Influence of Light Conditions on the Antibacterial Performance and Mechanism of Waterborne Fluorescent Coatings Based on Waterproof Long Afterglow Phosphors/PDMS Composites
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Preparation of WLAP/PDMS Composite Coatings
2.3. Characterization
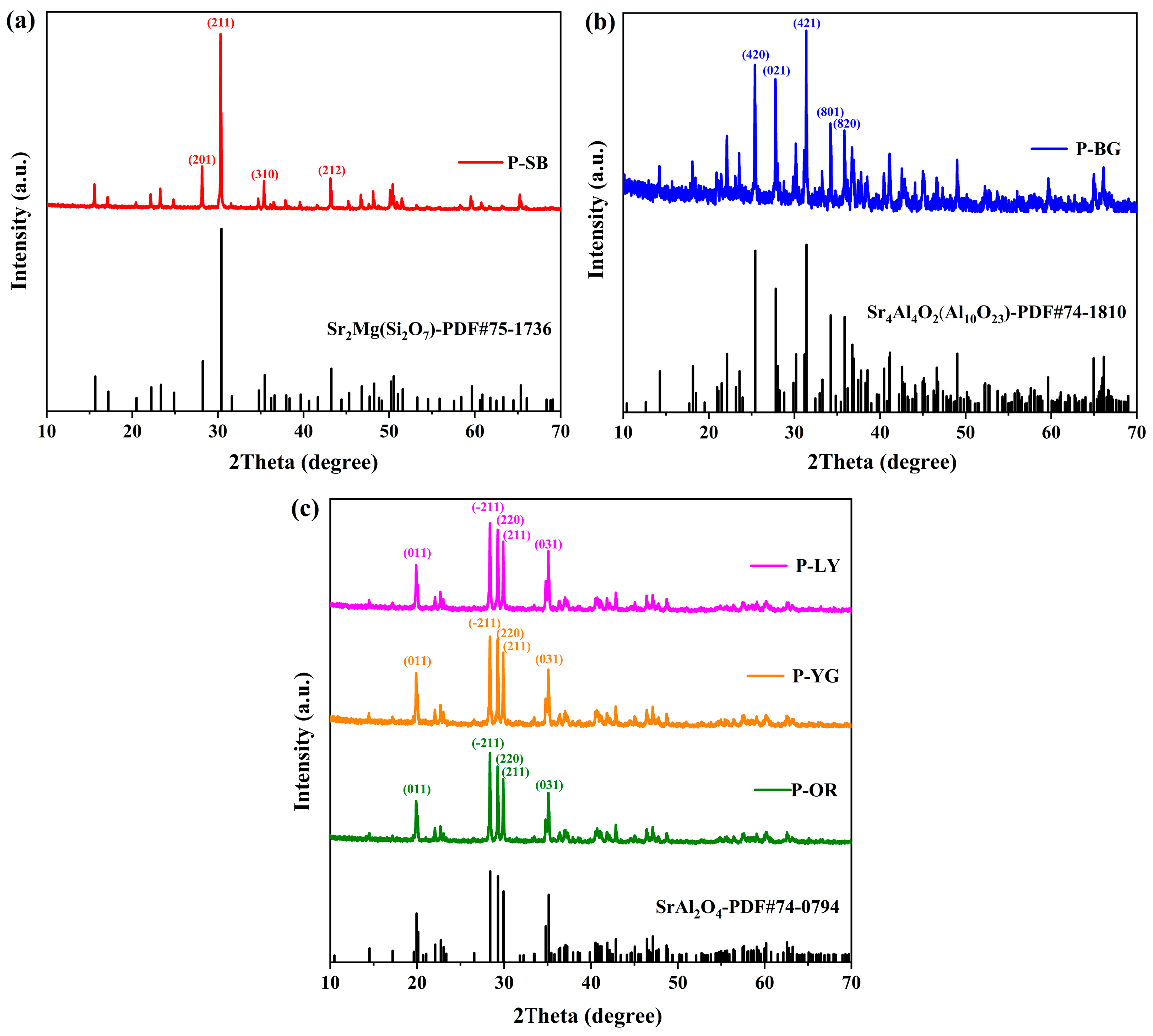
2.3.1. Crystal Structure
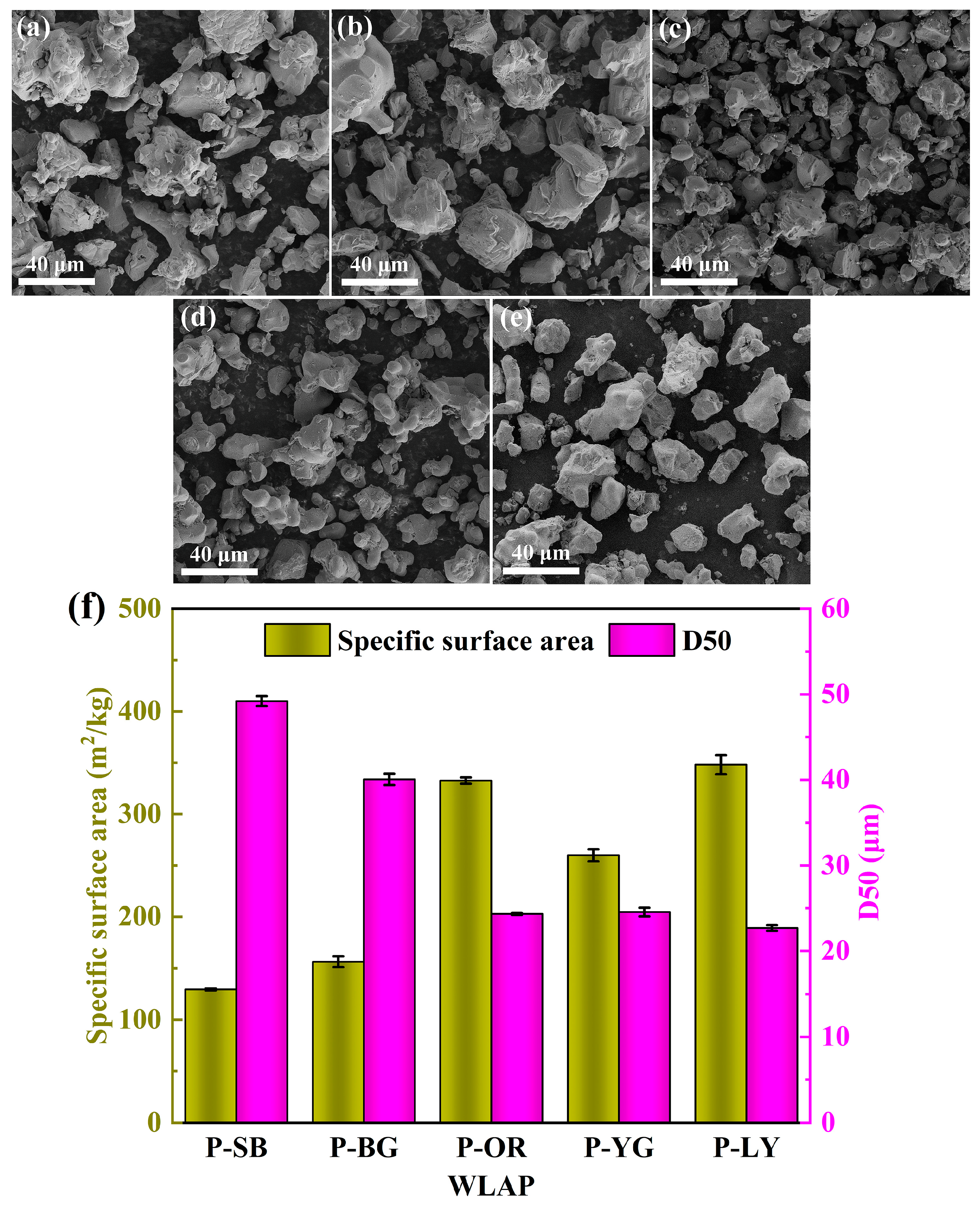
2.3.2. Surface Morphology, Chemical Composition and Roughness
2.3.3. Laser Particle Size Analysis
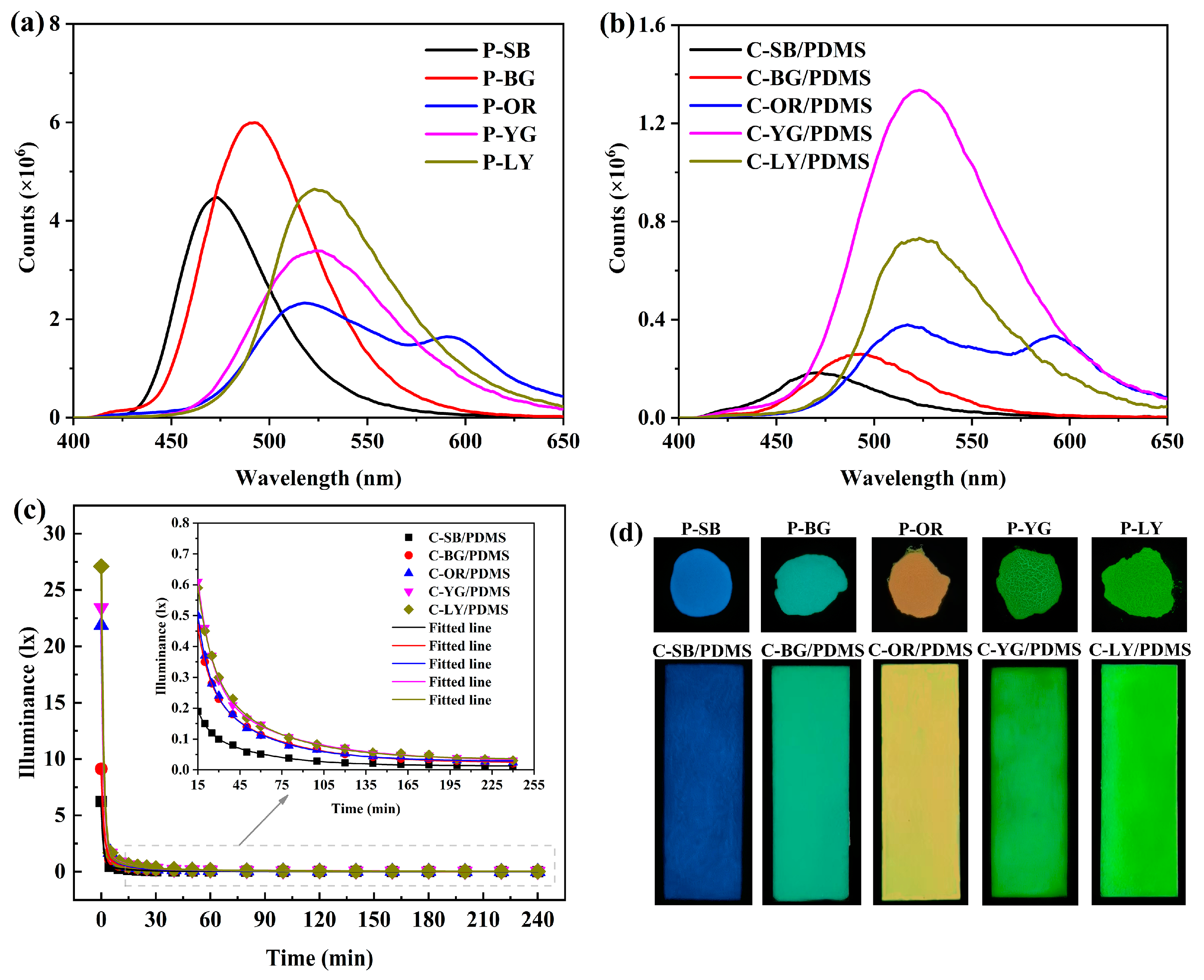
2.3.4. Luminescence Test
2.3.5. Contact Angle and SFE
2.3.6. Tensile Test
2.3.7. Evaluation of Antibacterial Effect
3. Results and Discussion
3.1. Crystal Phase Composition of WLAP
3.2. Surface Morphology and Particle Size
3.3. Surface Performance of Coatings
3.4. Mechanical Properties of Coatings
3.5. Luminescence Performance
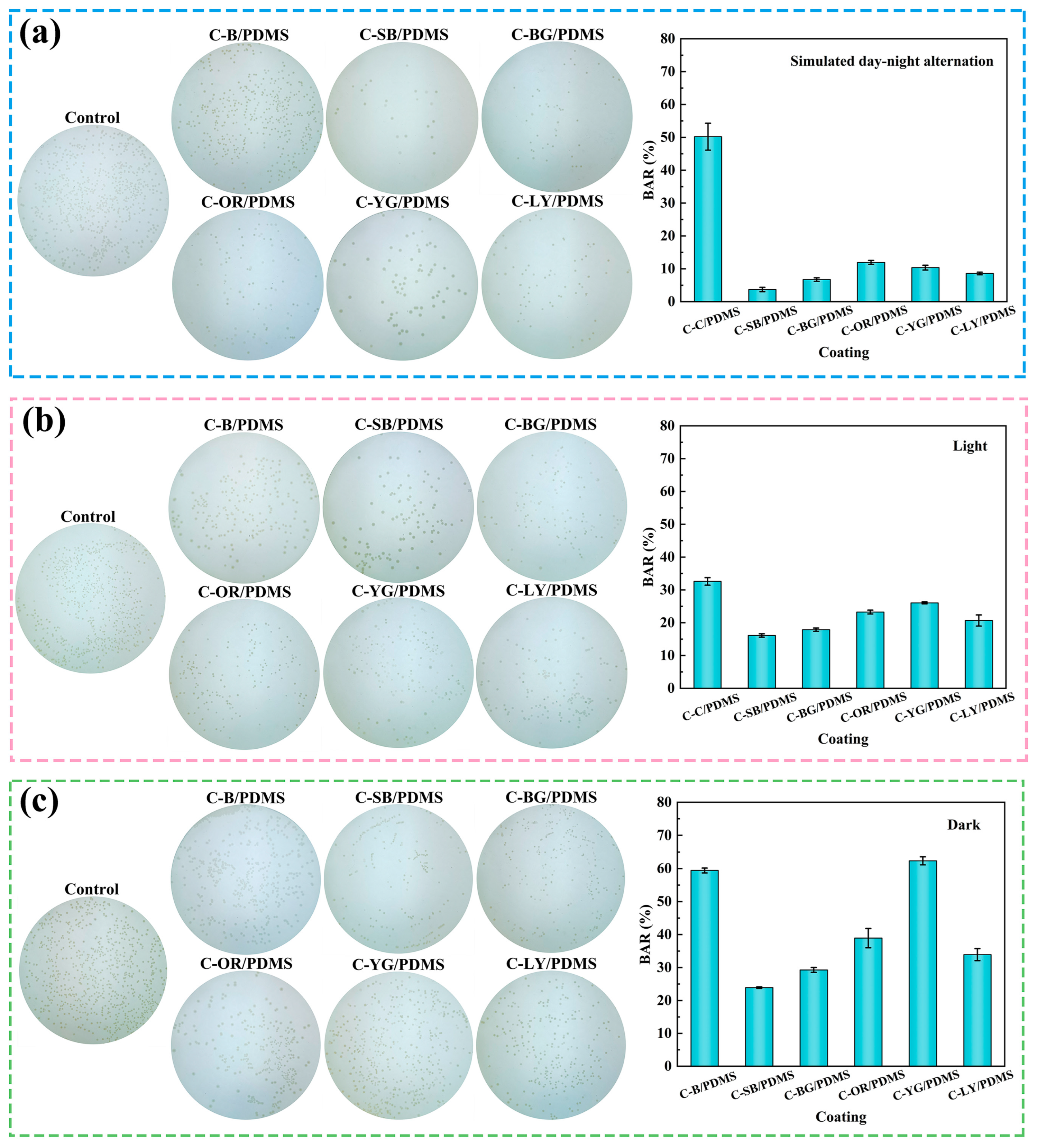
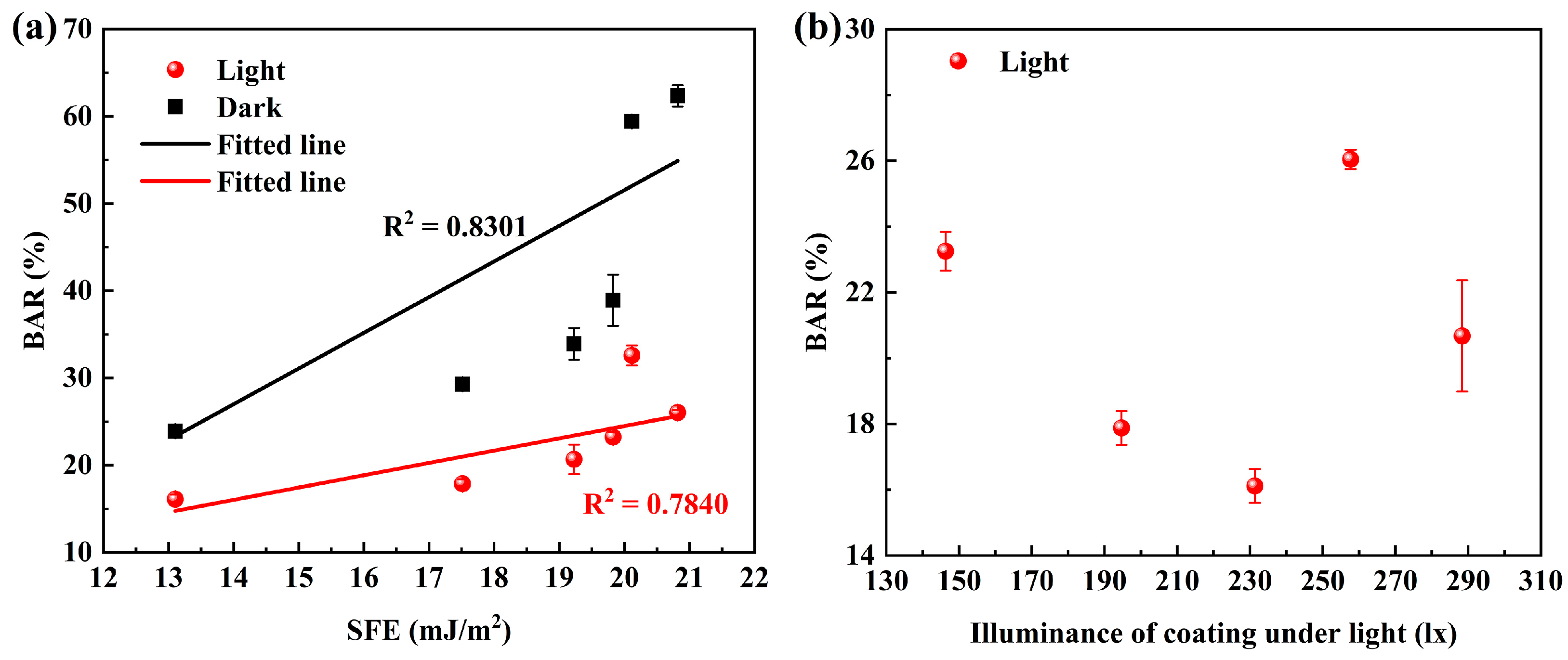
3.6. Antibacterial Performance
3.7. Analysis of Antibacterial Mechanism
3.7.1. Simulated Day-Night Alternation
3.7.2. Continuous Light or Darkness
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Conflicts of Interest
Abbreviation
| BAR | Bacterial adhesion rate |
| CLSM | Confocal laser scanning microscope |
| C-B/PDMS | PDMS control coating with no WLAP |
| C-BG/PDMS | Blue-green WLAP/PDMS composite coating |
| C-LY/PDMS | Lemon-yellow WLAP/PDMS composite coating |
| C-OR/PDMS | Orange-red WLAP/PDMS composite coating |
| C-SB/PDMS | Sky-blue WLAP/PDMS composite coating |
| C-YG/PDMS | Yellow-green WLAP/PDMS composite coating |
| DBTDL | Dibutyltin dilaurate |
| EPS | Extracellular polymeric substances |
| HT-SOE | Hydroxy-terminated silicone oil emulsion |
| FWHM | Full width at half maxima |
