Rational Design of Novel Conjugated Terpolymers Based on Diketopyrrolopyrrole and Their Applications to Organic Thin-Film Transistors
Abstract
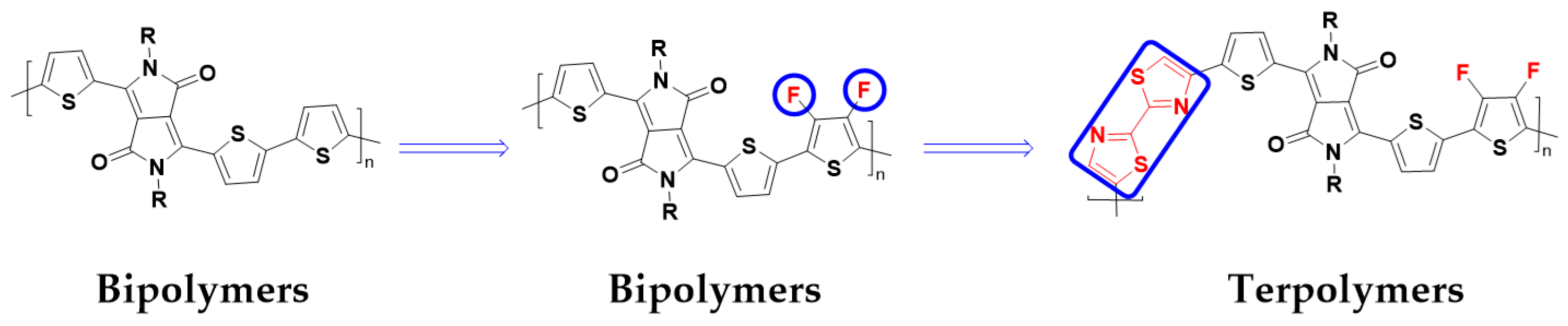
:1. Introduction
2. Materials and Methods
3. Results
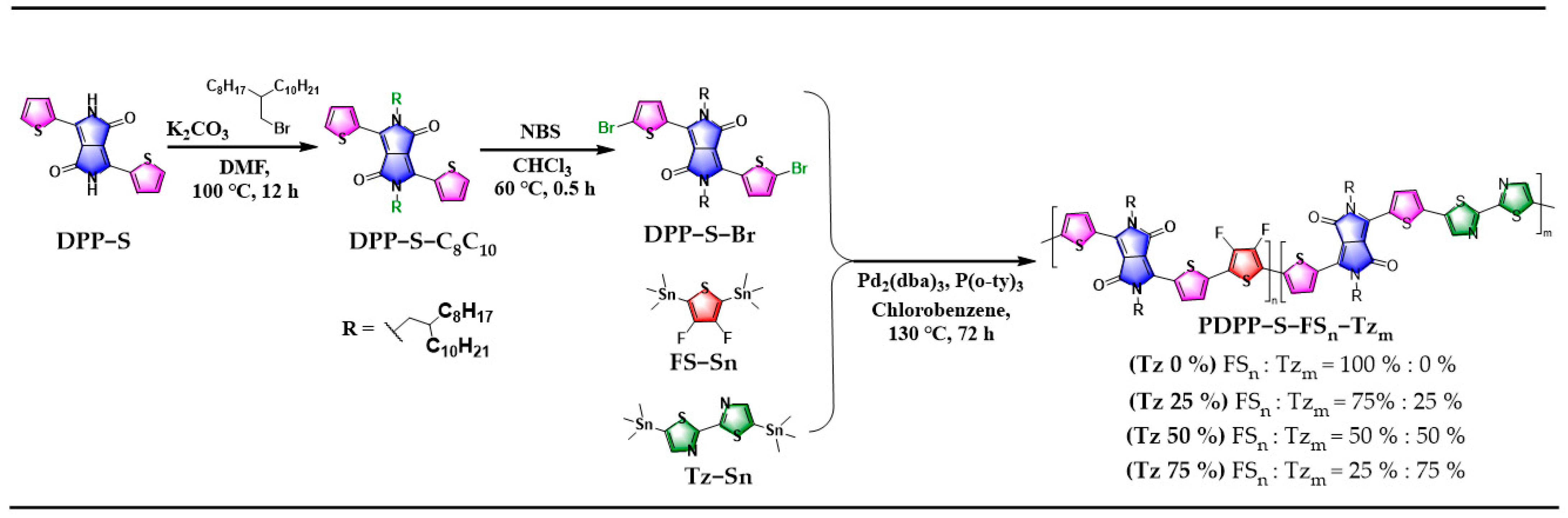
3.1. Synthesis and Structural Analysis
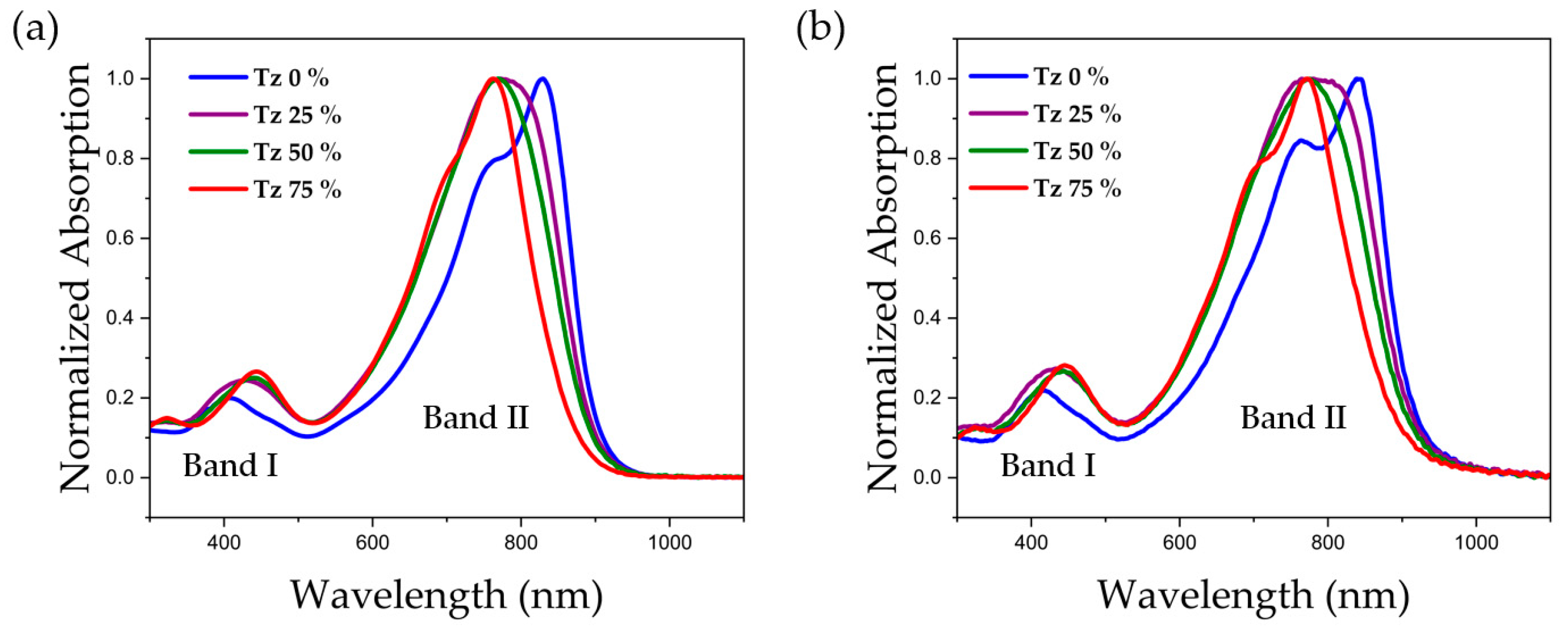
3.2. Photochemical Properties
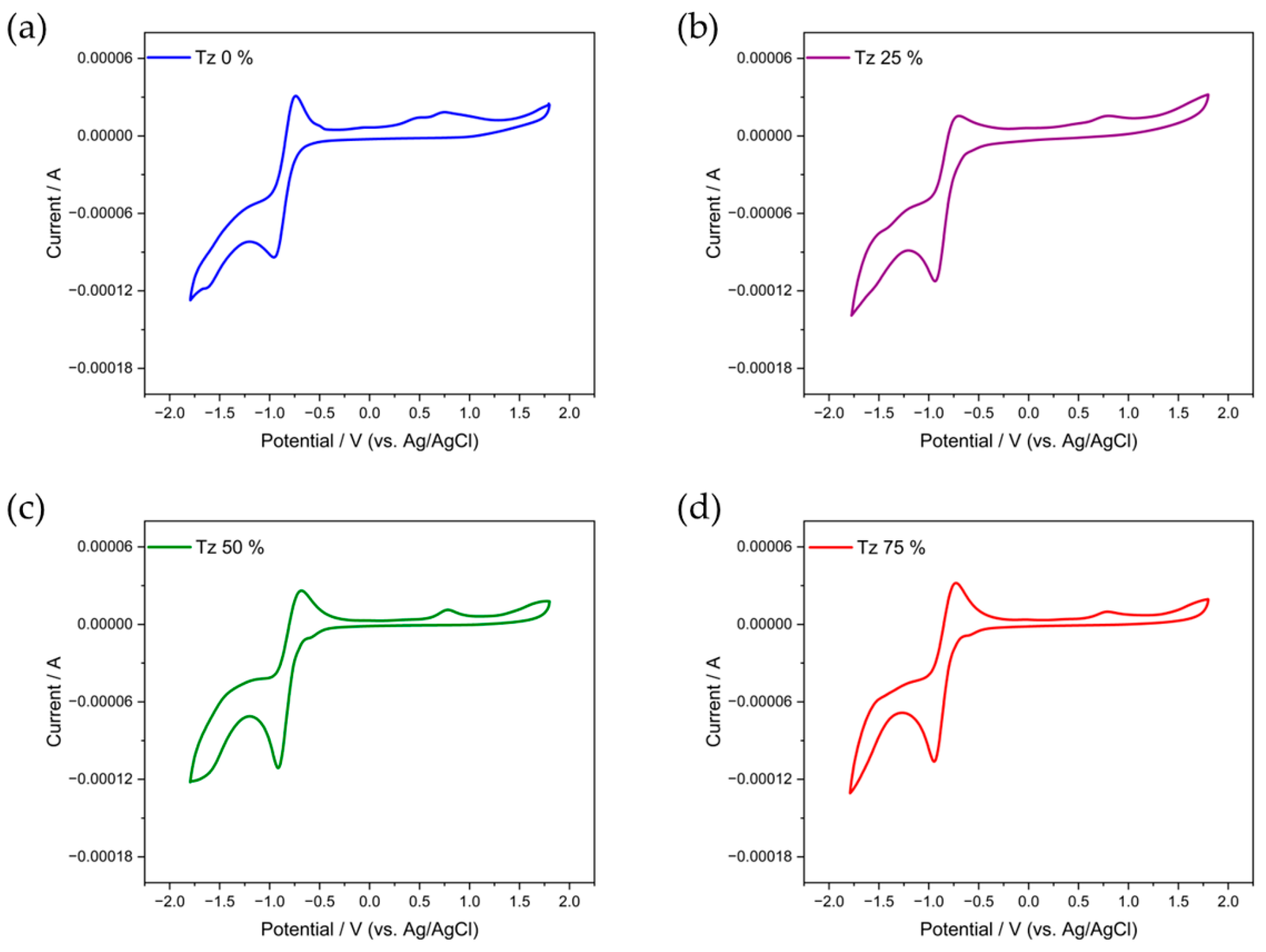
3.3. Electrochemical Properties
3.4. OTFT Performance
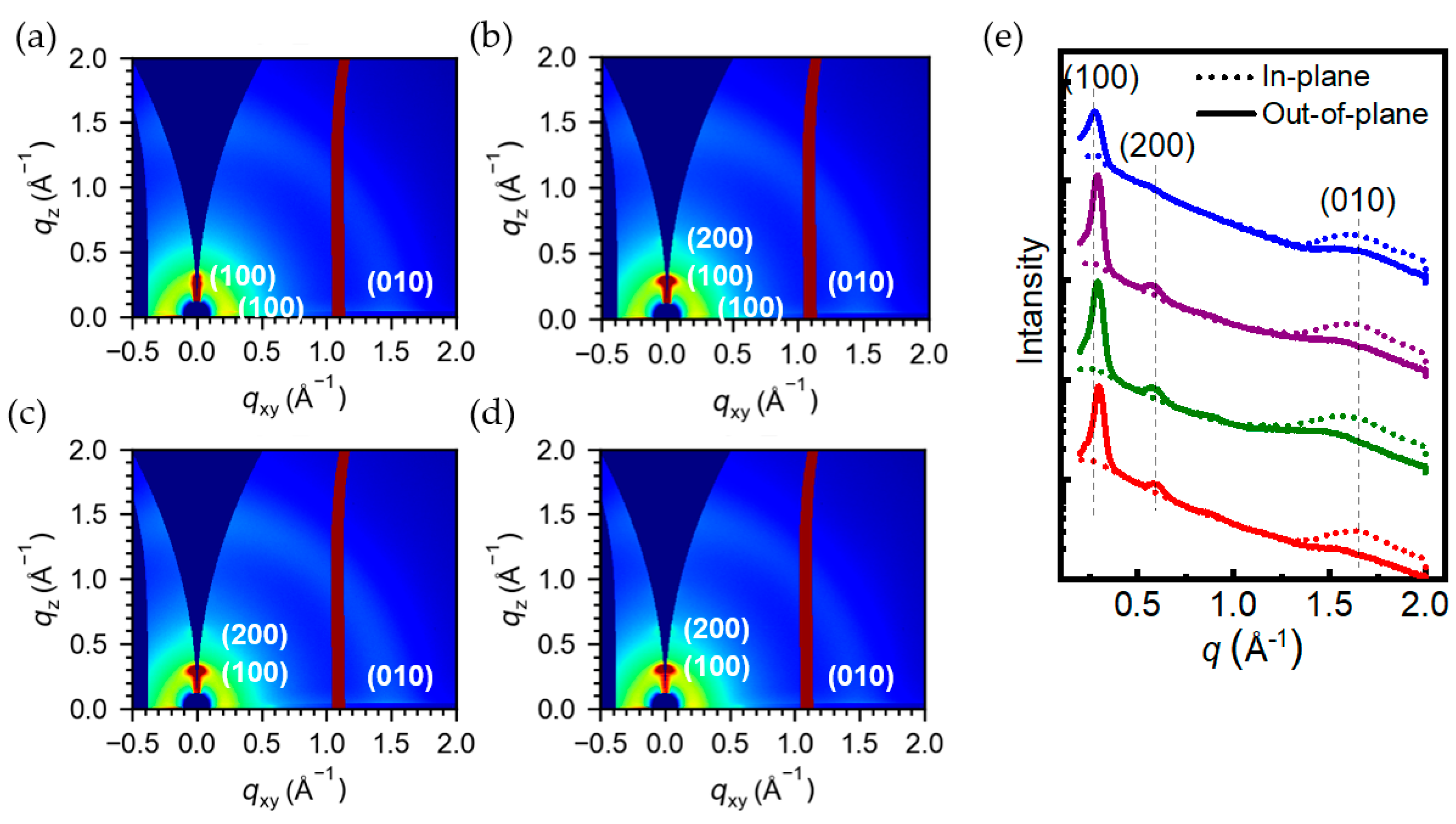
3.5. Crystallinity Analysis of Polymer Films
3.6. Morphological Analysis of Polymer Films
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Kim, M.; Ryu, S.U.; Park, S.A.; Choi, K.; Kim, T.; Chung, D.; Park, T. Donor–Acceptor-Conjugated Polymer for High-Performance Organic Field-Effect Transistors: A Progress Report. Adv. Funct. Mater. 2019, 30, 1904545. [Google Scholar] [CrossRef]
- Sirringhaus, H. 25th Anniversary Article: Organic Field-Effect Transistors: The Path Beyond Amorphous Silicon. Adv. Mater. 2014, 26, 1319–1335. [Google Scholar] [CrossRef]
- Tang, Z.; Wei, X.; Zhang, W.; Zhou, Y.; Wei, C.; Huang, J.; Chen, Z.; Wang, L.; Yu, G. An A−D−A’−D’ strategy enables perylenediimide-based polymer dyes exhibiting enhanced electron transport characteristics. Polymer 2019, 180, 121712. [Google Scholar] [CrossRef]
- Wu, W.; Liu, Y.; Zhu, D. π-Conjugated molecules with fused rings for organic field-effect transistors: Design, synthesis and applications. Chem. Soc. Rev. 2010, 39, 1489–1502. [Google Scholar] [CrossRef] [PubMed]
