Nanocomposites Based on Pyrolyzed Polyacrylonitrile Doped with FeCoCr/C Transition Metal Alloy Nanoparticles: Synthesis, Structure, and Electromagnetic Properties
Abstract
:1. Introduction
2. Materials and Methods
3. Results and Discussion
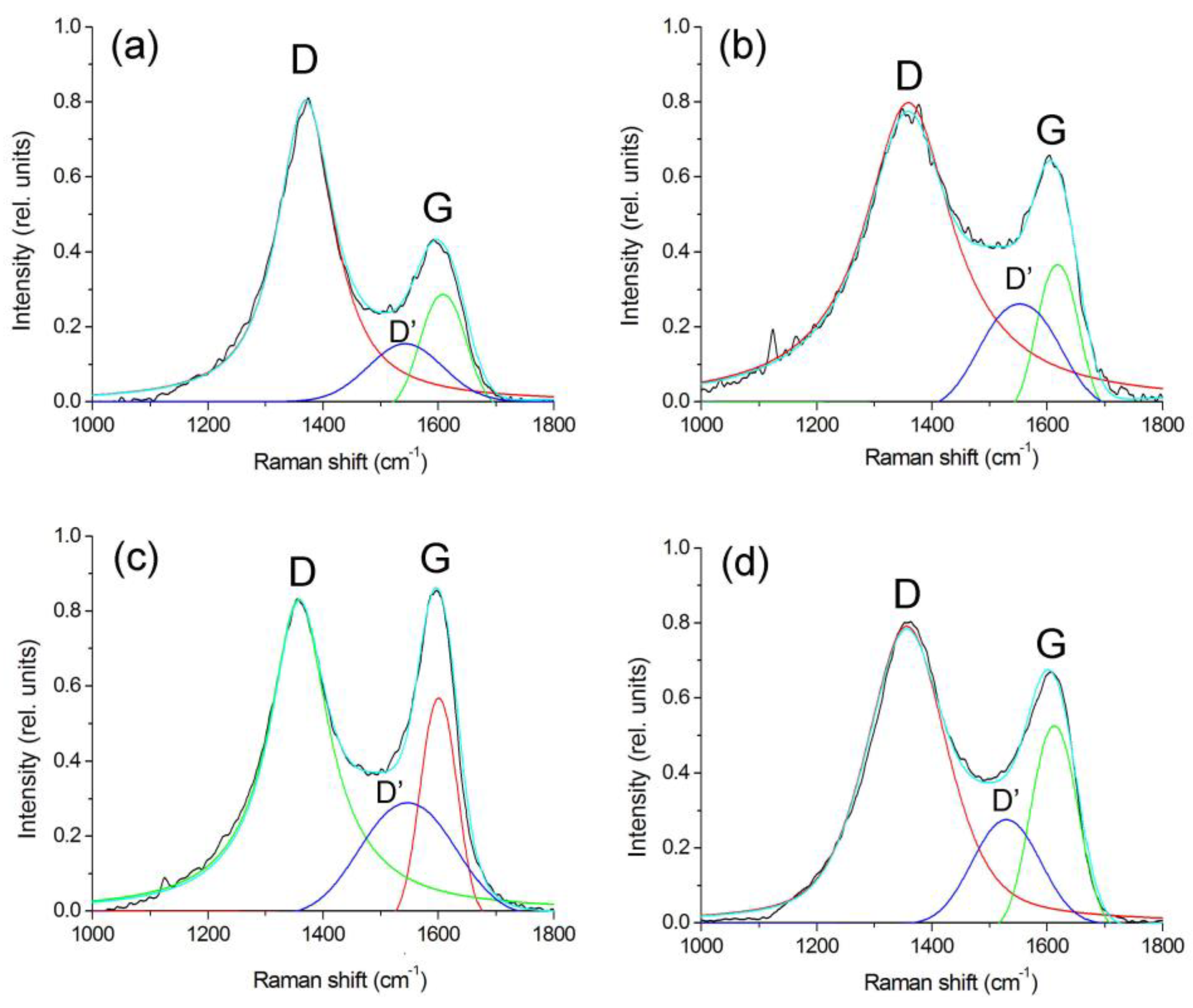
3.1. Structure and Composition of Nanocomposites
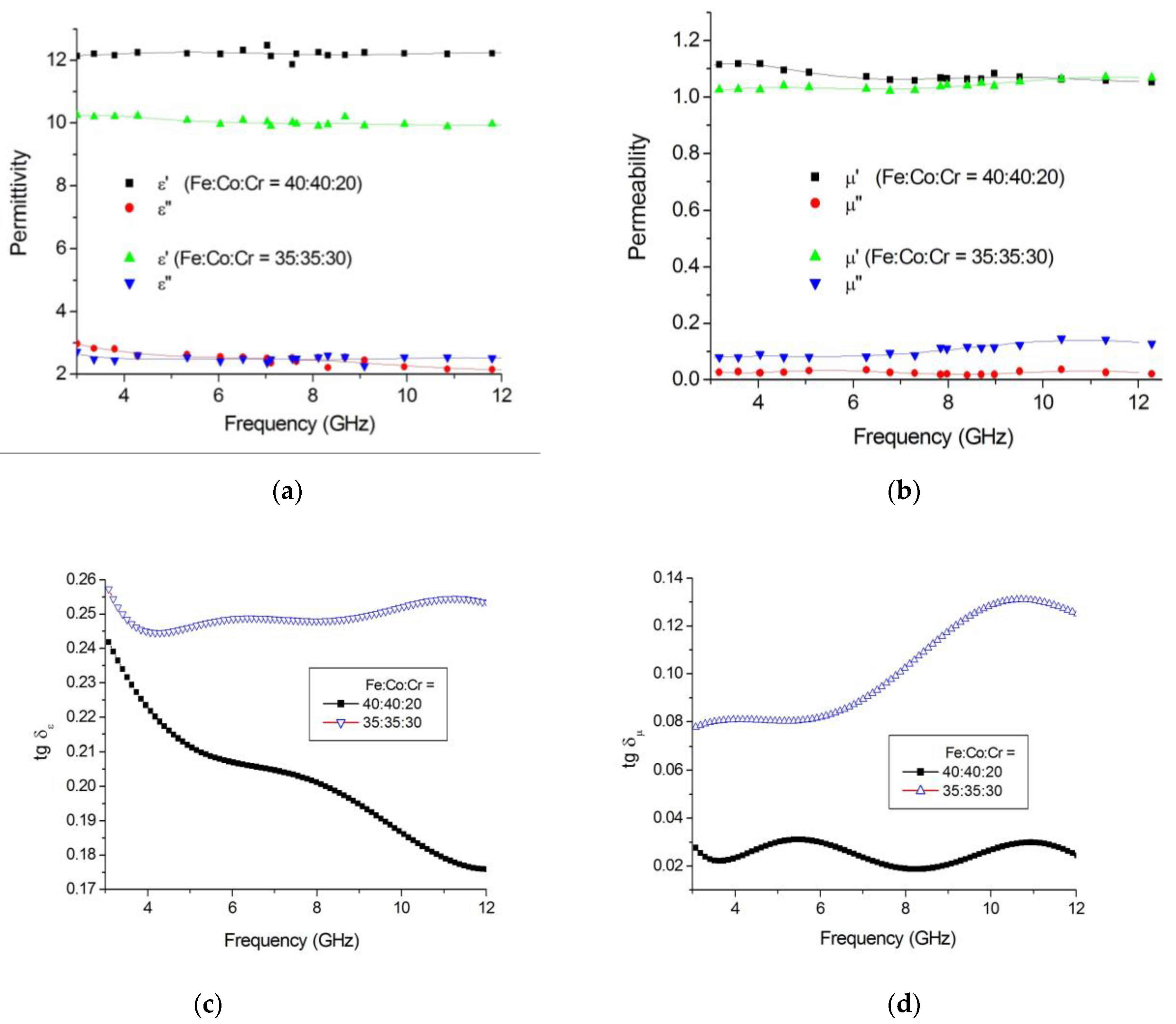
3.2. Magnetic Properties of Nanocomposites
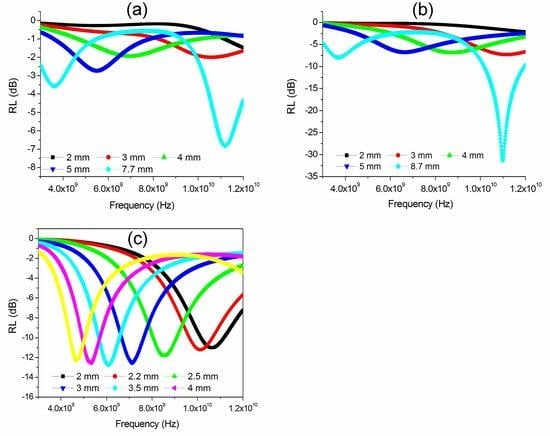
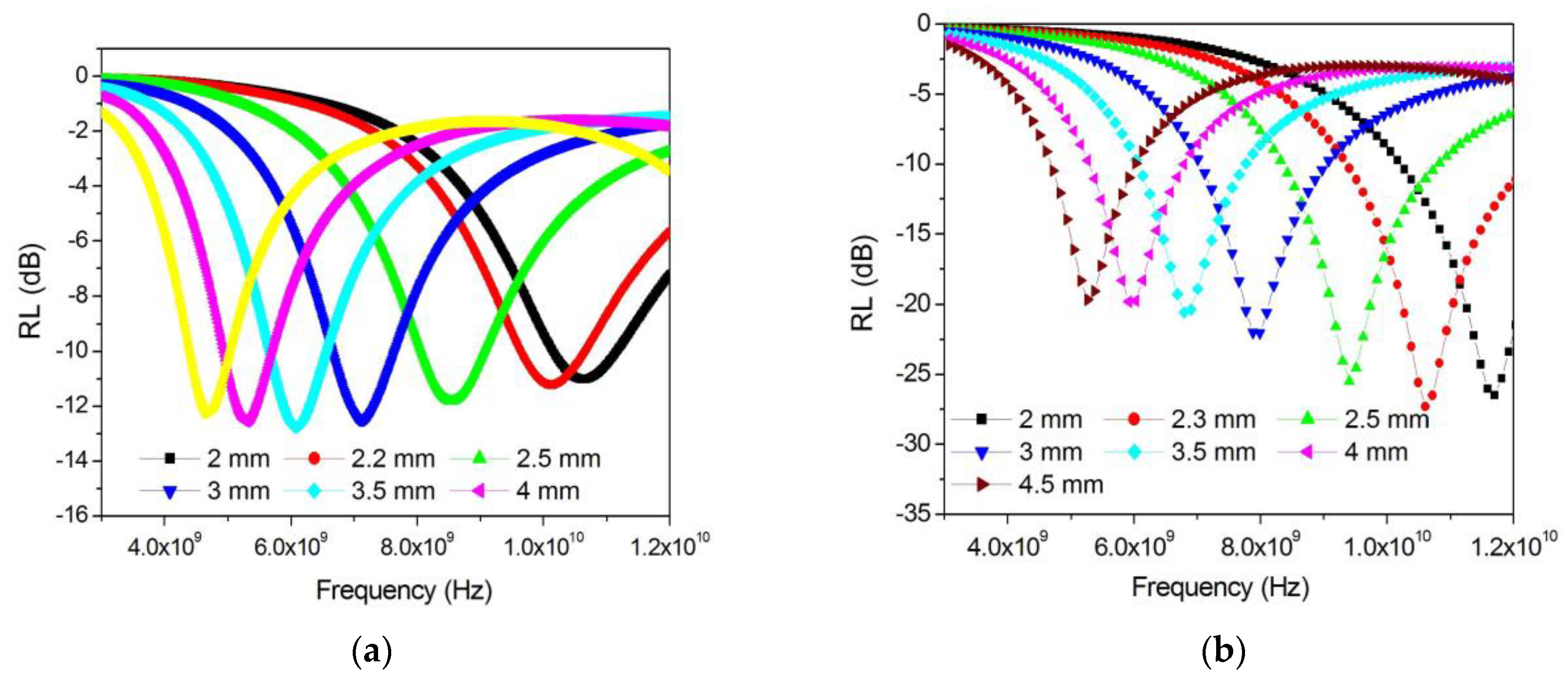
3.3. Radio-Absorbing Properties of FeCoCr/C Nanocomposites
3.4. Radio-Absorbing Properties of FeCoCr/C Nanocomposites
- -
- The method is versatile. As a wide range of polymers and metal salts can be used, the main condition is the possibility of joint dissolution in the same solvent.
- -
- The method uses simple equipment. In fact, for complex equipment, only an inert atmosphere furnace is needed.
- -
- The purity of reagents, which are chemically pure.
- -
- The simultaneous formation of nanoparticles and a matrix that stabilizes them but does not prevent the use of materials, for example, in catalysis.
- -
- IR heating for polymers is more efficient than resistive convective heating.
- -
- IR heating has a minimum of inertia as quick heating and cooling can be provided.
- -
- The impossibility of obtaining monodisperse nanoparticles: there will always be a normal-logarithmic distribution.
- -
- The furnace used for IR heating is structurally more complicated, due to the need to use a quartz reactor.
- -
- It is impossible to obtain metal nanoparticles that are strongly oxidized in contact with the air but are reduced at temperatures above 1200 °C.
