Characterization and Antifungal Activity of Lemongrass Essential Oil-Loaded Nanoemulsion Stabilized by Carboxylated Cellulose Nanofibrils and Surfactant
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Characterization of TEMPO-CNF
2.3. Preparation of Emulsion Stabilized by TEMPO-CNF and Tween 80
2.4. Thermodynamic Stability Testing of Emulsions
2.5. Determination of Emulsion Particle Size and Surface Charge (Zeta Potential)
2.6. Antifungal Activity Assay
2.6.1. Mycelial Growth Inhibition Assay
2.6.2. Conidial Germination Inhibition Assay
2.7. Statistical Analysis
3. Results and Discussion
3.1. XRD Characterization
3.2. FTIR Characterization
3.3. DSC and TGA Characterization
3.4. Thermodynamic Stability of Oil-in-Water Emulsions Stabilized by Tween 80 and TEMPO-CNF
3.5. Characterization of Emulsions
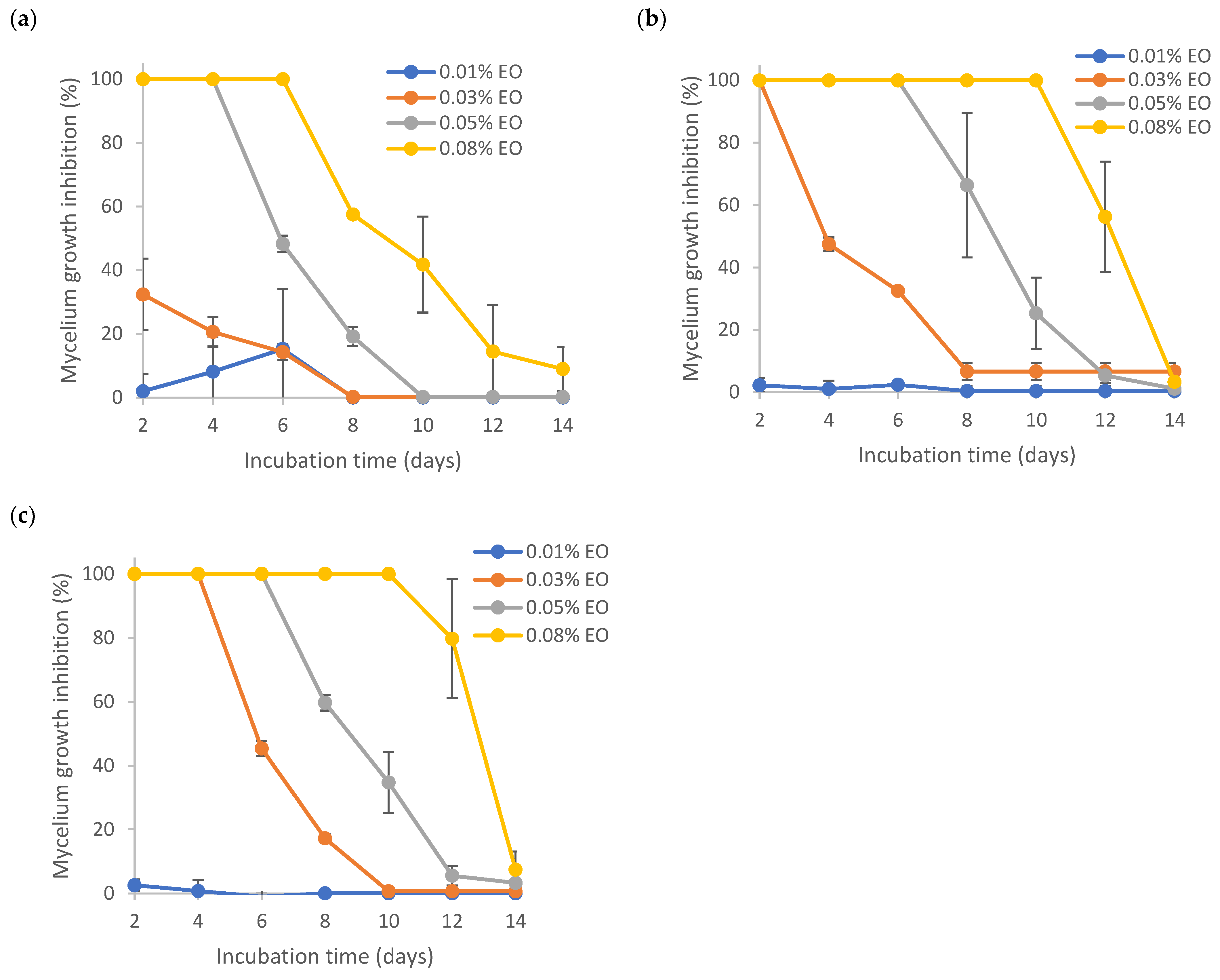
3.6. Antifungal Activity of Pure and Encapsulated Lemongrass Essential Oil
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Damalas, C.A.; Koutroubas, S.D. Current status and recent developments in biopesticide use. Agriculture 2018, 8, 13. [Google Scholar] [CrossRef]
- Baratta, M.T.; Dorman, H.D.; Deans, S.G.; Figueiredo, A.C.; Barroso, J.G.; Ruberto, G. Antimicrobial and antioxidant properties of some commercial essential oils. Flavour Fragr. J. 1998, 13, 235–244. [Google Scholar] [CrossRef]
- Wannissorn, B.; Jarikasem, S.; Siriwangchai, T.; Thubthimthed, S. Antibacterial properties of essential oils from Thai medicinal plants. Fitoterapia 2005, 76, 233–236. [Google Scholar] [CrossRef] [PubMed]
- Chao, S.C.; Young, D.G.; Oberg, C.J. Screening for inhibitory activity of essential oils on selected bacteria, fungi and viruses. J. Essent. Oil Res. 2000, 12, 639–649. [Google Scholar] [CrossRef]
- Montes-Belmont, R.; Carvajal, M. Control of Aspergillus flavus in maize with plant essential oils and their components. J. Food Prot. 1998, 61, 616–619. [Google Scholar] [CrossRef] [PubMed]
- Bahrami, G.; Sotoudehkia, S.; Hosseinzadeh, L.; Farzaei, M.; Shokoohinia, Y. Biochemical and histopathological evidence on the beneficial effects of essential oil of Pistacia atlantica oleoresin in acetic acid-induced colitis. J. Med. Plants 2019, 18, 192–201. [Google Scholar] [CrossRef]
- Luang-In, V.; Rossiter, J.T. Stability studies of isothiocyanates and nitriles in aqueous media. Songklanakarin J. Sci. Technol. 2015, 37, 625–630. [Google Scholar]
- Chedea, V.S.; Echim, C.; Braicu, C.; Andjelkovic, M.; Verhe, R.; Socaciu, C. Composition in polyphenols and stability of the aqueous grape seed extract from the Romanian variety “Merlot Recas”. J. Food Biochem. 2011, 35, 92–108. [Google Scholar] [CrossRef]
- Turek, C.; Stintzing, F.C. Stability of essential oils: A review. Compr. Rev. Food Sci. Food Saf. 2013, 12, 40–53. [Google Scholar] [CrossRef]
