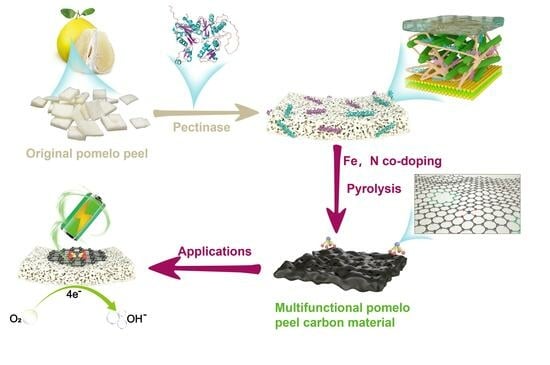
Pyrolysis Enzymolysis-Treated Pomelo Peel: Porous Carbon Materials with Fe−Nx Sites for High-Performance Supercapacitor and Efficient Oxygen Reduction Applications
Abstract
:1. Introduction
2. Experimental Section
2.1. Materials
2.2. Preparation of Carbon Precursors via Enzymatic Treatment of Pomelo Peel
2.3. Preparation of Hierarchical Porous Fe, N−Doped Carbon Materials
2.4. Electrochemical Measurement
3. Results and Discussion
3.1. Transformation of Pomelo Peel into PPE−FeNPC
3.2. Structural Characterization and Analysis
3.2.1. Morphological Characterization
3.2.2. Structure Analysis
3.3. Electrochemical Performance of Supercapacitors
3.4. Electrocatalytic Performance for Oxygen Reduction Reaction
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Conflicts of Interest
References
- Iqbal, M.Z.; Faisal, M.M.; Ali, S.R. Integration of supercapacitors and batteries towards high-performance hybrid energy storage devices. Int. J. Energy Res. 2021, 45, 1449–1479. [Google Scholar] [CrossRef]
- Qu, G.M.; Sun, P.X.; Xiang, G.T.; Yin, J.M.; Wei, Q.; Wang, C.G.; Xu, X.J. Moss-like nickel-cobalt phosphide nanostructures for highly flexible all-solid-state hybrid supercapacitors with excellent electrochemical performances. Appl. Mater. Today 2020, 20, 100713. [Google Scholar] [CrossRef]
- Xu, Z.H.; Sun, S.S.; Han, Y.; Wei, Z.P.; Cheng, Y.H.; Yin, S.G.; Cui, W. High-Energy-Density Asymmetric Supercapacitor Based on a Durable and Stable Manganese Molybdate Nanostructure Electrode for Energy Storage Systems. ACS Appl. Energy Mater. 2020, 3, 5393–5404. [Google Scholar] [CrossRef]
- Zhang, J.; Chen, J.W.; Luo, Y.; Chen, Y.H.; Zhang, C.Y.; Luo, Y.J.; Xue, Y.L.; Liu, H.G.; Wang, G.; Wang, R.L. EX Engineering heterointerfaces coupled with oxygen vacancies in lanthanum-based hollow microspheres for synergistically enhanced oxygen electrocatalysis. J. Energy Chem. 2021, 60, 503–511. [Google Scholar] [CrossRef]
- Yan, J.C.; Tang, Z.M.; Li, B.X.; Bi, D.; Lai, Q.X.; Liang, Y.Y. In Situ ZnO-Activated Hierarchical Porous Carbon Nanofibers as Self-Standing Electrodes for Flexible Zn-Air Batteries. ACS Sustain. Chem. Eng. 2019, 7, 17817–17824. [Google Scholar]
- Wang, D.; Li, B.; Tao, X.F.; Rao, S.S.; Li, J.H.; Wang, W.L.; Yang, J.; Zhou, Y.Z. Atomically dispersed iron atoms on nitrogen-doped porous carbon catalyst with high density and accessibility for oxygen reduction. J. Electroanal. Chem. 2021, 898, 115627. [Google Scholar] [CrossRef]
- Dong, Q.; Wang, H.; Ji, S.; Wang, X.Y.; Liu, Q.B.; Brett, D.J.L.; Linkov, V.; Wang, R.F. Mn Nanoparticles Encapsulated within Mesoporous Helical N-Doped Carbon Nanotubes as Highly Active Air Cathode for Zinc-Air Batteries. Adv. Sustain. Syst. 2019, 3, 1900085. [Google Scholar] [CrossRef]
- Jin, H.H.; Kou, Z.K.; Cai, W.W.; Zhou, H.; Ji, P.X.; Liu, B.S.; Radwan, A.; He, D.P.; Mu, S.C. P-Fe bond oxygen reduction catalysts toward high-efficiency metal-air batteries and fuel cells. J. Mater. Chem. A 2020, 8, 9121–9127. [Google Scholar] [CrossRef]
- Zaman, S.; Huang, L.; Douka, A.I.; Yang, H.; You, B.; Xia, B.Y. Oxygen Reduction Electrocatalysts toward Practical Fuel Cells: Progress and Perspectives. Angew. Chem.-Int. Ed. 2021, 60, 17832–17852. [Google Scholar] [CrossRef]
