Peculiarity of the Mechanism of Early Stages of Photo-Oxidative Degradation of Linear Low-Density Polyethylene Films in the Presence of Ferric Stearate
Abstract
:1. Introduction
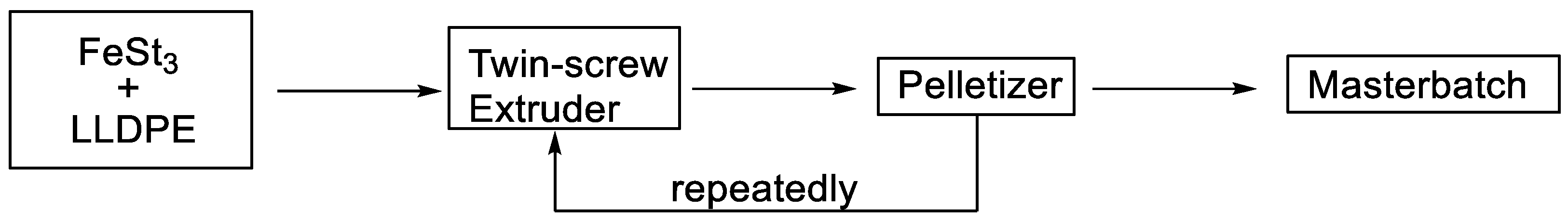
2. Experimental Sections
3. Results and Discussion
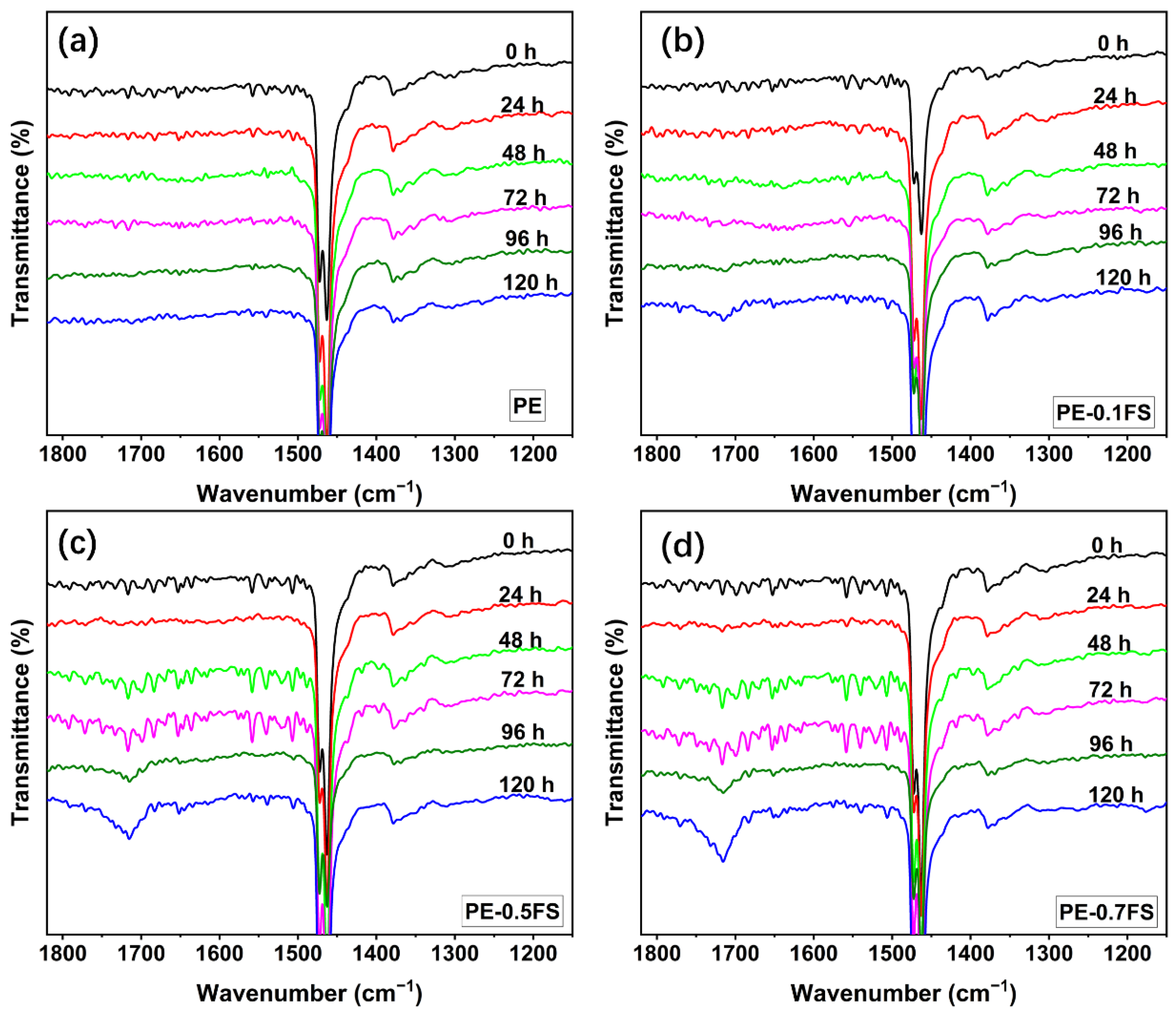
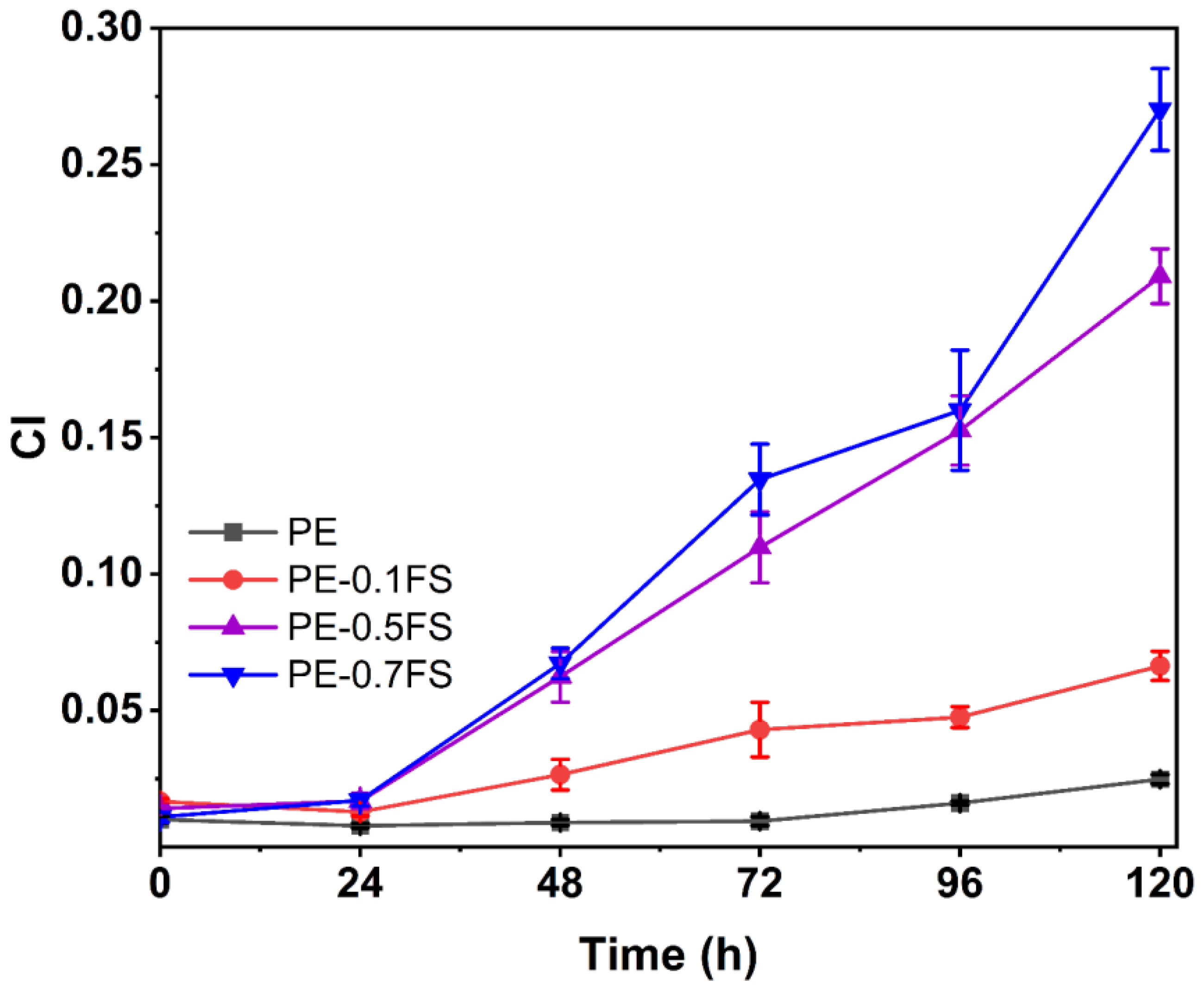
3.1. Analysis of Irradiation Test Results
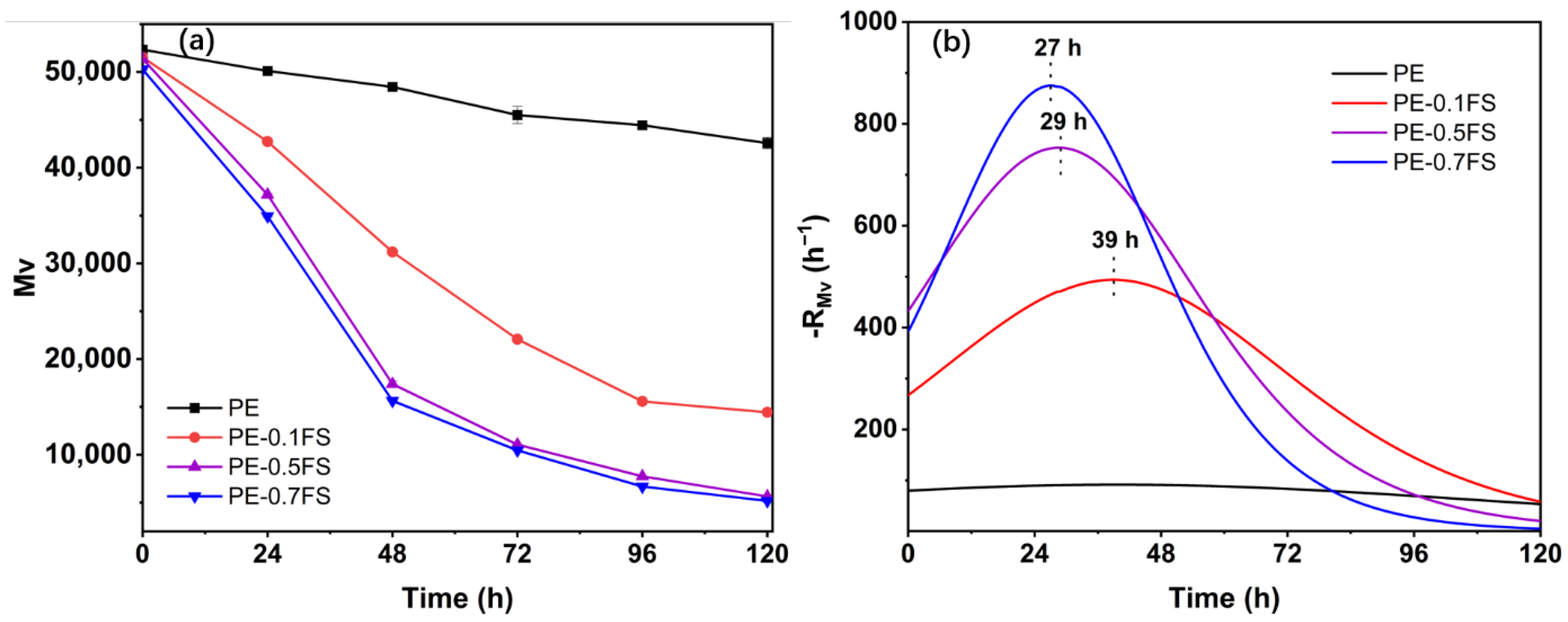
3.2. Analysis of Molecular Weight Changes
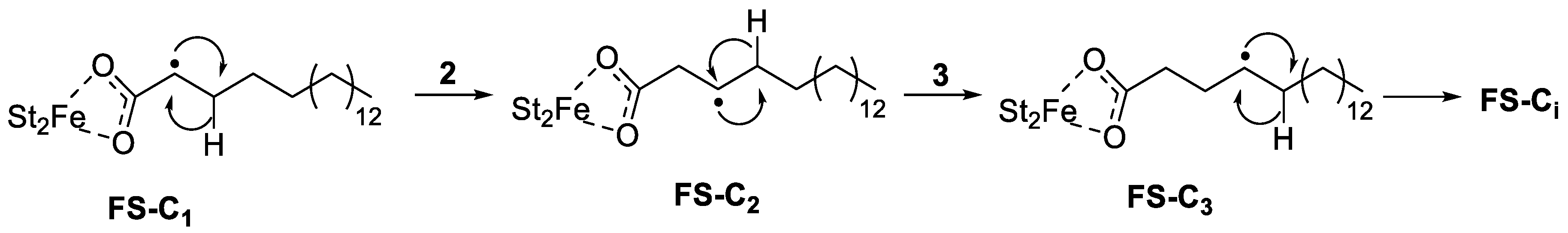
3.3. Study on the Enhancement of FeSt3 in the Initial Photo-Oxidative Degradation of Polyethylene
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Singh, B.; Sharma, N. Mechanistic Implications of Plastic Degradation. Polym. Degrad. Stab. 2008, 93, 561–584. [Google Scholar] [CrossRef]
- Peacock, A.J. Handbook of Polyethylene: Structures, Properties and Applications; Marcel Dekker: New York, NY, USA, 2000. [Google Scholar]
- Eriksen, M.; Mason, S.; Wilson, S.; Box, C.; Zellers, A.; Edwards, W.; Farley, H.; Amato, S. Microplastic pollution in the surface waters of the Laurentian Great Lakes. Mar. Pollut. Bull. 2013, 77, 177–182. [Google Scholar] [CrossRef]
- Medrano, D.E.; Thompson, R.C.; Aldridge, D.C. Microplastics in freshwater systems: A review of the emerging threats, iden-tification of knowledge gaps and prioritisation of research needs. Water Res. 2015, 75, 63–82. [Google Scholar] [CrossRef]
- Roy, P.K.; Hakkarainen, M.; Varma, I.K.; Albertsson, A.C. Degradable Polyethylene: Fantasy or Reality. Environ. Sci. Technol. 2011, 45, 4217–4227. [Google Scholar] [CrossRef]
