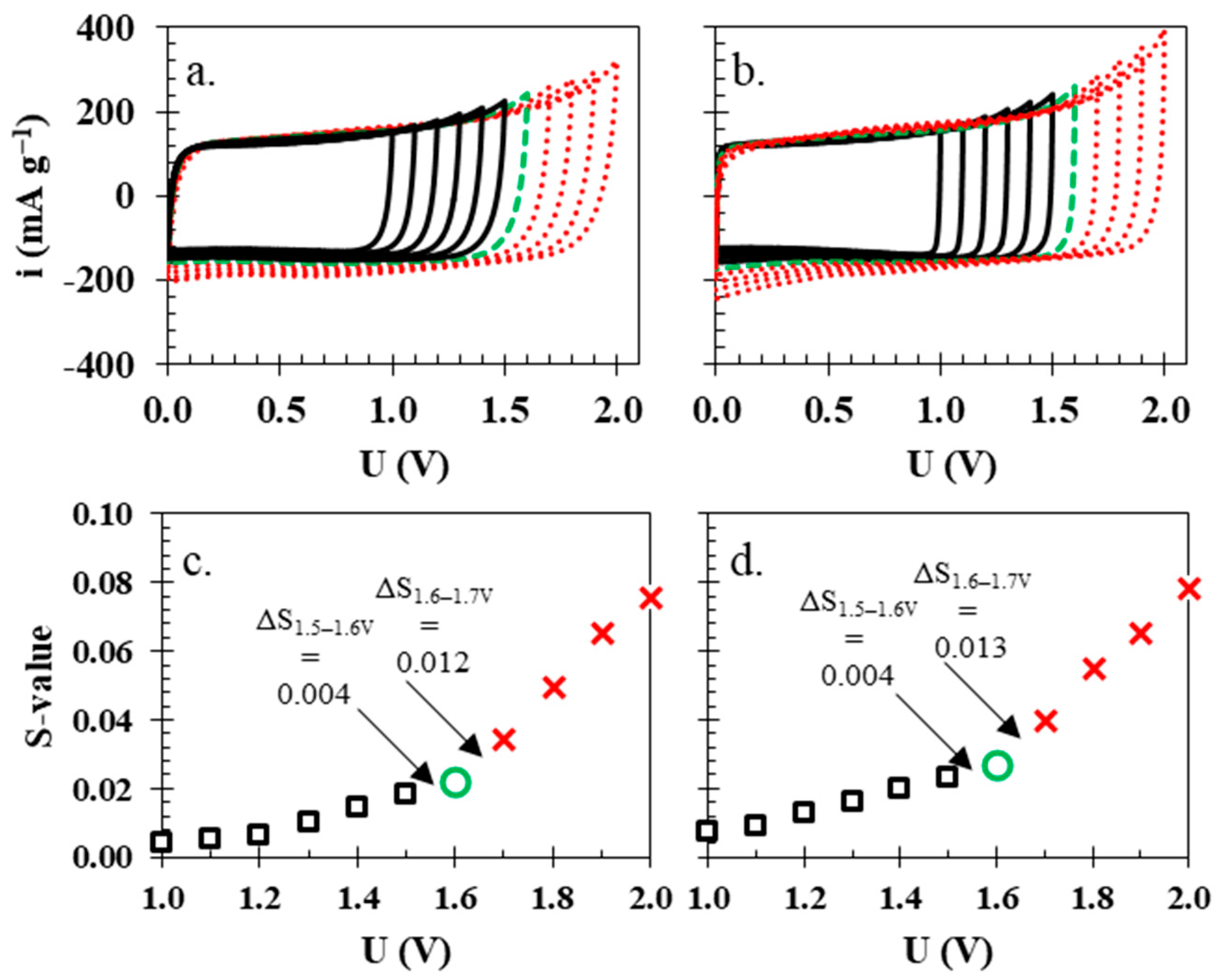
2.3. Electrochemical Testing Schedule
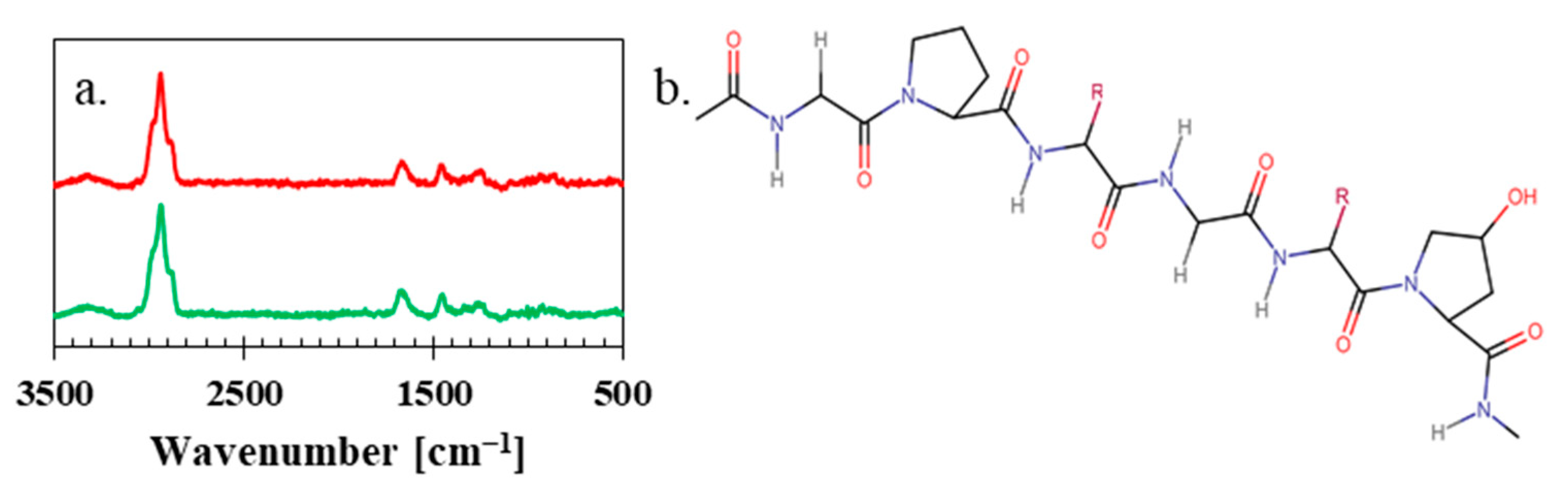
2.4. Raman Spectroscopy
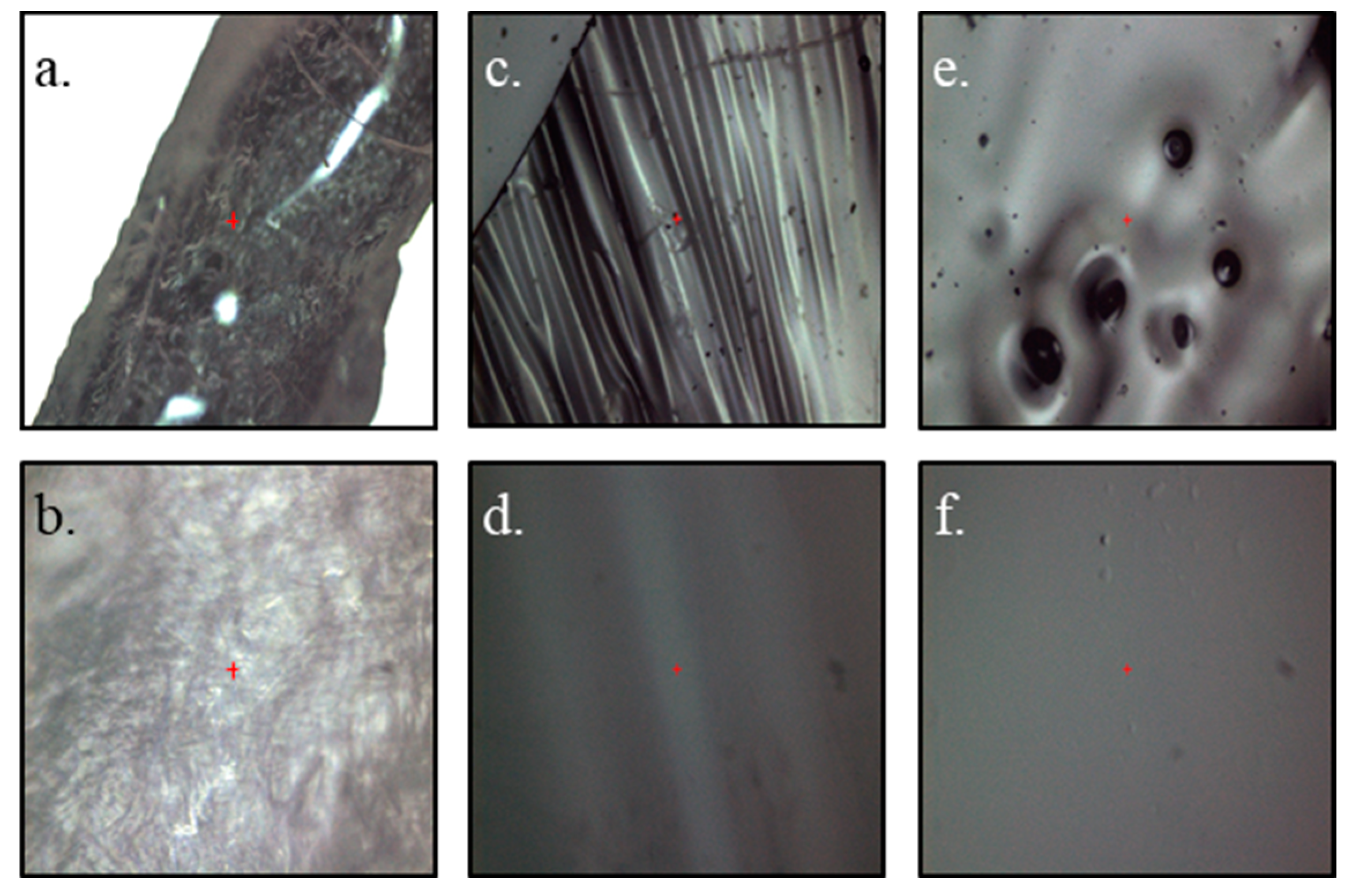
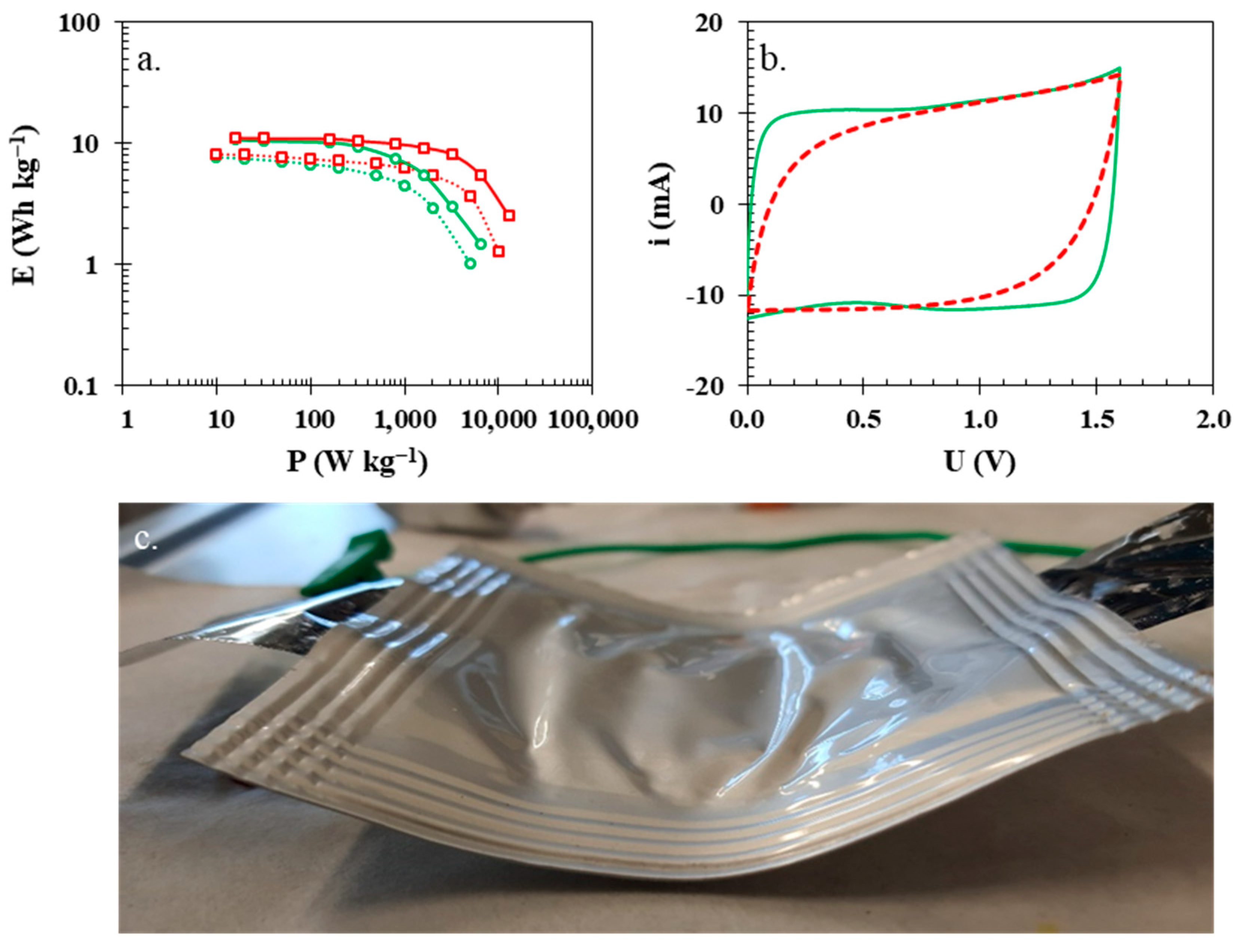
3. Results and Discussion
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Conflicts of Interest
References
- United Nations Environment. Drowning in Plastics: Marine Litter and Plastic Waste Vital Graphics; United Nations Environment: Nairobi, Kenya, 2021. [Google Scholar]
- Ritchie, H.; Roser, M. Plastic Pollution. Available online: https://ourworldindata.org/plastic-pollution (accessed on 24 July 2023).
- Hesterberg, T.W.; Chase, G.; Axten, C.; Miller, W.C.; Musselman, R.P.; Kamstrup, O.; Hadley, J.; Morscheidt, C.; Bernstein, D.M.; Thevenaz, P. Biopersistence of Synthetic Vitreous Fibers and Amosite Asbestos in the Rat Lung Following Inhalation. Toxicol. Appl. Pharmacol. 1998, 151, 262–275. [Google Scholar] [CrossRef] [PubMed]
- Li, D.; Shi, Y.; Yang, L.; Xiao, L.; Kehoe, D.K.; Gun’ko, Y.K.; Boland, J.J.; Wang, J.J. Microplastic release from the degradation of polypropylene feeding bottles during infant formula preparation. Nat. Food 2020, 1, 746–754. [Google Scholar] [CrossRef] [PubMed]
- Leja, K.; Lewandowicz, G. Polymer Biodegradation and Biodegradable Polymers—A Review. Polish J. Environ. Stud. 2010, 19, 255–266. [Google Scholar]
- Kasprzak, D.; Galiński, M. Chitin and chitin-cellulose composite hydrogels prepared by ionic liquid-based process as the novel electrolytes for electrochemical capacitors. J. Solid State Electrochem. 2021, 25, 2549–2563. [Google Scholar] [CrossRef]
- Kasprzak, D.; Galiński, M. Chitin as a universal and sustainable electrode binder for electrochemical capacitors. J. Power Sources 2023, 553, 232300. [Google Scholar] [CrossRef]
- Salleh, N.A.; Kheawhom, S.; Mohamad, A.A. Chitosan as biopolymer binder for graphene in supercapacitor electrode. Results Phys. 2021, 25, 104244. [Google Scholar] [CrossRef]
