A Light-Thin Chitosan Nanofiber Separator for High-Performance Lithium-Ion Batteries
Abstract
:1. Introduction
2. Experiments
2.1. Materials
2.2. Preparation of the Chitosan Nanofibers Separator
2.3. Characterization
2.3.1. Morphology of Separators Analyzed by SEM
2.3.2. Porosity
2.3.3. Mechanical Property
2.3.4. Wettability
2.3.5. Thermal Properties
2.3.6. Electrochemical Characterizations
3. Results and Discussion
3.1. Morphology of the New Chitosan Nanofiber Separators
3.2. Mechanical Property
3.3. Thermal Analysis
3.4. Wettability
3.5. Electrochemical Performances
| Samples | Ionic Conductivity (mS cm−1) | Li+ Transference Number | Rate Performance (2C, mAh g−1) | Battery Performance after 100 Cycles (%) | Reference |
|---|---|---|---|---|---|
| PSF | 2.93 | 0.42 | 120 | (0.5C) 95.1% | [60] |
| OBCS-200 | 2.9 | 0.56 | 116 | (0.5C) 83.2% | [36] |
| PU-PC | 1.59 | / | 115 | (0.2C) 95% | [61] |
| MSD-PI | 0.66 | 0.78 | / | (0.2C) 98.3% | [62] |
| UiO-66-NH2 | 3.1 | / | 85 | (1C) 81% | [63] |
| ZIF-67@CNFs | 1.55 | 0.45 | 125 | (0.5C) 88.4% | [64] |
| CME15 | 0.68 | 0.5 | 112 | (1C) 85% | This work |
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Conflicts of Interest
References
- Waqas, M.; Soomro, A.M.; Ali, S.; Ashraf, H.; Chan, A.S.; Kumar, S.; Choi, K.H. A highly efficient composite separator embedded with colloidal lanthanum oxide nanocrystals for high-temperature lithium-ion batteries. Int. J. Energy Res. 2021, 45, 11179–11192. [Google Scholar] [CrossRef]
- Huang, Z.; Chen, Y.; Han, Q.; Su, M.; Liu, Y.; Wang, S.; Wang, H. Vapor-induced phase inversion of poly (m-phenylene isophthalamide) modified polyethylene separator for high-performance lithium-ion batteries. Chem. Eng. J. 2022, 429, 132429. [Google Scholar] [CrossRef]
- Liu, X.; Qin, M.; Sun, W.; Zhang, D.; Jian, B.; Sun, Z.; Wang, S.; Li, X. Study on cellulose nanofibers/aramid fibers lithium-ion battery separators by the heterogeneous preparation method. Int. J. Biol. Macromol. 2023, 225, 1476–1486. [Google Scholar] [CrossRef] [PubMed]
- Jang, J.; Oh, J.; Jeong, H.; Kang, W.; Jo, C. A Review of Functional Separators for Lithium Metal Battery Applications. Materials 2020, 13, 4625. [Google Scholar] [CrossRef] [PubMed]
