Improving the Recyclability of an Epoxy Resin through the Addition of New Biobased Vitrimer
Abstract
:1. Introduction
2. Experimental Section
2.1. Reagents
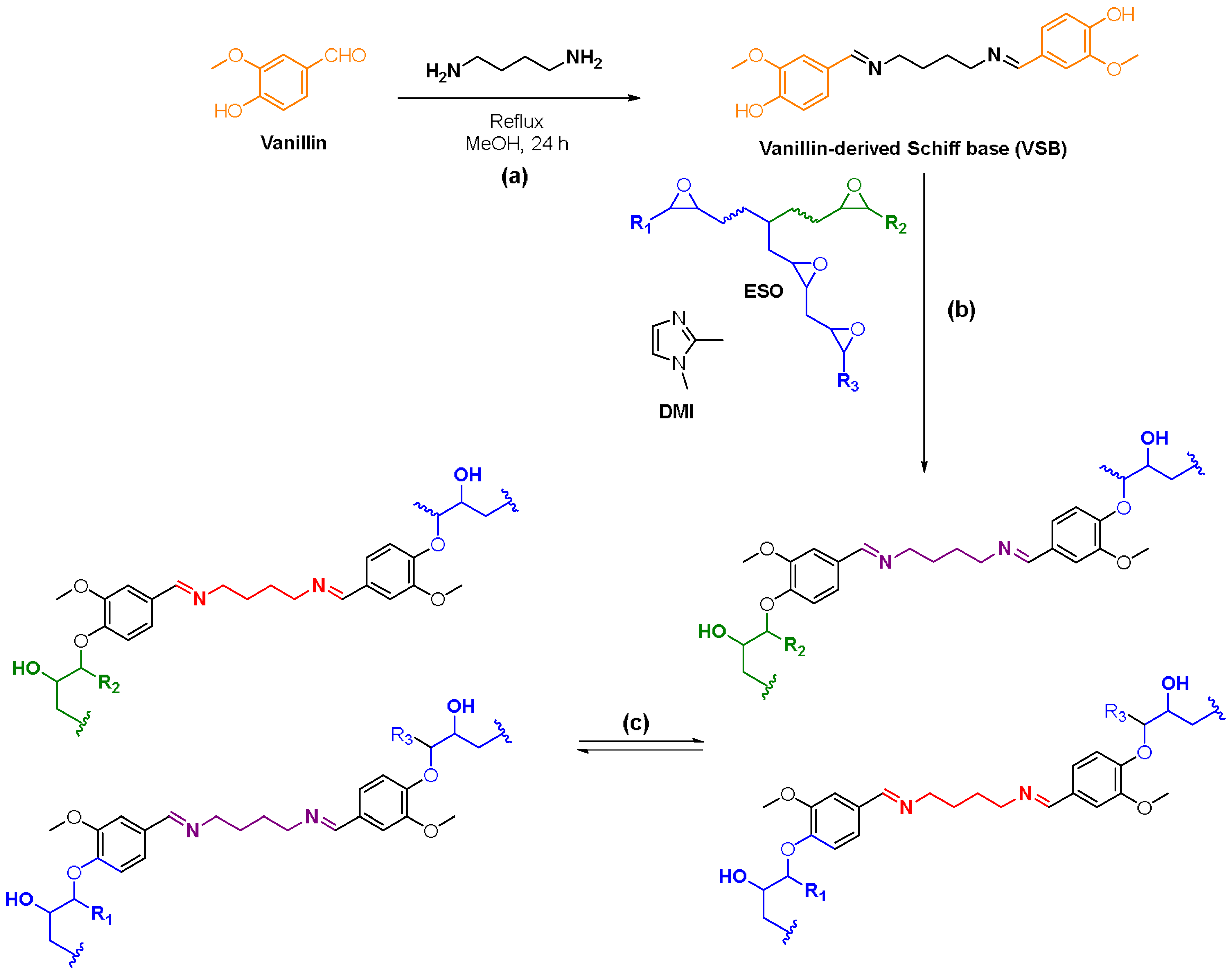
2.2. Synthesis of Vanillin-Derived Schiff Base Curing Agent
2.3. Synthesis of Vitrimers
3. Characterization
4. Results and Discussion
4.1. Synthesis and Characterization of Vanillin-Derived Schiff Base (VSB) Hardener
4.2. Synthesis and Characterization of VESOV, VESOV+ER (Different Percentages) and VSB+ER
4.3. Reprocessability of VESOV, VESOV+ER (Different Percentages) and VSB+ER
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| BDA | 1,4-butandiamine |
| CAN | Covalent Adaptable Network |
| CDCl3 | deuterated chloroform |
| DGEBA | diglycidyl ether of bisphenol A |
| DMA | Dynamic Mechanic Analysis |
| DMI | 1,2-dimethylimidazole |
| DSC | Differential Scanning Calorimetry |
| E’ | Storage modulus |
| E” | Loss modulus |
| ESO | Epoxidized Soybean Oil |
| ER | Epoxy resin |
| EVO | Epoxidized Vegetable Oil |
| FTIR | Fourier Transform Infrared |
| MeOH | Methanol |
| NMR | Nuclear Magnetic Resonance |
| tan δ | loss factor |
| T5% | temperature at 5% weight loss |
| T30% | temperature at 30% weight loss |
| Tg | Glass transition temperature |
| TGA | Thermogravimetric Analysis |
| To | onset decomposition temperature |
| Ts | statistic heat-resistant index temperature |
| Tv | Topology freezing point temperature |
| VAN | Vanillin |
| VESOV | VSB cured ESO vitrimer |
| VSB | Vanillin-derived Schiff Base |
References
- Jin, F.L.; Park, S.J.; Li, X.J. Synthesis and application of epoxy resins: A review. Ind. Eng. Chem. 2015, 29, 1–11. [Google Scholar] [CrossRef]
- Auvergne, R.; Caillol, S.; David, G.; Boutevin, B.; Pascault, J. Biobased thermosetting epoxy: Present and future. Chem. Rev. 2014, 114, 1082–1115. [Google Scholar] [CrossRef] [PubMed]
- Mullins, M.J.; Liu, D.; Sue, H.J. Mechanical properties of thermosets. In Thermosets: Structure, Properties and Applications, 2nd ed.; Guo, Q., Ed.; Fudan University: Shangai, China; Elsevier: Amsterdam, The Netherlands, 2018; pp. 35–68. [Google Scholar]