- Li, Y.; Teng, Z.; Chen, P.; Song, Y.; Luo, Y.; Wang, Q. Enhancement of aqueous stability of allyl isothiocyanate using nanoemulsions prepared by an emulsion inversion point method. J. Colloid Interface Sci. 2015, 438, 130–137. [Google Scholar] [CrossRef]
- Ghaderi-Ghahfarokhi, M.; Barzegar, M.; Sahari, M.A.; Azizi, M.H. Nanoencapsulation approach to improve antimicrobial and antioxidant activity of thyme essential oil in beef burgers during refrigerated storage. Food Bioprocess Technol. 2016, 9, 1187–1201. [Google Scholar] [CrossRef]
- Blanco-Padilla, A.; Soto, K.M.; Hernández Iturriaga, M.; Mendoza, S. Food antimicrobials nanocarriers. Sci. World J. 2014, 2014, 837215. [Google Scholar] [CrossRef] [PubMed]
- Kah, M.; Kookana, R.S.; Gogos, A.; Bucheli, T.D. A critical evaluation of nanopesticides and nanofertilizers against their conventional analogues. Nat. Nanotechnol. 2018, 13, 677–684. [Google Scholar] [CrossRef] [PubMed]
- Liu, Z.; Li, X.; Xie, W.; Deng, H. Extraction, isolation and characterization of nanocrystalline cellulose from industrial kelp (Laminaria japonica) waste. Carbohydr. Polym. 2017, 173, 353–359. [Google Scholar] [CrossRef] [PubMed]
- Lu, Z.; Fan, L.; Zheng, H.; Lu, Q.; Liao, Y.; Huang, B. Preparation, characterization and optimization of nanocellulose whiskers by simultaneously ultrasonic wave and microwave assisted. Bioresour. Technol. 2013, 146, 82–88. [Google Scholar] [CrossRef]
- Li, J.; Wei, X.; Wang, Q.; Chen, J.; Chang, G.; Kong, L.; Su, J.; Liu, Y. Homogeneous isolation of nanocellulose from sugarcane bagasse by high pressure homogenization. Carbohydr. Polym. 2012, 90, 1609–1613. [Google Scholar] [CrossRef]
- Anderson, S.R.; Esposito, D.; Gillette, W.; Zhu, J.; Baxa, U.; Mcneil, S.E. Enzymatic preparation of nanocrystalline and microcrystalline cellulose. Tappi J. 2014, 13, 35–42. [Google Scholar] [CrossRef]
- Khosravi-Darani, K.; Koller, M.; Akramzadeh, N.; Mortazavian, A.M. Bacterial nanocellulose: Biosynthesis and medical application. Biointerface Res. Appl. Chem. 2016, 6, 1511–1516. [Google Scholar]
- Winuprasith, T.; Khomein, P.; Mitbumrung, W.; Suphantharika, M.; Nitithamyong, A.; McClements, D.J. Encapsulation of vitamin D3 in pickering emulsions stabilized by nanofibrillated mangosteen cellulose: Impact on in vitro digestion and bioaccessibility. Food Hydrocoll. 2018, 83, 153–164. [Google Scholar] [CrossRef]
- Saelices, C.J.; Capron, I. Design of Pickering micro-and nanoemulsions based on the structural characteristics of nanocelluloses. Biomacromolecules 2018, 19, 460–469. [Google Scholar] [CrossRef]
- Costa, L.A.; Assis, D.d.J.; Gomes, G.V.; da Silva, J.B.; Fonsêca, A.F.; Druzian, J.I. Extraction and characterization of nanocellulose from corn stover. Mater. Today Proc. 2015, 2, 287–294. [Google Scholar] [CrossRef]
- Li, P.; Wang, Y.; Hou, Q.; Liu, H.; Liang, C.; Li, X. Effect of pretreatment on the structure and properties of nanofibrillated cellulose from soybean residues. BioResources 2019, 14, 554–560. [Google Scholar] [CrossRef]