- Ding, L.; Zhang, C.; Wu, T.; Yang, F.; Lan, F.; Cao, Y.; Xiang, M. Effect of temperature on compression behavior of polypropylene separator used for Lithium-ion battery. J. Power Sources 2020, 466, 228300. [Google Scholar] [CrossRef]
- Deng, J.; Cao, D.; Yang, X.; Zhang, G. Cross-linked cellulose/carboxylated polyimide nanofiber separator for lithium-ion battery application. Chem. Eng. J. 2022, 433, 133934. [Google Scholar] [CrossRef]
- Costa, C.M.; Silva, M.M.; Lanceros-Méndez, S. Battery separators based on vinylidene fluoride (VDF) polymers and copolymers for lithium ion battery applications. RSC Adv. 2013, 3, 11404–11417. [Google Scholar] [CrossRef]
- Liu, Y.; Zhou, T.; Zheng, Y.; He, Z.; Xiao, C.; Pang, W.K.; Tong, W.; Zou, Y.; Pan, B.; Guo, Z.; et al. Local Electric Field Facilitates High-Performance Li-Ion Batteries. ACS Nano 2017, 11, 8519–8526. [Google Scholar] [CrossRef]
- Xing, J.; Li, J.; Fan, W.; Zhao, T.; Chen, X.; Li, H.; Cui, Y.; Wei, Z.; Zhao, Y. A review on nanofibrous separators towards enhanced mechanical properties for lithium-ion batteries. Compos. Part B Eng. 2022, 243, 110105. [Google Scholar] [CrossRef]
- Lee, H.; Yanilmaz, M.; Toprakci, O.; Fu, K.; Zhang, X. A review of recent developments in membrane separators for rechargeable lithium-ion batteries. Energy Environ. Sci. 2014, 7, 3857–3886. [Google Scholar] [CrossRef]
- McCloskey, B.D. Attainable Gravimetric and Volumetric Energy Density of Li–S and Li Ion Battery Cells with Solid Separator-Protected Li Metal Anodes. J. Phys. Chem. Lett. 2015, 6, 4581–4588. [Google Scholar] [CrossRef] [PubMed]
- Cao, X.; Ma, C.; Luo, L.; Chen, L.; Cheng, H.; Orenstein, R.S.; Zhang, X. Nanofiber Materials for Lithium-Ion Batteries. Adv. Fiber Mater. 2023, 5, 1141–1197. [Google Scholar] [CrossRef]
- Guo, L.; Thornton, D.B.; Koronfel, M.A.; Stephens, I.E.L.; Ryan, M.P. Degradation in lithium ion battery current collectors. J. Phys. Energy 2021, 3, 032015. [Google Scholar] [CrossRef]
- Wang, H.; Xu, H.; Zhao, Z.; Wang, Q.; Jin, C.; Li, Y.; Sheng, J.; Li, K.; Du, Z.; Xu, C.; et al. An experimental analysis on thermal runaway and its propagation in Cell-to-Pack lithium-ion batteries. Appl. Therm. Eng. 2022, 211, 118418. [Google Scholar] [CrossRef]