- Hamad, K.; Kaseem, M.; Deri, F. Recycling of Waste From Polymer Materials: An Overview of the Recent Works. Polym. Degrad. Stab. 2013, 98, 2801–2812. [Google Scholar] [CrossRef]
- Serrano, D.P.; Aguado, J.; Escola, J.M. Developing Advanced Catalysts for the Conversion of PolyolefinicWaste Plastics into Fuels and Chemicals. ACS Catal. 2012, 2, 1924–1941. [Google Scholar] [CrossRef]
- He, P.; Chen, L.; Shao, L.; Zhang, H.; Lü, F. Municipal solid waste (MSW) landfill: A source of microplastics? -Evidence of microplastics in landfill leachate. Water. Res. 2019, 159, 38–45. [Google Scholar] [CrossRef]
- Ramisa, X.; Cadenatoa, A.; Salla, J.M.; Morancho, J.M.; Vallés, A.; Contat, L.; Ribes, A. Thermal degradation of polypropylene/starch-based materials with enhanced biodegradability. Polym. Degrad. Stab. 2004, 86, 483–491. [Google Scholar] [CrossRef]
- Abd El-Rehim, H.A.; Hegazy, E.A.; Ali, A.M.; Rabie, A.M. Synergistic effect of combining UV-sunlight–soil burial treatment on the biodegradation rate of LDPE/starch blends. J. Photoch. Photobio. A 2004, 163, 547–556. [Google Scholar] [CrossRef]
- Lin, Y.C. Study of ultraviolet photooxidative degradation of LDPE film containing cerium carboxylate photosensitizer. J. Appl. Polym. Sci. 1997, 63, 811–818. [Google Scholar] [CrossRef]
- Roy, P.K.; Surekha, P.; Rajagopal, C.; Choudhary, V.J. Accelerated aging of LDPE films containing cobalt complexes as prooxidants. Polym. Degrad. Stab. 2006, 91, 1791–1799. [Google Scholar] [CrossRef]