- Liu, L.; Gerard, G.; Peng, Z.; Yu, Z. The Use of Corn Stover-Derived Nanocellulose as a Stabilizer of Oil-in-Water Emulsion. Polymers 2023, 15, 757. [Google Scholar] [CrossRef] [PubMed]
- Lagogianni, C.S.; Tsitsigiannis, D.I. Effective biopesticides and biostimulants to reduce aflatoxins in maize fields. Front. Microbiol. 2019, 10, 2645. [Google Scholar] [CrossRef] [PubMed]
- Silvério, H.A.; Neto, W.P.F.; Dantas, N.O.; Pasquini, D. Extraction and characterization of cellulose nanocrystals from corncob for application as reinforcing agent in nanocomposites. Ind. Crops Prod. 2013, 44, 427–436. [Google Scholar] [CrossRef]
- Aloui, H.; Khwaldia, K.; Licciardello, F.; Mazzaglia, A.; Muratore, G.; Hamdi, M.; Restuccia, C. Efficacy of the combined application of chitosan and Locust Bean Gum with different citrus essential oils to control postharvest spoilage caused by Aspergillus flavus in dates. Int. J. Food Microbiol. 2014, 170, 21–28. [Google Scholar] [CrossRef] [PubMed]
- Kampeerapappun, P. Extraction and characterization of cellulose nanocrystals produced by acid hydrolysis from corn husk. J. Met. Mater. Miner. 2015, 25, 19–26. [Google Scholar]
- Liu, C.; Li, B.; Du, H.; Lv, D.; Zhang, Y.; Yu, G.; Mu, X.; Peng, H. Properties of nanocellulose isolated from corncob residue using sulfuric acid, formic acid, oxidative and mechanical methods. Carbohydr. Polym. 2016, 151, 716–724. [Google Scholar] [CrossRef]
- Saito, T.; Isogai, A. TEMPO-mediated oxidation of native cellulose. The effect of oxidation conditions on chemical and crystal structures of the water-insoluble fractions. Biomacromolecules 2004, 5, 1983–1989. [Google Scholar] [CrossRef]
- Isogai, A.; Saito, T.; Fukuzumi, H. TEMPO-oxidized cellulose nanofibers. Nanoscale 2011, 3, 71–85. [Google Scholar] [CrossRef]
- Mahendra, I.P.; Wirjosentono, B.; Ismail, H.; Mendez, J. Thermal and morphology properties of cellulose nanofiber from TEMPO-oxidized lower part of empty fruit bunches (LEFB). Open Chem. 2019, 17, 526–536. [Google Scholar]
- Rohaizu, R.; Wanrosli, W. Sono-assisted TEMPO oxidation of oil palm lignocellulosic biomass for isolation of nanocrystalline cellulose. Ultrason. Sonochemistry 2017, 34, 631–639. [Google Scholar] [CrossRef]
- Besbes, I.; Alila, S.; Boufi, S. Nanofibrillated cellulose from TEMPO-oxidized eucalyptus fibres: Effect of the carboxyl content. Carbohydr. Polym. 2011, 84, 975–983. [Google Scholar] [CrossRef]
- Jiang, F.; Kondo, T.; Hsieh, Y.-L. Rice straw cellulose nanofibrils via aqueous counter collision and differential centrifugation and their self-assembled structures. ACS Sustain. Chem. Eng. 2016, 4, 1697–1706. [Google Scholar] [CrossRef]
- Yang, X.; Reid, M.S.; Olsén, P.; Berglund, L.A. Eco-friendly cellulose nanofibrils designed by nature: Effects from preserving native state. ACS Nano 2019, 14, 724–735. [Google Scholar] [CrossRef] [PubMed]