- Zolin, L.; Chandesris, M.; Porcher, W.; Lestriez, B. An Innovative Process for Ultra-Thick Electrodes Elaboration: Toward Low-Cost and High-Energy Batteries. Energy Technol. 2019, 7, 1900025. [Google Scholar] [CrossRef]
- Wang, Z.; Pan, R.; Ruan, C.; Edstrom, K.; Stromme, M.; Nyholm, L. Redox-Active Separators for Lithium-Ion Batteries. Adv. Sci. 2018, 5, 1700663. [Google Scholar] [CrossRef]
- Francis, C.F.J.; Kyratzis, I.L.; Best, A.S. Lithium-Ion Battery Separators for Ionic-Liquid Electrolytes: A Review. Adv. Mater. 2020, 32, 1904205. [Google Scholar] [CrossRef]
- Lee, Y.; Park, J.; Jeon, H.; Yeon, D.; Kim, B.-H.; Cho, K.Y.; Ryou, M.-H.; Lee, Y.M. In-depth correlation of separator pore structure and electrochemical performance in lithium-ion batteries. J. Power Sources 2016, 325, 732–738. [Google Scholar] [CrossRef]
- Lim, S.-G.; Jo, H.-D.; Kim, C.; Kim, H.-T.; Chang, D.-R. Electro-Spun Poly(vinylidene fluoride) Nanofiber Web as Separator for Lithium Ion Batteries: Effect of Pore Structure and Thickness. J. Nanosci. Nanotechnol. 2016, 16, 956–961. [Google Scholar] [CrossRef]
- Horváth, D.V.; Tian, R.; Gabbett, C.; Nicolosi, V.; Coleman, J.N. Quantifying the Effect of Separator Thickness on Rate Performance in Lithium-Ion Batteries. J. Electrochem. Soc. 2022, 169, 030503. [Google Scholar] [CrossRef]
- Zhong, S.; Yuan, B.; Guang, Z.; Chen, D.; Li, Q.; Dong, L.; Ji, Y.; Dong, Y.; Han, J.; He, W. Recent progress in thin separators for upgraded lithium ion batteries. Energy Storage Mater. 2021, 41, 805–841. [Google Scholar] [CrossRef]
- Chen, S.; Niu, C.; Lee, H.; Li, Q.; Yu, L.; Xu, W.; Zhang, J.-G.; Dufek, E.J.; Whittingham, M.S.; Meng, S.; et al. Critical Parameters for Evaluating Coin Cells and Pouch Cells of Rechargeable Li-Metal Batteries. Joule 2019, 3, 1094–1105. [Google Scholar] [CrossRef]
- Zhu, G.-L.; He, Y.-Y.; Deng, Y.-L.; Wang, M.; Liu, X.-Y.; Wang, L.-P.; Gao, J. Dependence of Separator Thickness on Li-Ion Battery Energy Density. J. Electrochem. Soc. 2021, 168, 110545. [Google Scholar] [CrossRef]
