Comparative Evaluation of the In Vitro Cytotoxicity of a Series of Chitosans and Chitooligosaccharides Water-Soluble at Physiological pH
Abstract
:1. Introduction
2. Materials and Methods
2.1. Preparation of COS with Low DA (<1%) by Nitrous Acid Depolymerization of Chitosan
2.2. Preparation of CS and COS with High DA by Acetic Anhydride Reacetylation
2.2.1. Preparation of COS with High DA (From 35% to 57%)
2.2.2. Preparation of CS with High DA (~50%)
2.3. Characterization Methods of CS and COS
2.3.1. Proton Nuclear Magnetic Resonance Spectroscopy (1H NMR)
2.3.2. Size Exclusion Chromatography (SEC)
2.3.3. Thermogravimetric Analysis (TGA)
2.4. L929 Cell Culture
2.5. In Vitro Standard Cytotoxicity Test
2.6. Osmolality of Solutions
2.7. Statistical Analysis
3. Results
3.1. Structural Characterizations of Synthesized COS and CS and Determination of Their Osmolality
3.2. Cytotoxicity Analysis
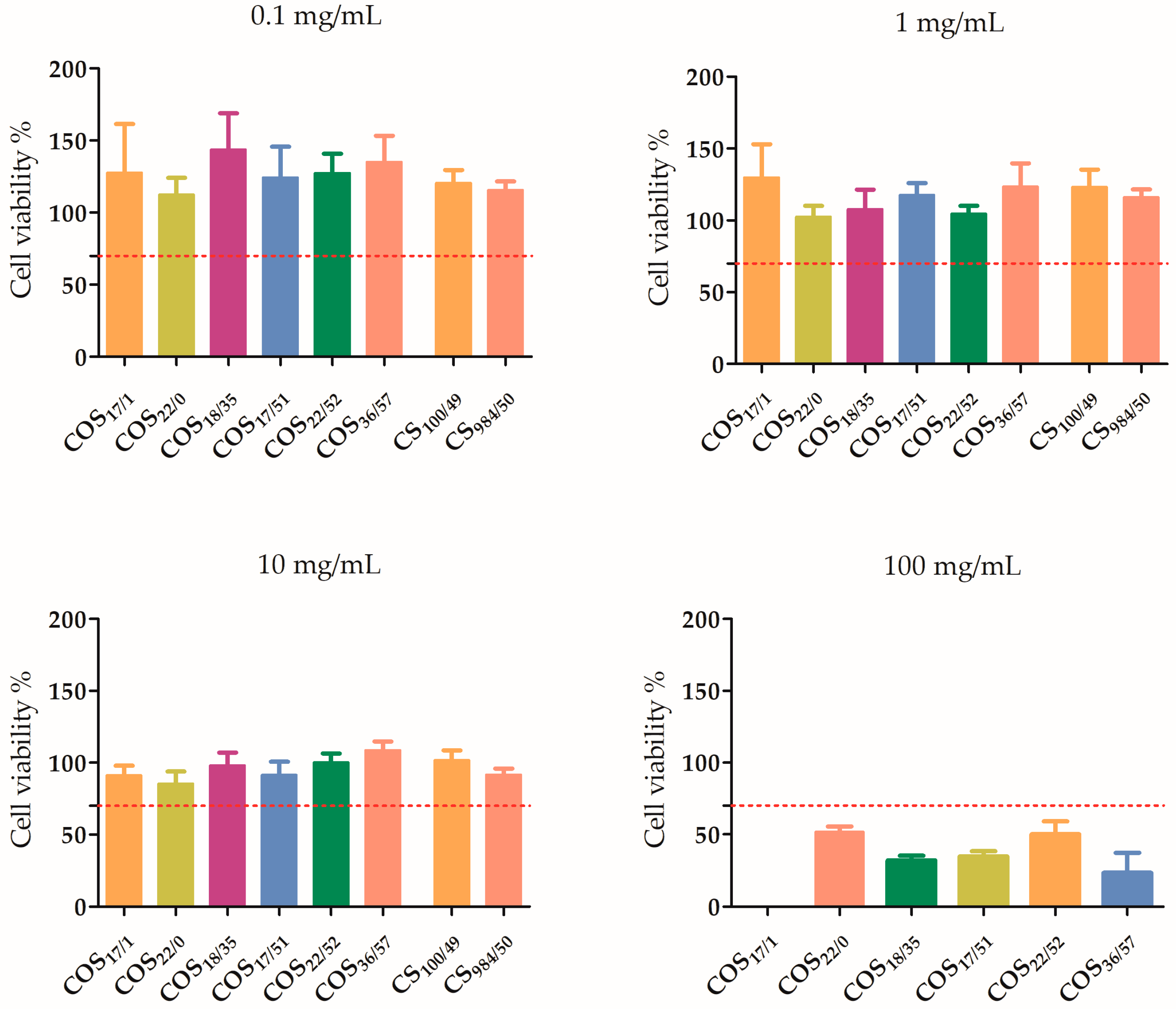
3.2.1. Influence of the Concentration
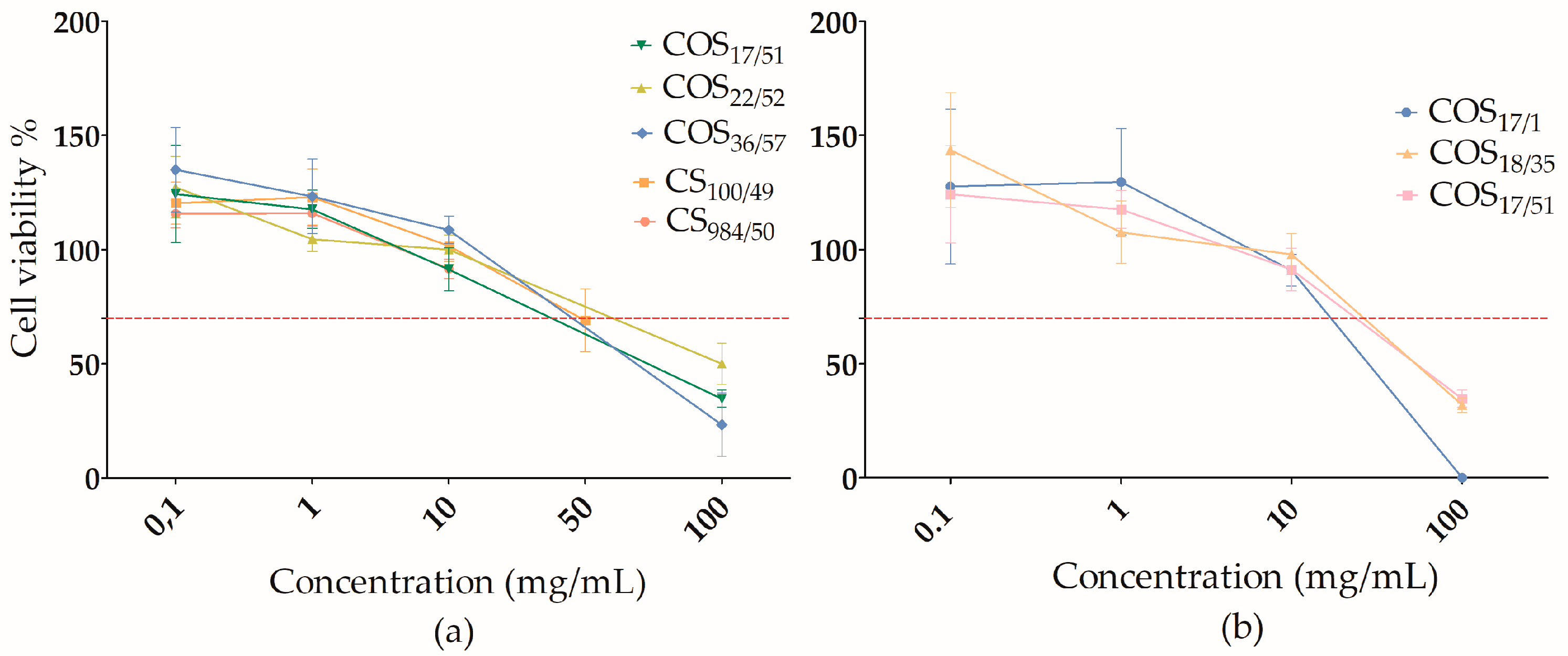
3.2.2. Influence of DP and DA
4. Discussion
4.1. Influence of the Concentration
4.2. Influence of DP and DA
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Fakhri, E.; Eslami, H.; Maroufi, P.; Pakdel, F.; Taghizadeh, S.; Ganbarov, K.; Yousefi, M.; Tanomand, A.; Yousefi, B.; Mahmoudi, S.; et al. Chitosan Biomaterials Application in Dentistry. Int. J. Biol. Macromol. 2020, 162, 956–974. [Google Scholar] [CrossRef]
- Muanprasat, C.; Chatsudthipong, V. Chitosan Oligosaccharide: Biological Activities and Potential Therapeutic Applications. Pharmacol. Ther. 2017, 170, 80–97. [Google Scholar] [CrossRef]
- Younes, I.; Rinaudo, M. Chitin and Chitosan Preparation from Marine Sources. Structure, Properties and Applications. Mar. Drugs 2015, 13, 1133–1174. [Google Scholar] [CrossRef] [PubMed]
- Yeul, V.S.; Rayalu, S.S. Unprecedented Chitin and Chitosan: A Chemical Overview. J. Polym. Environ. 2013, 21, 606–614. [Google Scholar] [CrossRef]
