Mechanical and Surface Properties of Edible Coatings Elaborated with Nanoliposomes Encapsulating Grape Seed Tannins and Polysaccharides
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Methods
2.2.1. Antioxidant Suspension (AS)
2.2.2. Filmogenic Suspension (FS)
2.2.3. Edible Compound Coatings (ECC)
2.2.4. Edible Compounds Coatings Solid (ECCS)
2.2.5. Surface Properties
Density
- ρ: Density of the sample at room temperature of 20 °C, [kg/m3].
- m: Mass of the ECC sample deposited in the pycnometer, [kg].
- V: Volume of the pycnometer, [m3].
Surface Tension
- : Cartesian coordinates at any point on the pendant drop.
- : Radius of curvature at the vertex.
- : Acceleration due to gravity.
- : Density difference between the sample and air at room temperature.
- : Angle between the axis of the droplet and the normal to the droplet interface.
- : Surface tension between the sample and air.
Surface Roughness
Contact Angles
- : Surface tension between the ECC and air at a temperature of 20 °C.
- : Angle between the axis of the droplet and the normal to the droplet interface.
Color
- : Total color difference between the ECC Sample and the base color standard FS.
- : Difference in luminosity between the ECC sample and the base color standard FS.
- : Shift in the green-red color between the ECC sample and the base color standard FS.
- : Shift in the blue-yellow color between the ECC sample and the base color standard FS.
Rheology
2.2.6. Mechanical Properties
Thickness
Tensile Testing
- F: Tensile load force (N).
- ΔL: Deformation, [m].
- L0: Initial distance, [m].
Puncture Testing
- Fmax: Maximum applied force [N].
- AT: Cross-sectional area of ECCS located in the cell (AT = 2re), [m2].
- r: Radius of ECCS, [m].
- e: Thickness of ECCS, [m].
- d: Displacement of the probe from the contact point with ECCS to the point of fracture, [m].
Effective Diffusivity (Def)
- Mt/M∞: Mass Fraction of tannins (catechin or epicatechin) that has been released at time t, [h].
- d: Average thickness of ECCS, [m].
- t: Time, [s].
Study of Tannin Release from ECCS
- Mt/M∞: Mass Fraction of tannin (catechin or epicatechin) that has been released at time t, [h].
- n: Exponent indicating the release mechanism of tannins.
- K1: Represents the contribution of the Fickian mechanism.
- K2: Represents the contribution to the relaxation mechanism of the polymeric chains.
- b: Represents the tannin immediately released upon contact with the simulant medium.
2.2.7. Statistical Analysis
3. Results
