Recent Advancements and Strategies for Overcoming the Blood–Brain Barrier Using Albumin-Based Drug Delivery Systems to Treat Brain Cancer, with a Focus on Glioblastoma
Abstract
:1. Introduction
2. Classification of Brain Tumors
3. Prevalence and Treatment
4. The Blood–Brain Barrier (BBB)
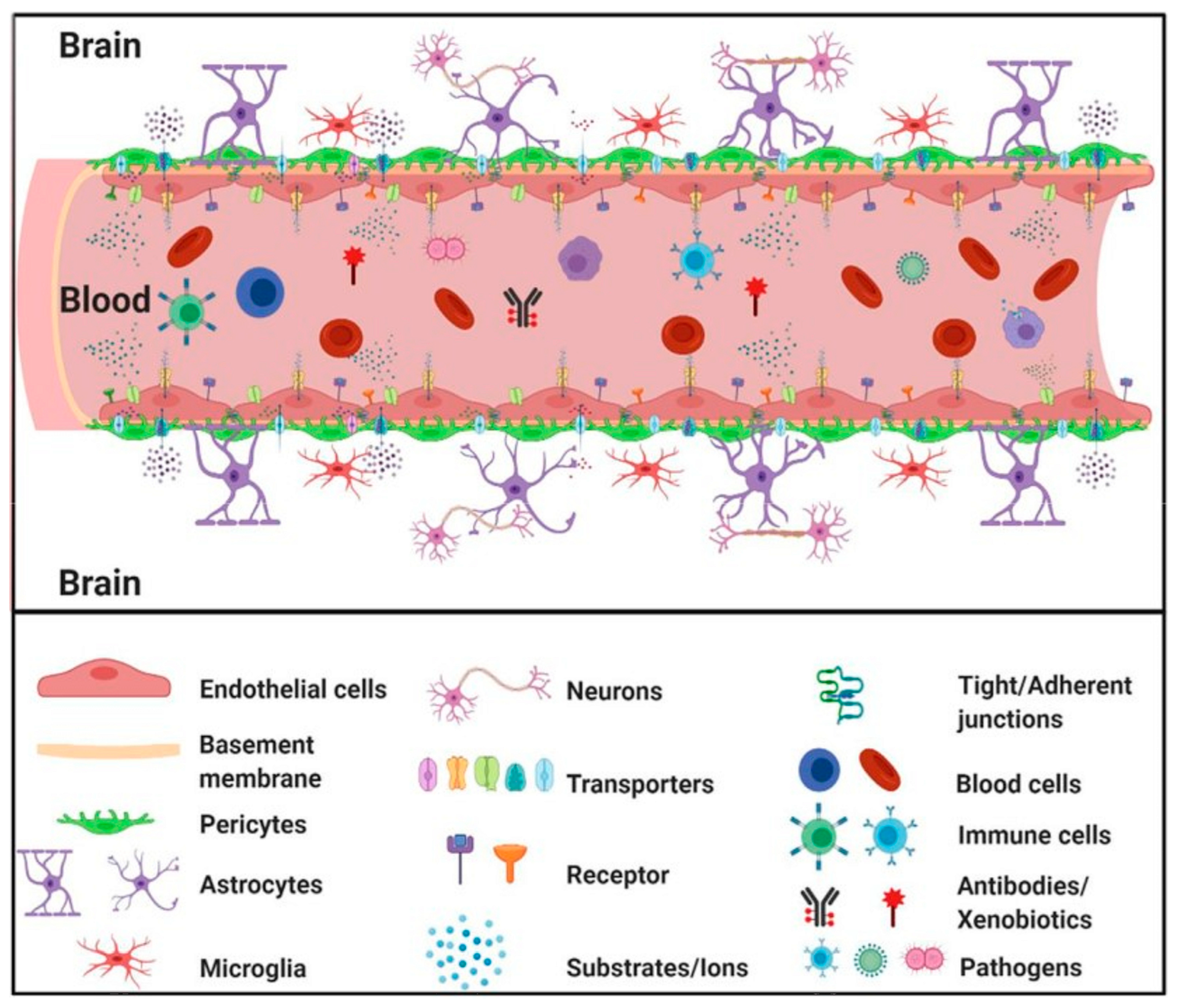
5. Other Central Nervous System Barriers
6. Transport through the BBB
7. Blood–Brain Tumor Barrier
8. Factors Influencing the Passage of Drug Molecules through the BBB
9. Challenges in Drug Delivery for the Treatment of Brain Tumors
10. The Albumin Structure and Properties
11. Albumin’s Ability to Target Cancer Cells
12. Techniques for the Preparation of Albumin-Based Release Nanosystems
13. Delivery Systems Based on Functionalized Nanoparticles Used in Cancer Treatment
13.1. Modification of the Albumin Nanoparticle Surface
13.2. Albumin Conjugates and Albumin-Coated Magnetic Nanoparticles Used as Theranostic Platforms
14. Albumin-Based Delivery Systems That Overcome the BBB and Treat Glioblastoma
15. Clinical Trials
16. Conclusions and Perspectives
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Ventero, M.P.; Fuentes-Baile, M.; Quereda, C.; Perez-Valeciano, E.; Alenda, C.; Garcia-Morales, P.; Esposito, D.; Dorado, P.; Barbera, V.M.; Saceda, M. Radiotherapy resistance acquisition in Glioblastoma. Role of SOCS1 and SOCS3. PLoS ONE 2019, 14, e0212581. [Google Scholar] [CrossRef] [PubMed]
- Grochans, S.; Cybulska, A.M.; Simińska, D.; Korbecki, J.; Kojder, K.; Chlubek, D.; Baranowska-Bosiacka, I. Epidemiology of Glioblastoma Multiforme–Literature Review. Cancers 2022, 14, 2412. [Google Scholar] [CrossRef]
- Dong, X. Current Strategies for Brain Drug Delivery. Theranostics 2018, 8, 1481–1493. [Google Scholar] [CrossRef] [PubMed]
- Geldenhuys, W.J.; Mohammad, A.S.; Adkins, C.E.; Lockman, P.R. Molecular Determinants of Blood-Brain Barrier Permeation. Ther. Deliv. 2015, 6, 961–971. [Google Scholar] [CrossRef] [PubMed]
- Banks, W.A. From blood-brain barrier to blood-brain interface: New opportunities for CNS drug delivery. Nat. Rev. Drug Discov. 2016, 15, 275–292. [Google Scholar] [CrossRef]
- Cruz, J.V.R.; Batista, C.; Afonso, B.D.H.; Alexandre-Moreira, M.S.; Dubois, L.G.; Pontes, B.; Moura Neto, V.; Mendes, F.d.A. Obstacles to Glioblastoma Treatment Two Decades after Temozolomide. Cancers 2022, 14, 3203. [Google Scholar] [CrossRef]
- Shi, H.; Sun, S.; Xu, H.; Zhao, Z.; Han, Z.; Jia, J.; Wu, D.; Lu, J.; Liu, H.; Yu, R. Combined Delivery of Temozolomide and siPLK1 Using Targeted Nanoparticles to Enhance Temozolomide Sensitivity in Glioma. Int. J. Nanomed. 2020, 15, 3347–3362. [Google Scholar] [CrossRef]
- Petrenko, D.; Chubarev, V.; Syzrantsev, N.; Ismail, N.; Merkulov, V.; Sologova, S.; Grigorevskikh, E.; Smolyarchuk, E.; Alyautdin, R. Temozolomide Efficacy and Metabolism: The Implicit Relevance of Nanoscale Delivery Systems. Molecules 2022, 27, 3507. [Google Scholar] [CrossRef]
- Fang, C.; Wang, K.; Stephen, Z.R.; Mu, Q.; Kievit, F.M.; Chiu, D.T.; Press, O.W.; Zhang, M. Temozolomide Nanoparticles for Targeted Glioblastoma Therapy. ACS Appl. Mater. Interfaces 2015, 7, 6674–6682. [Google Scholar] [CrossRef]
- Michael, J.S.; Lee, S.; Zhang, M.; Yu, J.S. Nanotechnology for Treatment of Glioblastoma Multiforme. J. Transl. Int. Med. 2018, 6, 128–133. [Google Scholar] [CrossRef]
- Kumari, S.; Ahsan, S.M.; Kumar, J.M.; Kondapi, A.K.; Rao, N.M. Overcoming blood-brain barrier with a dual purpose Temozolomide loaded Lactoferrin nanoparticles for combating glioma (SERP-17-12433). Sci. Rep. 2017, 7, 6602. [Google Scholar] [CrossRef] [PubMed]
- Alphandéry, E. Nano-Therapies for Glioblastoma Treatment. Cancers 2020, 12, 242. [Google Scholar] [CrossRef] [PubMed]
- Tapeinos, C.; Battaglini, M.; Ciofani, G. Advances in the design of solid lipid nanoparticles and nanostructured lipid carriers for targeting brain diseases. J. Control. Release 2017, 264, 306–332. [Google Scholar] [CrossRef] [PubMed]
- Kumar, A.; Badde, S.; Kamble, R.; Pokharkar, V. Development and characterization of liposomal drug delivery system for Nimesulide. Int. J. Pharm. Pharm. Sci. 2010, 2, 87–89. [Google Scholar]
- Satapathy, M.K.; Yen, T.L.; Jan, J.S.; Tang, R.D.; Wang, J.Y.; Taliyan, R.; Yang, C.H. Solid Lipid Nanoparticles (SLNs): An Advanced Drug Delivery System Targeting Brain through BBB. Pharmaceutics 2021, 13, 1183. [Google Scholar] [CrossRef]
- Patidar, A.; Thakur, D.S.; Kumar, P.; Verma, J. A review on novel lipid based nanocarriers. Int. J. Pharm. Pharm. Sci. 2010, 2, 30–35. [Google Scholar]
