1. Introduction
The goal of regenerative medicine is to regenerate or replace damaged or necrotic tissues with functional tissues inherent to the tissue type [
1]. To achieve this, tissue engineering requires a comprehensive understanding of the biological knowledge related to the proliferation and differentiation of cells within the context of biomaterials [
2,
3]. Given the three-dimensional nature of our bodies, one of the fundamental challenges in tissue engineering has been the cultivation of cells on 3D scaffolds. Conventional techniques used in scaffold fabrication include solvent casting, particulate leaching, gas foaming, fiber mesh/fiber bonding, phase separation, melt molding, emulsion freeze-drying, solution casting, and freeze-drying, among others. However, these existing methods often have limitations in achieving precise pore sizes [
3,
4,
5], pore shapes, high interconnectivity, and mechanical strength. Such conventional approaches often involve manually seeding cells, which can result in inconsistent cell incorporation, posing issues when precise cell alignment is required based on tissue demands and functions. For instance, achieving consistent alignment of endothelial cells to form blood vessels or arranging osteogenic cells to form mineralized clusters can be challenging. To overcome these limitations, advanced technologies such as 3D printing have been explored in research [
1]. Ultimately, this can lead to the production of matrix scaffolds that can more effectively promote the regeneration of functional tissues.
Three-dimensional bioprinting technology has the potential to address several challenges that have inhibited progress in tissue engineering applications, such as forming complex structures for tissue-specific needs, maintaining mass nutrient delivery in scaffolds, and subsequent tissue processing [
6]. This is important for modulating cell behavior under in vivo conditions by reproducing interactions between cells and the extracellular matrix (ECM) that are not seen in normal two-dimensional cell culture [
7]. Currently, a significant challenge in 3D culture is the inadequate supply of nutrients and oxygen to the core of the structure. To address this issue, research focusing on 3D bioprinting in mesh-like structures is underway. Furthermore, in pursuit of more comprehensive solutions, there have been reported instances of studies involving the integration of capsules that can provide oxygen and nutrients until neovascularization occurs [
8]. Bioprinting exhibits the capacity to replicate intricate tissue architectures, as documented in the literature. Its prowess lies in aligning cells and provoking favorable cellular responses through interactions with biomaterials. The advancement of technology in developing and regenerating complex tissue structures through such approaches is progressing steadily. The most commonly used bioprinting technologies include extrusion, particle fusion, photo-irradiation, and inkjet-based bioprinting [
9,
10]. Among them, extrusion-based bioprinting is one of the most popular technologies due to its compatibility with various bioinks, ease of operation, and relatively low cost [
11,
12]. An important technology for 3D bioprinting is the production of bioinks [
13]. An ideal bioink should support cell and tissue growth (cell adhesion and differentiation), adequate mechanical strength (sol–gel transition and self-standing), and mass metabolism (nutrient and growth factor transfer). And it must have a function similar to the minimal ECM [
14].
The field of 3D bioprinting utilizes various types of bioinks, which can be broadly categorized into synthetic polymers and naturally derived polymers [
15]. Synthetic polymers are man-made materials that offer precise control over their chemical composition and physical properties [
16]. Synthetic polymer bioinks commonly used in 3D bioprinting include polyethylene glycol (PEG) [
17], polycaprolactone (PCL) [
18], poly(lactic-co-glycolic acid) (PLGA) [
19], and polyvinyl alcohol (PVA) [
20]. These polymers offer customizability in terms of mechanical properties, degradation rates, and functional groups for biofunctionalization. They can be precisely engineered to mimic the extracellular matrix (ECM) and provide structural support for cells. However, synthetic polymers may lack inherent bioactivity and cell recognition motifs, requiring additional modifications to enhance interactions between cells and materials [
21]. On the other hand, naturally occurring polymers are derived from biological sources such as plants, animals, or microorganisms. These polymers often possess inherent bioactivity and biocompatibility, making them suitable for tissue engineering and regenerative medicine applications [
22]. Examples of naturally derived polymeric bioinks include alginate [
23], gelatin [
24], collagen [
25], fibroin [
26], chitosan [
27], and hyaluronic acid [
28]. Naturally derived polymers often possess intrinsic bioactivity and cell-adhesive properties that promote cell function and tissue regeneration. They can provide a more biomimetic environment for cells, allowing for better cell attachment, proliferation, and differentiation. However, naturally derived polymers can have limited mechanical strength and stability, requiring additional cross-linking or reinforcement strategies for 3D bioprinting [
29]. Hence, a silk-based hydrogel employing a physical crosslinking approach has emerged, and research on its utilization in 3D bioprinting has been documented.
