Investigation of Curing Process and Thermal Behavior of Copolymers Based on Polypropylene Glycol Fumarate and Acrylic Acid Using the Methods of DSC and TGA
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
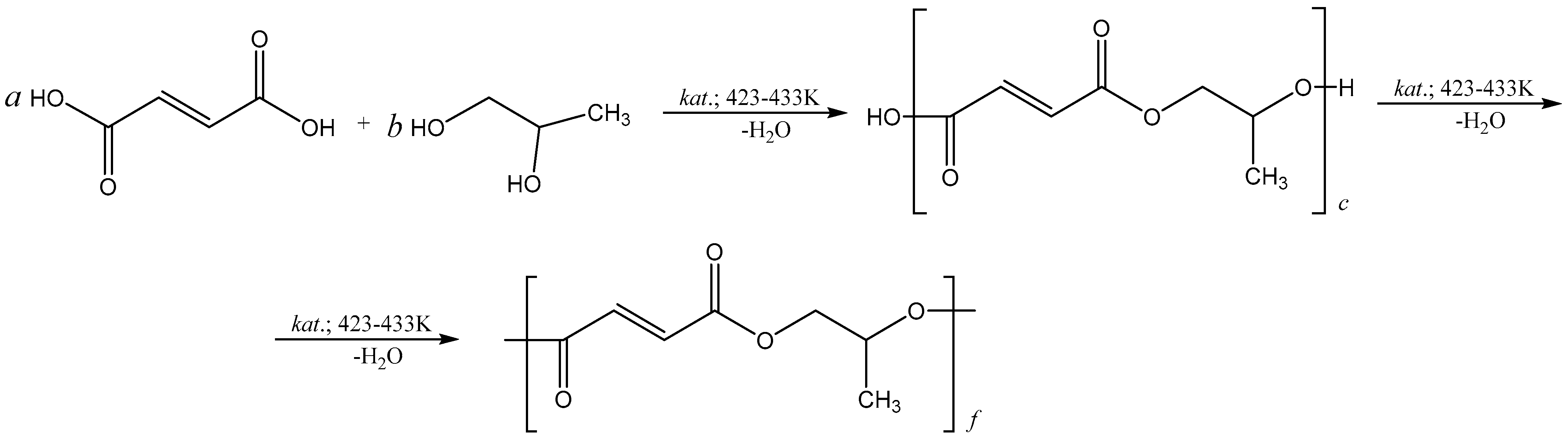
2.2. Preparation of Polypropylene Glycol Fumarate
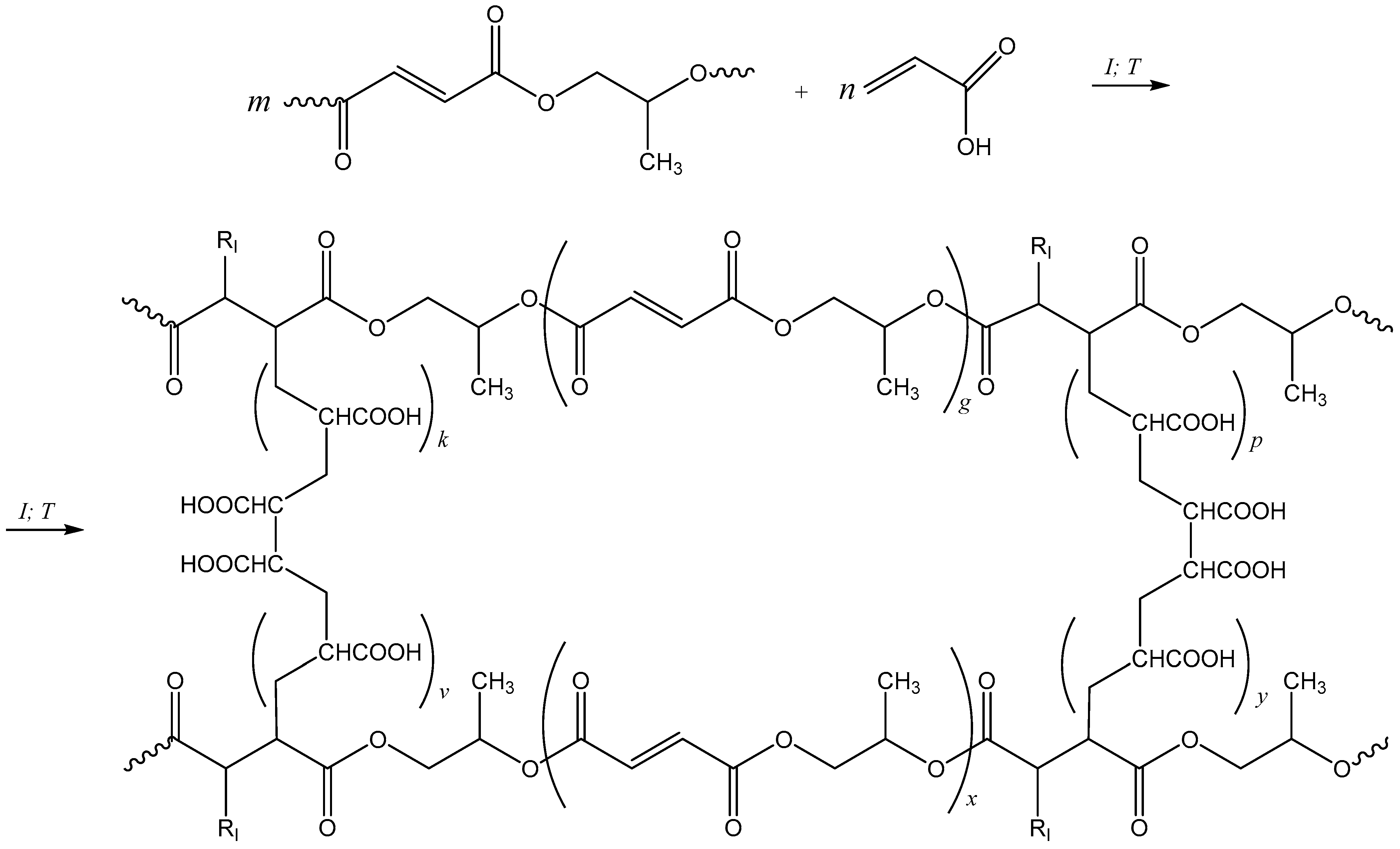
2.3. Curing of Polypropylene Glycol Fumarate
2.4. Methods
2.4.1. Gel-Permeation Chromatography
2.4.2. Differential Scanning Calorimetry (DSC)
2.4.3. Thermogravimetric Analysis (TGA)
2.4.4. HPLC Method, IR-Spectroscopy, Scanning Electron Microscope (SEM)
3. Results and Discussion
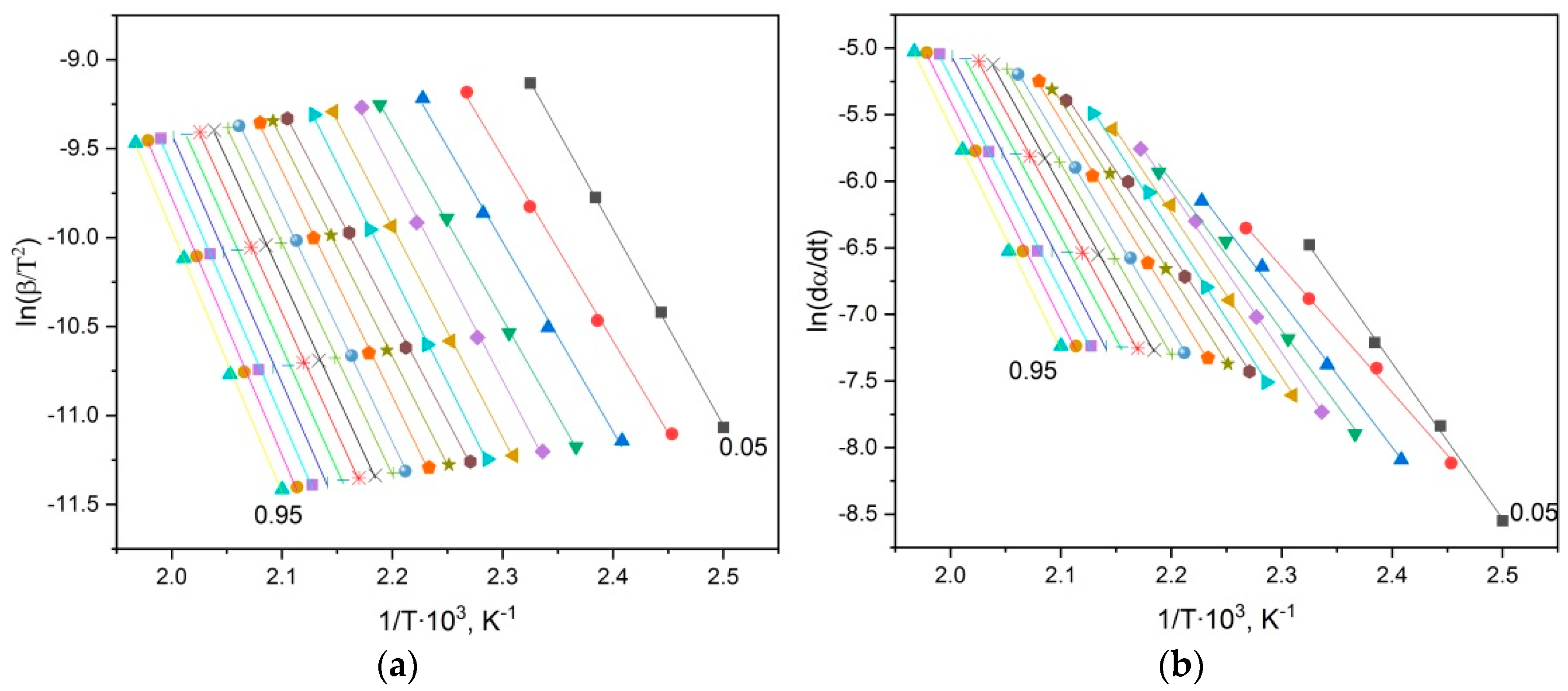
3.1. Curing of p-PGF-AA
Isothermal and Dynamic DSC Results
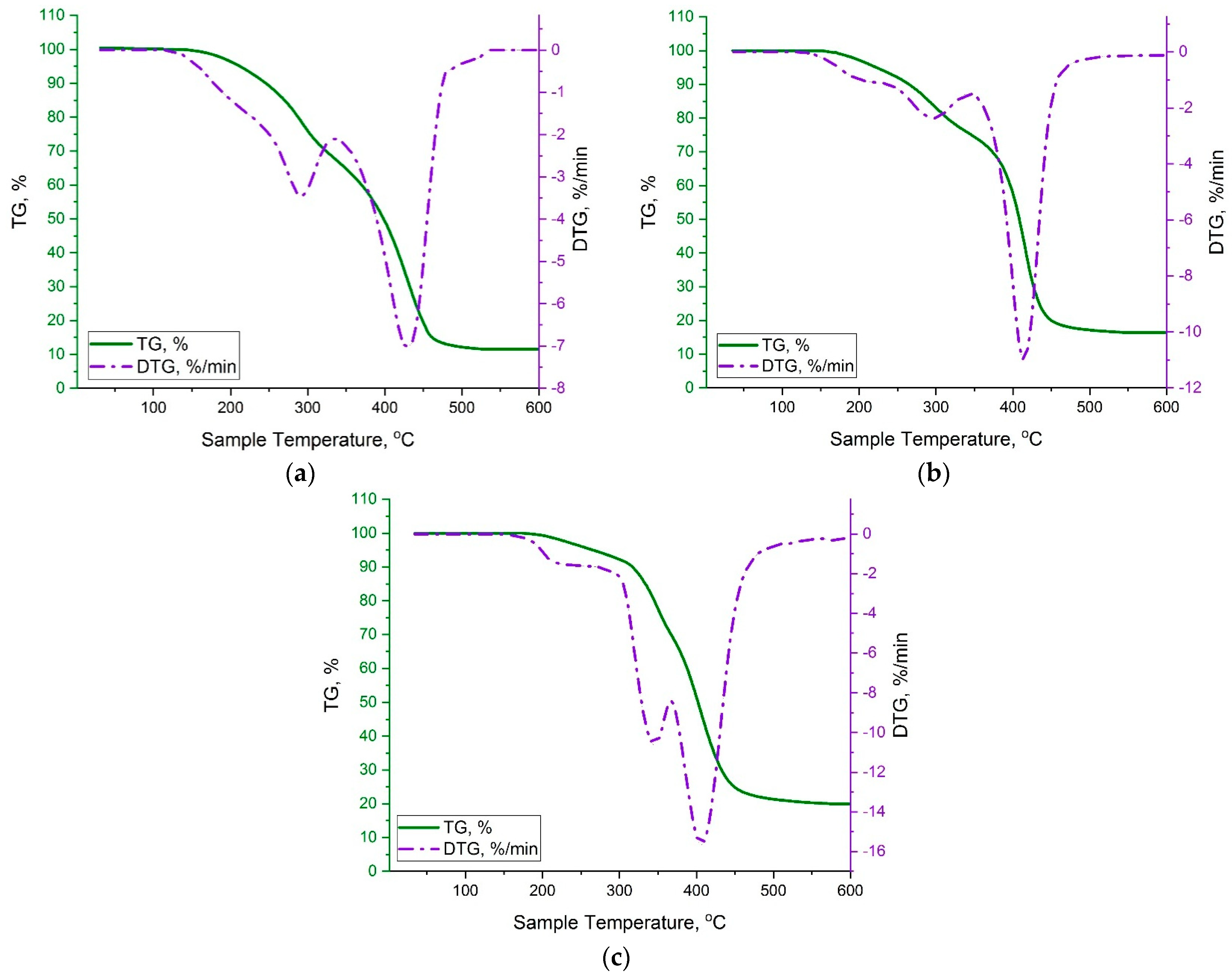
3.2. Thermogravimetric Analysis (TGA)