- Beloqui, A.; Solinís, M.Á.; Rodríguez-Gascón, A.; Almeida, A.J.; Préat, V. Nanostructured lipid carriers: Promising drug delivery systems for future clinics. Nanomedicine 2016, 12, 143–161. [Google Scholar] [CrossRef]
- Gaspar, D.P.; Faria, V.; Quintas, J.P.; Almeida, A.J. Targeted delivery of lipid nanoparticles by means of surface chemical modification. Curr. Org. Chem. 2017, 21, 2360–2375. [Google Scholar] [CrossRef]
- Bertrand, N.; Wu, J.; Xu, X.; Kamaly, N.; Farokhzad, O.C. Cancer nanotechnology: The impact of passive and active targeting in the era of modern cancer biology. Adv. Drug Deliv. Rev. 2014, 66, 2–25. [Google Scholar] [CrossRef]
- Ghasemiyeh, P.; Mohammadi-Samani, S. Solid lipid nanoparticles, and nanostructured lipid carriers as novel drug delivery systems: Applications, advantages and disadvantages. Res. Pharm. Sci. 2018, 13, 288–303. [Google Scholar] [CrossRef]
- Karimi, M.; Bahrami, S.; Ravari, S.B.; Zangabad, P.S.; Mirshekari, H.; Bozorgomid, M.; Shahreza, S.; Sori, M.; Hamblin, M.R. Albumin nanostructures as advanced drug delivery systems. Expert Opin. Drug Deliv. 2016, 13, 1609. [Google Scholar] [CrossRef]
- Hornok, V. Serum Albumin Nanoparticles: Problems and Prospects. Polymers 2021, 13, 3759. [Google Scholar] [CrossRef] [PubMed]
- An, F.; Zhang, H. Strategies for Preparing Albumin-based Nanoparticles for Multifunctional Bioimaging and Drug Delivery. Theranostics 2017, 7, 3667–3689. [Google Scholar] [CrossRef] [PubMed]
- Panico, S.; Capolla, S.; Bozzer, S.; Toffoli, G.; Dal Bo, M.; Macor, P. Biological Features of Nanoparticles: Protein Corona Formation and Interaction with the Immune System. Pharmaceutics 2022, 14, 2605. [Google Scholar] [CrossRef]
- Chubarov, A.S. Serum Albumin for Magnetic Nanoparticles Coating. Magnetochemistry 2022, 8, 13. [Google Scholar] [CrossRef]
- Ballinger, J.R. Challenges in Preparation of Albumin Nanoparticle-Based Radiopharmaceuticals. Molecules 2022, 27, 8596. [Google Scholar] [CrossRef]
- Hassanin, I.; Elzoghby, A. Albumin-based nanoparticles: A promising strategy to overcome cancer drug resistance. Cancer Drug Resist. 2020, 3, 930–946. [Google Scholar] [CrossRef]
- Shen, X.; Liu, X.; Li, T.; Chen, Y.; Chen, Y.; Wang, P.; Zheng, L.; Yang, H.; Wu, C.; Deng, S.; et al. Recent Advancements in Serum Albumin-Based Nanovehicles Toward Potential Cancer Diagnosis and Therapy. Front. Chem. 2021, 9, 746646. [Google Scholar] [CrossRef]
- Kunde, S.S.; Wairkar, S. Targeted delivery of albumin nanoarticles for breast cancer: A review. Colloids Surf. B Biointerfaces 2022, 213, 112422. [Google Scholar] [CrossRef]
- Chen, R.; Smith-Cohn, M.; Cohen, A.L.; Colman, H. Glioma subclassifications and their clinical significance. Neurotherapeutics 2017, 14, 284–297. [Google Scholar] [CrossRef]
- Cerna, T.; Stiborova, M.; Adam, V.; Kizek, R.; Eckschlager, T. Nanocarrier drugs in the treatment of brain tumors. J. Cancer Metastasis. Treat 2016, 2, 407–416. [Google Scholar] [CrossRef]
- Perry, A.; Wesseling, P. Histologic classification of gliomas. Handb. Clin. Neurol. 2016, 134, 71–95. [Google Scholar] [CrossRef] [PubMed]
- Batash, R.; Asna, N.; Schaffer, P.; Francis, N.; Schaffer, M. Glioblastoma Multiforme, Diagnosis, and Treatment; Recent Literature Review. Curr. Med. Chem. 2017, 24, 3002–3009. [Google Scholar] [CrossRef] [PubMed]
- Razavi, S.M.; Lee, K.E.; Jin, B.E.; Aujla, P.S.; Gholamin, S.; Li, G. Immune Evasion Strategies of Glioblastoma. Front. Surg. 2016, 3, 11. [Google Scholar] [CrossRef]
- Fabbro-Peray, P.; Zouaoui, S.; Darlix, A.; Fabbro, M.; Pallud, J.; Rigau, V.; Mathieu-Daude, H.; Bessaoud, F.; Bauchet, F.; Riondel, A.; et al. Association of patterns of care, prognostic factors, and use of radiotherapy–temozolomide therapy with survival in patients with newly diagnosed glioblastoma: A French national population-based study. J. Neurooncol. 2019, 142, 91–101. [Google Scholar] [CrossRef]
- Singla, A.K.; Madan, R.; Gupta, K.; Goyal, S.; Kumar, N.; Sahoo, S.K.; Uppal, D.K.; Ahuja, C.K. Clinical behaviour and outcome in pediatric glioblastoma: Current scenario. Radiat. Oncol. J. 2021, 39, 72–77. [Google Scholar] [CrossRef]
- Alimohammadi, E.; Bagheri, S.R.; Delfani, N.; Safari-Faramani, R.; Janatolmakan, M. Pediatric Non–Brain Stem High-Grade Glioma: A Single-Center Experience. Indian J. Neurosurg. 2020, 9, 162–169. [Google Scholar] [CrossRef]
- Haase, S.; Nuñez, F.M.; Gauss, J.C.; Thompson, S.; Brumley, E.; Lowenstein, P.; Castro, M.G. Hemispherical Pediatric High-Grade Glioma: Molecular Basis and Therapeutic Opportunities. Int. J. Mol. Sci. 2020, 21, 9654. [Google Scholar] [CrossRef]