- Roy, P.K.; Surekha, P.; Rajagopal, C.; Choudhary, V. Effect of cobalt carboxylates on the photo-oxidative degradation of low-density polyethylene. Part-I. Polym. Degrad. Stab. 2006, 91, 1980. [Google Scholar] [CrossRef]
- Roy, P.K.; Titus, S.; Surekha, P.; Tulsi, E.; Deshmukh, C.; Rajagopal, C. Degradation of abiotically aged LDPE films containing pro-oxidant by bacterial consortium. Polym. Degrad. Stab. 2008, 93, 1917. [Google Scholar] [CrossRef]
- Roy, P.K.; Surekha, P.; Raman, R.; Rajagopal, C. Investigating the role of metal oxidation state on the degradation behaviour of LDPE. Polym. Degrad. Stab. 2009, 94, 1033–1039. [Google Scholar] [CrossRef]
- Subramaniam, M.; Sharma, S.; Gupta, A.; Abdullah, N. Enhanced degradation properties of polypropylene integrated with iron and cobalt stearates and its synthetic application. J. Appl. Polym. Sci. 2018, 135, 46028. [Google Scholar] [CrossRef]
- Pablos, J.L.; Abrusci, C.; Marín, I.; López-Marín, J.; Catalina, F.; Espí, E.; Corrales, T. Photodegradation of polyethylenes: Comparative effect of Fe and Ca-stearates as pro-oxidant additives. Polym. Degrad. Stab. 2010, 95, 2057–2064. [Google Scholar] [CrossRef]
- Rajakumar, K.; Sarasvathy, V.; Thamaraichelvan, A.; Chitra, R.; Vijayakumar, C.T. Effect of Iron Carboxylates on the Photodegradability of Polypropylene. II. Artificial Weathering Studies. J. Appl. Poly. Sci. 2010, 118, 2601–2612. [Google Scholar] [CrossRef]
