Characterization and Application in Natural Rubber of Leucaena Leaf and Its Extracted Products
Abstract
:1. Introduction
2. Experimental
2.1. Materials
2.2. Preparation of LLP, DPP, and RP
2.3. Characterization of LLP, DPP, and RP
2.4. Rubber Compound Preparation and Testing
3. Results and Discussion
3.1. Basic Characterization of LLP, DPP, and RP
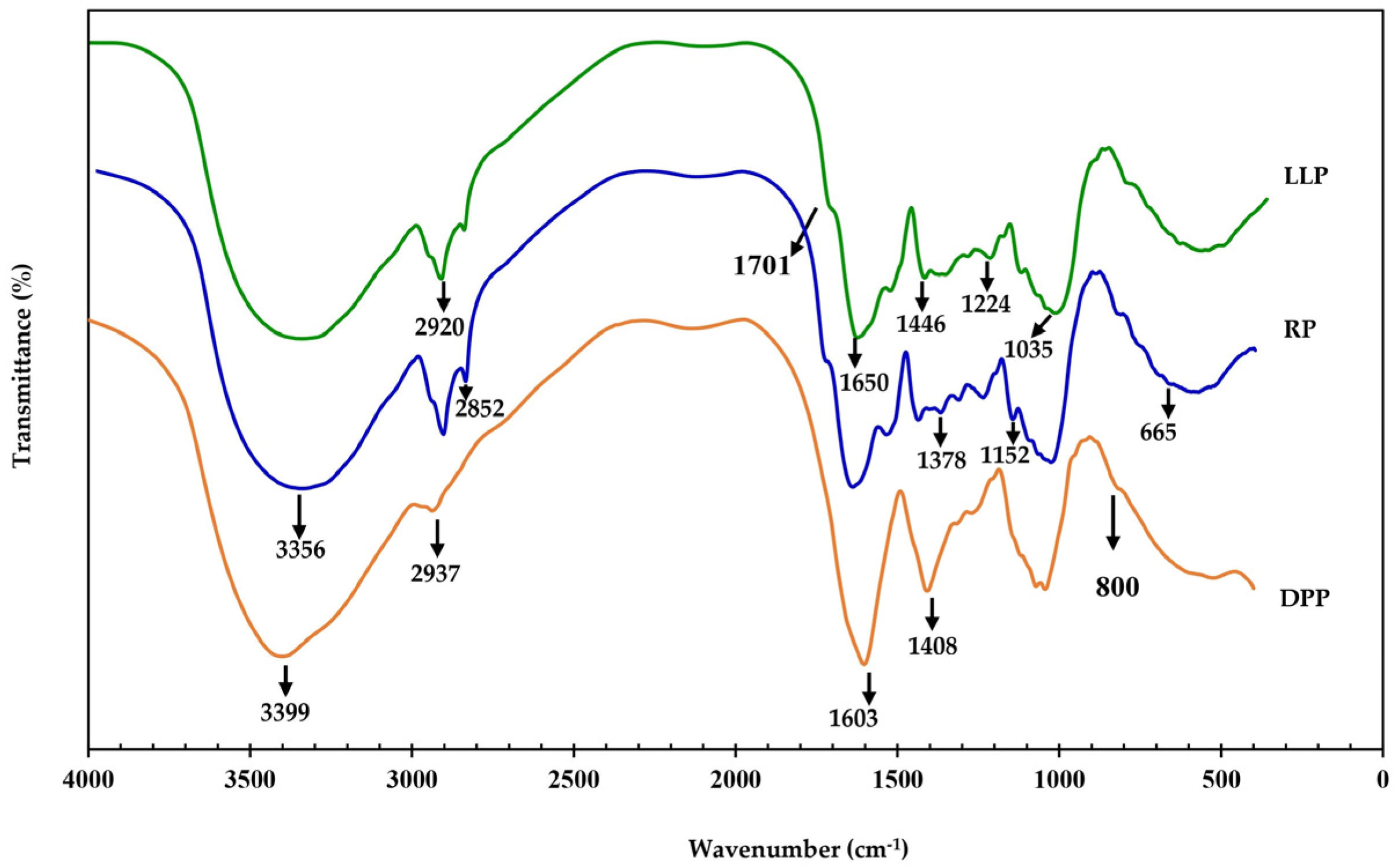
3.1.1. Fourier Transform Infrared Spectroscopy (FTIR)
3.1.2. Thermogravimetric Analysis (TGA)
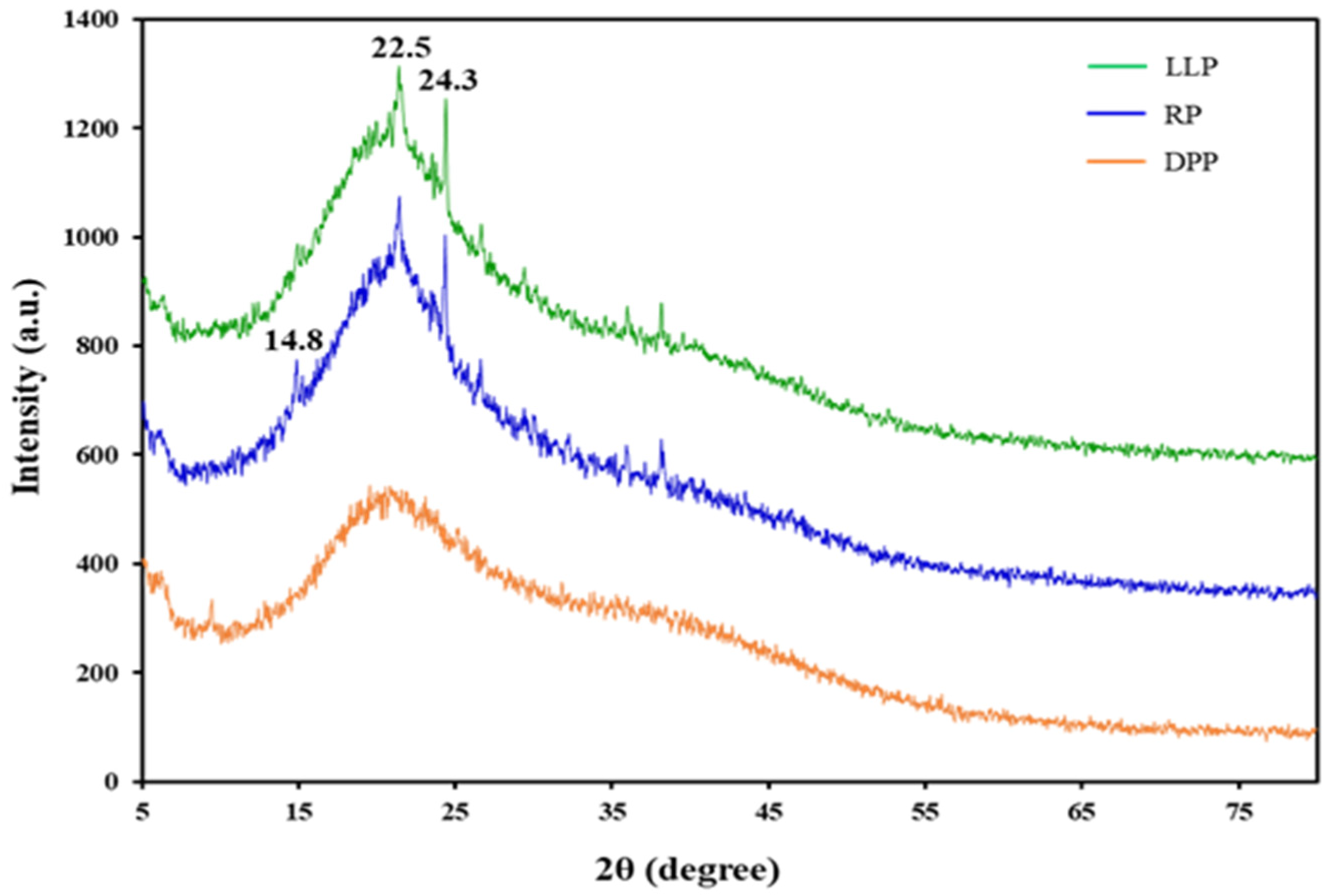
3.1.3. X-ray Diffraction Spectroscopy (XRD)
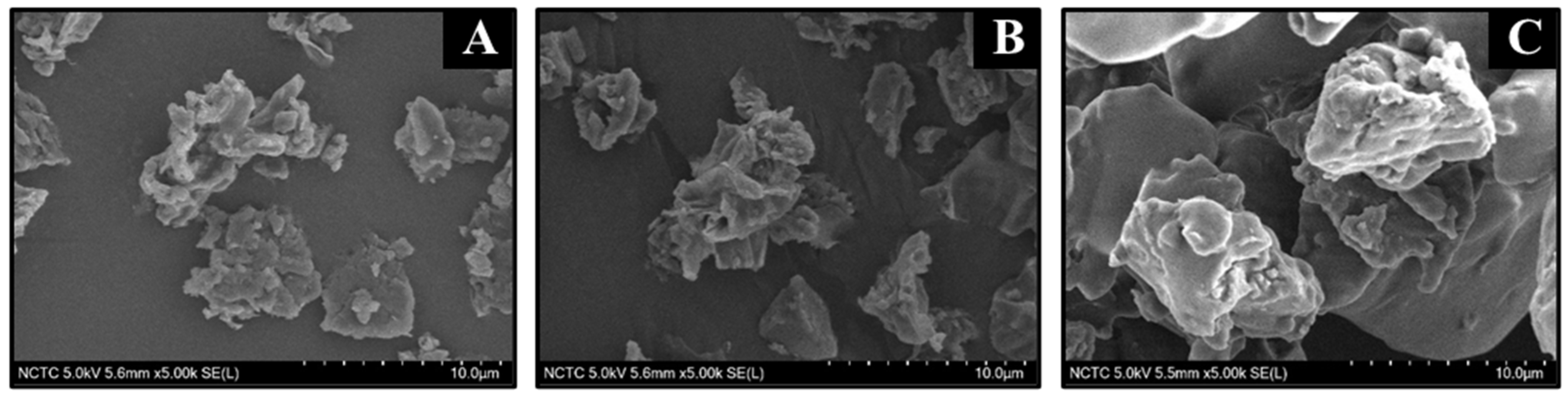
3.1.4. Scanning Electron Microscope (SEM)
3.1.5. Particle Size
3.1.6. Protein Analysis
3.2. Properties of Rubber Compounds
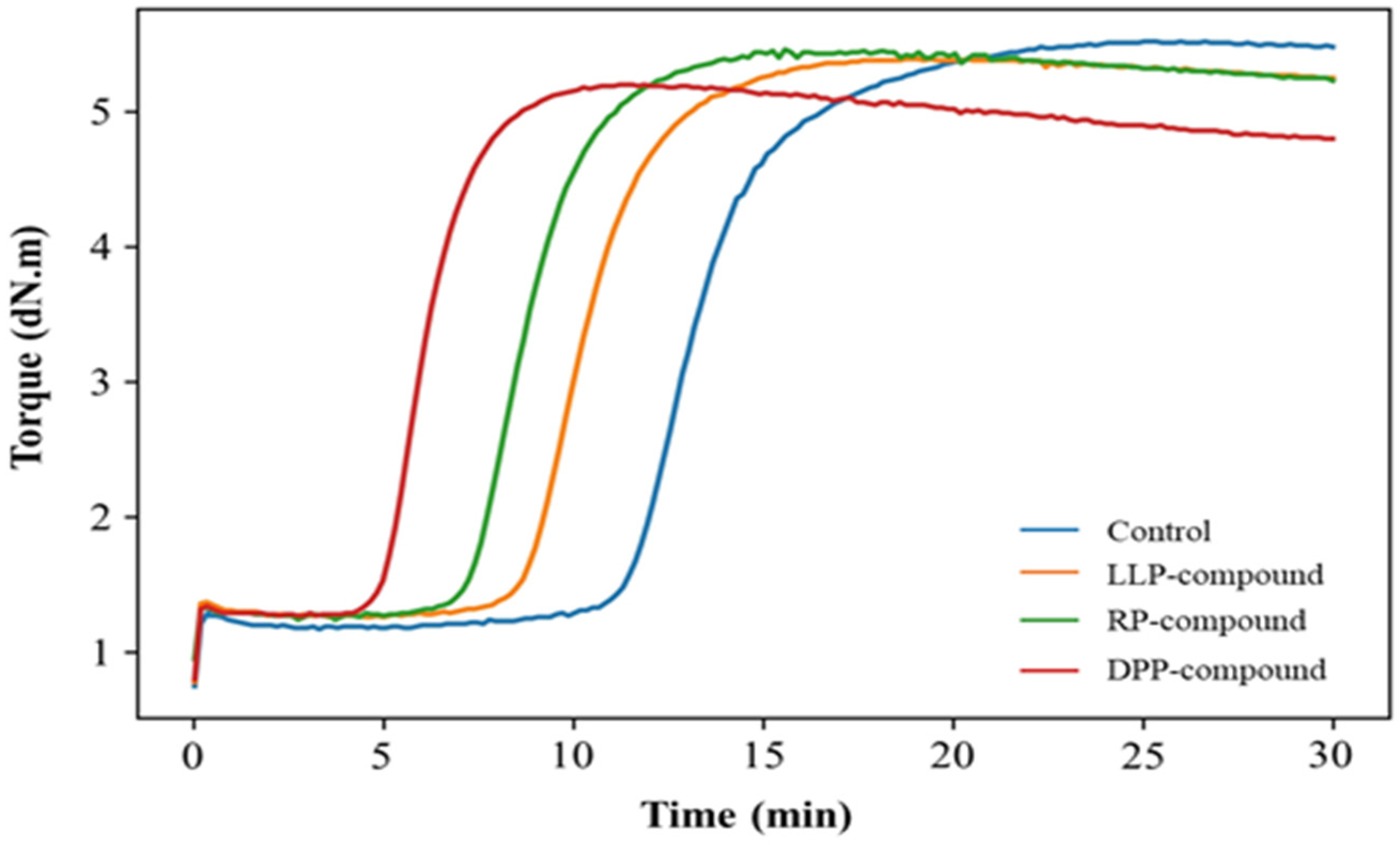
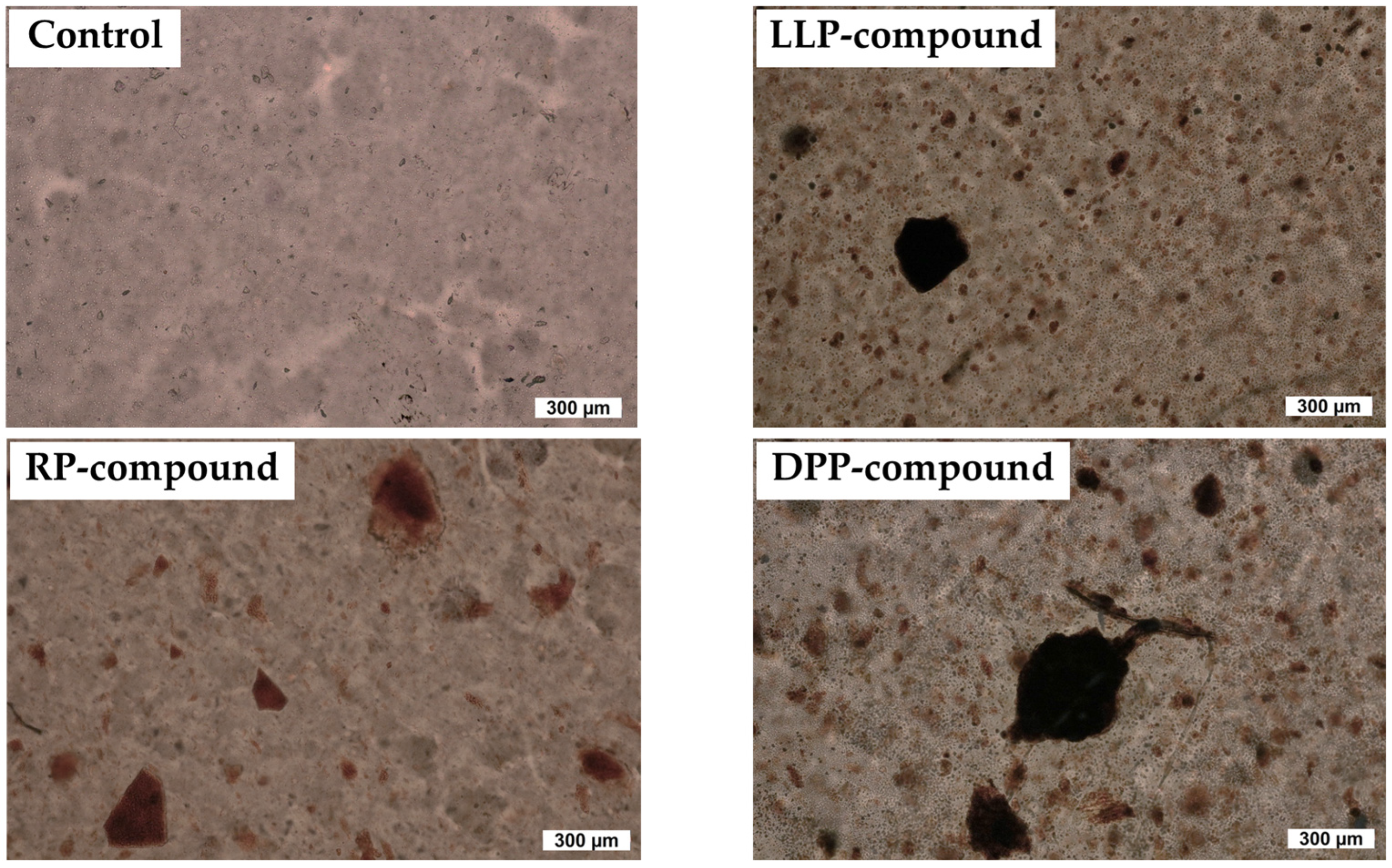
3.2.1. Cure Characteristics
3.2.2. Mechanical Properties
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Sethulekshmi, A.S.; Saritha, A.; Joseph, K. A comprehensive review on the recent advancements in natural rubber nanocomposites. Int. J. Biol. Macromol. 2022, 194, 819–842. [Google Scholar] [CrossRef] [PubMed]
- Shinde, S.A.; More, P.R.; Ingle, A.P. 16—Hazardous effects of nanomaterials on aquatic life. In Nanotechnology in Agriculture and Agroecosystems; Ingle, A.P., Ed.; Elsevier: Amsterdam, The Netherlands, 2023; pp. 423–450. [Google Scholar]
- Alam, M.D.; Kumar, V.; Park, S.S. Advances in Rubber Compounds Using ZnO and MgO as Co-Cure Activators. Polymers 2022, 14, 5289. [Google Scholar] [CrossRef] [PubMed]
- Alam, M.N.; Debnath, S.C.; Boondamnoen, O.; Kumar, K.N.; Kim, J.H.; Choi, J. Synergistic combination of 2-mercaptobenzothiazole (MBT) and nitrosoamine-safe thiuram disulfide as advanced rubber vulcanizing accelerators. J. Elastom. Plast. 2022, 54, 1061–1077. [Google Scholar] [CrossRef]
- Masłowski, M.; Miedzianowska, J.; Strzelec, K. Natural rubber biocomposites containing corn, barley and wheat straw. Polym. Test. 2017, 63, 84–91. [Google Scholar] [CrossRef]
- DeButts, B.L.; Thompson, R.V.; Barone, J.R. Hydrolyzed wheat protein as a self-assembled reinforcing filler in synthetic isoprene rubber vulcanizates. Ind. Crops Prod. 2019, 141, 111815. [Google Scholar] [CrossRef]