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Conflicts of Interest
References
- Qiang, R.; Du, Y.C.; Wang, Y.; Wang, N.; Tian, C.H.; Ma, J.; Xu, P.; Han, X.J. Rational design of yolk-shell C@C microspheres for the effective enhancement in microwave absorption. Carbon 2016, 98, 599–606. [Google Scholar] [CrossRef]
- Zhang, Y.; Huang, Y.; Chen, H.H.; Huang, Z.Y.; Yang, Y.; Xiao, P.S.; Zhou, Y.; Chen, Y.S. Composition and structure control of ultralight graphene foam for high-performance microwave absorption. Carbon 2016, 105, 438–447. [Google Scholar] [CrossRef]
- Zhu, H.P.; Zhang, H.Y.; Chen, Y.M.; Li, Z.H.; Zhang, D.F.; Zeng, G.X.; Huang, Y.X.; Wang, W.G.; Wu, Q.B.; Zhi, C.Y. The electromagnetic property and microwave absorption of wormhole-like mesoporous carbons with different surface areas. J. Mater. Sci. 2016, 51, 9723–9731. [Google Scholar] [CrossRef]
- Lu, M.M.; Cao, M.S.; Chen, Y.H.; Cao, W.Q.; Liu, J.; Shi, H.L.; Zhang, D.Q.; Wang, W.Z.; Yuan, J. Multiscale assembly of grapelike ferroferric oxide and carbon nanotubes: A smart absorber prototype varying temperature to tune intensities. ACS Appl. Mater. Interfaces 2015, 7, 19408–19415. [Google Scholar] [CrossRef]
- Xiang, J.; Li, J.L.; Zhang, X.H.; Ye, Q.; Xu, J.H.; Shen, X.Q. Magnetic carbon nanofibers containing uniformly dispersed Fe/Co/Ni nanoparticles as stable and high-performance electromagnetic wave absorbers. J. Mater. Chem. A 2014, 2, 16905–16914. [Google Scholar] [CrossRef]
- Lv, H.L.; Ji, G.B.; Liu, W.; Zhang, H.Q.; Du, Y.W. Achieving hierarchical hollow carbon@Fe@Fe304 nanospheres with superior microwave absorption properties and lightweight features. J. Mater. Chem. C 2015, 3, 10232–10241. [Google Scholar] [CrossRef]
- Wang, L.N.; Xia, X.L.; Li, Y.F.; Yang, F.; Zhang, L.Q.; Liu, L.P.; Ren, X.; Yang, H.T. Synthesis and microwave absorption property of flexible magnetic film based on graphene oxide/carbon nanotubes and Fe304 nanoparticles. J. Mater. Chem. A 2014, 2, 14940–14946. [Google Scholar] [CrossRef]
- Yuan, K.P.; Che, R.C.; Cao, Q.; Sun, Z.K.; Yue, Q.; Deng, Y.H. Designed fabrication and characterization of three-dimensionally ordered arrays of core-shell magnetic mesoporous carbon microspheres. ACS Appl. Mater. Interfaces 2015, 7, 5312–5319. [Google Scholar] [CrossRef]
- Li, G.M.; Wang, L.C.; Li, W.X.; Xu, Y. Fe-, Co-, and Ni- loaded porous activated carbon balls as lightweight microwave absorbents. ChemPhysChem 2015, 16, 3458–3467. [Google Scholar] [CrossRef]
