Organosilicon Compounds in Hot-Melt Adhesive Technologies
Abstract
:1. Introduction
1.1. Adhesive Bonding
1.2. Hot-Melt Adhesive
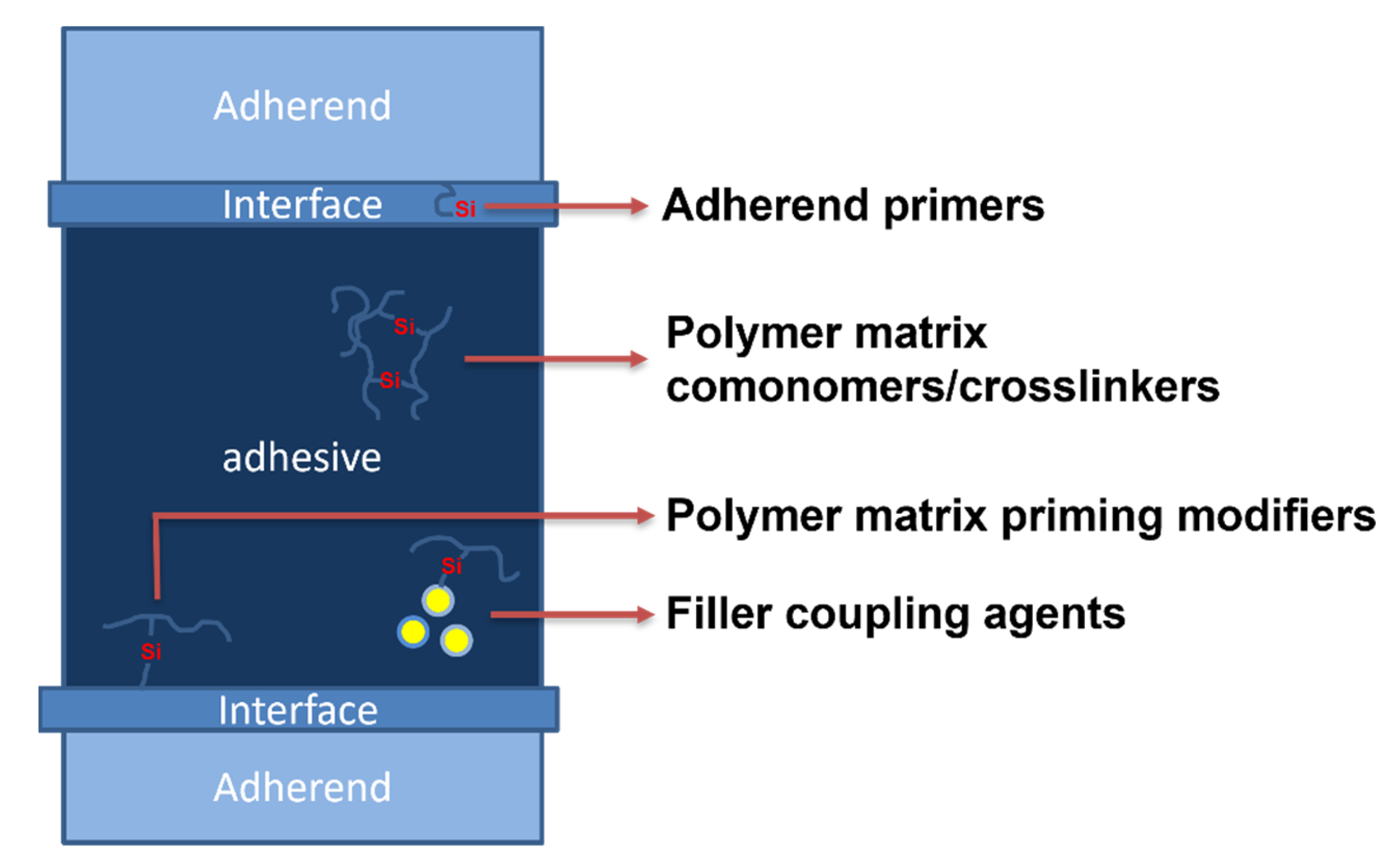
2. Hot-Melt Adhesive Composition
2.1. Primary Resins and Ingredients of Hot Melts
2.2. Organosilicon Compounds as Co-Monomers of Polymer Matrix
2.3. Organosilicon Compounds as a Polymer Matrix Additive
2.4. Organosilicon Compounds as Filler Coupling Agents
3. Materials Joined Using Hot Melts
3.1. Material Preparation
3.1.1. Physical Preparation (Sanding, Sandblasting, and Degreasing)
3.1.2. Chemical Preparation
3.2. Materials for Hot-Melt Bonding
Glass
3.3. Organosilicon Coupling Agents for Glass Treatment
3.3.1. Metals
3.3.2. Organosilicon Coupling Agents for Metal Treatment
3.3.3. Plastics and Composites
3.3.4. Organosilicon Treatment of Polymers
4. Future Trends in Hot-Melt Adhesives
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| 2-EHA | 2-ethylhexyl acrylate |
| AF | aramid fibers |
| AFM | Atomic Force Microscopy |
| APFR | alkylphenol–formaldehyde resin |
| A-POSS | acryloxypropyl-functional silsesquioxane |
| APTES | 3-aminopropyltrimethoxysilane |
| APTMOS | aminopropyltrimethoxysilane |
| ATH | aluminum trihydrate |
| ATRP | atom transfer radical polymerization |
| BCP | block copolymer |
| BR | butyl rubber |
| CF | carbon fiber |
| CNFs- | cellulose nanofibers |
| CS | carbon steel |
| DCP | dicumyl peroxide |
| EA | epoxyacrylate copolymer |
| EAA | ethylene–acrylic acid copolymer |
| EPDM | ethylene propylene diene monomers rubber |
| ETMOS | ethyltrimethoxysilane |
| EVA | ethylene–vinyl acetate |
| EVA-g-MA | ethylene–vinyl acetate grafted with maleic anhydride |
| EVA-g-VTEOS | ethylene–vinyl acetate grafted with vinyltriethoxysilane |
| EVM | ethylene–vinyl acetate copolymer rubber (an abbreviation used by some manufacturers) |
| FT-IR | Fourier-Transform Infrared |
| GPOSS | glycidoxypropyl-functional silsesquioxane |
| GPTMOS | 3-glycidyloxypropyltrimethoxysilane |
| HMA | hot-melt adhesives |
| ICPTEOS | 3-cyanatopropyltriethoxysilane |
| IPN | inter-penetrating network |
| LLDPE | linear low-density polyethylene |
| m-HCR | chemically modified hydrocarbon resin |
| MA-POSS | methacryloxypropylsilsesquioxane |
| MATMOS | methacryloxypropyltrimethoxysilane |
| MPTMOS | 3-mercaptopropyltrimethoxysilane |
| MTMOS | methyltrimethoxysilane |
| MTS | mechanics test system |
| NDM | N-dodecyl mercaptan |
| NR | natural rubber |
| OBC | olefin block copolymer |
| OBC-g-MA | olefin block copolymer grafted with maleic anhydride |
| P4HB | poly (4-hydroxybutyric acid) |
| PC | polycarbonate |
| PDA | polydopamine |
| PE | polyethylene |
| PET | poly(ethylene terephthalate) |
| PEW-g-PMMA | polyethylene wax grafted with methyl methacrylate |
| PGMA | poly(glycidyl methacrylate) |
| PHA | polyhydroxyalkanoate |
| POE | polyolefin elastomer |
| POSS | polyhedral oligomeric silsesquioxane (registered trademark of Hybrid Plastics) |
| PSA | pressure-sensitive adhesive |
| PV | photovoltaics |
| RFL | resorcinol formaldehyde latex |
| SAFT | shear adhesion failure test |
| SAT | self-adhesive tapes |
| SCCNFs | silanized cellulose nanofibers |
| STP | silane terminated prepolymers |
| TEOS | Tetraethoxysilane |
| tPA | ternary polyamide |
| UHMWPE | ultrahigh-molecular-weight polyethylene |
| UV | ultraviolet |
| VDPs | vibration damping plates |
| VTEOS | vinyltriethoxysilane |
| VTMOS | vinyltrimethoxysilane |
| XPS | X-ray Photoelectron Spectroscopy |
References
- Johnson, K.L.; Kendall, K.; Roberts, A.D. Surface energy and the contact of elastic solids. Proc. R. Soc. A Math. Phys. Eng. Sci. 1971, 324, 301–313. [Google Scholar]
- Liu, W.; Li, H.; Zhu, H.; Xu, P. The Interfacial Adhesion Performance and Mechanism of a Modified Asphalt–Steel Slag Aggregate. Materials 2020, 13, 1180. [Google Scholar] [CrossRef] [PubMed]
- Owens, D.K. Some thermodynamic aspects of polymer adhesion. J. Appl. Polym. Sci. 1970, 14, 1725–1730. [Google Scholar] [CrossRef]
- Wang, Y.; Guo, Z.; Liu, W. Adhesion behaviors on four special wettable surfaces: Natural sources, mechanisms, fabrications and applications. Soft Matter 2021, 17, 4895–4928. [Google Scholar] [CrossRef] [PubMed]
- Smith, M.J.; Dai, H.; Ramani, K. Wood–thermoplastic adhesive interface—Method of characterization and results. Int. J. Adhes. 2022, 22, 197–204. [Google Scholar] [CrossRef]