- Philips, A.; Henshaw, D.L.; Lamburn, G.; O’Carroll, M.J. Brain tumours: Rise in glioblastoma multiforme incidence in England 1995–2015 suggests an adverse environmental or lifestyle factor. Int. J. Environ. Res. Public Health 2018, 2018, 7910754. [Google Scholar] [CrossRef]
- Li, K.; Lu, D.; Guo, Y.; Wang, C.; Liu, X.; Liu, Y.; Liu, D. Trends and patterns of incidence of diffuse glioma in adults in the United States, 1973–2014. Cancer Med. 2018, 7, 5281–5290. [Google Scholar] [CrossRef]
- Li, X.; Li, R.; Ren, H.; Liu, H.; Liu, H. Predicting factors of tumor progression in adult patients with low-grade glioma within five years after surgery. Transl. Cancer Res. 2021, 10, 51466. [Google Scholar] [CrossRef] [PubMed]
- Ellor, S.V.; Pagano-Young, T.A.; Avgeropoulos, N.G. Glioblastoma: Background, standard treatment paradigms, and supportive care considerations. J. Law Med. Ethics 2014, 42, 171–182. [Google Scholar] [CrossRef]
- Forst, D.A.; Nahed, B.V.; Loeffler, J.S.; Batchelor, T.T. Low-grade gliomas. Oncologist 2014, 19, 403–413. [Google Scholar] [CrossRef] [PubMed]
- Ferraris, C.; Cavalli, R.; Panciani, P.P.; Battaglia, L. Overcoming the Blood–Brain Barrier: Successes and Challenges in Developing Nanoparticle-Mediated Drug Delivery Systems for the Treatment of Brain Tumours. Int. J. Nanomed. 2020, 15, 2999–3022. [Google Scholar] [CrossRef] [PubMed]
- Wilson, T.A.; Karajannis, M.A.; Harter, D.H. Glioblastoma multiforme: State of the art and future therapeutics. Surg. Neurol. Int. 2014, 5, 64. [Google Scholar] [CrossRef]
- Davis, M.E. Glioblastoma: Overview of Disease and Treatment. Clin. J. Oncol. Nurs. 2016, 20, 2–8. [Google Scholar] [CrossRef]
- Ostrom, Q.T.; Bauchet, L.; Davis, F.G.; Deltour, I.; Fischer, J.L.; Langer, C.E.; Barnholtz-Sloan, J.S. The epidemiology of glioma in adults: A “state of the science” review. Neuro-Oncology 2014, 16, 896–913. [Google Scholar] [CrossRef]
- Caffo, M.; Cardali, S.; Fazzari, E.; Barresi, V.; Caruso, G. Nanoparticles drug-delivery systems and antiangiogenic approaches in the treatment of gliomas. Glioma 2018, 1, 183. [Google Scholar] [CrossRef]
- Fisher, J.P.; Adamson, D.C. Current FDA-Approved Therapies for High-Grade Malignant Gliomas. Biomedicines 2021, 9, 324. [Google Scholar] [CrossRef]
- Darlix, A.; Mandonnet, E.; Freyschlag, C.F.; Pinggera, D.; Forster, M.T.; Voss, M.; Steinbach, J.; Loughrey, C.; Goodden, J.; Banna, G.; et al. Chemotherapy and diffuse low-grade gliomas: A survey within the European Low-Grade Glioma Network. Neurooncol. Pract. 2019, 6, 264–273. [Google Scholar] [CrossRef]
- Ozdemir-Kaynak, E.; Qutub, A.A.; Yesil-Celiktas, O. Advances in glioblastoma multiforme treatment: New models for nanoparticle therapy. Front. Physiol. 2018, 9, 1–14. [Google Scholar] [CrossRef] [PubMed]
- Pena, E.S.; Graham-Gurysh, E.G.; Bachelder, E.M.; Ainslie, K.M. Design of Biopolymer-Based Interstitial Therapies for the Treatment of Glioblastoma. Int. J. Mol. Sci. 2021, 22, 13160. [Google Scholar] [CrossRef] [PubMed]
- Bellettato, C.M.; Scarpa, M. Possible strategies to cross the blood–brain barrier. Ital. J. Pediatr. 2018, 44, 131. [Google Scholar] [CrossRef] [PubMed]
- Kaya, M.; Ahishali, B. Basic physiology of the blood-brain barrier in health and disease: A brief overview. Tissue Barriers 2021, 9, 1840913. [Google Scholar] [CrossRef] [PubMed]
- Gonschior, H.; Haucke, V.; Lehmann, M. Super-Resolution Imaging of Tight and Adherens Junctions: Challenges and Open Questions. Int. J. Mol. Sci. 2020, 21, 744. [Google Scholar] [CrossRef]
- Abbott, N.; Rönnbäck, L.; Hansson, E. Astrocyte–endothelial interactions at the blood-brain barrier. Nat. Rev. Neurosci. 2006, 7, 41–53. [Google Scholar] [CrossRef]
- Theodorakis, P.E.; Müller, E.A.; Craster, R.V.; Matar, O.K. Physical insights into the blood-brain barrier translocation mechanisms. Phys Biol. 2017, 14, 041001. [Google Scholar] [CrossRef]
- Delsing, L.; Herland, A.; Falk, A.; Hicks, R.; Synnergren, J.; Zetterberg, H. Models of the blood-brain barrier using iPSC-derived cells. Mol. Cell. Neurosci. 2020, 107, 103533. [Google Scholar] [CrossRef]
- Kucharz, K.; Kristensen, K.; Johnsen, K.B.; Lund, M.A.; Lønstrup, M.; Moos, T.; Andresen, T.L.; Lauritzen, M.J. Post-capillary venules are the key locus for transcytosis-mediated brain delivery of therapeutic nanoparticles. Nat. Comm. 2021, 12, 1–17. [Google Scholar] [CrossRef]
- Hashimoto, Y.; Campbell, M. Tight junction modulation at the blood-brain barrier: Current and future perspectives. Biochim. Biophys. Acta-Biomembr. 2020, 1862, 183298. [Google Scholar] [CrossRef]