- Guo, X.; Li, J.; Xing, J.; Zhang, K.; Zhou, Y.; Pan, C.; Wei, Z.; Zhao, Y. Silkworm Cocoon Layer with Gradient Structure as Separator for Lithium-Ion Battery. Energy Technol. 2022, 10, 2100996. [Google Scholar] [CrossRef]
- Xia, Y.; Li, X.; Zhuang, J.; Yuan, Y.; Wang, W. Cellulose microspheres enhanced polyvinyl alcohol separator for high-performance lithium-ion batteries. Carbohydr Polym. 2023, 300, 120231. [Google Scholar] [CrossRef]
- Kim, J.K.; Kim, D.H.; Joo, S.H.; Choi, B.; Cha, A.; Kim, K.M.; Kwon, T.H.; Kwak, S.K.; Kang, S.J.; Jin, J. Hierarchical Chitin Fibers with Aligned Nanofibrillar Architectures: A Nonwoven-Mat Separator for Lithium Metal Batteries. ACS Nano 2017, 11, 6114–6121. [Google Scholar] [CrossRef] [PubMed]
- Barbosa, J.C.; Reizabal, A.; Correia, D.M.; Fidalgo-Marijuan, A.; Gonçalves, R.; Silva, M.M.; Lanceros-Mendez, S.; Costa, C.M. Lithium-ion battery separator membranes based on poly(L-lactic acid) biopolymer. Mater. Today Energy 2020, 18, 100494. [Google Scholar] [CrossRef]
- Reizabal, A.; Fidalgo-Marijuan, A.; Goncalves, R.; Gutierrez-Pardo, A.; Aguesse, F.; Perez-Alvarez, L.; Vilas-Vilela, J.L.; Costa, C.M.; Lanceros-Mendez, S. Silk fibroin and sericin polymer blends for sustainable battery separators. J. Colloid Interface Sci. 2022, 611, 366–376. [Google Scholar] [CrossRef] [PubMed]
- Li, S.; Zhu, W.; Tang, Q.; Huang, Z.; Yu, P.; Gui, X.; Lin, S.; Hu, J.; Tu, Y. Mini Review on Cellulose-Based Composite Separators for Lithium-Ion Batteries: Recent Progress and Perspectives. Energy Fuels 2021, 35, 12938–12947. [Google Scholar] [CrossRef]
- Zhang, T.W.; Chen, J.L.; Tian, T.; Shen, B.; Peng, Y.D.; Song, Y.H.; Jiang, B.; Lu, L.L.; Yao, H.B.; Yu, S.H. Sustainable Separators for High-Performance Lithium Ion Batteries Enabled by Chemical Modifications. Adv. Funct. Mater. 2019, 29, 1902023. [Google Scholar] [CrossRef]
- Pan, R.; Wang, Z.; Sun, R.; Lindh, J.; Edström, K.; Strømme, M.; Nyholm, L. Thickness difference induced pore structure variations in cellulosic separators for lithium-ion batteries. Cellulose 2017, 24, 2903–2911. [Google Scholar] [CrossRef]
- Zhang, Z.; Fang, Z.; Xiang, Y.; Liu, D.; Xie, Z.; Qu, D.; Sun, M.; Tang, H.; Li, J. Cellulose-based material in lithium-sulfur batteries: A review. Carbohydr Polym. 2021, 255, 117469. [Google Scholar] [CrossRef]