- Cruz, J.; Kawasaki, M.; Gorski, W. Electrode Coatings Based on Chitosan Scaffolds. Anal. Chem. 2000, 72, 680–686. [Google Scholar] [CrossRef]
- Hoti, G.; Matencio, A.; Rubin Pedrazzo, A.; Cecone, C.; Appleton, S.L.; Khazaei Monfared, Y.; Caldera, F.; Trotta, F. Nutraceutical Concepts and Dextrin-Based Delivery Systems. Int. J. Mol. Sci. 2022, 23, 4102. [Google Scholar] [CrossRef]
- Abdulwahid, R.T.; Aziz, S.B.; Kadir, M.F.Z. Replacing synthetic polymer electrolytes in energy storage with flexible biodegradable alternatives: Sustainable green biopolymer blend electrolyte for supercapacitor device. Mater. Today Sustain. 2023, 23, 100472. [Google Scholar] [CrossRef]
- Aziz, S.; Hamsan, M.H.; Nofal, M.M.; Karim, W.O.; Brevik, I.; Brza, M.A.; Abdulwahid, R.T.; Al-Zangana, S.; Kadir, M.F.Z. Structural, Impedance and Electrochemical Characteristics of Electrical Double Layer Capacitor Devices Based on Chitosan: Dextran Biopolymer Blend Electrolytes. Polymers 2020, 12, 1411. [Google Scholar] [CrossRef]
- Aziz, S.B.; Ali, F.; Anuar, H.; Ahamad, T.; Kareem, W.O.; Brza, M.A.; Kadir, M.F.Z.; Abu Ali, O.A.; Saleh, D.I.; Asnawi, A.S.F.M.; et al. Structural and electrochemical studies of proton conducting biopolymer blend electrolytes based on MC: Dextran for EDLC device application with high energy density. Alex. Eng. J. 2022, 61, 3985–3997. [Google Scholar] [CrossRef]
- Raphael, E.; Avellaneda, C.O.; Manzolli, B.; Pawlicka, A. Agar-based films for application as polymer electrolytes. Electrochim. Acta 2010, 55, 1455–1459. [Google Scholar] [CrossRef]
- Zhang, F.; Liu, T.; Zhang, J.; Cui, E.; Yue, L.; Jiang, R.; Hou, G. The potassium hydroxide-urea synergy in improving the capacitive energy-storage performance of agar-derived carbon aerogels. Carbon 2019, 147, 451–459. [Google Scholar] [CrossRef]
- Espinoza-Acosta, J.L.; Torres-Chávez, P.I.; Olmedo-Martínez, J.L.; Vega-Rios, A.; Flores-Gallardo, S.; Zaragoza-Contreras, E.A. Lignin in storage and renewable energy applications: A review. J. Energy Chem. 2018, 27, 1422–1438. [Google Scholar] [CrossRef]