- Banks, W.A. Characteristics of compounds that cross the blood-brain barrier. BMC Neurol. 2009, 9 (Suppl. S1), S3. [Google Scholar] [CrossRef] [PubMed]
- Pardridge, W.M. Drug transport across the blood-brain barrier. J. Cereb. Blood Flow Metab. 2012, 32, 1959–1972. [Google Scholar] [CrossRef] [PubMed]
- Kinoshita, K. Traumatic brain injury: Pathophysiology for neurocritical care. J. Intensive Care 2016, 4, 29. [Google Scholar] [CrossRef]
- Andrew, W.; Mao, Y.; Amanda, L.; Jeffrey, R.; Dwight, B.; Peter, S. The blood-brain barrier: An engineering perspective. Front. Neuroeng. 2013, 6, 7. [Google Scholar] [CrossRef]
- Lajoie, J.M.; Shusta, E.V. Targeting receptor-mediated transport for delivery of biologics across the blood-brain barrier. Annu. Rev. Pharmacol. 2015, 55, 613. [Google Scholar] [CrossRef]
- Saunders, N.R.; Dreifuss, J.; Dziegielewska, K.M.; Johansson, P.A.; Habgood, M.D.; Møllgård, K.; Bauer, C. The rights and wrongs of blood-brain barrier permeability studies: A walk through 100 years of history. Front. Neurosci. 2014, 8, 404. [Google Scholar] [CrossRef]
- Hartsock, A.; Nelson, W.J. Adherens and Tight Junctions: Structure, Function and Connections to the Actin Cytoskeleton. Biochim. Biophys. Acta 2008, 1778, 660. [Google Scholar] [CrossRef]
- Marco, A.D.; Vignone, D.; Paz, O.G.; Fini, I.; Battista, M.R.; Cellucci, A.; Bracacel, E.; Auciello, G.; Veneziano, M.; Khetarpal, V.; et al. Establishment of an in Vitro Human Blood-Brain Barrier Model Derived from Induced Pluripotent Stem Cells and Comparison to a Porcine Cell-Based System. Cells 2020, 9, 994. [Google Scholar] [CrossRef]
- Frost, T.S.; Jiang, L.; Lynch, R.M.; Zohar, Y. Permeability of Epithelial/Endothelial Barriers in Transwells and Microfluidic Bilayer Devices. Micromachines 2019, 10, 533. [Google Scholar] [CrossRef]
- Thomsen, M.S.; Routhe, L.J.; Moos, T. The vascular basement membrane in the healthy and pathological brain. J. Cereb. Blood Flow Metab. 2017, 37, 3300–3317. [Google Scholar] [CrossRef]
- Wilhelm, I.; Molnár, J.; Fazakas, C.; Haskó, J.; Krizbai, I.A. Role of the Blood-Brain Barrier in the Formation of Brain Metastases. Int. J. Mol. Sci. 2013, 14, 1383–1411. [Google Scholar] [CrossRef] [PubMed]
- Menaceur, C.; Gosselet, F.; Fenart, L.; Saint-Pol, J. The Blood–Brain Barrier, an Evolving Concept Based on Technological Advances and Cell–Cell Communications. Cells 2022, 11, 133. [Google Scholar] [CrossRef]
- Engelhardt, S.; Huang, S.F.; Patkar, S.; Gassmann, M.; Ogunshola, O.O. Differential responses of blood-brain barrier associated cells to hypoxia and ischemia: A comparative study. Fluids Barriers CNS 2015, 12, 4. [Google Scholar] [CrossRef] [PubMed]
- Su, X.; Huang, L.; Qu, Y.; Xiao, D.; Mu, D. Pericytes in Cerebrovascular Diseases: An Emerging Therapeutic Target. Front. Cell. Neurosci. 2019, 13, 519. [Google Scholar] [CrossRef] [PubMed]
- Teleanu, R.I.; Preda, M.D.; Niculescu, G.; Vladâcenco, O.; Radu, C.I.; Grumezescu, A.M.; Teleanu, D.M. Current Strategies to Enhance Delivery of Drugs across the Blood–Brain Barrier. Pharmaceutics 2022, 14, 987. [Google Scholar] [CrossRef]
- Kadry, H.; Noorani, B.; Cucullo, L. A blood–brain barrier overview on structure, function, impairment, and biomarkers of integrity. Fluids Barriers CNS 2020, 17, 69. [Google Scholar] [CrossRef]
- Archie, S.R.; Shoyaib, A.A.; Cucullo, L. Blood-Brain Barrier Dysfunction in CNS Disorders and Putative Therapeutic Targets: An Overview. Pharmaceutics 2021, 13, 1779. [Google Scholar] [CrossRef]
- Daneman, R.; Prat, A. The Blood–Brain Barrier. Cold Spring Harb. Perspect. Biol. 2015, 7, a020412. [Google Scholar] [CrossRef]
- MacAulay, N.; Keep, R.F.; Zeuthen, T. Cerebrospinal fluid production by the choroid plexus: A century of barrier research revisited. Fluids Barriers CNS 2022, 19, 26. [Google Scholar] [CrossRef]
- Al Rihani, S.B.; Darakjian, L.I.; Deodhar, M.; Dow, P.; Turgeon, J.; Michaud, V. Disease-Induced Modulation of Drug Transporters at the Blood–Brain Barrier Level. Int. J. Mol. Sci. 2021, 22, 3742. [Google Scholar] [CrossRef]
- Brinker, T.; Stopa, E.; Morrison, J.; Klinge, P. A new look at cerebrospinal fluid circulation. Fluids Barriers CNS 2014, 11, 10. [Google Scholar] [CrossRef] [PubMed]
- Serlin, Y.; Shelef, I.; Knyazer, B.; Friedman, A. Anatomy and Physiology of the Blood-Brain Barrier. Semin. Cell Dev. Biol. 2015, 38, 2. [Google Scholar] [CrossRef] [PubMed]
- Cipolla, M.J. Barriers of the CNS. In The Cerebral Circulation; Cipolla, M.J., Ed.; Morgan & Claypool Life Sciences: San Rafael, CA, USA, 2009. Available online: https://www.ncbi.nlm.nih.gov/books/NBK53084/ (accessed on 12 September 2023).