- Liu, J.; Zeng, F.; Liu, M.; He, J.; Li, Q.; Song, F.; Hong, Z.; Chen, Y.; Bai, L.; Cheng, C.; et al. Amino-functionalized SiO2 modified porous polyetherimide separator for high performance Li-S batteries. Int. J. Energy Res. 2022, 46, 19480–19492. [Google Scholar] [CrossRef]
- Wang, Y.; Luo, J.; Chen, L.; Long, J.; Hu, J.; Meng, L. Effect of fibrillated fiber morphology on properties of paper-based separators for lithium-ion battery applications. J. Power Sources 2021, 482, 228899. [Google Scholar] [CrossRef]
- Sheng, J.; Chen, T.; Wang, R.; Zhang, Z.; Hua, F.; Yang, R. Ultra-light cellulose nanofibril membrane for lithium-ion batteries. J. Membr. Sci. 2020, 595, 117550. [Google Scholar] [CrossRef]
- Cheng, C.; Yang, R.; Wang, Y.; Fu, D.; Sheng, J.; Guo, X. A bacterial cellulose-based separator with tunable pore size for lithium-ion batteries. Carbohydr Polym. 2023, 304, 120489. [Google Scholar] [CrossRef]
- Jin, C.; Nai, J.; Sheng, O.; Yuan, H.; Zhang, W.; Tao, X.; Lou, X.W. Biomass-based materials for green lithium secondary batteries. Energy Environ. Sci. 2021, 14, 1326–1379. [Google Scholar] [CrossRef]
- Salehi, E.; Daraei, P.; Arabi Shamsabadi, A. A review on chitosan-based adsorptive membranes. Carbohydr Polym. 2016, 152, 419–432. [Google Scholar] [CrossRef]
- Salehi, E.; Khajavian, M.; Sahebjamee, N.; Mahmoudi, M.; Drioli, E.; Matsuura, T. Advances in nanocomposite and nanostructured chitosan membrane adsorbents for environmental remediation: A review. Desalination 2022, 527, 115565. [Google Scholar] [CrossRef]
- Zhai, Y.; Wang, X.; Chen, Y.; Sang, X.; Liu, H.; Sheng, J.; Wu, Y.; Wang, X.; Li, L. Multiscale-structured polyvinylidene fluoride/polyacrylonitrile/vermiculite nanosheets fibrous membrane with uniform Li+ flux distribution for lithium metal battery. J. Membr. Sci. 2021, 621, 118996. [Google Scholar] [CrossRef]
- Zhang, T.-W.; Shen, B.; Yao, H.-B.; Ma, T.; Lu, L.-L.; Zhou, F.; Yu, S.-H. Prawn Shell Derived Chitin Nanofiber Membranes as Advanced Sustainable Separators for Li/Na-Ion Batteries. Nano Lett. 2017, 17, 4894–4901. [Google Scholar] [CrossRef]
