Electrospun PVA Fibers for Drug Delivery: A Review
Abstract
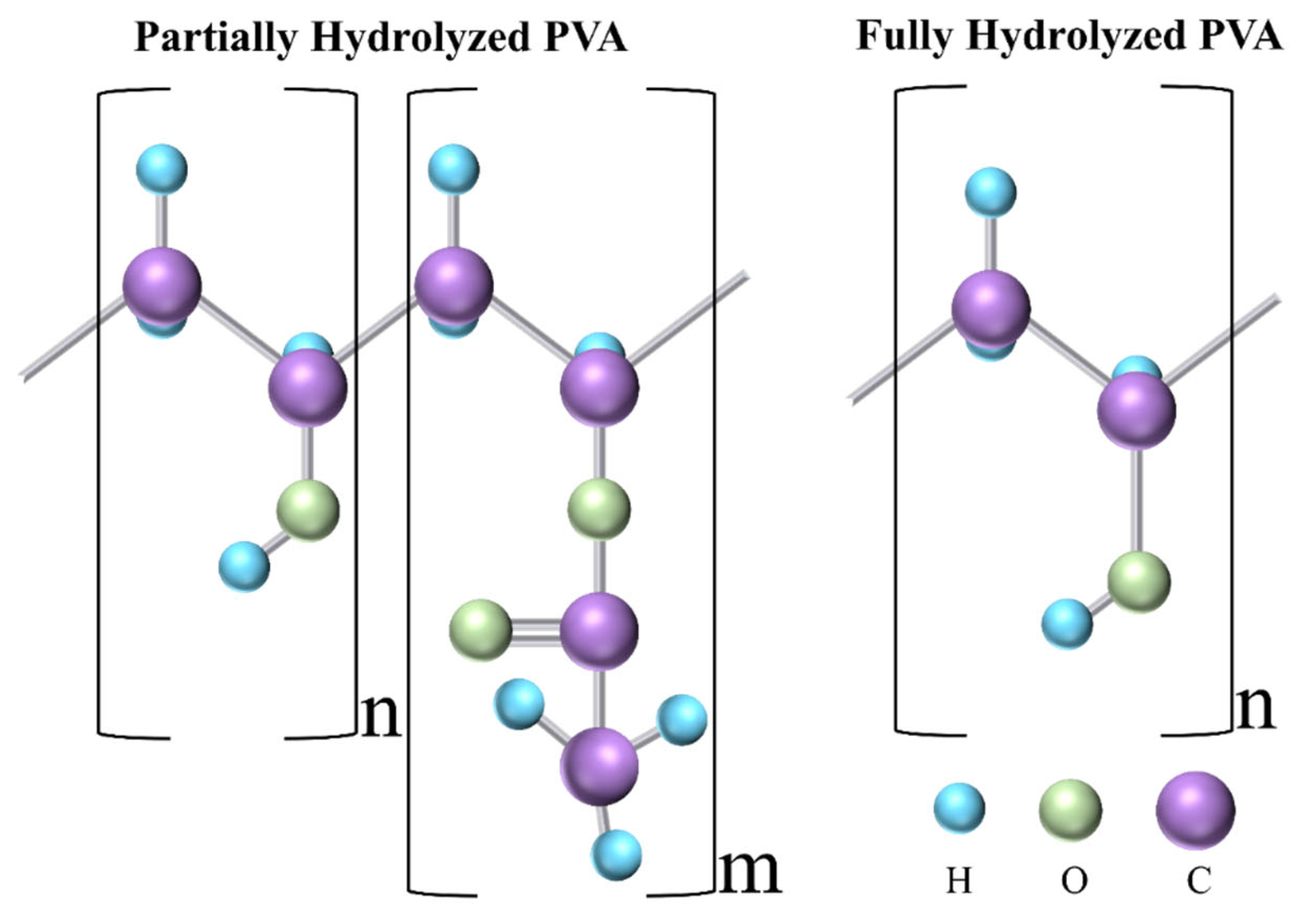
:1. Introduction
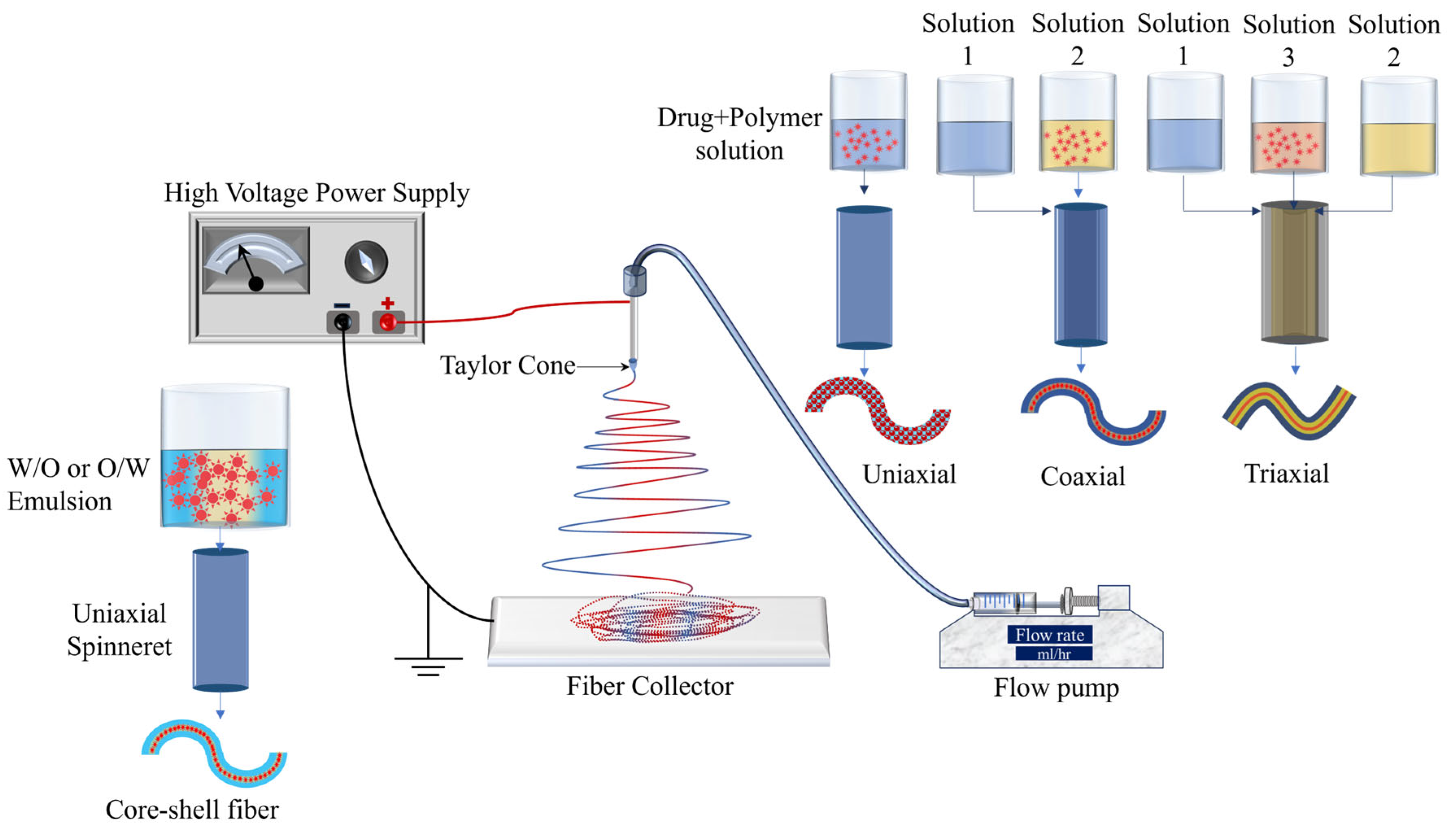
2. Electrospinning for DDSs
3. Electrospinning-Based PVA DDSs
3.1. Transdermal Drug Delivery Systems (TDDSs)
3.2. Electrospun PVA for Wound Dressing/Tissue Engineering
3.3. Electrospun PVA for Tissue Regeneration
3.4. Other Electrospun PVA-Based DDS
4. Conclusions
4.1. Developments
4.2. Challenges
4.3. Future Outlook
Funding
Institutional Review Board Statement
Data Availability Statement
Conflicts of Interest
References
- Guan, J.; Ferrell, N.; James Lee, L.; Hansford, D.J. Fabrication of Polymeric Microparticles for Drug Delivery by Soft Lithography. Biomaterials 2006, 27, 4034–4041. [Google Scholar] [CrossRef]
- Vlachopoulos, A.; Karlioti, G.; Balla, E.; Daniilidis, V.; Kalamas, T.; Stefanidou, M.; Bikiaris, N.D.; Christodoulou, E.; Koumentakou, I.; Karavas, E.; et al. Poly(Lactic Acid)-Based Microparticles for Drug Delivery Applications: An Overview of Recent Advances. Pharmaceutics 2022, 14, 359. [Google Scholar] [CrossRef]
- Croy, S.R.; Kwon, G.S. Polymeric Micelles for Drug Delivery. Curr. Pharm. Des. 2006, 12, 4669–4684. [Google Scholar] [CrossRef] [PubMed]
- Thang, N.H.; Chien, T.B.; Cuong, D.X. Polymer-Based Hydrogels Applied in Drug Delivery: An Overview. Gels 2023, 9, 523. [Google Scholar] [CrossRef] [PubMed]
- Stewart, S.A.; Domínguez-Robles, J.; Donnelly, R.F.; Larrañeta, E. Implantable Polymeric Drug Delivery Devices: Classification, Manufacture, Materials, and Clinical Applications. Polymers 2018, 10, 1379. [Google Scholar] [CrossRef]
- Nagati, V.; Tenugu, S.; Pasupulati, A.K. Chapter 4—Stability of Therapeutic Nano-Drugs during Storage and Transportation as Well as after Ingestion in the Human Body. In Nanotechnology in Biomedicine; Das Talukdar, A., Dey Sarker, S., Patra, J.K.B.T.-A., Eds.; Elsevier: Amsterdam, The Netherlands, 2022; pp. 83–102. ISBN 978-0-323-88450-1. [Google Scholar]
- Sharma, P.; Negi, P.; Mahindroo, N. Recent Advances in Polymeric Drug Delivery Carrier Systems. Adv. Polym. Biomed. Appl. 2018, 10, 369–388. [Google Scholar]
- Hadjianfar, M.; Semnani, D.; Varshosaz, J. Polycaprolactone/Chitosan Blend Nanofibers Loaded by 5-Fluorouracil: An Approach to Anticancer Drug Delivery System. Polym. Adv. Technol. 2018, 29, 2972–2981. [Google Scholar] [CrossRef]
- Desai, N.; Rana, D.; Salave, S.; Gupta, R.; Patel, P.; Karunakaran, B.; Sharma, A.; Giri, J.; Benival, D.; Kommineni, N. Chitosan: A Potential Biopolymer in Drug Delivery and Biomedical Applications. Pharmaceutics 2023, 15, 1313. [Google Scholar] [CrossRef]
- Gouda, M.; Khalaf, M.M.; Shaaban, S.; Abd El-Lateef, H.M. Fabrication of Chitosan Nanofibers Containing Some Steroidal Compounds as a Drug Delivery System. Polymers 2022, 14, 2094. [Google Scholar] [CrossRef]
- Mendes, A.C.; Gorzelanny, C.; Halter, N.; Schneider, S.W.; Chronakis, I.S. Hybrid Electrospun Chitosan-Phospholipids Nanofibers for Transdermal Drug Delivery. Int. J. Pharm. 2016, 510, 48–56. [Google Scholar] [CrossRef]
- Song, T.; Yao, C.; Li, X. Electrospinning of Zein/Chitosan Composite Fibrous Membranes. Chin. J. Polym. Sci. 2010, 28, 171–179. [Google Scholar] [CrossRef]
- Torres-Martinez, E.J.; Cornejo Bravo, J.M.; Serrano Medina, A.; Pérez González, G.L.; Villarreal Gómez, L.J. A Summary of Electrospun Nanofibers as Drug Delivery System: Drugs Loaded and Biopolymers Used as Matrices. Curr. Drug Deliv. 2018, 15, 1360–1374. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Z.; Qu, Q.; Li, J.; Zhou, S. The Effect of the Hydrophilic/Hydrophobic Ratio of Polymeric Micelles on Their Endocytosis Pathways into Cells. Macromol. Biosci. 2013, 13, 789–798. [Google Scholar] [CrossRef] [PubMed]
- Avramović, N.; Mandić, B.; Savić-Radojević, A.; Simić, T. Polymeric Nanocarriers of Drug Delivery Systems in Cancer Therapy. Pharmaceutics 2020, 12, 298. [Google Scholar] [CrossRef]
- Ma, S.; Feng, X.; Liu, F.; Wang, B.; Zhang, H.; Niu, X. The Pro-Inflammatory Response of Macrophages Regulated by Acid Degradation Products of Poly(Lactide-Co-Glycolide) Nanoparticles. Eng. Life Sci. 2021, 21, 709–720. [Google Scholar] [CrossRef] [PubMed]
- Mishra, D.; Gade, S.; Pathak, V.; Vora, L.; Mcloughlin, K.; Medina, R.; Donnelly, R.; Raghu Raj Singh, T. Ocular Application of Electrospun Materials for Drug Delivery and Cellular Therapies. Drug Discov. Today 2023, 28, 103676. [Google Scholar] [CrossRef]
- Gaaz, T.S.; Sulong, A.B.; Akhtar, M.N.; Kadhum, A.A.H.; Mohamad, A.B.; Al-Amiery, A.A.; McPhee, D.J. Properties and Applications of Polyvinyl Alcohol, Halloysite Nanotubes and Their Nanocomposites. Molecules 2015, 20, 22833–22847. [Google Scholar] [CrossRef]
- Teixeira, M.A.; Amorim, M.T.P.; Felgueiras, H.P. Poly(Vinyl Alcohol )-Based Nanofibrous Electrospun Scaffolds for Tissue Engineering Applications. Polymers 2019, 12, 7. [Google Scholar] [CrossRef]
- Rivera-Hernández, G.; Antunes-Ricardo, M.; Martínez-Morales, P.; Sánchez, M.L. Polyvinyl Alcohol Based-Drug Delivery Systems for Cancer Treatment. Int. J. Pharm. 2021, 600, 120478. [Google Scholar] [CrossRef]
- Luraghi, A.; Peri, F.; Moroni, L. Electrospinning for Drug Delivery Applications: A Review. J. Control. Release 2021, 334, 463–484. [Google Scholar] [CrossRef]
- Jafernik, K.; Ładniak, A.; Blicharska, E.; Czarnek, K.; Ekiert, H.; Wiącek, A.E.; Szopa, A. Chitosan-Based Nanoparticles as Effective Drug Delivery Systems—A Review. Molecules 2023, 28, 1963. [Google Scholar] [CrossRef] [PubMed]
- El-Seedi, H.R.; Said, N.S.; Yosri, N.; Hawash, H.B.; El-Sherif, D.M.; Abouzid, M.; Abdel-Daim, M.M.; Yaseen, M.; Omar, H.; Shou, Q.; et al. Gelatin Nanofibers: Recent Insights in Synthesis, Bio-Medical Applications and Limitations. Heliyon 2023, 9, e16228. [Google Scholar] [CrossRef] [PubMed]
- Milano, F.; Masi, A.; Madaghiele, M.; Sannino, A.; Salvatore, L.; Gallo, N. Current Trends in Gelatin-Based Drug Delivery Systems. Pharmaceutics 2023, 15, 1499. [Google Scholar] [CrossRef] [PubMed]
- Snetkov, P.; Morozkina, S.; Uspenskaya, M.; Olekhnovich, R. Hyaluronan-Based Nanofibers: Fabrication, Characterization and Application. Polymers 2019, 11, 2036. [Google Scholar] [CrossRef]
- Dovedytis, M.; Liu, Z.J.; Bartlett, S. Hyaluronic Acid and Its Biomedical Applications: A Review. Eng. Regen. 2020, 1, 102–113. [Google Scholar] [CrossRef]
- Humaira; Raza Bukhari, S.A.; Shakir, H.A.; Khan, M.; Saeed, S.; Ahmad, I.; Muzammil, K.; Franco, M.; Irfan, M.; Li, K. Hyaluronic Acid-Based Nanofibers: Electrospun Synthesis and Their Medical Applications; Recent Developments and Future Perspective. Front. Chem. 2022, 10, 1092123. [Google Scholar] [CrossRef]
- Fu, C.P.; Cai, X.Y.; Chen, S.L.; Yu, H.W.; Fang, Y.; Feng, X.C.; Zhang, L.M.; Li, C.Y. Hyaluronic Acid-Based Nanocarriers for Anticancer Drug Delivery. Polymers 2023, 15, 2317. [Google Scholar] [CrossRef]
- Law, J.X.; Liau, L.L.; Saim, A.; Yang, Y.; Idrus, R. Electrospun Collagen Nanofibers and Their Applications in Skin Tissue Engineering. Tissue Eng. Regen. Med. 2017, 14, 699–718. [Google Scholar] [CrossRef]
- Lee, C.H.; Singla, A.; Lee, Y. Biomedical Applications of Collagen. Int. J. Pharm. 2001, 221, 1–22. [Google Scholar] [CrossRef]
- Taemeh, M.A.; Shiravandi, A.; Korayem, M.A.; Daemi, H. Fabrication Challenges and Trends in Biomedical Applications of Alginate Electrospun Nanofibers. Carbohydr. Polym. 2020, 228, 115419. [Google Scholar] [CrossRef]
- Abasalizadeh, F.; Moghaddam, S.V.; Alizadeh, E.; Akbari, E.; Kashani, E.; Fazljou, S.M.B.; Torbati, M.; Akbarzadeh, A. Alginate-Based Hydrogels as Drug Delivery Vehicles in Cancer Treatment and Their Applications in Wound Dressing and 3D Bioprinting. J. Biol. Eng. 2020, 14, 8. [Google Scholar] [CrossRef]