- Rajakumar, K.; Sarasvathy, V.; Thamaraichelvan, A.; Chitra, R.; Vijayakumar, C.T. Effect of Iron Carboxylates on the Photodegradability of Polypropylene. I. Natural Weathering Studies. J. Appl. Poly. Sci. 2012, 123, 2968–2976. [Google Scholar] [CrossRef]
- Roy, P.K.; Surekha, P.; Rajagopal, C.; Chatterjee, S.N.; Choudhary, V. Effect of benzil and cobalt stearate on the aging of low-density polyethylene films. Polym. Degrad. Stab. 2005, 90, 577–585. [Google Scholar] [CrossRef]
- Roy, P.K.; Singh, P.; Kumar, D.; Rajagopal, C. Manganese Stearate Initiated Photo-Oxidative and Thermo-Oxidative Degradation of LDPE, LLDPE and Their Blends. J. Appl. Polym. Sci. 2010, 117, 524–533. [Google Scholar] [CrossRef]
- Koutny, M.; Lemaire, J.; Delort, A. Biodegradation of polyethylene films with prooxidant additives. Chemosphere 2006, 64, 1243–1252. [Google Scholar] [CrossRef] [PubMed]
- Ammala, A.; Bateman, S.; Dean, K.; Petinakis, E.; Sangwan, P.; Wong, S.; Yuan, Q.; Yu, L.; Patrick, C.; Leong, K.H. An overview of degradable and biodegradable polyolefins. Prog. Polym. Sci. 2011, 36, 1015–1049. [Google Scholar] [CrossRef]
- Costa, L.; Bracco, P. Mechanisms of Cross-Linking, Oxidative Degradation, and Stabilization of UHMWPE. In UHMWPE Biomateials Handbook Ultra High Molecular Weight Polyethylene in Total Joint Replacement and Medical Devices, 3rd ed.; Elsever Inc.: Amsterdam, The Netherlands, 2015; pp. 467–487. [Google Scholar]
- Bracco, P.; Costa, L.; Luda, M.P.; Billingham, N.A. Review of Experimental Studies of the Role of Free-Radicals in Polyethylene Oxidation. Polym. Degrad. Stab. 2018, 155, 67–83. [Google Scholar] [CrossRef]
