Microstructure and Water Retention Kinetics in Autogenous Cured Self-Compacting Concrete Blends Using Super Absorbent Polymer
Abstract
:1. Introduction
2. Materials and Methods
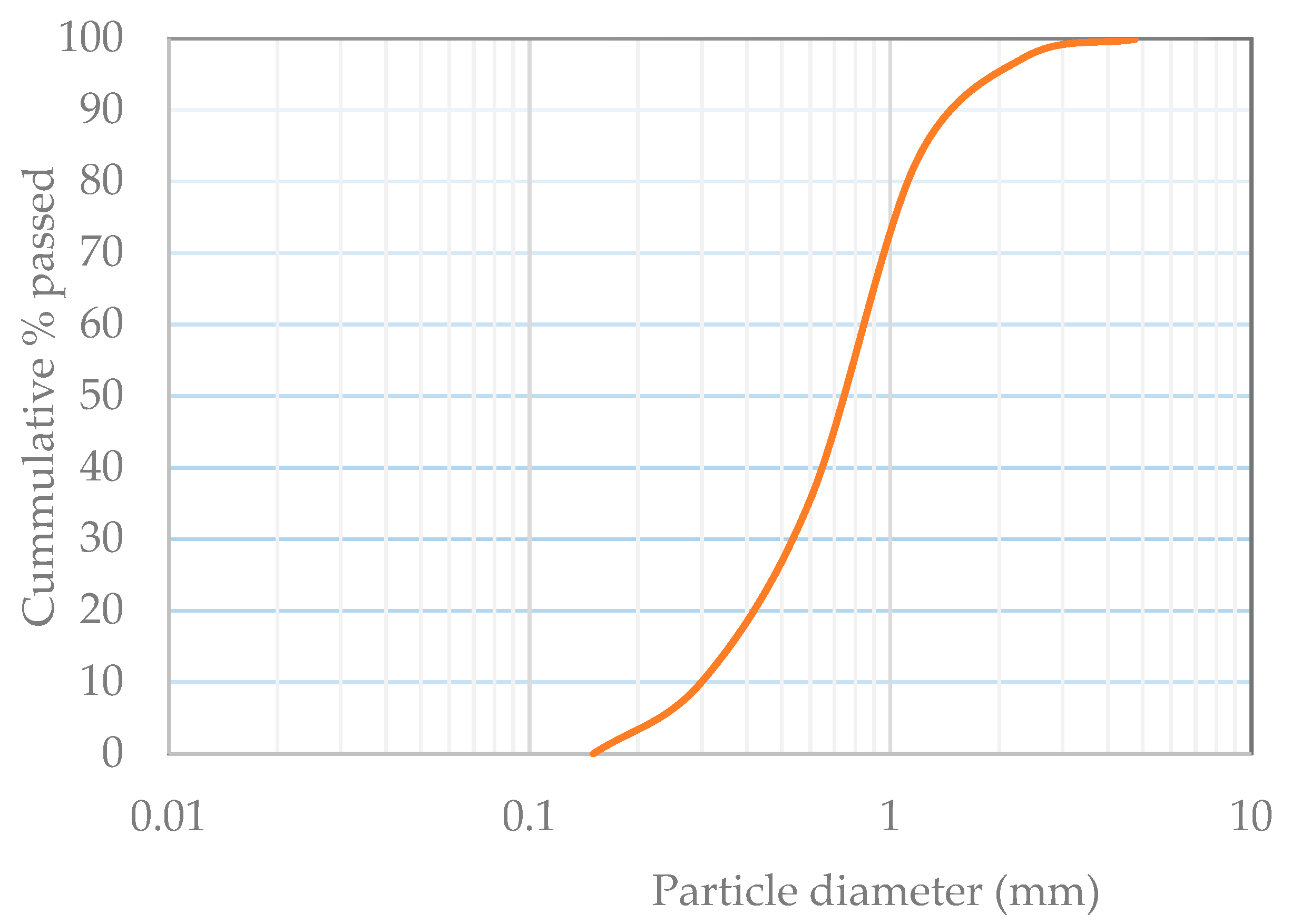
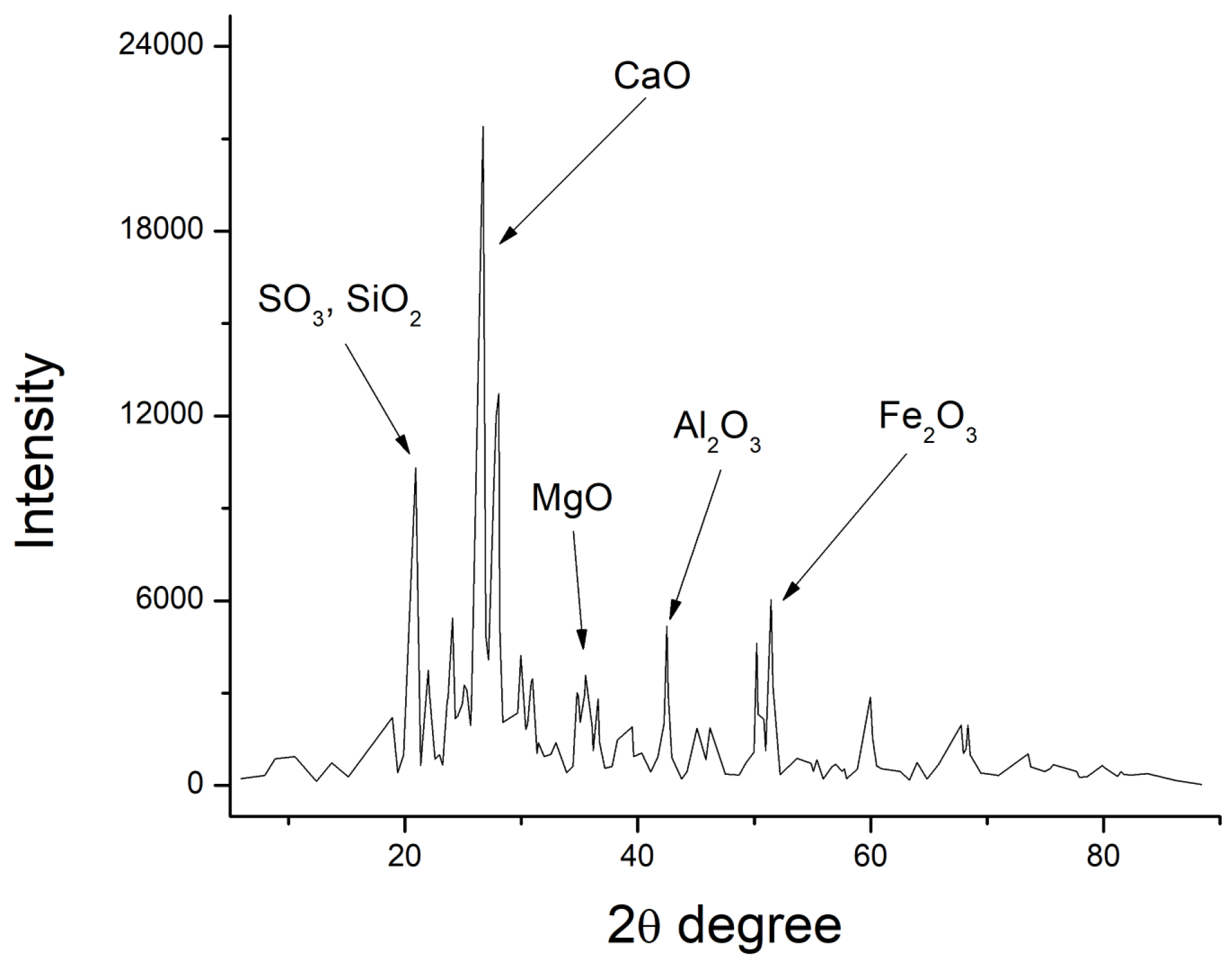
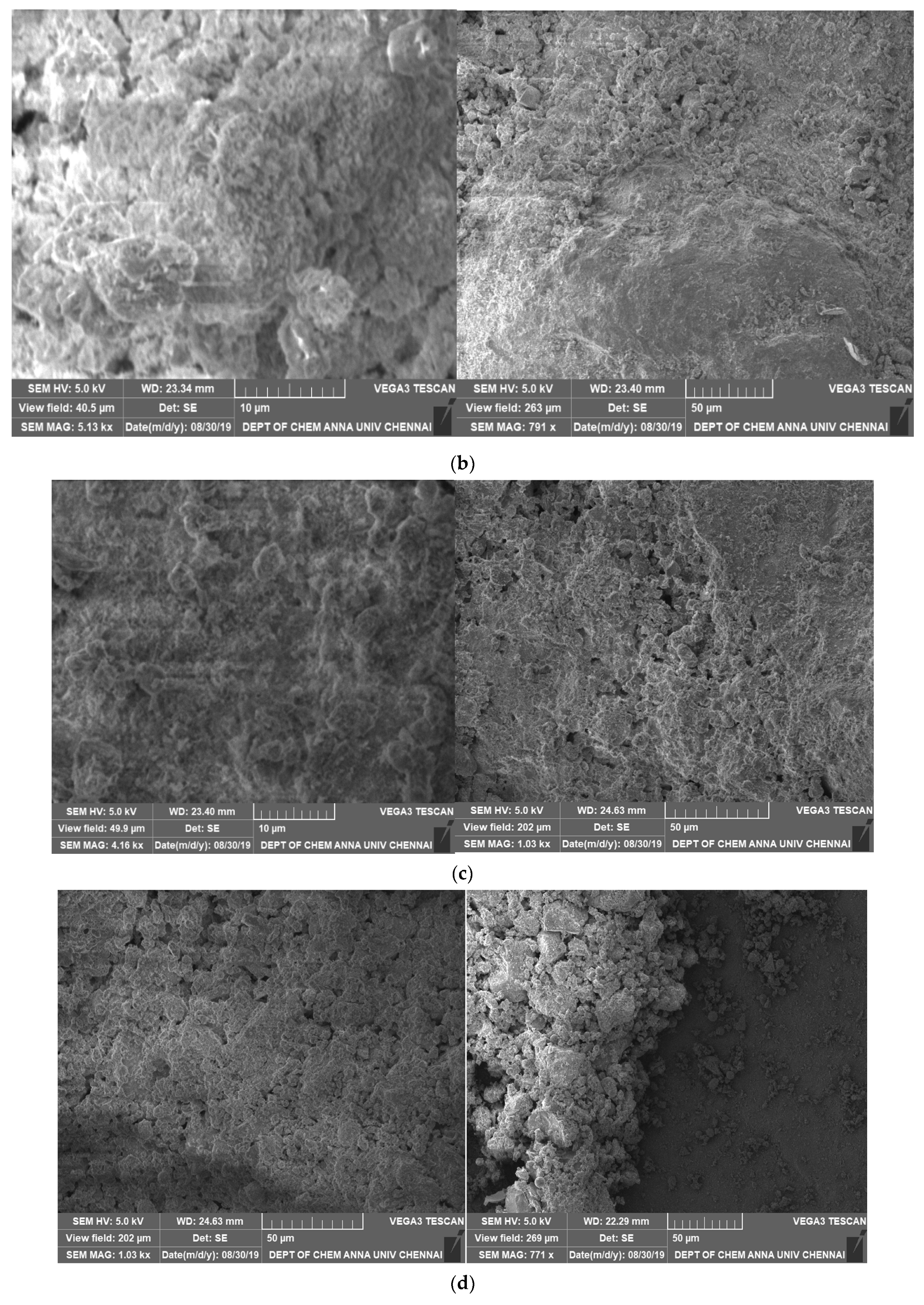
2.1. Chemical and Microstructural Properties
2.2. Water Retention Properties
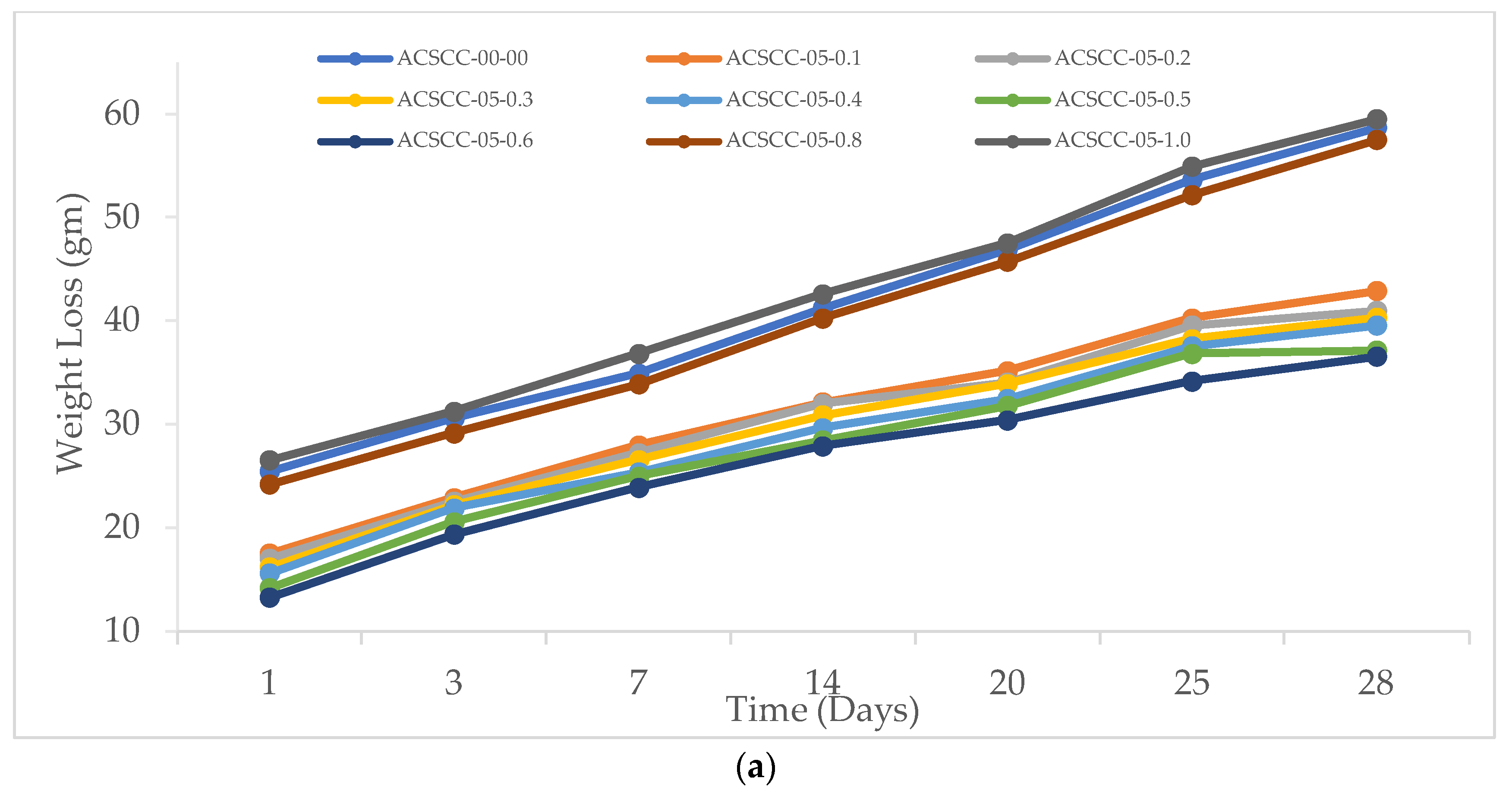
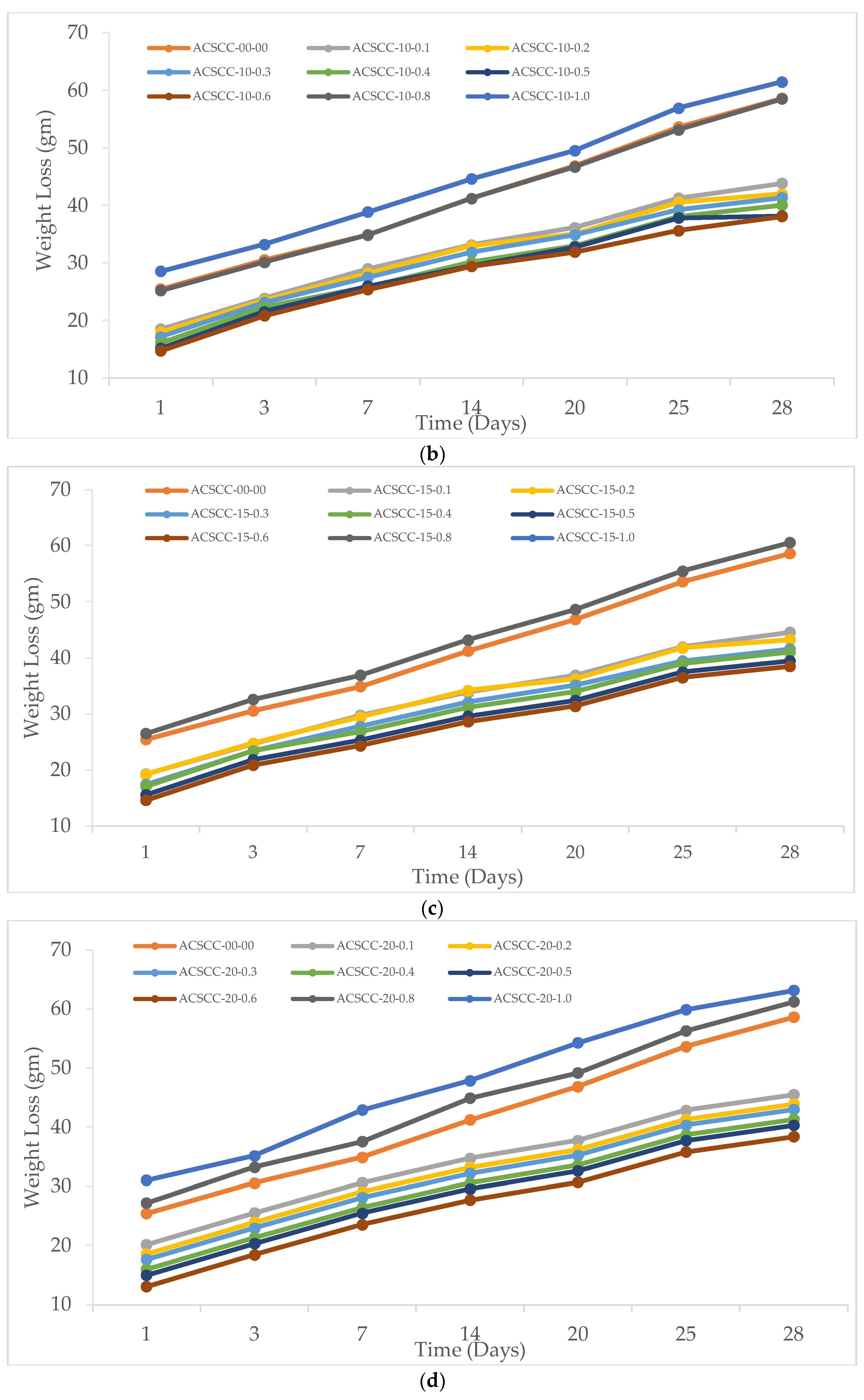
2.2.1. Weight Loss
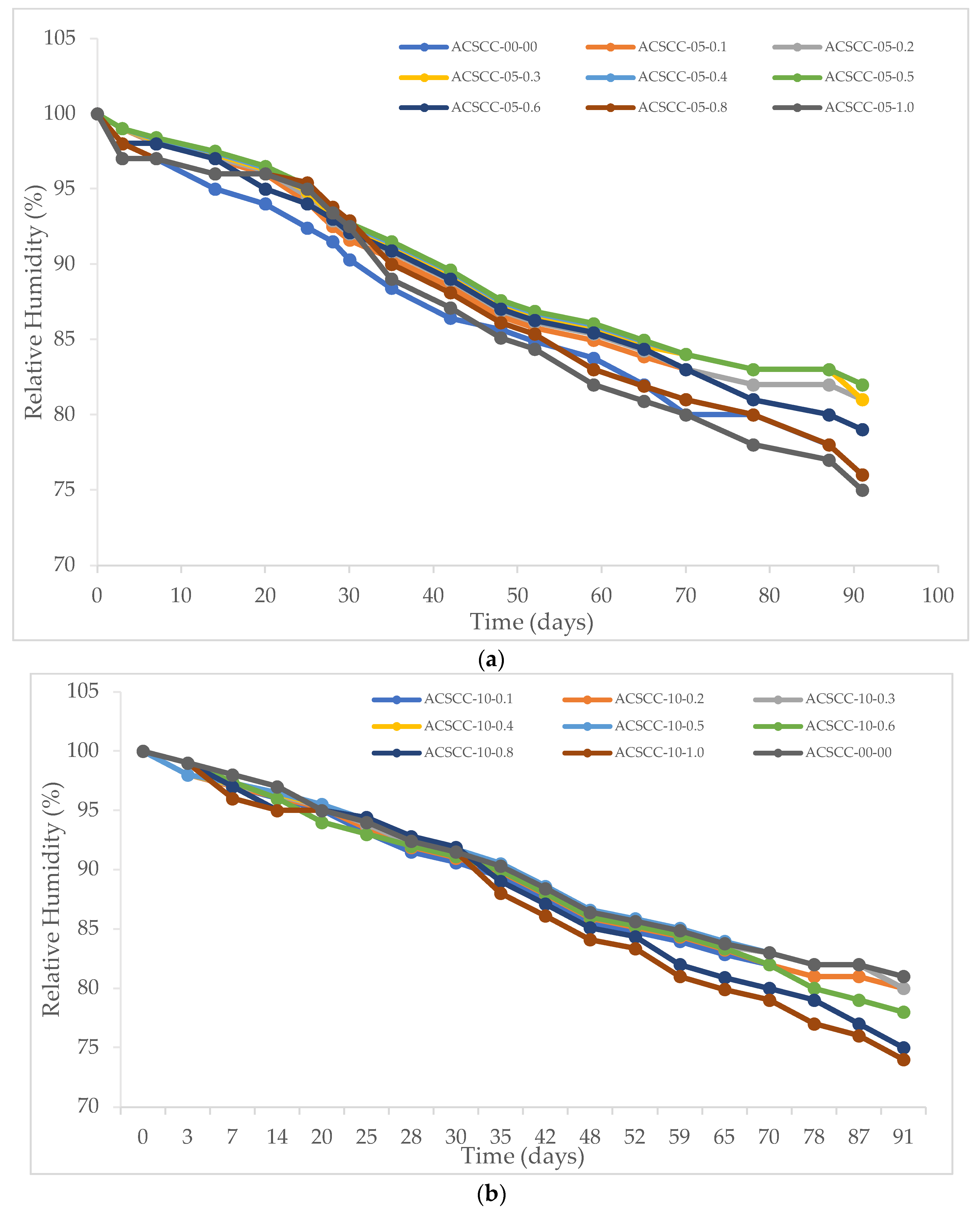
2.2.2. Internal Relative Humidity
2.3. Nonevaporable Water
2.4. Sorptivity
3. Results and Discussions
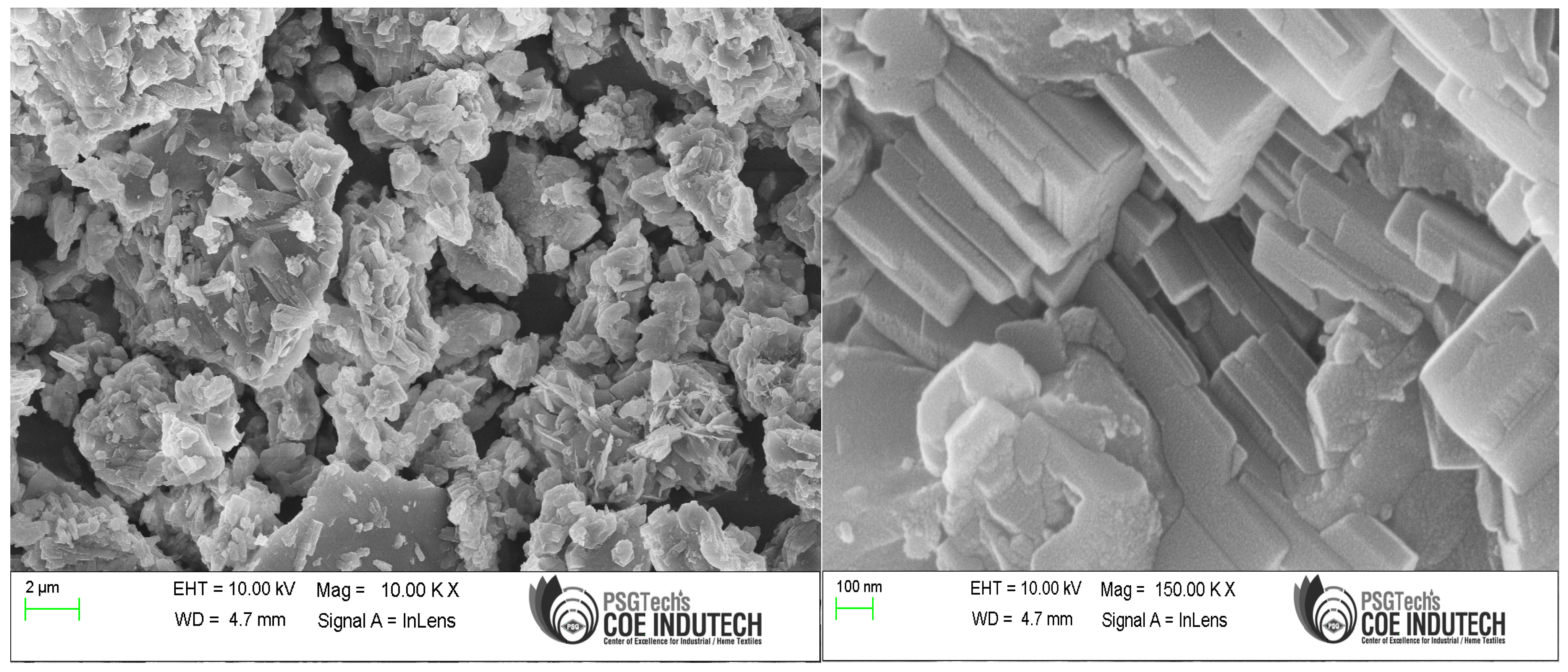
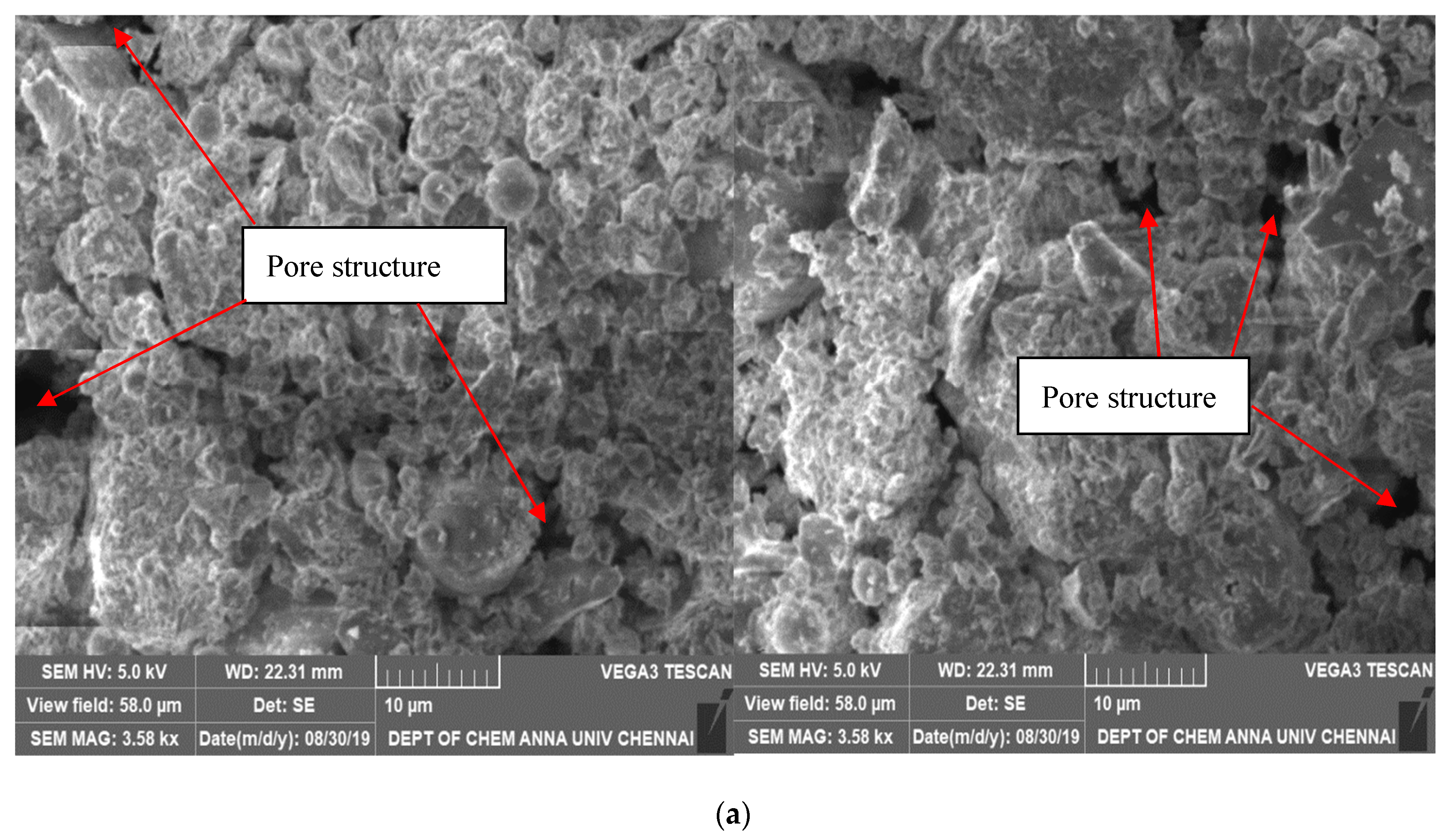