- Zargar, V.; Asghari, M.; Dashti, A. A Review on Chitin and Chitosan Polymers: Structure, Chemistry, Solubility, Derivatives, and Applications. ChemBioEng Rev. 2015, 2, 204–226. [Google Scholar] [CrossRef]
- Chatterjee, R.; Maity, M.; Hasnain, M.S.; Nayak, A.K. Chitosan: Source, Chemistry, and Properties. In Chitosan in Drug Delivery; Elsevier: Amsterdam, The Netherlands, 2022; pp. 1–22. ISBN 978-0-12-819336-5. [Google Scholar]
- Bhattarai, N.; Gunn, J.; Zhang, M. Chitosan-Based Hydrogels for Controlled, Localized Drug Delivery. Adv. Drug Deliv. Rev. 2010, 62, 83–99. [Google Scholar] [CrossRef] [PubMed]
- Elieh-Ali-Komi, D.; Hamblin, M.R. Chitin and Chitosan: Production and Application of Versatile Biomedical Nanomaterials. Int. J. Adv. Res. 2016, 4, 411–427. [Google Scholar]
- Ligler, F.S.; Lingerfelt, B.M.; Price, R.P.; Schoen, P.E. Development of Uniform Chitosan Thin-Film Layers on Silicon Chips. Langmuir 2001, 17, 5082–5084. [Google Scholar] [CrossRef]
- Cavallaro, G.; Micciulla, S.; Chiappisi, L.; Lazzara, G. Chitosan-Based Smart Hybrid Materials: A Physico-Chemical Perspective. J. Mater. Chem. B 2021, 9, 594–611. [Google Scholar] [CrossRef]
- Morin-Crini, N.; Lichtfouse, E.; Torri, G.; Crini, G. Applications of Chitosan in Food, Pharmaceuticals, Medicine, Cosmetics, Agriculture, Textiles, Pulp and Paper, Biotechnology, and Environmental Chemistry. Environ. Chem. Lett. 2019, 17, 1667–1692. [Google Scholar] [CrossRef]
- Hamed, I.; Özogul, F.; Regenstein, J.M. Industrial Applications of Crustacean By-Products (Chitin, Chitosan, and Chitooligosaccharides): A Review. Trends Food Sci. Technol. 2016, 48, 40–50. [Google Scholar] [CrossRef]
- Xia, W.; Liu, P.; Zhang, J.; Chen, J. Biological Activities of Chitosan and Chitooligosaccharides. Food Hydrocoll. 2011, 25, 170–179. [Google Scholar] [CrossRef]