- Ajjan, F.N.; Casado, N.; Rębiś, T.; Elfwing, A.; Solin, N.; Mecerreyes, D.; Inganäs, O. High performance PEDOT/lignin biopolymer composites for electrochemical supercapacitors. J. Mater. Chem. A 2016, 4, 1838–1847. [Google Scholar] [CrossRef]
- Aziz, S.B.; Brza, M.A.; Hamsan, M.H.; Kadir, M.F.Z.; Muzakir, S.K.; Abdulwahid, R.T. Effect of ohmic-drop on electrochemical performance of EDLC fabricated from PVA:dextran:NH4I based polymer blend electrolytes. J. Mater. Res. Technol. 2020, 9, 3734–3745. [Google Scholar] [CrossRef]
- Zainuddin, N.K.; Rasali, N.M.J.; Mazuki, N.F.; Saadiah, M.A.; Samsudin, A.S. Investigation on favourable ionic conduction based on CMC-K carrageenan proton conducting hybrid solid bio-polymer electrolytes for applications in EDLC. Int. J. Hydrogen Energy 2020, 45, 8727–8741. [Google Scholar] [CrossRef]
- Aziz, S.B.; Hamsan, M.H.; Nofal, M.M.; San, S.; Abdulwahid, R.T.; Raza Saeed, S.R.; Brza, M.A.; Kadir, M.F.Z.; Mohammed, S.J.; Al-Zangana, S. From Cellulose, Shrimp and Crab Shells to Energy Storage EDLC Cells: The Study of Structural and Electrochemical Properties of Proton Conducting Chitosan-Based Biopolymer Blend Electrolytes. Polymers 2020, 12, 1526. [Google Scholar] [CrossRef]
- Aziz, S.B.; Hamsan, M.H.; Abdullah, R.M.; Abdulwahid, R.T.; Brza, M.A.; Marif, A.S.; Kadir, M.F.Z. Protonic EDLC cell based on chitosan (CS): Methylcellulose (MC) solid polymer blend electrolytes. Ionics 2020, 26, 1829–1840. [Google Scholar] [CrossRef]
- Teoh, K.H.; Lim, C.-S.; Liew, C.-W.; Ramesh, S.; Ramesh, S. Electric double-layer capacitors with corn starch-based biopolymer electrolytes incorporating silica as filler. Ionics 2015, 21, 2061–2068. [Google Scholar] [CrossRef]
- Lizundia, E.; Kundu, D. Advances in Natural Biopolymer-Based Electrolytes and Separators for Battery Applications. Adv. Funct. Mater. 2021, 31, 2005646. [Google Scholar] [CrossRef]