- Goncalves, A.; Antonetti, D.A. Transgenic animal models to explore and modulate the blood brain and blood retinal barriers of the CNS. Fluids Barriers CNS 2022, 19, 86. [Google Scholar] [CrossRef] [PubMed]
- Johanson, C.E.; Stopa, E.G.; McMillan, P.N. The Blood–Cerebrospinal Fluid Barrier: Structure and Functional Significance. In The Blood-Brain and Other Neural Barriers. Methods in Molecular Biology; Nag, S., Ed.; Humana Press: Totowa, NJ, USA; Springer Nature: Cham, Switzerland, 2011; Volume 686, pp. 101–131. [Google Scholar] [CrossRef]
- Engelhardt, B.; Sorokin, L. The blood–brain and the blood-cerebrospinal fluid barriers: Function and dysfunction. Semin. Immunopathol. 2009, 31, 497–511. [Google Scholar] [CrossRef] [PubMed]
- Balslev, Y.; Saunders, N.R.; Møllgard, K. Ontogenetic development of diffusional restriction to protein at the pial surface of the rat brain: An electron microscopical study. J. Neurocytol. 1997, 26, 133–148. [Google Scholar] [CrossRef] [PubMed]
- Mitusova, K.; Peltek, O.O.; Karpov, T.E.; Muslimov, A.R.; Zyuzin, M.V.; Timin, A.S. Overcoming the blood-brain barrier for the therapy of malignant brain tumor: Current status and prospects of drug delivery approaches. J. Nanobiotechnol. 2022, 20, 412. [Google Scholar] [CrossRef]
- Lei, Y.; Han, H.; Yuan, F.; Javeed, A.; Zhao, Y. The brain interstitial system: Anatomy, modeling, in vivo measurement, and applications. Prog. Neurobiol. 2017, 157, 230–246. [Google Scholar] [CrossRef]
- Abbott, N.J. Astrocyte–endothelial interactions and blood–brain barrier permeability. J. Anat. 2002, 200, 629–638. [Google Scholar] [CrossRef]
- Lucke-Wold, B.P.; Logsdon, A.F.; Turner, R.C.; Rosen, C.L.; Huber, J.D. Aging, the metabolic syndrome, and ischemic stroke: Redefining the approach for studying the blood-brain barrier in complex neurological disease. Adv. Pharmacol. 2014, 71, 411–449. [Google Scholar] [CrossRef]
- Hladky, S.B.; Barrand, M.A. Fluid and ion transfer across the blood-brain and blood-cerebrospinal fluid barriers; a comparative account of mechanisms and roles. Fluids Barriers CNS 2016, 13, 19. [Google Scholar] [CrossRef]
- Ndemazie, N.B.; Inkoom, A.; Morfaw, E.F.; Smith, T.; Aghimien, M.; Ebesoh, D.; Agyare, E. Multi-disciplinary Approach for Drug and Gene Delivery Systems to the Brain. AAPS PharmSciTech 2022, 23, 11. [Google Scholar] [CrossRef] [PubMed]
- Boado, R.J.; Li, J.Y.; Nagaya, M.; Zhang, C.; Pardridge, W.M. Selective expression of the large neutral amino acid transporter at the blood–brain barrier. Proc. Natl. Acad. Sci. USA 1999, 96, 12079–12084. [Google Scholar] [CrossRef]
- Dienel, G.A. Brain Glucose Metabolism: Integration of Energetics with Function. Physiol. Rev. 2019, 99, 949–1045. [Google Scholar] [CrossRef] [PubMed]
- Sokoloff, L. The brain as a chemical machine. Prog. Brain Res. 1992, 94, 19–33. [Google Scholar] [CrossRef] [PubMed]
- Madsen, P.L.; Cruz, N.F.; Sokoloff, L.; Dienel, G.A. Cerebral oxygen/glucose ratio is low during sensory stimulation and rises above normal during recovery: Excess glucose consumption during stimulation is not accounted for by lactate efflux from or accumulation in brain tissue. J. Cereb. Blood Flow Metab. 1999, 19, 393–400. [Google Scholar] [CrossRef]
- Ball, K.K.; Cruz, N.F.; Mrak, R.E.; Dienel, G.A. Trafficking of glucose, lactate, and amyloid-beta from the inferior colliculus through perivascular routes. J. Cereb. Blood Flow Metab. 2010, 30, 162–176. [Google Scholar] [CrossRef]
- Mintun, M.A.; Lundstrom, B.N.; Snyder, A.Z.; Vlassenko, A.G.; Shulman, G.L.; Raichle, M.E. Blood flow and oxygen delivery to human brain during functional activity: Theoretical modelling and experimental data. Proc. Natl. Acad. Sci. USA 2001, 98, 6859–6864. [Google Scholar] [CrossRef]
- Powers, W.J.; Hirsch, I.B.; Cryer, P.E. Effect of stepped hypoglycemia on regional cerebral blood flow response to physiological brain activation. Am. J. Physiol. 1996, 270, H554–H559. [Google Scholar] [CrossRef]
- Ainslie, P.N.; Ogoh, S. Regulation of cerebral blood flow in mammals during chronic hypoxia: A matter of balance. Exp. Physiol. 2010, 95, 251–262. [Google Scholar] [CrossRef]
- Ainslie, P.N.; Duffin, J. Integration of cerebrovascular CO2 reactivity and chemoreflex control of breathing: Mechanisms of regulation, measurement, and interpretation. Am. J. Physiol. 2009, 296, 1473–1495. [Google Scholar] [CrossRef]
- Curley, G.; Kavanagh, B.P.; Laffey, J.G. Hypocapnia and the injured brain: More harm than benefit. Crit. Care Med. 2010, 38, 1348–1359. [Google Scholar] [CrossRef]
- Yudilevich, D.L.; de Rose, N. Blood–brain transfer of glucose and other molecules measured by rapid indicator dilution. Am. J. Physiol. 1971, 220, 841–846. [Google Scholar] [CrossRef]
- Lund-Andersen, H. Transport of glucose from blood to brain. Physiol. Rev. 1979, 59, 305–352. [Google Scholar] [CrossRef]
- Kubo, Y.; Ohtsuki, S.; Uchida, Y.; Terasaki, T. Quantitative determination of luminal and abluminal membrane distributions of transporters in porcine brain capillaries by plasma membrane fractionation and quantitative targeted proteomics. J. Pharm. Sci. 2015, 104, 3060–3068. [Google Scholar] [CrossRef] [PubMed]
- Cutler, R.W.; Sipe, J.C. Mediated transport of glucose between blood and brain in the cat. Am. J. Physiol. 1971, 220, 1182–1186. [Google Scholar] [CrossRef] [PubMed]