- Yang, J.; Zhao, Z.; Wang, S.; Guo, Y.; Liu, Y. Insight into High-Performance Conjugated Polymers for Organic Field-Effect Transistors. Chem 2018, 4, 2748–2785. [Google Scholar] [CrossRef]
- Anthony, J.E.; Facchetti, A.; Heeney, M.; Marder, S.R.; Zhan, X. n-Type organic semiconductors in organic electronics. Adv. Mater. 2010, 22, 3876–3892. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Wang, Y.; Gao, C.; Ni, Z.; Zhang, X.; Hu, W.; Dong, H. Recent advances in n-type and ambipolar organic semiconductors and their multi-functional applications. Chem. Soc. Rev. 2023, 52, 1331–1381. [Google Scholar] [CrossRef]
- Zhao, Y.; Guo, Y.; Liu, Y. 25th Anniversary Article: Recent Advances in n-Type and Ambipolar Organic Field-Effect Transistors. Adv. Mater. 2013, 25, 5372–5391. [Google Scholar] [CrossRef]
- Griggs, S.; Marks, A.; Bristow, H.; McCulloch, I. n-Type organic semiconducting polymers: Stability limitations, design considerations and applications. J. Mater. Chem. C Mater. 2021, 9, 8099–8128. [Google Scholar] [CrossRef]
- Park, K.H.; Go, J.Y.; Lim, B.; Noh, Y.Y. Recent progress in lactam-based polymer semiconductors for organic electronic devices. J. Polym. Sci. 2021, 60, 429–485. [Google Scholar] [CrossRef]
- Cheon, H.J.; An, T.K.; Kim, Y.-H. Diketopyrrolopyrrole (DPP)-Based Polymers and Their Organic Field-Effect Transistor Applications: A Review. Macromol. Res. 2022, 30, 71–84. [Google Scholar] [CrossRef]