- Tyler, B.; Gullotti, D.; Mangraviti, A.; Utsuki, T.; Brem, H. Polylactic Acid (PLA) Controlled Delivery Carriers for Biomedical Applications. Adv. Drug Deliv. Rev. 2016, 107, 163–175. [Google Scholar] [CrossRef] [PubMed]
- Liu, S.; Qin, S.; He, M.; Zhou, D.; Qin, Q.; Wang, H. Current Applications of Poly(Lactic Acid) Composites in Tissue Engineering and Drug Delivery. Compos. Part B Eng. 2020, 199, 108238. [Google Scholar] [CrossRef]
- Lu, Y.; Cheng, D.; Niu, B.; Wang, X.; Wu, X.; Wang, A. Properties of Poly (Lactic-Co-Glycolic Acid) and Progress of Poly (Lactic-Co-Glycolic Acid)-Based Biodegradable Materials in Biomedical Research. Pharmaceuticals 2023, 16, 454. [Google Scholar] [CrossRef] [PubMed]
- Yao, J.; Wang, Y.; Ma, W.; Dong, W.; Zhang, M.; Sun, D. Dual-Drug-Loaded Silk Fibroin/PLGA Scaffolds for Potential Bone Regeneration Applications. J. Nanomater. 2019, 2019, 8050413. [Google Scholar] [CrossRef]
- Homaeigohar, S.; Boccaccini, A.R. Nature-Derived and Synthetic Additives to Poly(ɛ-Caprolactone) Nanofibrous Systems for Biomedicine; an Updated Overview. Front. Chem. 2022, 9, 809676. [Google Scholar] [CrossRef]
- Bhadran, A.; Shah, T.; Babanyinah, G.K.; Polara, H.; Taslimy, S.; Biewer, M.C.; Stefan, M.C. Recent Advances in Polycaprolactones for Anticancer Drug Delivery. Pharmaceutics 2023, 15, 19–21. [Google Scholar] [CrossRef]
- Lobo, A.O.; Afewerki, S.; de Paula, M.M.M.; Ghannadian, P.; Marciano, F.R.; Zhang, Y.S.; Webster, T.J.; Khademhosseini, A. Electrospun Nanofiber Blend with Improved Mechanical and Biological Performance. Int. J. Nanomed. 2018, 13, 7891–7903. [Google Scholar] [CrossRef]
- Sun, S.; Cui, Y.; Yuan, B.; Dou, M.; Wang, G.; Xu, H.; Wang, J.; Yin, W.; Wu, D.; Peng, C. Drug Delivery Systems Based on Polyethylene Glycol Hydrogels for Enhanced Bone Regeneration. Front. Bioeng. Biotechnol. 2023, 11, 1117647. [Google Scholar] [CrossRef]
- Williams, G.R.; Raimi-Abraham, B.T.; Luo, C.J. Nanofibres in Drug Delivery Applications. Fibers 2023, 11, 21. [Google Scholar] [CrossRef]
- Liu, G.; Gu, Z.; Hong, Y.; Cheng, L.; Li, C. Electrospun Starch Nanofibers: Recent Advances, Challenges, and Strategies for Potential Pharmaceutical Applications. J. Control. Release 2017, 252, 95–107. [Google Scholar] [CrossRef]
- Amariei, N.; Manea, L.R.; Bertea, A.P.; Bertea, A.; Popa, A. The Influence of Polymer Solution on the Properties of Electrospun 3D Nanostructures. IOP Conf. Ser. Mater. Sci. Eng. 2017, 209, 12092. [Google Scholar] [CrossRef]
- Haider, A.; Haider, S.; Kang, I.K. A Comprehensive Review Summarizing the Effect of Electrospinning Parameters and Potential Applications of Nanofibers in Biomedical and Biotechnology. Arab. J. Chem. 2018, 11, 1165–1188. [Google Scholar] [CrossRef]
- Sun, B.; Long, Y.Z.; Zhang, H.D.; Li, M.M.; Duvail, J.L.; Jiang, X.Y.; Yin, H.L. Advances in Three-Dimensional Nanofibrous Macrostructures via Electrospinning. Prog. Polym. Sci. 2014, 39, 862–890. [Google Scholar] [CrossRef]
- Angammana, C.J.; Jayaram, S.H. Analysis of the Effects of Solution Conductivity on Electrospinning Process and Fiber Morphology. IEEE Trans. Ind. Appl. 2011, 47, 1109–1117. [Google Scholar] [CrossRef]
- Khalf, A.; Madihally, S.V. Recent Advances in Multiaxial Electrospinning for Drug Delivery. Eur. J. Pharm. Biopharm. 2017, 112, 1–17. [Google Scholar] [CrossRef]
- Dhandayuthapani, B.; Yoshida, Y.; Maekawa, T.; Kumar, D.S. Polymeric Scaffolds in Tissue Engineering Application: A Review. Int. J. Polym. Sci. 2011, 2011, 290602. [Google Scholar] [CrossRef]
- Alkilani, A.Z.; McCrudden, M.T.C.; Donnelly, R.F. Transdermal Drug Delivery: Innovative Pharmaceutical Developments Based on Disruption of the Barrier Properties of the Stratum Corneum. Pharmaceutics 2015, 7, 438–470. [Google Scholar] [CrossRef]
- Rujiravanit, R.; Kruaykitanon, S.; Jamieson, A.M.; Tokura, S. Preparation of Crosslinked Chitosan/Silk Fibroin Blend Films for Drug Delivery System. Macromol. Biosci. 2003, 3, 604–611. [Google Scholar] [CrossRef]
- Ngawhirunpat, T.; Opanasopit, P.; Rojanarata, T.; Akkaramongkolporn, P.; Ruktanonchai, U.; Supaphol, P. Development of Meloxicam-Loaded Electrospun Polyvinyl Alcohol Mats as a Transdermal Therapeutic Agent. Pharm. Dev. Technol. 2009, 14, 73–82. [Google Scholar] [CrossRef]
- Taepaiboon, P.; Rungsardthong, U.; Supaphol, P. Drug-Loaded Electrospun Mats of Poly(Vinyl Alcohol) Fibres and Their Release Characteristics of Four Model Drugs. Nanotechnology 2006, 17, 2317–2329. [Google Scholar] [CrossRef]
- Sa’adon, S.; Ansari, M.N.M.; Razak, S.I.A.; Anand, J.S.; Nayan, N.H.M.; Ismail, A.E.; Khan, M.U.A.; Haider, A. Preparation and Physicochemical Characterization of Sildenafil Cocrystals. Polymers 2021, 13, 2459. [Google Scholar] [CrossRef] [PubMed]
- Sa’adon, S.; Ansari, M.N.M.; Razak, S.I.A.; Yusof, A.H.M.; Faudzi, A.A.M.; Sagadevan, S.; Nayan, N.H.M.; Anand, J.S.; Amin, K.A.M. Electrospun Nanofiber and Cryogel of Polyvinyl Alcohol Transdermal Patch Containing Diclofenac Sodium: Preparation, Characterization and in Vitro Release Studies. Pharmaceutics 2021, 13, 1900. [Google Scholar] [CrossRef] [PubMed]
- Teng, G.; Zhang, X.; Zhang, C.; Chen, L.; Sun, W.; Qiu, T.; Zhang, J. Lappaconitine Trifluoroacetate Contained Polyvinyl Alcohol Nanofibrous Membranes: Characterization, Biological Activities and Transdermal Application. Mater. Sci. Eng. C 2020, 108, 110515. [Google Scholar] [CrossRef] [PubMed]
- Cui, Z.; Zheng, Z.; Lin, L.; Si, J.; Wang, Q.; Peng, X.; Chen, W. Electrospinning and Crosslinking of Polyvinyl Alcohol/Chitosan Composite Nanofiber for Transdermal Drug Delivery. Adv. Polym. Technol. 2018, 37, 1917–1928. [Google Scholar] [CrossRef]
- Charernsriwilaiwat, N.; Rojanarata, T.; Ngawhirunpat, T.; Opanasopit, P. Preparation of Chitosan-Thiamine Pyrophosphate/Polyvinyl Alcohol Blend Electrospun Nanofibers. Adv. Mater. Res. 2012, 506, 118–121. [Google Scholar] [CrossRef]
- Najafi-Taher, R.; Derakhshan, M.A.; Faridi-Majidi, R.; Amani, A. Preparation of an Ascorbic Acid/PVA-Chitosan Electrospun Mat: A Core/Shell Transdermal Delivery System. RSC Adv. 2015, 5, 50462–50469. [Google Scholar] [CrossRef]
- El Fawal, G.; Hong, H.; Song, X.; Wu, J.; Sun, M.; Zhang, L.; He, C.; Mo, X.; Wang, H. Polyvinyl Alcohol/Hydroxyethylcellulose Containing Ethosomes as a Scaffold for Transdermal Drug Delivery Applications. Appl. Biochem. Biotechnol. 2020, 191, 1624–1637. [Google Scholar] [CrossRef]
- Orasugh, J.T.; Sarkar, G.; Saha, N.R.; Das, B.; Bhattacharyya, A.; Das, S.; Mishra, R.; Roy, I.; Chattoapadhyay, A.; Ghosh, S.K.; et al. Effect of Cellulose Nanocrystals on the Performance of Drug Loaded in Situ Gelling Thermo-Responsive Ophthalmic Formulations. Int. J. Biol. Macromol. 2019, 124, 235–245. [Google Scholar] [CrossRef]
- Saska, S.; Teixeira, L.N.; de Castro Raucci, L.M.S.; Scarel-Caminaga, R.M.; Franchi, L.P.; dos Santos, R.A.; Santagneli, S.H.; Capela, M.V.; de Oliveira, P.T.; Takahashi, C.S.; et al. Nanocellulose-Collagen-Apatite Composite Associated with Osteogenic Growth Peptide for Bone Regeneration. Int. J. Biol. Macromol. 2017, 103, 467–476. [Google Scholar] [CrossRef] [PubMed]
- Samanta, A.P.; Ali, M.S.; Orasugh, J.T.; Ghosh, S.K.; Chattopadhyay, D. Crosslinked Nanocollagen-Cellulose Nanofibrils Reinforced Electrospun Polyvinyl Alcohol/Methylcellulose/Polyethylene Glycol Bionanocomposites: Study of Material Properties and Sustained Release of Ketorolac Tromethamine. Carbohydr. Polym. Technol. Appl. 2022, 3, 100195. [Google Scholar] [CrossRef]