- Zhou, Y.; Saito, T.; Bergström, L.; Isogai, A. Acid-free preparation of cellulose nanocrystals by TEMPO oxidation and subsequent cavitation. Biomacromolecules 2018, 19, 633–639. [Google Scholar] [CrossRef] [PubMed]
- Xu, J.; Krietemeyer, E.F.; Boddu, V.M.; Liu, S.X.; Liu, W.-C. Production and characterization of cellulose nanofibril (CNF) from agricultural waste corn stover. Carbohydr. Polym. 2018, 192, 202–207. [Google Scholar] [CrossRef] [PubMed]
- Huang, D.; Hong, H.; Huang, W.; Zhang, H.; Hong, X. Scalable preparation of cellulose nanofibers from office waste paper by an environment-friendly method. Polymers 2021, 13, 3119. [Google Scholar] [CrossRef]
- Wang, Q.; Wei, W.; Li, X.; Sun, J.; He, J.; He, M. Comparative study of alkali and acidic cellulose solvent pretreatment of corn stover for fermentable sugar production. BioResources 2016, 11, 482–491. [Google Scholar] [CrossRef]
- Li, B.; Xu, W.; Kronlund, D.; Määttänen, A.; Liu, J.; Smått, J.-H.; Peltonen, J.; Willför, S.; Mu, X.; Xu, C. Cellulose nanocrystals prepared via formic acid hydrolysis followed by TEMPO-mediated oxidation. Carbohydr. Polym. 2015, 133, 605–612. [Google Scholar] [CrossRef]
- Basu, P. Biomass Gasification, Pyrolysis and Torrefaction: Practical Design and Theory; Academic press: Cambridge, MA, USA, 2018. [Google Scholar]
- He, T.; Jiang, Z.; Wu, P.; Yi, J.; Li, J.; Hu, C. Fractionation for further conversion: From raw corn stover to lactic acid. Sci. Rep. 2016, 6, 38623. [Google Scholar] [CrossRef] [PubMed]
- Kumar, R.; Mago, G.; Balan, V.; Wyman, C.E. Physical and chemical characterizations of corn stover and poplar solids resulting from leading pretreatment technologies. Bioresour. Technol. 2009, 100, 3948–3962. [Google Scholar] [CrossRef] [PubMed]
- El Bakkari, M.; Bindiganavile, V.; Goncalves, J.; Boluk, Y. Preparation of cellulose nanofibers by TEMPO-oxidation of bleached chemi-thermomechanical pulp for cement applications. Carbohydr. Polym. 2019, 203, 238–245. [Google Scholar] [CrossRef]
- Fukuzumi, H.; Saito, T.; Okita, Y.; Isogai, A. Thermal stabilization of TEMPO-oxidized cellulose. Polym. Degrad. Stab. 2010, 95, 1502–1508. [Google Scholar] [CrossRef]
- Smyth, M.; García, A.; Rader, C.; Foster, E.J.; Bras, J. Extraction and process analysis of high aspect ratio cellulose nanocrystals from corn (Zea mays) agricultural residue. Ind. Crops Prod. 2017, 108, 257–266. [Google Scholar] [CrossRef]
- Al-Ahmed, Z.A.; Hassan, A.A.; El-Khouly, S.M.; El-Shafey, S.E. TEMPO-oxidized cellulose nanofibers/TiO2 nanocomposite as new adsorbent for Brilliant Blue dye removal. Polym. Bull. 2020, 77, 6213–6226. [Google Scholar]
- Sultana, T.; Sultana, S.; Nur, H.P.; Khan, M.W. Studies on mechanical, thermal and morphological properties of betel nut husk nano cellulose reinforced biodegradable polymer composites. J. Compos. Sci. 2020, 4, 83. [Google Scholar] [CrossRef]