- Kundu, A.; Mallick, S.; Ghora, S.; Raj, C.R. Advanced Oxygen Electrocatalyst for Air-Breathing Electrode in Zn-Air Batteries. ACS Appl. Mater. Interfaces 2021, 13, 40172–40199. [Google Scholar] [CrossRef]
- Abdelkareem, M.A.; Wilberforce, T.; Elsaid, K.; Sayed, E.T.; Abdelghani, E.A.M.; Olabi, A.G. Transition metal carbides and nitrides as oxygen reduction reaction catalyst or catalyst support in proton exchange membrane fuel cells (PEMFCs). Int. J. Hydrog. Energy 2021, 46, 23529–23547. [Google Scholar] [CrossRef]
- Jung, S.; Huong, P.T.; Sahani, S.; Tripathi, K.M.; Park, B.J.; Han, Y.H.; Kim, T. Biomass-Derived Graphene-Based Materials Embedded with Onion-Like Carbons for High Power Supercapacitors. J. Electrochem. Soc. 2022, 169, 010509. [Google Scholar] [CrossRef]
- Vijayakumar, M.; Sankar, A.B.; Rohita, D.S.; Rao, T.N.; Karthik, M. Conversion of Biomass Waste into High Performance Supercapacitor Electrodes for Real-Time Supercapacitor Applications. ACS Sustain. Chem. Eng. 2019, 7, 17175–17185. [Google Scholar] [CrossRef]
- Yadav, K.K.; Singh, H.; Rana, S.; Sunaina; Sammi, H.; Nishanthi, S.T.; Wadhwa, R.; Khan, N.; Jha, M. Utilization of waste coir fibre architecture to synthesize porous graphene oxide and their derivatives: An efficient energy storage material. J. Clean. Prod. 2020, 276, 124240. [Google Scholar] [CrossRef]
- Shah, A.; Singh, H.; Prajongtat, P.; Joshi, M.C.; Hannongbua, S.; Chattham, N.; Kim, Y.K.; Kumar, S.; Singh, D.P. Scalable production of reduced graphene oxide via biowaste valorisation: An efficient oxygen reduction reaction towards metal-free electrocatalysis. New J. Chem. 2023, 47, 1360–1370. [Google Scholar] [CrossRef]
- Xiao, L.; Ye, F.Y.; Zhou, Y.; Zhao, G.H. Utilization of pomelo peels to manufacture value-added products: A review. Food Chem. 2021, 351, 129247. [Google Scholar] [PubMed]
- Wang, F.; Cheong, J.Y.; Lee, J.; Ahn, J.; Duan, G.G.; Chen, H.L.; Zhang, Q.; Kim, I.D.; Jiang, S.H. Pyrolysis of Enzymolysis-Treated Wood: Hierarchically Assembled Porous Carbon Electrode for Advanced Energy Storage Devices. Adv. Funct. Mater. 2021, 31, 10. [Google Scholar] [CrossRef]
- Tan, Y.T.; Xu, Z.X.; He, L.J.; Li, H.B. Three-dimensional high graphitic porous biomass carbon from dandelion flower activated by K2FeO4 for supercapacitor electrode. J. Energy Storage 2022, 52, 104889. [Google Scholar] [CrossRef]
- Wang, C.J.; Wu, D.P.; Wang, H.J.; Gao, Z.Y.; Xu, F.; Jiang, K. Biomass derived nitrogen-doped hierarchical porous carbon sheets for supercapacitors with high performance. J. Colloid Interface Sci. 2018, 523, 133–143. [Google Scholar] [CrossRef]
- Yu, M.; Han, Y.Y.; Li, J.; Wang, L.J. CO2-activated porous carbon derived from cattail biomass for removal of malachite green dye and application as supercapacitors. Chem. Eng. J. 2017, 317, 493–502. [Google Scholar] [CrossRef]
- He, G.H.; Yan, G.P.; Song, Y.H.; Wang, L. Biomass Juncus Derived Nitrogen-Doped Porous Carbon Materials for Supercapacitor and Oxygen Reduction Reaction. Front. Chem. 2020, 8, 226. [Google Scholar]