- He, P.; Hou, Z.L.; Zhang, K.L.; Li, J.; Yin, K.; Feng, S.; Bi, S. Lightweight ferroferric oxide nanotubes with natural resonance property and design for broadband microwave absorption. J. Mater. Sci. 2017, 52, 8258–8267. [Google Scholar] [CrossRef]
- Lv, H.; Zhihong, Y.; Hongge, P. Electromagnetic absorption materials: Current progress and new frontiers. Prog. Mater. Sci. 2022, 127, 100946. [Google Scholar] [CrossRef]
- Feng, W.; Liu, Y.; Bi, Y.; Su, X.; Lu, C.; Han, X.; Ma, Y.; Feng, C.; Ma, M. Recent advancement of magnetic MOF composites in microwave absorption. Synth. Met. 2023, 294, 117307. [Google Scholar] [CrossRef]
- Wu, D.; Wang, Y.; Deng, S.; Lan, D.; Xiang, Z.; He, Q. Heterostructured CoFe@ N-doped carbon porous polyhedron for efficient microwave absorption. Nano Res. 2023, 16, 1859–1868. [Google Scholar] [CrossRef]
- Gai, L.; Zhao, H.; Wang, F.; Wang, P.; Liu, Y.; Han, X.; Du, Y. Advances in core—Shell engineering of carbon-based composites for electromagnetic wave absorption. Nano Res. 2022, 15, 9410–9419. [Google Scholar] [CrossRef]
- Ma, M.; Liao, Z.; Su, X.; Zheng, Q.; Liu, Y.; Wang, Y.; Ma, Y.; Wan, F. Magnetic CoNi alloy particles embedded N-doped carbon fibers with polypyrrole for excellent electromagnetic wave absorption. J. Colloid Interface Sci. 2022, 608, 2203–2212. [Google Scholar] [CrossRef]
- Wu, Y.; Peng, K.; Man, Z.; Zang, R.; Li, P.; Liu, S.; Wang, S.; Liu, P.; Li, P.; Cui, Y. A hierarchically three-dimensional CoNi/N-doped porous carbon nanosheets with high performance of electromagnetic wave absorption. Carbon 2022, 188, 503–512. [Google Scholar] [CrossRef]
- Ajia, S.; Asa, H.; Toyoda, Y.; Sato, M.; Matsuura, M.; Tezuka, N.; Sugimoto, S. Development of an alternative approach for electromagnetic wave absorbers using Fe–Cr–Co alloy powders. J. Alloys Compd. 2022, 903, 163920. [Google Scholar] [CrossRef]
- Sun, L.; Jia, Z.; Xu, S.; Ling, M.; Hu, D.; Liu, X.; Zhang, C.; Wu, G. Synthesis of NiCo2-0.5 xCr2O3@ C nanoparticles based on hydroxide with the heterogeneous interface for excellent electromagnetic wave absorption properties. Compos. Commun. 2022, 29, 100993. [Google Scholar] [CrossRef]
- Dai, B.; Ma, Y.; Dong, F.; Yu, J.; Ma, M.; Thabet, H.K.; El-Bahy, S.M.; Ibrahim, M.M.; Huang, M.; Seok, I.; et al. Overview of MXene and conducting polymer matrix composites for electromagnetic wave absorption. Adv. Compos. Hybrid Mater. 2022, 5, 704–754. [Google Scholar] [CrossRef]