3.1. Characterization of the Antioxidant Suspension (AS)
3.2. Optimization of the Filmogenic Suspension (FS)
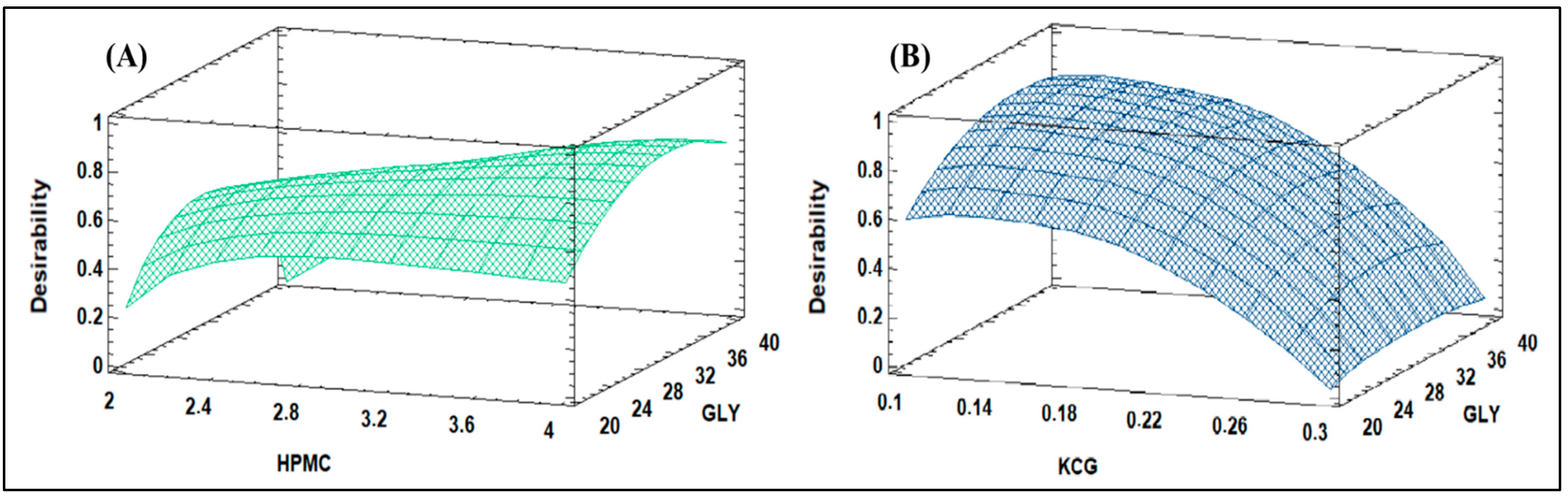
3.3. Design of Simplex Lattice Mixture
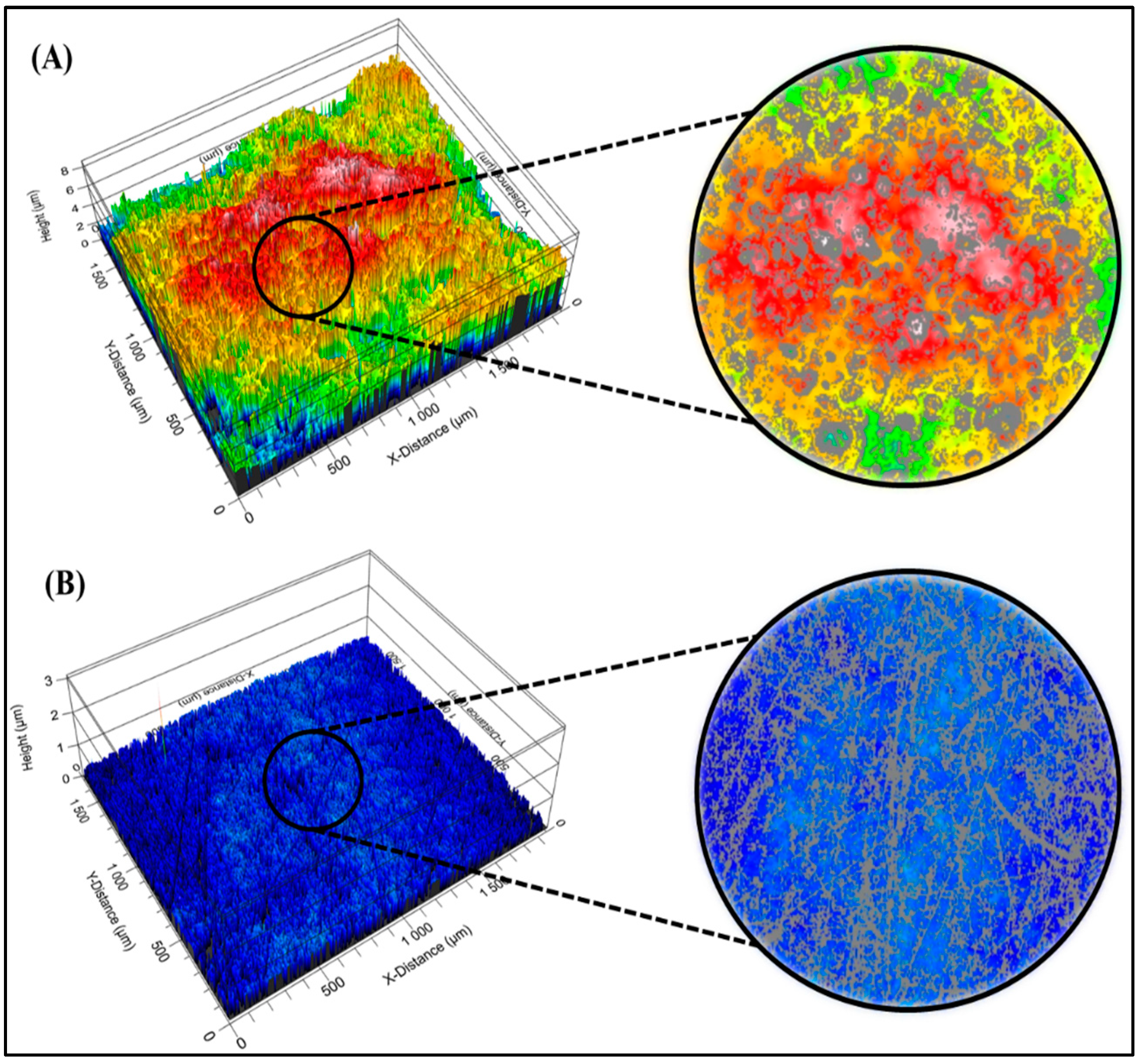
3.4. Surface Roughness
3.5. Surface Properties of ECC
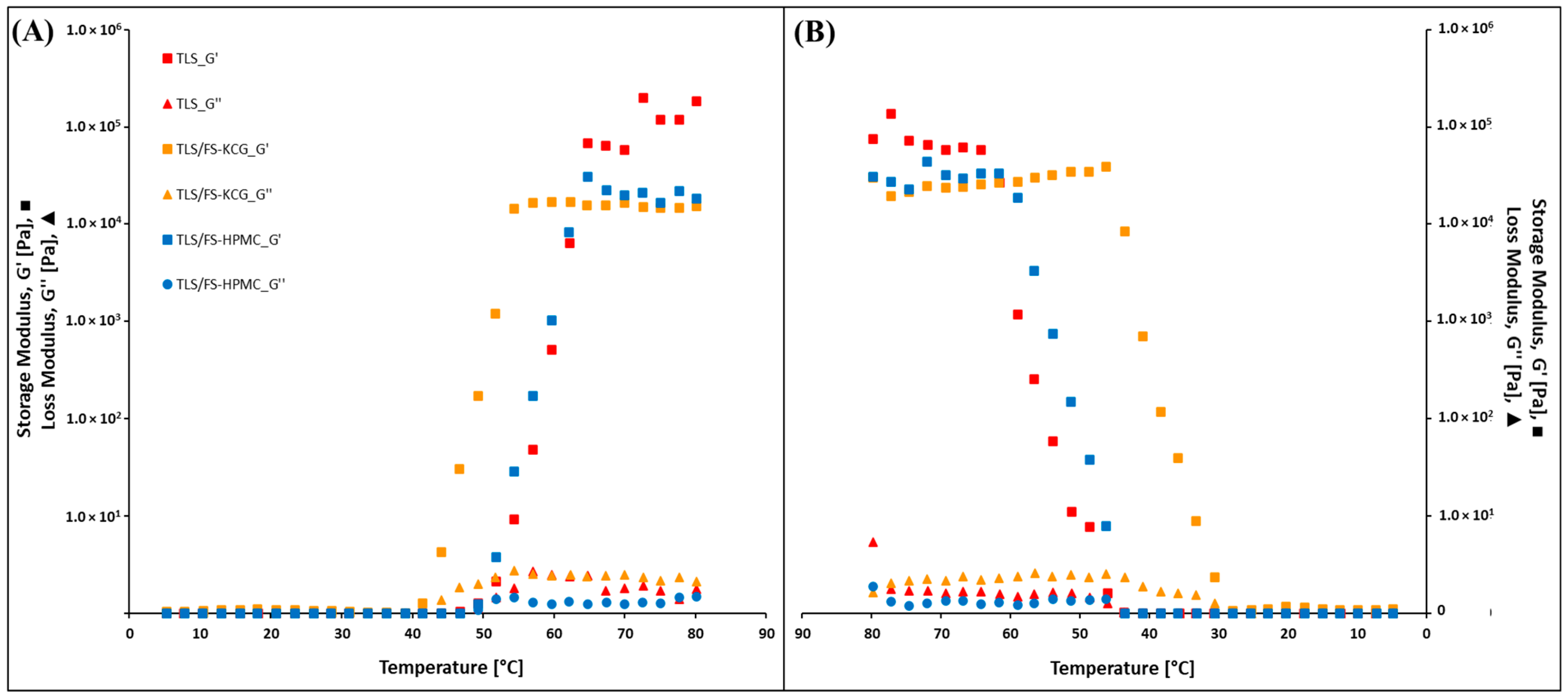
3.6. Rheological Properties of ECC
3.7. Mechanical Properties of ECCS
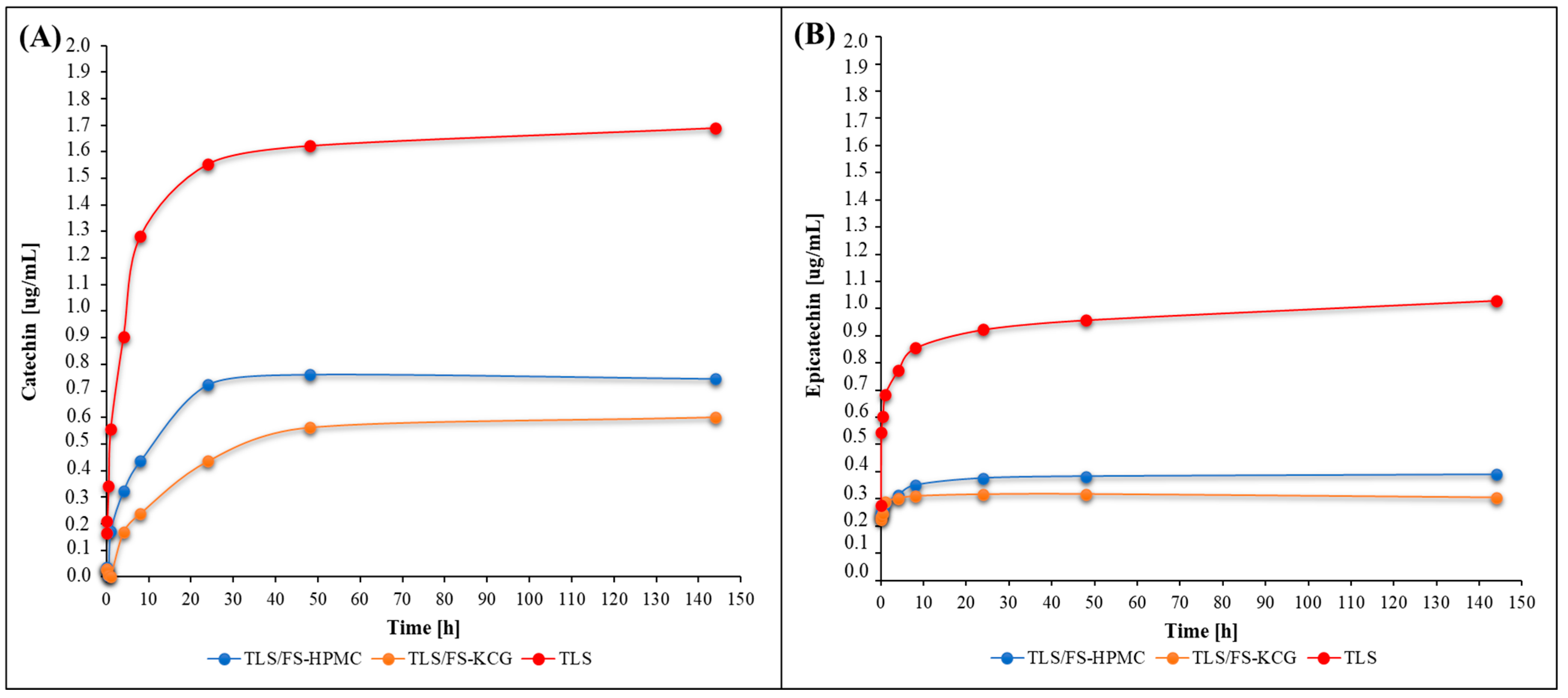
3.8. Tannins Release Study from ECCS
4. Discussion
4.1. Characterization of the Antioxidant Suspension (AS)
4.2. Optimization of the Filmogenic Suspension (FS)
4.3. Design of Simplex Lattice Mixture
4.4. Surface Roughness
4.5. Surface Properties of ECC
4.6. Rheological Properties
4.7. Mechanical Properties of ECCS
4.8. Tannins Release Study from ECCS
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Singh, D.P.; Packirisamy, G. Biopolymer based edible coating for enhancing the shelf life of horticulture products. Food Chem. Mol. Sci. 2022, 4, 100085. [Google Scholar] [CrossRef]
- Arya, S.S.; More, P.R.; Ladole, M.R.; Pegu, K.; Pandit, A.B. Non-thermal, energy efficient hydrodynamic cavitation for food processing, process intensification and extraction of natural bioactives: A review. Ultrason. Sonochemistry 2023, 98, 106504. [Google Scholar] [CrossRef] [PubMed]
- Mushtaq, A.; Wani, S.M.; Malik, A.; Gull, A.; Ramniwas, S.; Nayik, G.A.; Ercisli, S.; Marc, R.A.; Ullah, R.; Bari, A. Recent insights into Nanoemulsions: Their preparation, properties and applications. Food Chem. X 2023, 18, 100684. [Google Scholar] [CrossRef] [PubMed]
- de Rezende, L.P.; Barbosa, J.; Teixeira, P. Analysis of Alternative Shelf Life-Extending Protocols and Their Effect on the Preservation of Seafood Products. Foods 2022, 11, 1100. [Google Scholar] [CrossRef] [PubMed]
- Plaskova, A.; Mlcek, J. New insights of the application of water or ethanol-water plant extract rich in active compounds in food. Front. Nutr. 2023, 10, 1118761. [Google Scholar] [CrossRef] [PubMed]
- Prakoso, F.A.H.; Indiarto, R.; Utama, G.L. Edible Film Casting Techniques and Materials and Their Utilization for Meat-Based Product Packaging. Polymers 2023, 15, 2800. [Google Scholar] [CrossRef]
- Malik, G.K.; Khuntia, A.; Mitra, J. Comparative effect of different plasticizers on barrier, mechanical, optical, and sorption properties of hydroxypropyl methylcellulose (HPMC)—Based edible film. J. Biosyst. Eng. 2022, 47, 93–105. [Google Scholar] [CrossRef]