- Yu, B.-C.; Park, K.; Jang, J.-H.; Goodenough, J.B. Cellulose-Based Porous Membrane for Suppressing Li Dendrite Formation in Lithium–Sulfur Battery. ACS Energy Lett. 2016, 1, 633–637. [Google Scholar] [CrossRef]
- Ren, W.; Zheng, Y.; Cui, Z.; Tao, Y.; Li, B.; Wang, W. Recent progress of functional separators in dendrite inhibition for lithium metal batteries. Energy Storage Mater. 2021, 35, 157–168. [Google Scholar] [CrossRef]
- Xie, W.; Wu, L.; Liu, W.; Dang, Y.; Tang, A.; Luo, Y. Modelling electrolyte-immersed tensile property of polypropylene separator for lithium-ion battery. Mech. Mater. 2021, 152, 103667. [Google Scholar] [CrossRef]
- Lin, W.; Wang, F.; Wang, H.; Li, H.; Fan, Y.; Chan, D.; Chen, S.; Tang, Y.; Zhang, Y. Thermal-Stable Separators: Design Principles and Strategies Towards Safe Lithium-Ion Battery Operations. ChemSusChem 2022, 15, e202201464. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Chen, G.; Yang, F.; Luo, Z.; Yuan, B.; Chen, X.; Wang, L. Serendipity discovery of fire early warning function of chitosan film. Carbohydr. Polym. 2022, 277, 118884. [Google Scholar] [CrossRef] [PubMed]
- Li, H.; Wu, D.; Wu, J.; Dong, L.-Y.; Zhu, Y.-J.; Hu, X. Flexible, High-Wettability and Fire-Resistant Separators Based on Hydroxyapatite Nanowires for Advanced Lithium-Ion Batteries. Adv. Mater. 2017, 29, 1703548. [Google Scholar] [CrossRef]
- Li, J.; Zhang, Y.; Shang, R.; Cheng, C.; Cheng, Y.; Xing, J.; Wei, Z.; Zhao, Y. Recent advances in lithium-ion battery separators with reversible/irreversible thermal shutdown capability. Energy Storage Mater. 2021, 43, 143–157. [Google Scholar] [CrossRef]
- Banerjee, A.; Ziv, B.; Shilina, Y.; Luski, S.; Aurbach, D.; Halalay, I.C. Acid-Scavenging Separators: A Novel Route for Improving Li-Ion Batteries’ Durability. ACS Energy Lett. 2017, 2, 2388–2393. [Google Scholar] [CrossRef]
- Zou, M.; Luo, Y.; Ma, X.; Fan, J.; Niu, Y.; Liang, J.; Gong, G. High-performance electrospun membrane for lithium-ion batteries. J. Membr. Sci. 2021, 637, 119597. [Google Scholar] [CrossRef]
- Gu, H.; Wang, C.; Gong, S.; Mei, Y.; Li, H.; Ma, W. Investigation on contact angle measurement methods and wettability transition of porous surfaces. Surf. Coat. Technol. 2016, 292, 72–77. [Google Scholar] [CrossRef]