- Rajisha, K.R.; Maria, H.J.; Pothan, L.A.; Ahmad, Z.; Thomas, S. Preparation and characterization of potato starch nanocrystal reinforced natural rubber nanocomposites. Int. J. Biol. Macromol. 2014, 67, 147–153. [Google Scholar] [CrossRef]
- Thomas, S.K.; Dileep, P.; Begum, P.M.S. Green polymer nanocomposites based on natural rubber and nanocellulose whiskers from Acacia caesia: Mechanical, thermal, and diffusion properties. Mater. Today Proc. 2022, 51, 2444–2449. [Google Scholar] [CrossRef]
- Hirase, R.; Nagatani, A.; Yuguchi, Y. Development of powdering method for cellulose nanofibers assisted by zinc oxide for compounding reinforced natural rubber composite. CRGSC 2020, 3, 100005. [Google Scholar] [CrossRef]
- Lorwanishpaisarn, N.; Sae-Oui, P.; Amnuaypanich, S.; Siriwong, C. Fabrication of untreated and silane-treated carboxylated cellulose nanocrystals and their reinforcement in natural rubber biocomposites. Sci. Rep. 2023, 13, 2517. [Google Scholar] [CrossRef]
- Somseemee, O.; Sae-Oui, P.; Siriwong, C. Bio-based epoxidized natural rubber/chitosan/cellulose nanocrystal composites for enhancing mechanical properties, self-healing behavior and triboelectric nanogenerator performance. Cellulose 2022, 29, 8675–8693. [Google Scholar] [CrossRef]
- Somseemee, O.; Saeoui, P.; Schevenels, F.T.; Siriwong, C. Enhanced interfacial interaction between modified cellulose nanocrystals and epoxidized natural rubber via ultraviolet irradiation. Sci. Rep. 2022, 12, 6682. [Google Scholar] [CrossRef]
- Somseemee, O.; Sae-Oui, P.; Siriwong, C. Reinforcement of surface-modified cellulose nanofibrils extracted from Napier grass stem in natural rubber composites. Ind. Crops Prod. 2021, 171, 113881. [Google Scholar] [CrossRef]
- Ren, H.; Qu, Y.; Zhao, S. Reinforcement of Styrene-Butadiene Rubber with Silica Modified by Silane Coupling Agents: Experimental and Theoretical Chemistry Study. Chin. J. Chem. Eng. 2006, 14, 93–98. [Google Scholar] [CrossRef]
- Jantachum, P.; Khumpaitool, B.; Utara, S. Effect of silane coupling agent and cellulose nanocrystals loading on the properties of acrylonitrile butadiene rubber/natural rubber nanocomposites. Ind. Crops Prod. 2023, 195, 116407. [Google Scholar] [CrossRef]