- Dash, M.; Chiellini, F.; Ottenbrite, R.M.; Chiellini, E. Chitosan—A Versatile Semi-Synthetic Polymer in Biomedical Applications. Prog. Polym. Sci. 2011, 36, 981–1014. [Google Scholar] [CrossRef]
- Wang, W.; Xue, C.; Mao, X. Chitosan: Structural Modification, Biological Activity and Application. Int. J. Biol. Macromol. 2020, 164, 4532–4546. [Google Scholar] [CrossRef] [PubMed]
- Moussa, A.; Crépet, A.; Ladavière, C.; Trombotto, S. Reducing-End “Clickable” Functionalizations of Chitosan Oligomers for the Synthesis of Chitosan-Based Diblock Copolymers. Carbohydr. Polym. 2019, 219, 387–394. [Google Scholar] [CrossRef]
- Chapelle, C.; David, G.; Caillol, S.; Negrell, C.; Durand, G.; Le Foll, M.D.; Trombotto, S. Water-Soluble 2,5-Anhydro-d-Mannofuranose Chain End Chitosan Oligomers of a Very Low Molecular Weight: Synthesis and Characterization. Biomacromolecules 2019, 20, 4353–4360. [Google Scholar] [CrossRef]
- Naveed, M.; Phil, L.; Sohail, M.; Hasnat, M.; Baig, M.M.F.A.; Ihsan, A.U.; Shumzaid, M.; Kakar, M.U.; Khan, T.M.; Akabar, M.; et al. Chitosan Oligosaccharide (COS): An Overview. Int. J. Biol. Macromol. 2019, 129, 827–843. [Google Scholar] [CrossRef] [PubMed]
- Cord-Landwehr, S.; Richter, C.; Wattjes, J.; Sreekumar, S.; Singh, R.; Basa, S.; El Gueddari, N.E.; Moerschbacher, B.M. Patterns Matter Part 2: Chitosan Oligomers with Defined Patterns of Acetylation. React. Funct. Polym. 2020, 151, 104577. [Google Scholar] [CrossRef]
- Vanderzwalmen, P.; Ectors, F.; Panagiotidis, Y.; Schuff, M.; Murtinger, M.; Wirleitner, B. The Evolution of the Cryopreservation Techniques in Reproductive Medicine—Exploring the Character of the Vitrified State Intra- and Extracellularly to Better Understand Cell Survival after Cryopreservation. Reprod. Med. 2020, 1, 142–157. [Google Scholar] [CrossRef]
- Jang, T.H.; Park, S.C.; Yang, J.H.; Kim, J.Y.; Seok, J.H.; Park, U.S.; Choi, C.W.; Lee, S.R.; Han, J. Cryopreservation and Its Clinical Applications. Integr. Med. Res. 2017, 6, 12–18. [Google Scholar] [CrossRef]