Naturally derived proteins from silk fibers can be processed into diverse biomaterials, including 3D sponges, films, nanofibers, nanoparticles, and microparticles. Silk is characterized by its non-toxic, non-immunogenic, and biocompatible nature, and it has received approval from the US FDA for use in certain medical devices [
30]. Formulated with optimal rheological attributes, silk-based bioinks are suitable for precise 3D bioprinting, showcasing remarkable mechanical properties that facilitate the layer-by-layer construction of intricate tissue architectures and provide structural support akin to native tissues, mimicking their mechanical behavior. Additionally, silk-based hydrogels promote cell adhesion, proliferation, and differentiation within printed constructs, further enhancing tissue formation. The incorporation of bioactive agents, growth factors, or drugs into silk-based bioinks enables controlled and sustained release, which is advantageous for tissue regeneration and therapeutic applications. The inherent sol–gel property of silk protein’s polymer backbone, achieved through the physical crosslinking of β-sheet structures via hydrophobic interactions without involving chemical or photochemical crosslinking reactions [
31], positions silk as a promising candidate for 3D bioinks due to its biocompatibility and ample mechanical strength for additive manufacturing. The degree of β-sheet physical crosslinking influences gel mechanical properties and in vivo degradation rates. Although numerous studies have explored silk proteins as bioinks for tissue engineering [
32,
33,
34], some still employ chemical treatments, UV exposure, or ethanol [
33] for gel formation. Furthermore, research addressing the tailoring of mechanical properties to meet specific organizational requirements remains limited [
35].
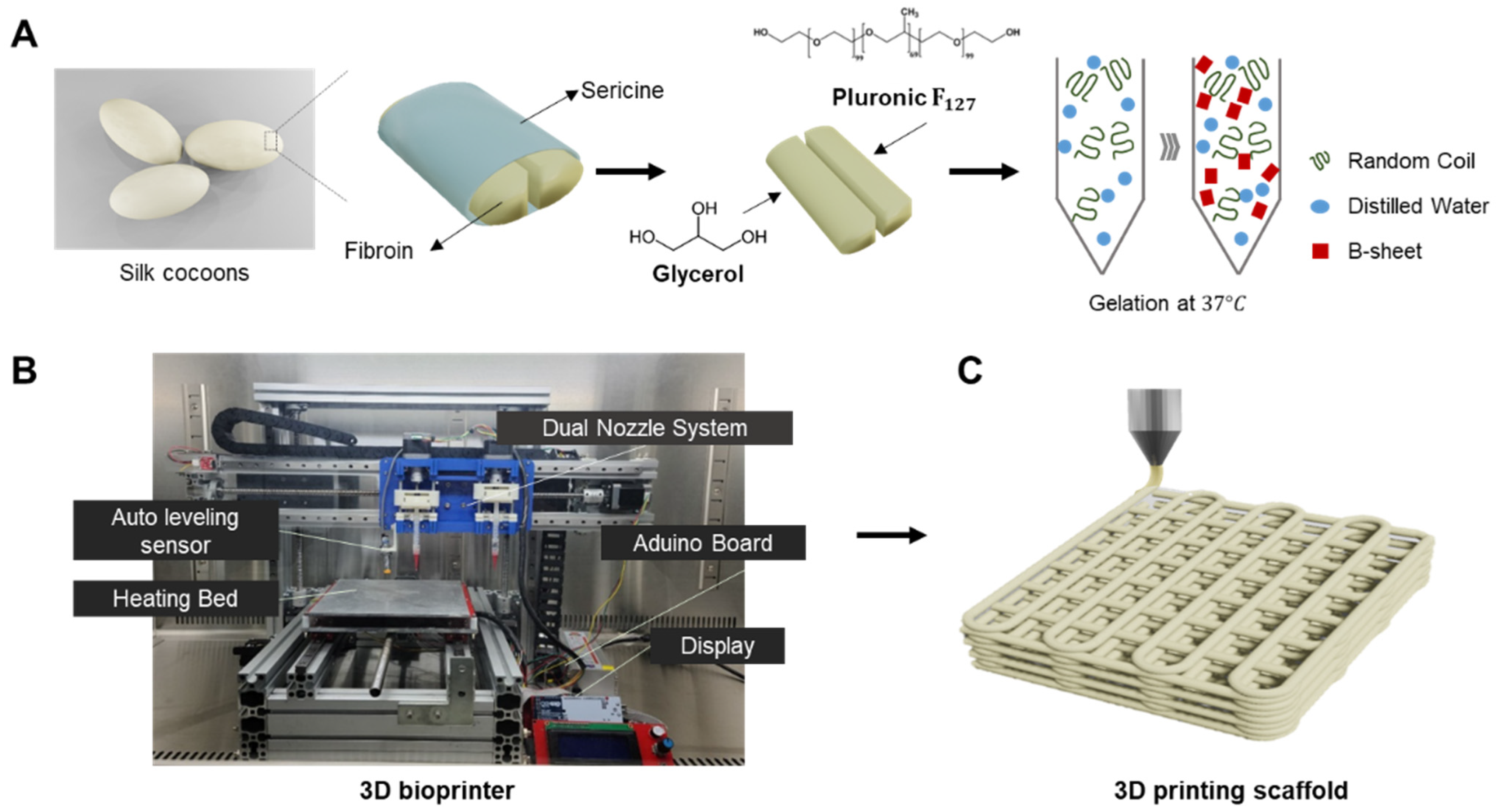
Therefore, we propose to develop a hybrid bioink that exploits property complementarity by combining synthetic polymers and naturally derived polymers. In this study, we developed silk fibroin-based thermosensitive 3D printing bioinks (SF-TPBs) that can control the degree of β-sheet formation using a thermosensitive polymer and glycerol. Prior to this experiment, a screening test was performed to find the optimal mixing ratio of the materials used in the hydrogel. Consistent with our hypothesis, gelation was performed at 37 °C by treatment with F
127, a thermosensitive polymer, and the gelation time decreased as the glycerol concentration increased. Thus, we have developed a bioink that can control gelation time and beta-sheet formation using a thermosensitive polymer and glycerol (