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Penczek, P.; Czub, P.; Pielichowski, J. Unsaturated Polyester Resins: Chemistry and Technology. In Crosslinking in Materials Science; Abe, A., Dus, K., Kobayashi, S., Eds.; Springer: Berlin/Heidelberg, Germany, 2005; pp. 1–95. [Google Scholar] [CrossRef]
- Boenig, H.V. Unsaturated Polyesters: Structure and Properties; Elsevier: Amsterdam, The Netherlands, 1968; pp. 251–253. Available online: https://www.studmed.ru/benig-g-nenasyschennye-poliefiry-stroenie-i-svoystva_44384a2212b.html(In Russian). (accessed on 28 July 2010). (In Russian)
- Feng, L.F.; Li, R.; Yang, H.; Chen, S.W.; Yang, W.B. The Hyperbranched Polyester Reinforced Unsaturated Polyester Resin. Polymers 2022, 14, 1127. [Google Scholar] [CrossRef] [PubMed]
- Abdelazim, A.A.A.; Nasr, E.S.; Farhat, M.S. Unsaturated polyester for large castings. Polym. J. 1994, 26, 423–429. [Google Scholar] [CrossRef]
- Burkeyev, M.Z.; Kovaleva, A.K.; Plocek, J.; Tazhbayev, E.M.; Burkeyeva, G.K.; Bolatbai, A.N.; Davrenbekov, S.Z. Synthesis and Properties of Poly(Propylene Glycol Maleate Phthalate)-Styrene Copolymers as a Base of Composite Materials. Russ. J. Appl. Chem. 2018, 91, 1742–1749. [Google Scholar] [CrossRef]
- Liu, C.G.; Dai, Y.; Hu, Y.; Shang, Q.Q.; Feng, G.D.; Zhou, J.; Zhou, Y.H. Highly Functional Unsaturated Ester Macromonomer Derived from Soybean Oil: Synthesis and Copolymerization with Styrene. Acs Sustain. Chem. Eng. 2016, 4, 4208–4216. [Google Scholar] [CrossRef]
- Dua, S.; McCullough, R.L.; Palmese, G.R. Copolymerization kinetics of styrene/vinyl-ester systems: Low temperature reactions. Polym. Compos. 1999, 20, 379–391. [Google Scholar] [CrossRef]
- Vinogradova, S.V.; Korshak, V.V.; Kul’chitskii, V.I.; Gribkova, P.N.; Danilov, V.G. Thermal stability of unsaturated polyesters and copolymers based on them. Polym. Sci. U.S.S.R. 1968, 10, 1757–1764. [Google Scholar] [CrossRef]
- Kicko-Walczak, E. Kinetics of thermal decomposition of unsaturated polyester resins with reduced flammability. J. Appl. Polym. Sci. 2003, 88, 2851–2857. [Google Scholar] [CrossRef]
- Toroptseva, A.V.; Belogorodskaya, K.V.; Bondarenko, V.M. Laboratory Course on Chemistry and Technology of Macromolecular Compounds; Khimiya Leningrad: Leningrad, Russia, 1972; pp. 231–233. [Google Scholar]
- Burkeyev, M.Z.; Kovaleva, A.K.; Tazhbayev, E.M. Method for Obtaining New Stimulus-Sensitive Moisture Sorbents Based on Polypropylene Glycol Maleate Phthalate with Acrylic. Acid. Patent 33759, 12 July 2019. Available online: https://gosreestr.kazpatent.kz/Invention/Details?docNumber=278663 (accessed on 12 July 2019).