- Ren, S.; Yassar, A. Recent Research Progress in Indophenine-Based-Functional Materials: Design, Synthesis, and Optoelectronic Applications. Materials 2023, 16, 2474–2505. [Google Scholar] [CrossRef] [PubMed]
- Chen, J.; Yang, J.; Guo, Y.; Liu, Y. Acceptor Modulation Strategies for Improving the Electron Transport in High-Performance Organic Field-Effect Transistors. Adv. Mater. 2021, 34, 2104325–2104355. [Google Scholar] [CrossRef] [PubMed]
- Ren, S.; Zhang, W.; Wang, Z.; Yassar, A.; Liao, Z.; Yi, Z. Synergistic Use of All-Acceptor Strategies for the Preparation of an Organic Semiconductor and the Realization of High Electron Transport Properties in Organic Field-Effect Transistors. Polymers 2023, 15, 3392–3404. [Google Scholar] [CrossRef] [PubMed]
- Kang, T.E.; Kim, K.-H.; Kim, B.J. Design of terpolymers as electron donors for highly efficient polymer solar cells. J. Mater. Chem. A 2014, 2, 15252–15267. [Google Scholar] [CrossRef]
- Dang, D.; Yu, D.; Wang, E. Conjugated Donor–Acceptor Terpolymers Toward High-Efficiency Polymer Solar Cells. Adv. Mater. 2019, 31, 1807019. [Google Scholar] [CrossRef] [PubMed]
- Luo, H.; Liu, Z.; Zhang, D. Conjugated D–A terpolymers for organic field-effect transistors and solar cells. Polym. J. 2017, 50, 21–31. [Google Scholar] [CrossRef]
- Mueller, C.J.; Singh, C.R.; Fried, M.; Huettner, S.; Thelakkat, M. High Bulk Electron Mobility Diketopyrrolopyrrole Copolymers with Perfluorothiophene. Adv. Funct. Mater. 2015, 25, 2725–2736. [Google Scholar] [CrossRef]
- Jiang, W.; Yu, X.; Li, C.; Zhang, X.; Zhang, G.; Liu, Z.; Zhang, D. Fluoro-substituted DPP-bisthiophene conjugated polymer with azides in the side chains as ambipolar semiconductor and photoresist. Sci. China Chem. 2022, 65, 1791–1797. [Google Scholar] [CrossRef]