Figure 1A). In addition, the physical properties, printability, and cytotoxicity of the glycerol hydrogels used as bioinks were systematically evaluated. These hybrid bioinks exhibit improved printability, mechanical strength, biocompatibility, and in vivo activity. SF-TPBs can be customized to meet the unique requirements of a desired tissue structure, including mechanical properties, biocompatibility, bioactivity, and desired cellular response [
36].
2. Materials and Methods
2.1. Preparation of Silk-Based Bioinks
Silk fibroin from B. mori cocoons was kindly provided by the Rural Development Administration (Jeonju, Republic of Korea). Briefly, the sliced silk cocoons were subjected to two rounds of degumming using Marseilles soap (0.5% of fiber weight) and sodium carbonate solution (0.3% of fiber weight) at 100 °C for 1 h. Subsequently, they were washed with distilled water to eliminate silk sericin. The silk fibroin was hydrolyzed using 6 M Hydrochloric acid (HCl, 7647-01-0, Duksan Chemical, Ansan, Republic of Korea) for 5 h, with the reaction being neutralized using Sodium hydroxide (NaOH, 1310-73-2, Duksan Chemical, Republic of Korea). An electrodialysis system was employed to extract salt from the silk peptide solution. Upon salt removal, the silk fibroin was transformed into powder form through freeze-drying. The resultant silk peptide had an approximate molecular weight of 3000 Da [
37]. Degummed silk fibroin and Pluronic F
127 (Sigma-Aldrich, St. Louis, MO, USA) were dissolved in 10% (
w/
v) distilled water at 4 °C. Once the silk and F
127 were completely dissolved, glycerol was added at various concentrations ranging from 15% to 55%. The resulting mixture was stored in a chamber at 37 °C until gelation occurred (
Figure 1A).
2.2. Secondary Structure of Silk
The formation of the β-sheet structure was confirmed by Fourier transform infrared (FTIR) spectrophotometry. In this study, attenuated total reflection Fourier transform infrared (ATR-FTIR) measurements were performed using a SPECTRUM TWO Fourier transform infrared spectrometer (PERKIN ELMER, Waltham, MA, USA) equipped with a MIRacleTM attenuated total reflection germanium crystal. When infrared radiation is passed through a sample, the sample absorbs a portion of the radiation and transmits the remainder. The absorbed and transmitted energy can be measured, and the resulting signal is converted into a spectrum specific to the molecular composition of the sample. Background and spectral scans were obtained in the wave number range of 1000 to 2000 cm−1 with a resolution of 4 cm−1, with 32 scans per sample. To investigate the effect of glycerol treatment, peaks before and after gelation were measured, and any differences between the spectra were analyzed. This analysis provided insight into the changes in molecular structure, particularly the formation of β-sheet structures characteristic of silk fibroin.