- Qiu, B.; Sun, T.; Li, M.; Chen, Y.; Zhou, S.; Liang, M.; Zou, H. High micromechanical interlocking graphene ox-ide/carboxymethyl cellulose composite architectures for enhancing the interface adhesion between carbon fiber and epoxy. Compos. A Appl. Sci. 2020, 139, 106092. [Google Scholar] [CrossRef]
- Lee, J.; Park, E.; Fujisawa, A.; Lee, H. Diatom Silica/Polysaccharide Elastomeric Hydrogels: Adhesion and Interlocking Synergy. ACS Appl. Mater. Interfaces 2021, 13, 21703–21713. [Google Scholar] [CrossRef]
- Seong, M.; Park, H.-H.; Hwang, I.; Jeong, H.E. Strong and Reversible Adhesion of Interlocked 3D-Microarchitectures. Coatings 2019, 9, 48. [Google Scholar] [CrossRef]
- Zhu, M.; Zhang, F.; Chen, X. Bioinspired Mechanically Interlocking Structures. Small Struct. 2020, 1, 2000045. [Google Scholar] [CrossRef]
- Hamilton, A.; Xu, Y.; Kartal, M.E.; Gadegaard, N.; Mulvihill, D.M. Enhancing strength and toughness of adhesive joints via micro-structured mechanical interlocking. Int. J. Adhes. Adhes. 2021, 105, 102775. [Google Scholar] [CrossRef]
- Yang, X.; Tu, Q.; Shen, X.; Jiang, C.; Pan, M.; Zhu, P.; Li, Y.; Hu, C.; Zhang, Q. Study on interfacial adhesion of the aramid fibers/rubber matrix by grafting mercapto hyperbranched polysiloxane. Polym. Test. 2020, 81, 106259. [Google Scholar] [CrossRef]
- Voiutskii, S.S. Autohesion and Adhesion of High Polymers; Interscience Publishers: New York, NY, USA, 1963; pp. 249–262. [Google Scholar]
- Daelemans, L.; Paepegem, W.V.; Clerck, K.D. Excellent Nanofiber Adhesion for Hybrid Polymer Materials with High Toughness Based on Matrix Interdiffusion During Chemical Conversion. Adv. Funct. Mater. 2018, 28, 1807434. [Google Scholar] [CrossRef]
- Rathner, R.; Leimhofer, C.; Roland, W.; Hammer, A.; Löw-Baselli, B.; Steinbichler, G.; Hild, S. Improving Layer Adhesion of Co-Extruded Polymer Sheets by Inducing Interfacial Flow Instabilities. Polymers 2022, 14, 587. [Google Scholar] [CrossRef] [PubMed]
- Shiraki, Y.; Saito, M.; Yamada, N.L.; Ito, K.; Yokoyama, H. Adhesion to Untreated Polyethylene and Polypropylene by Needle-like Polyolefin Crystals. Macromolecules 2023, 56, 2429–2436. [Google Scholar] [CrossRef]
- Padhye, N.; Vallabh, A. Deformation-induced bonding of polymer films below the glass transition temperature. J. Appl. Polym. Sci. 2021, 138, 50934. [Google Scholar] [CrossRef]
- Zaikov, G.E.; Yakh’yaeva, K.S.; Magomedov, G.M.; Kozlov, G.V. Polymer Adhesion Model. J. Nat. Sci. Sustain. Technol. 2020, 14, 223–239. [Google Scholar]
- Picard, L.; Phalip, P.; Fleury, E.; Ganachaud, F. Bonding of silicone rubbers on metal (2) physical chemistry of adhesion. Prog. Org. Coat. 2015, 87, 258–266. [Google Scholar] [CrossRef]
- Gutowski, W. Thermodynamics of Adhesion. In Fundamentals of Adhesion; Lee, L., Ed.; Springer: Boston, MA, USA, 1991; pp. 87–135. [Google Scholar]
- Comyn, J. Adhesion Science; Royal Society of Chemistry: London, UK, 2021. [Google Scholar]
- Cui, C.; Liu, W. Recent advances in wet adhesives: Adhesion mechanism, design principle and applications. Prog. Polym. Sci. 2021, 116, 101388. [Google Scholar] [CrossRef]
- Xie, L.; Gong, L.; Zhang, J.; Han, L.; Xiang, L.; Chen, J.; Liu, J.; Yan, B.; Zeng, H. A wet adhesion strategy via synergistic cation–π and hydrogen bonding interactions of antifouling zwitterions and mussel-inspired binding moieties. J. Mater. Chem. A 2021, 7, 21944–21952. [Google Scholar] [CrossRef]
- Xu, Y.Z.; Liu, J. Multiple H-bonding chain extender-based ultrastiff thermoplastic polyurethanes with autonomous self-healability, solvent-free adhesiveness, and AIE fluorescence. Adv. Funct. Mater. 2021, 31, 2006944. [Google Scholar]
- Zhang, Q.; Li, T.; Duan, A.; Dong, S.; Zhao, W.; Stang, P.J. Formation of a supramolecular polymeric adhesive via water-participant hydrogen bond formation. J. Am. Chem. Soc. 2019, 141, 8058–8063. [Google Scholar] [CrossRef] [PubMed]
- Wool, R.P.; Bunker, S.P. Polymer-Solid Interface Connectivity and Adhesion: Design of a Bio-Based Pressure Sensitive Adhesive. J. Adhes. 2007, 83, 907–926. [Google Scholar] [CrossRef]
- Malysheva, G.V.; Bodrykh, N.V. Hot-melt adhesives. Polym. Sci. -D 2011, 4, 301–303. [Google Scholar] [CrossRef]
- Benedek, I.; Feldstein, M.M. Fundamentals of Pressure Sensitivity, 1st ed.; CRC Press: Boca Raton, FL, USA, 2008; pp. 25–50. [Google Scholar]
- Li, Z.; Zhu, W.; Li, R.; Zeng, Z.; Han, T.; Ma, Z. Method for preparing polyester hot melt gel special for tin can seal, China. CN Patent CN102,690,624A, 26 September 2012. [Google Scholar]
