Designing an Oxygen Scavenger Multilayer System Including Volatile Organic Compound (VOC) Adsorbents for Potential Use in Food Packaging
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
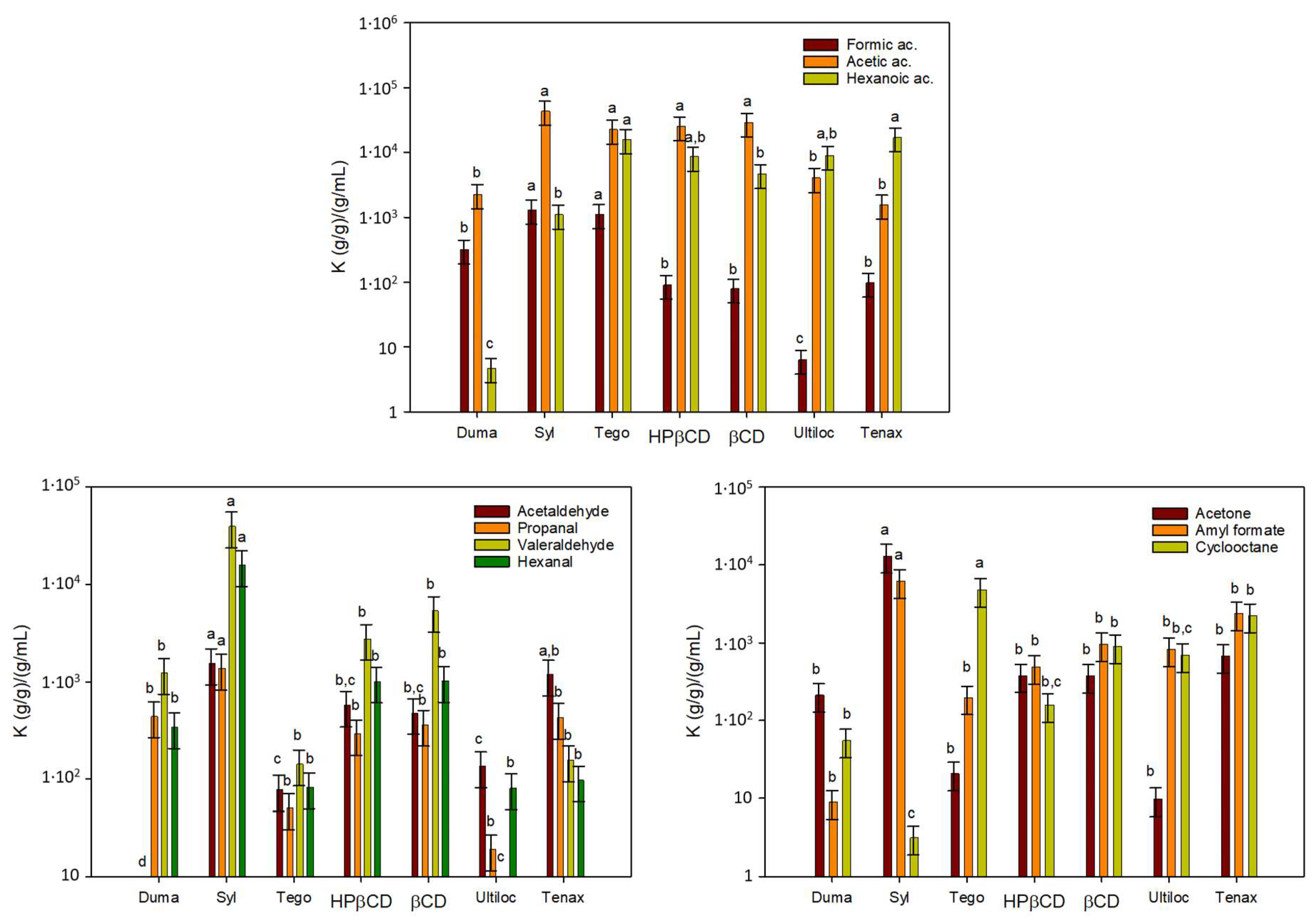
2.2. Selection of Adsorbents
2.2.1. Analysis of Retention Capacity of Adsorbents
2.2.2. Analysis of Retention Capacity of Adsorbents
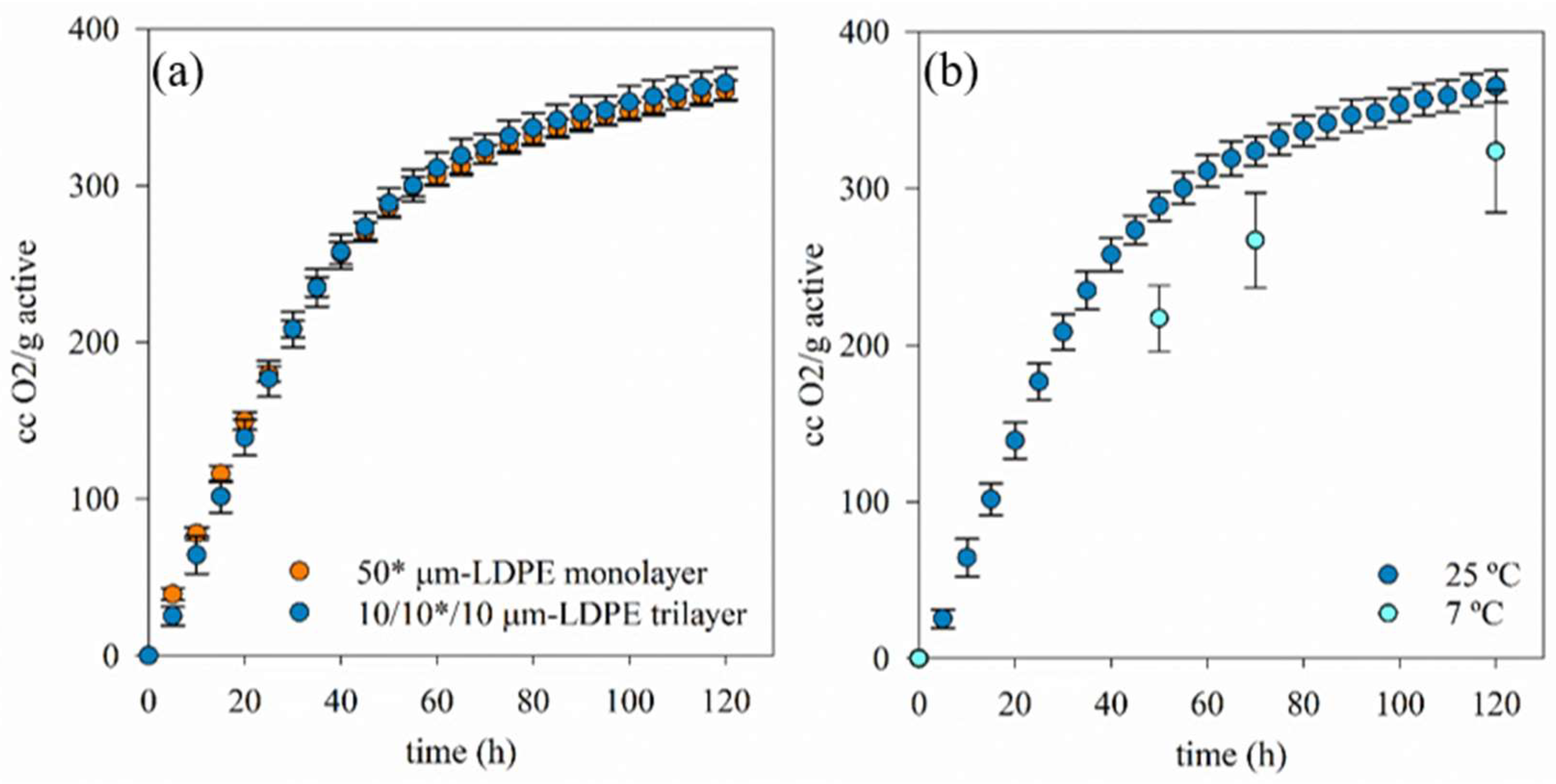
2.3. Assessment of the Oxygen Scavenging Activity of the TM Product
2.4. Analysis of the Capacity of Retention of VOCs Released by TM-Containing Films by Selected Adsorbents
2.5. Development of Films including Adsorbents