3.1. Chemical and Microstructural Properties
3.2. Water Retention Properties
3.2.1. Weight Loss
3.2.2. Internal Relative Humidity
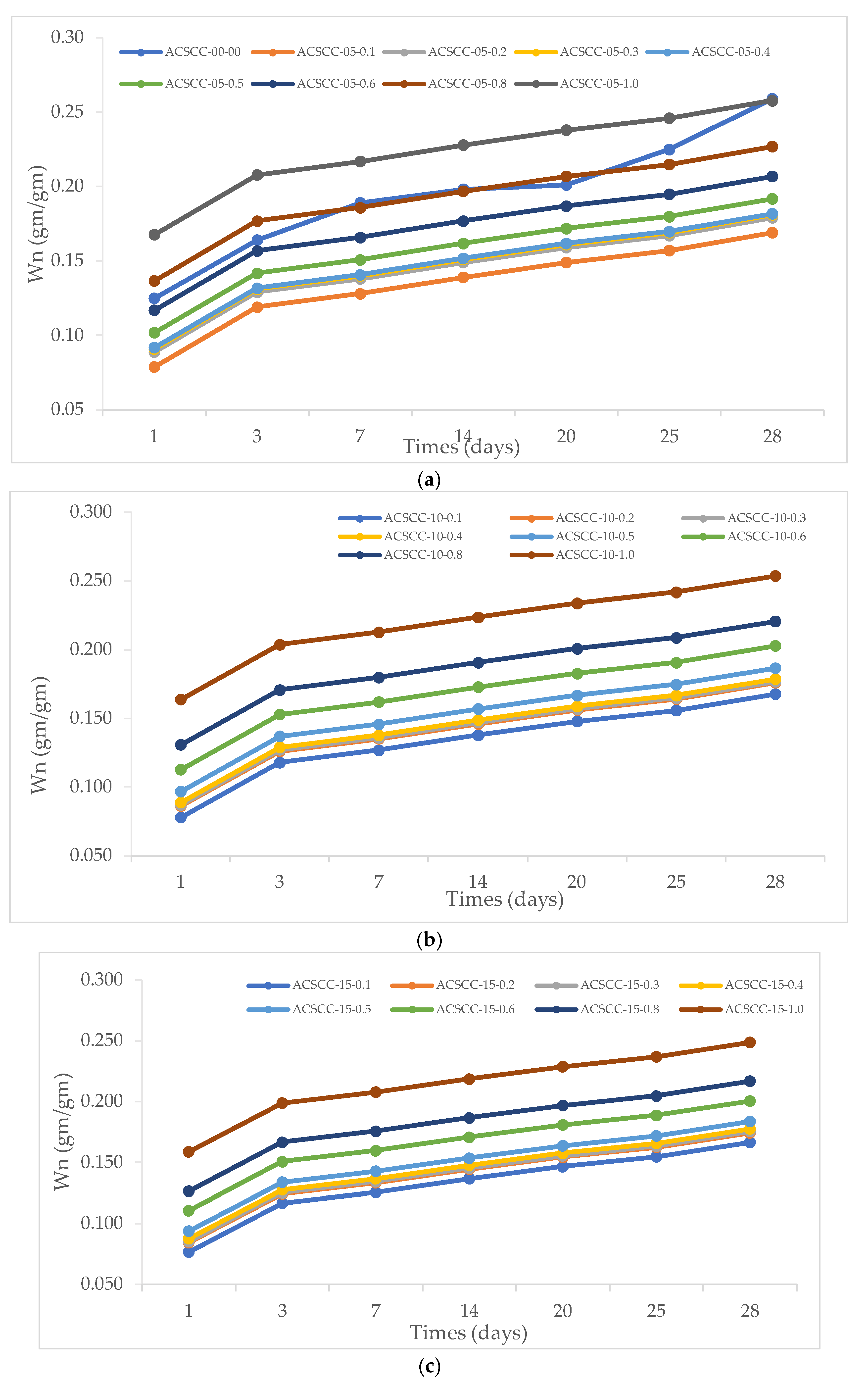
3.3. Nonevaporable Water
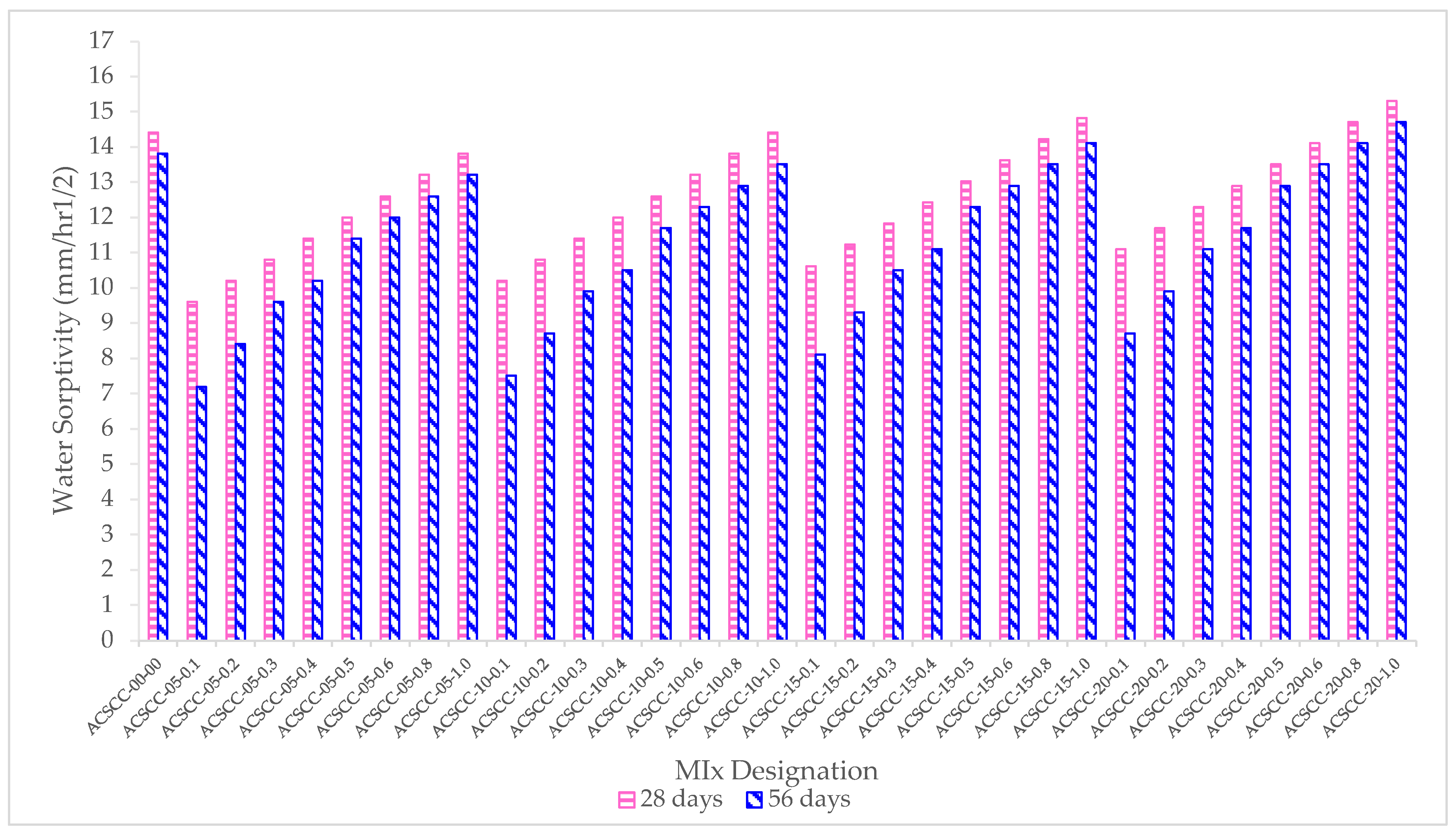
3.4. Sorptivity
4. Conclusions
- The core mineral and morphological features in the GP and cement specimens were comparable according to SEM-EDAX analysis.
- The microstructural studies showed that SAP addition up to 0.6% sped up the hydration process by supplying internal water and helped form the hydrated product.
- The inclusion of SAP and GP finally caused the weight reduction to halt. On the other hand, with time, when SAP addition was more than 0.6%, the weight loss quickened. For the GP-ACSCC combinations, weight loss was less than that for the control mixture.