- Zheng, J.; Han, D.; Ye, X.; Wu, X.; Wu, Y.; Wang, Y.; Zhang, L. Chemical and physical interaction between silane coupling agent with long arms and silica and its effect on silica/natural rubber composites. Polymer 2018, 135, 200–210. [Google Scholar] [CrossRef]
- Feng, Y.; Wang, Q.; Li, L.; Ma, Y.; Li, X. Multiscale analysis of silane coupling agent modified rubber-fiber concrete interfaces. Mater. Today Commun. 2023, 35, 105960. [Google Scholar] [CrossRef]
- Tapangnoi, P.; Sae-Oui, P.; Naebpetch, W.; Siriwong, C. Preparation of purified spent coffee ground and its reinforcement in natural rubber composite. Arab. J. Chem. 2022, 15, 103917. [Google Scholar] [CrossRef]
- Barana, D.; Orlandi, M.; Salanti, A.; Castellani, L.; Hanel, T.; Zoia, L. Simultaneous synthesis of cellulose nanocrystals and a lignin-silica biofiller from rice husk: Application for elastomeric compounds. Ind. Crops Prod. 2019, 141, 111822. [Google Scholar] [CrossRef]
- Sae-Oui, P.; Rakdee, C.; Thanmathorn, P. Use of rice husk ash as filler in natural rubber vulcanizates: In comparison with other commercial fillers. J. Appl. Polym. Sci. 2022, 83, 2485–2493. [Google Scholar] [CrossRef]
- Dominic, M.; Joseph, R.; Sabura Begum, P.M.; Kanoth, B.P.; Chandra, J.; Thomas, S. Green tire technology: Effect of rice husk derived nanocellulose (RHNC) in replacing carbon black (CB) in natural rubber (NR) compounding. Carbohydr. Polym. 2020, 230, 115620. [Google Scholar] [CrossRef]
- Krainoi, A.; Sripornsawat, B.; Toh-ae, P.; Kitisavetjit, W.; Pittayavinai, P.; Tangchirapat, W.; Kalkornsurapranee, E.; Johns, J.; Nakaramontri, Y. Utilization of high and low calcium oxide fly ashes as the alternative fillers for natural rubber composites: A waste to wealth approach. Ind. Crops Prod. 2022, 188, 115589. [Google Scholar] [CrossRef]
- Moonlek, B.; Saenboonruang, K. Mechanical and electrical properties of radiation-vulcanized natural rubber latex with waste eggshell powder as bio-fillers. Radiat. Eff. Defects Solids. 2019, 174, 452–466. [Google Scholar] [CrossRef]