2.6. Development and Efficiency of Oxygen Scavenger Packaging Systems including TM-Active Agent and VOC Adsorbers
2.7. Statistical Analysis
3. Results and Discussion
3.1. Adsorption Capacity of VOC Adsorbents
3.2. Oxygen Scavenging of TOR-Containing Films
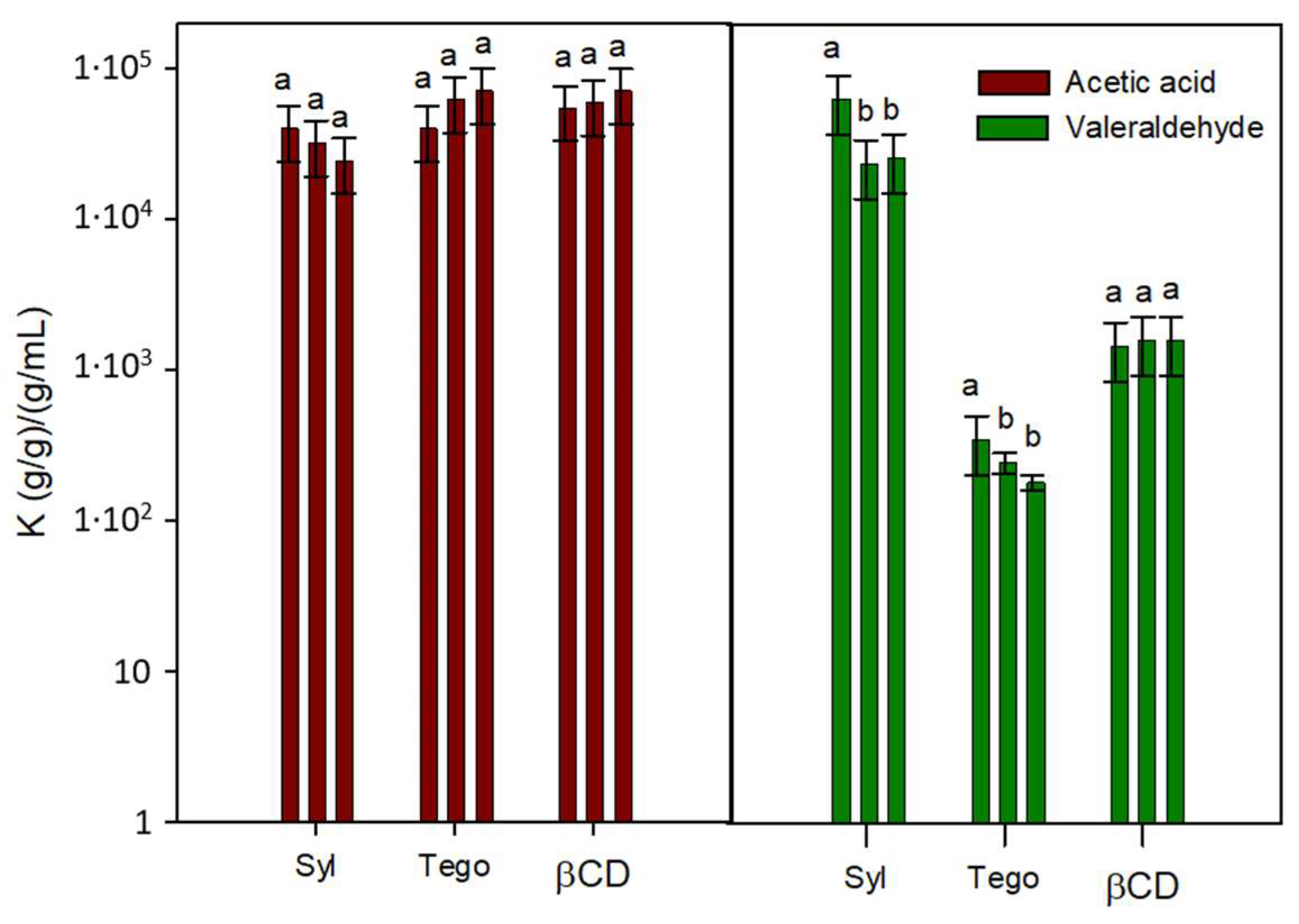
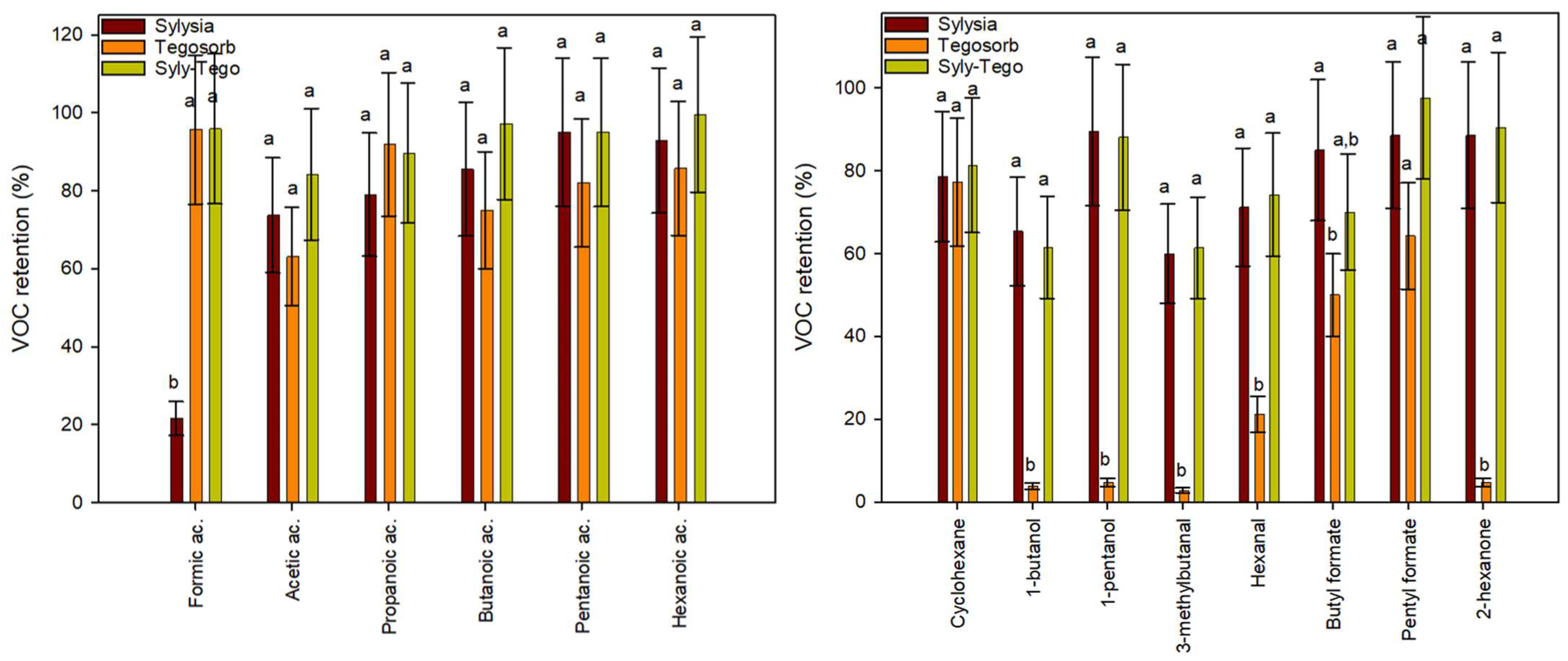
3.3. VOC Retention of Selected Adsorbents in Real Condition Testing
3.4. VOC Retention of Developed TM Adsorbents Prototypes
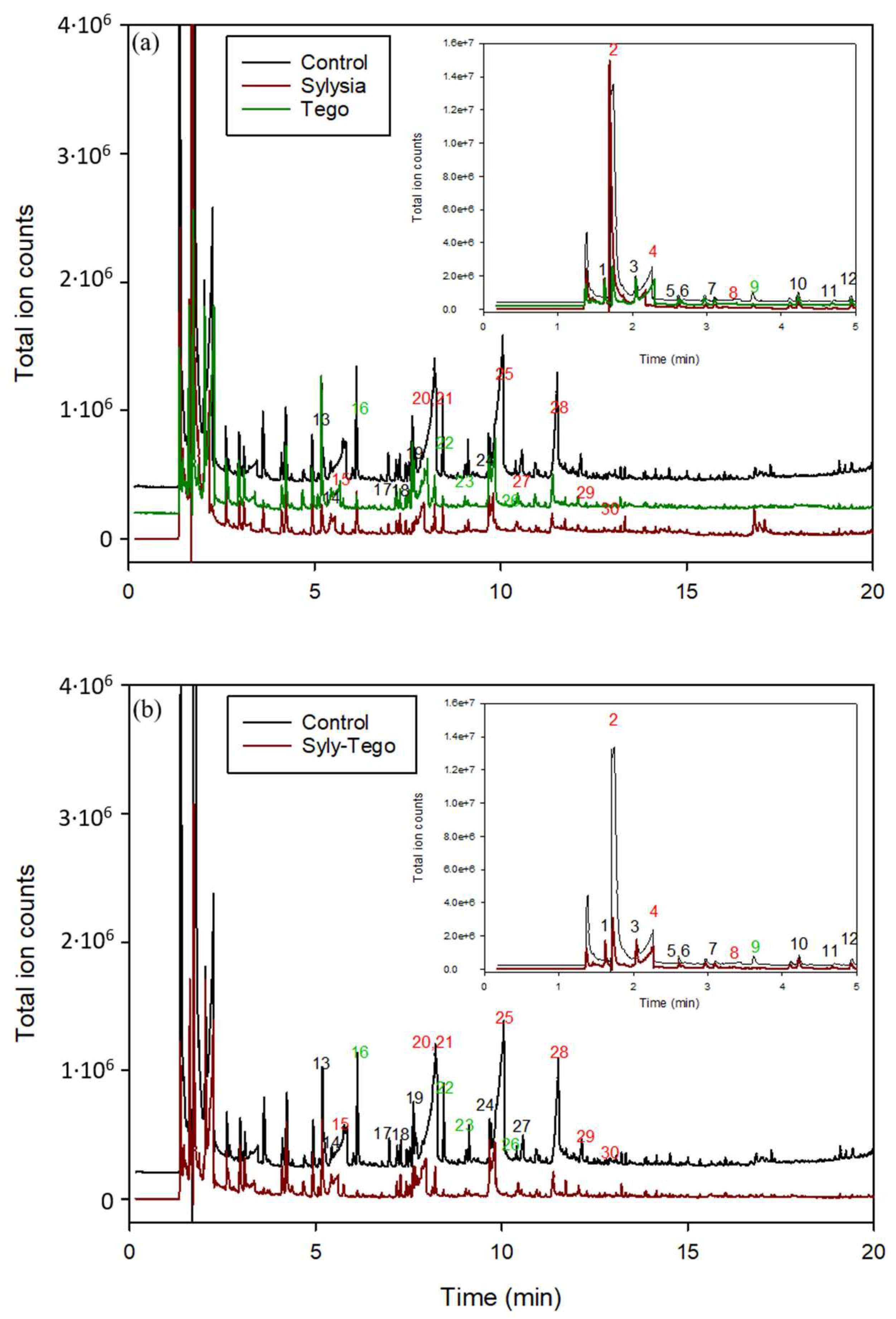
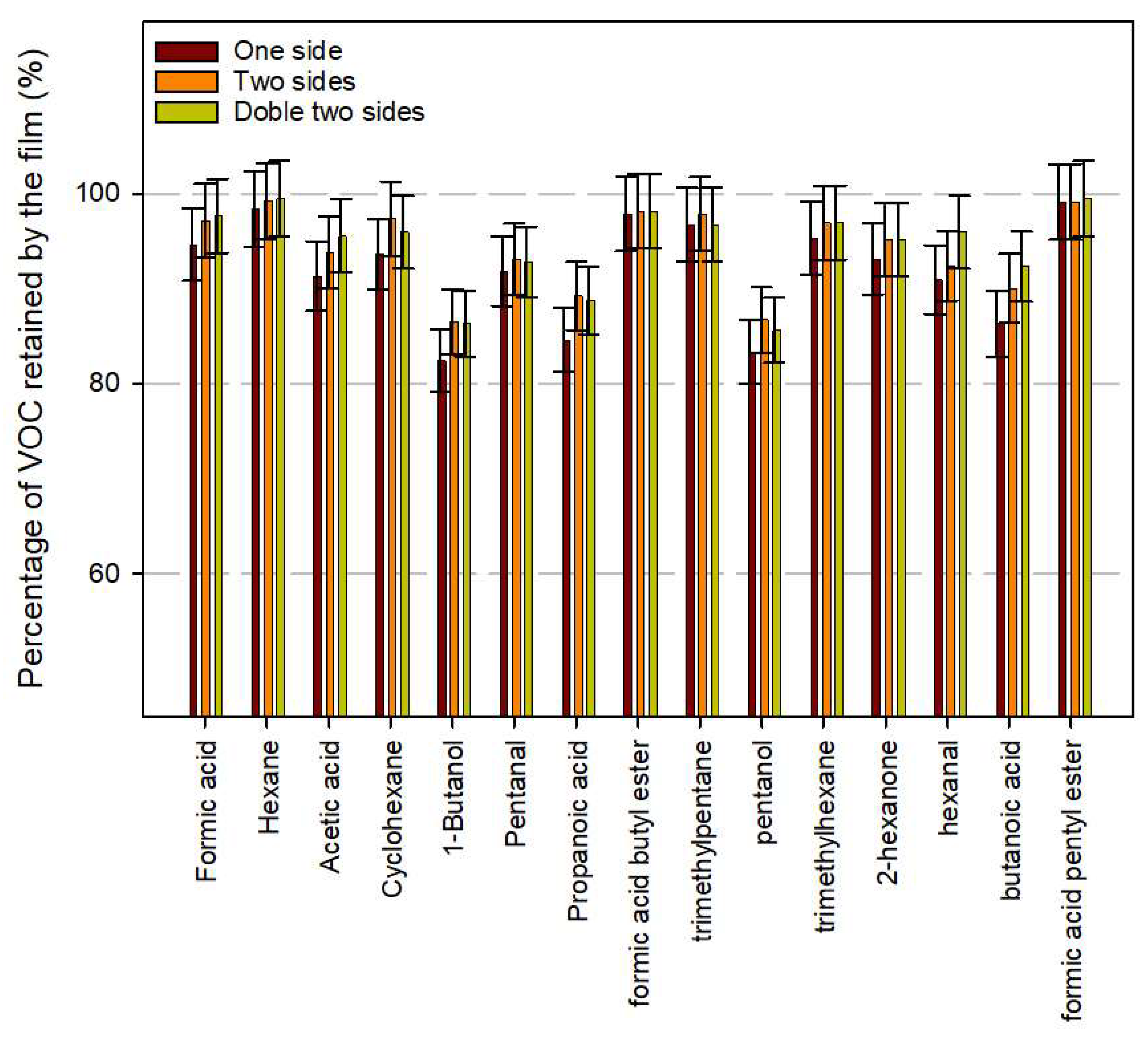
3.5. VOC Retention of Developed Multilayer Active Systems
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Firouz, M.S.; Mohi-Alden, K.; Omid, M. A critical review on intelligent and active