- Shiota, A.; Ober, C.K. Rigid rod and liquid crystalline thermosets. Prog. Polym. Sci. 1997, 22, 975–1000. [Google Scholar] [CrossRef]
- Raquez, J.; Deléglise, M.; Lacrampe, M.; Krawczak, P. Thermosetting (bio) materials derived from renewable resources: A critical review. Prog. Polym. Sci. 2010, 35, 487–509. [Google Scholar] [CrossRef]
- Rutz, B.H.; Berg, J.C. Thermosetting (bio) materials derived from renewable resources: A critical review. Adv. Colloid Interface Sci. 2010, 160, 56–75. [Google Scholar] [CrossRef]
- Wang, S.; Ma, S.; Xu, C.; Liu, Y.; Dai, J.; Wang, Z.; Liu, X.; Chen, J.; Shen, X.; Wei, J. Vanillin-derived high-performance flame retardant epoxy resins: Facile synthesis and properties. Macromolecules 2017, 50, 1892–1901. [Google Scholar] [CrossRef]
- Senthilkumar, K.; Saba, N.; Rajini, N.; Chandrasekar, M.; Jawaid, M.; Siengchin, S.; Alotman, O.Y. Mechanical properties evaluation of sisal fibre reinforced polymer composites: A review. Constr. Build. Mater. 2018, 174, 713–729. [Google Scholar] [CrossRef]
- Jensen, J.P.; Skelton, K. Wind turbine blade recycling: Experiences, challenges and possibilities in a circular economy. Renew. Sust. Energ. Rev. 2018, 97, 165–176. [Google Scholar] [CrossRef]
- O’Dea, R.M.; Willie, J.A.; Epps, T.H. 100th anniversary of macromolecular science viewpoint: Polymers from lignocellulosic biomass. Current challenges and future opportunities. ACS Macro Lett. 2020, 9, 476–493. [Google Scholar] [CrossRef] [PubMed]
- Jian, X.; An, X.; Li, Y.; Chen, J.; Wang, M.; Zeng, J. All plant oil derived epoxy thermosets with excellent comprehensive properties. Macromolecules 2017, 50, 5729–5738. [Google Scholar] [CrossRef]
- Vollmer, I.; Jenks, M.J.; Roelands, M.C.; White, R.J.; van Harmelen, T.; de Wild, P.; van Der Laan, G.P.; Meirer, F.; Keurentjes, J.T.; Weckhuysen, B.M. Beyond mechanical recycling: Giving new life to plastic waste. Angew. Chem. Int. Ed. 2020, 59, 15402–15423. [Google Scholar] [CrossRef] [PubMed]
- Hahladakis, J.N.; Iacovidou, E. An overview of the challenges and trade-offs in closing the loop of post-consumer plastic waste (PCPW): Focus on recycling. J. Hazard. Mater. 2019, 380, 120887–120897. [Google Scholar] [CrossRef] [PubMed]