- Wang, Y.; Jiang, S.; Chen, Y.; Qiu, D.; Weng, Y. Synthesis and Characterization of a Novel Composite Edible Film Based on Hydroxypropyl Methyl Cellulose Grafted with Gelatin. Gels 2023, 9, 332. [Google Scholar] [CrossRef] [PubMed]
- Bizymis, A.-P.; Giannou, V.; Tzia, C. Contribution of Hydroxypropyl Methylcellulose to the Composite Edible Films and Coatings Properties. Food Bioprocess Technol. 2023, 16, 1488–1501. [Google Scholar] [CrossRef]
- Abdillah, A.A.; Charles, A.L. Characterization of a natural biodegradable edible film obtained from arrowroot starch and iota-carrageenan and application in food packaging. Int. J. Biol. Macromol. 2021, 191, 618–626. [Google Scholar] [CrossRef]
- Bharti, S.K.; Pathak, V.; Arya, A.; Alam, T.; Rajkumar, V.; Verma, A.K. Packaging potential of Ipomoea batatas and κ-carrageenan biobased composite edible film: Its rheological, physicomechanical, barrier and optical characterization. J. Food Process. Preserv. 2021, 45, e15153. [Google Scholar] [CrossRef]
- Kusnadi, J.; Mahatmanto, T.; Marsheli, N.; Fawzia, N.; Rahmawani, D.E.; Alexander, K. Development of low-cost edible coatings based on polysaccharides with active lactic acid bacteria for the protection of fresh produce modeled using fresh cut apples. Food Sci. Technol. Int. 2022, 29, 287–298. [Google Scholar] [CrossRef] [PubMed]
- Galus, S.; Kibar, E.A.A.; Gniewosz, M.; Kraśniewska, K. Novel Materials in the Preparation of Edible Films and Coatings—A Review. Coatings 2020, 10, 674. [Google Scholar] [CrossRef]
- Lopez-Polo, J.; Silva-Weiss, A.; Giménez, B.; Cantero-López, P.; Vega, R.; Osorio, F.A. Effect of lyophilization on the physicochemical and rheological properties of food grade liposomes that encapsulate rutin. Food Res. Int. 2019, 130, 108967. [Google Scholar] [CrossRef] [PubMed]
- Fraga-Corral, M.; García-Oliveira, P.; Pereira, A.G.; Lourenço-Lopes, C.; Jimenez-Lopez, C.; Prieto, M.A.; Simal-Gandara, J. Technological Application of Tannin-Based Extracts. Molecules 2020, 25, 614. [Google Scholar] [CrossRef]
- Sani, I.K.; Masoudpour-Behabadi, M.; Sani, M.A.; Motalebinejad, H.; Juma, A.S.; Asdagh, A.; Mohammadi, F. Value-added utilization of fruit and vegetable processing by-products for the manufacture of biodegradable food packaging films. Food Chem. 2022, 405 Pt B, 134964. [Google Scholar]
- Li, P.; Li, X.; Nisar, T.; Yang, X.; Sun, J.; Yang, X.; Guo, Y. Structural characteristics of binary biopolymers-based emulsion-filled gels: A case of mixed sodium caseinate/methyl cellulose emulsion gels. Food Struct. 2021, 30, 100233. [Google Scholar] [CrossRef]