- Li, J.; Bai, H.; Feng, Z. Advances in the Modification of Silane-Based Sol-Gel Coating to Improve the Corrosion Resistance of Magnesium Alloys. Molecules 2023, 28, 2563. [Google Scholar] [CrossRef] [PubMed]
- Yan, W.; Chen, B.; Mahurin, S.M.; Hagaman, E.W.; Dai, S.; Overbury, S.H. Surface Sol−Gel Modification of Mesoporous Silica Materials with TiO2 for the Assembly of Ultrasmall Gold Nanoparticles. J. Phys. Chem. B 2004, 108, 2793–2796. [Google Scholar] [CrossRef]
- Chen, X.; Wilson, G.S. Electrochemical and Spectroscopic Characterization of Surface Sol−Gel Processes. Langmuir 2004, 20, 8762–8767. [Google Scholar] [CrossRef]
- Schmidt, H.; Scholze, H.; Tünker, G. Hot melt adhesives for glass containers by the sol-gel process. J. Non-Cryst. Solids 1986, 80, 557–563. [Google Scholar] [CrossRef]
- Feldstein, M.M.; Siegel, R.A. Molecular and nanoscale factors governing pressure-sensitive adhesion strength of viscoelastic polymers. J. Polym. Sci. B Polym. Phys. 2012, 50, 739–772. [Google Scholar] [CrossRef]
- Molecular Adhesion and Its Applications; Kluwer Academic Publishers: Alphen Aan den Rijn, The Netherlands, 2004.
- Fay, P.A. A History of Adhesive Bonding. In Adhesive Bonding; Elsevier: Amsterdam, The Netherlands, 2021; pp. 3–40. [Google Scholar] [CrossRef]
- Gadhave, R.V.I.; Gadhave, C.R. Adhesives for the Paper Packaging Industry: An Overview. Open J. Polym. Chem. 2022, 12, 55–79. [Google Scholar] [CrossRef]
- Gharde, S.; Sharma, G.; Kandasubramanian, B. Hot-Melt Adhesives: Fundamentals, Formulations, and Applications: A Critical Review. Prog. Adhes. Adhes. 2021, 6, 1–28. [Google Scholar]
- Orgilés-Calpena, E.; Arán-Aís, F.; Torró-Palau, A.M.; Sánchez, M.A.M. Adhesives in the footwear industry: A critical review. Rev. Adhes 2017, 7, 1. [Google Scholar]
- Vineeth, S.K.; Gadhave, R.V. Sustainable raw materials in hot melt adhesives: A review. Open J. Polym. Chem. 2020, 10, 49. [Google Scholar]
- Mittal, K.L.; Pizzi, A. Handbook of Sealant Technology; CRC Press: Boca Raton, FL, USA, 2020. [Google Scholar]
- Petrie, E.M. Handbook of Adhesives and Sealants, 3rd ed.; McGraw-Hill Education: New York, NY, USA, 2021. [Google Scholar]
- Yarusso, D.J. Effect of rheology on PSA performance. In Adhesion Science and Engineering; Elsevier: Amsterdam, The Netherlands, 2002; pp. 499–533. [Google Scholar] [CrossRef]
- Pocius, A.V. 11—Thermoplastic, Pseudothermoplastic, and Other Adhesives. In Adhesion and Adhesives Technology, 4th ed.; Pocius, A.V., Ed.; Hanser: Hardin, Montana, 2021; pp. 313–330. [Google Scholar]
- Moyano, M.A.; París, R.; Martín-Martínez, J.M. Viscoelastic and adhesion properties of hot-melts made with blends of ethylene-co-n-butyl acrylate (EBA) and ethylene-co-vinyl acetate (EVA) copolymers. Int. J. Adhes. Adhes. 2019, 88, 34–42. [Google Scholar] [CrossRef]
- Ciardiello, R.; Belingardi, G.; Martorana, B.; Brunella, V. Physical and mechanical properties of a reversible adhesive for automotive applications. Int. J. Adhes. Adhes. 2019, 89, 117–128. [Google Scholar] [CrossRef]
- Sharma, S.; Sharma, B.; Manral, A.; Bajpai, P.K.; Jain, P. Biopolymers in the automotive and adhesive industries. In Biopolymers and Their Industrial Applications; Elsevier: Amsterdam, The Netherlands, 2020; pp. 261–280. [Google Scholar] [CrossRef]
- Konoplin, A.Y.; Pushkarev, A.V.; Shakurov, A.V.; Baurova, N.I. Evaluation of Frost Resistance of Organosilicon Sealant at Ultralow Temperatures. Polym. Sci. Ser. D 2021, 14, 335–339. [Google Scholar] [CrossRef]
- Adkinson, D.K. Hot Melt Adhesives with Butyl Ionomer. European Patent Office, Patent EP3015521A1, 4 May 2016. [Google Scholar]
- Wang, S.; Liu, Z.; Zhang, L.; Guo, Y.; Song, J.; Lou, J.; Guan, Q.; He, C.; You, Z. Strong, detachable, and self-healing dynamic crosslinked hot melt polyurethane adhesive. Mater. Chem. Front. 2019, 3, 1833–1839. [Google Scholar] [CrossRef]
- Beaucarne, G.; Zelba, M.; Jadot, E.; Curon, J.; Gubbels, F.; Hayez, V.; Arenas, B.S.; Chambard, G.; Karoblis, R. Low Temperature Solar Cell Encapsulation with Novel Silicone Elastomer for Building Integrated Pv. In Proceedings of the 8th World Conference on Photo-Voltaic Energy Conversion, Milan, Italy, 26–30 September 2022. [Google Scholar]
- Ramírez, E.; Betancur, R.; Montoya, J.F.; Velilla, E.; Ramírez, D.; Jaramillo, F. Encapsulation against Extrinsic Degradation Factors and Stability Testing of Perovskite Solar Cells. In Recent Advances in Multifunctional Perovskite Materials; IntechOpen: London, UK, 2022. [Google Scholar] [CrossRef]