- Hayichelaeh, C.; Boonkerd, K. Utilization of palm oil as an alternative processing oil in carbon black-filled natural rubber compounds. Ind. Crops Prod. 2023, 194, 116270. [Google Scholar] [CrossRef]
- Sökmen, S.; Oßwald, K.; Reincke, K.; Ilisch, S. Influence of Treated Distillate Aromatic Extract (TDAE) Content and Addition Time on Rubber-Filler Interactions in Silica Filled SBR/BR Blends. Polymers 2021, 13, 698. [Google Scholar] [CrossRef]
- Sergio, R.-P.; Susana, R.-M.; Alberto, D.J.; Socorro, R.-M. Leucaena leucocephala extract has estrogenic and antiestrogenic actions on female rat reproduction. Physiol. Behav. 2019, 211, 112683. [Google Scholar] [CrossRef]
- Sereewatthanawut, I.; Prapintip, S.; Watchiraruji, K.; Goto, M.; Sasaki, M.; Shotipruk, A. Extraction of protein and amino acids from deoiled rice bran by subcritical water hydrolysis. Bioresour. Technol. 2008, 99, 555–561. [Google Scholar] [CrossRef]
- Rafi, N.M.; Halim, N.R.A.; Amin, A.M.; Sarbon, N.M. Response surface optimization of enzymatic hydrolysis conditions of lead tree (Leucaena leucocephala) seed hydrolysate. Int. Food Res. J. 2015, 22, 1015–1023. [Google Scholar]
- Balderas-León, I.; Baigts-Allende, D.; Cardador-Martínez, A. Antioxidant, angiotensin-converting enzyme, and α-amylase inhibitory activities of protein hydrolysates of Leucaena leucocephala seeds. CYTA J. Food. 2021, 19, 349–359. [Google Scholar] [CrossRef]
- Lowry, O.; Rosebrough, N.; Farr, A.L.; Randall, R. Protein measurement with the folin phenol reagent. J. Biol. Chem. 1951, 193, 265–275. [Google Scholar] [CrossRef]
- ISO 6502-3; Rubber—Measurement of Vulcanization Characteristics Using Curemeters—Part 3: Rotorless Curemeter. ISO: Geneva, Switzerland, 2018.
- ISO 48-4; Rubber, Vulcanized or Thermoplastic—Determination of Hardness—Part 4: Indentation Hardness by Durometer Method (Shore Hardness). ISO: Geneva, Switzerland, 2018.
- ISO 37; Rubber, Vulcanized or Thermoplastic—Determination of Tensile Stress-Strain Properties. ISO: Geneva, Switzerland, 2017.
- Gbassi, G.; Yolou, F.; Sarr, S.; Atheba, P.; Amin, C.; Ake, M. Whey proteins analysis in aqueous medium and in artificial gastric and intestinal fluids. Int. J. Biol. Chem. Sci. 2012, 6, 1828–1837. [Google Scholar] [CrossRef]