- Lee, M.; Kim, T.; Nguyen, H.V.T.; Cho, H.W.; Lee, K.-K.; Choi, J.-H.; Kim, B.; Kim, J.Y. Regio-regular alternating diketopyrrolopyrrole-based D1–A–D2–A terpolymers for the enhanced performance of polymer solar cells. RSC Adv. 2019, 9, 42096–42109. [Google Scholar] [CrossRef]
- Ly, J.T.; Burnett, E.K.; Thomas, S.; Aljarb, A.; Liu, Y.; Park, S.; Rosa, S.; Yi, Y.; Lee, H.; Emrick, T.; et al. Efficient Electron Mobility in an All-Acceptor Napthalenediimide-Bithiazole Polymer Semiconductor with Large Backbone Torsion. ACS Appl. Mater. Interfaces 2018, 10, 40070–40077. [Google Scholar] [CrossRef] [PubMed]
- Yuan, Z.; Buckley, C.; Thomas, S.; Zhang, G.; Bargigia, I.; Wang, G.; Fu, B.; Silva, C.; Brédas, J.-L.; Reichmanis, E. A Thiazole–Naphthalene Diimide Based n-Channel Donor–Acceptor Conjugated Polymer. Macromolecules 2018, 51, 7320–7328. [Google Scholar] [CrossRef]
- Yi, Z.; Yan, Y.; Wang, H.; Li, W.; Liu, K.; Zhao, Y.; Gu, G.; Liu, Y. Chain-Extending Polymerization for Significant Improvement in Organic Thin-Film Transistor Performance. ACS Appl. Mater. Interfaces 2022, 14, 36918–36926. [Google Scholar] [CrossRef] [PubMed]
- Bura, T.; Beaupré, S.; Ibraikulov, O.A.; Légaré, M.-A.; Quinn, J.; Lévêque, P.; Heiser, T.; Li, Y.; Leclerc, N.; Leclerc, M. New Fluorinated Dithienyldiketopyrrolopyrrole Monomers and Polymers for Organic Electronics. Macromolecules 2017, 50, 7080–7090. [Google Scholar] [CrossRef]