- da Silva, L.F.M.; Öchsner, A.; Adams, R.D. Handbook of Adhesion Technology; Springer International Publishing: New York, NY, USA, 2018. [Google Scholar]
- Pizzi, A.; Mittal, K.L. Handbook of Adhesive Technology; CRC Press: Boca Raton, FL, USA, 2017. [Google Scholar]
- Cherkashina, A.; Rassokha, O.; Mazhuga, O. Melting Adhesives with High Adhesion. Ph.D Thesis, Націoнальний університет цивільнoгo захисту України, Kharkiv, Ukraine, 2021. [Google Scholar]
- Robertson, D.; van Reenen, A.; Duveskog, H.; Brady, F. A comparative study of the application-based properties of hot melt adhesives (HMAs) formulated with different waxes. Int. J. Adhes. Adhes. 2021, 111, 102974. [Google Scholar] [CrossRef]
- Varga, L.J.; Bárány, T. Development of recyclable, lightweight polypropylene-based single polymer composites with amorphous poly-alpha-olefin matrices. Compos. Sci. Technol. 2021, 201, 108535. [Google Scholar] [CrossRef]
- Anderson, J.J. Styrenic Block Reinforcing Additives in Pressure Sensitive Adhesives; Lehigh University: Bethlehem, PE, USA, 2021. [Google Scholar]
- Maji, P.; Naskar, K. Styrenic block copolymer-based thermoplastic elastomers in smart applications: Advances in synthesis, microstructure, and structure-property relationships—A review. J. Appl. Polym. Sci. 2022, 139, e52942. [Google Scholar]
- Lisanevich, M.S.; Galimzyanova, R.Y.; Rusanova, S.N.; Khakimullin, Y.N.; Stoyanov, O.V. Hot-Melt Sealants of Curable Type Based on Butyl Rubber and Ethylene–Vinyl Acetate Copolymer. Polym. Sci. Ser. D 2018, 11, 359–362. [Google Scholar] [CrossRef]
- Behera, P.K.; Kumar, A.; Mohanty, S.; Gupta, V.K. Overview on Post-Polymerization Functionalization of Butyl Rubber and Properties. Ind. Eng. Chem. Res. 2022, 61, 16910–16923. [Google Scholar] [CrossRef]
- Pourali, M.; Peterson, A.M. A tale of two polyamides: Comparing the crystallization kinetics of a hot-melt adhesive and a PA 6/66 copolymer. Thermochim. Acta 2022, 710, 179176. [Google Scholar] [CrossRef]
- Hu, Y.; Paul, C.W. Block Copolymer-Based Hot-Melt Pressure-Sensitive Adhesives. In Feldstein Technology of Pressure-Sensitive Adhesives and Products; Benedek, M.M., Ed.; CRC Press: Boca Raton, FL, USA, 2009. [Google Scholar]
- Paul, C.W. Hot Melt Adhesives for Dermal Application. U.S. Patent 6,448,303, 10 September 2002. [Google Scholar]
- Gennari, C.G.M.; Quaroni, G.M.G.; Creton, C.; Minghetti, P.; Cilurzo, F. SEBS block copolymers as novel materials to design transdermal patches. Int. J. Pharm. 2020, 575, 118975. [Google Scholar] [CrossRef] [PubMed]
- Liu, Z.; Liu, Y.D.; Shi, Q.; Liang, Y. Electroactive dielectric polymer gels as new-generation soft actuators: A review. J. Mater. Sci. 2021, 56, 14943–14963. [Google Scholar] [CrossRef]
- Mineart, K.P.; Lin, Y.; Desai, S.C.; Krishnan, A.S.; Spontak, R.J.; Dickey, M.D. Ultrastretchable, cyclable and recyclable 1- and 2-dimensional conductors based on physically cross-linked thermoplastic elastomer gels. Soft Matter 2013, 9, 7695–7700. [Google Scholar] [CrossRef]
- Wu, M.; Liu, Y.; Du, P.; Wang, X.; Yang, B. Polyurethane hot melt adhesive based on Diels-Alder reaction. Int. J. Adhes. Adhes. 2020, 100, 102597. [Google Scholar] [CrossRef]
- Galimzyanova, R.Y.; Lisanevich, M.S.; Khakimullin, Y.N. The Effect of Adhesive Additives on the Properties of Uncured Sealants Based on Butyl Rubber. Polym. Sci. Ser. D 2021, 14, 319–322. [Google Scholar] [CrossRef]
- Galimzyanova, R.Y.; Lisanevich, M.S.; Khakimullin, Y.N. The Effect of Carbon Black on Characteristics of Nonhardening Sealants Based on Butyl Rubber. Polym. Sci. Ser. D 2021, 14, 42–46. [Google Scholar] [CrossRef]
- Czakaj, J. Otrzymywanie częściowo usieciowanych mieszanek uszczelniających za pomocą wytłaczarki dwuślimakowej współbieżnej. Przemysł Chem. 2018, 1, 185–187. [Google Scholar] [CrossRef]
- Czakaj, J. Kompozytowe termoplastyczne uszczelniacze butylowe o zwiększonej odporności na wysoką temperature. Przemysł Chem. 2018, 1, 176–178. [Google Scholar] [CrossRef]
- Brantseva, T.V.; Antonov, S.V.; Kostyuk, A.V.; Ignatenko, V.Y.; Smirnova, N.M.; Ilyin, S.O. PIB Pressure-sensitive Adhesives with Dispersed Nanofillers: Addressing the Cold Flow Problem. In Proceedings of the Conference: 11th European Adhesion Conference (EURADH 2016), Glasgow, Scotland, 21–23 September 2016. [Google Scholar]