- Vilas Dhumal, C.; Pal, K.; Sarkar, P. Synthesis, characterization, and antimicrobial efficacy of composite films from guar gum/sago starch/whey protein isolate loaded with carvacrol, citral and carvacrol-citral mixture. J. Mater. Sci. Mater. Med. 2019, 30, 117. [Google Scholar] [CrossRef]
- Barakat, A.; Gaillard, C.; Steyer, J.-P.; Carrere, H. Anaerobic Biodegradation of Cellulose–Xylan–Lignin Nanocomposites as Model Assemblies of Lignocellulosic Biomass. Waste Biomass Valoriz. 2014, 5, 293–304. [Google Scholar] [CrossRef]
- Reddy, N.; Yang, Y. Biofibers from agricultural byproducts for industrial applications. Trends Biotechnol. 2005, 23, 22–27. [Google Scholar] [CrossRef] [PubMed]
- Boopasiri, S.; Sae-Oui, P.; Siriwong, C. Fabrication of microcrystalline cellulose/zinc oxide hybrid composite by hydrothermal synthesis and its application in rubber compounding. J. Appl. Polym. Sci. 2022, 139, 52065. [Google Scholar] [CrossRef]
- Younis, U.; Rahi, A.A.; Danish, S.; Ali, M.A.; Ahmed, N.; Datta, R.; Fahad, S.; Holatko, J.; Hammerschmiedt, T.; Brtnicky, M.; et al. Fourier Transform Infrared Spectroscopy vibrational bands study of Spinacia oleracea and Trigonella corniculata under biochar amendment in naturally contaminated soil. PLoS ONE 2021, 16, e0253390. [Google Scholar] [CrossRef] [PubMed]
- Silverstein, R.M.; Webster, F.X.; Kiemle, D. Spectrometric Identification of Organic Compounds, 7th ed.; Wiley: Hoboken, NJ, USA, 2005. [Google Scholar]
- Manimaran, P.; Saravanakumar, S.S.; Mithun, N.K.; Senthamaraikannan, P. Physicochemical properties of new cellulosic fibers from the bark of Acacia arabica. Int. J. Polym. Anal. 2016, 21, 548–553. [Google Scholar] [CrossRef]
- Azmi, M.A.; Mokhtar, N.; Shoparwe, N.F.; Shukor, H. Biosorption of CU(II) Ions by Leucaena Leucocephala Leave from Aqueous Solution. IOP Conf. Ser. Earth Environ. Sci. 2021, 765, 012032. [Google Scholar] [CrossRef]
- Md Salim, R.; Asik, J.; Sarjadi, M.S. Chemical functional groups of extractives, cellulose and lignin extracted from native Leucaena leucocephala bark. Wood Sci. Technol. 2021, 55, 295–313. [Google Scholar] [CrossRef]