- Maiorana, A.; Spinella, S.; Gross, R.A. Bio-based alternative to the diglycidyl ether of bisphenol A with controlled materials properties. Biomacromolecules 2015, 16, 1021–1031. [Google Scholar] [CrossRef]
- Stemmelen, M.; Pessel, F.; Lapinte, V.; Caillol, S.; Habas, J.; Robin, J. A fully biobased epoxy resin from vegetable oils: From the synthesis of the precursors by thiol-ene reaction to the study of the final material. J. Polym. Sci. A Polym. Chem. 2011, 49, 2434–2444. [Google Scholar] [CrossRef]
- Dworakowska, S.; Cornille, A.; Bogdał, D.; Boutevin, B.; Caillol, S. Formulation of bio-based epoxy foams from epoxidized cardanol and vegetable oil amine. Eur. J. Lipid Sci. Technol. 2015, 117, 1893–1902. [Google Scholar] [CrossRef]
- Feng, X.; East, A.J.; Hammond, W.B.; Zhang, Y.; Jaffe, M. Overview of advances in sugar-based polymers. Polym. Adv. Technol. 2011, 22, 139–150. [Google Scholar] [CrossRef]
- Fache, M.; Viola, A.; Auvergne, R.; Boutevin, B.; Caillol, S. Biobased epoxy thermosets from vanillin-derived oligomers. Eur. Polym. J. 2015, 68, 526–535. [Google Scholar] [CrossRef]
- Fache, M.; Auvergne, R.; Boutevin, B.; Caillol, S. New vanillin-derived diepoxy monomers for the synthesis of biobased thermosets. Eur. Polym. J. 2015, 67, 527–538. [Google Scholar] [CrossRef]
- Tremblay-Parrado, K.; García-Astrain, C.; Avérous, L. Click chemistry for the synthesis of biobased polymers and networks derived from vegetable oils. Green Chem. 2021, 23, 4296–4327. [Google Scholar] [CrossRef]
- Wang, X.; Chen, L.; Wu, J.; Fu, T.; Wang, Y. Flame-retardant pressure-sensitive adhesives derived from epoxidized soybean oil and phosphorus-containing dicarboxylic acids. ACS Sustain. Chem. Eng. 2017, 5, 3353–3361. [Google Scholar] [CrossRef]
- Jian, X.; He, Y.; Li, Y.; Wang, M.; Zeng, J. Curing of epoxidized soybean oil with crystalline oligomeric poly (butylene succinate) towards high performance and sustainable epoxy resins. Chem. Eng. J. 2017, 326, 875–885. [Google Scholar] [CrossRef]