- Ren, R.; Li, Z.; Li, B.; Xu, J.; Jiang, X.; Liu, Y.; Zhang, K. Changes of Pollen Viability of Ornamental Plants after Long-Term Preservation in a Cryopreservation Pollen Bank. Cryobiology 2019, 89, 14–20. [Google Scholar] [CrossRef] [PubMed]
- Yong, K.W.; Laouar, L.; Elliott, J.A.W.; Jomha, N.M. Review of Non-Permeating Cryoprotectants as Supplements for Vitrification of Mammalian Tissues. Cryobiology 2020, 96, 1–11. [Google Scholar] [CrossRef]
- Le Tallec, B.; Ponsart, C.; Guienne, B.M.-L.; Guérina, B. Risks of Transmissible Diseases in Relation to Embryo Transfer. Reprod. Nutr. Dev. 2001, 41, 439–450. [Google Scholar] [CrossRef] [PubMed]
- Rienzi, L.; Gracia, C.; Maggiulli, R.; LaBarbera, A.R.; Kaser, D.J.; Ubaldi, F.M.; Vanderpoel, S.; Racowsky, C. Oocyte, Embryo and Blastocyst Cryopreservation in ART: Systematic Review and Meta-Analysis Comparing Slow-Freezing versus Vitrification to Produce Evidence for the Development of Global Guidance. Hum. Reprod. Update 2016, 23, 139–155. [Google Scholar] [CrossRef]
- Chang, C.-C.; Shapiro, D.B.; Nagy, Z.P. The Effects of Vitrification on Oocyte Quality. Biol. Reprod. 2022, 106, 316–327. [Google Scholar] [CrossRef] [PubMed]
- Amorim, C.A.; Curaba, M.; Van Langendonckt, A.; Dolmans, M.-M.; Donnez, J. Vitrification as an Alternative Means of Cryopreserving Ovarian Tissue. Reprod. BioMed Online 2011, 23, 160–186. [Google Scholar] [CrossRef]
- Murray, K.A.; Gibson, M.I. Chemical Approaches to Cryopreservation. Nat. Rev. Chem. 2022, 6, 579–593. [Google Scholar] [CrossRef]
- Sarmadi, F.; Kazemi, P.; Tirgar, P.; Fayazi, S.; Esfandiari, S.; Sotoodeh, L.; Molaeian, S.; Dashtizad, M. Using Natural Honey as an Anti-Oxidant and Thermodynamically Efficient Cryoprotectant in Embryo Vitrification. Cryobiology 2019, 91, 30–39. [Google Scholar] [CrossRef]