| PDMS | Polydimethylsiloxane |
| PL | Fluorescence emission spectra |
| PTFE | Polytetrafluoroethylene |
| P-BG | Blue-green WLAP |
| P-LY | Lemon-yellow WLAP |
| P-OR | Orange-red WLAP |
| P-SB | Sky-blue WLAP |
| P-YG | Yellow-green WLAP |
| REAP | Rare earth aluminate phosphor |
| ROS | Reactive oxygen species |
| SEM | Scanning electron microscope |
| SLIPS | Slippery liquid-infused porous surface |
| SSA | Specific surface area |
| TEOS | Tetraethyl orthosilicate |
| VOCs | Volatile organic compounds |
| WCA | Water contact angle |
| WLAP | Waterproof long afterglow phosphor |
| XRD | X-ray diffractometer |
References
- Jin, H.; Tian, L.; Bing, W.; Zhao, J.; Ren, L. Bioinspired marine antifouling coatings: Status, prospects, and future. Prog. Mater. Sci. 2022, 124, 100889. [Google Scholar] [CrossRef]
- Nurioglu, A.G.; Esteves, A.C.C.; de With, G. Non-toxic, non-biocide-release antifouling coatings based on molecular structure design for marine applications. J. Mater. Chem. B 2015, 32, 6547–6570. [Google Scholar] [CrossRef] [PubMed]
- Pan, J.; Ai, X.; Ma, C.; Zhang, G. Degradable vinyl polymers for combating marine biofouling. Acc. Chem. Res. 2022, 55, 1586–1598. [Google Scholar] [CrossRef] [PubMed]
- Hu, P.; Xie, Q.; Ma, C.; Zhang, G. Silicone-based fouling-release coatings for marine antifouling. Langmuir 2020, 36, 2170–2183. [Google Scholar] [CrossRef] [PubMed]
- Lejars, M.; Margaillan, A.; Bressy, C. Fouling release coatings: A nontoxic alternative to biocidal antifouling coatings. Chem. Rev. 2012, 112, 4347–4390. [Google Scholar] [CrossRef]
- Davidson, I.; Scianni, C.; Hewitt, C.; Everett, R.; Holm, E.; Tamburri, M.; Ruiz, G. Mini-review: Assessing the drivers of ship biofouling management--aligning industry and biosecurity goals. Biofouling 2016, 32, 411–428. [Google Scholar] [CrossRef]