- Mansaray, K.; Ghaly, A. Thermal degradation of rice husks in nitrogen atmosphere. Bioresour. Technol. 1998, 65, 13–20. [Google Scholar] [CrossRef]
- Rahaman, S.M.; Bardhan, A.; Mandal, T.; Chakraborty, M.; Karmakar, K.; Dhibar, S.; Sharma, S.; Chakravarty, M.; Ibrahim, S.M.; Saha, B. Understanding the effect of surfactants’ hydrophobicity on the growth of lanthanum sulfide nanospheres in water-in-oil microemulsions: A detailed dynamic light scattering, small angle X-ray scattering, and microscopy study. New J. Chem. 2023, 47, 10309–10321. [Google Scholar] [CrossRef]
- Hahn, A.; Mittal, K. Mechanism of demulsification of oil-in-water emulsion in the centrifuge. Colloid Polym. Sci. 1979, 257, 959–967. [Google Scholar] [CrossRef]
- Salvia-Trujillo, L.; Rojas-Graü, A.; Soliva-Fortuny, R.; Martín-Belloso, O. Physicochemical characterization of lemongrass essential oil–alginate nanoemulsions: Effect of ultrasound processing parameters. Food Bioprocess Technol. 2013, 6, 2439–2446. [Google Scholar] [CrossRef]
- da Silva, B.D.; Rosario, D.K.A.d.; Conte-Junior, C.A. Can droplet size influence antibacterial activity in ultrasound-prepared essential oil nanoemulsions? Crit. Rev. Food Sci. Nutr. 2022, 1–11. [Google Scholar] [CrossRef] [PubMed]
- Lu, G.W.; Gao, P. Emulsions and microemulsions for topical and transdermal drug delivery. In Handbook of Non-Invasive Drug Delivery Systems; Elsevier: Amsterdam, The Netherlands, 2010; pp. 59–94. [Google Scholar]
- Xu, M.; Zhang, W.; Jiang, J.; Pei, X.; Zhu, H.; Cui, Z.; Binks, B.P. Transition between a Pickering emulsion and an oil-in-dispersion emulsion costabilized by alumina nanoparticles and a cationic surfactant. Langmuir 2020, 36, 15543–15551. [Google Scholar] [CrossRef] [PubMed]
- Paranagama, P.; Abeysekera, K.; Abeywickrama, K.; Nugaliyadde, L. Fungicidal and anti-aflatoxigenic effects of the essential oil of Cymbopogon citratus (DC.) Stapf. (lemongrass) against Aspergillus flavus Link. isolated from stored rice. Lett. Appl. Microbiol. 2003, 37, 86–90. [Google Scholar] [CrossRef]
- Martinazzo, A.P.; de Oliveira, F.d.S.; de Souza Teodoro, C.E. Antifungal activity of Cymbopogon citratus essential oil against Aspergillus flavus. Ciência E Nat. 2019, 41, e20. [Google Scholar] [CrossRef]
- Xiang, F.; Zhao, Q.; Zhao, K.; Pei, H.; Tao, F. The efficacy of composite essential oils against aflatoxigenic fungus Aspergillus flavus in maize. Toxins 2020, 12, 562. [Google Scholar] [CrossRef]
- Wan, J.; Zhong, S.; Schwarz, P.; Chen, B.; Rao, J. Physical properties, antifungal and mycotoxin inhibitory activities of five essential oil nanoemulsions: Impact of oil compositions and processing parameters. Food Chem. 2019, 291, 199–206. [Google Scholar] [CrossRef]
- Schaneberg, B.T.; Khan, I.A. Comparison of extraction methods for marker compounds in the essential oil of lemon grass by GC. J. Agric. Food Chem. 2002, 50, 1345–1349. [Google Scholar] [CrossRef]