- Park, J.H.; Jung, E.H.; Jung, J.W.; Jo, W.H. A fluorinated phenylene unit as a building block for high-performance n-type semiconducting polymer. Adv. Mater. 2013, 25, 2583–2588. [Google Scholar] [CrossRef] [PubMed]
- Sui, Y.; Shi, Y.; Deng, Y.; Li, R.; Bai, J.; Wang, Z.; Dang, Y.; Han, Y.; Kirby, N.; Ye, L.; et al. Direct Arylation Polycondensation of Chlorinated Thiophene Derivatives to High-Mobility Conjugated Polymers. Macromolecules 2020, 53, 10147–10154. [Google Scholar] [CrossRef]
- Zhang, C.; Tan, W.L.; Liu, Z.; He, Q.; Li, Y.; Ma, J.; Chesman, A.S.R.; Han, Y.; McNeill, C.R.; Heeney, M.; et al. High-Performance Unipolar n-Type Conjugated Polymers Enabled by Highly Electron-Deficient Building Blocks Containing F and CN Groups. Macromolecules 2022, 55, 4429–4440. [Google Scholar] [CrossRef]
- Chen, J.; Jiang, Y.; Yang, J.; Sun, Y.; Shi, L.; Ran, Y.; Zhang, Q.; Yi, Y.; Wang, S.; Guo, Y.; et al. Copolymers of Bis-Diketopyrrolopyrrole and Benzothiadiazole Derivatives for High-Performance Ambipolar Field-Effect Transistors on Flexible Substrates. ACS Appl. Mater. Interfaces 2018, 10, 25858–25865. [Google Scholar] [CrossRef]
- Yi, Z.; Jiang, Y.; Xu, L.; Zhong, C.; Yang, J.; Wang, Q.; Xiao, J.; Liao, X.; Wang, S.; Guo, Y.; et al. Triple Acceptors in a Polymeric Architecture for Balanced Ambipolar Transistors and High-Gain Inverters. Adv. Mater. 2018, 30, 1801951–1801958. [Google Scholar] [CrossRef]