- Shahidi, F.; Dissanayaka, C.S. Phenolic-protein interactions: Insight from in-silico analyses—A review. Food Prod. Process. Nutr. 2023, 5, 2. [Google Scholar] [CrossRef]
- Velázquez-Martínez, R.I.; Criado, C.; Muñoz-González, C.; Crespo, J.; Pozo-Bayón, M. Evaluation of the Long-Lasting Flavour Perception after the Consumption of Wines Treated with Different Types of Oenological Additives Considering Individual 6-n-Propylthiouracil Taster Status. Foods 2023, 12, 2835. [Google Scholar] [CrossRef]
- Wang, S.; Mantilla, S.M.O.; Smith, P.A.; Stokes, J.R.; Smyth, H.E. Relationship between salivary lubrication and temporal sensory profiles of wine mouthfeel and astringency sub-qualities. Food Hydrocoll. 2023, 135, 108106. [Google Scholar] [CrossRef]
- Lombardo, D.; Kiselev, M.A. Methods of Liposomes Preparation: Formation and Control Factors of Versatile Nanocarriers for Biomedical and Nanomedicine Application. Pharmaceutics 2022, 14, 543. [Google Scholar] [CrossRef] [PubMed]
- Kong, I.; Degraeve, P.; Pui, L.P. Polysaccharide-Based Edible Films Incorporated with Essential Oil Nanoemulsions: Physico-Chemical, Mechanical Properties and Its Application in Food Preservation—A Review. Foods 2022, 11, 555. [Google Scholar] [CrossRef] [PubMed]
- Díaz, H.S.; Ríos-Gallardo, A.; Ortolani, D.; Díaz-Jara, E.; Flores, M.J.; Vera, I.; Monasterio, A.; Ortiz, F.C.; Brossard, N.; Osorio, F.; et al. Lipid-Encapsuled Grape Tannins Prevent Oxidative-Stress-Induced Neuronal Cell Death, Intracellular ROS Accumulation and Inflammation. Antioxidants 2022, 11, 1928. [Google Scholar] [CrossRef] [PubMed]
- Taladrid, D.; Marín, D.; Aleman, A.; Álvarez-Acero, I.; Montero, P.; Gómez-Guillén, M.C. Effect of chemical composition and sonication procedure on properties of food-grade soy lecithin liposomes with added glycerol. Food Res. Int. 2017, 100, 541–550. [Google Scholar] [CrossRef] [PubMed]
- Ghadiri, N.; Mirghazanfari, S.M.; Hadi, V.; Hadi, S.; Mohammadimehr, M.; Ardestani, M.M.; Talatappeh, H.D.; Mohajeri, M. Physicochemical properties and antioxidant activity of polyvinyl alcohol orally disintegrating films containing sweet almond oil nanoemulsion. J. Food Meas. Charact. 2023, 17, 4045–4059. [Google Scholar] [CrossRef]
- Dai, Y.; Ma, Y.; Liu, X.; Gao, R.; Min, H.; Zhang, S.; Hu, S. Formation Optimization, Characterization and Antioxidant Activity of Auricularia auricula-judae Polysaccharide Nanoparticles Obtained via Antisolvent Precipitation. Molecules 2022, 27, 7037. [Google Scholar] [CrossRef]