- Tang, X.; Shao, Y.-L.; Tang, Y.-J.; Zhou, W.-W. Antifungal activity of essential oil compounds (geraniol and citral) and inhibitory mechanisms on grain pathogens (Aspergillus flavus and Aspergillus ochraceus). Molecules 2018, 23, 2108. [Google Scholar] [CrossRef]
- Silva, C.d.B.d.; Guterres, S.S.; Weisheimer, V.; Schapoval, E.E. Antifungal activity of the lemongrass oil and citral against Candida spp. Braz. J. Infect. Dis. 2008, 12, 63–66. [Google Scholar] [CrossRef]
- Xiang, F.; Bai, J.; Tan, X.; Chen, T.; Yang, W.; He, F. Antimicrobial activities and mechanism of the essential oil from Artemisia argyi Levl. et Van. var. argyi cv. Qiai. Ind. Crops Prod. 2018, 125, 582–587. [Google Scholar] [CrossRef]
- Marei, G.I.K.; Rabea, E.I.; Badawy, M.E. Preparation and characterizations of chitosan/citral nanoemulsions and their antimicrobial activity. Appl. Food Biotechnol. 2018, 5, 69–78. [Google Scholar]
- Kapustová, M.; Granata, G.; Napoli, E.; Puškárová, A.; Bučková, M.; Pangallo, D.; Geraci, C. Nanoencapsulated essential oils with enhanced antifungal activity for potential application on agri-food, material and environmental fields. Antibiotics 2021, 10, 31. [Google Scholar] [CrossRef] [PubMed]
- Salvia-Trujillo, L.; Rojas-Graü, A.; Soliva-Fortuny, R.; Martín-Belloso, O. Physicochemical characterization and antimicrobial activity of food-grade emulsions and nanoemulsions incorporating essential oils. Food Hydrocoll. 2015, 43, 547–556. [Google Scholar] [CrossRef]
- Badr, M.M.; Badawy, M.E.; Taktak, N.E. Characterization, antimicrobial activity, and antioxidant activity of the nanoemulsions of Lavandula spica essential oil and its main monoterpenes. J. Drug Deliv. Sci. Technol. 2021, 65, 102732. [Google Scholar] [CrossRef]
- Donsì, F.; Annunziata, M.; Sessa, M.; Ferrari, G. Nanoencapsulation of essential oils to enhance their antimicrobial activity in foods. LWT-Food Sci. Technol. 2011, 44, 1908–1914. [Google Scholar] [CrossRef]
- Liolios, C.; Gortzi, O.; Lalas, S.; Tsaknis, J.; Chinou, I. Liposomal incorporation of carvacrol and thymol isolated from the essential oil of Origanum dictamnus L. and in vitro antimicrobial activity. Food Chem. 2009, 112, 77–83. [Google Scholar] [CrossRef]
- Prakash, B.; Kujur, A.; Yadav, A.; Kumar, A.; Singh, P.P.; Dubey, N. Nanoencapsulation: An efficient technology to boost the antimicrobial potential of plant essential oils in food system. Food Control 2018, 89, 1–11. [Google Scholar] [CrossRef]
- Mikulcová, V.; Bordes, R.; Kašpárková, V. On the preparation and antibacterial activity of emulsions stabilized with nanocellulose particles. Food Hydrocoll. 2016, 61, 780–792. [Google Scholar] [CrossRef]
- Negi, A.; Kesari, K.K. Chitosan nanoparticle encapsulation of antibacterial essential oils. Micromachines 2022, 13, 1265. [Google Scholar] [CrossRef]