- Qiu, H.; Feng, K.; Gapeeva, A.; Meurisch, K.; Kaps, S.; Li, X.; Yu, L.; Mishra, Y.K.; Adelung, R.; Baum, M. Functional polymer materials for modern marine biofouling control. Prog. Polym. Sci. 2022, 127, 101516. [Google Scholar] [CrossRef]
- Buskens, P.; Wouters, M.; Rentrop, C.; Vroon, Z. A brief review of environmentally benign antifouling and foul-release coatings for marine applications. J. Coat. Technol. Res. 2012, 10, 29–36. [Google Scholar] [CrossRef]
- Yebra, D.M.; Kiil, S.; Dam-Johansen, K. Antifouling technology-past, present and future steps towards efficient and environmentally friendly antifouling coatings. Prog. Org. Coat. 2004, 50, 75–104. [Google Scholar] [CrossRef]
- Jensen, P.R.; Fenical, W. Strategies for the discovery of secondary metabolites from marine bacteria: Ecological perspectives. Annu. Rev. Microbiol. 1994, 48, 559–584. [Google Scholar] [CrossRef] [PubMed]
- Stincone, P.; Brandelli, A. Marine bacteria as source of antimicrobial compounds. Crit. Rev. Biotechnol. 2020, 40, 306–319. [Google Scholar] [CrossRef]
- Ba, M.; Zhang, Z.; Qi, Y. Fouling release coatings based on polydimethylsiloxane with the incorporation of phenylmethylsilicone oil. Coatings 2018, 8, 153. [Google Scholar] [CrossRef]
- Galhenage, T.P.; Hoffman, D.; Silbert, S.D.; Stafslien, S.J.; Daniels, J.; Miljkovic, T.; Finlay, J.A.; Franco, S.C.; Clare, A.S.; Nedved, B.T.; et al. Fouling-release performance of silicone oil-modified siloxane-polyurethane coatings. ACS Appl. Mater. Interfaces 2016, 8, 29025–29036. [Google Scholar] [CrossRef] [PubMed]