- Lamch, Ł. Membrane-assisted core-shell entrapment technique as a powerful tool for curcumin encapsulation. Colloids Surfaces A: Physicochem. Eng. Asp. 2023, 661, 130938. [Google Scholar] [CrossRef]
- Domínguez-Rodríguez, G.; Marina, M.L.; Plaza, M. Strategies for the extraction and analysis of non-extractable polyphenols from plants. J. Chromatogr. A 2017, 1514, 1–15. [Google Scholar] [CrossRef]
- Gitea, M.A.; Gitea, D.; Tit, D.M.; Bungau, S.G.; Bogdan, M.A.; Radu, A.-F.; Dulf, F.V.; Pasca, M.B. Organically Cultivated Vine Varieties—Distinctive Qualities of the Oils Obtained from Grape Seeds. Sustainability 2023, 15, 11037. [Google Scholar] [CrossRef]
- Sridhar, K.; Charles, A.L. In vitro antioxidant activity of Kyoho grape extracts in DPPH and ABTS assays: Estimation methods for EC50 using advanced statistical programs. Food Chem. 2018, 275, 41–49. [Google Scholar] [CrossRef]
- Ou, B.; Hampsch-Woodill, M.; Prior, R.L. Development and Validation of an Improved Oxygen Radical Absorbance Capacity Assay Using Fluorescein as the Fluorescent Probe. J. Agric. Food Chem. 2001, 49, 4619–4626. [Google Scholar] [CrossRef] [PubMed]
- Skurtys, O.; Velásquez, P.; Henriquez, O.; Matiacevich, S.; Enrione, J.; Osorio, F. Wetting behavior of chitosan solutions on blueberry epicarp with or without epicuticular waxes. LWT-Food Sci. Technol. 2011, 44, 1449–1457. [Google Scholar] [CrossRef]
- ISO 25178-1:2016; Geometrical Product Specifications (GPS)—Surface Texture: Areal—Part 1: Indication of Surface Texture. International Organization for Standardization: Ginebra, Suiza, 2016. Available online: https://www.iso.org/standard/46065.html (accessed on 5 July 2023).
- Osorio, F.; Valdés, G.; Skurtys, O.; Andrade, R.; Villalobos-Carvajal, R.; Silva-Weiss, A.; Silva-Vera, W.; Giménez, B.; Zamorano, M.; Lopez, J. Surface Free Energy Utilization to Evaluate Wettability of Hydrocolloid Suspension on Different Vegetable Epicarps. Coatings 2017, 8, 16. [Google Scholar] [CrossRef]
- Lopez-Polo, J.; Silva-Weiss, A.; Zamorano, M.; Osorio, F.A. Humectability and physical properties of hydroxypropyl methylcellulose coatings with liposome-cellulose nanofibers: Food application. Carbohydr. Polym. 2019, 231, 115702. [Google Scholar] [CrossRef] [PubMed]
- ASTM D882-18; Standard Test Method for Tensile Properties of Thin Plastic Sheeting. American Society for Testing and Materials: West Consshohocken, PA, USA, 2018. Available online: https://www.compass.astm.org/document/?contentCode=ASTM%7CD0882-18%7Cen-US (accessed on 4 September 2023).