- Aboutalebi Anaraki, N.; Roshanfekr Rad, L.; Irani, M.; Haririan, I. Fabrication of PLA/PEG/MWCNT Electrospun Nanofibrous Scaffolds for Anticancer Drug Delivery. J. Appl. Polym. Sci. 2015, 132, 41286. [Google Scholar] [CrossRef]
- Riaz, T.; Khenoussi, N.; Rata, D.M.; Atanase, L.I.; Adolphe, D.C.; Delaite, C. Blend Electrospinning of Poly(ϵ-Caprolactone) and Poly(Ethylene Glycol-400) Nanofibers Loaded with Ibuprofen as a Potential Drug Delivery System for Wound Dressings. Autex Res. J. 2023, 23, 66–76. [Google Scholar] [CrossRef]
- Dilawar, N.; Ur-Rehman, T.; Shah, K.U.; Fatima, H.; Alhodaib, A. Development and Evaluation of PLGA Nanoparticle-Loaded Organogel for the Transdermal Delivery of Risperidone. Gels 2022, 8, 709. [Google Scholar] [CrossRef] [PubMed]
- Patel, M.; Jha, A.; Patel, R. Potential Application of PLGA Microsphere for Tissue Engineering. J. Polym. Res. 2021, 28, 214. [Google Scholar] [CrossRef]
- Qvist, M.H.; Hoeck, U.; Kreilgaard, B.; Madsen, F.; Frokjaer, S. Release of Chemical Permeation Enhancers from Drug-in-Adhesive Transdermal Patches. Int. J. Pharm. 2002, 231, 253–263. [Google Scholar] [CrossRef]
- Yun, J.; Im, J.S.; Lee, Y.S.; Kim, H. Il Electro-Responsive Transdermal Drug Delivery Behavior of PVA/PAA/MWCNT Nanofibers. Eur. Polym. J. 2011, 47, 1893–1902. [Google Scholar] [CrossRef]
- Yoo, H.-J.; Kim, H.-D. Characteristics of Waterborne Polyurethane/Poly(N-Vinylpyrrolidone) Composite Films for Wound-Healing Dressings. J. Appl. Polym. Sci. 2008, 107, 331–338. [Google Scholar] [CrossRef]
- Rahmani, F.; Ziyadi, H.; Baghali, M.; Luo, H.; Ramakrishna, S. Electrospun Pvp/Pva Nanofiber Mat as a Novel Potential Transdermal Drug-Delivery System for Buprenorphine: A Solution Needed for Pain Management. Appl. Sci. 2021, 11, 2779. [Google Scholar] [CrossRef]
- Pilehvar-Soltanahmadi, Y.; Dadashpour, M.; Mohajeri, A.; Fattahi, A.; Sheervalilou, R.; Zarghami, N. An Overview on Application of Natural Substances Incorporated with Electrospun Nanofibrous Scaffolds to Development of Innovative Wound Dressings. Mini-Reviews Med. Chem. 2018, 18, 414–427. [Google Scholar] [CrossRef] [PubMed]
- Azimi, B.; Maleki, H.; Zavagna, L.; de la Ossa, J.G.; Linari, S.; Lazzeri, A.; Danti, S. Bio-Based Electrospun Fibers for Wound Healing. J. Funct. Biomater. 2020, 11, 67. [Google Scholar] [CrossRef] [PubMed]
- Juncos Bombin, A.D.; Dunne, N.J.; McCarthy, H.O. Electrospinning of Natural Polymers for the Production of Nanofibres for Wound Healing Applications. Mater. Sci. Eng. C 2020, 114, 110994. [Google Scholar] [CrossRef] [PubMed]
- Fatehi, P.; Abbasi, M. Medicinal Plants Used in Wound Dressings Made of Electrospun Nanofibers. J. Tissue Eng. Regen. Med. 2020, 14, 1527–1548. [Google Scholar] [CrossRef] [PubMed]
- Kalva, S.N.; Augustine, R.; Al Mamun, A.; Dalvi, Y.B.; Vijay, N.; Hasan, A. Active Agents Loaded Extracellular Matrix Mimetic Electrospun Membranes for Wound Healing Applications. J. Drug Deliv. Sci. Technol. 2021, 63, 102500. [Google Scholar] [CrossRef]
- Sell, S.; Barnes, C.; Smith, M.; McClure, M.; Madurantakam, P.; Grant, J.; McManus, M.; Bowlin, G. Extracellular Matrix Regenerated: Tissue Engineering via Electrospun Biomimetic Nanofibers. Polym. Int. 2007, 56, 1349–1360. [Google Scholar] [CrossRef]
- Yang, X.; Fan, L.; Ma, L.; Wang, Y.; Lin, S.; Yu, F.; Pan, X.; Luo, G.; Zhang, D.; Wang, H. Green Electrospun Manuka Honey/Silk Fibroin Fibrous Matrices as Potential Wound Dressing. Mater. Des. 2017, 119, 76–84. [Google Scholar] [CrossRef]
- Herrmann, I.; Supriyanto, E.; Jaganathan, S.K.; Manikandan, A. Advanced Nanofibrous Textile-Based Dressing Material for Treating Chronic Wounds. Bull. Mater. Sci. 2018, 41, 18. [Google Scholar] [CrossRef]
- Safdari, M.; Shakiba, E.; Kiaie, S.H.; Fattahi, A. Preparation and Characterization of Ceftazidime Loaded Electrospun Silk Fibroin/Gelatin Mat for Wound Dressing. Fibers Polym. 2016, 17, 744–750. [Google Scholar] [CrossRef]
- Alven, S.; Peter, S.; Mbese, Z.; Aderibigbe, B.A. Polymer-Based Wound Dressing Materials Loaded with Bioactive Agents: Potential Materials for the Treatment of Diabetic Wounds. Polymers 2022, 14, 724. [Google Scholar] [CrossRef]
- Kalaycıoğlu, Z.; Kahya, N.; Adımcılar, V.; Kaygusuz, H.; Torlak, E.; Akın-Evingür, G.; Erim, F.B. Antibacterial Nano Cerium Oxide/Chitosan/Cellulose Acetate Composite Films as Potential Wound Dressing. Eur. Polym. J. 2020, 133, 109777. [Google Scholar] [CrossRef]
- Li, S.; Li, L.; Guo, C.; Qin, H.; Yu, X. A Promising Wound Dressing Material with Excellent Cytocompatibility and Proangiogenesis Action for Wound Healing: Strontium Loaded Silk Fibroin/Sodium Alginate (SF/SA) Blend Films. Int. J. Biol. Macromol. 2017, 104, 969–978. [Google Scholar] [CrossRef] [PubMed]
- Wei, S.; You, Y.; Ma, Y.; Huang, W.; Liang, X.; Zhang, A.; Lin, Y. Bi-Layer Supramolecular Polydimethylsiloxane Elastomer Film: Synthesis, Characterization, and Application in Wound Dressing on Normal and Diabetic Rat. React. Funct. Polym. 2019, 141, 21–32. [Google Scholar] [CrossRef]
- García-Hernández, A.B.; Morales-Sánchez, E.; Berdeja-Martínez, B.M.; Escamilla-García, M.; Salgado-Cruz, M.P.; Rentería-Ortega, M.; Farrera-Rebollo, R.R.; Vega-Cuellar, M.A.; Calderón-Domínguez, G. PVA-Based Electrospun Biomembranes with Hydrolyzed Collagen and Ethanolic Extract of Hypericum Perforatum for Potential Use as Wound Dressing: Fabrication and Characterization. Polymers 2022, 14, 1981. [Google Scholar] [CrossRef] [PubMed]
- Serbezeanu, D.; Bargan, A.; Homocianu, M.; Aflori, M.; Rîmbu, C.M.; Enache, A.A.; Vlad-Bubulac, T. Electrospun Polyvinyl Alcohol Loaded with Phytotherapeutic Agents for Wound Healing Applications. Nanomaterials 2021, 11, 3336. [Google Scholar] [CrossRef]
- Mouro, C.; Gomes, A.P.; Ahonen, M.; Fangueiro, R.; Gouveia, I.C. Chelidonium Majus l. Incorporated Emulsion Electrospun Pcl/Pva_pec Nanofibrous Meshes for Antibacterial Wound Dressing Applications. Nanomaterials 2021, 11, 1785. [Google Scholar] [CrossRef]
- Mata, G.C.d.; Morais, M.S.; de Oliveira, W.P.; Aguiar, M.L. Composition Effects on the Morphology of PVA/Chitosan Electrospun Nanofibers. Polymers 2022, 14, 4856. [Google Scholar] [CrossRef]
- Stoica Oprea, A.E.; Albuleț, D.; Bîrcă, A.C.; Iordache, F.; Ficai, A.; Grumezescu, A.M.; Vasile, B.Ș.; Andronescu, E.; Marinescu, F.; Holban, A.M. Electrospun Nanofibrous Mesh Based on PVA, Chitosan, and Usnic Acid for Applications in Wound Healing. Int. J. Mol. Sci. 2023, 24, 11037. [Google Scholar] [CrossRef] [PubMed]
- Francolini, I.; Norris, P.; Piozzi, A.; Donelli, G.; Stoodley, P. Usnic Acid, a Natural Antimicrobial Agent Able to Inhibit Bacterial Biofilm Formation on Polymer Surfaces. Antimicrob. Agents Chemother. 2004, 48, 4360–4365. [Google Scholar] [CrossRef]
- Pagano, C.; Ceccarini, M.R.; Calarco, P.; Scuota, S.; Conte, C.; Primavilla, S.; Ricci, M.; Perioli, L. Bioadhesive Polymeric Films Based on Usnic Acid for Burn Wound Treatment: Antibacterial and Cytotoxicity Studies. Colloids Surf. B Biointerfaces 2019, 178, 488–499. [Google Scholar] [CrossRef]
- Zhang, D.; Yang, S.; Chen, Y.; Liu, S.; Zhao, H.; Gu, J. 60Co γ-Ray Irradiation Crosslinking of Chitosan/Graphene Oxide Composite Film: Swelling, Thermal Stability, Mechanical, and Antibacterial Properties. Polymers 2018, 10, 294. [Google Scholar] [CrossRef]
- Yang, S.; Zhang, X.; Zhang, D. Electrospun Chitosan/Poly (Vinyl Alcohol)/Graphene Oxide Nanofibrous Membrane with Ciprofloxacin Antibiotic Drug for Potential Wound Dressing Application. Int. J. Mol. Sci. 2019, 20, 4395. [Google Scholar] [CrossRef]