- Tao, X.; Li, W.; Wu, Q.; Wei, H.; Yan, Y.; Zhao, L.; Hu, Y.; Zhao, Y.; Chen, H.; Liu, Y. Ladder-Like Difluoroindacenodithiophene-4,9-dione Derivative: A New Acceptor System for High-Mobility n-Type Polymer Semiconductors. Adv. Funct. Mater. 2022, 33, 2210846–2210857. [Google Scholar] [CrossRef]
- Cao, X.; Li, H.; Hu, J.; Tian, H.; Han, Y.; Meng, B.; Liu, J.; Wang, L. An Amorphous n-Type Conjugated Polymer with an Ultra-Rigid Planar Backbone. Angew. Chem. Int. Ed. Engl. 2023, 62, e202212979–e202212986. [Google Scholar] [CrossRef] [PubMed]
- Zhang, W.; Shi, K.; Lai, J.; Zhou, Y.; Wei, X.; Che, Q.; Wei, J.; Wang, L.; Yu, G. Record-High Electron Mobility Exceeding 16 cm2 V−1 s−1 in Bisisoindigo-Based Polymer Semiconductor with a Fully Locked Conjugated Backbone. Adv. Mater. 2023, 35, e2300145–e2300155. [Google Scholar] [CrossRef] [PubMed]
- Shi, Y.; Guo, H.; Qin, M.; Zhao, J.; Wang, Y.; Wang, H.; Wang, Y.; Facchetti, A.; Lu, X.; Guo, X. Thiazole Imide-Based All-Acceptor Homopolymer: Achieving High-Performance Unipolar Electron Transport in Organic Thin-Film Transistors. Adv. Mater. 2018, 30, 1705745–1705753. [Google Scholar] [CrossRef] [PubMed]

| Mn | Mw | PDI | C 1 | H 1 | N 1 | S 1 | |
|---|---|---|---|---|---|---|---|
| (%) | (%) | (%) | (%) | ||||
| Tz 0% | 24,541 | 85,717 | 3.49 | 71.20 | 9.10 | 2.65 | 9.90 |
| Tz 25% | 21,933 | 96,864 | 4.41 | 70.85 | 9.01 | 3.41 | 10.69 |
| Tz 50% | 28,506 | 139,502 | 4.89 | 70.73 | 8.85 | 4.04 | 11.08 |
| Tz 75% | 24,390 | 107,112 | 4.39 | 70.19 | 8.82 | 4.57 | 11.83 |
| λmax (nm) 1 | λmax (nm) 1 | λonset (nm) 1 | λmax (nm) 2 | λmax (nm) 2 | λonset (nm) 2 | Egsoln (eV) 3 | Egfilm (eV) 4 | |