- Sun, X.; Wu, H.; Long, R. Thermomechanics of a temperature sensitive covalent adaptable polymer with bond exchange reactions. Soft Matter 2016, 12, 8847–8860. [Google Scholar] [CrossRef] [PubMed]
- Murphy, E.B.; Bolanos, E.; Schaffner-Hamann, C.; Wudl, F.; Nutt, S.R.; Auad, M.L. Synthesis and characterization of a single-component thermally remendable polymer network: Staudinger and Stille revisited. Macromolecules 2008, 41, 5203–5209. [Google Scholar] [CrossRef]
- Lendlein, A.; Jiang, H.; Juenger, O.; Langer, R. Light-induced shape-memory polymers. Nature 2005, 434, 879–882. [Google Scholar] [CrossRef]
- Zheng, J.; Png, Z.M.; Ng, S.H.; Tham, G.X.; Ye, E.; Goh, S.S.; Loh, X.J.; Li, Z. Vitrimers: Current research trends and their emerging applications. Mater. Today Commun. 2021, 51, 586–625. [Google Scholar] [CrossRef]
- Pepels, M.; Filot, I.; Klumperman, B.; Goossens, H. Self-healing systems based on disulfide–thiol exchange reactions. Polym. Chem. 2013, 4, 4955–4965. [Google Scholar] [CrossRef]
- Leibler, L.; Tournilhac, F.; Capelot, M.; Montarnal, D. Silica-like malleable materials from permanent organic networks. Science 2011, 334, 965–968. [Google Scholar]
- Huang, Z.; Wang, Y.; Zhu, J.; Yu, J.; Hu, Z. Surface engineering of nanosilica for vitrimer composites. Compos. Sci. Technol. 2018, 154, 18–27. [Google Scholar] [CrossRef]
- Li, H.; Zhang, B.; Yu, K.; Yuan, C.; Zhou, C.; Dunn, M.L.; Qi, H.J.; Shi, Q.; Wei, Q.; Liu, J. Influence of treating parameters on thermomechanical properties of recycled epoxy-acid vitrimers. Soft Matter 2020, 16, 1668–1677. [Google Scholar] [CrossRef]
- Snyder, R.L.; Fortman, D.J.; De Hoe, G.X.; Hillmyer, M.A.; Dichtel, W.R. Reprocessable acid-degradable polycarbonate vitrimers. Macromolecules 2018, 51, 389–397. [Google Scholar] [CrossRef]
- Zhou, Y.; Goossens, J.G.; Sijbesma, R.P.; Heuts, J.P. Poly (butylene terephthalate)/glycerol-based vitrimers via solid-state polymerization. Macromolecules 2017, 50, 6742–6751. [Google Scholar] [CrossRef]
- Stukenbroeker, T.; Wang, W.; Winne, J.M.; Du Prez, F.E.; Nicolaÿ, R.; Leibler, L. Polydimethylsiloxane quenchable vitrimers. Polym. Chem. 2017, 8, 6590–6593. [Google Scholar] [CrossRef]