- Qiang, R.; Du, Y.C.; Zhao, H.T.; Wang, Y.; Tian, C.H.; Li, Z.G.; Han, X.J.; Xu, P. Metal organic framework-derived Fe/C nanocubes toward efficient microwave absorption. J. Mater. Chem. A 2015, 3, 13426–13434. [Google Scholar] [CrossRef]
- Wang, J.C.; Zhou, H.; Zhuang, J.D.; Liu, Q. Magnetic gamma-Fe203, Fe304, and Fe nanoparticles confined within ordered mesoporous carbons as efficient microwave absorbers. Phys. Chem. Chem. Phys. 2015, 17, 3802–3812. [Google Scholar] [CrossRef] [PubMed]
- Wu, G.L.; Cheng, Y.H.; Ren, Y.Y.; Wang, Y.Q.; Wang, Z.D.; Wu, H.J. Synthesis and characterization of c-Fe203@C nanorod-carbon sphere composite and its application as microwave absorbing material. J. Alloys Compd. 2015, 652, 346–350. [Google Scholar] [CrossRef]
- Yang, H.; Wang, A.; Feng, X.; Dong, H.; Zhuang, T.; Sui, J.; Zhao, S.; Sun, C. PPyNT/NR/NBR Composites with Excellent Microwave Absorbing Performance in X-Band. Polymers 2023, 15, 1866. [Google Scholar] [CrossRef]
- Liu, P.; Ming, V.; Ng, H.; Yao, Z.; Zhou, J.; Lei, Y.; Yang, Z.; Lv, H.; Bing, L. Facile synthesis and hierarchical assembly of flowerlike NiO structures with enhanced dielectric and microwave absorption properties. ACS Appl. Mater. Interfaces 2017, 9, 16404–16416. [Google Scholar] [CrossRef]
- Berlin, A.; Geyderikh, M.; Davydov, B.; Kargin, V.; Karpacheva, G.; Krenzel, B.; Khukhareva, G. Chemistry of Polyconjoint Systems; Chemistry: Moscow, Russia, 1972; 272p. [Google Scholar]
- Liu, M.; Wu, L.; Fan, B.; Tong, G.; Chen, D.B.; Wu, W. Governing the Ni content and size of 2D layered C/Ni nanoparticle composites for enhanced electromagnetic wave absorption. Appl. Surf. Sci. 2022, 571, 151273. [Google Scholar] [CrossRef]
- Ren, X.; Gao, Z.; Wu, G. Tunable nano-effect of Cu clusters derived from MOF-on-MOF hybrids for electromagnetic wave absorption. Compos. Commun. 2022, 35, 101292. [Google Scholar] [CrossRef]
- Ghorpade, R.V.; Cho, D.W.; Hong, S.C. Effect of controlled tacticity of polyacrylonitrile (co)polymers on their thermal oxidative stabilization behaviors and the properties of resulting carbon films. Carbon 2017, 121, 502–511. [Google Scholar] [CrossRef]
- Li, G.X.; Guo, Y.X.; Sun, X.; Wang, T.; Zhou, J.H.; He, J.P. Synthesis and microwave absorbing properties of FeNi alloy incorporated ordered mesoporous carbon-silica nanocomposite. J. Phys. Chem. Solids 2012, 73, 1268–1273. [Google Scholar] [CrossRef]
- Han, Z.; Li, D.; Wang, H.; Liu, X.G.; Geng, D.Y.; Zhang, Z.D. Broadband electromagnetic-wave absorption by FeCo/C nanocapsules. Appl. Phys. Lett. 2009, 95, 023114. [Google Scholar] [CrossRef]