- Kumar, K.D.; Tsou, A.H.; Bhowmick, A.K. Unique Tackification Behavior of Needle-like Sepiolite Nanoclay in Brominated Isobutylene-co-p-methylstyrene (BIMS) Rubber. Macromolecules 2010, 43, 4184–4193. [Google Scholar] [CrossRef]
- Kostyuk, A.; Ignatenko, V.; Smirnova, N.; Brantseva, T.; Ilyin, S.; Antonov, S. Rheology and adhesive properties of filled PIB-based pressure-sensitive adhesives. I. Rheology and shear resistance. J. Adhes. Sci. Technol. 2014, 29, 1831–1848. [Google Scholar] [CrossRef]
- Jones, R.G.; Ando, W.; Chojnowski, J. Silicon-Containing Polymers: The Science and Technology of Their Synthesis and Applications; Springer Science & Business Media: New York, NY, USA, 2013. [Google Scholar]
- Muzafarov, A.M. Silicon Polymers; Springer Science & Business Media: New York, NY, USA, 2010; Volume 235. [Google Scholar]
- Shi, H.; Yang, J.; Li, Z.; He, C. Introduction of Organosilicon Materials. In Silicon Containing Hybrid Copolymers; Wiley: Hoboken, NJ, USA, 2020; pp. 1–21. [Google Scholar] [CrossRef]
- Kowalczyk, A.; Kowalczyk, K.; Gziut, K. Synthesis of Monoacryloxypropyl-POSS-based Hybrid Epoxyacrylate Copolymers and Their Application in Thermally Curable Structural Self-Adhesive Tapes. Polymers 2019, 11, 2058. [Google Scholar] [CrossRef] [PubMed]
- Ma, Y.; Wu, H.; Shen, Y. Dual-functional linear and star POSS-containing organic-inorganic hybrid block copolymers: Synthesis, self-assembly, and film property. J. Mater. Sci. 2022, 57, 7791–7803. [Google Scholar] [CrossRef]
- Hanifpour, A.; Bahri-Laleh, N.; Nekoomanesh-Haghighi, M. Methacrylate-functionalized POSS as an efficient adhesion promoter in olefin-based adhesives. Polym. Eng. Sci. 2020, 60, 2991–3000. [Google Scholar] [CrossRef]
- Bilgin, E.T.; Dülgar, C.A.; Serhatlı, İ.E. Incorporation of vinyl silane and epoxy silane oligomer into 2-EHA-based poly-acrylate latexes via mini-emulsion polymerization and investigation of pressure-sensitive adhesive properties on polar and nonpolar surfaces. Polym. Bull. 2019, 76, 5773–5789. [Google Scholar] [CrossRef]
- Park, H.-W.; Seo, H.-S.; Lee, J.-H.; Shin, S. Adhesion improvement of the acrylic pressure-sensitive adhesive to low-surface-energy substrates using silicone urethane dimethacrylates. Eur. Polym. J. 2020, 137, 109949. [Google Scholar] [CrossRef]
- Wu, Z.; Shangguan, Y.; Zhang, C.; Zheng, Q. Effects of Crosslinking and Silicone Coupling Agent on Properties of EVA Composite Hot Melt Adhesive. Polymers 2021, 13, 4101. [Google Scholar] [CrossRef]
- Yazıcı, N.; Dursun, S.; Yarıcı, T.; Kılıç, B.; Arıcan, M.O.; Mert, O.; Karaağaç, B.; Özkoç, G.; Kodal, M. The outstanding interfacial adhesion between acrylo-POSS/natural rubber composites and polyamide-based cords: An environmentally friendly alternative to resorcinol-formaldehyde latex coating. Polymer 2021, 228, 123880. [Google Scholar] [CrossRef]
- Murtazina, I.; Akhmedgoraeva, L.; Galimzyanova, A.R.; Khakimullin, R.Y. Construction Sealants Based on EPDM Modified with Silane-Terminated Urethane Prepolymers. IOP Conf. Ser. Mater. Sci. Eng. 2020, 753, 4. [Google Scholar] [CrossRef]
- SLai, M.; Li, C.-H.; Kao, H.-C.; Liu, L.-C. Shape Memory Properties of Melt-Blended Olefin Block Copolymer (OBC)/Ethylene-Vinyl Acetate Blends. J. Macromol. Sci. B 2019, 58, 174–191. [Google Scholar]
- Doganci, E. Improving adhesion between polyester cord and rubber by using glycidyl-POSS. J. Appl. Polym. Sci. 2021, 138, 49681. [Google Scholar] [CrossRef]
- Ahmed, J.; Mushtaq, S. Effects of silane-modified Al2O3 and its hybrid filler on thermal stability and mechanical properties of ethylene–vinyl acetate copolymer/polyamide composites. Iran. Polym. J. 2022, 31, 1571–1581. [Google Scholar] [CrossRef]
- Bi, W.; Hoch, M.; Yu, G.; Goegelein, C.; Kirchhoff, J.; Zhao, S. Investigation of Silane Coupling Agents on the Filler-Filler and Filler-Rubber Interaction and Mechanical Properties of EVM/ATH Composites. IOP Conf. Ser. Mater. Sci. Eng. 2019, 493, 012089. [Google Scholar] [CrossRef]
- Jo, J.; Jeong, S.-Y.; Lee, J.; Park, C.; Koo, B. Green and Sustainable Hot Melt Adhesive (HMA) Based on Polyhydroxyal-kanoate (PHA) and Silanized Cellulose Nanofibers (SCNFs). Polymers 2022, 14, 5284. [Google Scholar] [CrossRef]
- Li, J.; Li, Y.; Huang, M.; Xiang, Y.; Liao, Y. Improvement of aluminum lithium alloy adhesion performance based on sand-blasting techniques. Int. J. Adhes. 2018, 84, 307–316. [Google Scholar] [CrossRef]
- Baldan, A. Adhesion phenomena in bonded joints. Int. J. Adhes. Adhes. 2012, 38, 95–116. [Google Scholar] [CrossRef]