- Hinsken, H.; Moss, S.; Pauquet, J.; Zweifel, H. Degradation of Polyolefins during Melt Processing. Polym. Degrad. Stab. 1991, 34, 279–293. [Google Scholar] [CrossRef]
- Wang, Z.M.; Chen, H.M.; Zhang, Y.P.; Wang, Q.Z. Study on the Mechanism of Molecular Weight Reduction of Polyethylene Based on Fe-Montmorillonite and Its Potential Application. Polymers 2023, 15, 1429. [Google Scholar] [CrossRef] [PubMed]
- Abrusci, C.; Pablos, J.L.; Marín, I.; Espí, E.; Corrales, T.; Catalina, F. Comparative effect of metal stearates as pro-oxidant additives on bacterial biodegradation of thermal- and photo-degraded low density polyethylene mulching films. Int. Biodeter. Biodegr. 2013, 83, 25–32. [Google Scholar] [CrossRef]
- Yeh, C.L.; Nikolić, M.A.L.; Gomes, B.; Gauthier, E.; Laycock, B.; Halley, P.; Bottle, S.E.; Colwell, J.M. The effect of common agrichemicals on the environmental stability of polyethylene films. Polym. Degrad. Stab. 2015, 120, 53–60. [Google Scholar] [CrossRef]
- Maalihan, R.D.; Pajarito, B.B. Effect of colorant, thickness, and pro-oxidant loading on degradation of low-density polyethylene films during thermal aging. J. Plast. Film Sheeting 2016, 32, 124–139. [Google Scholar] [CrossRef]
- Gulmine, J.V.; Janissek, P.R.; Heise, H.M.; Akcelrud, L. Degradation Profile of Polyethylene After Artificial Accelerated Weathering. Polym. Degrad. Stab. 2003, 79, 385–397. [Google Scholar] [CrossRef]
- Brandrup, J.; Immergut, E.H. Polymer Handbook; Wiley: Toronto, ON, Canada, 1986; Volume II. [Google Scholar]