- A significant increase in relative humidity was noticed for 91 days compared to the conventional mixture for almost all GP-ACSCC mixtures.
- SAP addition up to 0.6% demonstrated a considerable increase in water retention qualities among the 32 GP-ACSCC mixtures.
- Compared to control mixtures, the addition of SAP up to 0.6% and the substitution of cement with GP up to 15% had favorable impacts on all water kinetics parameters.
- Larger quantities of nonevaporable water were produced as a result of the GP-ACSCC mixture’s capacity to store water, which in turn led to higher levels of hydration.
- Both the GP-ACSCC blends and the conventional concrete mixture sorptivity values were observed to decrease over time, while most of the GP-ACSCC blends fell under the “very good” and “good” criteria as per the code provision.
- If SAP is evenly distributed across the hydration zones, it will more effectively function as an internal curing substance. The GP-ACSCC mixture’s small particle size and structure enabled the SAP to be distributed evenly and improved the pore structure. Self-desiccation was prevented by the available curing water at the small reservoirs of the super absorbent polymers. The high water retention property and improved kinetics of water was proven by the increase in nonevaporable water, increase in relative humidity, and decrease in percentage of weight loss. It was obvious that the GP-ACSCC combination concrete was more densely packed, homogeneous, and had a more refined pore structure in comparison with the control specimen. This produced a higher water retention property.
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- World Business Council for Sustainable Development (WBCSD). The Cement Sustainability Initiative Recycling Concrete; World Business Council for Sustainable Developments: Geneva, Switzerland, 2016. [Google Scholar]
- CEMBUREAU—The European Cement Association. Key Facts Figures. Available online: https://www.cembureau.be/about-cement/key-facts-figures (accessed on 8 July 2016).