- Barros, L.F.; Bittner, C.X.; Loaiza, A.; Porras, O.H. A quantitative overview of glucose dynamics in the gliovascular unit. Glia 2007, 55, 1222–1237. [Google Scholar] [CrossRef]
- Leybaert, L.; de Bock, M.; van Moorhem, M.; Decrock, E.; de Vuyst, E. Neurobarrier coupling in the brain: Adjusting glucose entry with demand. J. Neurosci. Res. 2007, 85, 3213–3220. [Google Scholar] [CrossRef]
- Paulson, O.B.; Hasselbalch, S.G.; Rostrup, E.; Knudsen, G.M.; Pelligrino, D. Cerebral blood flow response to functional activation. J. Cereb. Blood Flow Metab. 2010, 30, 2–14. [Google Scholar] [CrossRef] [PubMed]
- Betz, A.L.; Goldstein, G.W. Polarity of the blood-brain barrier: Neutral amino acid transport into isolated brain capillaries. Science 1978, 202, 225–227. [Google Scholar] [CrossRef] [PubMed]
- Mann, G.E.; Yudilevich, D.L.; Sobrevia, L. Regulation of amino acid and glucose transporters in endothelial and smooth muscle cells. Physiol. Rev. 2003, 83, 183–252. [Google Scholar] [CrossRef]
- Oldendorf, W.H. Brain uptake of radiolabeled amino acids, amines, and hexoses after arterial injection. Am. J. Physiol. 1971, 221, 1629–1639. [Google Scholar] [CrossRef] [PubMed]
- Zaragozá, R. Transport of Amino Acids across the Blood-Brain Barrier. Front. Physiol. 2020, 11, 973. [Google Scholar] [CrossRef] [PubMed]
- Morawski, M.; Reinert, T.; Meyer-Klaucke, W.; Wagner, F.E.; Tröger, W.; Reinert, A.; Jäger, C.; Brückner, G.; Arendt, T. Ion exchanger in the brain: Quantitative analysis of perineuronally fixed anionic binding sites suggests diffusion barriers with ion sorting properties. Sci. Rep. 2015, 5, 16471. [Google Scholar] [CrossRef] [PubMed]
- Seo, S.; Kim, H.; Sung, J.H.; Choi, N.; Lee, K.; Kim, H.N. Microphysiological systems for recapitulating physiology and function of blood-brain barrier. Biomaterials 2020, 232, 119732. [Google Scholar] [CrossRef]
- Stoica, R.; Rusu, C.M.; Staicu, C.E.; Burlacu, A.E.; Radu, M.; Radu, B.M. Ca2+ homeostasis in brain microvascular endothelial cells. Int. Rev. Cell. Mol. Biol. 2021, 362, 55–110. [Google Scholar] [CrossRef]
- Hervé, F.; Ghinea, N.; Scherrmann, M. CNS Delivery Via Adsorptive Transcytosis. AAPS J. 2008, 10, 455–472. [Google Scholar] [CrossRef]
- Song, J.; Lu, C.; Leszek, J.; Zhang, J. Design and Development of Nanomaterial-Based Drug Carriers to Overcome the Blood–Brain Barrier by Using Different Transport Mechanisms. Int. J. Mol. Sci. 2021, 22, 10118. [Google Scholar] [CrossRef]
- Lo, E.H.; Singhal, A.B.; Torchilin, V.P.; Abbott, N.J. Drug Delivery to Damaged Brain. Brain Res. Rev. 2001, 38, 140–148. [Google Scholar] [CrossRef]
- Wanjale, M.V.; Kumar, G.S.V. Peptides as a therapeutic avenue for nanocarrier-aided targeting of glioma. Expert Opin. Drug Deliv. 2017, 14, 811–824. [Google Scholar] [CrossRef]
- Sharma, H.S.; Muresanu, D.F.; Castellani, R.J.; Nozari, A.; Lafuente, J.V.; Tian, Z.R.; Sahib, S.; Bryukhovetskiy, I.; Bryukhovetskiy, A.; Buzoianu, A.D.; et al. Chapter One—Pathophysiology of blood-brain barrier in brain tumor. Novel therapeutic advances using nanomedicine. In International Review of Neurobiology; Bryukhovetskiy, I., Sharma, A., Zhang, Z., Sharma, H.S., Eds.; Academic Press: Cambridge, MA, USA; Elsevier: Amsterdam, The Netherlands, 2020; Volume 151, pp. 1–66. [Google Scholar] [CrossRef]
- Alexander, A.; Agrawal, M.; Uddin, A.; Siddique, S.; Shehata, A.M.; Shaker, M.A.; Ur Rahman, S.A.; Abdul, M.I.; Shaker, M.A. Recent expansions of novel strategies towards the drug targeting into the brain. Int. J. Nanomed. 2019, 14, 5895–5909. [Google Scholar] [CrossRef]
- Kabanov, A.V.; Batrakova, E.V. New technologies for drug delivery across the blood brain barrier. Curr. Pharm. Des. 2004, 10, 1355–1363. [Google Scholar] [CrossRef] [PubMed]
- Misra, A.; Ganesh, S.; Shahiwala, A.; Shah, S.P. Drug delivery to the central nervous system: A review. J. Pharm. Pharm. Sci. 2003, 6, 252–273. [Google Scholar] [PubMed]
- Grubb, J.H.; Vogler, C.; Levy, B.; Galvin, N.; Tan, Y.; Sly, W.S. Chemically modified β-glucuronidase crosses blood–brain barrier and clears neuronal storage in murine mucopolysaccharidosis VII. Proc. Natl. Acad. Sci. USA 2008, 105, 2616–2621. [Google Scholar] [CrossRef] [PubMed]
- Mitragotri, S.; Burke, P.; Langer, R. Overcoming the challenges in administering biopharmaceuticals: Formulation and delivery strategies. Nat. Rev. Drug Discov. 2014, 13, 655–672. [Google Scholar] [CrossRef]
- de Boer, A.G.; Gaillard, P.J. Drug targeting to the brain. Annu. Rev. Pharmacol. Toxicol. 2007, 47, 323–355. [Google Scholar] [CrossRef]
- Lu, C.T.; Zhao, Y.Z.; Wong, H.L.; Cai, J.; Peng, L.; Tian, X.Q. Current approaches to enhance CNS delivery of drugs across the brain barriers. Int. J. Nanomed. 2014, 9, 2241–2257. [Google Scholar] [CrossRef]
- Wolak, D.J.; Thorne, R.G. Diffusion of Macromolecules in the Brain: Implications for Drug Delivery. Mol. Pharm. 2013, 10, 1492. [Google Scholar] [CrossRef]
- Obermeier, B.; Daneman, R.; Ransohoff, R. Development, maintenance and disruption of the blood-brain barrier. Nat. Med. 2013, 19, 1584–1596. [Google Scholar] [CrossRef]
- Hamann, G.F.; Liebetrau, M.; Martens, H.; Burggraf, D.; Kloss, C.U.; Bültemeier, G.; Wunderlich, N.; Jäger, G.; Pfefferkorn, T. Microvascular basal lamina injury after experimental focal cerebral ischemia and reperfusion in the rat. J. Cereb. Blood Flow Metab. 2002, 22, 526–533. [Google Scholar] [CrossRef]