- Kuleshova, L.L.; MacFarlane, D.R.; Trounson, A.O.; Shaw, J.M. Sugars Exert a Major Influence on the Vitrification Properties of Ethylene Glycol-Based Solutions and Have Low Toxicity to Embryos and Oocytes. Cryobiology 1999, 38, 119–130. [Google Scholar] [CrossRef]
- Herrick, J.R.; Wang, C.; Machaty, Z. The Effects of Permeating Cryoprotectants on Intracellular Free-Calcium Concentrations and Developmental Potential of in Vitro-Matured Feline Oocytes. Reprod. Fertil. Dev. 2016, 28, 599. [Google Scholar] [CrossRef]
- ISO 10993-5; Biological Evaluation of Medical Devices—Part 5: Tests for In Vitro Cytotoxicity. ISO: Geneva, Switzerland, 2009.
- Yang, Y.; Yu, B. Recent Advances in the Synthesis of Chitooligosaccharides and Congeners. Tetrahedron 2014, 70, 1023–1046. [Google Scholar] [CrossRef]
- Tømmeraas, K.; Vårum, K.M.; Christensen, B.E.; Smidsrød, O. Preparation and Characterisation of Oligosaccharides Produced by Nitrous Acid Depolymerisation of Chitosans. Carbohydr. Res. 2001, 333, 137–144. [Google Scholar] [CrossRef]
- Abla, M.; Marmuse, L.; Delolme, F.; Vors, J.-P.; Ladavière, C.; Trombotto, S. Access to Tetra-N-Acetyl-Chitopentaose by Chemical N-Acetylation of Glucosamine Pentamer. Carbohydr. Polym. 2013, 98, 770–777. [Google Scholar] [CrossRef]
- Lamarque, G.; Lucas, J.-M.; Viton, C.; Domard, A. Physicochemical Behavior of Homogeneous Series of Acetylated Chitosans in Aqueous Solution: Role of Various Structural Parameters. Biomacromolecules 2005, 6, 131–142. [Google Scholar] [CrossRef]
- Hirai, A.; Odani, H.; Nakajima, A. Determination of Degree of Deacetylation of Chitosan by 1H NMR Spectroscopy. Polym. Bull. 1991, 26, 87–94. [Google Scholar] [CrossRef]
- Schatz, C.; Viton, C.; Delair, T.; Pichot, C.; Domard, A. Typical Physicochemical Behaviors of Chitosan in Aqueous Solution. Biomacromolecules 2003, 4, 641–648. [Google Scholar] [CrossRef] [PubMed]
- ISO 10993-12; Biological Evaluation of Medical Devices—Part 12: Sample Preparation and Reference Materials. ISO: Geneva, Switzerland, 2012.