- Zhang, T.-W.; Tian, T.; Shen, B.; Song, Y.-H.; Yao, H.-B. Recent advances on biopolymer fiber based membranes for lithium-ion battery separators. Compos. Commun. 2019, 14, 7–14. [Google Scholar] [CrossRef]
- Uddin, M.-J.; Alaboina, P.K.; Zhang, L.; Cho, S.-J. A low-cost, environment-friendly lignin-polyvinyl alcohol nanofiber separator using a water-based method for safer and faster lithium-ion batteries. Mater. Sci. Eng. B 2017, 223, 84–90. [Google Scholar] [CrossRef]
- Aziz, S.B.; Hadi, J.M.; Dannoun, E.M.A.; Abdulwahid, R.T.; Saeed, S.R.; Shahab Marf, A.; Karim, W.O.; Kadir, M.F.Z. The Study of Plasticized Amorphous Biopolymer Blend Electrolytes Based on Polyvinyl Alcohol (PVA): Chitosan with High Ion Conductivity for Energy Storage Electrical Double-Layer Capacitors (EDLC) Device Application. Polymers 2020, 12, 1938. [Google Scholar] [CrossRef] [PubMed]
- Spina, G.E.; Poli, F.; Brilloni, A.; Marchese, D.; Soavi, F. Natural Polymers for Green Supercapacitors. Energies 2020, 13, 3115. [Google Scholar] [CrossRef]
- Kasprzak, D.; Stępniak, I.; Galiński, M. Electrodes and hydrogel electrolytes based on cellulose: Fabrication and characterization as EDLC components. J. Solid State Electrochem. 2018, 22, 3035–3047. [Google Scholar] [CrossRef]
- Jeżowski, P.; Kowalczewski, P.Ł. Starch as a Green Binder for the Formulation of Conducting Glue in Supercapacitors. Polymers 2019, 11, 1648. [Google Scholar] [CrossRef]
- Menzel, J.; Frąckowiak, E.; Fic, K. Agar-based aqueous electrolytes for electrochemical capacitors with reduced self-discharge. Electrochim. Acta 2020, 332, 135435. [Google Scholar] [CrossRef]
- Wang, Q.; Cao, Q.; Wang, X.; Jing, B.; Kuang, H.; Zhou, L. A high-capacity carbon prepared from renewable chicken feather biopolymer for supercapacitors. J. Power Sources 2013, 225, 101–107. [Google Scholar] [CrossRef]
- Suzanowicz, A.M.; Lee, Y.; Schultz, A.; Marques, O.J.J.; Lin, H.; Segre, C.U.; Mandal, B.K. Synthesis and Electrochemical Properties of Lignin-Derived High Surface Area Carbons. Surfaces 2022, 5, 265–279. [Google Scholar] [CrossRef]