- Khabbaz, F.; Albertsson, A.C.; Karlsson, S. Chemical and Morphological Changes of Environmentally Degradable Polyethylene Films Exposed to Thermo-Oxidation. Polym. Degrad. Stab. 1999, 63, 127–138. [Google Scholar] [CrossRef]
- Setnescu, R.; Jipa, S.; Osawa, Z. Chemiluminescence Study on the Oxidation of Several Polyolefins-I. Thermal-Induced Degradation of Additive-Free Polyolefins. Polym. Degrad. Stab. 1998, 60, 377–383. [Google Scholar] [CrossRef]
- Valadez-Gonzalez, A.; Cervantes-Uc, J.M.; Veleva, L. Mineral Filler Influence on the Photo-Oxidation of High Density Polyethylene: I. Accelerated UV Chamber Exposure Test. Polym. Degrad. Stab. 1999, 63, 253–260. [Google Scholar] [CrossRef]
- Liu, X.; Gao, C.; Sangwan, P.; Yu, L.; Tong, Z. Accelerating the Degradation of Polyolefins Through Additives and Blending. J. Appl. Polym. Sci. 2014, 131, 40750. [Google Scholar] [CrossRef]
- Abrusci, C.; Pablos, J.L.; Corrales, T.; López-Marín, J.; Marín, I.; Catalina, F. Biodegradation of photo-degraded mulching films based on polyethylenes and stearates of calcium and iron as pro-oxidant additives. Int. Biodeter. Biodegr. 2011, 65, 451–459. [Google Scholar] [CrossRef]
- Muthukumar, T.; Aravinthan, A.; Mukesh, D. Effect of environment on the degradation of starch and pro-oxidant blended polyolefins. Polym. Degrad. Stab. 2010, 95, 1988–1993. [Google Scholar] [CrossRef]
- Andrady, A.L. Wavelength Sensitivity in Polymer Photodegradation. Adv. Polym. Sci. 1997, 128, 47–94. [Google Scholar]

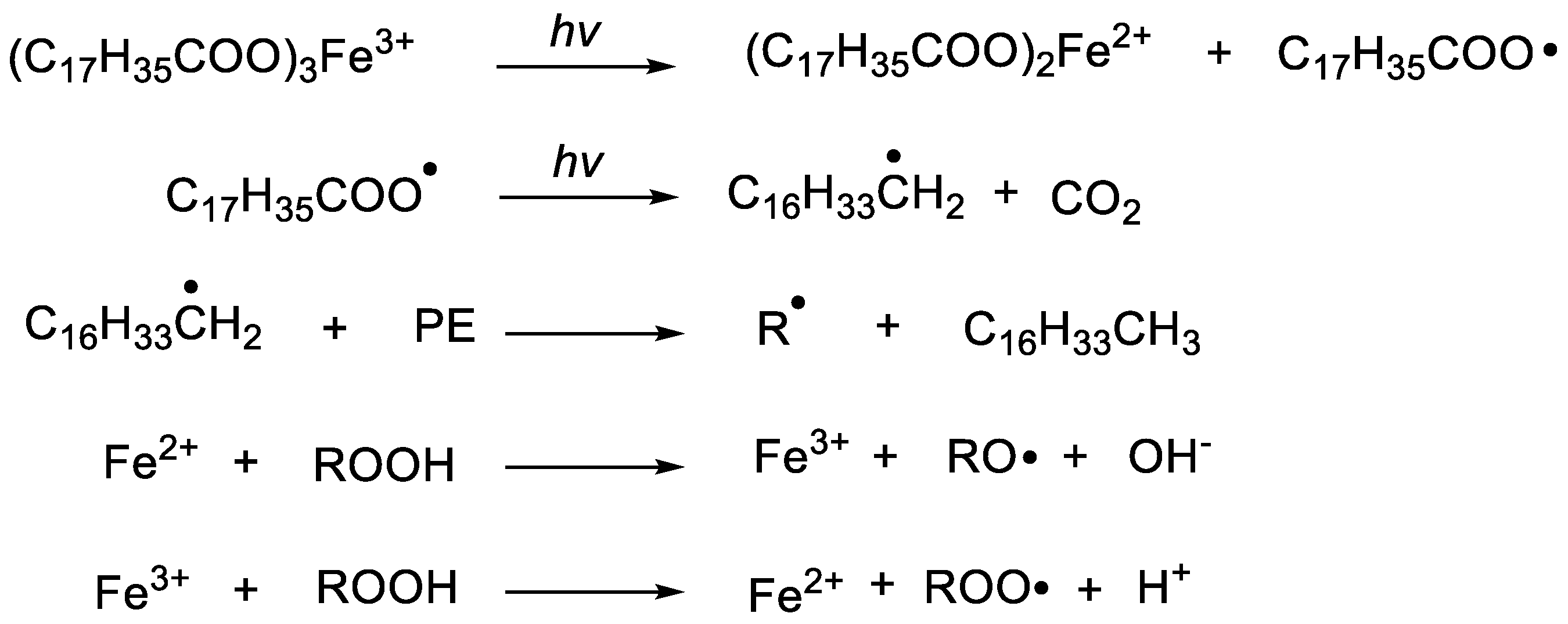





