Preparation and Characterization of TiO2-Coated Hollow Glass Beads for Functionalization of Deproteinized Natural Rubber Latex via UVA-Activated Photocatalytic Degradation
Abstract
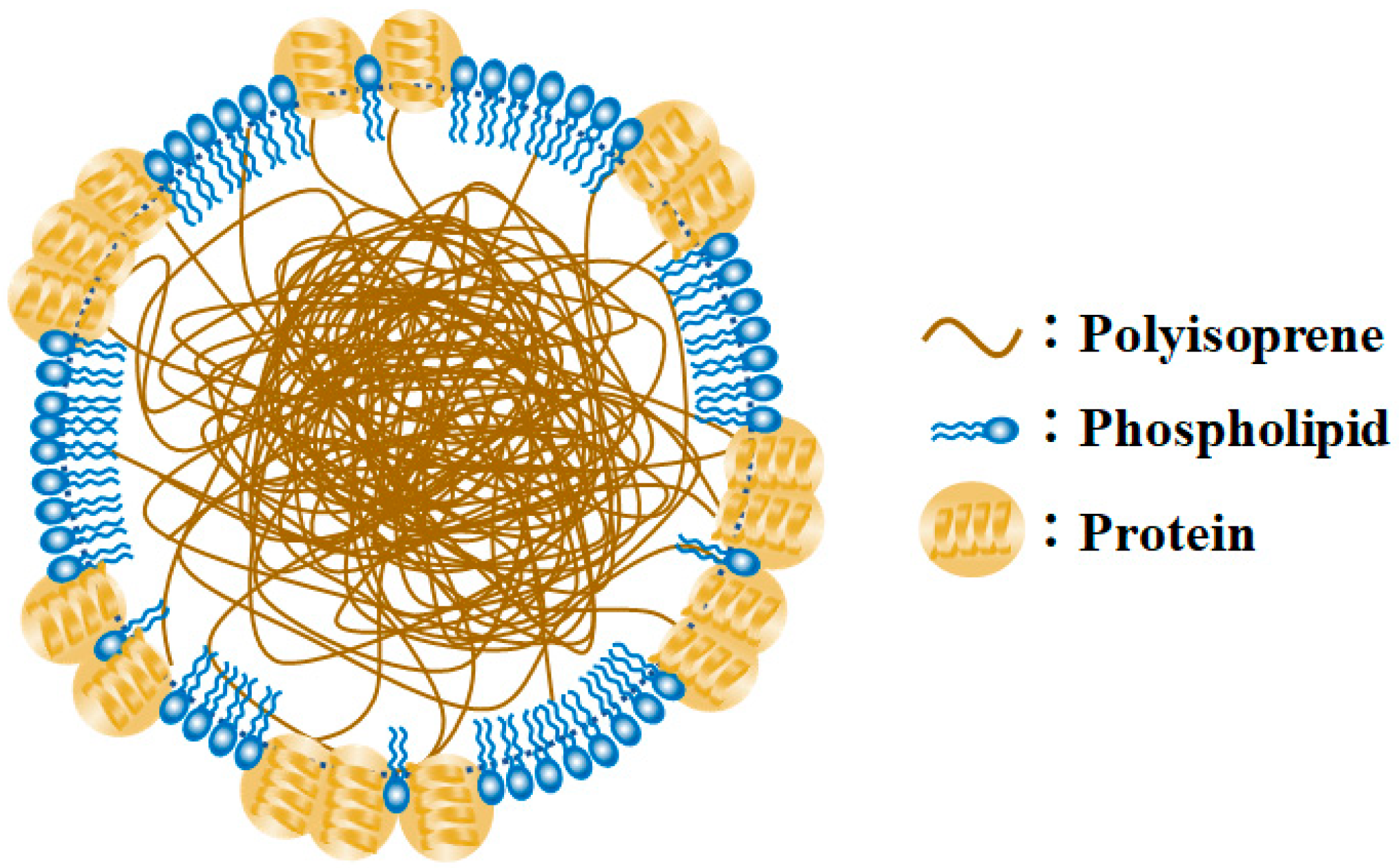
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Preparation of TiO2-Coated HGBs
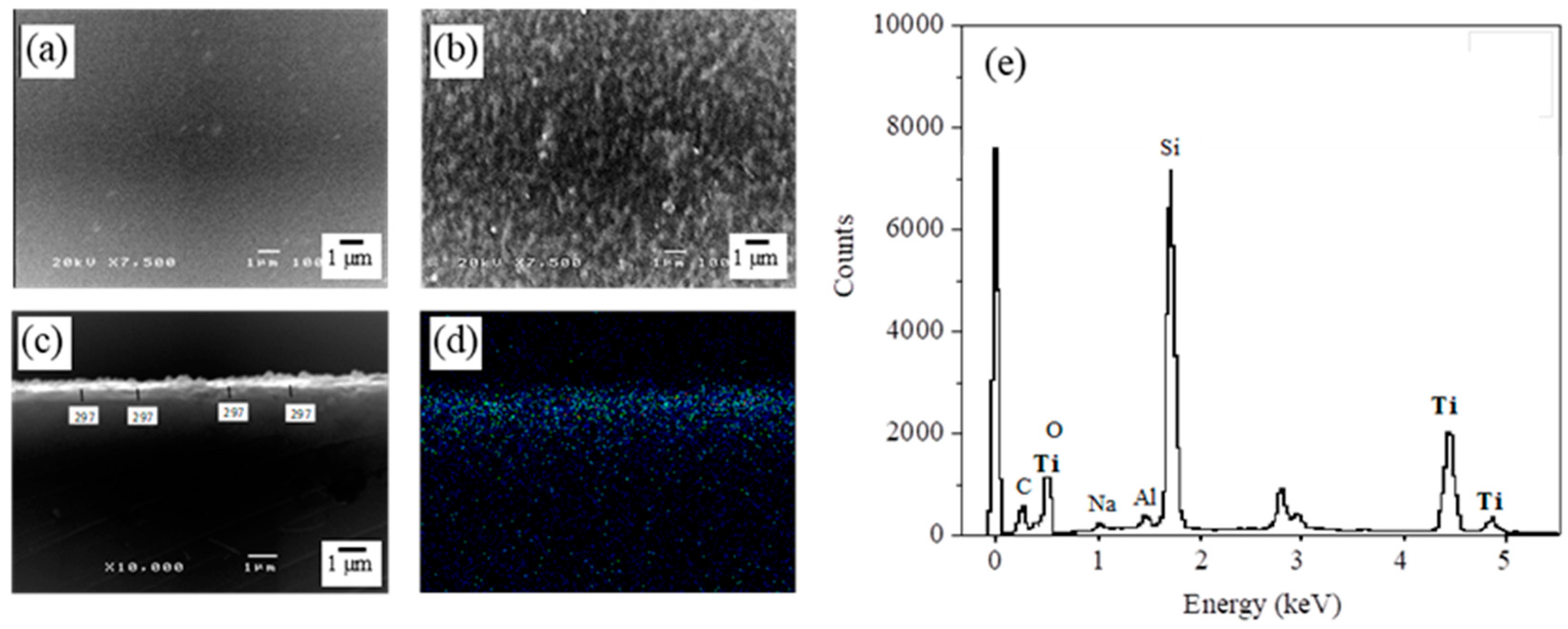
2.3. Characterization of TiO2-HGBs
2.4. Preparation of FLNR Latex
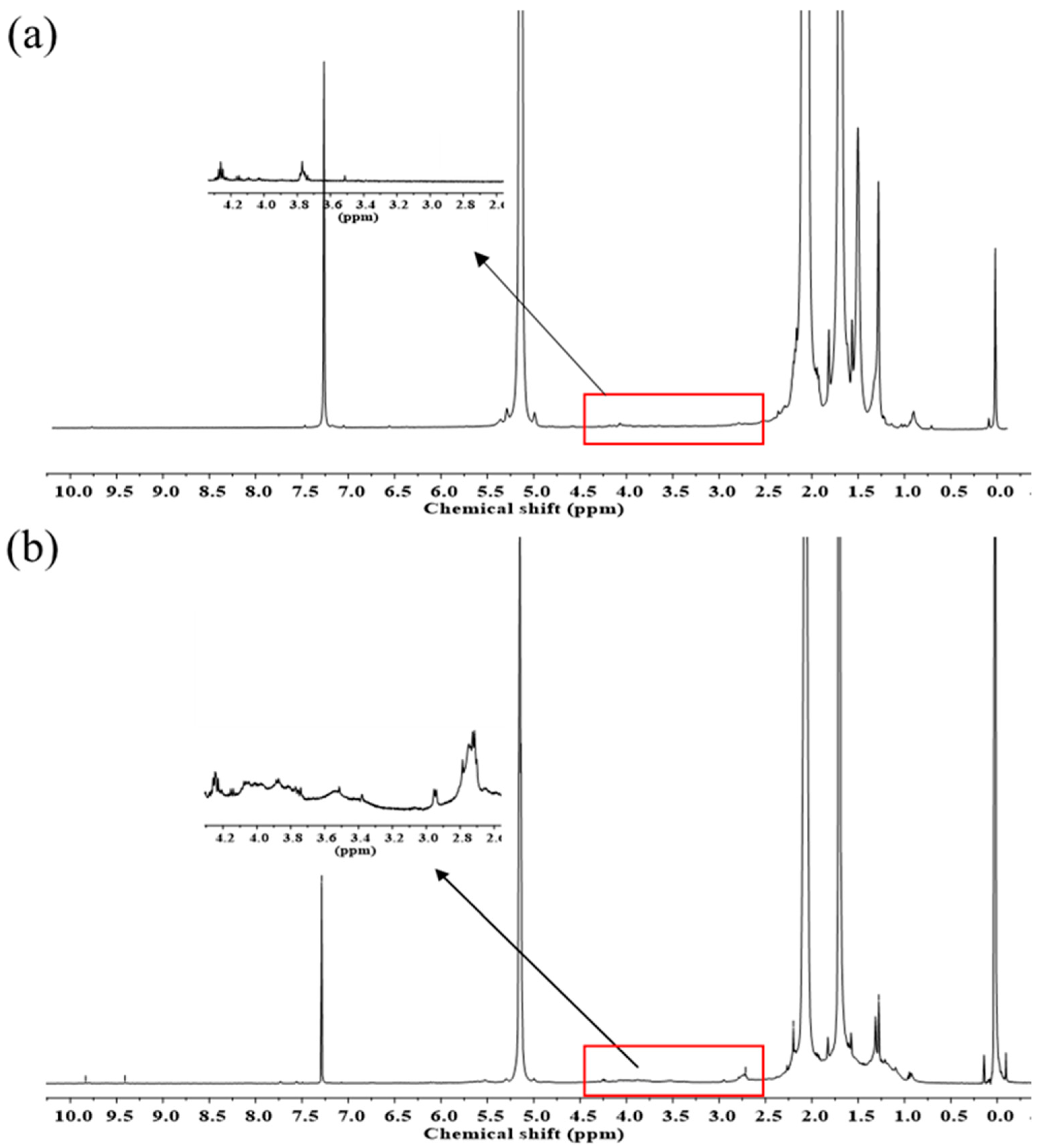
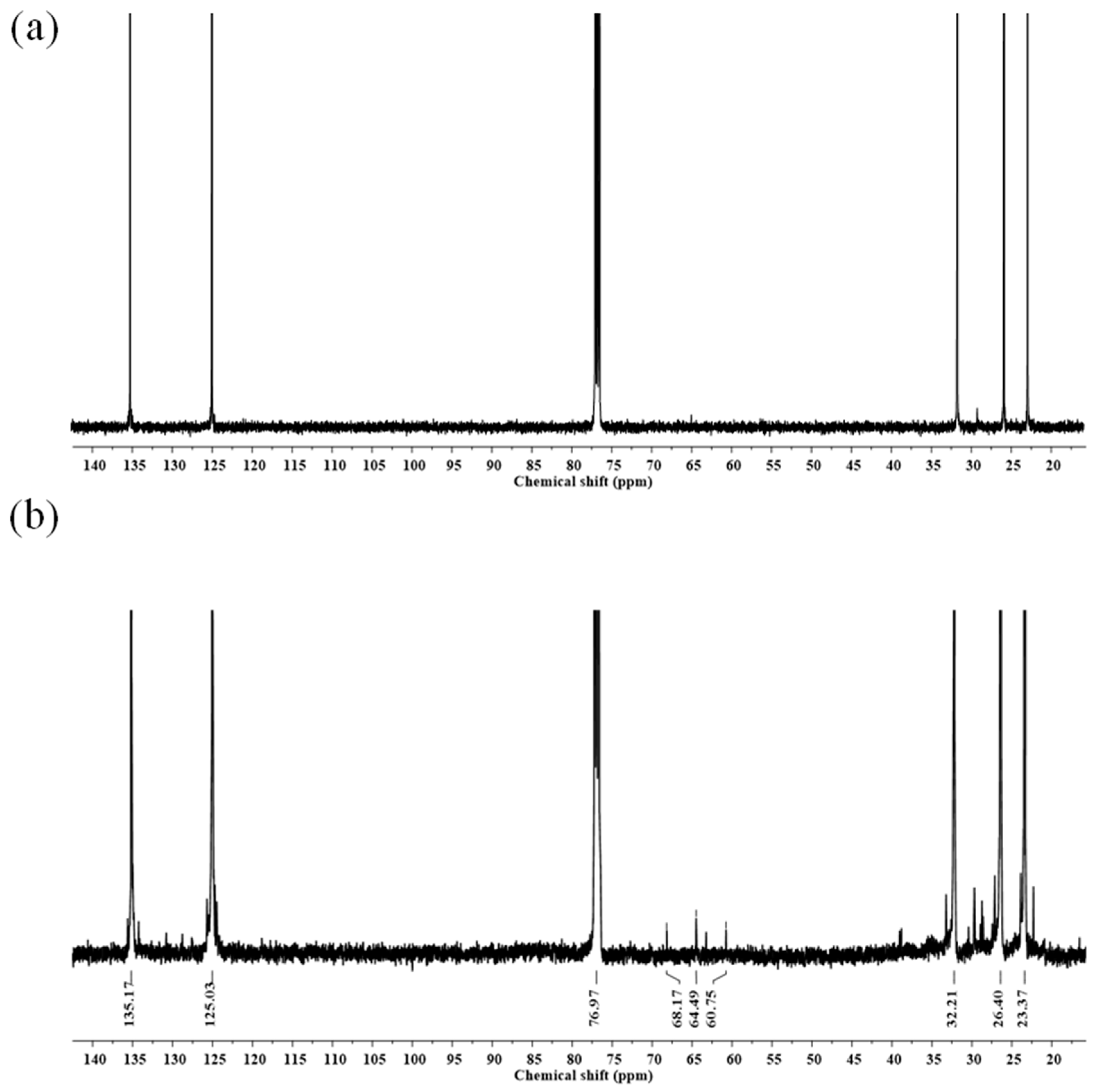
2.5. Characterization of FLNR Latex
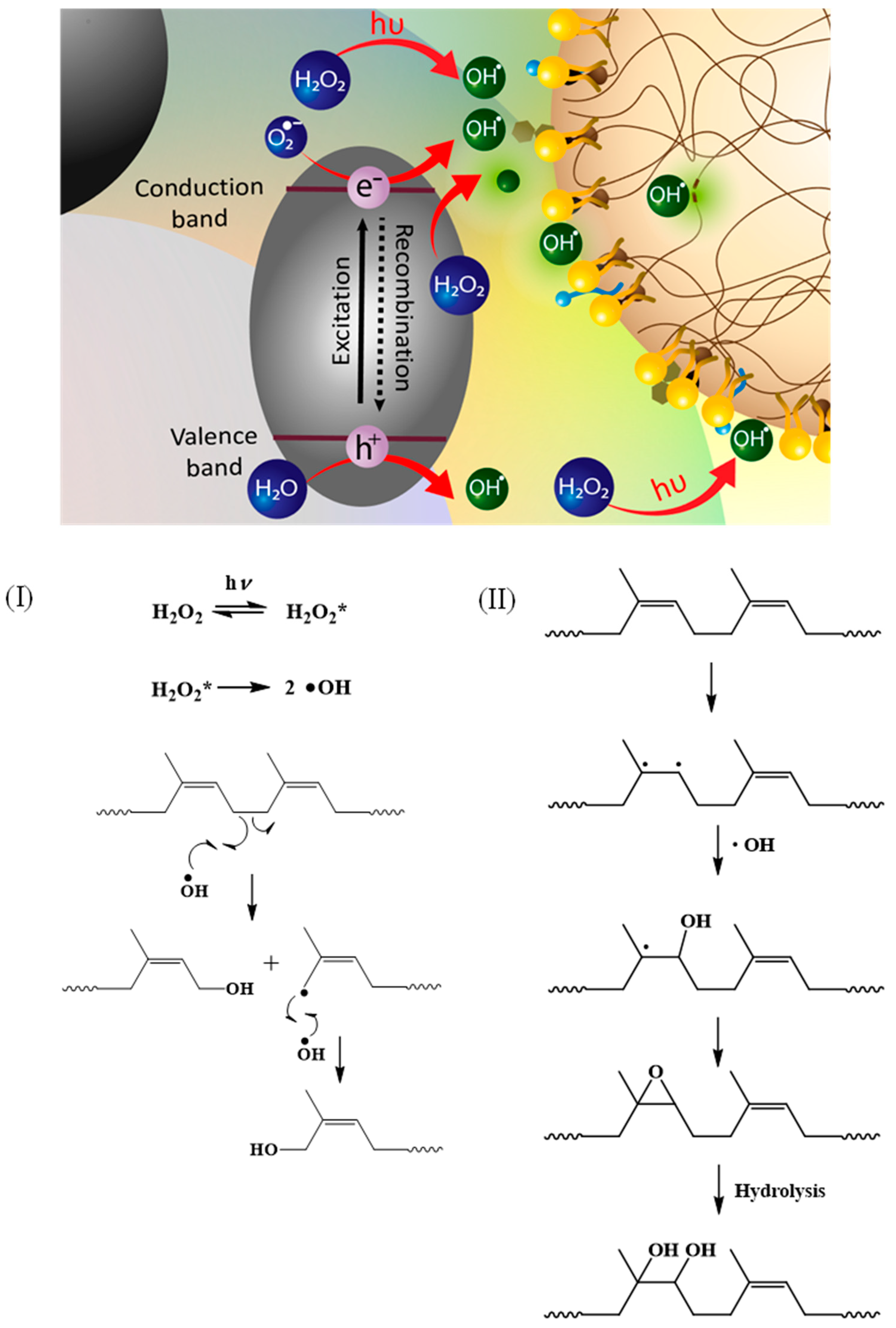
3. Results
3.1. Characterization of TiO2-Coated HGBs
3.2. Preparation of FLNR Latex
3.2.1. Effect of H2O2 Concentration
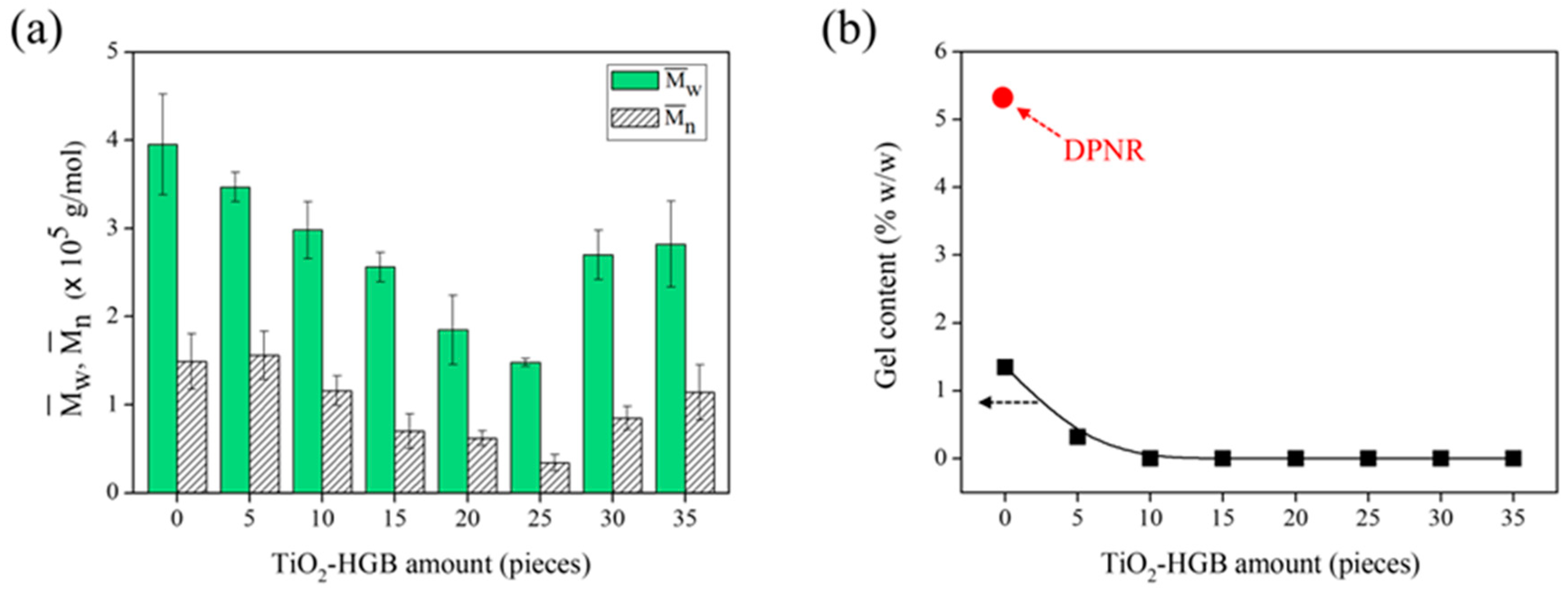
3.2.2. Effect of TiO2-HGB Quantity
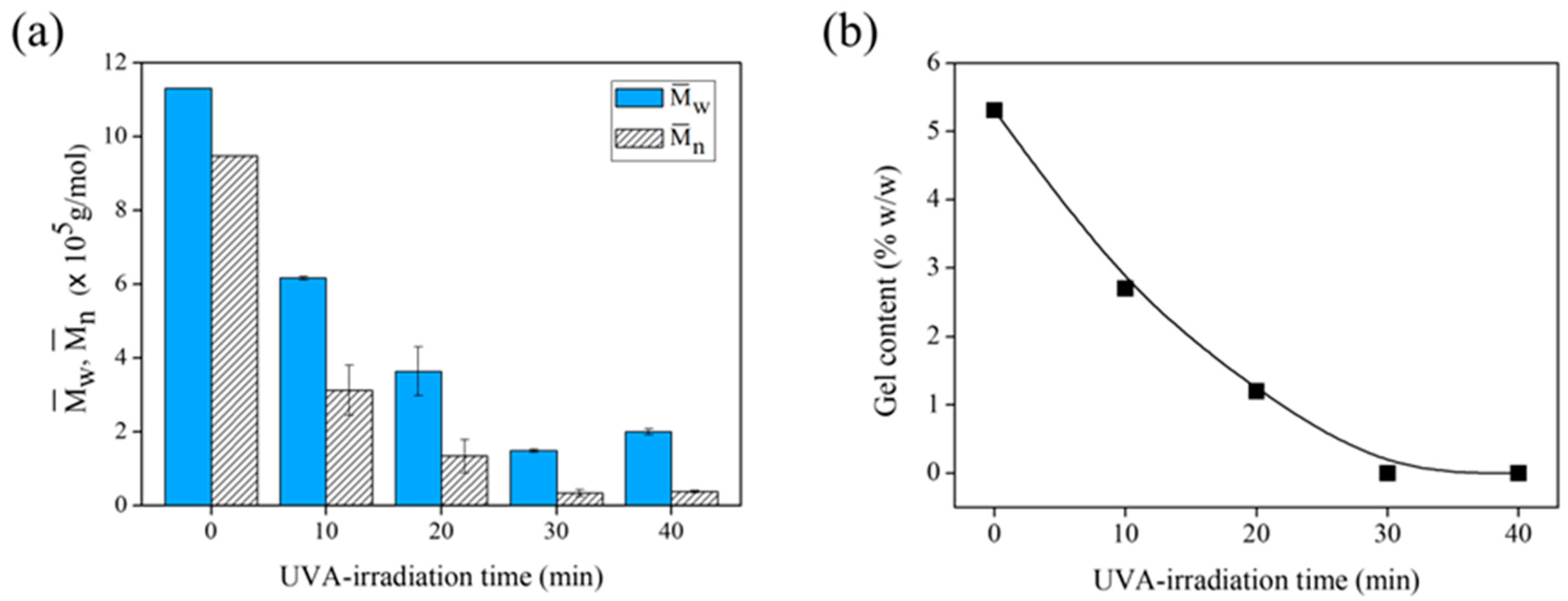
3.2.3. Effect of UVA-Irradiation Time
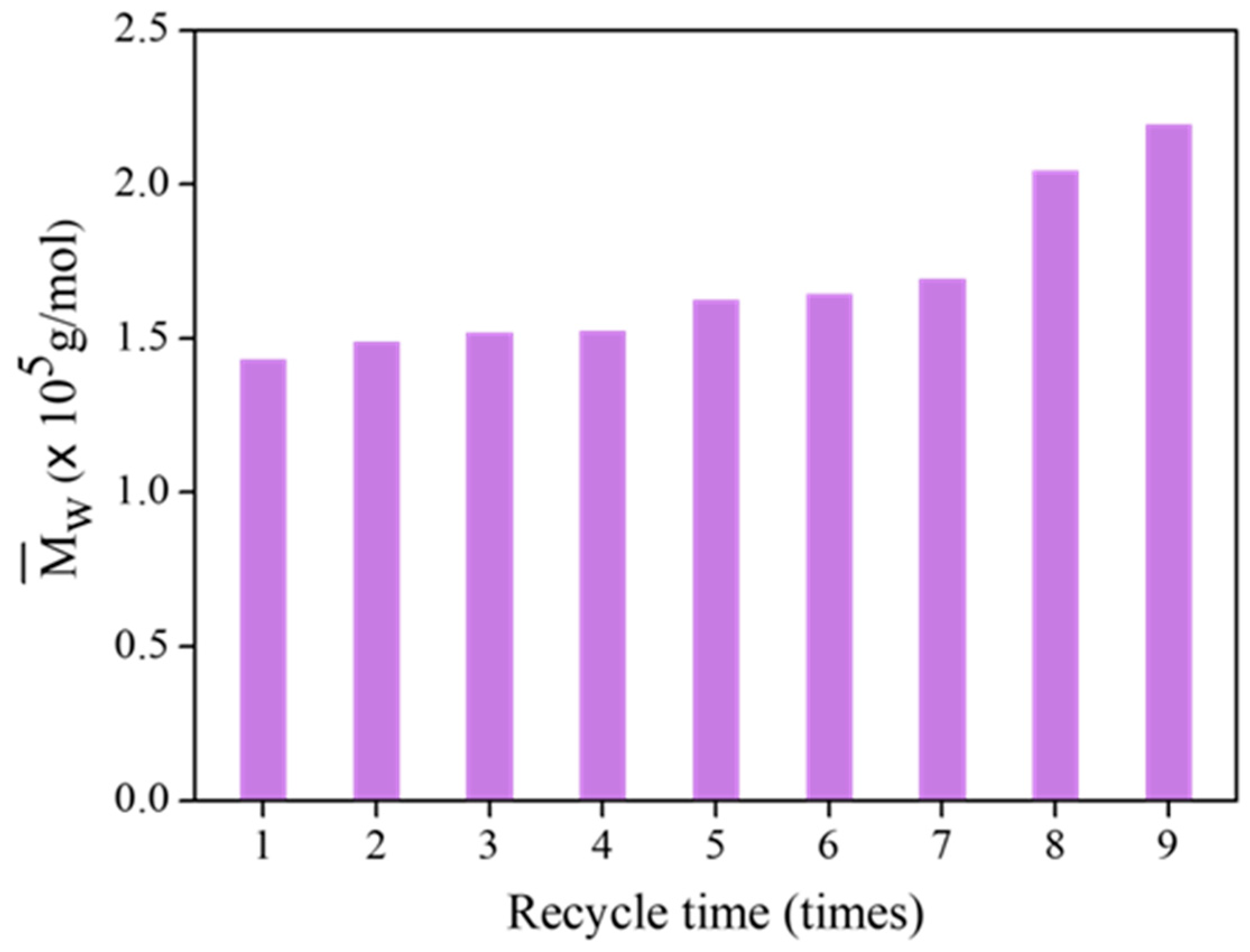
3.3. Reusability of TiO2-HGBs
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Sansatsadeekul, J.; Sakdapipanich, J.; Rojruthai, P. Characterization of associated proteins and phospholipids in natural rubber latex. J. Biosci. Bioeng. 2011, 111, 628–634. [Google Scholar] [CrossRef]
- Srilathakutty, R.; John, N.; Joseph, R.; George, K.E. Use of amine terminated liquid natural rubber as a plasticiser in filled NR and NBR compounds. Int. J. Polym. Mater. Polym. Biomater. 1996, 32, 235–246. [Google Scholar] [CrossRef]
- Dahlan, H.M.; Zaman, M.D.K.; Ibrahim, A. Liquid natural rubber (LNR) as a compatibiliser in NR/LLDPE blends-II: The effects of electron-beam (EB) irradiation. Radiat. Phys. Chem. 2002, 64, 429–436. [Google Scholar] [CrossRef]