- Allen, K. Silanes as the interphase in adhesive bonds. J. Adhes. Sci. Technol. 1992, 6, 23–32. [Google Scholar] [CrossRef]
- Pujol, J.-M.; Prébet, C. Functional silanes: Crosslinkers for silicone elastomers. J. Adhes. Sci. Technol. 2003, 17, 261–275. [Google Scholar] [CrossRef]
- Li, M.; Zhang, L.; Li, X.; Wang, R.; Wu, X.; Zhang, D.; Chen, Y. Improvements of adhesion strength of water-based epoxy resin on carbon fiber reinforced polymer (CFRP) composites via building surface roughness using modified silica particles. Compos. Part A Appl. Sci. Manuf. 2023, 169, 107511. [Google Scholar] [CrossRef]
- Chen, L.; Jin, H.; Xu, Z.; Shan, M.; Tian, X.; Yang, C.; Wang, Z.; Cheng, B. A design of gradient interphase reinforced by silanized graphene oxide and its effect on carbon fiber/epoxy interface. Mater. Chem. Phys. 2014, 145, 186–196. [Google Scholar] [CrossRef]
- Hatefi, A.; Mohagheghi, S.; Kianvash, A. The effect of silane layer drying temperature on epoxy coating adhesion on silane-pretreated aluminum substrate. J. Coat. Technol. Res. 2013, 10, 743–747. [Google Scholar] [CrossRef]
- Bajat, J.; Milošev, I.; Jovanović, Z.; Mišković-Stan, V. Studies on Adhesion Characteristics and Corrosion Behaviour of Vinyltriethoxysilane/Epoxy Coating Protective System on Aluminium. Appl. Surf. Sci. 2010, 256, 3508–3517. [Google Scholar] [CrossRef]
- Hench, L.L. 2–Sol-Gel Kinetics. In Sol-Gel Silica; Hench, L.L., Ed.; William Andrew Publishing: Westwood, NJ, USA, 1998; pp. 8–23. [Google Scholar]
- Klein, L.C. Sol-Gel Processing of Silicates. Annu. Rev. Mater. Sci. 1985, 15, 227–248. [Google Scholar] [CrossRef]
- Pierre, A.C. Introduction to Sol-Gel Processing; Alain, C.P., Ed.; Kluwer Academic Publishers: Alphen Aan den Rijn, The Netherlands, 1998. [Google Scholar]
- Musgraves, J.D.; Hu, J.; Calvez, L. Springer Handbook of Glass; Springer International Publishing: New York, NY, USA, 2019. [Google Scholar]
- Hamzaoui, H.E.; Bouazaoui, M.; Capoen, B. Sol–gel materials for optical fibers. In Sol-Gel Derived Optical and Photonic Materials; Elsevier: Amsterdam, The Netherlands, 2020; pp. 315–346. [Google Scholar]
- Atkins, A.G. Intermittent bonding for high toughness/ high strength composites. J. Mater. Sci. 1975, 10, 819–832. [Google Scholar] [CrossRef]
- Outwater, J.O.; Murphy, M.C. The Influences of Environment and Glass Finishes on the Fracture Energy of Glass-Epoxy Joints. J. Adhes. 1970, 2, 242–253. [Google Scholar] [CrossRef]
- Plueddemann, E.P. Reminiscing on silane coupling agents. J. Adhes. Sci. Technol. 1991, 5, 261–277. [Google Scholar] [CrossRef]
- Matisons, J.G. Silanes and Siloxanes as Coupling Agents to Glass: A Perspective. In Advances in Silicon Science; Springer: New York, NY, USA, 2012; pp. 281–298. [Google Scholar] [CrossRef]
- Angst, D.L.; Simmons, G.W. Moisture absorption characteristics of organosiloxane self-assembled monolayers. Langmuir 1991, 7, 2236–2242. [Google Scholar] [CrossRef]
- Brook, M.A. Brook and Others, Silicon in Organic, Organometallic, and Polymer Chemistry; Wiley: New York, NY, USA, 2000; Volume 123. [Google Scholar]
- Pantano, C.G.; Wittberg, T.N. XPS analysis of silane coupling agents and silane-treated E-glass fibers. Surf. Interface Anal. 1990, 15, 498–501. [Google Scholar] [CrossRef]
- Drown, E.; Al Moussawi, H.; Drzal, L. Glass fiber ‘sizings’ and their role in fiber-matrix adhesion. J. Adhes. Sci. Technol. 1992, 5, 865–881. [Google Scholar] [CrossRef]
- Wang, D.; Jones, F.; Denison, P. TOF SIMS and XPS study of the interaction of hydrolysed γ-aminopropyltriethoxysilane with E-glass surfaces. J. Adhes. Sci. Technol. 1992, 6, 79–98. [Google Scholar] [CrossRef]
- Feller, J.; Grohens, Y. Coupling ability of silane grafted poly(propene) at glass fibers/poly(propene) interface. Compos. Part A Appl. Sci. Manuf. 2004, 35, 1–10. [Google Scholar] [CrossRef]
- Cech, V.; Prikryl, R.; Balkova, R.; Vanek, J.; Grycova, A. The influence of surface modifications of glass on glass fiber/polyester interphase properties. J. Adhes. Sci. Technol. 2003, 17, 1299–1320. [Google Scholar] [CrossRef]
- Luo, N.; Zhong, H.; Yang, M.; Yuan, X.; Fan, Y. Modifying glass fiber surface with grafting acrylamide by UV-grafting copolymerization for preparation of glass fiber reinforced PVDF composite membrane. J. Environ. Sci. 2016, 39, 208–217. [Google Scholar] [CrossRef]
- Chen, J.; Zhao, D.; Jin, X.; Wang, C.; Wang, D.; Ge, H. Modifying glass fibers with graphene oxide: Towards high-performance polymer composites. Compos. Sci. Technol. 2014, 97, 41–45. [Google Scholar] [CrossRef]