- Islam, M.A.; Ong, H.L.; Villagracia, A.R.; Halim, K.A.A.; Ganganboina, A.B.; Doong, R.-A. Biomass–derived cellulose nanofibrils membrane from rice straw as sustainable separator for high performance supercapacitor. Ind. Crops Prod. 2021, 170, 113694. [Google Scholar] [CrossRef]
- Nowacki, K.; Galiński, M.; Stępniak, I. Synthesis and Characterization of Chitosan/Sodium Alginate Blend Membrane for Application in an Electrochemical Capacitor. Prog. Chem. Appl. Chitin Deriv. 2020, XXV, 174–191. [Google Scholar] [CrossRef]
- Nowacki, K.; Galiński, M.; Stępniak, I. Synthesis and characterization of modified chitosan membranes for applications in electrochemical capacitor. Electrochim. Acta 2019, 320, 134632. [Google Scholar] [CrossRef]
- Wysokowski, M.; Nowacki, K.; Jaworski, F.; Niemczak, M.; Bartczak, P.; Sandomierski, M.; Piasecki, A.; Galiński, M.; Jesionowski, T. Ionic liquid-assisted synthesis of chitin–ethylene glycol hydrogels as electrolyte membranes for sustainable electrochemical capacitors. Sci. Rep. 2022, 12, 8861. [Google Scholar] [CrossRef]
- Yang, H.; Liu, Y.; Kong, L.; Kang, L.; Ran, F. Biopolymer-based carboxylated chitosan hydrogel film crosslinked by HCl as gel polymer electrolyte for all-solid-sate supercapacitors. J. Power Sources 2019, 426, 47–54. [Google Scholar] [CrossRef]
- Kasturi, P.R.; Ramasamy, H.; Meyrick, D.; Sung Lee, Y.; Kalai Selvan, R. Preparation of starch-based porous carbon electrode and biopolymer electrolyte for all solid-state electric double layer capacitor. J. Colloid Interface Sci. 2019, 554, 142–156. [Google Scholar] [CrossRef]
- Skunik-Nuckowska, M.; Wisińska, N.H.; Garbacz, P.; Dyjak, S.; Wieczorek, W.; Kulesza, P.J. Polysaccharide-Based Hydrogel Electrolytes Enriched with Poly(Norepinephrine) for Sustainable Aqueous Electrochemical Capacitors. SSRN Electron. J. 2022, 11, 109346. [Google Scholar] [CrossRef]