- Kuo, S.W.; Lin, H.C.; Huang, W.J.; Huang, C.F.; Chang, F.C. Hydrogen bonding interactions and miscibility between phenolic resin and octa(acetoxystyryl) polyhedral oligomeric silsesquioxane (AS-POSS) nanocomposites. J. Polym. Sci. Part B-Polym. Phys. 2006, 44, 673–686. [Google Scholar] [CrossRef]
- Vyazovkin, S.; Sbirrazzuoli, N. Isoconversional kinetic analysis of thermally stimulated processes in polymers. Macromol. Rapid Commun. 2006, 27, 1515–1532. [Google Scholar] [CrossRef]
- Friedman, H.L. Kinetics of thermal degradation of charforming plastics from thermogravimetry. Application to a phenolic plastic. J. Polym. Sci. 1964, 6, 183–185. [Google Scholar] [CrossRef]
- Kissinger, H.E. Reaction kinetics in differential thermal analysis. Anal. Chem. 1957, 29, 1702–1706. [Google Scholar] [CrossRef]
- Akahira, T.; Sunose, T. Method of determining activation deterioration constant of electrical insulating materials. Res. Rep. Chiba Inst. Technol. (Sci. Technol.) 1971, 16, 22–31. Available online: https://scholar.google.com/scholar_lookup?journal=Res.+Rep.+Chiba+Inst.+Technol.&title=Method+of+determining+activation+deterioration+constant+of+electrical+insulating+materials&author=T.+Akahira&author=T.+Sunose&volume=16&publication_year=1971&pages=22-31& (accessed on 18 June 2020).
- Grunden, B.L.; Sung, C.S.P. Cure characterization of unsaturated polyester resin by fluorescence spectroscopy. J. Appl. Polym. Sci. 2004, 94, 2446–2450. [Google Scholar] [CrossRef]
- Zimmermann, B.; Vrsaljko, D. IR spectroscopy based thermal analysis of polymers. Polym. Test. 2010, 29, 849–856. [Google Scholar] [CrossRef]
- Burkeev, M.Z.; Kudaibergen, G.K.; Burkeeva, G.K.; Seilkhanov, T.M.; Tazhbaev, E.M.; Hranicek, J.; Omasheva, A.V.; Davrenbekov, S.Z. New Polyampholyte Polymers Based on Polypropylene Glycol Fumarate with Acrylic Acid and Dimethylaminoethyl Methacrylate. Russ. J. Appl. Chem. 2018, 91, 1145–1152. [Google Scholar] [CrossRef]