- Chen, L.; Duan, Y.; Cui, M.; Huang, R.; Su, R.; Qi, W.; He, Z. Biomimetic surface coatings for marine antifouling: Natural antifoulants, synthetic polymers and surface microtopography. Sci. Total Environ. 2021, 766, 144469. [Google Scholar] [CrossRef] [PubMed]
- Saha, M.; Goecke, F.; Bhadury, P. Minireview: Algal natural compounds and extracts as antifoulants. J. Appl. Phycol. 2018, 30, 1859–1874. [Google Scholar] [CrossRef] [PubMed]
- Tong, Z.; Song, L.; Chen, S.; Hu, J.; Hou, Y.; Liu, Q.; Ren, Y.; Zhan, X.; Zhang, Q. Hagfish-inspired smart SLIPS marine antifouling coating based on supramolecular: Lubrication modes responsively switching and self-healing properties. Adv. Funct. Mater. 2022, 32, 2201290. [Google Scholar] [CrossRef]
- Wong, T.-S.; Kang, S.H.; Tang, S.K.Y.; Smythe, E.J.; Hatton, B.D.; Grinthal, A.; Aizenberg, J. Bioinspired self-repairing slippery surfaces with pressure-stable omniphobicity. Nature 2011, 477, 443–447. [Google Scholar] [CrossRef]
- Soliman, Y.A.A.; Brahim, A.M.; Moustafa, A.H.; Hamed, M.A.F. Antifouling evaluation of extracts from Red Sea soft corals against primary biofilm and biofouling. Asian Pac. J. Trop. Biomed. 2017, 7, 991–997. [Google Scholar] [CrossRef]
- Wang, J.; Su, P.; Gu, Q.; Li, W.D.; Guo, J.L.; Qiao, W.; Feng, D.Q.; Tang, S.A. Antifouling activity against bryozoan and barnacle by cembrane diterpenes from the soft coral Sinularia flexibilis. Int. Biodeter. Biodegr. 2017, 120, 97–103. [Google Scholar] [CrossRef]
- Zhang, J.; Tang, X.; Han, X.; Feng, D.; Luo, X.; van Ofwegen, L.; Li, P.; Li, G. Sarcoglaucins A-I, new antifouling cembrane-type diterpenes from the South China Sea soft coral Sarcophyton glaucum. Inorg. Chem. Front. 2019, 6, 2004–2013. [Google Scholar] [CrossRef]
- Salih, A.; Larkum, A.; Cox, G.; Kuhl, M.; Hoegh-Guldberg, O. Fluorescent pigments in corals are photoprotective. Nature 2000, 408, 850–853. [Google Scholar] [CrossRef] [PubMed]