- Mounir, A.; Darwish, N.A.; Shehata, A. Effect of maleic anhydride and liquid natural rubber as compatibilizers on the mechanical properties and impact resistance of the NR-NBR blend. Polym. Adv. Technol. 2004, 15, 209–213. [Google Scholar] [CrossRef]
- Wayakron, P.C.; Bumee, R.; Namahoot, J.; Ruamcharoen, J.; Ruamcharoen, P. Polyurethane polyester elastomer: Innovative environmental friendly wood adhesive from modified PETs and hydroxyl liquid natural rubber polyols. Int. J. Adhes. Adhes. 2013, 41, 127–131. [Google Scholar] [CrossRef]
- Kébir, N.; Campistron, I.; Laguerre, A.; Pilard, J.F.; Bunel, C. New cross-linked polyurethane elastomers with various physical properties from natural rubber derivatives. J. Appl. Polym. Sci. 2011, 122, 1677–1687. [Google Scholar] [CrossRef]
- Saetung, A.; Kaenhin, L.; Klinpitukda, P.; Rungvichaniwat, A.; Tulyapitak, T.; Munleh, S.; Campistron, I.; Pilard, J.F. Synthesis, characteristic, and properties of waterborne polyurethane based on natural rubber. J. Appl. Polym. Sci. 2012, 124, 2742–2752. [Google Scholar] [CrossRef]
- Pilard, K.F. Waterborne Polyurethane: Effect of functional groups in aromatic isocyanate and the chain length of hydroxyl terminated natural rubber. Adv. Mater. Res. 2012, 415–417, 2032–2035. [Google Scholar]
- Ly, P.H. Reinforcement of natural rubber from hydroxyl-terminated liquid natural rubber grafted carbon black. I. Grafting of acyl chloride capped liquid natural rubber onto carbon black. J. Macromol. Sci. A. 1996, 33, 1931–1937. [Google Scholar] [CrossRef]