- Kebelmann, K.; Hornung, A.; Karsten, U.; Griffiths, G. Intermediate pyrolysis and product identification by TGA and Py-GC/MS of green microalgae and their extracted protein and lipid components. Biomass Bioenergy 2013, 49, 38–48. [Google Scholar] [CrossRef]
- Jagadeesh, D.; Jeevan Prasad Reddy, D.; Varada Rajulu, A. Preparation and Properties of Biodegradable Films from Wheat Protein Isolate. J. Polym. Environ. 2011, 19, 248–253. [Google Scholar] [CrossRef]
- Francisca Gómez-Rico, M.; Font, R.; Fullana, A.; Martín-Gullón, I. Thermogravimetric study of different sewage sludges and their relationship with the nitrogen content. J. Anal. Appl. Pyrolysis 2005, 74, 421–428. [Google Scholar] [CrossRef]
- Schaberg, A.; Wroblowski, R.; Goertz, R. Comparative study of the thermal decomposition behaviour of different amino acids and peptides. J. Phys. Conf. Ser. 2018, 1107, 032013. [Google Scholar] [CrossRef]
- Ishiwatari, M.; Ishiwatari, R.; Sakashita, H.; Tatsumi, T. Thermogravimetry and pyrolysis-GC of chlorophyll-a: With a special emphasis on thermal behavior of its phytyl chain. J. Anal. Appl. Pyrolysis 1995, 35, 237–247. [Google Scholar] [CrossRef]
- Moghadam, M.; Salami, M.; Mohammadian, M.; Delphi, L.; Sepehri, H.; Emam-Djomeh, Z.; Moosavi-Movahedi, A.A. Walnut protein–curcumin complexes: Fabrication, structural characterization, antioxidant properties, and in vitro anticancer activity. J. Food Meas. Charact. 2020, 14, 876–885. [Google Scholar] [CrossRef]
- De Angelis, A.; Gasco, L.; Parisi, G.; Danieli, P.P. A Multipurpose Leguminous Plant for the Mediterranean Countries: Leucaena leucocephala as an Alternative Protein Source: A Review. Animals 2021, 11, 2230. [Google Scholar] [CrossRef]
- Zhan, Y.-H.; Wei, Y.-C.; Tian, J.-j.; Gao, Y.-Y.; Luo, M.-C.; Liao, S. Effect of protein on the thermogenesis performance of natural rubber matrix. Sci. Rep. 2020, 10, 16417. [Google Scholar] [CrossRef] [PubMed]
- Lhamo, D.; McMahan, C. Effect of protein addition on properties of guayule natural rubber. Rubber Chem. Technol. 2017, 90, 387–404. [Google Scholar] [CrossRef]