- Konwar, S.; Singh, D.; Strzałkowski, K.; Masri, M.N.B.; Yahya, M.Z.A.; Diantoro, M.; Savilov, S.V.; Singh, P.K. Stable and Efficient Dye-Sensitized Solar Cells and Supercapacitors Developed Using Ionic-Liquid-Doped Biopolymer Electrolytes. Molecules 2023, 28, 5099. [Google Scholar] [CrossRef]
- Asnawi, A.S.F.M.; Hamsan, M.H.; Aziz, S.B.; Kadir, M.F.Z.; Matmin, J.; Yusof, Y.M. Impregnation of [Emim]Br ionic liquid as plasticizer in biopolymer electrolytes for EDLC application. Electrochim. Acta 2021, 375, 137923. [Google Scholar] [CrossRef]
- Sun, P.; Chen, J.; Huang, Y.; Tian, J.-H.; Li, S.; Wang, G.; Zhang, Q.; Tian, Z.; Zhang, L. High-Strength agarose gel electrolyte enables long-endurance wearable Al-air batteries with greatly suppressed self-corrosion. Energy Storage Mater. 2021, 34, 427–435. [Google Scholar] [CrossRef]
- Scardecchia, S.; Vita, A.; Santulli, C.; Forcellese, A. A material proposed for re-use of hemp shives as a waste from fiber production. Mater. Today Proc. 2020, 31, S213–S216. [Google Scholar] [CrossRef]
- Nazeer, R.A.; Joshi, I.; Mahankali, S.; Mazumdar, A.; Sridharan, B.; Sankar, S.J. Is Marine Waste a Boon or Bane? An Insight on Its Source, Production, Disposal Consequences, and Utilization. In Applied Biotechnology for Emerging Pollutants Remediation and Energy Conversion; Springer Nature: Singapore, 2023; pp. 231–250. [Google Scholar]
- Schellmann, N.C. Animal glues: A review of their key properties relevant to conservation. Stud. Conserv. 2007, 52, 55–66. [Google Scholar] [CrossRef]
- Li, L.; Lu, F.; Wang, C.; Zhang, F.; Liang, W.; Kuga, S.; Dong, Z.; Zhao, Y.; Huang, Y.; Wu, M. Flexible double-cross-linked cellulose-based hydrogel and aerogel membrane for supercapacitor separator. J. Mater. Chem. A 2018, 6, 24468–24478. [Google Scholar] [CrossRef]
- Weingarth, D.; Noh, H.; Foelske-Schmitz, A.; Wokaun, A.; Kötz, R. A reliable determination method of stability limits for electrochemical double layer capacitors. Electrochim. Acta 2013, 103, 119–124. [Google Scholar] [CrossRef]