- Kaipannan, S.; Ganesh, P.A.; Manickavasakam, K.; Sundaramoorthy, S.; Govindarajan, K.; Mayavan, S.; Marappan, S. Waste engine oil derived porous carbon/ZnS Nanocomposite as Bi-functional electrocatalyst for supercapacitor and oxygen reduction. J. Energy Storage 2020, 32, 101774. [Google Scholar] [CrossRef]
- Shao, S.; Wang, G.B.; Gong, Z.M.; Wang, M.J.; Hu, J.H.; Peng, J.B.; Lu, K.; Gao, S.X. Insights into the role of hydroxyl group on carboxyl-modified MWCNTs in accelerating atenolol removal by Fe(III)/H2O2 system. Chem. Eng. J. 2021, 425, 130581. [Google Scholar] [CrossRef]
- Hou, J.H.; Cao, C.B.; Idrees, F.; Ma, X.L. Hierarchical Porous Nitrogen-Doped Carbon Nanosheets Derived from Silk for Ultrahigh-Capacity Battery Anodes and Supercapacitors. Acs Nano. 2015, 9, 2556–2564. [Google Scholar]
- Niu, W.H.; Li, L.G.; Liu, X.J.; Wang, N.; Liu, J.; Zhou, W.J.; Tang, Z.H.; Chen, S.W. Mesoporous N-Doped Carbons Prepared with Thermally Removable Nanoparticle Templates: An Efficient Electrocatalyst for Oxygen Reduction Reaction. J. Am. Chem. Soc. 2015, 137, 5555–5562. [Google Scholar] [CrossRef]
- Li, G.F.; Li, Y.W.; Chen, X.F.; Hou, X.Y.; Lin, H.T.; Jia, L.S. One step synthesis of N, P co-doped hierarchical porous carbon nanosheets derived from pomelo peel for high performance supercapacitors. J. Colloid Interface Sci. 2022, 605, 71–81. [Google Scholar] [CrossRef] [PubMed]
- Xue, B.C.; Xu, J.H.; Xiao, R. Ice template-assisting activation strategy to prepare biomass-derived porous carbon cages for high-performance Zn-ion hybrid supercapacitors. Chem. Eng. J. 2023, 454, 140192. [Google Scholar] [CrossRef]
- Han, P.; Cheng, M.S.; Luo, D.H.; Cui, W.; Liu, H.C.; Du, J.G.; Wang, M.L.; Zhao, Y.P.; Chen, L.; Zhu, C.Z.; et al. Selective etching of C-N bonds for preparation of porous carbon with ultrahigh specific surface area and superior capacitive performance. Energy Stor. Mater. 2020, 24, 486–494. [Google Scholar] [CrossRef]
- Ma, D.; Wu, G.; Wan, J.F.; Ma, F.W.; Geng, W.D.; Song, S.J. Oxygen-enriched hierarchical porous carbon derived from biowaste sunflower heads for high-performance supercapacitors. RSC Adv. 2015, 5, 107785–107792. [Google Scholar]
- Wang, D.; Zhou, Q.; Fu, H.; Lian, Y.; Zhang, H. A Fe2(SO4)3 assisted approach towards green synthesis of cuttlefish ink-derived carbon nanospheres for high-performance supercapacitors. J Colloid Interface Sci. 2023, 638, 695–708. [Google Scholar] [CrossRef] [PubMed]
- Gao, S.Y.; Li, X.G.; Li, L.Y.; Wei, X.J. A versatile biomass derived carbon material for oxygen reduction reaction, supercapacitors and oil/water separation. Nano Energy 2017, 33, 334–342. [Google Scholar]
- Lu, S.S.; Yang, W.S.; Zhou, M.; Qiu, L.R.; Tao, B.F.; Zhao, Q.; Wang, X.H.; Zhang, L.; Xie, Q.; Ruan, Y.J. Nitrogen- and oxygen-doped carbon with abundant micropores derived from biomass waste for all-solid-state flexible supercapacitors. J. Colloid Interface Sci. 2022, 610, 1088–1099. [Google Scholar]
- Wu, X.; Dong, J.C.; Qiu, M.; Li, Y.; Zhang, Y.F.; Zhang, H.B.; Zhang, J. Subnanometer iron clusters confined in a porous carbon matrix for highly efficient zinc-air batteries. Nanoscale Horiz. 2020, 5, 359–365. [Google Scholar] [CrossRef]