- Porath, L.E.; Evans, C.M. Importance of broad temperature windows and multiple rheological approaches for probing viscoelasticity and entropic elasticity in vitrimers. Macromolecules 2021, 54, 4782–4791. [Google Scholar] [CrossRef]
- Meng, F.; Saed, M.O.; Terentjev, E.M. Elasticity and relaxation in full and partial vitrimer networks. Macromolecules 2019, 52, 7423–7429. [Google Scholar] [CrossRef]
- Li, L.; Chen, X.; Jin, K.; Rusayyis, M.B.; Torkelson, J.M. Arresting Elevated-Temperature Creep and Achieving Full Cross-Link Density Recovery in Reprocessable Polymer Networks and Network Composites via Nitroxide-Mediated Dynamic Chemistry. Macromolecules 2021, 54, 1452–1464. [Google Scholar] [CrossRef]
- Lessard, J.J.; Scheutz, G.M.; Sung, S.H.; Lantz, K.A.; Epps, T.H., III; Sumerlin, B.S. Block copolymer vitrimers. J. Am. Chem. Soc. 2020, 142, 283–289. [Google Scholar] [CrossRef]
- Ling, F.; Liu, Z.; Chen, M.; Wang, H.; Zhu, Y.; Ma, C.; Wu, J.; Huang, G. Compatibility driven self-strengthening during the radical-responsive remolding process of poly-isoprene vitrimers. J. Mater. Chem. A 2019, 7, 25324–25332. [Google Scholar] [CrossRef]
- Jourdain, A.; Asbai, R.; Anaya, O.; Chehimi, M.M.; Drockenmuller, E.; Montarnal, D. Rheological properties of covalent adaptable networks with 1, 2, 3-triazolium cross-links: The missing link between vitrimers and dissociative networks. Macromolecules 2020, 53, 1884–1900. [Google Scholar] [CrossRef]
- Elling, B.R.; Dichtel, W.R. Reprocessable cross-linked polymer networks: Are associative exchange mechanisms desirable? ACS Cent. Sci. 2020, 6, 1488–1496. [Google Scholar] [CrossRef]
- Guerre, M.; Taplan, C.; Winne, J.M.; Du Prez, F.E. Vitrimers: Directing chemical reactivity to control material properties. Chem. Sci. 2020, 11, 4855–4870. [Google Scholar] [CrossRef]
- Dyre, J.C. Colloquium: The glass transition and elastic models of glass-forming liquids. Rev. Mod. Phys. 2006, 78, 953–972. [Google Scholar] [CrossRef]