- Cao, S.; Wang, J.; Zhang, Y.; Chen, D. The effectiveness of an antifouling compound coating based on a silicone elastomer and colored phosphor powder against Navicula species diatom. J. Coat. Technol. Res. 2012, 10, 397–406. [Google Scholar] [CrossRef]
- Guo, H.; Song, L.; Hu, J.; Lin, T.; Li, X.; Yu, H.; Cheng, D.; Hou, Y.; Zhan, X.; Zhang, Q. Enhanced antifouling strategy with a strong synergistic effect of fluorescent antifouling and contact bacteriostasis using 7-amino-4-methylcoumarin. Chem. Eng. J. 2021, 420, 127676. [Google Scholar] [CrossRef]
- Kim, Y.K.; Sharker, S.M.; In, I.; Park, S.Y. Surface coated fluorescent carbon nanoparticles/TiO2 as visible-light sensitive photocatalytic complexes for antifouling activity. Carbon 2016, 103, 412–420. [Google Scholar] [CrossRef]
- Yan, D.; Wu, X.; Pei, J.; Wu, C.; Wang, X.; Zhao, H. Construction of g-C3N4/TiO2/Ag composites with enhanced visible-light photocatalytic activity and antibacterial properties. Ceram. Int. 2020, 46, 696–702. [Google Scholar] [CrossRef]
- Zhang, N.; Qiao, S.; Wu, H.; Fakhri, A.; Gupta, V.K. Sustainable nano-composites polyglutamic acid functionalized Ag/g-C3N4/SiC for the ultrasensitive colorimetric assay, visible light irradiated photocatalysis and antibacterial efficiency. Opt. Mater. 2021, 120, 111452. [Google Scholar] [CrossRef]
- Thejo Kalyani, N.; Jain, A.; Dhoble, S.J. Persistent phosphors for luminous paints: A review. Luminescence 2022, 37, 524–542. [Google Scholar] [CrossRef]
- Capone, A.; Digaetano, T.; Grimaldi, A.; Habel, R.; Lo Presti, D.; Migneco, E.; Masullo, R.; Moro, F.; Petruccetti, M.; Petta, C.; et al. Measurements of light transmission in deep sea with the AC9 trasmissometer. Nucl. Instrum. Methods Phys. Res. Sect. A Accel. Spectrom. Detect. Assoc. Equip. 2022, 487, 423–434. [Google Scholar] [CrossRef]