- Kodama, D.; Shinoda, K.; Kasuya, R.; Doi, M.; Tohji, K.; Jeyadevan, B. Potential of sub-micron-sized Fe-Co particles for antenna applications. J. Appl. Phys. 2012, 111, 07A331. [Google Scholar] [CrossRef]
- Yu, Z.X.; Zhang, N.; Yao, Z.P.; Han, X.J.; Jiang, Z.H. Synthesis of hierarchical dendritic micro-nano structure Cox-Fel-x alloy with tunable electromagnetic absorption performance. J. Mater. Chem. A 2013, 1, 12462–12470. [Google Scholar] [CrossRef]
- Wang, X.Q.; Liang, C.D.; Dai, S. Facile synthesis of ordered mesoporous carbons with high thermal stability by self-assembly of resorcinol-formaldehyde and block copolymers under highly acidic conditions. Langmiur 2008, 24, 7500–7505. [Google Scholar] [CrossRef]
- Karpenkov, D.Y.u.; Kozitov, L.V.; Skokov, K.P.; Karpenkov, A.Y.u.; Popkova, A.V.; Gutfleisch, O. Infrared heating mediated synthesis and characterization of FeCo/C nanocomposites. J. Magn. Magn. Mater. 2017, 429, 94–101. [Google Scholar] [CrossRef]
- Kozitov, L.V.; Muratov, D.G.; Kostishin, V.G.; Suslyaev, V.I.; Korovin, E.Y.U.; Popkova, A.V. FeCo/C Nanocomposites: Synthesis, Magnetic and Electromagnetic Properties. Russ. J. Inorg. Chem. 2017, 62, 1499–1507. [Google Scholar] [CrossRef]
- Yakushko, E.V.; Kozhitov, L.V.; Muratov, D.G.; Kostishin, V.G. Synthesis and magnetic properties of NiCo/C nanocomposites. J. Inorg. Chem. 2016, 61, 1653–1657. [Google Scholar] [CrossRef]
- Muratov, D.G.; Kozhitov, L.V.; Emelyanov, S.G.; Vasilyev, A.V.; Popkova, A.V. The syntesis of nanoparticles of ternary alloys Fe-Co-Ni encapsulated in the carbon matrix of nanocomposites Fe-Co-Ni/C. J. Nano-Electron. Phys. 2016, 3, 03037. [Google Scholar]
- Patterson, A. The Scherrer Formula for X-Ray Particle Size Determination. Phys. Rev. J. 1939, 56, 978–982. [Google Scholar] [CrossRef]
- Bannykh, O.; Budberg, P.; Alisova, S. Diagrams of the State of Double and Multicomponent Systems Based on Iron; Metallurgy: Moscow, Russia, 1986; 440p. [Google Scholar]
- Ferrari, A.; Robertson, J. Interpretation of Raman spectra of disordered and amorphous carbon. Phys. Rev. B Condens. Matter Mater. Phys. 2000, 61, 14095–14107. [Google Scholar] [CrossRef]
- Ferrari, A.C. Raman spectroscopy of graphene and graphite: Disorder, electron-phonon coupling, doping and nonadiabatic effects. Solid State Commun. 2007, 143, 47–57. [Google Scholar] [CrossRef]
- Tunistra, F.; Koenig, J. Raman spectrum of graphite. J. Chem. Phys. 1970, 53, 1126–1130. [Google Scholar] [CrossRef]