- Zhao, J.; Burke, A.F. Electrochemical Capacitors: Performance Metrics and Evaluation by Testing and Analysis. Adv. Energy Mater. 2021, 11, 2002192. [Google Scholar] [CrossRef]
- Połomska, M.; Kubisz, L.; Wolak, J.; Hojan-Jezierska, D. Effects of Temperature on the FT NIR Raman Spectra of Fish Skin Collagen. Appl. Sci. 2021, 11, 8358. [Google Scholar] [CrossRef]
- Senadheera, T.R.L.; Dave, D.; Shahidi, F. Sea Cucumber Derived Type I Collagen: A Comprehensive Review. Mar. Drugs 2020, 18, 471. [Google Scholar] [CrossRef]
- Wu, T.-H.; Hsu, C.-T.; Hu, C.-C.; Hardwick, L.J. Important parameters affecting the cell voltage of aqueous electrical double-layer capacitors. J. Power Sources 2013, 242, 289–298. [Google Scholar] [CrossRef]
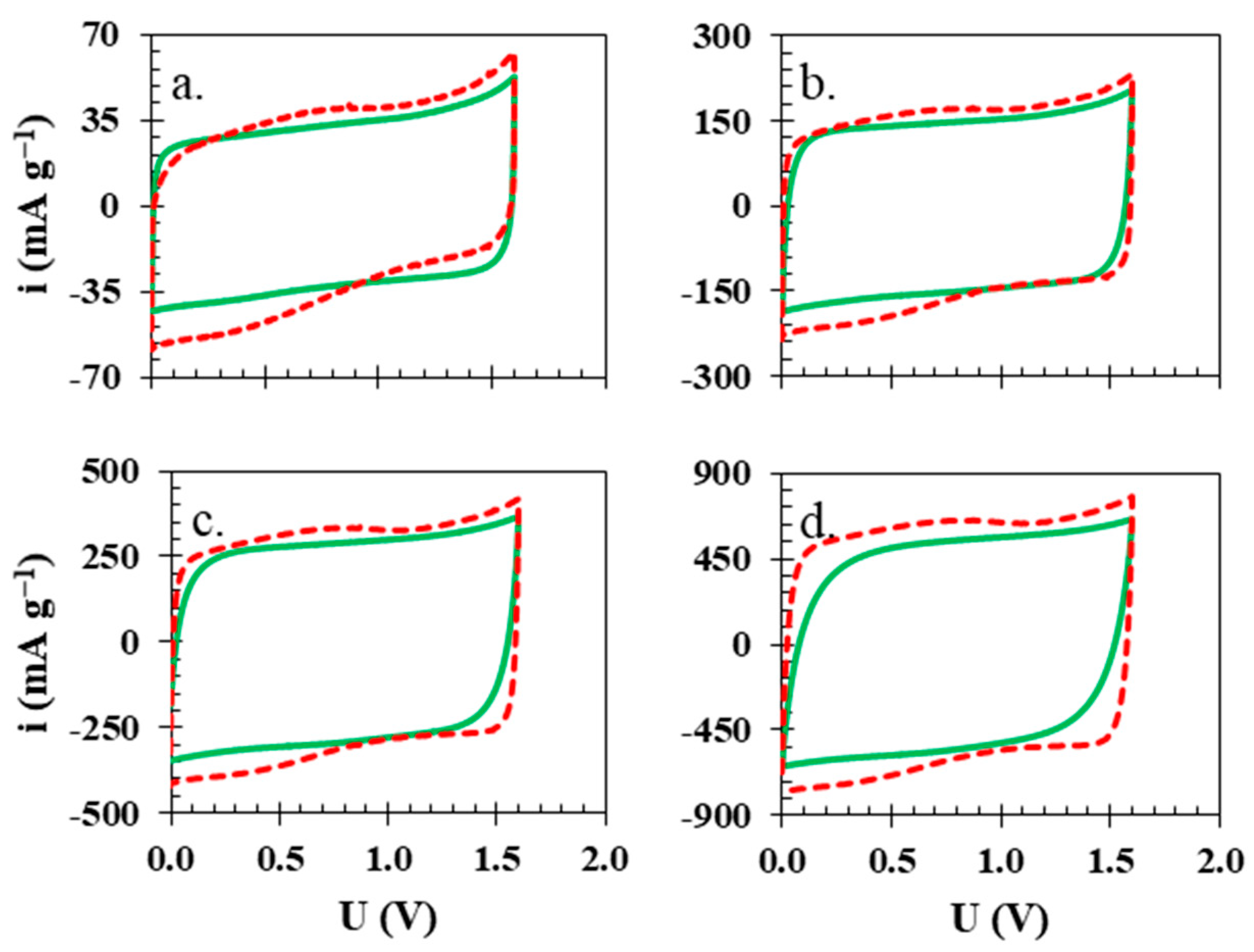

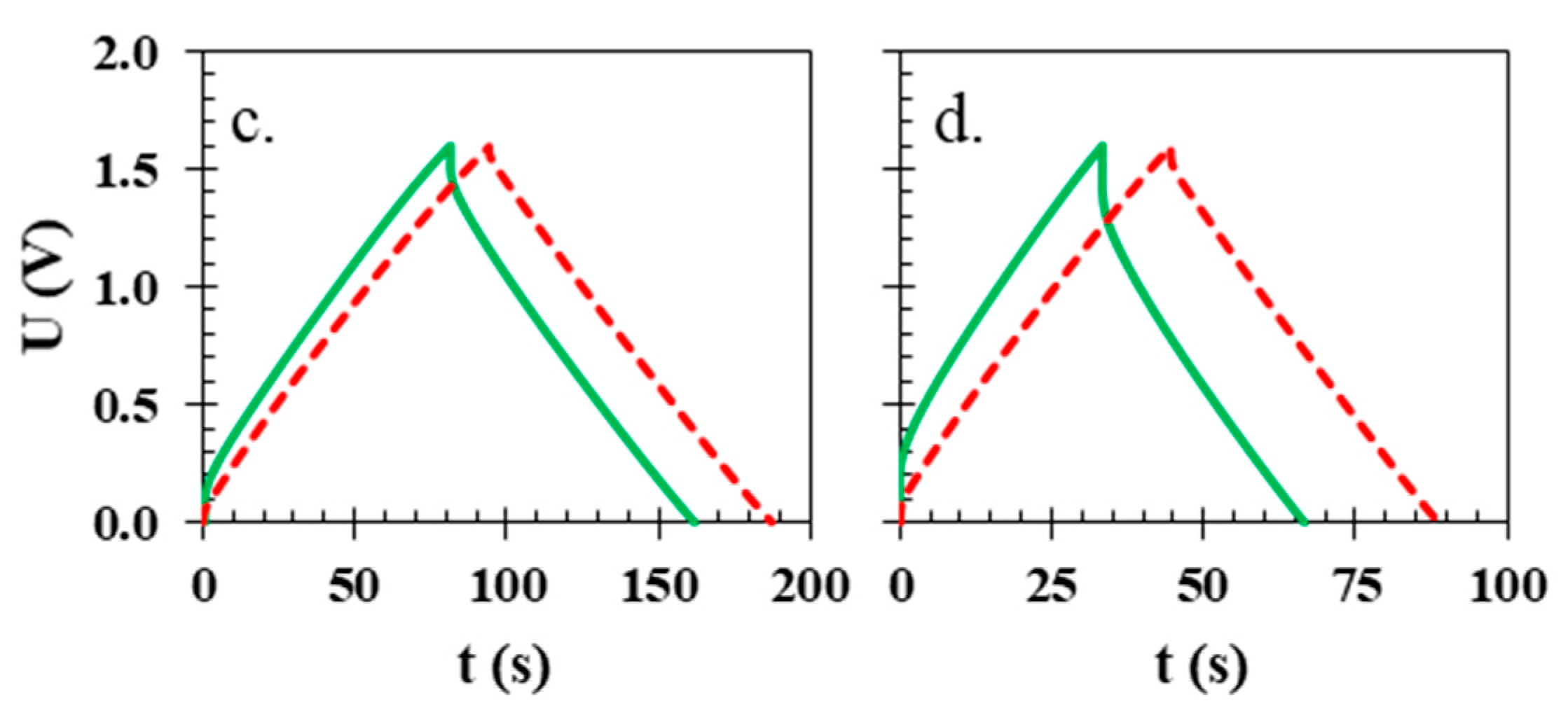
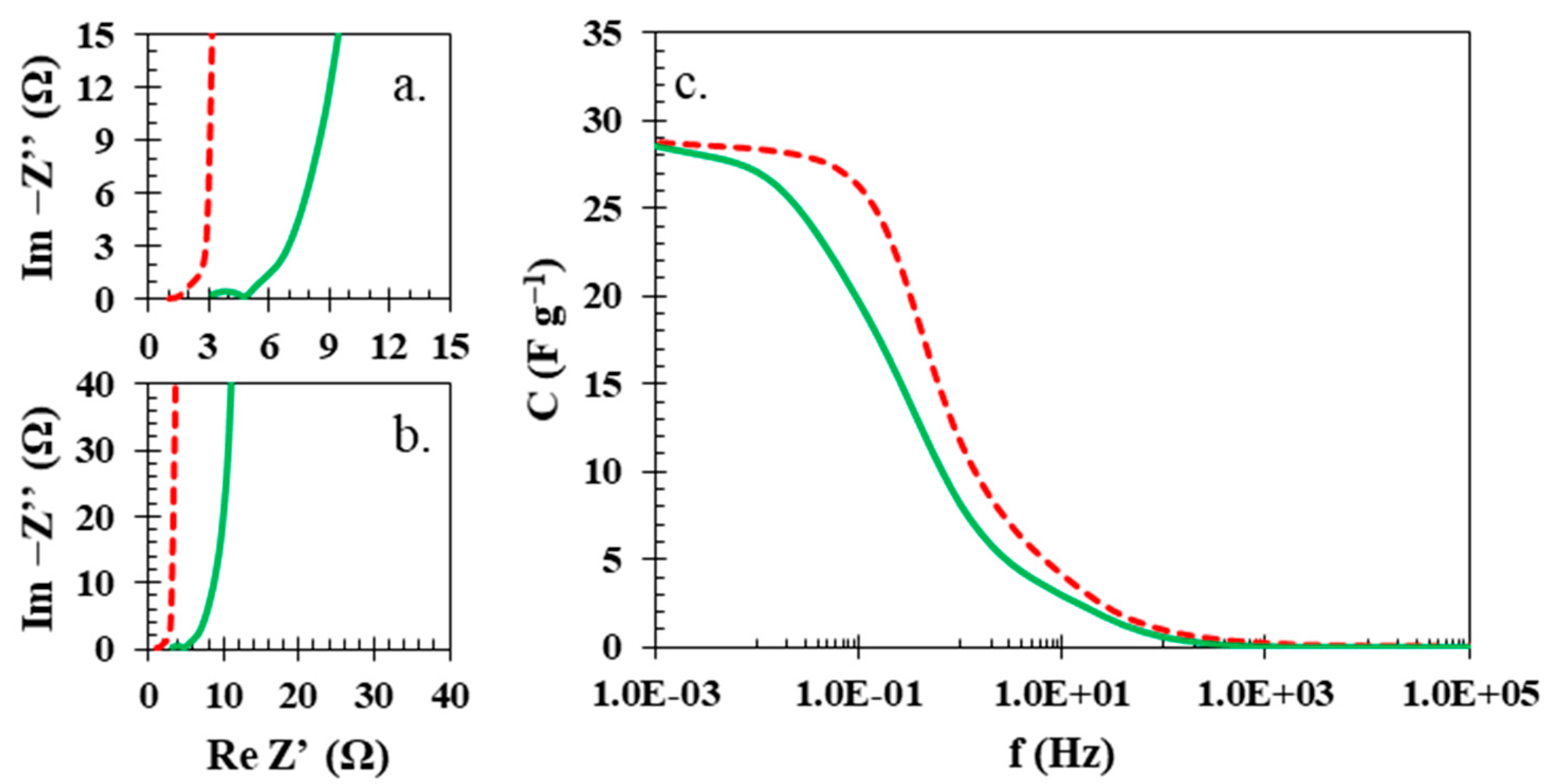
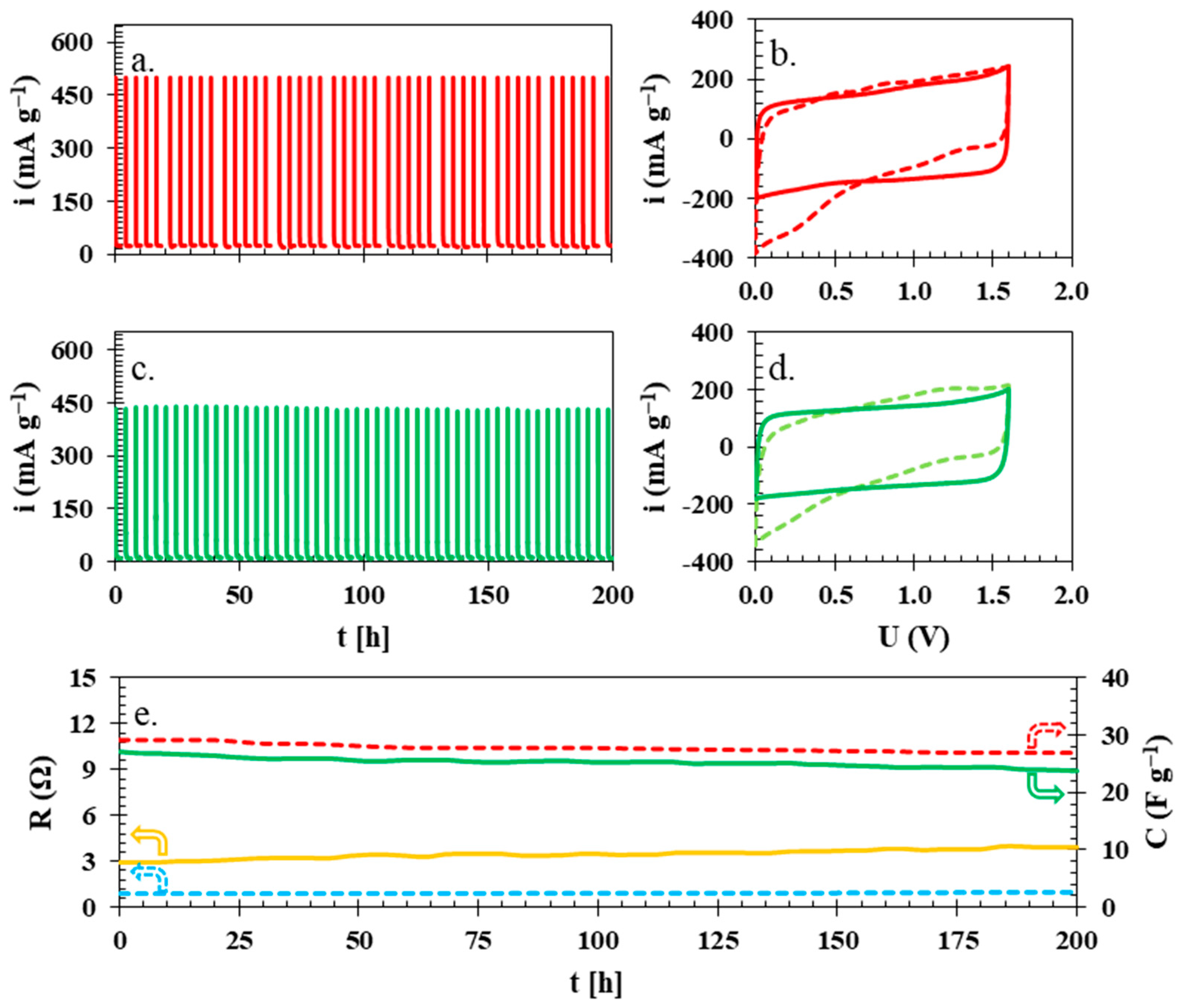

| Ref. | Membrane | Electrolyte | Electrode Active Material | U [V] | ESR [Ω] | EDR [Ω] | C [F g−1] | E [Wh kg−1] | Longevity |
|---|---|---|---|---|---|---|---|---|---|
| [6] | Chitin/ Cellulose | 1.0 mol L−1 lithium sulphate | AC Kynol ACC-507-20 | 0.8 | 1 | 1.7 | 90 * | 2 | 20 k cycles, 5% capacitance drop |
| [28] | Cellulose | 2.0 mol L−1 lithium acetate | AC Norti DLC Supra 30 AC Kynol ACC-507-20 | 0.8 | 5 | 15 | 20-25 | 2 | 10 k cycles, no observable drop |
| [33] | Cellulose nanofibrils | 1.0 mol L−1 sodium sulphate | AC (not specified) | 1.2 | 1–3 | 12–22 | 80–100 * | 23–27 * | 5 k cycles, up to 5% capacitance drop |
| [34] | Chitosan/Sodium alginate | 2.0 mol L−1 lithium sulphate | AC Kynol ACC-507-20 | 1.6 | 1 | 3–5 | 125 * | 8–10 | 1 k cycles, 10% capacitance drop |
| [35] | Chitosan/NaOH /glutaraldehyde | 2.0 mol L−1 lithium acetate | AC Kynol ACC-507-20 | 0.8 | 1–3 | 5–10 | 100 * | 2 | 10 k cycles, no observable drop |
| [36] | Chitin | 2.0 mol L−1 lithium acetate | AC Kynol ACC-507-20 | 0.8 | 1 | 5 | 100 * | 2 | 10 k cycles, no observable drop |
| [37] | Carboxylated chitosan | 1.0 mol L−1 hydrochloric acid | AC Shenyang Kejing | 0.9 | 1 | 8 | 40 | 3 | (not specified) |