- Jin, H.; Bing, W.; Jin, E.; Tian, L.; Jiang, Y. Bioinspired PDMS–phosphor–silicone rubber sandwich-structure coatings for combating biofouling. Adv. Mater. Interfaces 2020, 7, 1901577. [Google Scholar] [CrossRef]
- Faccini, M.; Bautista, L.; Soldi, L.; Escobar, A.M.; Altavilla, M.; Calvet, M.; Domènech, A.; Domínguez, E. Environmentally friendly anticorrosive polymeric coatings. Appl. Sci. Basel 2021, 11, 3446. [Google Scholar] [CrossRef]
- Liu, Z.; Ye, W.; Little, J.C. Predicting emissions of volatile and semivolatile organic compounds from building materials: A review. Build. Environ. 2013, 64, 7–25. [Google Scholar] [CrossRef]
- Owens, D.K.; Wendt, R.C. Estimation of the surface free energy of polymers. J. Appl. Polym. Sci. 1969, 13, 1741–1747. [Google Scholar] [CrossRef]
- Cheng, X.; Ye, Y.; Li, Z.; Chen, X.; Bai, Q.; Wang, K.; Zhang, Y.; Drioli, E.; Ma, J. Constructing environmental-friendly “Oil-diode” janus membrane for oil/water separation. ACS Nano 2022, 16, 4684–4692. [Google Scholar] [CrossRef]
- Chen, R.; Xie, Q.; Zeng, H.; Ma, C.; Zhang, G. Non-elastic glassy coating with fouling release and resistance abilities. J. Mater. Chem. A 2020, 8, 380–387. [Google Scholar] [CrossRef]
- Brady, R.F.; Singer, I.L. Mechanical factors favoring release from fouling release coatings. Biofouling 2000, 15, 73–81. [Google Scholar] [CrossRef] [PubMed]
- Liu, C.; Ma, C.; Xie, Q.; Zhang, G. Self-repairing silicone coatings for marine anti-biofouling. J. Mater. Chem. A 2017, 5, 15855–15861. [Google Scholar] [CrossRef]