- Gu, Q.Q.; Fu, C.L.; Sun, Z.Y. Resin-silica composite nanoparticle grafted polyethylene membranes for lithium ion batteries. J. Appl. Polym. Sci. 2021, 138, 50713. [Google Scholar] [CrossRef]
- Gu, Q.-Q.; Xue, H.-J.; Li, Z.-W.; Song, J.-C.; Sun, Z.-Y. High-performance polyethylene separators for lithium-ion batteries modified by phenolic resin. J. Power Sources 2021, 483, 229155. [Google Scholar] [CrossRef]
- Han, W.-W.; Ardhi, R.E.A.; Liu, G.-C. Dual impact of superior SEI and separator wettability to inhibit lithium dendrite growth. Rare Met. 2021, 41, 353–355. [Google Scholar] [CrossRef]
- Zeng, X.; Liu, Y.; He, R.; Li, T.; Hu, Y.; Wang, C.; Xu, J.; Wang, L.; Wang, H. Tissue paper-based composite separator using nano-SiO2 hybrid crosslinked polymer electrolyte as coating layer for lithium ion battery with superior security and cycle stability. Cellulose 2022, 29, 3985–4000. [Google Scholar] [CrossRef]
- Wang, Z.; Guo, F.; Chen, C.; Shi, L.; Yuan, S.; Sun, L.; Zhu, J. Self-assembly of PEI/SiO2 on polyethylene separators for Li-ion batteries with enhanced rate capability. ACS Appl. Mater. Interfaces 2015, 7, 3314–3322. [Google Scholar] [CrossRef]
- Chen, C.; Hu, L. Nanocellulose toward Advanced Energy Storage Devices: Structure and Electrochemistry. Acc. Chem. Res. 2018, 51, 3154–3165. [Google Scholar] [CrossRef] [PubMed]
- Zuo, X.; Wu, J.; Ma, X.; Deng, X.; Cai, J.; Chen, Q.; Liu, J.; Nan, J. A poly(vinylidene fluoride)/ethyl cellulose and amino-functionalized nano-SiO2 composite coated separator for 5 V high-voltage lithium-ion batteries with enhanced performance. J. Power Sources 2018, 407, 44–52. [Google Scholar] [CrossRef]
- Sharma, L.; Nakamoto, K.; Okada, S.; Barpanda, P. Tavorite LiFePO4OH hydroxyphosphate as an anode for aqueous lithium-ion batteries. J. Power Sources 2019, 429, 17–21. [Google Scholar] [CrossRef]
- Li, Z.; Wang, W.; Liang, X.; Wang, J.; Xu, Y.; Li, W. Fiber swelling to improve cycle performance of paper-based separator for lithium-ion batteries application. J. Energy Chem. 2023, 79, 92–100. [Google Scholar] [CrossRef]