- Fortman, D.J.; Brutman, J.P.; De Hoe, G.X.; Snyder, R.L.; Dichtel, W.R.; Hillmyer, M.A. Approaches to sustainable and continually recyclable cross-linked polymers. ACS Sustain. Chem. Eng. 2018, 6, 11145–11159. [Google Scholar] [CrossRef]
- Hayashi, M. Implantation of Recyclability and Healability into Cross-Linked Commercial Polymers by Applying the Vitrimer Concept. Polymers 2020, 12, 1322. [Google Scholar] [CrossRef]
- Alabiso, W.; Schlögl, S. The Impact of Vitrimers on the Industry of the Future: Chemistry, Properties and Sustainable Forward-Looking Applications. Polymers 2020, 12, 1660. [Google Scholar] [CrossRef] [PubMed]
- Denissen, W.; Rivero, G.; Nicolaÿ, R.; Leibler, L.; Winne, J.M.; Du Prez, F.E. Vinylogous urethane vitrimers. Adv. Funct. Mater. 2015, 25, 2451–2457. [Google Scholar] [CrossRef]
- Obadia, M.M.; Mudraboyina, B.P.; Serghei, A.; Montarnal, D.; Drockenmuller, E. Reprocessing and recycling of highly cross-linked ion-conducting networks through transalkylation exchanges of C–N bonds. J. Am. Chem. Soc. 2015, 137, 6078–6083. [Google Scholar] [CrossRef]
- de Luzuriaga, A.R.; Martin, R.; Markaide, N.; Rekondo, A.; Cabañero, G.; Rodríguez, J.; Odriozola, I. Epoxy resin with exchangeable disulfide crosslinks to obtain reprocessable, repairable and recyclable fiber-reinforced thermoset composites. Mater. Horiz. 2016, 3, 241–247. [Google Scholar] [CrossRef]
- Liu, H.; Zhang, H.; Wang, H.; Huang, X.; Huang, G.; Wu, J. Weldable, malleable and programmable epoxy vitrimers with high mechanical properties and water insensitivity. Chem. Eng. J. 2019, 368, 61–70. [Google Scholar] [CrossRef]
- Wang, H.; Zhang, Y.; Possanza, C.M.; Zimmerman, S.C.; Cheng, J.; Moore, J.S.; Harris, K.; Katz, J.S. Trigger chemistries for better industrial formulations. ACS Appl. Mater. Interfaces 2015, 7, 6369–6382. [Google Scholar] [CrossRef]
- Zhao, S.; Abu-Omar, M.M. Recyclable and malleable epoxy thermoset bearing aromatic imine bonds. Macromolecules 2018, 51, 9816–9824. [Google Scholar] [CrossRef]
- Yu, Q.; Peng, X.; Wang, Y.; Geng, H.; Xu, A.; Zhang, X.; Xu, W.; Ye, D. Vanillin-based degradable epoxy vitrimers: Reprocessability and mechanical properties study. Eur. Polym. J. 2019, 117, 55–63. [Google Scholar] [CrossRef]