- Casillo, A.; Lanzetta, R.; Parrilli, M.; Corsaro, M.M. Exopolysaccharides from marine and marine extremophilic bacteria: Structures, properties, ecological roles and applications. Mar. Drugs 2018, 16, 69. [Google Scholar] [CrossRef]
- Jin, J.; Wu, G.; Zhang, Z.; Guan, Y. Effect of extracellular polymeric substances on corrosion of cast iron in the reclaimed wastewater. Bioresour. Technol. 2014, 165, 162–165. [Google Scholar] [CrossRef] [PubMed]
- Chebath-Taub, D.; Steinberg, D.; Featherstone, J.D.; Feuerstein, O. Influence of blue light on Streptococcus mutans re-organization in biofilm. J. Photochem. Photobiol. B Biol. 2022, 116, 75–78. [Google Scholar] [CrossRef]
- Sousa, N.T.; Santos, M.F.; Gomes, R.C.; Brandino, H.E.; Martinez, R.; de Jesus Guirro, R.R. Blue laser inhibits bacterial growth of Staphylococcus aureus, Escherichia coli, and Pseudomonas aeruginosa. Photomed. Laser Surg. 2015, 33, 278–282. [Google Scholar] [CrossRef] [PubMed]
- Higuchi-Takeuchi, M.; Numata, K. Marine purple photosynthetic bacteria as sustainable microbial Production Hosts. Front. Bioeng. Biotechnol. 2019, 7, 258. [Google Scholar] [CrossRef] [PubMed]
- Stojiljkovic, I.; Evavold, B.D.; Kumar, V. Antimicrobial properties of porphyrins. Expert Opin. Investig. Drugs 2001, 10, 309–320. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Yang, X.; Song, H.; Liao, W.; Zhuo, L.; Wang, G.; Wei, H.; Yang, Y.; Luo, S.; Zhou, Z. Visible light-induced biocidal activities and mechanistic study of neutral porphyrin derivatives against S. aureus and E. coli. J. Photochem. Photobiol. B Biol. 2018, 185, 199–205. [Google Scholar] [CrossRef] [PubMed]
- Hoenes, K.; Wenzel, U.; Spellerberg, B.; Hessling, M. Photoinactivation sensitivity of Staphylococcus carnosus to visible-light irradiation as a function of wavelength. Photochem. Photobiol. 2020, 96, 156–169. [Google Scholar] [CrossRef]