- Van Breugel, K. Is there a market for self-healing cement-based materials. In Proceedings of the First International Conference on Self-Healing Materials, Noordwijk aan Zee, The Netherlands, 18–20 April 2007. [Google Scholar]
- Mahasenan, N.; Smith, S.; Humphreys, K.; Kaya, Y. The cement industry and global climate change: Current and potential future cement industry CO2 emissions. In Proceedings of the 6th International Conference on Greenhouse Gas Control Technologies, Kyoto, Japan, 1–4 October 2002. [Google Scholar]
- Meyer, C. The greening of the concrete industry. Cem. Concr. Compos. 2009, 31, 601–605. [Google Scholar]
- Amran, M.; Fediuk, R.; Murali, G.; Avudaiappan, S.; Ozbakkaloglu, T.; Vatin, N.; Karelina, M.; Klyuev, S.; Gholampour, A. Fly Ash-Based Eco-Efficient Concretes: A Comprehensive Review of the Short-Term Properties. Materials 2021, 14, 4264. [Google Scholar] [PubMed]
- Prakash, R.; Divyah, N.; Srividhya, S.; Avudaiappan, S.; Amran, M.; Raman, S.N.; Guindos, P.; Vatin, N.I.; Fediuk, R. Effect of Steel Fiber on the Strength and Flexural Characteristics of Coconut Shell Concrete Partially Blended with Fly Ash. Materials 2022, 15, 4272. [Google Scholar] [PubMed]
- Arularasi, V.; Thamilselvi, P.; Avudaiappan, S.; Saavedra Flores, E.I.; Amran, M.; Fediuk, R.; Vatin, N.; Karelina, M. Rheological Behavior and Strength Characteristics of Cement Paste and Mortar with Fly Ash and GGBS Admixtures. Sustainability 2021, 13, 9600. [Google Scholar]
- Joseph, H.S.; Thamilselvi, P.; Avudaiappan, S.; Maureira-Carsalade, N.; Roco-Videla, A.; Guindos, P.; Pablo, F.; Parra, A. Comprehensive Review on Recycling of Construction Demolition Waste in Concrete. Sustainability 2021, 15, 4932. [Google Scholar]
- Arularasi, V.; Pachiappan, T.; Avudaiappan, S.; Raman, S.N.; Guindos, P.; Amran, M.; Fediuk, R.; Vatin, N.I. Effects of Admixtures on Energy Consumption in the Process of Ready-Mixed Concrete Mixing. Materials 2022, 15, 4143. [Google Scholar]
- Aggoune, S.; Imache, R.; Khadraoui, A.; Mezghiche, M. Evaluation of e-government information systems agility in the perspective of sustainability. In Electronic Government and the Information Systems Perspective; Springer: New York, NY, USA, 2011; pp. 315–329. [Google Scholar]
- Sellevold, E.J.; Bjontegaard, O. Coefficient of thermal expansion of cement paste and concrete: Mechanisms of moisture interaction. Mater. Struct. 2006, 39, 809–815. [Google Scholar]
- Bentz, D.P.; Jensen, O.M. Mitigation strategies for autogenous shrinkage cracking. Cem. Concr. Compos. 2004, 26, 677–685. [Google Scholar]
- Snoeck, D.; Jensen, O.M.; De Belie, N. The influence of superabsorbent polymers on the autogenous shrinkage properties of cement pastes with supplementary cementitious materials. Cem. Concr. Compos. 2015, 74, 59–67. [Google Scholar]
- Lija, R.L.; Philips, J.; Vandhana, D.V. Influence of waste foundry sand on microstructural and mechanical behavior of self-consolidated concrete filled steel column. Mater. Today: Proc. 2023. [Google Scholar] [CrossRef]
- Weber, S.; Reinhardt, H.W. A new generation of high performance concrete: Concrete with autogenous curing. Adv. Cem. Based Mater. 1997, 6, 59–68. [Google Scholar]
- Shen, D.; Jiangm, J.; Shen, J.; Yao, P.; Jiang, G. Influence of curing temperature on autogenous shrinkage and cracking resistance of high-performance concrete at an early age. Constr. Build. Mater. 2016, 103, 67–76. [Google Scholar]
- Jensen, O.M.; Hansen, P.F. Water-entrained cement-based materials: I. Principles and theoretical background. Cem. Concr. Res. 2001, 31, 647–654. [Google Scholar]
- Tazawa, E.I.; Miyazawa, S. Influence of cement and admixture on autogenous shrinkage of cement paste. Cem. Concr. Res. 1995, 25, 281–287. [Google Scholar]
- Leemann, A.; Lura, P.; Loser, R. Shrinkage and creep of SCC—The influence of paste volume and binder composition. Constr. Build. Mater. 2011, 25, 2283–2289. [Google Scholar]
- Abraham, S.; Lija, R.L. Behavior of self-cured self-compacting concrete filled short channel columns under axial compression. Mater. Today: Proc. 2023. [Google Scholar] [CrossRef]
- Jensen, O.M.; Hansen, P.F. Water-entrained cement-based materials II: Experimental observations. Cem. Concr. Res. 2002, 32, 973–978. [Google Scholar]
- Jensen, O.M.; Lura, P. Techniques for internal water curing of concrete. Mater. Struct. 2006, 39, 817–825. [Google Scholar]
- Lura, P.; Jensen, O.M.; Igarashi, S.I. Experimental observation of internal water curing of concrete. Mater. Struct. 2007, 40, 211–220. [Google Scholar]
- Arunachalam, K.P.; Avudaiappan, S.; Maureira, N.; Da Costa Garcia Filho, F.; Monteiro, S.N.; Batista, I.D.; de Azevedo, A.R.G. Innovative use of copper mine tailing as an additive in cement mortar. J. Mater. Res. Technol. 2023, 25, 2261–2274. [Google Scholar] [CrossRef]
- Igarashi, S.I.; Aragane, N.; Koike, Y. Effects of spatial structure of superabsorbent polymer particles on autogenous shrinkage behavior of cement paste. In International RILEM Conference on Use of Superabsorbent Polymers and Other New Additives in Concrete; RILEM Publications SARL: Champs-sur-Marne, France, 2010; pp. 137–147. [Google Scholar]
- Kavitha, S.A.; Priya, R.K.; Arunachalam, K.P.; Avudaiappan, S.; Maureira-Carsalade, N.; Roco-Videla, Á. Investigation on Properties of Raw and Alkali Treated Novel Cellulosic Root Fibres of Zea Mays for Polymeric Composites. Polymers 2023, 15, 1802. [Google Scholar]
- IS 12269:1987; Grade53 Grade Ordinary Portland Cement-Specification. Bureau of Indian Standards: New Delhi, India, 1987.
- IS 4031:1988; Methods of Physical Test for Cement. Bureau of Indian Standards: New Delhi, India, 1988.
- EFNARC. Specification and Guidelines for Self-Compacting Concrete; EFNARC: Surrey, UK, 2002; ISBN 0953973344. [Google Scholar]
- IS 2386:1963; Methods of Test for Aggregates for Concrete. Bureau of Indian Standards: New Delhi, India, 1963.
- IS 3812:2003; Pulverized Fuel Ash-Specification. Bureau of Indian Standards: New Delhi, India, 2003.
- IS: 9103-1999; Indian Standard Concrete Admixtures-Specification. Bureau of Indian Standards: New Delhi, India, 1999.
- Laila, L.R.; Gurupatham, B.G.A.; Roy, K.; Lim, J.B.P. Influence of super absorbent polymer on mechanical, rheological, durability, and microstructural properties of self-compacting concrete using non-biodegradable granite pulver. Struct. Concr. 2020, 22, 1093–1116. [Google Scholar]
- Deepankar Kumar, A.; Surender Kumar, V. An overview on mixture design of self-compacting concrete. Struct. Concr. 2018, 20, 371–395. [Google Scholar]
- Arunachalam, K.P.; Avudaiappan, S.; Flores, E.I.S.; Parra, P.F. Experimental Study on the Mechanical Properties and Microstructures of Cenosphere Concrete. Materials 2023, 16, 3518. [Google Scholar] [CrossRef] [PubMed]
- Avudaiappan, S.; Cendoya, P.; Arunachalam, K.P.; Maureira-Carsalade, N.; Canales, C.; Amran, M.; Parra, P.F. Innovative Use of Single-Use Face Mask Fibers for the Production of a Sustainable Cement Mortar. J. Compos. Sci. 2023, 7, 214. [Google Scholar] [CrossRef]
- Hall, C. Water sorptivity of mortars and concretes: A review. Mag. Concr. Res. 1989, 41, 51–61. [Google Scholar]
- El-Dieb, A.S. Effect of sorptivity test time on the variation of test results. Ain Shams Univ. Faculty Eng. Sci. Bull 1999, 34, 41–51. [Google Scholar]
- Laila, L.R.; Gurupatham, B.G.A.; Roy, K.; Lim, J.B.P. Effect of super absorbent polymer on microstructural and mechanical properties of concrete blends using granite pulver. Struct. Concr. 2020, 22, 898–915. [Google Scholar]
- Marmol, I.; Ballester, P.; Cerro, S.; Monros, G.; Morales, J.; Sanchez, L. Use of Granite Sludge wastes for the production of coloured cement based mortars. Cem. Concr. Compos. 2010, 32, 617–622. [Google Scholar]
- Igarashi, S.; Watanabe, A. Experimental study on prevention of autogenous deformation by internal curing using super-absorbent polymer particles. In International RILEM Conference on Volume Changes of Hardening Concrete; RILEM Publications SARL: Champs-sur-Marne, France, 2006; pp. 77–86. [Google Scholar]
- Craeye, B.; Geirnaert, M.; De Schutter, G. Super absorbing polymers as an internal curing agent for mitigation of early-age cracking of high-performance concrete bridge decks. Constr. Build. Mater. 2010, 25, 1–13. [Google Scholar]
- Reinhardt, H.W.; Assmann, A. Enhanced durability of concrete by superabsorbent polymers. In International Symposium Brittle Matrix Composites; Woodhead Publishing: Warsaw, Poland, 2009; pp. 291–300. [Google Scholar]
- Mjornell, K. Self-Desiccation in concrete. Chalmers Univ. Technol. 1994, 2, 94. [Google Scholar]
- McGrath, P.F. Internal Self-Desiccation of Silica Fume Concrete. Master’s Thesis, Civil Engineering Department, University of Toronto, Toronto, ON, Canada, 1989. [Google Scholar]
- Wyrzykowski, M.; Lura, P. Effect of relative humidity decrease due to self-desiccation on the hydration kinetics of cement. Cem. Concr. Res. 2016, 85, 75–81. [Google Scholar] [CrossRef]
- Miller, A.E.; Barrett, T.J.; Weiss, W. Evaluation of superabsorbent polymers for use in cementitious systems for the purpose of mitigating autogenous shrinkage. In Proceedings of the International RILEM Conference on the Application of Superabsorbent Polymers and Other New Admixtures in Concrete Construction, Dresden, Germany, 14–17 September 2014. [Google Scholar]
- Zhutovsky, S.; Kovler, K. Hydration kinetics of high performance cementitious systems under different curing conditions. Mater. Struct. 2013, 46, 1599–1611. [Google Scholar] [CrossRef]
- ASTM C-1585-04; Measurement of Rate of Absorption of Water by Hydraulic Cement Concretes. ASTM International: West Conshohocken, PA, USA, 2004.