|---|---|---|---|---|---|---|---|---|
| Tz 0% | 829 | 406 | 906 | 841 | 421 | 925 | 1.34 | 1.31 |
| Tz 25% | 775 | 427 | 899 | 782 | 433 | 921 | 1.35 | 1.32 |
| Tz 50% | 769 | 441 | 895 | 775 | 442 | 914 | 1.36 | 1.33 |
| Tz 75% | 762 | 444 | 878 | 772 | 446 | 900 | 1.38 | 1.36 |
| Ered (V) | Eredonset (V) | LUMO (eV) 5 | Eox (V) | Eoxonset (V) | HOMO (eV) 6 | Eg cv (eV) 7 | |
|---|---|---|---|---|---|---|---|
| Tz 0% | −0.96 | −0.75 | −3.66 | 0.49 | 0.29 | −4.70 | 1.04 |
| Tz 25% | −0.94 | −0.72 | −3.69 | 0.79 | 0.58 | −4.99 | 1.30 |
| Tz 50% | −0.91 | −0.71 | −3.70 | 0.79 | 0.60 | −5.01 | 1.31 |
| Tz 75% | −0.94 | −0.76 | −3.65 | 0.78 | 0.62 | −5.03 | 1.38 |
| Coating Speed (mm/s) | Annealing (°C) | Max Electron Mobilities (cm2/(V s)) | Electron Mobilities 1 (cm2/(V s)) | Max Hole Mobilities 1 (cm2/(V s)) | Hole Mobilities 1 (cm2/(V s)) | |
|---|---|---|---|---|---|---|
| Tz 0% | 2000 | 200 | 0.38 | 0.25 ± 0.11 | 0.070 | 0.053 ± 0.013 |
| Tz 25% | 2000 | 200 | 0.48 | 0.36 ± 0.08 | 0.064 | 0.050 ± 0.011 |
| Tz 50% | 2000 | 200 | 0.69 | 0.50 ± 0.08 | 0.036 | 0.022 ± 0.014 |
| Tz 75% | 2000 | 200 | 0.57 | 0.42 ± 0.10 | 0.051 | 0.035 ± 0.016 |
| In-Plane (010) Peak Position (Å−1) | In-Plane (010) π-Spacing (Å) | In-Plane (100) Peak Position (Å−1) | In-Plane (100) d-Spacing (Å) | Out-of-Plane (100) Peak Position (Å−1) | Out-of-Plane (100) d-Spacing (Å) | |
|---|---|---|---|---|---|---|
| Tz 0% | 1.58 | 3.98 | 0.27 | 23.26 | 0.28 | 22.43 |
| Tz 25% | 1.58 | 3.98 | 0.26 | 24.15 | 0.29 | 21.65 |
| Tz 50% | 1.59 | 3.95 | − | − | 0.30 | 20.93 |