- Iqbal, H.; Khan, B.A.; Khan, Z.U.; Razzaq, A.; Khan, N.U.; Menaa, B.; Menaa, F. Fabrication, Physical Characterizations and in Vitro Antibacterial Activity of Cefadroxil-Loaded Chitosan/Poly(Vinyl Alcohol) Nanofibers against Staphylococcus Aureus Clinical Isolates. Int. J. Biol. Macromol. 2020, 144, 921–931. [Google Scholar] [CrossRef] [PubMed]
- Kataria, K.; Gupta, A.; Rath, G.; Mathur, R.B.; Dhakate, S.R. In Vivo Wound Healing Performance of Drug Loaded Electrospun Composite Nanofibers Transdermal Patch. Int. J. Pharm. 2014, 469, 102–110. [Google Scholar] [CrossRef] [PubMed]
- Fathi, A.; Khanmohammadi, M.; Goodarzi, A.; Foroutani, L.; Mobarakeh, Z.T.; Saremi, J.; Arabpour, Z.; Ai, J. Fabrication of Chitosan-Polyvinyl Alcohol and Silk Electrospun Fiber Seeded with Differentiated Keratinocyte for Skin Tissue Regeneration in Animal Wound Model. J. Biol. Eng. 2020, 14, 27. [Google Scholar] [CrossRef] [PubMed]
- Norouzi, Z.; Abdouss, M. Electrospun Nanofibers Using β-Cyclodextrin Grafted Chitosan Macromolecules Loaded with Indomethacin as an Innovative Drug Delivery System. Int. J. Biol. Macromol. 2023, 233, 123518. [Google Scholar] [CrossRef] [PubMed]
- Mirmajidi, T.; Chogan, F.; Rezayan, A.H.; Sharifi, A.M. In Vitro and in Vivo Evaluation of a Nanofiber Wound Dressing Loaded with Melatonin. Int. J. Pharm. 2021, 596, 120213. [Google Scholar] [CrossRef]
- Kan, Y.; Bondareva, J.V.; Statnik, E.S.; Cvjetinovic, J.; Lipovskikh, S.; Abdurashitov, A.S.; Kirsanova, M.A.; Sukhorukhov, G.B.; Evlashin, S.A.; Salimon, A.I.; et al. Effect of Graphene Oxide and Nanosilica Modifications on Electrospun Core-Shell PVA–PEG–SiO2@PVA–GO Fiber Mats. Nanomaterials 2022, 12, 998. [Google Scholar] [CrossRef]
- Abdul Hameed, M.M.; Mohamed Khan, S.A.P.; Thamer, B.M.; Al-Enizi, A.; Aldalbahi, A.; El-Hamshary, H.; El-Newehy, M.H. Core-Shell Nanofibers from Poly(Vinyl Alcohol) Based Biopolymers Using Emulsion Electrospinning as Drug Delivery System for Cephalexin Drug. J. Macromol. Sci. Part A Pure Appl. Chem. 2020, 58, 130–144. [Google Scholar] [CrossRef]
- Prezotti, F.G.; Boni, F.I.; Ferreira, N.N.; de Souza e Silva, D.; Campana-Filho, S.P.; Almeida, A.; Vasconcelos, T.; Gremião, M.P.D.; Cury, B.S.F.; Sarmento, B. Gellan Gum/Pectin Beads Are Safe and Efficient for the Targeted Colonic Delivery of Resveratrol. Polymers 2018, 10, 50. [Google Scholar] [CrossRef]
- Carrêlo, H.; Cidade, M.T.; Borges, J.P.; Soares, P. Gellan Gum/Alginate Microparticles as Drug Delivery Vehicles: DOE Production Optimization and Drug Delivery. Pharmaceuticals 2023, 16, 1029. [Google Scholar] [CrossRef]
- Palumbo, F.S.; Federico, S.; Pitarresi, G.; Fiorica, C.; Giammona, G. Gellan Gum-Based Delivery Systems of Therapeutic Agents and Cells. Carbohydr. Polym. 2020, 229, 115430. [Google Scholar] [CrossRef]
- Vashisth, P.; Pruthi, P.A.; Singh, R.P.; Pruthi, V. Process Optimization for Fabrication of Gellan Based Electrospun Nanofibers. Carbohydr. Polym. 2014, 109, 16–21. [Google Scholar] [CrossRef] [PubMed]
- Stijnman, A.C.; Bodnar, I.; Hans Tromp, R. Electrospinning of Food-Grade Polysaccharides. Food Hydrocoll. 2011, 25, 1393–1398. [Google Scholar] [CrossRef]
- Vashisth, P.; Srivastava, A.K.; Nagar, H.; Raghuwanshi, N.; Sharan, S.; Nikhil, K.; Pruthi, P.A.; Singh, R.P.; Roy, P.; Pruthi, V. Drug Functionalized Microbial Polysaccharide Based Nanofibers as Transdermal Substitute. Nanomed. Nanotechnol. Biol. Med. 2016, 12, 1375–1385. [Google Scholar] [CrossRef] [PubMed]
- Nayak, D.; Tripathi, N.; Kathuria, D.; Siddharth, S.; Nayak, A.; Bharatam, P.V.; Kundu, C. Quinacrine and Curcumin Synergistically Increased the Breast Cancer Stem Cells Death by Inhibiting ABCG2 and Modulating DNA Damage Repair Pathway. Int. J. Biochem. Cell Biol. 2020, 119, 105682. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Chang, X.; Zhan, H.; Zhang, Q.; Li, C.; Gao, Q.; Yang, M.; Luo, Z.; Li, S.; Sun, Y. Curcumin and Baicalin Ameliorate Ethanol-Induced Liver Oxidative Damage via the Nrf2/HO-1 Pathway. J. Food Biochem. 2020, 44, e13425. [Google Scholar] [CrossRef]
- Al-dossari, M.H.; Fadda, L.M.; Attia, H.A.; Hasan, I.H.; Mahmoud, A.M. Curcumin and Selenium Prevent Lipopolysaccharide/Diclofenac-Induced Liver Injury by Suppressing Inflammation and Oxidative Stress. Biol. Trace Elem. Res. 2020, 196, 173–183. [Google Scholar] [CrossRef] [PubMed]
- Rathinavel, S.; Indrakumar, J.; Korrapati, P.S.; Dharmalingam, S. Synthesis and Fabrication of Amine Functionalized SBA-15 Incorporated PVA/Curcumin Nanofiber for Skin Wound Healing Application. Colloids Surf. A Physicochem. Eng. Asp. 2022, 637, 128185. [Google Scholar] [CrossRef]
- Salami, M.S.; Bahrami, G.; Arkan, E.; Izadi, Z.; Miraghaee, S.; Samadian, H. Co-Electrospun Nanofibrous Mats Loaded with Bitter Gourd (Momordica Charantia) Extract as the Wound Dressing Materials: In Vitro and in Vivo Study. BMC Complement. Med. Ther. 2021, 21, 111. [Google Scholar] [CrossRef]
- Andra, S.; Balu, S.k.; Ramamoorthy, R.; Muthalagu, M.; Sampath, D.; Sivagnanam, K.; Arumugam, G. Synthesis, Characterization, and Antimicrobial Properties of Novel Dual Drug Loaded Electrospun Mat for Wound Dressing Applications. J. Bioact. Compat. Polym. 2021, 36, 431–443. [Google Scholar] [CrossRef]
- Rai, M.; Ingle, A.P.; Gupta, I.; Brandelli, A. Bioactivity of Noble Metal Nanoparticles Decorated with Biopolymers and Their Application in Drug Delivery. Int. J. Pharm. 2015, 496, 159–172. [Google Scholar] [CrossRef] [PubMed]
- Chao, S.; Zhang, Y.; Cheng, S.; Shao, X.; Liu, S.; Lu, W.; Wang, Y.; Zhang, P.; Yao, Q. Ibuprofen-Loaded ZnO Nanoparticle/Polyacrylonitrile Nanofibers for Dual-Stimulus Sustained Release of Drugs. ACS Appl. Nano Mater. 2023, 6, 5535–5544. [Google Scholar] [CrossRef]
- Hussein, M.A.M.; Ulag, S.; Dena, A.S.A.; Sahin, A.; Grinholc, M.; Gunduz, O.; El-Sherbiny, I.; Megahed, M. Chitosan/Gold Hybrid Nanoparticles Enriched Electrospun Pva Nanofibrous Mats for the Topical Delivery of Punica Granatum l. Extract: Synthesis, Characterization, Biocompatibility and Antibacterial Properties. Int. J. Nanomed. 2021, 16, 5133–5151. [Google Scholar] [CrossRef] [PubMed]
- Yadav, S.; Arya, D.K.; Pandey, P.; Anand, S.; Gautam, A.K.; Ranjan, S.; Saraf, S.A.; Rajamanickam, V.M.; Singh, S.; Chidambaram, K.; et al. ECM Mimicking Biodegradable Nanofibrous Scaffold Enriched with Curcumin/ZnO to Accelerate Diabetic Wound Healing via Multifunctional Bioactivity. Int. J. Nanomed. 2022, 17, 6843–6859. [Google Scholar] [CrossRef]
- Rahmani, E.; Pourmadadi, M.; Zandi, N.; Rahdar, A.; Baino, F. PH-Responsive PVA-Based Nanofibers Containing GO Modified with Ag Nanoparticles: Physico-Chemical Characterization, Wound Dressing, and Drug Delivery. Micromachines 2022, 13, 1847. [Google Scholar] [CrossRef]
- Sandoval-Herrera, I.; Romero-García, J.; Ledezma-Pérez, A.; Alvarado-Canché, C.; Torres-Lubian, R.; De-León, A. Controlled Release of Chlorogenic Acid from Polyvinyl Alcohol/Poly(γ-Glutamic Acid) Blended Electrospun Nanofiber Mats with Potential Applications in Diabetic Foot Treatment. Polymers 2021, 13, 2943. [Google Scholar] [CrossRef]
- Yue, Y.; Liu, X.; Pang, L.; Liu, Y.; Lin, Y.; Xiang, T.; Li, J.; Liao, S.; Jiang, Y. Astragalus Polysaccharides/PVA Nanofiber Membranes Containing Astragaloside IV-Loaded Liposomes and Their Potential Use for Wound Healing. Evid.-Based Complement. Altern. Med. 2022, 2022, 9716271. [Google Scholar] [CrossRef]
- El-attar, A.A.; El-wakil, H.B.; Hassanin, A.H.; Bakr, B.A.; Almutairi, T.M.; Hagar, M.; Elwakil, B.H.; Olama, Z.A. Silver/Snail Mucous PVA Nanofibers: Electrospun Synthesis and Antibacterial and Wound Healing Activities. Membranes 2022, 12, 536. [Google Scholar] [CrossRef]