- Pizarro, R.D.A.; Skurtys, O.; Osorio-Lira, F. Effect of cellulose nanofibers concentration on mechanical, optical, and barrier properties of gelatin-based edible films. Dyna 2015, 82, 219–226. [Google Scholar] [CrossRef]
- Gontard, N.; Guilbert, S.; Cuq, J.-L. Edible Wheat Gluten Films: Influence of the Main Process Variables on Film Properties using Response Surface Methodology. J. Food Sci. 1992, 57, 190–195. [Google Scholar] [CrossRef]
- Foulady-Dehaghi, R.; Sohrabnezhad, S.; Hadavi, M. Drug delivery with solvent-free synthesized polyimide-COF/amino-functionalized MCM-41 nanohybrid. J. Drug Deliv. Sci. Technol. 2023, 81, 104283. [Google Scholar] [CrossRef]
- Prasetyaningrum, A.; Wicaksono, B.S.; Hakiim, A.; Ashianti, A.D.; Manalu SF, C.; Rokhati, N.; Utomo, D.P.; Djaeni, M. Ultrasound-Assisted Encapsulation of Citronella Oil in Alginate/Carrageenan Beads: Characterization and Kinetic Models. ChemEngineering 2023, 7, 10. [Google Scholar] [CrossRef]
- Neter, J.; Wasserman, W.; Kunter, M. Applied Linear Statistical Models, 2nd ed.; Richard D. Irwin, Inc.: Homewood, IL, USA, 1985; pp. 491–505. [Google Scholar]
- Li, S.; Wilkinson, K.L.; Mierczynska-Vasilev, A.; Bindon, K.A. Applying Nanoparticle Tracking Analysis to Characterize the Polydispersity of Aggregates Resulting from Tannin–Polysaccharide Interactions in Wine-Like Media. Molecules 2019, 24, 2100. [Google Scholar] [CrossRef]
- Natolino, A.; Celotti, E. Ultrasound treatment of red wine: Effect on polyphenols, mathematical modeling, and scale-up considerations. LWT 2021, 154, 112843. [Google Scholar] [CrossRef]
- Wu, H.; Oliveira, G.; Lila, M.A. Protein-binding approaches for improving bioaccessibility and bioavailability of anthocyanins. Compr. Rev. Food Sci. Food Saf. 2023, 22, 333–354. [Google Scholar] [CrossRef] [PubMed]
- Cong, L.; Wang, J.; Lu, H.; Tian, M.; Ying, R.; Huang, M. Influence of different anionic polysaccharide coating on the properties and delivery performance of nanoliposomes for quercetin. Food Chem. 2023, 409, 135270. [Google Scholar] [CrossRef] [PubMed]
- Casanova, F.; Pereira, C.F.; Ribeiro, A.B.; Costa, E.M.; Freixo, R.; Castro, P.M.; Fernandes, J.C.; Pintado, M.; Ramos, L. Design of Innovative Biocompatible Cellulose Nanostructures for the Delivery and Sustained Release of Curcumin. Pharmaceutics 2023, 15, 981. [Google Scholar] [CrossRef] [PubMed]
- Gorjian, H.; Amiri, Z.R.; Milani, J.M.; Khaligh, N.G. Influence of Nanovesicle Type, Nanoliposome and Nanoniosome, on Antioxidant and Antimicrobial Activities of Encapsulated Myrtle Extract: A Comparative Study. Food Bioprocess Technol. 2022, 15, 144–164. [Google Scholar] [CrossRef]
- Gil, K.A.; Nowicka, P.; Wojdyło, A.; Serreli, G.; Deiana, M.; Tuberoso, C.I.G. Antioxidant Activity and Inhibition of Digestive Enzymes of New Strawberry Tree Fruit/Apple Smoothies. Antioxidants 2023, 12, 805. [Google Scholar] [CrossRef] [PubMed]
- Osterberg, M.; Henn, K.A.; Farooq, M.; Valle-Delgado, J.J. Biobased Nanomaterials—The Role of Interfacial Interactions for Advanced Materials. Chem. Rev. 2023, 123, 2200–2241. [Google Scholar] [CrossRef]
- Pinem, M.P.; Wardhono, E.Y.; Clausse, D.; Saleh, K.; Guénin, E. Droplet behavior of chitosan film-forming solution on the solid surface. South Afr. J. Chem. Eng. 2022, 41, 26–33. [Google Scholar] [CrossRef]
- Sobhani, S.; Bakhshandeh, E.; Jafari, R.; Momen, G. Mechanical properties, icephobicity, and durability assessment of HT-PDMS nanocomposites: Effectiveness of sol–gel silica precipitation content. J. Sol-Gel Sci. Technol. 2023, 105, 348–359. [Google Scholar] [CrossRef]
- Dopilka, A.; Gu, Y.; Larson, J.M.; Zorba, V.; Kostecki, R. Nano-FTIR Spectroscopy of the Solid Electrolyte Interphase Layer on a Thin-Film Silicon Li-Ion Anode. ACS Appl. Mater. Interfaces 2023, 15, 6755–6767. [Google Scholar] [CrossRef]
- Lin, C.; Jung, J.; Zhao, Y. Cellulose nanofiber-based emulsion coatings with enhanced hydrophobicity and surface adhesion for preserving anthocyanins within thermally processed blueberries packed in aqueous media. J. Food Process. Eng. 2023, 46, e14277. [Google Scholar] [CrossRef]