| Mixture Designation | Relative Humidity (%) | |||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Days | 0 | 3 | 7 | 14 | 20 | 25 | 28 | 30 | 35 | 42 | 48 | 52 | 59 | 65 | 70 | 78 | 87 | 91 |
| ACSCC-00-00 | 100 | 98 | 97 | 95 | 94 | 92 | 92 | 90 | 88 | 86 | 86 | 85 | 84 | 82 | 80 | 80 | 78 | 77 |
| ACSCC-05-0.1 | 100 | 99 | 98 | 97 | 96 | 94 | 93 | 92 | 90 | 89 | 87 | 86 | 85 | 84 | 83 | 82 | 82 | 81 |
| ACSCC-05-0.2 | 100 | 99 | 98 | 97 | 96 | 95 | 93 | 92 | 91 | 89 | 87 | 86 | 85 | 84 | 83 | 82 | 82 | 81 |
| ACSCC-05-0.3 | 100 | 99 | 98 | 97 | 96 | 95 | 93 | 92 | 91 | 89 | 87 | 86 | 86 | 85 | 84 | 83 | 83 | 81 |
| ACSCC-05-0.4 | 100 | 99 | 98 | 97 | 96 | 95 | 94 | 93 | 91 | 90 | 88 | 87 | 86 | 85 | 84 | 83 | 83 | 82 |
| ACSCC-05-0.5 | 100 | 99 | 98 | 98 | 97 | 95 | 94 | 93 | 92 | 90 | 88 | 87 | 86 | 85 | 84 | 83 | 83 | 82 |
| ACSCC-05-0.6 | 100 | 98 | 98 | 97 | 95 | 94 | 93 | 92 | 91 | 89 | 87 | 86 | 85 | 84 | 83 | 81 | 80 | 79 |
| ACSCC-05-0.8 | 100 | 98 | 97 | 96 | 96 | 95 | 94 | 93 | 90 | 88 | 86 | 85 | 83 | 82 | 81 | 80 | 78 | 76 |
| ACSCC-05-1.0 | 100 | 97 | 97 | 96 | 96 | 95 | 93 | 93 | 89 | 87 | 85 | 84 | 82 | 81 | 80 | 78 | 77 | 75 |
| ACSCC-10-0.1 | 100 | 99 | 98 | 97 | 95 | 94 | 92 | 92 | 90 | 88 | 86 | 86 | 85 | 84 | 83 | 82 | 82 | 81 |
| ACSCC-10-0.2 | 100 | 98 | 97 | 96 | 95 | 93 | 92 | 91 | 89 | 88 | 86 | 85 | 84 | 83 | 82 | 81 | 81 | 80 |
| ACSCC-10-0.3 | 100 | 98 | 97 | 96 | 95 | 94 | 92 | 91 | 90 | 88 | 86 | 85 | 84 | 83 | 82 | 81 | 81 | 80 |
| ACSCC-10-0.4 | 100 | 98 | 97 | 96 | 95 | 94 | 92 | 91 | 90 | 88 | 86 | 85 | 85 | 84 | 83 | 82 | 82 | 80 |
| ACSCC-10-0.5 | 100 | 98 | 97 | 96 | 95 | 94 | 93 | 92 | 90 | 89 | 87 | 86 | 85 | 84 | 83 | 82 | 82 | 81 |
| ACSCC-10-0.6 | 100 | 98 | 97 | 97 | 96 | 94 | 93 | 92 | 91 | 89 | 87 | 86 | 85 | 84 | 83 | 82 | 82 | 81 |
| ACSCC-10-0.8 | 100 | 99 | 97 | 96 | 94 | 93 | 92 | 91 | 90 | 88 | 86 | 85 | 84 | 83 | 82 | 80 | 79 | 78 |
| ACSCC-10-1.0 | 100 | 99 | 97 | 95 | 95 | 94 | 93 | 92 | 89 | 87 | 85 | 84 | 82 | 81 | 80 | 79 | 77 | 75 |
| ACSCC-15-0.1 | 100 | 99 | 98 | 97 | 95 | 94 | 92 | 92 | 90 | 88 | 86 | 86 | 85 | 84 | 83 | 82 | 82 | 81 |
| ACSCC-15-0.2 | 100 | 97 | 96 | 95 | 94 | 92 | 91 | 90 | 88 | 87 | 85 | 84 | 83 | 82 | 81 | 80 | 80 | 80 |
| ACSCC-15-0.3 | 100 | 97 | 96 | 95 | 94 | 93 | 91 | 90 | 89 | 87 | 85 | 84 | 83 | 82 | 81 | 80 | 80 | 80 |
| ACSCC-15-0.4 | 100 | 97 | 96 | 95 | 94 | 93 | 91 | 90 | 89 | 87 | 85 | 84 | 84 | 83 | 82 | 81 | 81 | 80 |
| ACSCC-15-0.5 | 100 | 97 | 96 | 95 | 94 | 93 | 92 | 91 | 89 | 88 | 86 | 85 | 84 | 83 | 82 | 81 | 81 | 80 |
| ACSCC-15-0.6 | 100 | 97 | 96 | 96 | 95 | 93 | 92 | 91 | 90 | 88 | 86 | 85 | 84 | 83 | 82 | 81 | 81 | 80 |
| ACSCC-15-0.8 | 100 | 98 | 96 | 95 | 93 | 92 | 91 | 90 | 89 | 87 | 85 | 84 | 83 | 82 | 81 | 79 | 78 | 77 |
| ACSCC-15-1.0 | 100 | 98 | 96 | 94 | 94 | 93 | 92 | 91 | 88 | 86 | 84 | 83 | 81 | 80 | 79 | 78 | 76 | 74 |
| ACSCC-20-0.1 | 100 | 99 | 98 | 97 | 95 | 94 | 92 | 92 | 90 | 88 | 86 | 86 | 85 | 84 | 83 | 82 | 82 | 81 |
| ACSCC-20-0.2 | 100 | 97 | 95 | 94 | 93 | 91 | 90 | 89 | 87 | 86 | 84 | 83 | 82 | 81 | 80 | 80 | 80 | 80 |
| ACSCC-20-0.3 | 100 | 97 | 95 | 94 | 93 | 92 | 90 | 89 | 88 | 86 | 84 | 83 | 82 | 81 | 80 | 80 | 80 | 80 |
| ACSCC-20-0.4 | 100 | 97 | 95 | 94 | 93 | 92 | 90 | 89 | 88 | 86 | 84 | 83 | 83 | 82 | 81 | 80 | 80 | 79 |
| ACSCC-20-0.5 | 100 | 97 | 95 | 94 | 93 | 92 | 91 | 90 | 88 | 87 | 85 | 84 | 83 | 82 | 81 | 80 | 80 | 79 |
| ACSCC-20-0.6 | 100 | 97 | 95 | 95 | 94 | 92 | 91 | 90 | 89 | 87 | 85 | 84 | 83 | 82 | 81 | 80 | 80 | 79 |
| ACSCC-20-0.8 | 100 | 96 | 95 | 94 | 92 | 91 | 90 | 89 | 88 | 86 | 84 | 83 | 82 | 81 | 80 | 78 | 77 | 76 |
| ACSCC-20-1.0 | 100 | 96 | 95 | 93 | 93 | 92 | 91 | 90 | 87 | 85 | 83 | 82 | 80 | 79 | 78 | 77 | 75 | 73 |
| Mixture Designation | Nonevaporable Water | ||||||
|---|---|---|---|---|---|---|---|
| Days | 1 | 3 | 7 | 14 | 20 | 25 | 28 |
| ACSCC-00-00 | 0.070 | 0.110 | 0.120 | 0.130 | 0.145 | 0.150 | 0.168 |
| ACSCC-05-0.1 | 0.079 | 0.119 | 0.128 | 0.139 | 0.149 | 0.157 | 0.169 |
| ACSCC-05-0.2 | 0.089 | 0.129 | 0.138 | 0.149 | 0.159 | 0.167 | 0.179 |
| ACSCC-05-0.3 | 0.091 | 0.131 | 0.140 | 0.151 | 0.161 | 0.169 | 0.181 |
| ACSCC-05-0.4 | 0.092 | 0.132 | 0.141 | 0.152 | 0.162 | 0.170 | 0.182 |