- Rodriguez, E.L. The effect of free-radical initiators and fillers on the cure of unsaturated polyester resins. Polym. Eng. Sci. 1991, 31, 1022–1028. [Google Scholar] [CrossRef]
- Swan, S.R.; Creighton, C.; Griffin, J.M.; Gashi, B.V.; Varley, R.J. Aromatic tetra-glycidyl ether versus tetra-glycidyl amine epoxy networks: Influence of monomer structure and epoxide conversion. Polymer 2022, 239, 124401. [Google Scholar] [CrossRef]

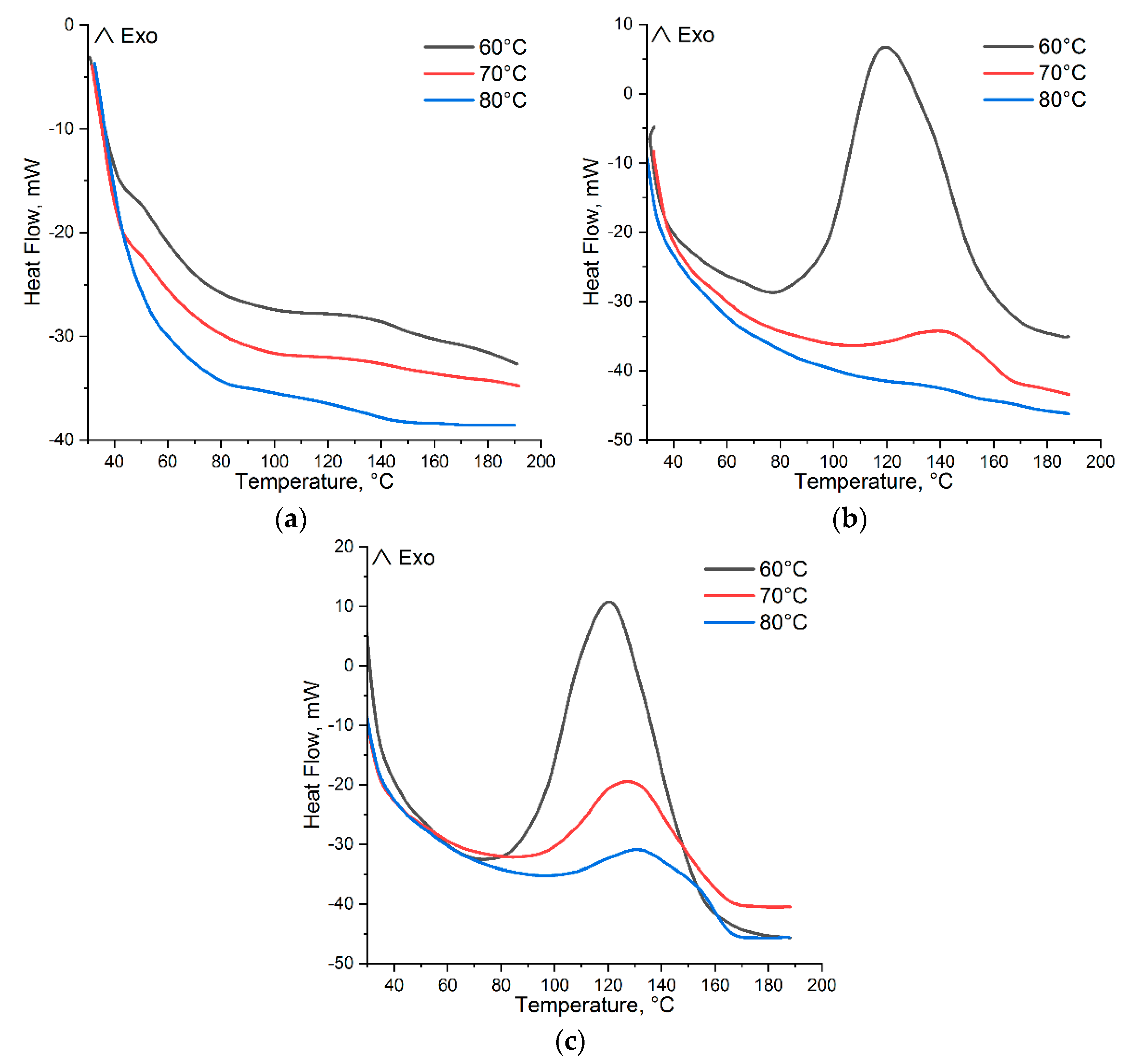




| Sample, mol.% | Temperature, °C | ti, min | tcuring, min | tv, min | |
|---|---|---|---|---|---|
| p-PGF | AA | ||||
| 30.05 | 69.95 | 60 | 11.30 | 15.10 | 21.30 |
| 70 | 04.40 | 05.70 | 10.20 | ||
| 80 | 04.40 | 05.27 | 09.40 | ||
| 45.13 | 54.87 | 60 | 15.10 | 24.40 | 39.80 |
| 70 | 09.50 | 13.48 | 19.10 | ||
| 80 | 04.80 | 06.24 | 10.90 | ||
| 61.21 | 39.79 | 60 | 27.40 | 36.28 | 59.00 |
| 70 | 13.40 | 18.71 | 31.40 | ||
| 80 | 05.70 | 08.01 | 16.40 | ||
| Sample, mol.% | Temperature, °C | −∆Hiso, J/g | −∆Hr, J/g | −∆Htot, J/g | |
|---|---|---|---|---|---|
| p-PGF | AA | ||||
| 30.05 | 69.95 | 60 | 374.318 | 1.687 | 376.005 |
| 70 | 393.180 | 0.827 | 394.007 | ||
| 80 | 410.006 | 0.078 | 410.084 | ||
| 45.13 | 54.87 | 60 | 286.880 | 51.115 | 337.995 |
| 70 | 354.397 | 8.894 | 363.291 | ||
| 80 | 377.947 | 0.933 | 378.880 | ||
| 61.21 | 39.79 | 60 | 226.339 | 98.605 | 324.944 |
| 70 | 308.479 | 37.599 | 346.078 | ||
| 80 | 330.987 | 22.098 | 353.085 | ||
| Sample, mol.% | Temperature, °C | α, % | k/103, s−1 | Ea, kJ/mol | |
|---|---|---|---|---|---|
| p-PGF | AA | ||||
| 30.05 | 69.95 | 60 | 99.55 | 6.13 | 27.4 |
| 70 | 99.79 | 8.01 | |||
| 80 | 99.98 | 10.73 | |||
| 45.13 | 54.87 | 60 | 84.87 | 3.06 | 48.4 |
| 70 | 97.55 | 5.85 | |||
| 80 | 99.75 | 8.25 | |||
| 61.21 | 39.79 | 60 | 69.66 | 1.11 | 77.3 |
| 70 | 89.14 | 2.79 | |||
| 80 | 93.74 | 5.41 | |||
| Sample, mol.% | Temperature Range, °C | dTGmax, %/min | Residue, % | |||
|---|---|---|---|---|---|---|
| p-PGF | AA | I Stage | II Stage | III Stage | ||
| 30.05 | 69.95 | 140–230 | 230–345 | 345–530 | 7 | 11 |