- Turrión, S.G.; Olmos, D.; González-Benito, J. Complementary characterization by fluorescence and AFM of poly-aminosiloxane glass fibers coatings. Polym. Test. 2005, 24, 301–308. [Google Scholar] [CrossRef]
- Sarr, M.M.; Inoue, H.; Kosaka, T. Study on the improvement of interfacial strength between glass fiber and matrix resin by grafting cellulose nanofibers. Compos. Sci. Technol. 2021, 211, 108853. [Google Scholar] [CrossRef]
- Mattson, B.; Aksnes, E.; Huse, J.; Tveten, C.; Redford, K.; Stori, A. Silane-modified polymers as interphases in glass-fibre-reinforced composites: 2. Grafting and crosslinking of interphase materials. Compos. Interfaces 1996, 4, 77–94. [Google Scholar] [CrossRef]
- Sorokin, A.E.; Petrova, G.N. Lubricants and Coupling Agents in the Processes of the Liquid-Phase Modification of the Surface of Carbon and Glass Fiber Fillers in the Production of Structural Materials: A Review. Theor. Found. Chem. Eng. 2020, 54, 737–744. [Google Scholar] [CrossRef]
- Park, J.H.; Hwang, S.-H. Construction and Characterization of Polyolefin Elastomer Blends with Chemically Modified Hydrocarbon Resin as a Photovoltaic Module Encapsulant. Polymers 2022, 14, 4620. [Google Scholar] [CrossRef]
- Baiamonte, M.; Morici, E.; Colletti, C.; Dintcheva, N.T. Polar Wax as Adhesion Promoter in Polymeric Blend Films for Durable Photovoltaic Encapsulants. Materials 2022, 15, 6751. [Google Scholar] [CrossRef]
- McMahon, T.J.; Jorgensen, G.J. Adhesion and Thin-Film Module Reliability. In Proceedings of the 2006 IEEE 4th World Conference on Photovoltaic Energy Conference, Waikoloa, HI, USA, 7–12 May 2006. [Google Scholar]
- Miller, D.C.; Annigoni, E.; Ballion, A.; Bokria, J.G.; Bruckman, L.S.; Burns, D.M.; Chen, X.; Feng, J.; French, R.H.; Fowler, S.; et al. Degradation in PV encapsulant strength of attachment: An interlaboratory study towards a climate-specific test. In Proceedings of the 2016 IEEE 43rd Photovoltaic Specialists Conference (PVSC), Portland, OR, USA, 5–10 June 2016. [Google Scholar]
- Kalbe, K.; Piikov, H.; Kesti, J.; Honkakoski, E.; Kurnitski, J.; Kalamees, T. Moisture dry-out from steel faced insulated sandwich panels. E3S Web Conf. 2020, 172, 17007. [Google Scholar] [CrossRef]
- King, A. Flash into View. Build. Connect. 2018, 2, 22–23. [Google Scholar]
- Watts, A. Silicone-Sealed Glazing and Rooflights. In Modern Construction Envelopes; Springer: Vienna, Austria, 2011; pp. 348–357. [Google Scholar] [CrossRef]
- Pochivalov, K.V.; Shilov, A.N.; Lebedeva, T.N.; Ilyasova, A.N.; Golovanov, R.Y.; Basko, A.V.; Kudryavtsev, Y.V. Development of vibration damping materials based on butyl rubber: A study of the phase equilibrium, rheological, and dynamic properties of compositions. J. Appl. Polym. Sci. 2020, 138, 50196. [Google Scholar] [CrossRef]
- Li, Z.; Crocker, M.J. A Review on Vibration Damping in Sandwich Composite Structures. Int. J. Acoust. Vib. 2005, 10, 159–169. [Google Scholar] [CrossRef]
- Lu, J.; Wang, L. Production and application of high strength steel sheet for automobile. Automob. Techno Mat 2004, 2, 1–6. [Google Scholar]
- Roth, R.; Clark, J.; Kelkar, A. Automobile bodies: Can aluminum be an economical alternative to steel? JOM 2001, 53, 28–32. [Google Scholar] [CrossRef]
- Boon, J.E.; Isaacs, J.A.; Gupta, S.M. Economic Impact of Aluminum-Intensive Vehicles on the U.S. Automotive Recycling Infrastructure. J. Ind. Ecol. 2000, 4, 117–134. [Google Scholar] [CrossRef]
- Wang, F.; Xu, J.; Luo, H.; Wang, J.; Wang, Q. A New Organofunctional Ethoxysilane Self-Assembly Monolayer for Promoting Adhesion of Rubber to Aluminum. Molecules 2009, 14, 4087–4097. [Google Scholar] [CrossRef]
- Plueddemann, E.P. Adhesion Through Silane Coupling Agents. J. Adhes. 1970, 2, 184–201. [Google Scholar] [CrossRef]
- Thiedmanu, W.; Tolan, F.C.; Pearce, P.J.; Morris, C.E.M. Silane Coupling Agents as Adhesion Promoters for Aerospace Structural Film Adhesives. J. Adhes. 1987, 22, 197–210. [Google Scholar] [CrossRef]
- Crook, R.A.; Sinclair, J.W.; Poulter, L.W.; Schulte, K.J. An Environmentally-Friendly Process for Bonding Aluminum Using Aqueous Metasilicate Sol-Gel and Silane Adhesion Promoters. J. Adhes. 1998, 68, 315–329. [Google Scholar] [CrossRef]
- Chen, H.; Wang, J.; Huo, Q. Self-assembled monolayer of 3-aminopropyltrimethoxysilane for improved adhesion between aluminum alloy substrate and polyurethane coating. Thin Solid Films 2007, 515, 7181–7189. [Google Scholar] [CrossRef]