2.3. Rheological Properties
Shear thinning is a rheological behavior exhibited by non-Newtonian fluids in which the viscosity of the fluid decreases as the shear rate increases. This behavior is often described as being similar to pseudoplasticity and is characterized by a lack of time dependence, such as thixotropy. In hydrogel printing, the shear thinning property plays a critical role in achieving accurate printing results. The hydrogel must maintain its fluidity while passing through the printing nozzle and then quickly recover its shape after extrusion. To quantitatively assess the gel and solution states of the hydrogel, rheological properties were measured. Rheological analysis of the bioinks was performed using an Advanced Rheometer AR 1500ex (TA Instruments, West Sussex, UK) equipped with a Peltier plate. The dynamic storage modulus (G’) and loss modulus (G”) of the materials were measured in oscillatory mode at a temperature of 37 °C. These measurements provide valuable information about the viscoelastic behavior of the material and its ability to undergo shear thinning and subsequent recovery.
2.4. Three-Dimensional Printing with a Silk-Based Thermosensitive Hydrogel
The 3D printer setup included essential components such as a controller board, stepper motor driver, LCD controller, and sensors. These electronic devices communicated with various sensors and executed commands to control the actuators. Printer operation was performed using the Repeater Firmware Configuration Tool (version 1.0.3), which allowed easy adjustment of parameters such as print speed and ink extrusion to achieve desired settings. The design of all 3D-printed structures was created using the Inventor program and saved as stereolithography (STL) files. These files were then converted to G-code files using the Cura 15.04.6 slicing program, which generated the necessary coordinates and paths for the 3D printer nozzles during printing.
To evaluate the physical properties of silk-based inks with different glycerol concentrations (15–55%) for 3D printing, we fabricated 3D structures of different sizes. The printed structures were used to compare the actual dimensions, including height and width, with the intended design dimensions. The printing process involved preparing a mixture of silk and F127 dissolved in a 10% solution, which was loaded into a syringe. Each concentration of glycerol (15–55%) was added to a syringe of equal volume and mixed through a syringe mixing tube. The resulting hydrogel mixture was then allowed to gel and stored in a chamber at 37 °C. After gelation, the hydrogel-filled syringe was attached to the 3D printer head. Prior to the extrusion of the silk-based mixture, the heated bed temperature was set at 37 °C. In the context of printing, a 22-gauge needle, corresponding to an inner diameter range of 420 μm, was utilized. The printed layer demonstrated a height of 200 μm. Tailored to the unique attributes of each silk-based mixture, the dispensing feed rate was established at 100 mm/min, and the printing speed was maintained at 5 mm/s for inks containing 15 to 25% glycerol. In the case of inks featuring 35% glycerol, the dispensing feed rate was adjusted to 120 mm/min, and the printing speed was set to 7 mm/s. Similarly, for inks comprising 45–55% glycerol, the dispensing feed rate and printing speed were configured at 105 mm/min and 5 mm/s, respectively.
2.5. Cell Viability Assay
MC3T3-E1 cells were cultured in α-MEM supplemented with 10% fetal bovine serum (FBS, Corning Cellgro, Corning, NY, USA) and 1% penicillin-streptomycin (Fisher Scientific, Waltham, MA, USA) under a 5% CO2 atmosphere at 37 °C. In addition, we assessed cell viability using LIVE/DEAD assays performed on silk-based hydrogels. The LIVE/DEAD cytotoxicity test kit provides a two-color fluorescence-based assessment of cell viability by simultaneously determining the proportion of live and dead cells. This method relies on two probes: cellular esterase activity and plasma membrane integrity. It provides an alternative to traditional methods such as trypan blue exclusion and 51Cr release for assessing cell viability and cytotoxicity. Cell viability is determined based on the ubiquitous presence of esterase activity in living cells, which catalyzes the enzymatic conversion of the virtually non-fluorescent cell-permeant calcein AM to a fluorescent product. This fluorescence-based method is faster, less expensive, safer, and more sensitive in detecting cytotoxic events than other approaches. Live cells retain the polyanionic dye calcein and exhibit a strong and uniform green fluorescence. Conversely, dead cells with compromised membranes allow the entry of EthD-1, a dye that undergoes a 40-fold increase in fluorescence upon binding to nucleic acids. This results in bright red fluorescence in dead cells because EthD-1 is unable to penetrate the intact plasma membrane of living cells. For 3D bioprinting of silk-based hydrogels, MC3T3-E1 cells were incorporated at a concentration of 7 × 106 cells. Cells were harvested at 90% confluence and resuspended in supplemented α-MEM for encapsulation. Following the bioprinting of the MC3T3-E1-incorporated silk-based hydrogel onto a tissue culture dish, a 3 mL volume of α-MEM was introduced for subsequent cultivation. After 1, 3, and 7 days of culture, 100–150 µL of optimized concentrations of LIVE/DEAD test reagents were added to the silk hydrogels to ensure complete submersion of all cells in the solution. After incubation for 1 h at room temperature, labeled cells were visualized using a fluorescence microscope. The viability was calculated as a percentage by dividing the number of live cells by the total number of cells after counting cells using image J in the acquired image.