packaging in the food industry: Research and development. Food Res. Int. 2021, 141, 110113. [Google Scholar] [CrossRef] [PubMed]
- Ahari, H.; Soufiani, S.P. Smart and Active Food Packaging: Insights in Novel Food Packaging. Front. Microbiol. 2021, 12, 1366. [Google Scholar] [CrossRef] [PubMed]
- Rüegg, N.; Röcker, B.; Yildirim, S. Application of palladium-based oxygen scavenger to extend the mould free shelf life of bakery products. Food Packag. Shelf Life 2022, 31, 100771. [Google Scholar] [CrossRef]
- Pourshahbazi, H.; Javanmard dakheli, M.; Salehirad, A.S. farhadi, Novel oxygen scavenger screw-cap for shelf-life improvement in virgin olive oil packaging during storage. J. Food Meas. Charact. 2022, 2022, 1–7. [Google Scholar] [CrossRef]
- Ramakanth, D.; Akhila, K.; Gaikwad, K.K.; Maji, P.K. UV-activated oxygen scavenging system based on natural rubber latex from Hevea brasiliensis for active packaging applications. Ind. Crops Prod. 2022, 178, 114658. [Google Scholar] [CrossRef]
- di Giuseppe, F.; Coffigniez, F.; Aouf, C.; Guillard, V.; Torrieri, E. Activated gallic acid as radical and oxygen scavenger in biodegradable packaging film. Food Packag. Shelf Life 2022, 31, 100811. [Google Scholar] [CrossRef]
- Martín-Mateos, M.J.; Amaro-Blanco, G.; Manzano, R.; Andrés, A.I.; Ramírez, R. Efficacy of modified active packaging with oxygen scavengers for the preservation of sliced Iberian dry-cured shoulder. Food Sci. Technol. Int. 2022, 29, 318–330. [Google Scholar] [CrossRef]
- Kruijf, D.D.; Beest, V.V.; Rijk, R.; Sipiläinen-Malm, T.; Losada, P.P.; Meulenaer, D.D. Active and intelligent packaging: Applications and regulatory aspects. Food Addit. Contam. 2002, 19, 144–162. [Google Scholar] [CrossRef]
- Dey, A.; Neogi, S. Oxygen scavengers for food packaging applications: A review. Trends Food Sci. Technol. 2019, 90, 26–34. [Google Scholar] [CrossRef]
- US5211875A—Methods and Compositions for Oxygen Scavenging—Google Patents. Available online: https://patents.google.com/patent/US5211875A/en (accessed on 27 May 2022).