- Voirin, A.C.; Perek, N.; Roche, F. Inflammatory stress induced by a combination of cytokines (IL-6, IL-17, TNF-α) leads to a loss of integrity on bEnd.3 endothelial cells in vitro BBB model. Brain Res. 2020, 1730, 146647. [Google Scholar] [CrossRef]
- Lichota, J.; Skjørringe, T.; Thomsen, L.B.; Moos, T. Macromolecular drug transport into the brain using targeted therapy. J. Neurochem. 2010, 113, 1–13. [Google Scholar] [CrossRef] [PubMed]
- Rautio, J.; Laine, K.; Gynther, M.; Savolainen, J. Prodrug Approaches for CNS Delivery. AAPS J. 2008, 10, 92–102. [Google Scholar] [CrossRef] [PubMed]
- Lentz, T.B.; Gray, S.J.; Samulski, R.J. Viral Vectors for Gene Delivery to the Central Nervous System. Neurobiol. Dis. 2012, 48, 179. [Google Scholar] [CrossRef] [PubMed]
- Woodworth, G.F.; Dunn, G.P.; Nance, E.A.; Hanes, J.; Brem, H. Emerging Insights into Barriers to Effective Brain Tumor Therapeutics. Front. Oncol. 2014, 4, 126. [Google Scholar] [CrossRef]
- Batrakova, E.V.; Gendelman, H.E.; Kabanov, A.V. Cell-Mediated Drugs Delivery. Expert Opin. Drug Deliv. 2011, 8, 415. [Google Scholar] [CrossRef]
- Bourdenx, M.; Dutheil, N.; Bezard, E.; Dehay, B. Systemic gene delivery to the central nervous system using Adeno-associated virus. Front. Mol. Neurosci. 2014, 7, 50. [Google Scholar] [CrossRef]
- Oller-Salvia, B.; Sánchez-Navarro, M.; Giralt, E.; Teixidó, M. Blood-brain barrier shuttle peptides: An emerging paradigm for brain delivery. Chem. Soc. Rev. 2016, 45, 4690–4707. [Google Scholar] [CrossRef]
- Altun, I.; Sonkaya, A. The most common side effects experienced by patients were receiving first cycle of chemotherapy. Iran. J. Public Health 2018, 47, 1218–1219. [Google Scholar]
- Pearce, A.; Haas, M.; Viney, R.; Pearson, S.-A.; Haywood, P.; Brown, C.; Ward, R. Incidence and severity of self-reported chemotherapy side effects in routine care: A prospective cohort study. PLoS ONE 2017, 12, e0184360. [Google Scholar] [CrossRef]
- Angeli, E.; Nguyen, T.T.; Janin, A.; Bousquet, G. How to make anticancer drugs cross the blood–Brain barrier to treat brain metastases. Int. J. Mol. Sci. 2019, 21, 22. [Google Scholar] [CrossRef]
- Deeken, J.F.; Löscher, W. The blood-brain barrier and cancer: Transporters, treatment, and trojan horses. Clin. Cancer Res. 2007, 13, 1663–1674. [Google Scholar] [CrossRef]
- El-Awady, R.; Saleh, E.; Hashim, A.; Soliman, N.; Dallah, A.; Elrasheed, A.; Elakraa, G. The Role of Eukaryotic and Prokaryotic ABC transporter family in failure of chemotherapy. Front. Pharmacol. 2017, 7, 535. [Google Scholar] [CrossRef]
- Choi, C.-H. ABC transporters as multidrug resistance mechanisms and the development of chemosensitizers for their reversal. Cancer Cell Int. 2005, 5, 30. [Google Scholar] [CrossRef]
- Banks, W.A. The Blood–Brain Barrier and CNS Drug Delivery. In Burger’s Medicinal Chemistry and Drug Discovery; Wiley: Hoboken, NJ, USA, 2021; pp. 1–22. [Google Scholar]
- Myers, R.; Ghosh, C. Drug Delivery Challenges in Brain Disorders across the Blood–Brain Barrier: Novel Methods and Future Considerations for Improved Therapy. Biomedicines 2021, 9, 1834. [Google Scholar] [CrossRef]
- Bors, L.A.; Erdö, F. Overcoming the Blood–Brain Barrier. Challenges and Tricks for CNS Drug Delivery. Sci. Pharm. 2019, 87, 6. [Google Scholar] [CrossRef]
- Wu, D.; Chen, Q.; Chen, X.; Han, F.; Chen, Z.; Wang, Y. The Blood–Brain Barrier: Structure, Regulation, and Drug Delivery. Signal Transduct. Target. Ther. 2023, 8, 217. [Google Scholar] [CrossRef]
- Ribeiro, M.M.B.; Domingues, M.M.; Freire, J.M.; Santos, N.C.; Castanho, M.A.R.B. Translocating the blood-brain barrier using electrostatics. Front. Cell. Neurosci. 2012, 6, 44. [Google Scholar] [CrossRef]
- Zhang, L.; Fan, J.; Li, G.; Yin, Z.; Fu, B.M. Transcellular Model for Neutral and Charged Nanoparticles across an In Vitro Blood–Brain Barrier. Cardiovasc. Eng. Technol. 2020, 11, 607–620. [Google Scholar] [CrossRef]
- Lockman, P.; Koziara, J.M.; Mumper, R.J.; Allen, D.D. Nanoparticle Surface Charges Alter Blood–Brain Barrier Integrity and Permeability. J. Drug Target. 2004, 12, 635–641. [Google Scholar] [CrossRef]
- Knudsen, K.B.; Northeved, H.; Ek, P.K.; Permin, A.; Andresen, T.L.; Larsen, S.; Wegener, K.M.; Lam, H.R.; Lykkesfeldt, J. Differential toxicological response to positively and negatively charged nanoparticles in the rat brain. Nanotoxicology 2014, 8, 764–774. [Google Scholar] [CrossRef]
- Yu, Z.; Li, Q.; Wang, J.; Yu, Y.; Wang, Y.; Zhou, Q.; Li, P. Reactive Oxygen Species-Related Nanoparticle Toxicity in the Biomedical Field. Nanoscale Res. Lett. 2020, 15, 115. [Google Scholar] [CrossRef]
- Fu, P.P.; Xia, Q.; Hwang, H.-M.; Ray, P.C.; Yu, H. Mechanisms of nanotoxicity: Generation of reactive oxygen species. J. Food Drug Anal. 2014, 22, 64–75. [Google Scholar] [CrossRef]
- Guo, Y.; Lee, H.; Fang, Z.; Velalopoulou, A.; Kim, J.; Thomas, M.B.; Liu, J.; Abramowitz, R.G.; Kim, Y.T.; Coskun, A.F.; et al. Single-cell analysis reveals effective siRNA delivery in brain tumors with microbubble-enhanced ultrasound and cationic nanoparticles. Sci. Adv. 2021, 7, eabf7390. [Google Scholar] [CrossRef] [PubMed]
- Ingusci, S.; Verlengia, G.; Soukupova, M.; Zucchini, S.; Simonato, M. Gene Therapy Tools for Brain Diseases. Front. Pharmacol. 2019, 10, 724. [Google Scholar] [CrossRef] [PubMed]