2.6. Releasing Test and Degradation Behavior
One of the important functions of inks used for bioprinting is the sufficient diffusion of oxygen, nutrients, and waste during culture. Therefore, we analyzed the release properties of silk-based hydrogels using bovine serum albumin (BSA) and bicinchonic acid (BCA). The BCA quantification method utilizes the property that proteins can reduce copper ions (Cu 2+, Cu 1+). Copper ions reduced by the protein react with the BCA solution to form purple compounds, and their absorbance is measured. Briefly, 1 mL of the silk-based mixture was combined with a 0.2% BSA solution, followed by dispensing the resulting mixture into Eppendorf tubes. The tubes were then incubated at 37 °C to facilitate gelation. Upon the transition of the mixture from a solution to a gel state, PBS was applied to it. The sample was subsequently placed in a controlled environment at 37 °C, and the absorbance was measured at regular intervals. The measuring solution was prepared by mixing a 50:1 mixture of PBS and BCA with PBS poured 1:1 on the sample and incubating for 1 h.
The degradation properties of bioinks offer crucial insights into their transient in vivo degradation and modifications. The assessment of degradation properties aids in comprehending the temporal degradation of inks within the environment and contributes to the evaluation of the sustainability and potential environmental consequences of products based on bioink. Specific bioinks confer distinctive functionalities or fulfill specific roles. A comprehensive comprehension of degradation characteristics enables the anticipation and management of alterations in ink performance over time. Deterioration measurements were conducted by transferring 200 μL of the silk-based mixture into Eppendorf tubes and incubating them at 37 °C for one day. Following this, 200 μL of PBS at 37 °C was added to each gelated sample, and the weight of the silk-based composite was determined by gently removing the PBS on the specified day. The measured weight was then calculated based on the dry weight of the lyophilized silk composite. This procedure was replicated with five samples over a span of approximately three months, and the measured weights were then arithmetically averaged.
4. Discussion
Selecting an appropriate material is a critical step in using 3D printing technology to develop tissue regeneration constructs. The selected material must have several key characteristics. First, it should be printable, meaning it should have suitable viscoelastic properties that allow extrusion through a small nozzle and subsequent recovery without compromising its structural integrity. In addition, the material should be biocompatible, ensuring that it does not cause damage or adverse immune responses when in contact with living tissue. It is also essential that the material has the ability to encapsulate and support viable cells throughout the printing process. In the field of tissue engineering, the development of a hydrogel material that can be 3D-bioprinted to create scaffolds with uniform cell distribution has significant potential to promote improved tissue healing compared to conventional scaffolds. Silk fibroin (SF) is emerging as an ideal candidate for 3D bioprinting due to its unique properties. SF offers a controllable gelation process that allows precise control over scaffold formation. In addition, SF has a natural hydrogel structure that closely resembles the native extracellular matrix found in living tissues. By leveraging the controllable gelation and natural hydrogel properties of SF, it is possible to create 3D-printed constructs with tailored architecture and mechanical properties that provide an environment conducive to cell proliferation, differentiation, and tissue regeneration.