- Zubareva, A.; Shagdarova, B.; Varlamov, V.; Kashirina, E.; Svirshchevskaya, E. Penetration and Toxicity of Chitosan and Its Derivatives. Eur. Polym. J. 2017, 93, 743–749. [Google Scholar] [CrossRef]
- Kean, T.; Thanou, M. Biodegradation, Biodistribution and Toxicity of Chitosan. Adv. Drug Deliv. Rev. 2010, 62, 3–11. [Google Scholar] [CrossRef]
- Mourya, V.K.; Inamdar, N.N.; Choudhari, Y.M. Chitooligosaccharides: Synthesis, Characterization and Applications. Polym. Sci. Ser. A 2011, 53, 583–612. [Google Scholar] [CrossRef]
- Allan, G.G.; Peyron, M. Molecular Weight Manipulation of Chitosan I: Kinetics of Depolymerization by Nitrous Acid. Carbohydr. Res. 1995, 277, 257–272. [Google Scholar] [CrossRef]
- De Oliveira, P.N.; Moussa, A.; Milhau, N.; Dosciatti Bini, R.; Prouillac, C.; Ferraz De Oliveira, B.; Dias, G.S.; Santos, I.A.; Morfin, I.; Sudre, G.; et al. In Situ Synthesis of Fe3O4 Nanoparticles Coated by Chito-Oligosaccharides: Physico-Chemical Characterizations and Cytotoxicity Evaluation for Biomedical Applications. Nanotechnology 2020, 31, 175602. [Google Scholar] [CrossRef] [PubMed]
- Salehi, F.; Behboudi, H.; Kavoosi, G.; Ardestani, S.K. Chitosan Promotes ROS-Mediated Apoptosis and S Phase Cell Cycle Arrest in Triple-Negative Breast Cancer Cells: Evidence for Intercalative Interaction with Genomic DNA. RSC Adv. 2017, 7, 43141–43150. [Google Scholar] [CrossRef]
- Schimpf, U.; Nachmann, G.; Trombotto, S.; Houska, P.; Yan, H.; Björndahl, L.; Crouzier, T. Assessment of Oligo-Chitosan Biocompatibility toward Human Spermatozoa. ACS Appl. Mater. Interfaces 2019, 11, 46572–46584. [Google Scholar] [CrossRef] [PubMed]
- Mao, S.; Shuai, X.; Unger, F.; Simon, M.; Bi, D.; Kissel, T. The Depolymerization of Chitosan: Effects on Physicochemical and Biological Properties. Int. J. Pharm. 2004, 281, 45–54. [Google Scholar] [CrossRef]
- Fernandes, J.C.; Borges, M.; Nascimento, H.; Bronze-da-Rocha, E.; Ramos, O.S.; Pintado, M.E.; Malcata, F.X.; Santos-Silva, A. Cytotoxicity and Genotoxicity of Chitooligosaccharides upon Lymphocytes. Int. J. Biol. Macromol. 2011, 49, 433–438. [Google Scholar] [CrossRef] [PubMed]
- Chae, S.Y.; Jang, M.-K.; Nah, J.-W. Influence of Molecular Weight on Oral Absorption of Water Soluble Chitosans. J. Control. Release 2005, 102, 383–394. [Google Scholar] [CrossRef]
- Huang, M.; Khor, E.; Lim, L.-Y. Uptake and Cytotoxicity of Chitosan Molecules and Nanoparticles: Effects of Molecular Weight and Degree of Deacetylation. Pharm. Res. 2004, 21, 344–353. [Google Scholar] [CrossRef]
- Schipper, N.G.M.; Vårum, K.M.; Artursson, P. Chitosans as Absorption Enhancers for Poorly Absorbable Drugs. 1: Influence of Molecular Weight and Degree of Acetylation on Drug Transport across Human Intestinal Epithelial (Caco-2) Cells. Pharm. Res. 1996, 13, 1686–1692. [Google Scholar] [CrossRef]