- Liu, X.-X.; He, M.-F.; Luo, M.-C.; Wei, Y.-C.; Liao, S. The role of natural rubber endogenous proteins in promoting the formation of vulcanization networks. e-Polymers 2022, 22, 445–453. [Google Scholar] [CrossRef]
- Smitthipong, W.; Tantatherdtam, R.; Rungsanthien, K.; Suwanruji, P.; Klanarong, S.; Radabutra, S.; Thanawan, S.; Vallat, M.F.; Nardin, M.; Mougin, K.; et al. Effect of Non-Rubber Components on Properties of Sulphur Crosslinked Natural Rubbers. Adv. Mat. Res. 2014, 844, 345–348. [Google Scholar] [CrossRef]
- Wei, Y.C.; Liu, G.X.; Zhang, L.; Zhao, F. Exploring the unique characteristics of natural rubber induced by coordination interaction between proteins and Zn2+. Polymer 2022, 193, 122357. [Google Scholar] [CrossRef]
- Coran, A.Y. Vulcanization. Part VI. A model and treatment for scorch delay kinetics. Rubber Chem. Technol. 1964, 37, 679–688. [Google Scholar] [CrossRef]
- Qiao, H.-Q.; Gao, W.-J.; Xu, C.; Liu, Y.-X.; Chen, X.-L.; Xu, H.-T.; Ning, Y.-H.; Li, Y.-J.; Liu, Y.-C. The interaction of Chlorophyll a with ZnO Nanoparticles Synthesized by Sol-gel Method. Chin. J. Lumin. 2008, 29, 591–595. [Google Scholar]
- Akahori, Y.; Kawahara, S. Effect of water on the accelerated sulfur vulcanization of natural rubber. Polym. Test. 2023, 123, 108030. [Google Scholar] [CrossRef]
- Butler, J.; Freakley, P.K. Effect of Humidity and Water Content on the Cure Behavior of a Natural-Rubber Accelerated Sulfur Compound. Rubber Chem. Technol. 1992, 65, 374–384. [Google Scholar] [CrossRef]



| Chemicals | Supplier |
|---|---|
| Sodium hydroxide (NaOH) | Merck (Darmstadt, Germany) |
| Proteolytic enzyme (KP3939) | Kao (Tokyo, Japan) |
| Hydrochloric acid (HCl) | SPS Lab Co., Ltd. (Nonthaburi, Thailand) |
| Zinc oxide (ZnO) | Thai-Lysaght Co., Ltd. (Ayutthaya, Thailand) |
| Stearic acid | Kij Paiboon Chemical LP (Bangkok, Thailand) |
| N-tert-butyl-2-benzothiazylsulfenamide (TBBS) | Monflex Pte. Ltd. (Singapore) |
| Sulfur | Siam Chemical Industry Co., Ltd. (Muang, Samutprakarn, Thailand) |
| Ingredients | Control | LLP-Compound | DPP-Compound | RP-Compound |
|---|---|---|---|---|
| Natural rubber (STR 5 L) | 100.0 | 100.0 | 100.0 | 100.0 |