- WO1996040799 Compositions Having Ethylenic Backbone and Benzylic, Allylic, or Ether-Containing Side-Chains, Oxygen Scavenging Compositions Containing Same, and Process for Making These Compositions by Esterification or Transesterification of a Polymer Melt. Available online: https://patentscope.wipo.int/search/en/detail.jsf?docId=WO1996040799 (accessed on 27 May 2022).
- Li, H.; Tung, K.K.; Paul, D.R.; Freeman, B.D.; Stewart, M.E.; Jenkins, J.C. Characterization of Oxygen Scavenging Films Based on 1,4-Polybutadiene. Ind. Eng. Chem. Res. 2012, 51, 7138–7145. [Google Scholar] [CrossRef]
- US6908652B1—Poly(lactic acid) in Oxygen Scavenging Article—Google Patents. Available online: https://patents.google.com/patent/US6908652B1/en (accessed on 24 July 2023).
- Peri, J.B.; Hensley, A.L. The surface structure of silica gel. J. Phys. Chem. 1968, 72, 2926–2933. [Google Scholar] [CrossRef]
- Shen, X.; Du, X.; Yang, D.; Ran, J.; Yang, Z.; Chen, Y. Influence of physical structures and chemical modification on VOCs adsorption characteristics of molecular sieves. J. Environ. Chem. Eng. 2021, 9, 106729. [Google Scholar] [CrossRef]
- Hou, S.; Tang, Y.; Zhu, T.; Huang, Z.H.; Liu, Y.; Sun, Y.; Li, X.; Shen, F. The molecular simulation and experimental investigation of toluene and naphthalene adsorption on ordered porous silica. Chem. Eng. J. 2022, 435, 134844. [Google Scholar] [CrossRef]
- WO2007053790A1—Fabric Softening Dryer Sheet with Odor Control—Google Patents. Available online: https://patents.google.com/patent/WO2007053790A1/en (accessed on 20 June 2022).
- WO2007057043 Absorbent Articles Comprising Acidic Superabsorber and an Organic Zinc Salt. Available online: https://patentscope.wipo.int/search/en/detail.jsf?docId=WO2007057043 (accessed on 20 June 2022).
- Mura, P. Advantages of the combined use of cyclodextrins and nanocarriers in drug delivery: A review. Int. J. Pharm. 2020, 579. [Google Scholar] [CrossRef] [PubMed]
- Cid-Samamed, A.; Rakmai, J.; Mejuto, J.C.; Simal-Gandara, J.; Astray, G. Cyclodextrins inclusion complex: Preparation methods, analytical techniques and food industry applications. Food Chem. 2022, 384, 132467. [Google Scholar] [CrossRef] [PubMed]
- Zhang, G.; Yuan, C.; Sun, Y. Effect of Selective Encapsulation of Hydroxypropyl-β-cyclodextrin on Components and Antibacterial Properties of Star Anise Essential Oil. Mol. A J. Synth. Chem. Nat. Prod. Chem. 2018, 23, 1126. [Google Scholar] [CrossRef]
- Alopaeus, J.F.; Göbel, A.; Breitkreutz, J.; Sande, S.A.; Tho, I. Investigation of hydroxypropyl-β-cyclodextrin inclusion complexation of two poorly soluble model drugs and their taste-sensation—Effect of electrolytes, freeze-drying and incorporation into oral film formulations. J. Drug Deliv. Sci. Technol. 2021, 61, 102245. [Google Scholar] [CrossRef]
- Higueras, L.; López-Carballo, G.; Gavara, R.; Hernández-Muñoz, P. Reversible Covalent Immobilization of Cinnamaldehyde on Chitosan Films via Schiff Base Formation and Their Application in Active Food Packaging. Food Bioprocess Technol. 2015, 8, 526–538. [Google Scholar] [CrossRef]
- Balaguer, M.P.; Gómez-Estaca, J.; Gavara, R.; Hernandez-Munoz, P. Biochemical properties of bioplastics made from wheat gliadins cross-linked with cinnamaldehyde. J. Agric. Food Chem. 2011, 59, 13212–13220. [Google Scholar] [CrossRef]
- US20160311949A1—Epoxy-Terminated Polybutadiene as Oxygen Scavenger—Google Patents. Available online: https://patents.google.com/patent/US20160311949A1/en (accessed on 25 July 2023).