- Wong, Y.L.; Pandey, M.; Choudhury, H.; Lim, W.M.; Bhattamisra, S.K.; Gorain, B. Development of In-Situ Spray for Local Delivery of Antibacterial Drug for Hidradenitis Suppurativa: Investigation of Alternative Formulation. Polymers 2021, 13, 2770. [Google Scholar] [CrossRef]
- Luo, Z.; Zhou, J.; Lu, Z.; Wei, H.; Yu, Y. Natural Polysaccharides as Multifunctional Components for One-Step 3D Printing Tough Hydrogels. Macromol. Mater. Eng. 2021, 306, 2100433. [Google Scholar] [CrossRef]
- Kouser, F.; Kumar, S.; Bhat, H.F.; Hassoun, A.; Bekhit, A.E.-D.A.; Bhat, Z.F. Aloe barbadensis Based Bioactive Edible Film Improved Lipid Stability and Microbial Quality of the Cheese. Foods 2023, 12, 229. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Lin, Q.; Li, Z. Effect of Surface Finishes on the Welding of Sn58Bi Solder. J. Electron. Mater. 2021, 51, 1106–1115. [Google Scholar] [CrossRef]
- Xie, D.; Guo, D.; Guo, Z.; Hu, X.; Luo, S.; Liu, C. Reduction of oil uptake of fried food by coatings: A review. Int. J. Food Sci. Technol. 2022, 57, 3268–3277. [Google Scholar] [CrossRef]
- Pham, T.T.; Nguyen, L.L.P.; Dam, M.S.; Baranyai, L. Application of Edible Coating in Extension of Fruit Shelf Life: Review. Agriengineering 2023, 5, 520–536. [Google Scholar] [CrossRef]
- Moon, C.H.; Yasmeen, S.; Park, K.; Gaiji, H.; Chung, C.; Kim, H.; Moon, H.-S.; Choi, J.W.; Lee, H.-B. Icephobic Coating through a Self-Formed Superhydrophobic Surface Using a Polymer and Microsized Particles. ACS Appl. Mater. Interfaces 2022, 14, 3334–3343. [Google Scholar] [CrossRef]
- Rabbi, K.F.; Ho, J.Y.; Yan, X.; Ma, J.; Hoque, M.J.; Sett, S.; Miljkovic, N. Polydimethylsiloxane-Silane Synergy enables Dropwise Condensation of Low Surface Tension Liquids. Adv. Funct. Mater. 2022, 32, 2112837. [Google Scholar] [CrossRef]
- Kedrina-Okutan, O.; Novello, V.; Hoffmann, T.; Hadersdorfer, J.; Occhipinti, A.; Schwab, W.; Ferrandino, A. Constitutive polyphenols in blades and veins of grapevine (Vitis vinifera L.) healthy leaves. J. Agric. Food Chem. 2018, 66, 10977–10990. [Google Scholar] [CrossRef]
- Cebrián, P.; Pérez-Sienes, L.; Sanz-Vicente, I.; López-Molinero, Á.; de Marcos, S.; Galbán, J. Solving Color Reproducibility between Digital Devices: A Robust Approach of Smartphones Color Management for Chemical (Bio) Sensors. Biosensors 2022, 12, 341. [Google Scholar] [CrossRef]
- Yang, N.; Huang, Y.; Hou, J.; Zhang, Y.; Tian, L.; Chen, Z.; Jin, Z.; Shen, Y.; Guo, S. Rheological behaviors and texture properties of semi-interpenetrating networks of hydroxypropyl methylcellulose and gellan. Food Hydrocoll. 2021, 122, 107097. [Google Scholar] [CrossRef]
- Tohamy HA, S.; El-Sakhawy, M.; Strachota, B.; Strachota, A.; Pavlova, E.; Mares Barbosa, S.; Kamel, S. Temperature-and pH-Responsive Super-Absorbent Hydrogel Based on Grafted Cellulose and Capable of Heavy Metal Removal from Aqueous Solutions. Gels 2023, 9, 296. [Google Scholar] [CrossRef]
- Huang, M.; Theng, A.H.P.; Yang, D.; Yang, H. Influence of κ-carrageenan on the rheological behaviour of a model cake flour system. LWT 2020, 136, 110324. [Google Scholar] [CrossRef]
- Homayoonfal, M.; Mousavi, M.; Kiani, H.; Askari, G.; Desobry, S.; Arab-Tehrany, E. Modifying the Stability and Surface Characteristic of Anthocyanin Compounds Incorporated in the Nanoliposome by Chitosan Biopolymer. Pharmaceutics 2022, 14, 1622. [Google Scholar] [CrossRef]
- Li, M.; Wu, Q.; Moon, R.J.; Hubbe, M.A.; Bortner, M.J. Rheological Aspects of Cellulose Nanomaterials: Governing Factors and Emerging Applications. Adv. Mater. 2021, 33, e2006052. [Google Scholar] [CrossRef] [PubMed]
- Goyanes, A.; Buanz, A.B.M.; Basit, A.W.; Gaisford, S. Fused-filament 3D printing (3DP) for fabrication of tablets. Int. J. Pharm. 2014, 476, 88–92. [Google Scholar] [CrossRef]
- Guo, J.; Dong, S.; Ye, M.; Wu, X.; Lv, X.; Xu, H.; Li, M. Effects of Hydroxypropyl Methylcellulose on Physicochemical Properties and Microstructure of κ-Carrageenan Film. Foods 2022, 11, 3023. [Google Scholar] [CrossRef] [PubMed]