| Sample Name | LLDPE (g) | Masterbatch (g) | FeSt3 (wt%) |
|---|---|---|---|
| PE | 600 | 0 | 0 |
| PE-0.1FS | 596 | 4 | 0.1 |
| PE-0.5FS | 580 | 20 | 0.5 |
| PE-0.7FS | 572 | 28 | 0.7 |
| Wavenumber (cm−1) | Attribution |
|---|---|
| 1780 | lactones |
| 1733 | aldehydes |
| 1716 | ketones |
| 1701 | carboxylic acids |
| Sample | Mv at UV Irradiation Time | |||||
|---|---|---|---|---|---|---|
| 0 h | 24 h | 48 h | 72 h | 96 h | 120 h | |
| PE | 52,336 | 50,111 | 48,449 | 45,502 | 44,448 | 42,571 |
| PE-0.1FS | 51,533 | 42,733 | 31,198 | 22,067 | 15,575 | 14,428 |
| PE-0.5FS | 51,376 | 37,182 | 17,386 | 11,054 | 7739 | 6674 |
| PE-0.7FS | 50,274 | 34,929 | 15,626 | 10,454 | 5643 | 5174 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wang, Z.; Wang, Z.; Liu, D.; Wang, Q. Peculiarity of the Mechanism of Early Stages of Photo-Oxidative Degradation of Linear Low-Density Polyethylene Films in the Presence of Ferric Stearate. Polymers 2023, 15, 3672. https://doi.org/10.3390/polym15183672
Wang Z, Wang Z, Liu D, Wang Q. Peculiarity of the Mechanism of Early Stages of Photo-Oxidative Degradation of Linear Low-Density Polyethylene Films in the Presence of Ferric Stearate. Polymers. 2023; 15(18):3672. https://doi.org/10.3390/polym15183672
Chicago/Turabian StyleWang, Zhiming, Zhongwei Wang, Dayong Liu, and Qingzhao Wang. 2023. "Peculiarity of the Mechanism of Early Stages of Photo-Oxidative Degradation of Linear Low-Density Polyethylene Films in the Presence of Ferric Stearate" Polymers 15, no. 18: 3672. https://doi.org/10.3390/polym15183672