| Sample | DP a | DA (%) b | Mn (kg/mol) a | Mw (kg/mol) a | Ð a | Water Content (% w/w) c | Ash Content (% w/w) c | Solubility Threshold (mg/mL) |
|---|---|---|---|---|---|---|---|---|
| COS | ||||||||
| COS17/1 | 17 | 1 | 2.68 | 3.19 | 1.19 | 5.1 | 1.3 | 100 |
| COS22/0 | 22 | 0 | 3.53 | 5.08 | 1.44 | 6.4 | 0 | 100 |
| COS18/35 | 18 | 35 | 3.09 | 4.97 | 1.61 | 9.8 | 0.5 | 100 |
| COS17/51 | 17 | 51 | 3.17 | 5.05 | 1.60 | 6.5 | 0.4 | 100 |
| COS22/52 | 22 | 52 | 3.94 | 5.17 | 1.31 | 16.5 | 0 | 100 |
| COS36/57 | 36 | 57 | 6.60 | 12.1 | 1.83 | 11.6 | 0 | 100 |
| CS | ||||||||
| CS100/49 | 100 | 49 | 18.3 | 25.6 | 1.40 | 10.2 | 0.8 | 50 |
| CS984/50 | 984 | 50 | 179 | 318 | 1.77 | 12.9 | 0 | 10 |
| Solution | Concentration (mg/mL) | |||||
|---|---|---|---|---|---|---|
| 0 | 0.1 | 1 | 10 | 50 | 100 | |
| DMEM | 341.2 ± 25.3 | |||||
| DMEM + COS17/1 | 323.1 ± 12.0 | 323.1 ± 12.0 | 349.9 ± 38.0 | 431.6 ± 48.0 * | ||
| DMEM + COS22/0 | 320.3 ± 14.6 | 325.2 ± 11.6 | 349.4 ± 10.3 | 479.8 ± 62.2 *** | ||
| DMEM + COS18/35 | 320.4 ± 10.7 | 324.7 ± 12.5 | 359.1 ± 36.7 | 465.4 ± 18.6 *** | ||
| DMEM + COS17/51 | 325.3 ± 11.8 | 325.7 ± 10.8 | 366.0 ± 32.4 | 455.2 ± 16.4 ** | ||
| DMEM + COS22/52 | 344.6 ± 30.3 | 332.1 ± 21.0 | 353.1 ± 14.4 | 387.0 ± 23.3 | ||
| DMEM + COS36/57 | 319.1 ± 8.3 | 324.6 ± 8.2 | 329.0 ± 33.6 | 403.3 ± 47.5 | ||
| DMEM + CS100/49 | 320.9 ± 25.1 | 330.9 ± 25.1 | 341.3 ± 14.2 | 355.5 ± 70.0 a | ||
| DMEM + CS984/50 | 321.4 ± 18.1 | 324.8 ± 11.1 | 358.4 ± 40.0 | |||
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Dias, C.; Commin, L.; Bonnefont-Rebeix, C.; Buff, S.; Bruyère, P.; Trombotto, S. Comparative Evaluation of the In Vitro Cytotoxicity of a Series of Chitosans and Chitooligosaccharides Water-Soluble at Physiological pH. Polymers 2023, 15, 3679. https://doi.org/10.3390/polym15183679
Dias C, Commin L, Bonnefont-Rebeix C, Buff S, Bruyère P, Trombotto S. Comparative Evaluation of the In Vitro Cytotoxicity of a Series of Chitosans and Chitooligosaccharides Water-Soluble at Physiological pH. Polymers. 2023; 15(18):3679. https://doi.org/10.3390/polym15183679
Chicago/Turabian StyleDias, Catia, Loris Commin, Catherine Bonnefont-Rebeix, Samuel Buff, Pierre Bruyère, and Stéphane Trombotto. 2023. "Comparative Evaluation of the In Vitro Cytotoxicity of a Series of Chitosans and Chitooligosaccharides Water-Soluble at Physiological pH" Polymers 15, no. 18: 3679. https://doi.org/10.3390/polym15183679
APA StyleDias, C., Commin, L., Bonnefont-Rebeix, C., Buff, S., Bruyère, P., & Trombotto, S. (2023). Comparative Evaluation of the In Vitro Cytotoxicity of a Series of Chitosans and Chitooligosaccharides Water-Soluble at Physiological pH. Polymers, 15(18), 3679. https://doi.org/10.3390/polym15183679