- Zhou, X.; Liu, X.; Liao, W.; Wang, Q.; Xia, W. Chitosan/bacterial cellulose films incorporated with tea polyphenol nanoliposomes for silver carp preservation. Carbohydr. Polym. 2022, 297, 120048. [Google Scholar] [CrossRef]
- Solanki, P.; Ansari, D.; Sultana, Y. Nanostructured Carrageenan as Drug Carrier. Nanoeng. Biomater. 2022, 3, 523–542. [Google Scholar]
- Dorofte, A.L.; Dima, C.; Ceoromila, A.; Botezatu, A.; Dinica, R.; Bleoanca, I.; Borda, D. Controlled Release of β-CD-Encapsulated Thyme Essential Oil from Whey Protein Edible Packaging. Coatings 2023, 13, 5088. [Google Scholar]
- Bercea, M.; Plugariu, I.-A.; Gradinaru, L.M.; Avadanei, M.; Doroftei, F.; Gradinaru, V.R. Hybrid Hydrogels for Neomycin Delivery: Synergistic Effects of Natural/Synthetic Polymers and Proteins. Polymers 2023, 15, 630. [Google Scholar] [CrossRef] [PubMed]

| Factors | Low | High | Levels | Units | Response | Units |
|---|---|---|---|---|---|---|
| HPMC | 2 | 4 | 3 | [%w/v] | Density | [kg/m3] |
| KCG | 0.1 | 0.3 | 3 | [%w/v] | Surface tension | [mN/m] |
| Gly | 20 | 40 | 3 | [%w/w] relative to polysaccharide |
| Block | TLS | FS-HPMC | TLS | FS-KCG | Response | Units |
|---|---|---|---|---|---|---|
| 1 | 70 | 30 | 90 | 10 | Density | [kg/m3] |
| 1 | 10 | 90 | 10 | 90 | Surface tension | [mN/m] |
| 1 | 50 | 50 | 30 | 70 | Mean particle size | [nm] |
| 1 | 90 | 10 | 70 | 30 | ||
| 1 | 30 | 70 | 50 | 50 | ||
| 1 | 90 | 10 | 50 | 50 | ||
| 1 | 10 | 90 | 90 | 10 | ||
| 1 | 90 | 10 | 50 | 50 |
| MPS [nm] | PDI | ξ [mV] | Catechin [%] | Epicatechin [%] | TPC [mg AG/100 g] | ABTS [μmoles TE/100 g] | ORAC [μmoles TE/100 g] |
|---|---|---|---|---|---|---|---|
| 259.7 ± 5.10 | 0.26 ± 0.03 | −41.8 ± 1.50 | 91 ± 0.02 | 79 ± 0.01 | 77.96 ± 2.42 | 12.09±0.034 | 1187.14 ± 1.24 |
| Sample | Density [kg/m3] | ) [mN/m] | Scattering Coefficient (S1), HPS [mN/m] | Scattering Coefficient (S2), PDMS [mN/m] | ∆E2000 |
|---|---|---|---|---|---|
| TLS | 1041 ± 0.577 d | 55.49 ± 1.247 d | −2.935 ± 0.087 e | −17.642 ± 0.482 d | 9.98 ± 0.007 a |
| HPMC | 1010 ± 0.577 a | 47.88 ± 0.745 b | −15.281 ± 0.060 b | −18.033 ± 0.048 d | - |
| TLS/FS-HPMC | 1050 ± 0.577 e | 42.42 ± 1.040 a | −8.601 ± 0.268 d | −22.788 ± 0.573 c | 20.46 ± 0.263 c |
| KCG | 1021 ± 1.155 b | 53.74 ± 1.453 c | −9.820 ± 0.370 c | −30.484 ± 0.945 b | - |
| TLS/FS-KCG | 1030 ± 1.528 e | 66.09 ± 0.241 d | −17.626 ± 0.184 a | −40.958 ± 0.379 a | 14.74 ± 0.036 b |
| Sample | Thickness [mm] | Young’s Modulus [Pa] | EAB [%] | RP [Pa] | Ep [%] | |
|---|---|---|---|---|---|---|
| TLS | 0.16 ± 0.012 a | 6.00 × 107 a | 3.00 × 106 a,b | 93.73 ± 1.533 b | 2.06 ± 0.211 a | 147.86 ± 4.295 b |
| HPMC | 0.16 ± 0.022 a | 2.00 × 108 d | 1.00 × 107 c | 91.06 ± 0.354 a | 18.18 ± 0.294 d | 14.69 ± 1.441 a |
| TLS/FS-HPMC | 0.16 ± 0.016 a | 2.00 × 108 d | 9.00 × 106 c | 94.71 ± 0.296 b,c | 13.74 ± 0.944 c | 16.25 ± 2.350 a |
| KCG | 0.17 ± 0.011 a | 8.00 × 107 b | 3.00 × 106 a | 94.74 ± 2.019 b,c | 7.40 ± 1.199 b | 17.36 ± 4.678 a |
| TLS/FS-KCG | 0.17 ± 0.036 a | 1.00 × 108 c | 4.00 × 106 b | 96.49 ± 0.839 c | 7.02 ± 0.838 b | 21.85 ± 5.108 a |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Monasterio, A.; Núñez, E.; Brossard, N.; Vega, R.; Osorio, F.A. Mechanical and Surface Properties of Edible Coatings Elaborated with Nanoliposomes Encapsulating Grape Seed Tannins and Polysaccharides. Polymers 2023, 15, 3774. https://doi.org/10.3390/polym15183774
Monasterio A, Núñez E, Brossard N, Vega R, Osorio FA. Mechanical and Surface Properties of Edible Coatings Elaborated with Nanoliposomes Encapsulating Grape Seed Tannins and Polysaccharides. Polymers. 2023; 15(18):3774. https://doi.org/10.3390/polym15183774
Chicago/Turabian StyleMonasterio, Angela, Emerson Núñez, Natalia Brossard, Ricardo Vega, and Fernando A. Osorio. 2023. "Mechanical and Surface Properties of Edible Coatings Elaborated with Nanoliposomes Encapsulating Grape Seed Tannins and Polysaccharides" Polymers 15, no. 18: 3774. https://doi.org/10.3390/polym15183774