- Bac, N.V.; Terlemezyan, L.; Mihailov, M. Epoxidation of natural rubber in latex in the presence of a reducing agent. J. Appl. Polym. Sci. 1993, 50, 845–849. [Google Scholar] [CrossRef]
- Ibrahim, S.; Daik, R.; Abdullah, I. Functionalization of liquid natural rubber via oxidative degradation of natural rubber. Polymers 2014, 6, 2928. [Google Scholar] [CrossRef]
- Ravindran, T.; Nayar, M.R.G.; Francis, D.J. Production of hydroxyl-terminated liquid natural rubber-mechanism of photochemical depolymerization and hydroxylation. J. Appl. Polym. Sci. 1988, 35, 1227–1239. [Google Scholar] [CrossRef]
- Shamraiz, U.; Hussain, R.A.; Badshah, A.; Raza, B.; Saba, S. Functional metal sulfides and selenides for the removal of hazardous dyes from Water. J. Photochem. Photobiol. B Biol. 2016, 159, 33–41. [Google Scholar] [CrossRef]
- Juabrum, S.; Chankhanittha, T.; Nanan, S. Hydrothermally grown SDS-capped ZnO photocatalyst for degradation of RR141 azo dye. Mater. Lett. 2019, 245, 1–5. [Google Scholar] [CrossRef]
- Moon, J.; Yun, C.Y.; Chung, K.W.; Kang, M.S.; Yi, J. Photocatalytic activation of TiO2 under visible light using Acid Red 44. Catal. Today 2003, 87, 77–86. [Google Scholar] [CrossRef]
- Dong, P.; Hou, G.; Xi, X.; Shao, R.; Dong, F. WO3-based photocatalysts: Morphology control, activity enhancement and multifunctional applications. Environ. Sci. Nano 2017, 4, 539–557. [Google Scholar] [CrossRef]
- Van Cuong, N. Bifunctionalcore–shell nanocomposite Mn-doped ZnO/Fe3O4 for photodegradation of reactive blue 198 dye. Adv. Nat. Sci. Nanosci. Nanotechnol. 2014, 5, 035014. [Google Scholar]