- Maege, I.; Jaehne, E.; Henke, A.; Adler, H.-J.P.; Bram, C.; Jung, C.; Stratmann, M. Self-assembling adhesion promoters for corrosion resistant metal polymer interfaces. Prog. Org. Coat. 1998, 34, 1–12. [Google Scholar] [CrossRef]
- Li, X.; Wang, P.; Long, S.; Huang, Y.; Li, H.; Hu, C. Interfacial adhesion and water resistance of stainless steel–polyolefin improved by functionalized silane. Polym. Eng. Sci. 2019, 59, 1866–1873. [Google Scholar] [CrossRef]
- Lee, S.R.; Bae, K.M.; Baek, J.J.; Kang, M.C.; Lee, T.I. Adhesion enhancement between aluminum and butyl rubber by (3-mercaptopropyl) trimethoxy silane for vibration damping plate. J. Adhes. Sci. Technol. 2021, 35, 1114–1124. [Google Scholar] [CrossRef]
- Sang, J.; Aisawa, S.; Miura, K.; Hirahara, H.; Jan, O.; Jozef, P.; Pavol, M. Adhesion of carbon steel and natural rubber by functionalized silane coupling agents. Int. J. Adhes. Adhes. 2017, 72, 70–74. [Google Scholar] [CrossRef]
- Nützel, O.; Saul, R. Long-term corrosion protection for bridge cables with butyl rubber tapes using the ATIS Cableskin® system. Steel Constr. 2015, 8, 59–64. [Google Scholar] [CrossRef]
- Saul, R.; Nützel, O. Wrapping with Butyl Rubber Tapes—An Innovative Corrosion Protection for Bridge Cables. Struct. Eng. Int. 2012, 22, 330–335. [Google Scholar] [CrossRef]
- Rajkiewicz, M.; Slaczka, M.; Czakaj, J. Adhesive Properties of The Butyl Rubber Compounds. Adv. Sustain. Pet. Eng. Sci. 2014, 6, 123. [Google Scholar]
- Rajkiewicz, M.; Ślączka, M.; Czakaj, J. Part I—The Butyl Rubber Compounds. Adhesive Properties; Apple Academic Press: Palm Bay, FL, USA, 2014; pp. 409–422. [Google Scholar]
- Thomas, S.; Dechant, D.A. 2-Layer Polyethylene Extruded Factory-Applied Pipe Corrosion Coating. In Pipelines 2019; American Society of Civil Engineers: Reston, VA, USA, 2019. [Google Scholar] [CrossRef]
- Okyere, M.S. Corrosion Protection for the Oil and Gas Industry: Pipelines, Subsea Equipment, and Structures; CRC Press: Boca Raton, FL, USA, 2019. [Google Scholar]
- Papavinasam, S.; Attard, M.; Revie, R.W. Evolution of External Pipeline Coatings for Corrosion Protection—A Review. Corros. Rev. 2008, 26, 373–438. [Google Scholar] [CrossRef]
- Perova, M.; Galimzyanova, R.; Khakimullin, Y.; Vol’Fson, S. Influence of the Molecular Weight of Oligoisobutylenes on the Properties of Uncured Sealants. Int. Polym. Sci. Technol. 2011, 38, 9–11. [Google Scholar] [CrossRef]
- Akhmedgoraeva, A.R.; Stytsenkov, A.A.; Galimzyanova, R.Y.; Khakimullin, Y.N. The Influence of Thickness and Reinforcement on the Properties of Sealing Tapes of Incongealable Type on the Basis of Butyl Rubber Depending on the Nature of the Glued Substrate. Polym. Sci. Ser. D 2019, 12, 137–141. [Google Scholar] [CrossRef]
- Kempe, M.D.; Dameron, A.A.; Reese, M.O. Evaluation of moisture ingress from the perimeter of photovoltaic modules. Prog. Photovolt. Res. Appl. 2013, 22, 1159–1171. [Google Scholar] [CrossRef]
- Tian, Y.; Guo, L. Adhesion performance of UHMWPE fiber treated with polyethylene wax grafted methyl methacrylate alone or in conjunction with silane coupling agent. J. Adhes. Sci. Technol. 2020, 35, 1219–1235. [Google Scholar] [CrossRef]
- Guo, Y.; Li, Y.; Wang, S.; Liu, Z.-X.; Cai, B.; Wang, P.-C. Effect of silane treatment on adhesion of adhesive-bonded carbon fiber reinforced nylon 6 composite. Int. J. Adhes. Adhes. 2019, 91, 102–115. [Google Scholar] [CrossRef]
- Lee, J.W.; Heo, J.H.; Lee, B.; Cho, H.H.; Kim, T.; Lee, J.H. Enhancement in the adhesion properties of polycarbonate surfaces through chemical functionalization with organosilicon coupling agents. J. Mater. Sci. Mater. Electron. 2019, 30, 17773–17779. [Google Scholar] [CrossRef]




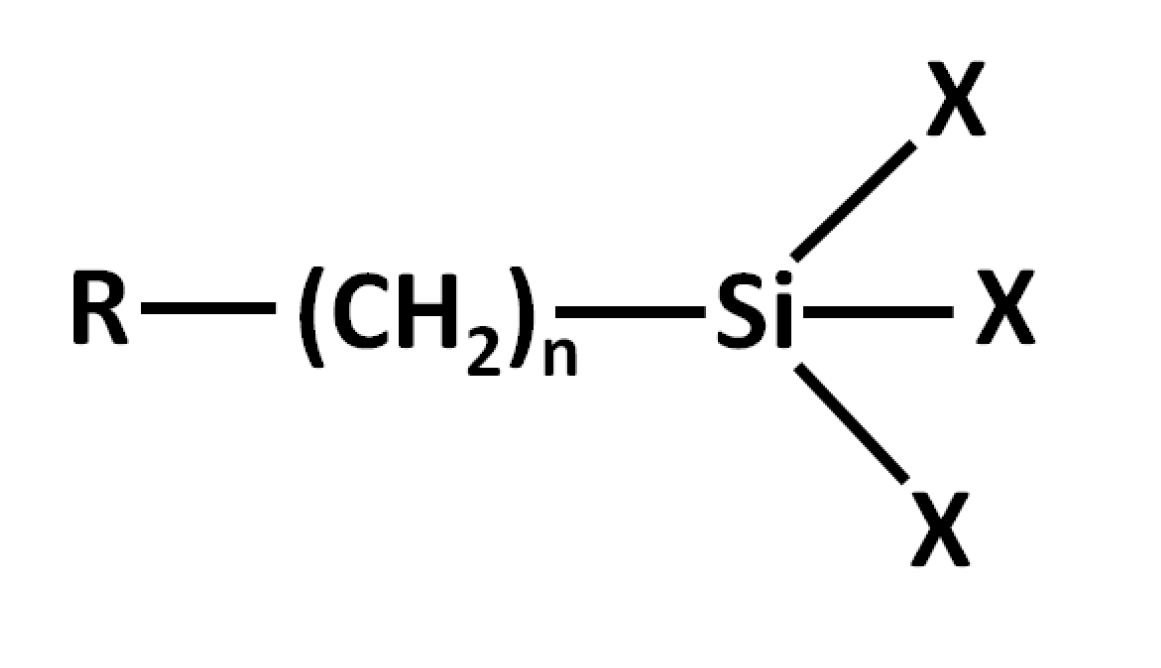
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Czakaj, J.; Sztorch, B.; Romanczuk-Ruszuk, E.; Brząkalski, D.; Przekop, R.E. Organosilicon Compounds in Hot-Melt Adhesive Technologies. Polymers 2023, 15, 3708. https://doi.org/10.3390/polym15183708
Czakaj J, Sztorch B, Romanczuk-Ruszuk E, Brząkalski D, Przekop RE. Organosilicon Compounds in Hot-Melt Adhesive Technologies. Polymers. 2023; 15(18):3708. https://doi.org/10.3390/polym15183708
Chicago/Turabian StyleCzakaj, Jakub, Bogna Sztorch, Eliza Romanczuk-Ruszuk, Dariusz Brząkalski, and Robert E. Przekop. 2023. "Organosilicon Compounds in Hot-Melt Adhesive Technologies" Polymers 15, no. 18: 3708. https://doi.org/10.3390/polym15183708