| Zinc oxide | 1.0 | 1.0 | 1.0 | 1.0 |
| Stearic acid | 1.0 | 1.0 | 1.0 | 1.0 |
| LLP | - | 3.0 | - | - |
| DPP | - | - | 3.0 | - |
| RP | - | - | - | 3.0 |
| TBBS | 1.0 | 1.0 | 1.0 | 1.0 |
| Sulfur | 1.0 | 1.0 | 1.0 | 1.0 |
| Sample | Protein Content (%w/w) |
|---|---|
| LLP | 21.3 |
| DPP | 49.0 |
| RP | 7.3 |
| Compound | ts1 (min) | tc95 (min) | ML (dN.m) | MH (dN.m) | Swelling Ratio (%) |
|---|---|---|---|---|---|
| Control | 11.45 | 17.14 | 1.16 | 5.53 | 411.0 ± 1.3 |
| LLP-compound | 7.84 | 11.87 | 1.24 | 5.31 | 427.6 ± 0.1 |
| DPP-compound | 4.75 | 7.56 | 1.22 | 5.05 | 430.9 ± 0.9 |
| RP-compound | 6.72 | 10.25 | 1.24 | 5.19 | 425.7 ± 0.2 |
| Sample | Mechanical Properties | |||
|---|---|---|---|---|
| Hardness (Shore A) | Tensile Strength (MPa) | 100% Modulus (MPa) | Elongation at Break (%) | |
| Control | 34.8 ± 0.2 | 22.0 ± 1.1 | 0.69 ± 0.01 | 707 ± 19 |
| LLP-compound | 34.1 ± 0.3 | 20.3 ± 1.6 | 0.67 ± 0.02 | 680 ± 7 |
| RP-compound | 33.9 ± 0.4 | 21.5 ± 0.6 | 0.67 ± 0.01 | 685 ± 19 |
| DPP-compound | 34.2 ± 0.1 | 19.6 ± 1.5 | 0.67 ± 0.01 | 677 ± 16 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Klongklaew, P.; Khamjapo, P.; Sae-Oui, P.; Jittham, P.; Loykulnant, S.; Intiya, W. Characterization and Application in Natural Rubber of Leucaena Leaf and Its Extracted Products. Polymers 2023, 15, 3698. https://doi.org/10.3390/polym15183698
Klongklaew P, Khamjapo P, Sae-Oui P, Jittham P, Loykulnant S, Intiya W. Characterization and Application in Natural Rubber of Leucaena Leaf and Its Extracted Products. Polymers. 2023; 15(18):3698. https://doi.org/10.3390/polym15183698
Chicago/Turabian StyleKlongklaew, Pattamaporn, Phimthong Khamjapo, Pongdhorn Sae-Oui, Pairote Jittham, Surapich Loykulnant, and Weenusarin Intiya. 2023. "Characterization and Application in Natural Rubber of Leucaena Leaf and Its Extracted Products" Polymers 15, no. 18: 3698. https://doi.org/10.3390/polym15183698
APA StyleKlongklaew, P., Khamjapo, P., Sae-Oui, P., Jittham, P., Loykulnant, S., & Intiya, W. (2023). Characterization and Application in Natural Rubber of Leucaena Leaf and Its Extracted Products. Polymers, 15(18), 3698. https://doi.org/10.3390/polym15183698