- Zhao, L.; Zhang, Y.; Huang, L.B.; Liu, X.Z.; Zhang, Q.H.; He, C.; Wu, Z.Y.; Zhang, L.J.; Wu, J.P.; Yang, W.L.; et al. Cascade anchoring strategy for general mass production of high-loading single-atomic metal-nitrogen catalysts. Nat. Commun. 2019, 10, 1278. [Google Scholar] [PubMed]
- Zhou, H.F.; Deng, Z.B.; Liu, T.B.; Liu, T.; Zhang, L.J.; Su, X.T.; Lin, Z. In situ controlled synthesis of porous Fe-N-C materials from oily sludge by chlorinating calcination and their novel application in supercapacitors. Environ. Sci. Nano. 2020, 7, 3814–3823. [Google Scholar]
- Zhou, H.; Wu, S.M.; Wang, H.; Li, Y.H.; Liu, X.Q.; Zhou, Y.M. The preparation of porous carbon materials derived from bio-protic ionic liquid with application in flexible solid-state supercapacitors. J. Hazard. Mater. 2021, 402, 124023. [Google Scholar] [CrossRef]
- Wang, C.; Wang, H.W.; Yang, C.X.; Dang, B.K.; Li, C.C.; Sun, Q.F. A multilevel gradient structural carbon derived from naturally preprocessed biomass. Carbon 2020, 168, 624–632. [Google Scholar] [CrossRef]
- Ran, F.T.; Yang, X.B.; Xu, X.Q.; Li, S.W.; Liu, Y.Y.; Shao, L. Green activation of sustainable resources to synthesize nitrogen-doped oxygen-riched porous carbon nanosheets towards high-performance supercapacitor. Chem. Eng. J. 2021, 412, 128673. [Google Scholar]
- Wang, J.G.; Liu, H.Z.; Zhang, X.Y.; Li, X.; Liu, X.R.; Kang, F.Y. Green Synthesis of Hierarchically Porous Carbon Nanotubes as Advanced Materials for High-Efficient Energy Storage. Small 2018, 14, e1703950. [Google Scholar] [CrossRef]
- Sun, Y.K.; Xu, D.; Wang, S.R. Self-assembly of biomass derivatives into multiple heteroatom-doped 3D-interconnected porous carbon for advanced supercapacitors. Carbon 2022, 199, 258–267. [Google Scholar]
- Chen, B.; Wu, D.; Wang, T.; Yuan, F.; Jia, D. Rapid preparation of porous carbon by flame burning carbonization method for supercapacitor. Chem. Eng. J. 2023, 462, 142163. [Google Scholar] [CrossRef]
- Dong, Y.; Zhang, S.; Du, X.; Hong, S.; Zhao, S.N.; Chen, Y.X.; Chen, X.H.; Song, H.H. Boosting the Electrical Double-Layer Capacitance of Graphene by Self-Doped Defects through Ball-Milling. Adv. Funct. Mater. 2019, 29, 1901127. [Google Scholar] [CrossRef]
- Zhou, J.Q.; Xu, L.R.; Li, L.J.; Li, X. Polytetrafluoroethylene-assisted N/F co-doped hierarchically porous carbon as a high performance electrode for supercapacitors. J. Colloid Interface Sci. 2019, 545, 25–34. [Google Scholar] [CrossRef] [PubMed]
- Fu, F.B.; Yang, D.J.; Zhang, W.L.; Wang, H.; Qiu, X.Q. Green self-assembly synthesis of porous lignin-derived carbon quasi-nanosheets for high-performance supercapacitors. Chem. Eng. J. 2020, 392, 123721. [Google Scholar] [CrossRef]
- Tarimo, D.J.; Mirghni, A.A.; Oyedotun, K.O.; Rutavi, G.; Kitenge, V.N.; Manyala, N. Recycling of biomass wastes from amarula husk by a modified facile economical water salt method for high energy density ultracapacitor application. J. Energy Storage. 2022, 53, 105166. [Google Scholar] [CrossRef]
- Li, D.; Guo, Y.C.A.; Li, Y.; Liu, Z.G.; Chen, Z.L. Waste-biomass tar functionalized carbon spheres with N/P Co-doping and hierarchical pores as sustainable low-cost energy storage materials. Renew. Energ. 2022, 188, 61–69. [Google Scholar]