- Motasadizadeh, H.; Azizi, S.; Shaabani, A.; Sarvestani, M.G.; Sedghi, R.; Dinarvand, R. Development of PVA/Chitosan-g-Poly (N-Vinyl Imidazole)/TiO2/Curcumin Nanofibers as High-Performance Wound Dressing. Carbohydr. Polym. 2022, 296, 119956. [Google Scholar] [CrossRef]
- Mouro, C.; Simões, M.; Gouveia, I.C.; Xu, B. Emulsion Electrospun Fiber Mats of PCL/PVA/Chitosan and Eugenol for Wound Dressing Applications. Adv. Polym. Technol. 2019, 2019, 9859506. [Google Scholar] [CrossRef]
- Najafiasl, M.; Osfouri, S.; Zaeri, S. Evaluation of Physicochemical Properties, Release Kinetics, and in Vitro/in Vivo Wound Healing Activity of the Electrospun Nanofibres Loaded with the Natural Antioxidant Oil from Pistacia Atlantica. J. Drug Deliv. Sci. Technol. 2023, 84, 104512. [Google Scholar] [CrossRef]
- Golchin, A.; Nourani, M.R. Effects of Bilayer Nanofibrillar Scaffolds Containing Epidermal Growth Factor on Full-Thickness Wound Healing. Polym. Adv. Technol. 2020, 31, 2443–2452. [Google Scholar] [CrossRef]
- Eakwaropas, P.; Ngawhirunpat, T.; Rojanarata, T.; Akkaramongkolporn, P.; Opanasopit, P.; Patrojanasophon, P. Fabrication of Electrospun Hydrogels Loaded with Ipomoea Pes-Caprae (L.) R. Br Extract for Infected Wound. J. Drug Deliv. Sci. Technol. 2020, 55, 101478. [Google Scholar] [CrossRef]
- Sequeira, R.S.; Miguel, S.P.; Cabral, C.S.D.; Moreira, A.F.; Ferreira, P.; Correia, I.J. Development of a Poly(Vinyl Alcohol)/Lysine Electrospun Membrane-Based Drug Delivery System for Improved Skin Regeneration. Int. J. Pharm. 2019, 570, 118640. [Google Scholar] [CrossRef]
- Xie, J.; MacEwan, M.R.; Ray, W.Z.; Liu, W.; Siewe, D.Y.; Xia, Y. Radially Aligned, Electrospun Nanofibers as Dural Substitutes for Wound Closure and Tissue Regeneration Applications. ACS Nano 2010, 4, 5027–5036. [Google Scholar] [CrossRef]
- Ru, C.; Wang, F.; Pang, M.; Sun, L.; Chen, R.; Sun, Y. Suspended, Shrinkage-Free, Electrospun PLGA Nanofibrous Scaffold for Skin Tissue Engineering. ACS Appl. Mater. Interfaces 2015, 7, 10872–10877. [Google Scholar] [CrossRef]
- Zhu, C.; Ma, X.; Xian, L.; Zhou, Y.; Fan, D. Characterization of a Co-Electrospun Scaffold of HLC/CS/PLA for Vascular Tissue Engineering. Biomed. Mater. Eng. 2014, 24, 1999–2005. [Google Scholar] [CrossRef]
- Socci, M.C.; Rodríguez, G.; Oliva, E.; Fushimi, S.; Takabatake, K.; Nagatsuka, H.; Felice, C.J.; Rodríguez, A.P. Polymeric Materials, Advances and Applications in Tissue Engineering: A Review. Bioengineering 2023, 10, 218. [Google Scholar] [CrossRef]
- Yan, X.; Yao, H.; Luo, J.; Li, Z.; Wei, J. Functionalization of Electrospun Nanofiber for Bone Tissue Engineering. Polymers 2022, 14, 2940. [Google Scholar] [CrossRef]
- Rahmati, M.; Mills, D.K.; Urbanska, A.M.; Saeb, M.R.; Venugopal, J.R.; Ramakrishna, S.; Mozafari, M. Electrospinning for Tissue Engineering Applications. Prog. Mater. Sci. 2021, 117, 100721. [Google Scholar] [CrossRef]
- Su, Y.; Toftdal, M.S.; Le Friec, A.; Dong, M.; Han, X.; Chen, M. 3D Electrospun Synthetic Extracellular Matrix for Tissue Regeneration. Small Sci. 2021, 1, 2100003. [Google Scholar] [CrossRef]
- Peranidze, K.; Safronova, T.V.; Kildeeva, N.R. Fibrous Polymer-Based Composites Obtained by Electrospinning for Bone Tissue Engineering. Polymers 2022, 14, 96. [Google Scholar] [CrossRef]
- Anjum, S.; Rahman, F.; Pandey, P.; Arya, D.K.; Alam, M.; Rajinikanth, P.S.; Ao, Q. Electrospun Biomimetic Nanofibrous Scaffolds: A Promising Prospect for Bone Tissue Engineering and Regenerative Medicine. Int. J. Mol. Sci. 2022, 23, 9206. [Google Scholar] [CrossRef] [PubMed]
- Boda, R.; Lázár, I.; Keczánné-Üveges, A.; Bakó, J.; Tóth, F.; Trencsényi, G.; Kálmán-Szabó, I.; Béresová, M.; Sajtos, Z.; Tóth, E.D.; et al. β-Tricalcium Phosphate-Modified Aerogel Containing PVA/Chitosan Hybrid Nanospun Scaffolds for Bone Regeneration. Int. J. Mol. Sci. 2023, 24, 7562. [Google Scholar] [CrossRef]
- dos Santos, D.M.; Chagas, P.A.M.; Leite, I.S.; Inada, N.M.; de Annunzio, S.R.; Fontana, C.R.; Campana-Filho, S.P.; Correa, D.S. Core-Sheath Nanostructured Chitosan-Based Nonwovens as a Potential Drug Delivery System for Periodontitis Treatment. Int. J. Biol. Macromol. 2020, 142, 521–534. [Google Scholar] [CrossRef] [PubMed]
- Shadjou, N.; Hasanzadeh, M. Bone tissue engineering using silica-based mesoporous nanobiomaterials: Recent progress. Mater. Sci. Eng. C 2015, 55, 401–409. [Google Scholar] [CrossRef] [PubMed]
- Zhou, X.; Weng, W.; Chen, B.; Feng, W.; Wang, W.; Nie, W.; Chen, L.; Mo, X.; Su, J.; He, C. Mesoporous Silica Nanoparticles/Gelatin Porous Composite Scaffolds with Localized and Sustained Release of Vancomycin for Treatment of Infected Bone Defects. J. Mater. Chem. B 2018, 6, 740–752. [Google Scholar] [CrossRef]
- Shao, L.; Li, Y.; Huang, F.; Wang, X.; Lu, J.; Jia, F.; Pan, Z.; Cui, X.; Ge, G.; Deng, X.; et al. Complementary Autophagy Inhibition and Glucose Metabolism with Rattle-Structured Polydopamine@mesoporous Silica Nanoparticles for Augmented Low-Temperature Photothermal Therapy and in Vivo Photoacoustic Imaging. Theranostics. Theranostics 2020, 10, 7273–7286. [Google Scholar] [CrossRef]
- Mi, H.Y.; Jing, X.; Napiwocki, B.N.; Li, Z.T.; Turng, L.S.; Huang, H.X. Fabrication of Fibrous Silica Sponges by Self-Assembly Electrospinning and Their Application in Tissue Engineering for Three-Dimensional Tissue Regeneration. Chem. Eng. J. 2018, 331, 652–662. [Google Scholar] [CrossRef]
- Stoica (Oprea), A.E.; Bîrcă, A.C.; Gherasim, O.; Ficai, A.; Grumezescu, A.M.; Oprea, O.-C.; Vasile, B.Ș.; Balta, C.; Andronescu, E.; Hermenean, A.O. Electrospun Fibrous Silica for Bone Tissue Engineering Applications. Pharmaceutics 2023, 15, 1728. [Google Scholar] [CrossRef]
- Pathmanapan, S.; Sekar, M.; Pandurangan, A.K.; Anandasadagopan, S.K. Fabrication of Mesoporous Silica Nanoparticle–Incorporated Coaxial Nanofiber for Evaluating the In Vitro Osteogenic Potential. Appl. Biochem. Biotechnol. 2022, 194, 302–322. [Google Scholar] [CrossRef]
- Takahama, H.; Minamino, T.; Asanuma, H.; Fujita, M.; Asai, T.; Wakeno, M.; Sasaki, H.; Kikuchi, H.; Hashimoto, K.; Oku, N.; et al. Prolonged Targeting of Ischemic/Reperfused Myocardium by Liposomal Adenosine Augments Cardioprotection in Rats. J. Am. Coll. Cardiol. 2009, 53, 709–717. [Google Scholar] [CrossRef]
- Boison, D.; Scheurer, L.; Zumsteg, V.; Rülicke, T.; Litynski, P.; Fowler, B.; Brandner, S.; Mohler, H. Neonatal Hepatic Steatosis by Disruption of the Adenosine Kinase Gene. Proc. Natl. Acad. Sci. USA 2002, 99, 6985–6990. [Google Scholar] [CrossRef]
- Dunwiddie, T. V Adenosine and Suppression of Seizures. Adv. Neurol. 1999, 79, 1001–1010. [Google Scholar]
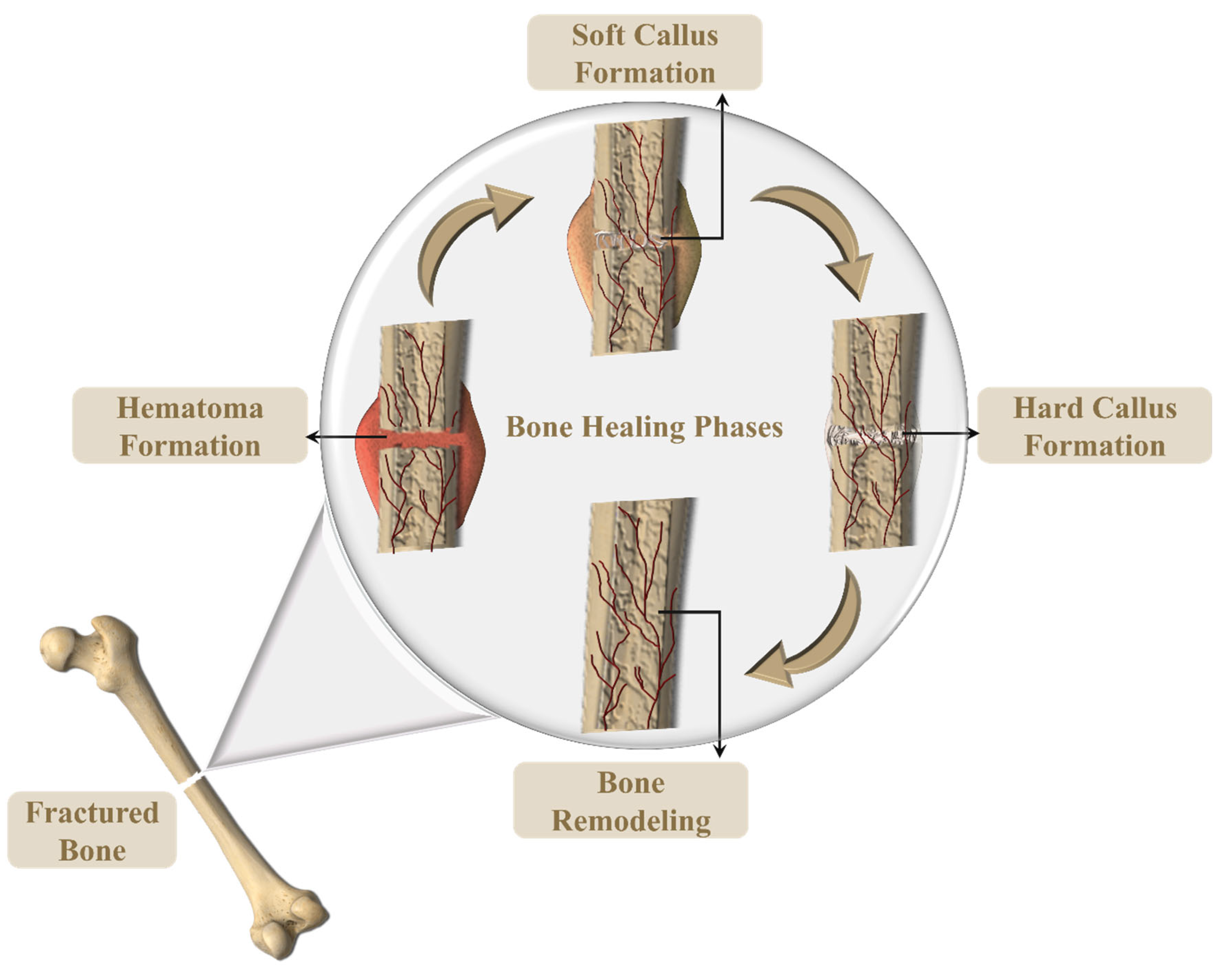
- Cheng, X.; Cheng, G.; Xing, X.; Yin, C.; Cheng, Y.; Zhou, X.; Jiang, S.; Tao, F.; Deng, H.; Li, Z. Controlled Release of Adenosine from Core-Shell Nanofibers to Promote Bone Regeneration through STAT3 Signaling Pathway. J. Control. Release 2020, 319, 234–245. [Google Scholar] [CrossRef]
- Chen, X.; Gleeson, S.E.; Yu, T.; Khan, N.; Yucha, R.W.; Marcolongo, M.; Li, C.Y. Hierarchically Ordered Polymer Nanofiber Shish Kebabs as a Bone Scaffold Material. J. Biomed. Mater. Res. Part A 2017, 105, 1786–1798. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Salick, M.R.; Wang, X.; Cordie, T.; Han, W.; Peng, Y.; Li, Q.; Turng, L.-S. Poly(ε-Caprolactone) Nanofibers with a Self-Induced Nanohybrid Shish-Kebab Structure Mimicking Collagen Fibrils. Biomacromolecules 2013, 14, 3557–3569. [Google Scholar] [CrossRef] [PubMed]