- Fu, Q.; Zhang, W.; Muhammad, I.P.; Chen, X.; Zeng, Y.; Wang, B.; Zhang, S. Coaxially electrospun PAN/HCNFs@PVDF/UiO-66 composite separator with high strength and thermal stability for lithium-ion battery. Microporous Mesoporous Mater. 2021, 311, 110724. [Google Scholar] [CrossRef]
- Liao, C.; Wang, W.; Wang, J.; Han, L.; Qiu, S.; Song, L.; Gui, Z.; Kan, Y.; Hu, Y. Magnetron sputtering deposition of silicon nitride on polyimide separator for high-temperature lithium-ion batteries. J. Energy Chem. 2021, 56, 1–10. [Google Scholar] [CrossRef]
- Barbosa, J.C.; Gonçalves, R.; Valverde, A.; Martins, P.M.; Petrenko, V.I.; Márton, M.; Fidalgo-Marijuan, A.; Fernández de Luis, R.; Costa, C.M.; Lanceros-Méndez, S. Metal organic framework modified poly(vinylidene fluoride-co-hexafluoropropylene) separator membranes to improve lithium-ion battery capacity fading. Chem. Eng. J. 2022, 443, 136329. [Google Scholar] [CrossRef]
- Sun, X.; Xu, W.; Zhang, X.; Lei, T.; Lee, S.-Y.; Wu, Q. ZIF-67@Cellulose nanofiber hybrid membrane with controlled porosity for use as Li-ion battery separator. J. Energy Chem. 2021, 52, 170–180. [Google Scholar] [CrossRef]


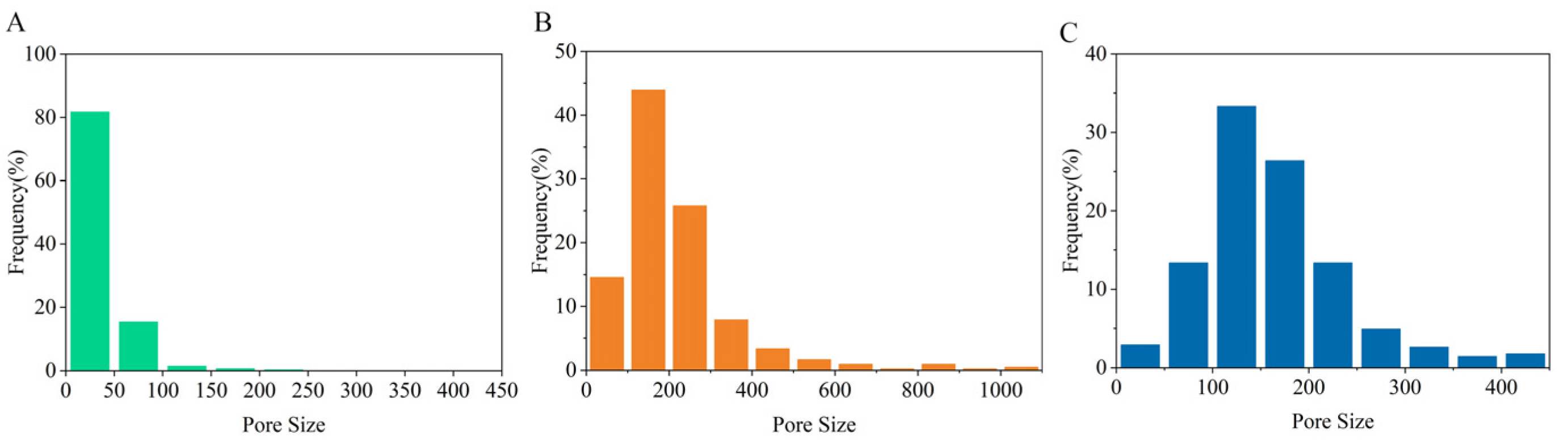

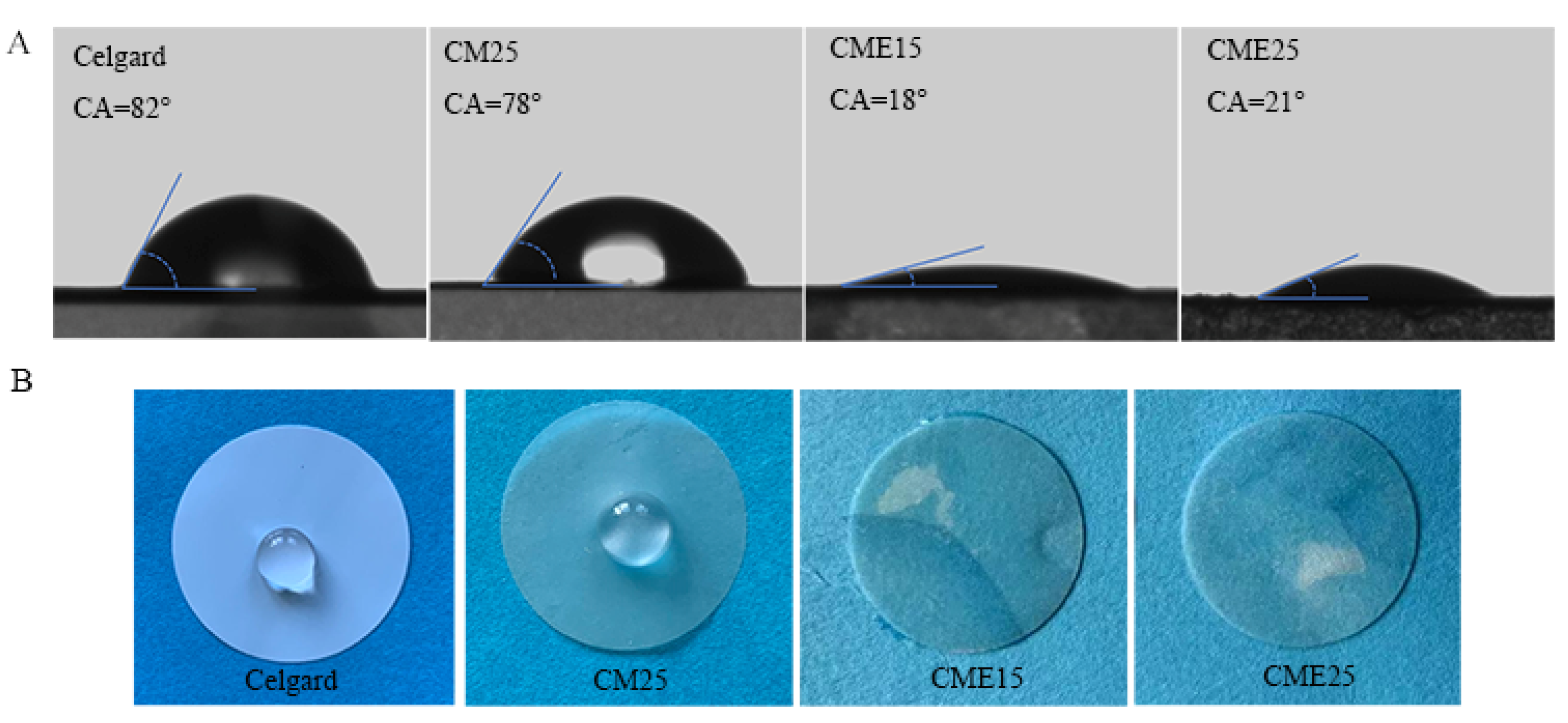
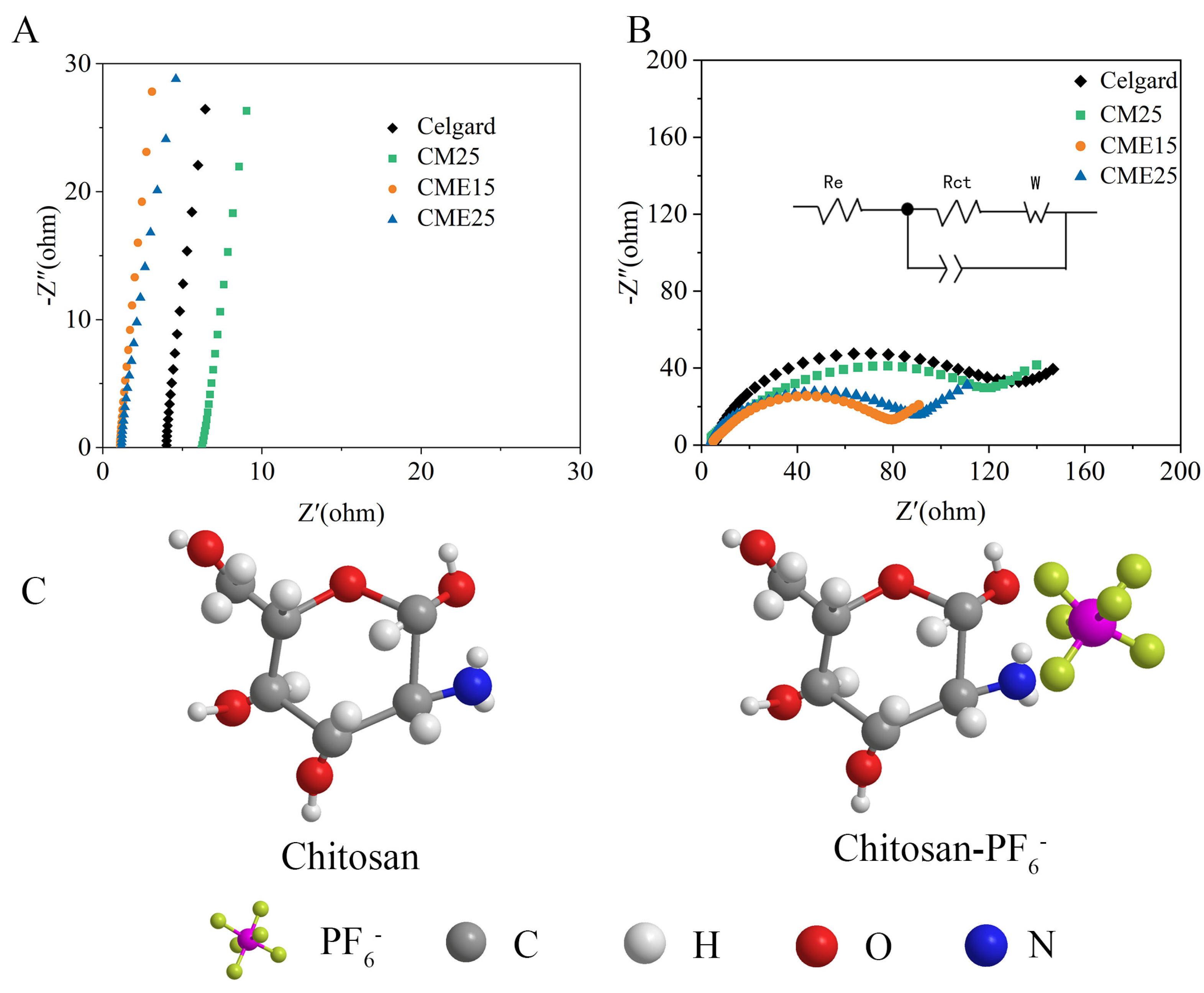
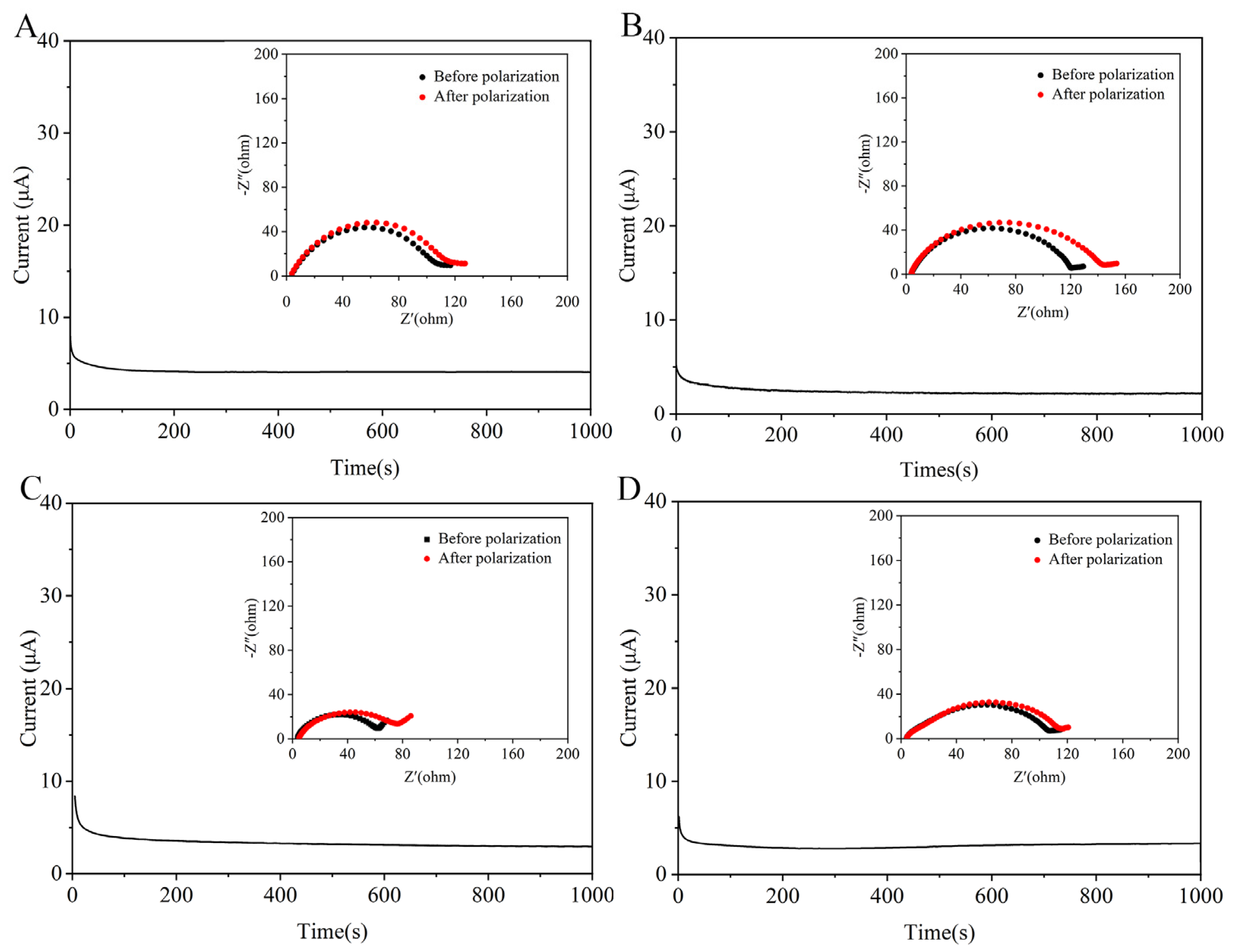

| Samples | Thickness (μm) | Density (g cm−3) | Porosity (%) | Gurley (s) | Tensile Strength (MPa) | Uptake (%) |
|---|---|---|---|---|---|---|
| CM25 | 25 | 0.91 | 34 | - | 47 | 98 |
| CME15 | 15 | 0.54 | 69 | 379 | 32 | 324 |
| CME25 | 25 | 0.71 | 54 | 480 | 37 | 267 |
| Celgard | 25 | 0.59 | 39 | 549 | 153/11 | 124 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Song, Y.; Zhao, G.; Zhang, S.; Xie, C.; Li, X. A Light-Thin Chitosan Nanofiber Separator for High-Performance Lithium-Ion Batteries. Polymers 2023, 15, 3654. https://doi.org/10.3390/polym15183654
Song Y, Zhao G, Zhang S, Xie C, Li X. A Light-Thin Chitosan Nanofiber Separator for High-Performance Lithium-Ion Batteries. Polymers. 2023; 15(18):3654. https://doi.org/10.3390/polym15183654
Chicago/Turabian StyleSong, Yanghui, Guanglei Zhao, Sihan Zhang, Chong Xie, and Xiaofeng Li. 2023. "A Light-Thin Chitosan Nanofiber Separator for High-Performance Lithium-Ion Batteries" Polymers 15, no. 18: 3654. https://doi.org/10.3390/polym15183654