| Tz 75% | 1.58 | 3.97 | − | − | 0.30 | 20.93 |
| Materials | Electron Mobilities (cm2/(V s) | Hole Mobilities (cm2/(V s) | Device Structure | Average Electron Mobilities (cm2/(V s) |
|---|---|---|---|---|
| DPP-T | − | 0.094 | BGBC | − |
| DPP-FDT | 0.51 | 0.80 | BGBC | 0.50 |
| DPP-F1Ph | 0.26 | 0.20 | BGTC | 0.30 |
| DPPTh-4ClBT | 0.82 | 0.73 | TGBC | − |
| F2CNTVT-DPP | 2.03 | − | TGBC | 1.50 |
| P2DPP-2FBTz | 0.68 | − | TGBC | 0.61 |
| DPP-2T-DPP-TBT | 3.84 | 3.01 | TGBC | 3.00 |
| PFIDTO-T | 0.27 | − | TGBC | 0.03 |
| PBN-27 | 0.34 | − | TGBC | 0.32 |
| PNFFN-DTE | 1.82 | − | TGBC | 1.30 |
| PNFFN-FDTE | 10.74 | − | TGBC | 10.56 |
| PDTzNTI | 1.22 | − | TGBC | 0.87 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ren, S.; Ding, Y.; Zhang, W.; Wang, Z.; Wang, S.; Yi, Z. Rational Design of Novel Conjugated Terpolymers Based on Diketopyrrolopyrrole and Their Applications to Organic Thin-Film Transistors. Polymers 2023, 15, 3803. https://doi.org/10.3390/polym15183803
Ren S, Ding Y, Zhang W, Wang Z, Wang S, Yi Z. Rational Design of Novel Conjugated Terpolymers Based on Diketopyrrolopyrrole and Their Applications to Organic Thin-Film Transistors. Polymers. 2023; 15(18):3803. https://doi.org/10.3390/polym15183803
Chicago/Turabian StyleRen, Shiwei, Yubing Ding, Wenqing Zhang, Zhuoer Wang, Sichun Wang, and Zhengran Yi. 2023. "Rational Design of Novel Conjugated Terpolymers Based on Diketopyrrolopyrrole and Their Applications to Organic Thin-Film Transistors" Polymers 15, no. 18: 3803. https://doi.org/10.3390/polym15183803