- Jia, J.X.; Yao, Z.L.; Zhao, L.X.; Xie, T.; Sun, Y.X.; Tian, L.W.; Huo, L.L.; Liu, Z.D. Functionalization of supercapacitors electrodes oriented hydrochar from cornstalk: A new vision via biomass fraction. Biomass Bioenergy. 2023, 175, 106858. [Google Scholar]
- Agrawal, A.; Gaur, A.; Kumar, A. Fabrication of Phyllanthus emblica leaves derived high-performance activated carbon-based symmetric supercapacitor with excellent cyclic stability. J. Energy Storage. 2023, 66, 107395. [Google Scholar] [CrossRef]
- Chen, L.; Xiang, L.Y.; Hu, B.; Zhang, H.; He, G.; Yin, X.; Cao, X. Hierarchical porous carbons with honeycomb-like macrostructure derived from steamed-rice for high performance supercapacitors. Mater. Today Sustainability. 2023, 24, 100480. [Google Scholar] [CrossRef]
- Liu, S.; Yin, Y.; Ni, D.X.; Hui, K.S.; Hui, K.N.; Lee, S.; Ouyang, C.Y.; Jun, S.C. Phosphorous-containing oxygen-deficient cobalt molybdate as an advanced electrode material for supercapacitors. Energy Stor. Mater. 2019, 19, 186–196. [Google Scholar]
- Wang, T.; Guo, J.; Guo, Y.; Feng, J.; Wu, D.L. Nitrogen-Doped Carbon Derived from Deep Eutectic Solvent as a High-Performance Supercapacitor. ACS Appl. Energy Mater. 2021, 4, 2190–2200. [Google Scholar] [CrossRef]
- Hang, X.X.; Xue, Y.D.; Du, M.; Yang, R.; Zhao, J.W.; Pang, H. Controlled synthesis of a cobalt-organic framework: Hierarchical micro/nanospheres for high-performance supercapacitors. Inorg. Chem. Front. 2022, 9, 2845–2851. [Google Scholar]
- Chen, R.W.; Tang, H.; He, P.; Zhang, W.; Dai, Y.H.; Zong, W.; Guo, F.; He, G.J.; Wang, X.H. Interface Engineering of Biomass-Derived Carbon used as Ultrahigh-Energy-Density and Practical Mass-Loading Supercapacitor Electrodes. Adv. Funct. Mater. 2023, 33, 2212078. [Google Scholar] [CrossRef]
- Zhu, W.; Shen, D.; Xie, H. Combination of chemical activation and nitrogen doping toward hierarchical porous carbon from houttuynia cordata for supercapacitors. J. Energy Storage. 2023, 60, 106595. [Google Scholar] [CrossRef]
- Liu, D.; Xu, G.Y.; Yuan, X.Q.; Ding, Y.G.; Fan, B.M. Pore size distribution modulation of waste cotton-derived carbon materials via citrate activator to boost supercapacitive performance. Fuel 2023, 332, 126044. [Google Scholar] [CrossRef]
- Dong, D.; Zhang, Y.S.; Xiao, Y.; Wang, T.; Wang, J.W.; Romero, C.E.; Pan, W.P. High performance aqueous supercapacitor based on nitrogen-doped coal-based activated carbon electrode materials. J. Colloid Interface Sci. 2020, 580, 77–87. [Google Scholar]
- Dong, D.; Zhang, Y.S.; Xiao, Y.; Wang, T.; Wang, J.W.; Gao, W. Oxygen-enriched coal-based porous carbon under plasma-assisted MgCO3 activation as supercapacitor electrodes. Fuel 2022, 309, 122168. [Google Scholar] [CrossRef]
- Jiang, Y.C.; He, Z.F.; Du, Y.Y.; Wan, J.F.; Liu, Y.F.; Ma, F.W. In-situ ZnO template preparation of coal tar pitch-based porous carbon-sheet microsphere for supercapacitor. J. Colloid Interface Sci. 2021, 602, 721–731. [Google Scholar] [CrossRef]