- Fisher, J.; Egerton, T.A. Titanium Compounds, Inorganic. Kirk-Othmer Encyclopedia of Chemical Technology; John Wiley & Sons, Inc.: New York, NY, USA, 2000; pp. 1–76. [Google Scholar]
- Reddy, M.K.; Manorama, S.V.; Reddy, A.R. Band gap studies on anatase titanium oxide nanoparticles. Mater. Chem. Phys. 2003, 78, 239–245. [Google Scholar] [CrossRef]
- Hanaor, D.A.H.; Sorrell, C.C. Review of the anatase to rutile phase transformation. J. Mater. Sci. 2011, 46, 855–874. [Google Scholar] [CrossRef]
- Franch, M.I.; Peral, J.; Domenech, X.; Ayllon, J.A. Aluminium(iii) adsorption: A soft and simple method to prevent TiO2 deactivation during salicylic acid photodegradation. Chem. Commun. 2005, 14, 1851–1853. [Google Scholar] [CrossRef]
- Haick, H.; Paz, Y. Remote photocatalytic activity as probed by measuring the degradation of self-assembled monolayers anchored near microdomains of titanium oxide. J. Phys. Chem. B. 2001, 105, 3045–3051. [Google Scholar] [CrossRef]
- Ni, M.; Leung, M.K.H.; Leung, D.Y.C.; Sumathy, K. A review and recent developments in photocatalytic water-splitting using for hydrogen production. Renew. Sustain. Energy Rev. 2007, 11, 401–425. [Google Scholar] [CrossRef]
- Pozzo, R.L.; Baltanás, M.A.; Cassano, A.E. Supported titanium oxide as photocatalyst in water decontamination: State of the art. Catal. Today 1997, 39, 219–231. [Google Scholar] [CrossRef]
- Baram, N.; Starosvetsky, D.; Starosvetsky, J.; Epshtein, M.; Armon, R.; Ein, E.Y. Enhanced photo-efficiency of immobilized TiO2 catalyst via intense anodic bias. Electrochem. Commun. 2007, 9, 1684–1688. [Google Scholar] [CrossRef]