- Simionescu, O.G.; Popa, R.C.; Avram, A.; Dinescu, G. Thin films of nanocrystalline graphene/graphite: An overview of synthesis and applications. Plasma Process. Polym. 2020, 17, 1–22. [Google Scholar] [CrossRef]
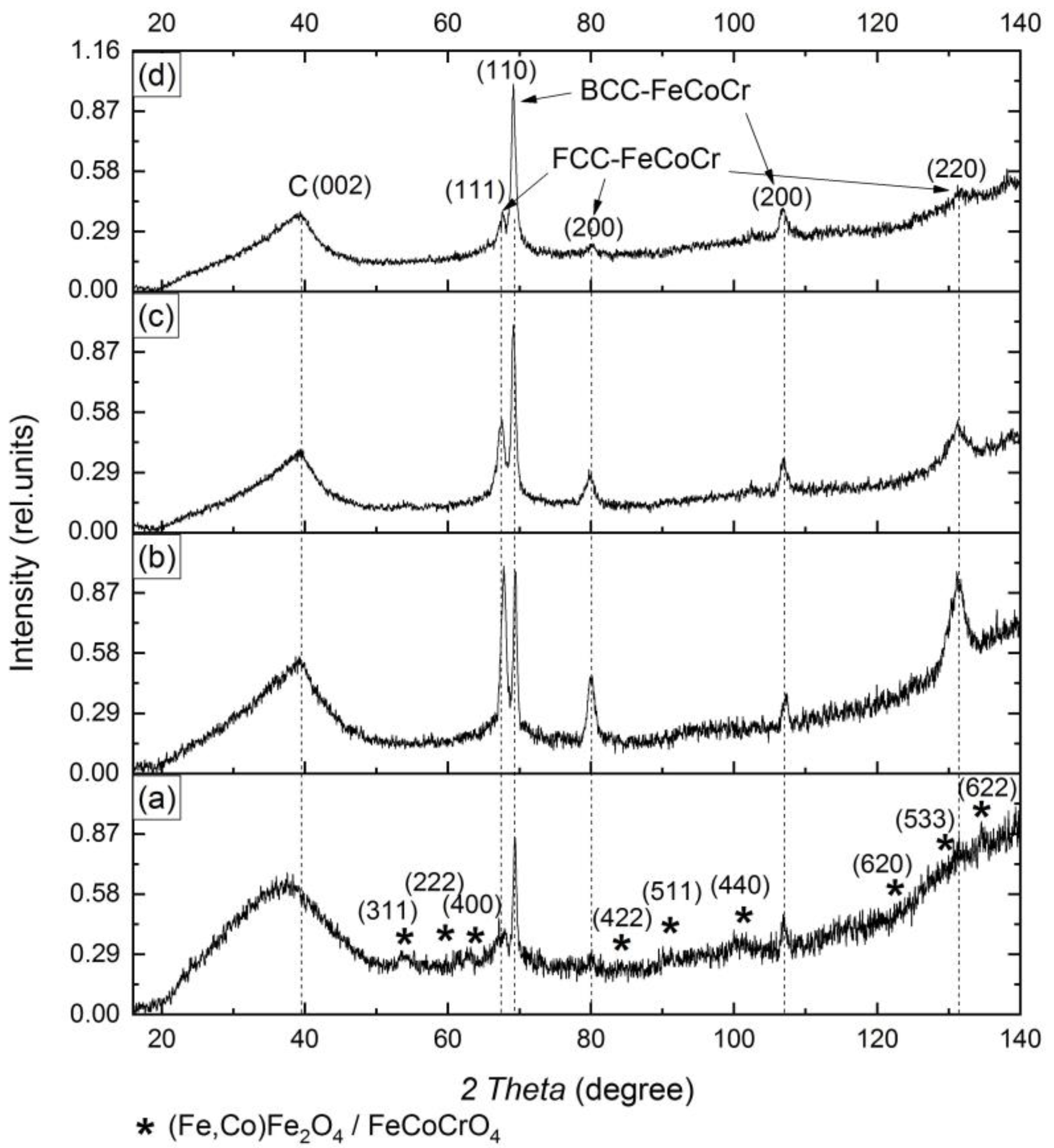


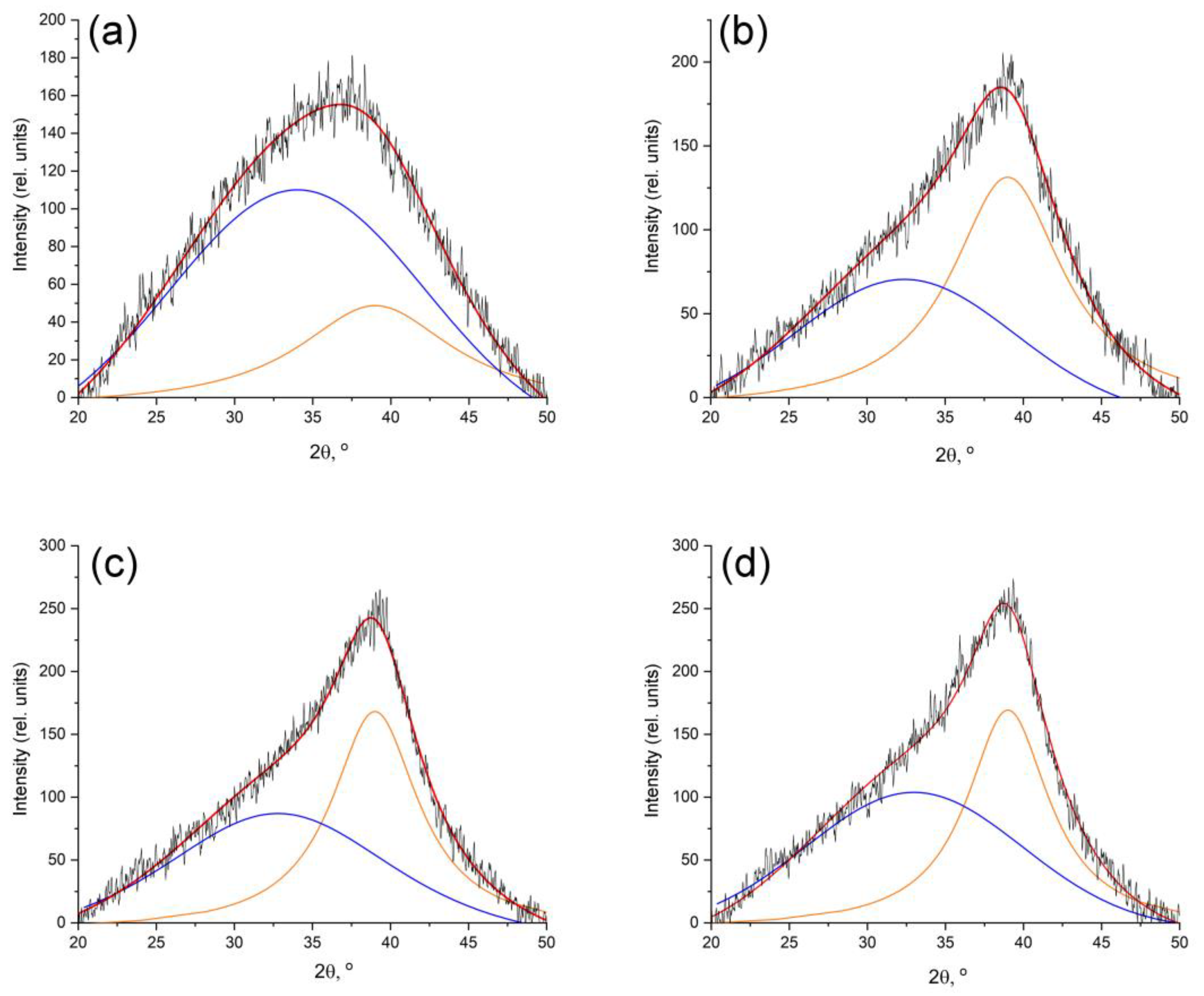






| Tsynth., °C | Ratio Fe:Co:Cr | Phases FeCoCr | CSR np 1, nm | CSR m 2, nm | A(Cam)/A(Cg) 3 |
|---|---|---|---|---|---|
| 500 | 40:40:20 | FCC BCC | 9 22 | 1.3 | 3.4 |
| 600 | FCC BCC | 14 23 | 1.8 | 1.3 | |
| 700 | FCC BCC | 16 24 | 2.6 | 1.1 | |
| 700 | 35:35:30 | FCC BCC | 12 28 | 2.2 | 1.2 |
| Tsynth., °C | Fe:Co:Cr | ν(ID), sm−1 | ν(IG), sm−1 | ν(ID’), sm−1 | ID/IG | ID’/IG | La, nm |
|---|---|---|---|---|---|---|---|
| 500 | 40:40:20 | 1370 | 1608 | 1531 | 2.87 | 0.56 | 1.5 |
| 600 | 1359 | 1618 | 1553 | 1.77 | 0.53 | 2.5 | |
| 700 | 1358 | 1601 | 1547 | 1.47 | 0.49 | 3.0 | |
| 700 | 35:35:30 | 1356 | 1612 | 1530 | 1.68 | 0.52 | 2.6 |
| Tsynth., °C | Fe:Co:Cr | Ms, A·m2·kg−1 | Mr, A·m2·kg−1 | Hc, O | Mr/Ms | Dnp, nm (FCC/BCC) |
|---|---|---|---|---|---|---|
| 500 | 40:40:20 | 1.3 | 0.05 | 168 | 0.04 | 9/22 |
| 600 | 2.5 | 0.18 | 205 | 0.07 | 14/23 | |
| 700 | 11.2 | 1.89 | 424 | 0.17 | 16/24 | |
| 700 | 35:35:30 | 7.6 | 2.4 | 620 | 0.32 | 12/28 |
| 700 | 50:50:0 | 25.8 | 7.8 | 562 | 0.30 | 0/14 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zaporotskova, I.; Muratov, D.; Kozhitov, L.; Popkova, A.; Boroznina, N.; Boroznin, S.; Vasiliev, A.; Tarala, V.; Korovin, E. Nanocomposites Based on Pyrolyzed Polyacrylonitrile Doped with FeCoCr/C Transition Metal Alloy Nanoparticles: Synthesis, Structure, and Electromagnetic Properties. Polymers 2023, 15, 3596. https://doi.org/10.3390/polym15173596
Zaporotskova I, Muratov D, Kozhitov L, Popkova A, Boroznina N, Boroznin S, Vasiliev A, Tarala V, Korovin E. Nanocomposites Based on Pyrolyzed Polyacrylonitrile Doped with FeCoCr/C Transition Metal Alloy Nanoparticles: Synthesis, Structure, and Electromagnetic Properties. Polymers. 2023; 15(17):3596. https://doi.org/10.3390/polym15173596
Chicago/Turabian StyleZaporotskova, Irina, Dmitriy Muratov, Lev Kozhitov, Alena Popkova, Natalia Boroznina, Sergey Boroznin, Andrey Vasiliev, Vitaly Tarala, and Evgeny Korovin. 2023. "Nanocomposites Based on Pyrolyzed Polyacrylonitrile Doped with FeCoCr/C Transition Metal Alloy Nanoparticles: Synthesis, Structure, and Electromagnetic Properties" Polymers 15, no. 17: 3596. https://doi.org/10.3390/polym15173596