- Granata, G.; Stracquadanio, S.; Leonardi, M.; Napoli, E.; Consoli, G.M.L.; Cafiso, V.; Stefani, S.; Geraci, C. Essential oils encapsulated in polymer-based nanocapsules as potential candidates for application in food preservation. Food Chem. 2018, 269, 286–292. [Google Scholar] [CrossRef] [PubMed]







| Sample | Crystallinity Index (%) |
|---|---|
| Corn stover after washing (CS) | 57.07 |
| Corn stover after bleaching treatment (CS-BT) | 67.20 |
| Corn stover after bleaching and alkaline treatment (CS-BAT) | 69.95 |
| Corn stover after TEMPO oxidation (TEMPO-CNF) | 69.50 |
| Oil/Tween Ratio | Lemongrass Essential Oil (wt%) | Tween 80 (wt%) | TEMPO-CNF (wt%) | After Centrifugation | After 2 Freeze–Thaw Cycles | After 30 Days Storage |
|---|---|---|---|---|---|---|
| 3:1 | 2.5 | 0.83 | 0 | PS 1 | PS | NPS 2 |
| 5 | 1.67 | 0 | PS | PS | NPS | |
| 10 | 3.33 | 0 | PS | PS | NPS | |
| 20 | 6.67 | 0 | PS | PS | NPS | |
| 2.5 | 0.83 | 0.3 | PS | NPS | NPS | |
| 5 | 1.67 | 0.3 | PS | NPS | NPS | |
| 10 | 3.33 | 0.3 | PS | NPS | NPS | |
| 20 | 6.67 | 0.3 | PS | NPS | NPS | |
| 1:1 | 2.5 | 2.5 | 0 | NPS | PS | NPS |
| 5 | 5 | 0 | NPS | PS | NPS | |
| 10 | 10 | 0 | NPS | PS | NPS | |
| 20 | 20 | 0 | NPS | PS | NPS | |
| 2.5 | 2.5 | 0.3 | NPS | NPS | NPS | |
| 5 | 5 | 0.3 | NPS | NPS | NPS | |
| 10 | 10 | 0.3 | NPS | NPS | NPS | |
| 20 | 20 | 0.3 | NPS | NPS | NPS | |
| 1:3 | 2.5 | 7.5 | 0.3 | NPS 3 | NPS 3 | NPS |
| 5 | 15 | 0.3 | NPS 3 | NPS 3 | NPS |
| Sample Label | Lemongrass Essential Oil (wt%) | Tween 80 (wt%) | TEMPO-CNF (wt%) | Mean Particle Size (nm) | Polydispersity Index | Zeta Potential (mV) |
|---|---|---|---|---|---|---|
| EO + W | 2.5 | 0 | 0 | 619 ± 86 | 0.69 ± 0.09 | −53 ± 4 |
| EO + T80 | 2.5 | 7.5 | 0 | 19 ± 2 | 0.46 ± 0.06 | −21 ± 9 |
| EO + T80 + TCNF | 2.5 | 7.5 | 0.3 | 19 ± 2 | 0.63 ± 0.08 | −34 ± 6 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Liu, L.; Fisher, K.D.; Friest, M.A.; Gerard, G. Characterization and Antifungal Activity of Lemongrass Essential Oil-Loaded Nanoemulsion Stabilized by Carboxylated Cellulose Nanofibrils and Surfactant. Polymers 2023, 15, 3946. https://doi.org/10.3390/polym15193946
Liu L, Fisher KD, Friest MA, Gerard G. Characterization and Antifungal Activity of Lemongrass Essential Oil-Loaded Nanoemulsion Stabilized by Carboxylated Cellulose Nanofibrils and Surfactant. Polymers. 2023; 15(19):3946. https://doi.org/10.3390/polym15193946
Chicago/Turabian StyleLiu, Lingling, Kaleb D. Fisher, Mason A. Friest, and Gina Gerard. 2023. "Characterization and Antifungal Activity of Lemongrass Essential Oil-Loaded Nanoemulsion Stabilized by Carboxylated Cellulose Nanofibrils and Surfactant" Polymers 15, no. 19: 3946. https://doi.org/10.3390/polym15193946
APA StyleLiu, L., Fisher, K. D., Friest, M. A., & Gerard, G. (2023). Characterization and Antifungal Activity of Lemongrass Essential Oil-Loaded Nanoemulsion Stabilized by Carboxylated Cellulose Nanofibrils and Surfactant. Polymers, 15(19), 3946. https://doi.org/10.3390/polym15193946