- Kordjazi, Z.; Ajji, A.; Tabatabaei, H. Development of Oxygen Scavenging Polymeric Systems for Food Packaging Applications. Ph.D. Thesis, Polytechnic University Montreal, Montreal, QC, Canada, 2019. [Google Scholar]
- Kordjazi, Z.; Ajji, A. Development of TiO2 catalyzed HTPB based oxygen scavenging films for food packaging applications. Food Control 2021, 121, 107639. [Google Scholar] [CrossRef]
- Pan, L.; Zhang, M.; Lu, L.; Ou, B.; Chen, X. A polyethylene base moisture activating oxygen scavenging film co-extruded with tea polyphenols-β-cyclodextrin inclusion complex. Materials 2020, 13, 3857. [Google Scholar] [CrossRef] [PubMed]
- US4968438A—Gallic Acid as an Oxygen Scavenger—Google Patents. Available online: https://patents.google.com/patent/US4968438A/en (accessed on 27 June 2022).
- Coquillat, M.; Verdu, J.; Colin, X.; Audouin, L.; Nevière, R. Thermal oxidation of polybutadiene. Part 1: Effect of temperature, oxygen pressure and sample thickness on the thermal oxidation of hydroxyl-terminated polybutadiene. Polym. Degrad. Stab. 2007, 92, 1326–1333. [Google Scholar] [CrossRef]
- Senthilnathan, J.; Kim, K.H.; Kim, J.C.; Lee, J.H.; Song, H.N. Indoor air pollution; sorbent selection, and analytical techniques for volatile organic compounds. Asian J. Atmos. Environ. 2018, 12, 289–310. [Google Scholar] [CrossRef]
- Idris, S.A.; Robertson, C.; Morris, M.A.; Gibson, L.T. A comparative study of selected sorbents for sampling of aromatic VOCs from indoor air. Anal. Methods 2010, 2, 1803–1809. [Google Scholar] [CrossRef]
- Gibson, L.T. Mesosilica materials and organic pollutant adsorption: Part A removal from air. Chem. Soc. Rev. 2014, 43, 5163. [Google Scholar] [CrossRef]
- Ewlad-Ahmed, A.M.; Morris, M.; Holmes, J.; Belton, D.J.; Patwardhan, S.V.; Gibson, L.T. Green Nanosilicas for Monoaromatic Hydrocarbons Removal from Air. Silicon 2022, 14, 1447–1454. [Google Scholar] [CrossRef]


| Screw Speed (rpm) | T1 1 (°C) | T2 1 (°C) | T3 1 (°C) | T4 1 (°C) | T5 1 (°C) | COEX (°C) | DIE (°C) | |
|---|---|---|---|---|---|---|---|---|
| Extruder A | 120 | 165 | 180 | 180 | 180 | 180 | 210 | 200 |
| Extruder B | 25 | 165 | 175 | 175 | 180 | 185 | ||
| Extruder C | 55 | 205 | 220 | 220 | 220 | 220 | ||
| Scheme | 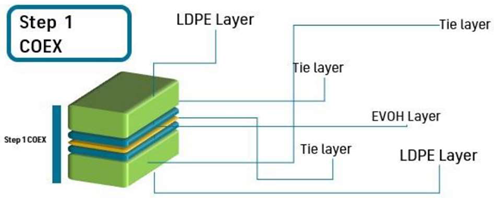 | |||||||
| Screw Speed (rpm) | T1 1 (°C) | T2 1 (°C) | T3 1 (°C) | T4 1 (°C) | T5 1 (°C) | COEX (°C) | DIE (°C) | |
|---|---|---|---|---|---|---|---|---|
| Extruder A | 210 | 200 | ||||||
| Extruder B | 5 | 190 | 215 | 225 | 240 | 245 | ||
| Extruder C | 80 | 210 | 240 | 240 | 240 | 240 | ||
| Scheme | 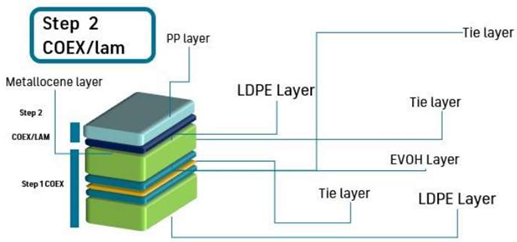 | |||||||
| Screw Speed (rpm) | T1 1 (°C) | T2 1 (°C) | T3 1 (°C) | T4 1 (°C) | T5 1 (°C) | COEX (°C) | DIE (°C) | |
|---|---|---|---|---|---|---|---|---|
| Extruder A | 120 | 165 | 190 | 190 | 195 | 200 | 205 | 205 |
| Extruder B | - | - | - | - | - | - | ||