- Lipovsky, A.; Nitzan, Y.; Gedanken, A.; Lubart, R. Visible light-induced killing of bacteria as a function of wavelength: Implication for wound healing. Lasers Surg. Med. 2010, 42, 467–472. [Google Scholar] [CrossRef]
- Plavskii, V.Y.; Mikulich, A.V.; Tretyakova, A.I.; Leusenka, I.A.; Plavskaya, L.G.; Kazyuchits, O.A.; Dobysh, I.I.; Krasnenkova, T.P. Porphyrins and flavins as endogenous acceptors of optical radiation of blue spectral region determining photoinactivation of microbial cells. J. Photochem. Photobiol. B Biol. 2018, 183, 172–183. [Google Scholar] [CrossRef]



Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hao, S.; Qi, Y.; Zhang, Z. Influence of Light Conditions on the Antibacterial Performance and Mechanism of Waterborne Fluorescent Coatings Based on Waterproof Long Afterglow Phosphors/PDMS Composites. Polymers 2023, 15, 3873. https://doi.org/10.3390/polym15193873
Hao S, Qi Y, Zhang Z. Influence of Light Conditions on the Antibacterial Performance and Mechanism of Waterborne Fluorescent Coatings Based on Waterproof Long Afterglow Phosphors/PDMS Composites. Polymers. 2023; 15(19):3873. https://doi.org/10.3390/polym15193873
Chicago/Turabian StyleHao, Sinan, Yuhong Qi, and Zhanping Zhang. 2023. "Influence of Light Conditions on the Antibacterial Performance and Mechanism of Waterborne Fluorescent Coatings Based on Waterproof Long Afterglow Phosphors/PDMS Composites" Polymers 15, no. 19: 3873. https://doi.org/10.3390/polym15193873
APA StyleHao, S., Qi, Y., & Zhang, Z. (2023). Influence of Light Conditions on the Antibacterial Performance and Mechanism of Waterborne Fluorescent Coatings Based on Waterproof Long Afterglow Phosphors/PDMS Composites. Polymers, 15(19), 3873. https://doi.org/10.3390/polym15193873