- Jing, X.; Mi, H.-Y.; Wang, X.-C.; Peng, X.-F.; Turng, L.-S. Shish-Kebab-Structured Poly(ε-Caprolactone) Nanofibers Hierarchically Decorated with Chitosan–Poly(ε-Caprolactone) Copolymers for Bone Tissue Engineering. ACS Appl. Mater. Interfaces 2015, 7, 6955–6965. [Google Scholar] [CrossRef] [PubMed]
- Jing, X.; Jin, E.; Mi, H.-Y.; Li, W.-J.; Peng, X.-F.; Turng, L.-S. Hierarchically Decorated Electrospun Poly(Epsilon-Caprolactone)/Nanohydroxyapatite Composite Nanofibers for Bone Tissue Engineering. J. Mater. Sci. 2015, 50, 4174–4186. [Google Scholar] [CrossRef]
- Huang, C.; Yang, G.; Zhou, S.; Luo, E.; Pan, J.; Bao, C.; Liu, X. Controlled Delivery of Growth Factor by Hierarchical Nanostructured Core-Shell Nanofibers for the Efficient Repair of Critical-Sized Rat Calvarial Defect. ACS Biomater. Sci. Eng. 2020, 6, 5758–5770. [Google Scholar] [CrossRef]
- Rama, M.; Vijayalakshmi, U. Influence of Silk Fibroin on the Preparation of Nanofibrous Scaffolds for the Effective Use in Osteoregenerative Applications. J. Drug Deliv. Sci. Technol. 2021, 61, 102182. [Google Scholar] [CrossRef]
- Cheng, G.; Yin, C.; Tu, H.; Jiang, S.; Wang, Q.; Zhou, X.; Xing, X.; Xie, C.; Shi, X.; Du, Y.; et al. Controlled Co-Delivery of Growth Factors through Layer-by-Layer Assembly of Core Shell Nanofibers for Improving Bone Regeneration. ACS Nano 2019, 13, 6372–6382. [Google Scholar] [CrossRef]
- Marín, J.A.T.; Londoño, S.R.; Delgado, J.; Porras, D.P.N.; Zapata, M.E.V.; Hernandez, J.H.M.; Valencia, C.H.; Tovar, C.D.G. Biocompatible and Antimicrobial Electrospun Membranes Based on Nanocomposites of Chitosan/Poly (Vinyl Alcohol)/Graphene Oxide. Int. J. Mol. Sci. 2019, 20, 2987. [Google Scholar] [CrossRef]
- Rezk, A.I.; Rajan Unnithan, A.; Hee Park, C.; Sang Kim, C. Rational Design of Bone Extracellular Matrix Mimicking Tri-Layered Composite Nanofibers for Bone Tissue Regeneration. Chem. Eng. J. 2018, 350, 812–823. [Google Scholar] [CrossRef]
- Yan, E.; Cao, M.; Wang, Y.; Meng, Y.; Zheng, H.; Hao, X.; Yu, Z.; Ba, X.; Gu, X.; Zhang, D. Degradable Polyvinyl Alcohol/Poly(Butylene Carbonate) Core-Shell Nanofibers for Chemotherapy and Tissue Engineering. Mater. Lett. 2016, 167, 13–17. [Google Scholar] [CrossRef]
- Srisuk, P.; Bishi, D.K.; Berti, F.V.; Silva, C.J.R.; Kwon, I.K.; Correlo, V.M.; Reis, R.L. Eumelanin Nanoparticle-Incorporated Polyvinyl Alcohol Nanofibrous Composite as an Electroconductive Scaffold for Skeletal Muscle Tissue Engineering. ACS Appl. Bio Mater. 2018, 1, 1893–1905. [Google Scholar] [CrossRef] [PubMed]
- Gao, C.; Gao, Q.; Li, Y.; Rahaman, M.N.; Teramoto, A.; Abe, K. Preparation and in Vitro Characterization of Electrospun PVA Scaffolds Coated with Bioactive Glass for Bone Regeneration. J. Biomed. Mater. Res. Part A 2012, 100, 1324–1334. [Google Scholar] [CrossRef]
- Balashanmugam, P.; Sucharithra, G.S.M.T.S. Efficacy of Biopolymeric PVA-AuNPs and PCL-Curcumin Loaded Electrospun Nanofibers and Their Anticancer Activity against A431 Skin Cancer Cell Line. Mater. Today Commun. 2020, 25, 101276. [Google Scholar] [CrossRef]
- Chen, S.; Li, Y.; Yan, E.; Lu, H.; Gao, J.; Wang, Y. A Novel Polyhydroxyalkanoate/Polyvinyl Alcohol Composite Porous Membrane via Electrospinning and Spin Coating as Potential Application for Chemotherapy and Tissue Engineering. Polym. Adv. Technol. 2023, 34, 3154–3163. [Google Scholar] [CrossRef]
- Yadav, B.K.; Patel, G. Fabrication and Characterization of 5-FLUOROURACIL Loaded Multilayered Electrospun Nanofiber for the Treatment OF SKIN Cancer. J. Drug Deliv. Sci. Technol. 2023, 86, 104582. [Google Scholar] [CrossRef]
- Irani, M.; Nodeh, S.M. PVA/κ-Carrageenan/Au/Camptothecin/Pegylated-Polyurethane/Paclitaxel Nanofibers against Lung Cancer Treatment. RSC Adv. 2022, 12, 16310–16318. [Google Scholar] [CrossRef]
- Abasalta, M.; Asefnejad, A.; Khorasani, M.T.; Saadatabadi, A.R.; Irani, M. Adsorption and Sustained Release of Doxorubicin from N-Carboxymethyl Chitosan/Polyvinyl Alcohol/Poly(ε-Caprolactone) Composite and Core-Shell Nanofibers. J. Drug Deliv. Sci. Technol. 2022, 67, 102937. [Google Scholar] [CrossRef]
- Wu, Z.; Kong, B.; Liu, R.; Sun, W.; Mi, S. Engineering of Corneal Tissue through an Aligned PVA/Collagen Composite Nanofibrous Electrospun Scaffold. Nanomaterials 2018, 8, 124. [Google Scholar] [CrossRef] [PubMed]
- Limoee, M.; Allahdad, M.; Samadian, H.; Bahrami, G.; Pourmanouchehri, Z.; Hosseinzadeh, L.; Mohammadi, B.; Vosoughi, A.; Forouhar, K.; Behbood, L. Preparation and Evaluation of Extended-Release Nanofibers Loaded with Pramipexole as a Novel Oral Drug Delivery System: Hybridization of Hydrophilic and Hydrophobic Polymers. J. Pharm. Innov. 2023, 18, 287–299. [Google Scholar] [CrossRef]
- Emerine, R.; Chou, S.-F. Fast Delivery of Melatonin from Electrospun Blend Polyvinyl Alcohol and Polyethylene Oxide (PVA/PEO) Fibers. AIMS Bioeng. 2022, 9, 178–196. [Google Scholar] [CrossRef]


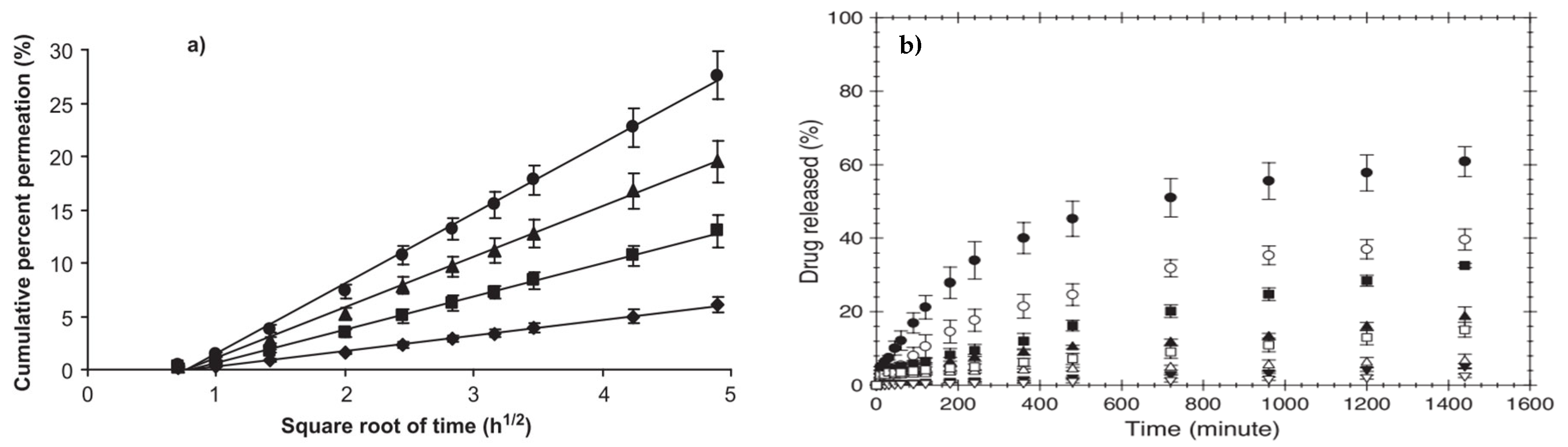
 ) represents 0.5% GA crosslinked PVA/CS fibers and (b) concentration of GA (%), on drug loading efficiency (%) [56].
) represents 0.5% GA crosslinked PVA/CS fibers and (b) concentration of GA (%), on drug loading efficiency (%) [56].
 ) represents 0.5% GA crosslinked PVA/CS fibers and (b) concentration of GA (%), on drug loading efficiency (%) [56].
) represents 0.5% GA crosslinked PVA/CS fibers and (b) concentration of GA (%), on drug loading efficiency (%) [56].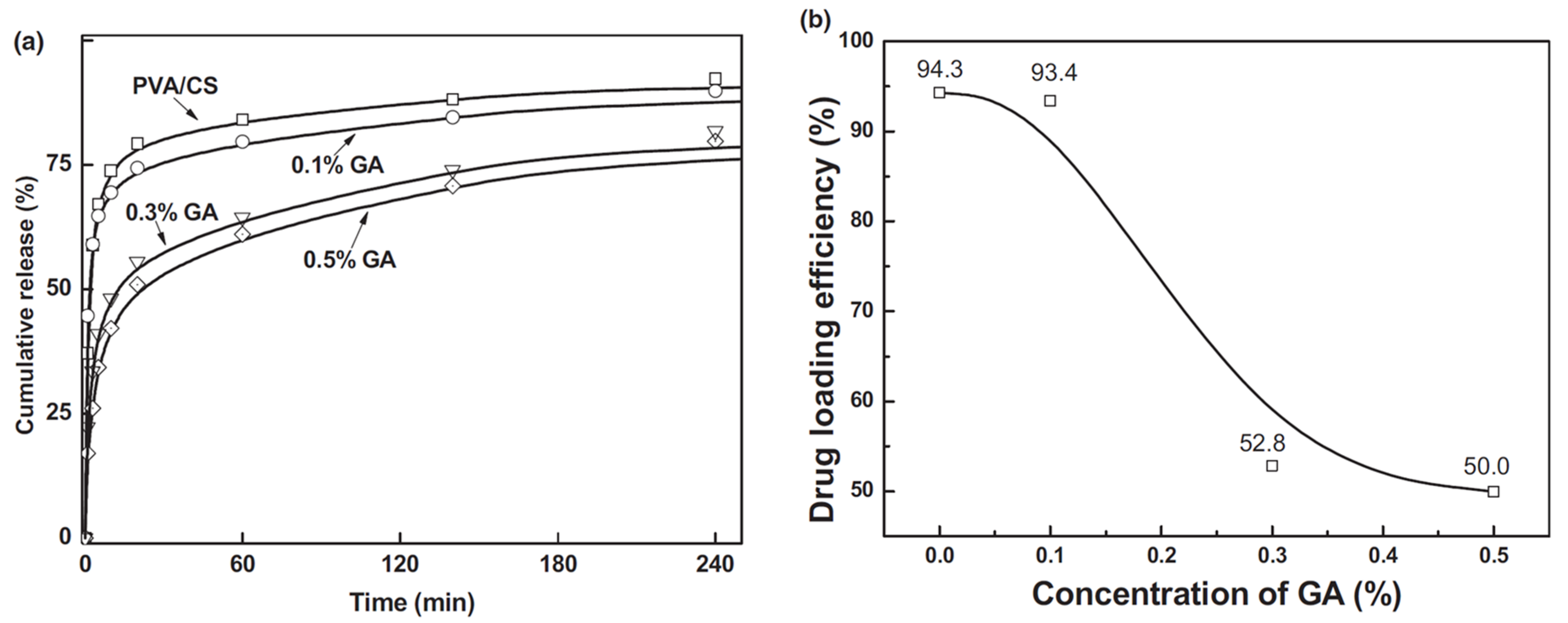




| Polymer, Type of Polymer | Limitations | Advantages | Ref. |
|---|---|---|---|
| Chitosan Natural | Extremely hydrophilic which leads to loss of nanofibrous structure, high degradation rate, and poor mechanical strength | Non-toxic, and biodegradable qualities make it biocompatible with a wide range of organs, tissues, and cells. | [9,22] |
| Gelatin Natural | Rapid degradation, poor mechanical strength, and complete dissolution | Intrinsic bioactivity, high biocompatibility, cell adhesion, biodegradability, low immunogenicity | [23,24] |
| Hyaluronic Acid Natural | High viscosity, high surface tension, low evaporability, high electrical conductivity that may lead to electrospinning circuit failure | Biocompatibility, non-immunogenicity, biodegradability, excellent tumor-targeting ability | [25,26,27,28] |
| Collagen Natural | Variability in enzymatic degradation rate (depending on enzyme concentration), difficult to maintain its dimension in vivo due to swelling, poor mechanical strength, in vivo (not suitable for load-bearing tissues) | Biocompatible, non-antigenic, non-toxic, biodegradable (degradation can be regulated via crosslinking), compatible with synthetic polymers, promotes blood coagulation | [29,30] |
| Alginate Natural | Low solubility, high viscosity due to high MW, high density of hydrogen bonding, polyelectrolyte nature of aqueous solution, and lack of appropriate organic solvent. | High water content, nontoxicity, soft consistency, biocompatibility, biodegradability, low immunogenicity | [31,32] |
| PLA Natural | Poor mechanical strength, low cell adhesion because of its hydrophobicity, biological inertness, acidic degradation products, inflammation in vivo | Biocompatible, biodegradable by hydrolysis and enzymatic activity, low immunogenicity | [33,34] |
| PLGA Synthetic | Poor hydrophilicity, poor cell adhesion, higher viscosity, production of acids upon degradation | Strong biodegradability, suitable for controlled-release drug delivery of medicines, peptides, proteins, and other substances | [35,36] |
| PCL Synthetic | High hydrophobicity, poor bioactivity, low mechanical strength, and higher amount of PCL reduces the swelling capability of DDS | Slower degradation rate, shorter in vivo adsorbable time, generation of a minimal acidic environment during degradation | [37,38] |