| Extruder C | 100 | 190 | 190 | 190 | 195 | 200 | ||
| Scheme | 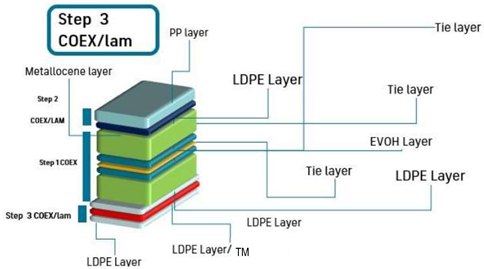 | |||||||
| Peak | Retention Time (min) | Volatile Compound | Ion Mass | Peak | Retention Time (min) | Volatile Compound | Ion Mass |
|---|---|---|---|---|---|---|---|
| 1 | 1.63 | pentane | 43 | 17 | 6.98 | 2-pentenol | 68 |
| 2 | 1.73 | formic acid | 46 | 18 | 7.63 | 2-heptanone | 43 |
| 3 | 2.05 | hexane | 57.1 | 19 | 7.69 | cyclohexanone | 55 |
| 4 | 2.24 | acetic acid | 60 | 20 | 8.1 | pentanoic acid | 60 |
| 5 | 2.65 | cyclohexane | 84.1 | 21 | 8.23 | cyclooctane | 56 |
| 6 | 2.69 | butanol | 56 | 22 | 8.44 | hexyl formate | 56.1 |
| 7 | 3.11 | 3-methylbutanal | 57 | 23 | 9.13 | cyclohexyl formate | 67 |
| 8 | 3.4 | propanoic acid | 74 | 24 | 9.73 | 2-octanone | 43 |
| 9 | 3.62 | Butyl formate | 56.1 | 25 | 9.96 | hexanoic acid | 60 |
| 10 | 4.12 | trimethylpentane | 71.1 | 26 | 10.41 | heptyl formate | 70.1 |
| 11 | 4.72 | pentanol | 55 | 27 | 10.53 | cyclopentyl carboxilic acid | 73 |
| 12 | 4.94 | trimethylhexane | 57.1 | 28 | 11.48 | heptanoic acid | 60 |
| 13 | 5.1 | 3-hexanone | 57 | 29 | 12.16 | cyclohexane carboxilic acid | 55.1 |
| 5.19 | 2-hexanone | 43 | 30 | 12.92 | octanoic acid | 60 | |
| 14 | 5.44 | hexanal | 56 | 31 | 16.18 | tetradecanal | 82.1 |
| 15 | 5.75 | butanoic acid | 60 | 32 | 16.93 | 1-phenyl 1-hexanone | 105 |
| 16 | 6.13 | Pentyl formate | 70.1 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
López-de-Dicastillo, C.; López-Carballo, G.; Vázquez, P.; Schwager, F.; Aragón-Gutiérrez, A.; Alonso, J.M.; Hernández-Muñoz, P.; Gavara, R. Designing an Oxygen Scavenger Multilayer System Including Volatile Organic Compound (VOC) Adsorbents for Potential Use in Food Packaging. Polymers 2023, 15, 3899. https://doi.org/10.3390/polym15193899
López-de-Dicastillo C, López-Carballo G, Vázquez P, Schwager F, Aragón-Gutiérrez A, Alonso JM, Hernández-Muñoz P, Gavara R. Designing an Oxygen Scavenger Multilayer System Including Volatile Organic Compound (VOC) Adsorbents for Potential Use in Food Packaging. Polymers. 2023; 15(19):3899. https://doi.org/10.3390/polym15193899
Chicago/Turabian StyleLópez-de-Dicastillo, Carol, Gracia López-Carballo, Pedro Vázquez, Florian Schwager, Alejandro Aragón-Gutiérrez, José M. Alonso, Pilar Hernández-Muñoz, and Rafael Gavara. 2023. "Designing an Oxygen Scavenger Multilayer System Including Volatile Organic Compound (VOC) Adsorbents for Potential Use in Food Packaging" Polymers 15, no. 19: 3899. https://doi.org/10.3390/polym15193899
APA StyleLópez-de-Dicastillo, C., López-Carballo, G., Vázquez, P., Schwager, F., Aragón-Gutiérrez, A., Alonso, J. M., Hernández-Muñoz, P., & Gavara, R. (2023). Designing an Oxygen Scavenger Multilayer System Including Volatile Organic Compound (VOC) Adsorbents for Potential Use in Food Packaging. Polymers, 15(19), 3899. https://doi.org/10.3390/polym15193899