- O’Regan, B.; Gratzel, M. A low-cost, high-efficiency solar cell based on dye-sensitized colloidal TiO2 films. Nature. 1991, 353, 737–740. [Google Scholar] [CrossRef]
- Huang, S.Y.; Schlichthörl, G.; Nozik, A.J.; Grätzel, M.; Frank, A.J. Charge recombination in dye-sensitized nanocrystalline TiO2 solar cells. J. Phys. Chem. B. 1997, 101, 2576–2582. [Google Scholar] [CrossRef]
- Fujishima, A.; Zhang, X.; Tryk, D.A. TiO2 photocatalysis and related surface phenomena. Surf. Sci. Rep. 2008, 63, 515–582. [Google Scholar] [CrossRef]
- Kisch, H.; Burgeth, G.; Macyk, W. Visible light photocatalysis by a titania transition metal complex. In Advances in Inorganic Chemistry; Van Eldik, R., Ed.; Academic Press: London, UK, 2004; Volume 56, pp. 241–259. [Google Scholar]
- Ohno, T.; Sarukawa, K.; Tokieda, K.; Matsumura, M. Morphology of a TiO2 photocatalyst (Degussa, P-25) consisting of anatase and rutile crystalline phases. J. Catal. 2001, 203, 82–86. [Google Scholar] [CrossRef]
- Cornish, B.J.P.A.; Lawton, L.A.; Robertson, P.K.J. Hydrogen peroxide enhanced photocatalytic oxidation of microcystin-LR using titanium oxide. Appl. Catal. B Environ. 2000, 25, 59–67. [Google Scholar] [CrossRef]
- Sakdapipanich, J.; Suksawad, P.; Insom, K.; Kawahara, S. Preparation of functionalized low molecular weight natural rubber latex using solid nanometric TiO2 film as a photocatalyst. Rubber Chem. Technol. 2005, 78, 597–605. [Google Scholar] [CrossRef]