- Memon, H.; Liu, H.; Rashid, M.A.; Chen, L.; Jiang, Q.; Zhang, L.; Wei, Y.; Liu, W.; Qiu, Y. Vanillin-based epoxy vitrimer with high performance and closed-loop recyclability. Macromolecules 2020, 53, 621–630. [Google Scholar] [CrossRef]
- Fache, M.; Boutevin, B.; Caillol, S. Vanillin production from lignin and its use as a renewable chemical. ACS Sustain. Chem. Eng. 2016, 4, 35–46. [Google Scholar] [CrossRef]
- Liu, W.; Schmidt, D.F.; Reynaud, E. Catalyst selection, creep, and stress relaxation in high-performance epoxy vitrimers. Ind. Eng. Chem. Res. 2017, 56, 2667–2672. [Google Scholar] [CrossRef]
- Snijkers, F.; Pasquino, R.; Maffezzoli, A. Curing and viscoelasticity of vitrimers. Soft Matter 2017, 13, 258–268. [Google Scholar] [CrossRef]
- Winne, J.M.; Leibler, L.; Du Prez, F.E. Dynamic covalent chemistry in polymer networks: A mechanistic perspective. Polym. Chem. 2019, 10, 6091–6108. [Google Scholar] [CrossRef]
- Li, L.; Chen, X.; Jin, K.; Torkelson, J.M. Vitrimers designed both to strongly suppress creep and to recover original cross-link density after reprocessing: Quantitative theory and experiments. Macromolecules 2018, 51, 5537–5546. [Google Scholar] [CrossRef]
- Sun, Y.; Sheng, D.; Wu, H.; Tian, X.; Xie, H.; Shi, B.; Yang, Y. Bio-based vitrimer-like polyurethane based on dynamic imine bond with high-strength, reprocessability, rapid-degradability and antibacterial ability. Polymer 2021, 233, 124208. [Google Scholar] [CrossRef]
- Zhao, X.; Liu, Y.; Weng, Y.; Li, Y.; Zeng, J. Sustainable epoxy vitrimers from epoxidized soybean oil and vanillin. ACS Sustain. Chem. Eng. 2020, 8, 15020–15029. [Google Scholar] [CrossRef]
- Zeng, R.; Wu, Y.; Li, Y.; Wang, M.; Zeng, J. Curing behavior of epoxidized soybean oil with biobased dicarboxylic acids. Polym. Test. 2017, 57, 281–287. [Google Scholar] [CrossRef]
- Di Mauro, C.; Malburet, S.; Genua, A.; Graillot, A.; Mija, A. Sustainable series of new epoxidized vegetable oil-based thermosets with chemical recycling properties. Biomacromolecules 2020, 21, 3923–3935. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.Y.; He, J.; Li, Y.D.; Zhao, X.L.; Zeng, J.B. Biobased, reprocessable and weldable epoxy vitrimers from epoxidized soybean oil. Ind. Crops Prod. 2020, 153, 112576–112584. [Google Scholar] [CrossRef]