The gelation mechanism of silk fibroin (SF) has been extensively investigated, and it was found that SF, unlike other bioinks derived from natural materials, could undergo gelation through the formation of beta sheets. Remarkably, this gelation process did not require the use of chemical cross-linking methods. Silk protein is primarily composed of amino acids such as alanine (Ala), glycine (Gly), serine (Ser), and others. These amino acids form hydrogen bonds within the silk molecule, which is a crucial factor in determining the strength and stability of silk. Hydrogen bonds can take various forms within the silk molecule, and among them, the -OH (hydroxyl) groups play a significant role in hydrogen bond formation. Glycerol serves to evenly disperse silk within a hydrogel and plays a significant role in the hydrogen bond formation of silk-based hydrogels through its hydroxyl groups. These hydroxyl groups within the silk molecule induce interactions between protein chains by forming hydrogen bonds. Such interactions can induce gel formation. Hydrogen bonding between silk protein chains leads to the creation of a grid-like 3D structure, ultimately forming the solid structure of the gel. Leveraging the OH groups within silk molecules to form hydrogen bonds and induce gelation can lead to the development of materials that mimic physiological conditions, enhancing interactions with living systems. The rate of gelation could be controlled by adjusting the concentration of glycerol in the SF solution. By modulating the glycerol concentration, the rate of beta-sheet formation and, consequently, the gelation process could be controlled. Furthermore, when evaluating the rheological properties, which are crucial for determining the printability of hydrogels during the 3D printing process, the SF-based hydrogels exhibited shear-thinning behavior [
38,
39]. This means that the viscosity of the hydrogel decreases with increasing shear rates. As the hydrogel is extruded through the print nozzle, the shear rate increases, resulting in a decrease in viscosity. This shear-thinning behavior allows the hydrogel to flow more easily, facilitating the deposition of continuous strands during the printing process. Overall, the ability to control the gelation rate and shear thinning behavior of SF-based hydrogels through the incorporation of glycerol provides valuable control over the printability and rheological properties of the bioink. This knowledge is essential for optimizing the 3D printing process and achieving the desired structural fidelity and mechanical properties in the printed constructs.
The cylindrical scaffold design was chosen as a model system to determine the optimal formulation for achieving high morphological fidelity in 3D printing. These optimal formulation parameters can be used to print larger and more complex designs, which will be investigated in future studies. In our laboratory, we proposed to develop hydrogels optimized for 3D printing by utilizing SF-based hydrogel formulations with well-defined gelation times suitable for surgical applications. To achieve this, the glycerol content in the base formulation was modified to create an ideal formulation for 3D printing, taking into account our understanding of the gelation mechanism of SF. The gelled SF-based bioink showed excellent printability, as indicated by the storage coefficient G’, which was higher than the loss coefficient G’’ in all areas. However, the group treated with 15% glycerol exhibited prolonged gelation time and insufficient hydrogel viscosity, resulting in lateral spreading beyond the designed cylinder diameter and poor printing precision. On the other hand, the group treated with 55% glycerol showed a significantly reduced gelation time. However, the hydrogel exhibited excessive viscosity, resulting in excessive extrusion of the hydrogel due to residual shear forces, which compromised the quality of the printed scaffold. The range of 25–45% glycerol showed reasonable printing accuracy, with good results also observed in terms of cytotoxicity. Therefore, the optimal formulation is considered to be a bio-ink containing 25–45% glycerol. Overall, the results highlight the importance of optimizing the glycerol concentration in SF-based hydrogel formulations to achieve suitable gelation times and viscosities for 3D printing. The bioink formulation with 25–45% glycerol is considered optimal, offering good printability and compatibility, making it a promising candidate for bioprinting applications.
The MC3T3-E1 cells were successfully incorporated into the optimal SF-TPB hydrogel formulation and 3D-printed as scaffolds with high morphological fidelity. The printability of the system was not affected by cell culture medium or cell suspension. The encapsulation of cells within the hydrogel matrix offers advantages for tissue healing by allowing uniform cell infiltration and avoiding post-fabrication seeding processes that can hinder tissue regeneration. The MC3T3 cell line has the potential to induce uniform tissue formation throughout the encapsulated scaffold. To assess the viability and proliferation of the encapsulated cells, immediate post-print viability was evaluated after incubation in a calcium bath. Proliferation of the encapsulated cells was confirmed in the optimal SF-TPBs formulation, indicating that the printing process was not harmful to the cells and that the hydrogel formulation provided effective cell protection. However, the group treated with 55% glycerol showed low cell proliferation, which is consistent with literature reports suggesting that excessive glycerol can lead to cell death. However, as cell viability remains relatively high in other groups, the surviving cells retain the potential for recovery and proliferation through further in vitro culture, which will be investigated in future studies. In conclusion, the incorporation of appropriate glycerol concentrations not only increases the viscosity of the hydrogel formulation, but also indirectly improves cell viability after printing. The optimal SF-TPB formulation demonstrates the ability to support cell proliferation within the 3D-printed scaffolds, indicating its potential to promote tissue regeneration. This highlights the suitability of SF as a material for 3D bioprinting applications in tissue engineering and underscores its potential to advance the field of regenerative medicine.