- Sillapasuwan, A.; Saekhow, P.; Rojruthai, P.; Sakdapipanich, J. The preparation of hydroxyl-terminated deproteinized natural rubber latex by photochemical reaction utilizing nanometric TiO2 depositing on quartz substrate as a photocatalyst. Polymers 2022, 14, 2877. [Google Scholar] [CrossRef]
- Nijpanich, S.; Nimpaiboon, A.; Sakdapipanich, J. Functionalization of styrene-butadiene rubber and skim latex by photocatalytic reaction using nanometric TiO2 film as a photocatalyst. Key Eng. Mater. 2015, 659, 409–413. [Google Scholar] [CrossRef]
- Kim, S.C.; Lee, D.K. Preparation of TiO2-coated hollow glass beads and their application to the control of algal growth in eutrophic water. Microchem. J. 2005, 80, 227–232. [Google Scholar] [CrossRef]
- Nawamawat, K.; Sakdapipanich, J.T.; Ho, C.C.; Ma, Y.; Song, J.; Vancso, J.G. Surface nanostructure of Hevea brasiliensis natural rubber latex particles. Colloids Surf. A Physicochem. Eng. Asp. 2011, 390, 157–166. [Google Scholar] [CrossRef]
- Karunakaran, C.; Gomathisankar, P.; Manikandan, G. Solar photocatalytic detoxification of cyanide by different forms of TiO2. Korean J. Chem. Eng. 2011, 28, 1214–1220. [Google Scholar] [CrossRef]
- Yu, J.; Yu, H.; Cheng, B.; Zhou, M.; Zhao, X. Enhanced photocatalytic activity of TiO2 powder (P25) by hydrothermal treatment. J. Mol. Catal. A Chem. 2006, 253, 112–118. [Google Scholar] [CrossRef]
- Klinklai, W.; Saito, T.; Kawahara, S.; Tashiro, K.; Suzuki, Y.; Sakdapipanich, J.T.; Isono, Y. Hyperdeproteinized natural rubber prepared with urea. J. Appl. Polym. Sci. 2004, 93, 555–559. [Google Scholar] [CrossRef]