| [38] | Starch | 1.0 mol L−1 sulphuric acid | AC (as prepared) | 0.8 | 0.5 | 9–63 | 100–250 * | 10–20 * | 2 k cycles, 3% capacitance drop |
| [39] | Cellulose/Agarose | 1.0 mol L−1 sulphuric acid 1.0 mol L−1 sodium sulphate | AC Kurary YP-80F | 0.8 | 1–14 | 1–30 | 100–120 * | 2 | 10 k cycles, 10% capacitance drop |
| [30] | Agar | 0.5 mol L−1 potassium sulphate | AC Kynol ACC-507-20 | 1.6 | 1 | 2 | 80–110 * | 7 | 10 k cycles, up to 8% capacitance drop |
| This work | Isinglass | 1.0 mol L−1 sodium sulphate | AC Kynol ACC-507-20 | 1.6 | 2 | 7 | 25 | 8–10 | 10 k cycles, up to 5% capacitance drop |
| Glass fibre | 1.0 mol L−1 sodium sulphate | AC Kynol ACC-507-20 | 1.6 | 1 | 3 | 28 | 8–10 | 10 k cycles, up to 4% capacitance drop |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Jeżowski, P.; Kowalczewski, P.Ł. Isinglass as an Alternative Biopolymer Membrane for Green Electrochemical Devices: Initial Studies of Application in Electric Double-Layer Capacitors and Future Perspectives. Polymers 2023, 15, 3557. https://doi.org/10.3390/polym15173557
Jeżowski P, Kowalczewski PŁ. Isinglass as an Alternative Biopolymer Membrane for Green Electrochemical Devices: Initial Studies of Application in Electric Double-Layer Capacitors and Future Perspectives. Polymers. 2023; 15(17):3557. https://doi.org/10.3390/polym15173557
Chicago/Turabian StyleJeżowski, Paweł, and Przemysław Łukasz Kowalczewski. 2023. "Isinglass as an Alternative Biopolymer Membrane for Green Electrochemical Devices: Initial Studies of Application in Electric Double-Layer Capacitors and Future Perspectives" Polymers 15, no. 17: 3557. https://doi.org/10.3390/polym15173557
APA StyleJeżowski, P., & Kowalczewski, P. Ł. (2023). Isinglass as an Alternative Biopolymer Membrane for Green Electrochemical Devices: Initial Studies of Application in Electric Double-Layer Capacitors and Future Perspectives. Polymers, 15(17), 3557. https://doi.org/10.3390/polym15173557