| PEG Synthetic | Low molecular weight makes it challenging to electrospin | Non-toxic, non-immunogenicity, good biocompatibility, and anti-protein adsorption | [39,40] |
| Formulations | Electrospinning Type and Morphology | DDS Type | Conclusive Remarks | Ref |
|---|---|---|---|---|
|
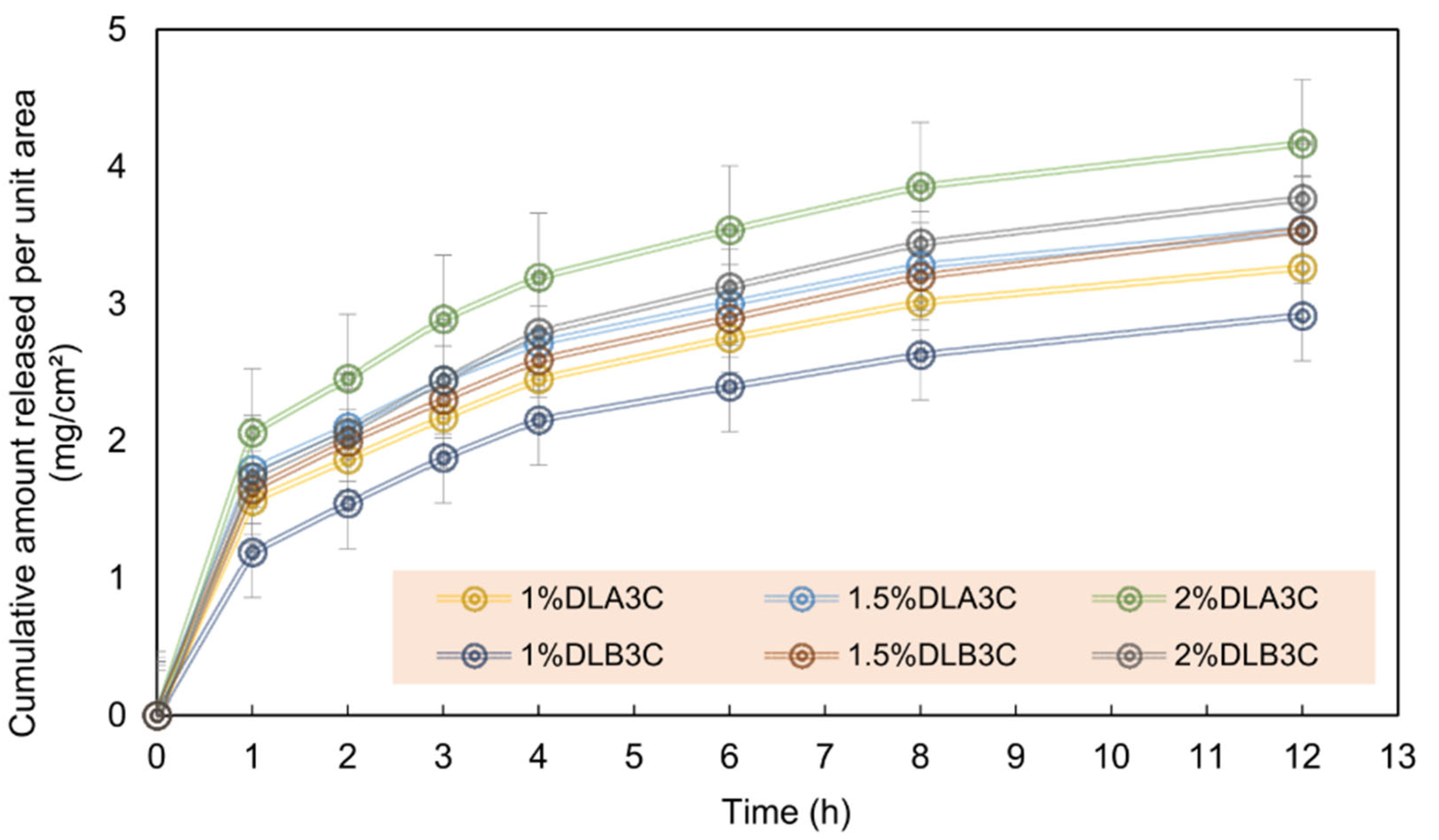
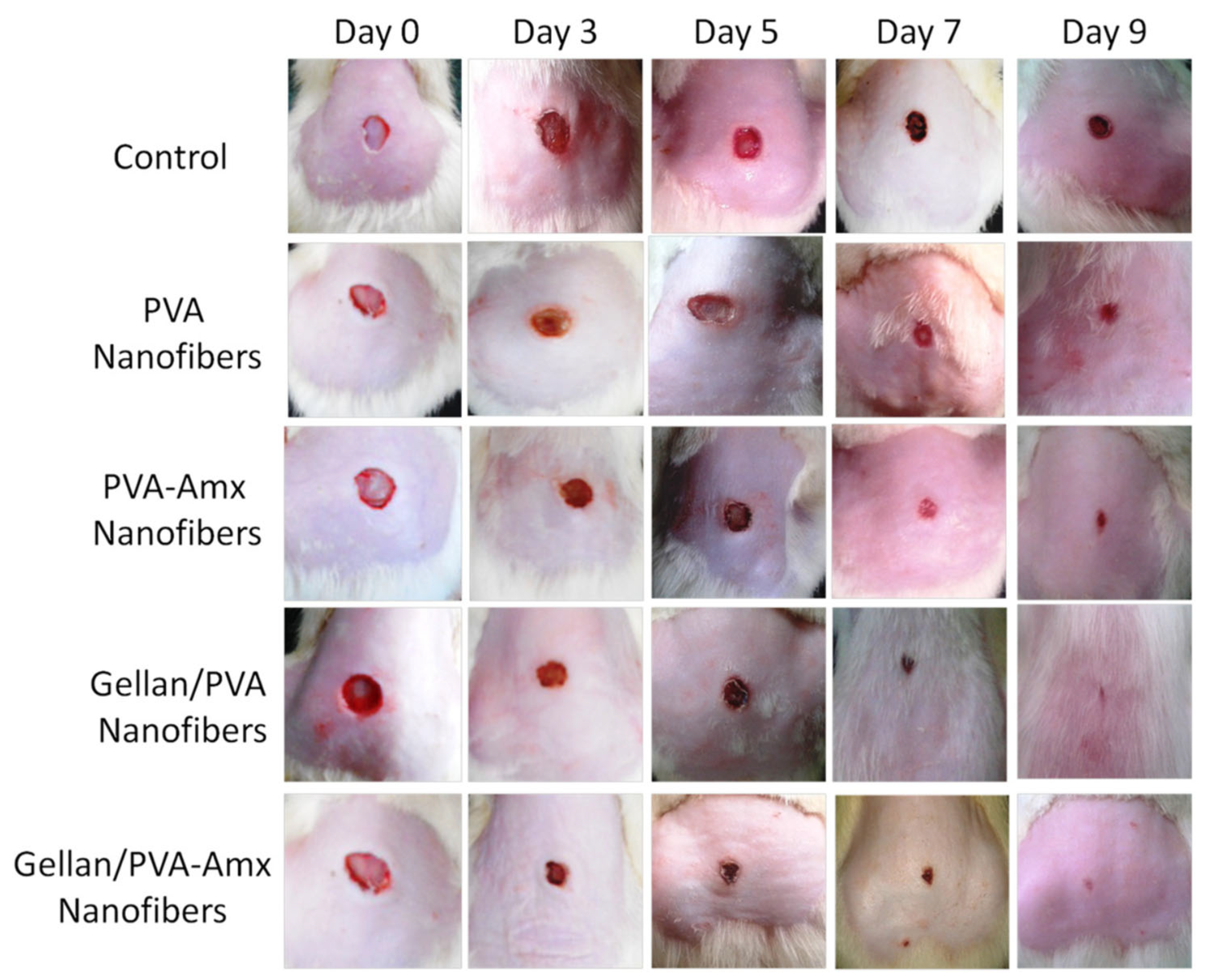
PVA, PVA/astragalus polysaccharide (APS), PVA/APS/astragaloside IV (ASL) | Uniaxial Fine fiber morphology with average diameters of 210.56 ± 91.30, 138.679 ± 93.616, and 145.68 ± 66.856 nm, respectively. | Diabetic wound healing in vivo | ASL/APS/PVA showed outstanding results in inhibiting inflammation, assisting collagen deposition, and better wound re-epithelialization. Large area healing (94.5 ± 6.1%), basal congestion at the center of the wound, massive tissue proliferation with no infection. PVA alone did not show a promising wound healing rate. | [118] |
|
PVA, PVA/Snail Mucus (SM), PVA/Ag-SM | Uniaxial Bead-free fine morphology and homogeneous fiber mats for all formulations with average diameters of 170, 126, and 110 nm, respectively. | Wound Healing In vitro/In vivo | After a sharp release for an initial 6 h, sustained drug release was observed for 72 h. Significantly high cell viability of HSF-PI 18 fibroblast cells. PVA/Ag-SM inhibited bacterial growth and enhanced the wound-healing process. | [119] |
| PVA/CS-g-Poly (N-vinyl imidazole) /TiO2/CUR | Uniaxial PVA/CS-g-Poly (N-vinyl imidazole) /18.5%TiO2/25%CUR formulation showed fine fiber morphology with an average diameter of 245 ± 40 nm. PVA/CS-g-Poly (N-vinyl imidazole)/97%TiO2/150%CUR exhibited fine fiber morphology with average diameter of 319 ± 50 nm | Wound Healing In vitro/In vivo | Heated PVA/CS-g-Poly (N-vinyl imidazole) /97%TiO2/150%CUR formulation avoided burst release and slower drug release characteristics. Superior antibacterial activity against S. aureus (99.9% in 24 h) and E. coli (85% in 24 h) with no toxicity to healthy fibroblasts. PVA/CS-g-Poly (N-vinyl imidazole)/18.5%TiO2/25%CUR formulation showed good mechanical strength and complete wound healing in 14 days. | [120] |
| 5% (w/w) Eugenol (EUG)-incorporated PCL/PVA/CS | W/O and O/W Emulsion W/O emulsion with 5% EUG showed fiber morphology with high bead density. O/W emulsion with 5% EUG showed fewer beads with uniform fiber formation. The average diameters of fibers produced from W/O and O/W were 387.07 ± 179.51 nm and 174.47 ± 38.93 nm, respectively. | Wound healing In vitro | W/O emulsion showed better inhibiting properties against S. aureus (92.43%) and P. aeruginosa (94.68%) as compared to O/W emulsion (83.08% and 87.85%, respectively). O/W exhibited superior in vitro drug release properties. | [121] |
| 0.0%, 1.5 and 2.5% (w/v) Pistacia atlantica oil (PAO) in PVA/sodium alginate (ALG) | Uniaxial Bead-free fine fiber morphology with average diameters of 191 ± 15, 237 ± 18, and 259 ± 10 nm. Fiber diameters increase with %PAO | Wound healing In vitro/In vivo | Mean fiber diameter increased with %PAO. PVA/ALG-1.5% (w/v) PAO showed suitability for wound dressings due to its promising antimicrobial activity and providing moisture to the wound site while allowing oxygen exchange. In vivo study showed 92.07% wound healing. | [122] |
| Bilayer fibrillar scaffold immobilized with epidermal growth factor
(EGF)
PCL as upper layer, CS/PVA as lower layer | Uniaxial Randomly aligned, bead-free, fine fibers with an average diameter of 238.36 ± 36.99 nm for the CS/PVA layer, 1271.79 ± 428.49 nm for the PCL layer | Wound healing In vitro/In vivo | In vitro analysis showed that a bilayer design immobilized with EGF possessed suitable biological properties for wound dressing applications. A 14-day In vitro analysis confirmed that EGF immobilized scaffold promoted wound healing, similar to commercial wound dressing. | [123] |
| 40% (w/w) Ipomoea pes-caprae (IPC) leaf-extract-loaded PVA (10% (w/w)) | Uniaxial Homogeneous and smooth fiber morphology with an average diameter of 100 nm. | Wound healing In vitro | Electrospun hydrogel showed swelling 102 ± 7.45% as compared to conventional hydrogel 68.60 ± 6.72% which is attributed to the greater surface area of electrospun hydrogels. Higher drug loading capacity was observed for electrospun hydrogels. IPC leaf-extract-loaded electrospun hydrogels demonstrated desirable antimicrobial activity against S. aureus. | [124] |
| Lysine
(Lys)-loaded PVA IBP-Lys -loaded PVA Lavender oil (LO)-Lys-loaded PVA | Uniaxial Bead-free uniform fiber morphology was observed for all samples. Average diameters were 474.22 ± 144.85 nm, 385.03 ± 108.21 nm, and 487.14 ± 155.81 nm, respectively. | Skin Regeneration Antimicrobial | All the electrospun membranes presented suitable morphological, mechanical, physiochemical, and biological properties to be used as wound dressings. The LO incorporation on PVA_Lys membranes mediated a strong antibacterial effect against both S. aureus and P. aeruginosa. | [125] |
| Formulations | Electrospinning Type and Morphology | DDS type | Conclusive Remarks | Ref |
|---|---|---|---|---|
| Platelet-rich plasma
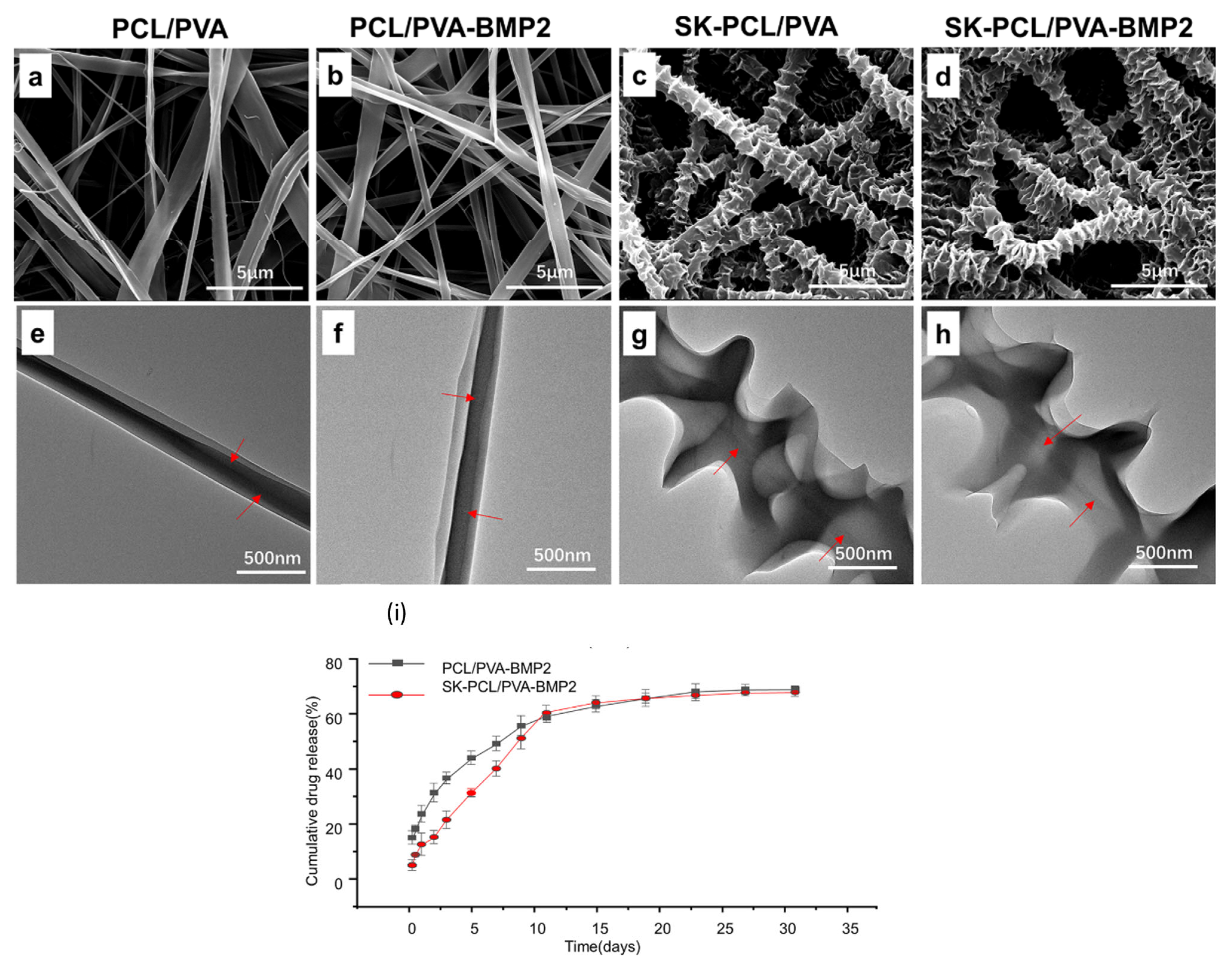
(PRP)-incorporated SF/PCL/PVA PRP:PVA (10:0, 9:1, 8:2, and 7:3) for the core | Coaxial SF/PCL/ (PRP-PVA)7:3 nanofibrous scaffolds showed uniform morphology among all formulations, with an average diameter of 385.9 ± 84.6 nm. | Bone Tissue Engineering In vitro/In vivo | The PRP-derived growth factors, released from the SF/PCL/(PRP-PVA)7:3 scaffolds, exhibited sustained release for nearly 30 days and positively influenced the proliferation, migration, and osteogenesis of BMSCs in vitro and in vivo. | [154] |
| Tri-layer fibers Layer I: PCL Layer II: PCL/Cellulose acetate (CA)-loaded with 5 wt% beta-tri calcium phosphate (β-tcp) Layer III: PVA/Poly(vinyl Acetate) (PVAc)-loaded with 5 wt% simvastatin (SIM) | Uniaxial Fine fiber morphology was observed for all layers with average diameters of 736.052 nm (Layer I), 668.28 nm (Layer II), and 281.14 nm (Layer III). | Bone Tissue Regeneration In vitro | The fabricated ECM mimicking composite nanofibers loaded with β-tcp, SIM shows excellent bioactivity inducing precipitation of bone-like apatite minerals on its surface under simulated physiological conditions. In vitro cell culture test revealed that the incorporation of β-tcp and SIM into the composite nanofiber enhanced osteoblast cell adhesion and proliferation than the control fiber. The characteristics depicted the potential of the Tri-layered fibrous structures in bone regeneration. | [155] |
| Doxorubicin (DOX)-loaded PVA/poly(butylene carbonate)(PBC) | Coaxial DOX + PVA as core, PBC as shell Lower and equal feed rate ratios for PVA/PBC (1:1.3, 1:1) showed non-uniformity in fiber diameter. Fine morphology was observed for feed rate 1:0.7 with an average diameter of 42 nm. | Chemotherapy/Tissue engineering In vitro | In vitro analysis revealed that DOX-loaded core–shell PVA/PBC nanofibers were effective in prohibiting SKOV3 ovary cell attachment and proliferation. The prepared fibers were degraded in a physiological environment | [156] |
| Eumelanin nanoparticles (EUNp)/PVA | Uniaxial Bead-free fine fiber morphology was observed with an average fiber diameter of 161.40 ± 8.86 nm. | Skeletal Muscle Tissue Engineering In vitro | EUNp/PVA nanofibrous scaffolds exhibit inherent physiochemical characteristics along with high electrical conductivity and structural integrity. The composites promoted guided reorganization of C2C12 myoblasts towards myotube-like structure formation within a week. | [157] |