| 45.13 | 54.87 | 155–250 | 250–340 | 340–550 | 11 | 15 |
| 61.21 | 39.79 | 190–285 | 285–370 | 370–560 | 16 | 20 |
| Sample, mol.% | Eaave, kJ/mol | ||||
|---|---|---|---|---|---|
| p-PGF | AA | I Stage | II Stage | III Stage | Σ |
| Method of Kissinger–Akakhira–Sunose | |||||
| 30.05 | 69.95 | 140 ± 5.3 | 117 ± 3.7 | 199 ± 6.1 | 456 ± 15.1 |
| 45.13 | 54.87 | 104 ± 1.8 | 146 ± 3.1 | 227 ± 7.2 | 477 ± 12.1 |
| 61.21 | 39.79 | 98 ± 1.7 | 140 ± 2.3 | 270 ± 9.5 | 508 ± 13.5 |
| Method of Friedman | |||||
| 30.05 | 69.95 | 140 ± 7.7 | 120 ± 5.6 | 226 ± 8.1 | 486 ± 21.4 |
| 45.13 | 54.87 | 112 ± 2.6 | 163 ± 4.9 | 250 ± 3.4 | 525 ± 10.9 |
| 61.21 | 39.79 | 112 ± 3.1 | 164 ± 3.4 | 272 ± 12.2 | 548 ± 18.7 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Burkeyeva, G.; Kovaleva, A.; Tazhbayev, Y.; Ibrayeva, Z.; Zhaparova, L. Investigation of Curing Process and Thermal Behavior of Copolymers Based on Polypropylene Glycol Fumarate and Acrylic Acid Using the Methods of DSC and TGA. Polymers 2023, 15, 3753. https://doi.org/10.3390/polym15183753
Burkeyeva G, Kovaleva A, Tazhbayev Y, Ibrayeva Z, Zhaparova L. Investigation of Curing Process and Thermal Behavior of Copolymers Based on Polypropylene Glycol Fumarate and Acrylic Acid Using the Methods of DSC and TGA. Polymers. 2023; 15(18):3753. https://doi.org/10.3390/polym15183753
Chicago/Turabian StyleBurkeyeva, Gulsym, Anna Kovaleva, Yerkeblan Tazhbayev, Zhansaya Ibrayeva, and Lyazzat Zhaparova. 2023. "Investigation of Curing Process and Thermal Behavior of Copolymers Based on Polypropylene Glycol Fumarate and Acrylic Acid Using the Methods of DSC and TGA" Polymers 15, no. 18: 3753. https://doi.org/10.3390/polym15183753
APA StyleBurkeyeva, G., Kovaleva, A., Tazhbayev, Y., Ibrayeva, Z., & Zhaparova, L. (2023). Investigation of Curing Process and Thermal Behavior of Copolymers Based on Polypropylene Glycol Fumarate and Acrylic Acid Using the Methods of DSC and TGA. Polymers, 15(18), 3753. https://doi.org/10.3390/polym15183753