- Liu, H.W.; Wang, Y.Z.; Lv, L.; Liu, X.; Wang, Z.Q.; Liu, J. Oxygen-enriched hierarchical porous carbons derived from lignite for high-performance supercapacitors. Energy 2023, 269, 126707. [Google Scholar]
- Yang, N.N.; Ji, L.; Fu, H.C.; Shen, Y.F.; Wang, M.J.; Liu, J.H.; Chang, L.P.; Lv, Y.K. Hierarchical porous carbon derived from coal-based carbon foam for high-performance supercapacitors. Chin. Chem. Lett. 2022, 33, 3961–3967. [Google Scholar] [CrossRef]
- Gao, Q.; Wang, Y.; Yang, M.; Shen, W.; Jiang, Y.M.; He, R.X.; Li, M. N, S-codoped porous carbon as metal-free electrocatalyst for oxygen reduction reaction. J. Solid State Electrochem. 2021, 25, 1765–1773. [Google Scholar] [CrossRef]
- Luo, H.; Jiang, W.J.; Zhang, Y.; Niu, S.; Tang, T.; Huang, L.B.; Chen, Y.Y.; Wei, Z.D.; Hu, J.S. Self-terminated activation for high-yield production of N,P-codoped nanoporous carbon as an efficient metal-free electrocatalyst for Zn-air battery. Carbon. 2018, 128, 97–105. [Google Scholar] [CrossRef]
- Begum, H.; Ahmed, M.S.; Kim, Y.B. Nitrogen-rich graphitic-carbon (R) graphene as a metal-free electrocatalyst for oxygen reduction reaction. Sci. Rep. 2020, 10, 12431. [Google Scholar]
- Sun, T.; Wang, J.; Qiu, C.T.; Ling, X.; Tian, B.B.; Chen, W.; Su, C.L. B, N Codoped and Defect-Rich Nanocarbon Material as a Metal-Free Bifunctional Electrocatalyst for Oxygen Reduction and Evolution Reactions. Adv. Sci. 2018, 5, 1800036. [Google Scholar] [CrossRef]
- Najam, T.; Shah, S.S.A.; Ali, H.; Song, Z.Q.; Sun, H.H.; Peng, Z.C.; Cai, X.K. A metal free electrocatalyst for high-performance zinc-air battery application with good resistance towards poisoning species. Carbon 2020, 164, 12–18. [Google Scholar] [CrossRef]
- Zong, L.B.; Wu, W.C.; Liu, S.L.; Yin, H.J.; Chen, Y.N.; Liu, C.; Fan, K.C.; Zhao, X.X.; Chen, X.; Wang, F.M.; et al. Metal-free, active nitrogen-enriched, efficient bifunctional oxygen electrocatalyst for ultrastable zinc-air batteries. Energy Stor. Mater. 2020, 27, 514–521. [Google Scholar]









Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chen, X.; Ma, J.; Sun, X.; Zhao, C.; Li, J.; Li, H. Pyrolysis Enzymolysis-Treated Pomelo Peel: Porous Carbon Materials with Fe−Nx Sites for High-Performance Supercapacitor and Efficient Oxygen Reduction Applications. Polymers 2023, 15, 3879. https://doi.org/10.3390/polym15193879
Chen X, Ma J, Sun X, Zhao C, Li J, Li H. Pyrolysis Enzymolysis-Treated Pomelo Peel: Porous Carbon Materials with Fe−Nx Sites for High-Performance Supercapacitor and Efficient Oxygen Reduction Applications. Polymers. 2023; 15(19):3879. https://doi.org/10.3390/polym15193879
Chicago/Turabian StyleChen, Xiangyu, Jiahua Ma, Xiaoshuai Sun, Chuanshan Zhao, Jiehua Li, and Hui Li. 2023. "Pyrolysis Enzymolysis-Treated Pomelo Peel: Porous Carbon Materials with Fe−Nx Sites for High-Performance Supercapacitor and Efficient Oxygen Reduction Applications" Polymers 15, no. 19: 3879. https://doi.org/10.3390/polym15193879
APA StyleChen, X., Ma, J., Sun, X., Zhao, C., Li, J., & Li, H. (2023). Pyrolysis Enzymolysis-Treated Pomelo Peel: Porous Carbon Materials with Fe−Nx Sites for High-Performance Supercapacitor and Efficient Oxygen Reduction Applications. Polymers, 15(19), 3879. https://doi.org/10.3390/polym15193879