- Addamo, M.; Augugliaro, V.; Di, P.A.; García, L.E.; Loddo, V.; Marci, G.; Molinari, R.; Palmisano, L.; Schiavello, M. Preparation, characterization, and photoactivity of polycrystalline nanostructured TiO2 Catalysts. J. Phys. Chem. B. 2004, 108, 3303–3310. [Google Scholar] [CrossRef]
- Guo, B.; Liu, Z.; Hong, L.; Jiang, H.; Lee, J.Y. Photocatalytic effect of the sol-gel derived nanoporous TiO2 transparent thin films. Thin Solid Films 2005, 479, 310–315. [Google Scholar] [CrossRef]
- Nimpaiboon, A.; Amnuaypornsri, S.; Sakdapipanich, J. Influence of gel content on the physical properties of unfilled and carbon black filled natural rubber vulcanizates. Polym. Test. 2013, 32, 1135–1144. [Google Scholar] [CrossRef]
- De, A.K.; Chaudhuri, B.; Bhattacharjee, S.; Dutta, B.K. Estimation of •OH radical reaction rate constants for phenol and chlorinated phenols using UV/H2O2 photooxidation. J. Hazard. Mater. 1999, 64, 91–104. [Google Scholar] [CrossRef] [PubMed]
- Rippel, M.M.; Lee, L.-T.; Leite, C.A.P.; Galembeck, F. Skim and cream natural rubber particles: Colloidal properties, coalescence and film formation. J. Colloid Interface Sci. 2003, 268, 330–340. [Google Scholar] [CrossRef]
- Isa, S.Z.; Yahya, R.; Hassan, A.; Tahir, M. The influence of temperature and reaction time in the degradation of natural rubber latex. Malays. J. Anal. Sci. 2007, 11, 42–47. [Google Scholar]
- Vilar, W.D.; Menezes, S.M.C.; Akcelrud, L. Characterization of hydroxyl-terminated polybutadiene. Polym. Bull. 1994, 33, 557–561. [Google Scholar] [CrossRef]
- Anancharoenwong, E. Synthesis and Characterization of cis-1, 4-Polyisoprene-Based Polyurethane Coatings; Study of Their Adhesive Properties on Metal Surface. Ph.D. Thesis, Université du Maine, Le Mans, France, 21 September 2011. [Google Scholar]





Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Nijpanich, S.; Nimpaiboon, A.; Rojruthai, P.; Park, J.-H.; Hagio, T.; Ichino, R.; Sakdapipanich, J. Preparation and Characterization of TiO2-Coated Hollow Glass Beads for Functionalization of Deproteinized Natural Rubber Latex via UVA-Activated Photocatalytic Degradation. Polymers 2023, 15, 3885. https://doi.org/10.3390/polym15193885
Nijpanich S, Nimpaiboon A, Rojruthai P, Park J-H, Hagio T, Ichino R, Sakdapipanich J. Preparation and Characterization of TiO2-Coated Hollow Glass Beads for Functionalization of Deproteinized Natural Rubber Latex via UVA-Activated Photocatalytic Degradation. Polymers. 2023; 15(19):3885. https://doi.org/10.3390/polym15193885
Chicago/Turabian StyleNijpanich, Supinya, Adun Nimpaiboon, Porntip Rojruthai, Jae-Hyeok Park, Takeshi Hagio, Ryoichi Ichino, and Jitladda Sakdapipanich. 2023. "Preparation and Characterization of TiO2-Coated Hollow Glass Beads for Functionalization of Deproteinized Natural Rubber Latex via UVA-Activated Photocatalytic Degradation" Polymers 15, no. 19: 3885. https://doi.org/10.3390/polym15193885
APA StyleNijpanich, S., Nimpaiboon, A., Rojruthai, P., Park, J.-H., Hagio, T., Ichino, R., & Sakdapipanich, J. (2023). Preparation and Characterization of TiO2-Coated Hollow Glass Beads for Functionalization of Deproteinized Natural Rubber Latex via UVA-Activated Photocatalytic Degradation. Polymers, 15(19), 3885. https://doi.org/10.3390/polym15193885