|
PVA, Bioactive glass (BG)-coated PVA scaffolds | Uniaxial Bead-free and homogeneous morphology for PVA was observed with an average diameter of 286 ± 14 nm. For BG-coated PVA scaffolds homogeneity of the fibers was reduced, however, no bead formation was observed. The average diameter was 318 ± 36 nm. | Bone Regeneration In vitro | BG-coated PVA scaffolds revealed superior mechanical properties as compared to PVA fibers. In vitro, the BG-coated PVA scaffolds showed a better capacity to support the proliferation of osteogenic MC3T3-E1 cells, ALP activity, and mineralization. | [158] |
| Formulations | Electrospinning Type and Morphology | DDS Type | Conclusive Remarks | Ref |
|---|---|---|---|---|
| Gold nanoparticle (AuNP)-loaded PVA CUR-loaded PCL | Uniaxial Uniform bead-free fiber morphology for both formulations was observed with diameters in the range of 300 nm, and 600–800 nm, respectively. | Skin Cancer In vitro | The anticancer activity on skin cancer cell lines by the preliminary in vitro assay of the drug was confirmed. The cell line studies revealed that the treatment of nanofibers in cancer cells exhibited more cytotoxicity than in the normal cells where similar concentrations were used thereby proving selective toxicity. | [159] |
| Doxorubicin hydrochloride (DOX)-loaded Polyhydroxyalkanoate (PHA)/PVA | Uniaxial for DOX-loaded PVA(1:100) fibers. Spin coating for PHA on DOX-loaded PVA fibers to obtain porous membrane. | Chemotherapy for Colon cancer In vitro | The composite membranes had an outstanding pH sensitivity for the DOX release, which was desirable for clinical applications. The Caco-2 cells were almost apoptotic after being cultured for 6 days. The results suggested a high potential of the prepared membranes treating colonic carcinoma. | [160] |
|
Tri layered nanofibers Layer I: 5-fluorouracil (5-FU)loaded PCL Layer II: 5-FU-loaded PVA/methyl cellulose(MC) Layer III: 5-FU-loaded PCL | Uniaxial Bead-free fiber formation was observed for drug-loaded layers with an average diameter of 258.6 nm | Skin Cancer In vitro | Controlled drug release was obtained by incorporating 5-FU into multilayered nanofibers. The prepared formulation revealed regulated drug release’s greater capacity to prevent negative side effects, which is a characteristic of anticancer drugs. Moreover, the multilayer structure allows for straightforward but efficient dosage modifications. | [161] |
| Uniaxial for composite fibers Coaxial for core–shell fibers The bead-free morphology was observed with a mean fiber diameter of 225 nm at optimized process parameters for composite fibers. The increase in shell feed rate (0.3 mL/h to 0.7 mL/h) increases the fiber diameter from 330 nm to 640 nm at 20 kV. | Lung Cancer In vitro/In vivo | The higher DEE (drug entrapment efficiency) than 95% for PTX and CMPT confirmed an effective loading of anticancer drugs into the nanofibers. The maximum cytotoxicity was 75% in the presence of PVA/k-carrageenan/CMPT/Au/pegylated-PU/PTX core–shell nanofiber. In vivo release studies indicated that in rats fed with core–shell nanofibers, the blood concentration of CMPT and PTX reached the highest values of 26.8 ± 0.04 µg/mL and 26.5 ± 0.05 µg/mL in 36 h and 24 h and kept in the constant values between 36 and 84 h, and 24 and 48 and finally reduced after 84 h and 48 h, respectively. In vivo antitumor efficacy results of A549 tumor-bearing mice treated with composite and core–shell nanofibers demonstrated the best effect on the reduction in tumor volume and enhancement in tumor inhibition | [162] |
| Doxorubicin (DOX)-loaded N-carboxymethyl CS (N-CMCS)-PVA/PCL composite and core–shell fibers | Uniaxial for composite fibers Coaxial for core–shell fibers Beaded fiber morphology was observed for composite fibers. Bead-free morphology was observed for core–shell fibers with an average diameter of 410 nm. | Breast Cancer In vitro | DEE for core–shell nanofibers was found to be higher than composite fibers. Initial burst release of DOX was observed for composite for 11 days and 3 days, and physiological and acidic pH. Sustained drug release for core–shell fibers without initial burst release for 20 days and 10 days at pH 5.5 and 7.4, respectively. Core–shell nanofibers exhibited higher drug encapsulation efficiency, sustained release of DOX, lower adsorption capacity, higher cytotoxicity of MCF-7 breast cancer, and high biocompatibility. | [163] |
| (i) Non-aligned fibers; 7% collagen (COL), 10%PVA, 7%PVA/Collagen (COL), 9% PVA/Collagen (COL) (ii) Well-aligned fibers; 7% Collagen (COL), 10%PVA, 7%PVA/COL, 9% PVA/COL | Uniaxial For non-aligned fibers, average diameters were 301.5 ± 74.2, 302 ± 37.9, 211.6± 142.5, and 262.9 ± 199.3 nm For aligned fibers, average diameters were 431.8 ± 72.6,204 ± 55, 183.3 ± 96.7, and 163.1 ± 103.2 nm. Fine morphology for both non-aligned and aligned fibers was observed | Corneal Tissue Engineering In vitro | Human keratocytes (HKs) and human corneal epithelial cells (HCECs) exhibited good adhesion and proliferation when cultured on electrospun scaffolds made of aligned and random PVA/COL nanofibers. The aligned nanofibers promoted organized growth in HKs, suggesting that the designed PVA/COL composite nanofibrous electrospun scaffold holds promise for use in tissue-engineered cornea. | [164] |
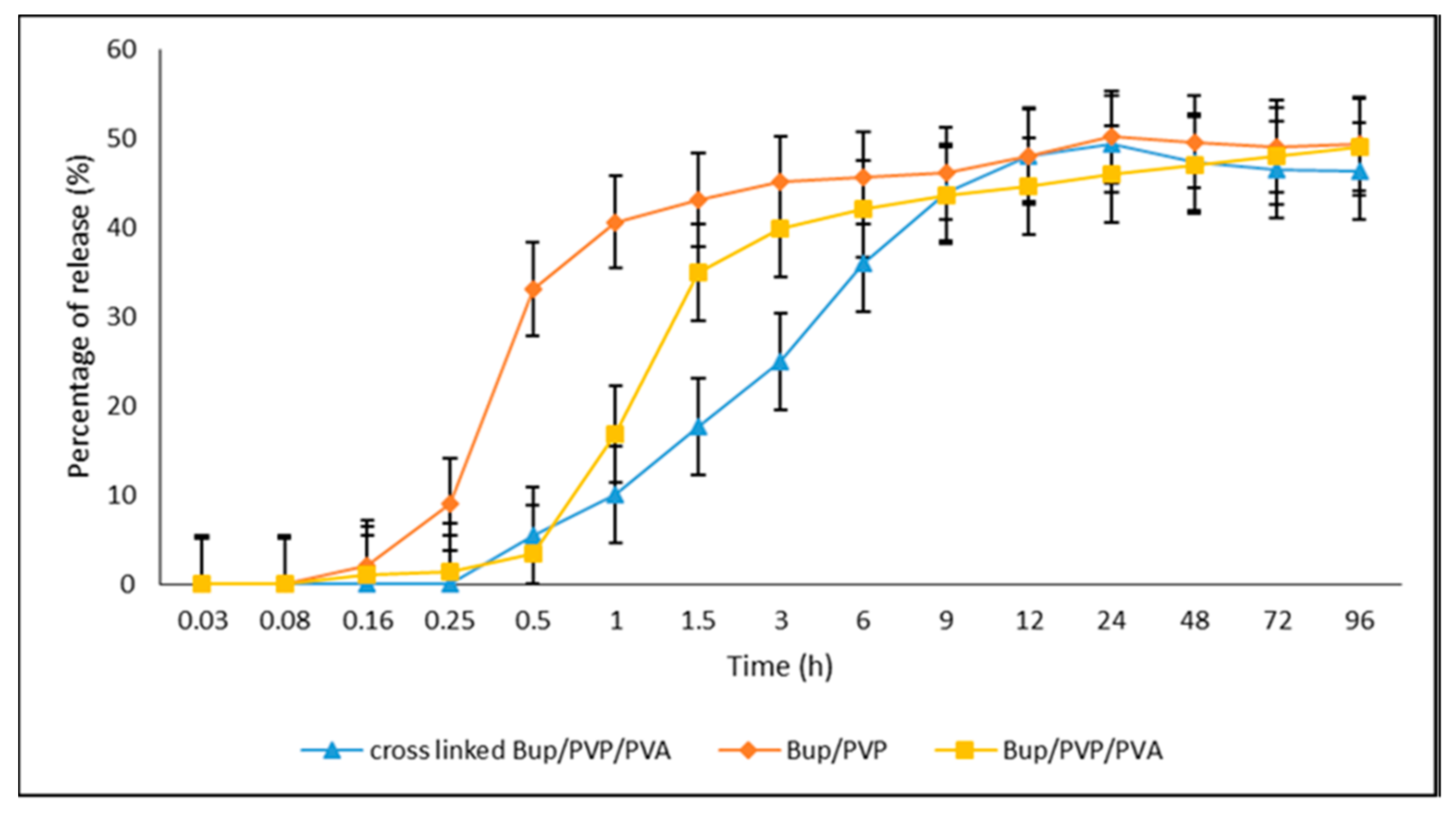
| 8 combinations of Pramipexole (Prami)-loaded PVA/carboxymethylcellulose (CMC) + PCL hybrid fibers GA crosslinking was performed | Uniaxial (Co-electrospinning) Fibers with a diameter smaller than 500 nm for PVA/CMC/Prami fibers, and a diameter larger than 500 nm for PCL nanofibers; the average diameter of fibers in this hybrid nanofibers is 931 ± 618 nm | Oral drug delivery for Parkinson’s Disease In vitro | Nanofiber PCL/PVA/CMC, which underwent a 12-h exposure to GA vapors, exhibited the most favorable release profile among the eight nanofibers tested. This particular formulation demonstrated the longest duration of drug release during the initial 8-h period, while also exhibiting an acceptable level of cytotoxicity. The nanofibers can be concentrated into a new capsule formulation to decrease the amounts of additives used in tablet form and simplify the manufacturing process. | [165] |
| 5%(w/w) Melatonin (MLT)-loaded (5%, 6%, 7 wt% (w/v) PVA/Polyethylene oxide (PEO) | Uniaxial Bead-free fine morphology of drug-loaded fibers (7 wt%) was observed with a diameter in the range of 300–700 nm | Oral Drug Delivery In vitro | The electrospun blend PVA/PEO fibers loaded with MLT showed great compatibility with human umbilical vein endothelial cells (HUVECs) for a period of 24 h, indicating their potential application in topical and/or mucoadhesive drug delivery. | [166] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zahra, F.T.; Quick, Q.; Mu, R. Electrospun PVA Fibers for Drug Delivery: A Review. Polymers 2023, 15, 3837. https://doi.org/10.3390/polym15183837
Zahra FT, Quick Q, Mu R. Electrospun PVA Fibers for Drug Delivery: A Review. Polymers. 2023; 15(18):3837. https://doi.org/10.3390/polym15183837
Chicago/Turabian StyleZahra, Fatima T., Quincy Quick, and Richard Mu. 2023. "Electrospun PVA Fibers for Drug Delivery: A Review" Polymers 15, no. 18: 3837. https://doi.org/10.3390/polym15183837
APA StyleZahra, F. T., Quick, Q., & Mu, R. (2023). Electrospun PVA Fibers for Drug Delivery: A Review. Polymers, 15(18), 3837. https://doi.org/10.3390/polym15183837