- Ma, Z.; Wang, Y.; Zhu, J.; Yu, J.; Hu, Z.J. Bio-based epoxy vitrimers: Reprocessibility, controllable shape memory, and degradability. Polym. Sci. A Polym. Chem. 2017, 55, 1790–1799. [Google Scholar] [CrossRef]
- Pascault, J.P.; Williams, R.J.J. Glass transition temperature versus conversion relationships for thermosetting polymers. J. Polym. Sci. B Polym. Phys. 1990, 28, 85–95. [Google Scholar] [CrossRef]


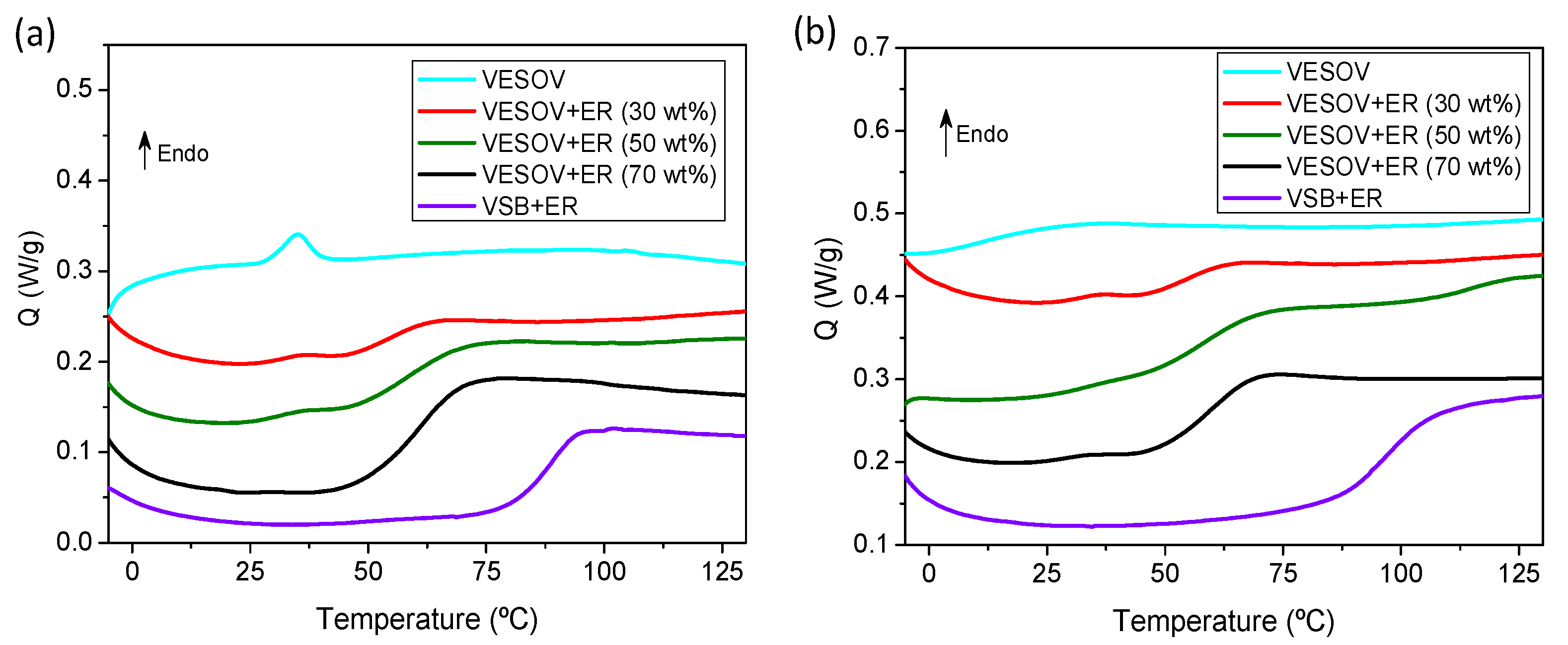

| Sample | To (°C) | Ts (°C) |
|---|---|---|
| VESOV | 297.3 | 169.3 |
| VESOV+ER (30 wt%) | 278.3 | 160.5 |
| VESOV+ER (50 wt%) | 282.4 | 161.4 |
| VESOV+ER (70 wt%) | 272.2 | 157.8 |
| VSB+ER | 277.6 | 158.5 |
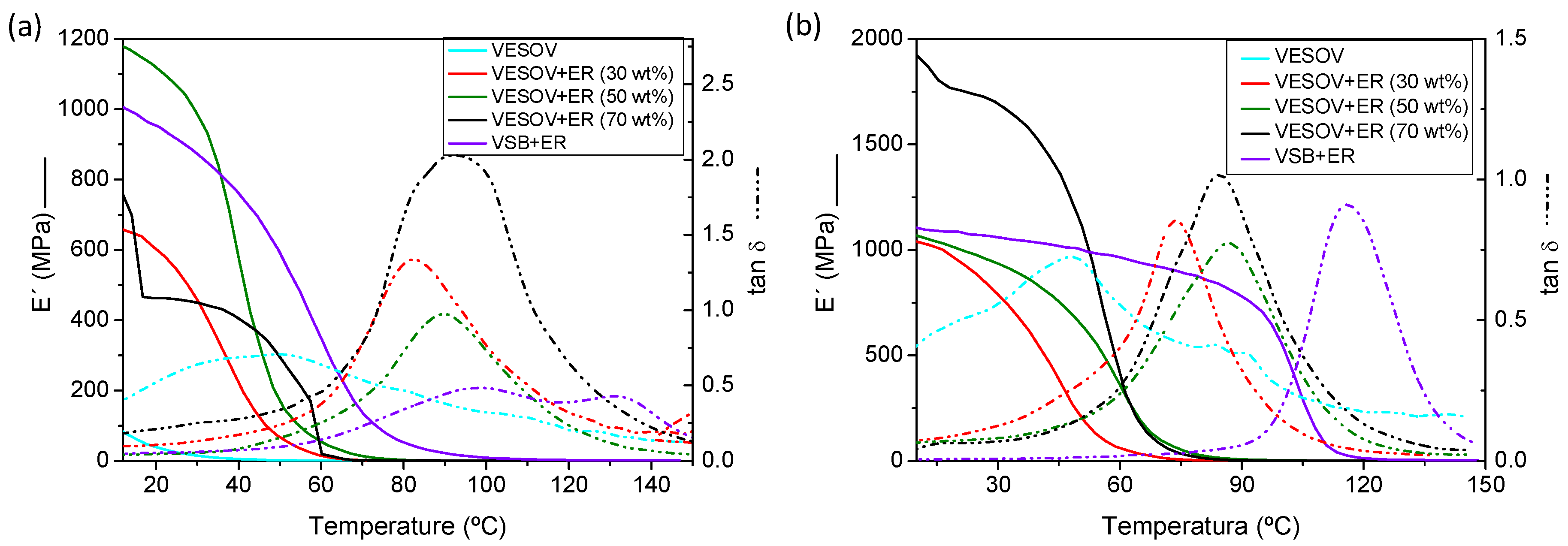
| Sample | DSC Tg (°C) | DMA Tg (°C) | ||
|---|---|---|---|---|
| Original | Reprocessed | Original | Reprocessed | |
| VESOV | 28.7 | 6.8 | 44.0 | 50.7 |
| VESOV+ER (30 wt%) | 48.2 | 45.1 | 70.2 | 75.1 |
| VESOV+ER (50 wt%) | 48.7 | 55.0 | 78.0 | 88.4 |
| VESOV+ER (70 wt%) | 50.3 | 50.6 | 78.7 | 85.3 |
| VSB+ER | 78.6 | 91.5 | 111.5 | 116.1 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Veloso-Fernández, A.; Ruiz-Rubio, L.; Yugueros, I.; Moreno-Benítez, M.I.; Laza, J.M.; Vilas-Vilela, J.L. Improving the Recyclability of an Epoxy Resin through the Addition of New Biobased Vitrimer. Polymers 2023, 15, 3737. https://doi.org/10.3390/polym15183737
Veloso-Fernández A, Ruiz-Rubio L, Yugueros I, Moreno-Benítez MI, Laza JM, Vilas-Vilela JL. Improving the Recyclability of an Epoxy Resin through the Addition of New Biobased Vitrimer. Polymers. 2023; 15(18):3737. https://doi.org/10.3390/polym15183737
Chicago/Turabian StyleVeloso-Fernández, Antonio, Leire Ruiz-Rubio, Imanol Yugueros, M. Isabel Moreno-Benítez, José Manuel Laza, and José Luis Vilas-Vilela. 2023. "Improving the Recyclability of an Epoxy Resin through the Addition of New Biobased Vitrimer" Polymers 15, no. 18: 3737. https://doi.org/10.3390/polym15183737