| ACSCC-05-0.5 | 0.102 | 0.142 | 0.151 | 0.162 | 0.172 | 0.180 | 0.192 |
| ACSCC-05-0.6 | 0.117 | 0.157 | 0.166 | 0.177 | 0.187 | 0.195 | 0.207 |
| ACSCC-05-0.8 | 0.137 | 0.177 | 0.186 | 0.197 | 0.207 | 0.215 | 0.227 |
| ACSCC-05-1.0 | 0.168 | 0.208 | 0.217 | 0.228 | 0.238 | 0.246 | 0.258 |
| ACSCC-10-0.1 | 0.078 | 0.118 | 0.127 | 0.138 | 0.148 | 0.156 | 0.168 |
| ACSCC-10-0.2 | 0.086 | 0.126 | 0.135 | 0.146 | 0.156 | 0.164 | 0.176 |
| ACSCC-10-0.3 | 0.087 | 0.127 | 0.136 | 0.147 | 0.157 | 0.165 | 0.177 |
| ACSCC-10-0.4 | 0.089 | 0.129 | 0.138 | 0.149 | 0.159 | 0.167 | 0.179 |
| ACSCC-10-0.5 | 0.097 | 0.137 | 0.146 | 0.157 | 0.167 | 0.175 | 0.187 |
| ACSCC-10-0.6 | 0.113 | 0.153 | 0.162 | 0.173 | 0.183 | 0.191 | 0.203 |
| ACSCC-10-0.8 | 0.131 | 0.171 | 0.180 | 0.191 | 0.201 | 0.209 | 0.221 |
| ACSCC-10-1.0 | 0.164 | 0.204 | 0.213 | 0.224 | 0.234 | 0.242 | 0.254 |
| ACSCC-15-0.1 | 0.077 | 0.117 | 0.126 | 0.137 | 0.147 | 0.155 | 0.167 |
| ACSCC-15-0.2 | 0.085 | 0.125 | 0.134 | 0.145 | 0.155 | 0.163 | 0.175 |
| ACSCC-15-0.3 | 0.086 | 0.126 | 0.135 | 0.146 | 0.156 | 0.164 | 0.176 |
| ACSCC-15-0.4 | 0.088 | 0.128 | 0.137 | 0.148 | 0.158 | 0.166 | 0.178 |
| ACSCC-15-0.5 | 0.094 | 0.134 | 0.143 | 0.154 | 0.164 | 0.172 | 0.184 |
| ACSCC-15-0.6 | 0.111 | 0.151 | 0.160 | 0.171 | 0.181 | 0.189 | 0.201 |
| ACSCC-15-0.8 | 0.127 | 0.167 | 0.176 | 0.187 | 0.197 | 0.205 | 0.217 |
| ACSCC-15-1.0 | 0.159 | 0.199 | 0.208 | 0.219 | 0.229 | 0.237 | 0.249 |
| ACSCC-20-0.1 | 0.075 | 0.115 | 0.124 | 0.135 | 0.145 | 0.153 | 0.165 |
| ACSCC-20-0.2 | 0.082 | 0.122 | 0.131 | 0.142 | 0.152 | 0.160 | 0.172 |
| ACSCC-20-0.3 | 0.083 | 0.123 | 0.132 | 0.143 | 0.153 | 0.161 | 0.173 |
| ACSCC-20-0.4 | 0.086 | 0.126 | 0.135 | 0.146 | 0.156 | 0.164 | 0.176 |
| ACSCC-20-0.5 | 0.091 | 0.131 | 0.140 | 0.151 | 0.161 | 0.169 | 0.181 |
| ACSCC-20-0.6 | 0.108 | 0.148 | 0.157 | 0.168 | 0.178 | 0.186 | 0.198 |
| ACSCC-20-0.8 | 0.124 | 0.164 | 0.173 | 0.184 | 0.194 | 0.202 | 0.214 |
| ACSCC-20-1.0 | 0.155 | 0.195 | 0.204 | 0.215 | 0.225 | 0.233 | 0.245 |
| Mixture Designation | Sorptivity INDEX (mm/min1/2) | |
|---|---|---|
| 28 Days | 56 Days | |
| ACSCC-00-00 | 0.24 | 0.23 |
| ACSCC-05-0.1 | 0.16 | 0.12 |
| ACSCC-05-0.2 | 0.17 | 0.14 |
| ACSCC-05-0.3 | 0.18 | 0.16 |
| ACSCC-05-0.4 | 0.19 | 0.17 |
| ACSCC-05-0.5 | 0.20 | 0.19 |
| ACSCC-05-0.6 | 0.21 | 0.2 |
| ACSCC-05-0.8 | 0.22 | 0.21 |
| ACSCC-05-1.0 | 0.23 | 0.22 |
| ACSCC-10-0.1 | 0.17 | 0.13 |
| ACSCC-10-0.2 | 0.18 | 0.15 |
| ACSCC-10-0.3 | 0.19 | 0.17 |
| ACSCC-10-0.4 | 0.20 | 0.18 |
| ACSCC-10-0.5 | 0.21 | 0.20 |
| ACSCC-10-0.6 | 0.22 | 0.21 |
| ACSCC-10-0.8 | 0.23 | 0.22 |
| ACSCC-10-1.0 | 0.24 | 0.23 |
| ACSCC-15-0.1 | 0.18 | 0.14 |
| ACSCC-15-0.2 | 0.19 | 0.16 |
| ACSCC-15-0.3 | 0.20 | 0.18 |
| ACSCC-15-0.4 | 0.21 | 0.19 |
| ACSCC-15-0.5 | 0.22 | 0.21 |
| ACSCC-15-0.6 | 0.23 | 0.22 |
| ACSCC-15-0.8 | 0.24 | 0.23 |
| ACSCC-15-1.0 | 0.25 | 0.24 |
| ACSCC-20-0.1 | 0.19 | 0.15 |
| ACSCC-20-0.2 | 0.20 | 0.17 |
| ACSCC-20-0.3 | 0.21 | 0.19 |
| ACSCC-20-0.4 | 0.22 | 0.20 |
| ACSCC-20-0.5 | 0.23 | 0.22 |
| ACSCC-20-0.6 | 0.24 | 0.23 |
| ACSCC-20-0.8 | 0.25 | 0.24 |
| ACSCC-20-1.0 | 0.26 | 0.25 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Laila, L.R.; Karmegam, A.; Avudaiappan, S.; Flores, E.I.S. Microstructure and Water Retention Kinetics in Autogenous Cured Self-Compacting Concrete Blends Using Super Absorbent Polymer. Polymers 2023, 15, 3720. https://doi.org/10.3390/polym15183720
Laila LR, Karmegam A, Avudaiappan S, Flores EIS. Microstructure and Water Retention Kinetics in Autogenous Cured Self-Compacting Concrete Blends Using Super Absorbent Polymer. Polymers. 2023; 15(18):3720. https://doi.org/10.3390/polym15183720
Chicago/Turabian StyleLaila, Lija Rajamony, Aarthi Karmegam, Siva Avudaiappan, and Erick I. Saavedra Flores. 2023. "Microstructure and Water Retention Kinetics in Autogenous Cured Self-Compacting Concrete Blends Using Super Absorbent Polymer" Polymers 15, no. 18: 3720. https://doi.org/10.3390/polym15183720
APA StyleLaila, L. R., Karmegam, A., Avudaiappan, S., & Flores, E. I. S. (2023). Microstructure and Water Retention Kinetics in Autogenous Cured Self-Compacting Concrete Blends Using Super Absorbent Polymer. Polymers, 15(18), 3720. https://doi.org/10.3390/polym15183720