- Hersh, A.M.; Alomari, S.; Tyler, B.M. Crossing the Blood-Brain Barrier: Advances in Nanoparticle Technology for Drug Delivery in Neuro-Oncology. Int. J. Mol. Sci. 2022, 23, 4153. [Google Scholar] [CrossRef] [PubMed]
- Wanat, K. Biological barriers, and the influence of protein binding on the passage of drugs across them. Mol. Biol. Rep. 2020, 47, 3221–3231. [Google Scholar] [CrossRef]
- Raju, R.; Abuwatfa, W.H.; Pitt, W.G.; Husseini, G.A. Liposomes for the Treatment of Brain Cancer—A Review. Pharmaceuticals 2023, 16, 1056. [Google Scholar] [CrossRef]
- Kinoshita, M.; McDannold, N.; Jolesz, F.A.; Hynynen, K. Noninvasive localized delivery of Herceptin to the mouse brain by MRI-guided focused ultrasound-induced blood–brain barrier disruption. Proc. Natl. Acad. Sci. USA 2006, 103, 11719–11723. [Google Scholar] [CrossRef]
- Bhowmik, A.; Khan, R.; Ghosh, M.K. Blood brain barrier: A challenge for effectual therapy of brain tumors. Biomed. Res. Int. 2015, 2015, 320941. [Google Scholar] [CrossRef]
- Keller, A.; Merkel, O.; Popp, A. Intranasal drug delivery: Opportunities and toxicologic challenges during drug development. Drug Deliv. Transl. Res. 2022, 12, 735–757. [Google Scholar] [CrossRef]
- Kim, D.G.; Bynoe, M.S. A2A adenosine receptor modulates drug efflux transporter P-glycoprotein at the blood-brain barrier. J. Clin. Investig. 2016, 126, 1717–1733. [Google Scholar] [CrossRef] [PubMed]
- Xhima, K.; Weber-Adrian, D.; Silburt, J. Glutamate Induces Blood–Brain Barrier Permeability through Activation of N-Methyl-D-Aspartate Receptors. J. Neurosci. 2016, 36, 12296–12298. [Google Scholar] [CrossRef] [PubMed]
- Seelig, A. P-Glycoprotein: One Mechanism, Many Tasks and the Consequences for Pharmacotherapy of Cancers. Front. Oncol. 2020, 10, 576559. [Google Scholar] [CrossRef]
- Leweke, F.; Damian, M.S.; Schindler, C.; Schachenmayr, W. Multidrug resistance in glioblastoma. Chemosensitivity testing and immunohistochemical demonstration of P-glycoprotein. Pathol. Res. Pract. 1998, 194, 149–155. [Google Scholar] [CrossRef] [PubMed]
- Doyle, L.A.; Ross, D.D. Multidrug resistance mediated by the breast cancer resistance protein BCRP (ABCG2). Oncogene 2003, 22, 7340–7358. [Google Scholar] [CrossRef]
- Bates, S.F.; Chen, C.; Robey, R.; Kang, M.; Figg, W.D.; Fojo, T. Reversal of multidrug resistance: Lessons from clinical oncology. Novartis. Found. Symp. 2002, 243, 83–185. [Google Scholar] [CrossRef]
- Thomas, H.; Coley, H.M. Overcoming multidrug resistance in cancer: An update on the clinical strategy of inhibiting P-glycoprotein. Cancer Control 2003, 10, 159–165. [Google Scholar] [CrossRef]
- Roe, M.; Folkes, A.; Ashworth, P.; Brumwell, J.; Chima, L.; Hunjan, S.; Pretswell, I.; Dangerfield, W.; Ryder, H.; Charlton, P. Reversal of P-glycoprotein mediated multidrug resistance by novel anthranilamide derivatives. Bioorg. Med. Chem. Lett. 1999, 9, 595–600. [Google Scholar] [CrossRef]
- Dantzig, A.H.; Shepard, R.L.; Law, K.L.; Tabas, L.; Pratt, S.; Gillespie, J.S.; Binkley, S.N.; Kuhfeld, M.T.; Starling, J.J.; Wrighton, S.A. Selectivity of the multidrug resistance modulator, LY335979, for P-glycoprotein and effect on cytochrome P-450 activities. J. Pharm. Exp. Ther. 1999, 290, 854–862. [Google Scholar]
- van Zuylen, L.; Sparreboom, A.; van der Gaast, A.; van der Burg, M.E.; van Beurden, V.; Bol, C.J.; Woestenborghs, R.; Palmer, P.A.; Verweij, J. The orally administered P-glycoprotein inhibitor R101933 does not alter the plasma pharmacokinetics of docetaxel. Clin. Cancer Res. 2000, 6, 1365–1371. [Google Scholar]
- Kemper, E.M.; van Zandbergen, A.E.; Cleypool, C.; Mos, H.A.; Boogerd, W.; Beijnen, J.H.; van Tellingen, O. Increased penetration of paclitaxel into the brain by inhibition of P-glycoprotein. Clin. Cancer Res. 2003, 9, 2849–2855. [Google Scholar]
- Fricker, G.; Miller, D.S. Modulation of drug transporters at the blood-brain barrier. Pharmacology 2004, 70, 169–176. [Google Scholar] [CrossRef]
- Tournier, N.; Goutal, S.; Auvity, S.; Traxl, A.; Mairinger, S.; Wanek, T.; Helal, O.B.; Buvat, I.; Soussan, M.; Caillé, F.; et al. Strategies to Inhibit ABCB1- and ABCG2-Mediated Efflux Transport of Erlotinib at the Blood-Brain Barrier: A PET Study on Nonhuman Primates. J. Nucl. Med. 2017, 58, 117–122. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.; Agarwal, S.; Shaik, N.M.; Chen, C.; Yang, Z.; Elmquist, W.F. P-glycoprotein and breast cancer resistance protein influence brain distribution of dasatinib. J. Pharm. Exp. Ther. 2009, 330, 956–963. [Google Scholar] [CrossRef] [PubMed]
- Agarwal, S.; Sane, R.; Gallardo, J.L.; Ohlfest, J.R.; Elmquist, W.F. Distribution of gefitinib to the brain is limited by P-glycoprotein (ABCB1) and breast cancer resistance protein (ABCG2)-mediated active efflux. J. Pharm. Exp. Ther. 2010, 334, 147–155. [Google Scholar] [CrossRef] [PubMed]
- Agarwal, S.; Sane, R.; Ohlfest, J.R.; Elmquist, W.F. The role of the breast cancer resistance protein (ABCG2) in the distribution of sorafenib to the brain. J. Pharm. Exp. Ther. 2011, 336, 223–233. [Google Scholar] [CrossRef]
- Fallacara, A.L.; Zamperini, C.; Podolski-Renić, A.; Dinić, J.; Stanković, T.; Stepanović, M.; Mancini, A.; Rango, E.; Iovenitti, G.; Molinari, A.; et al. A New Strategy for Glioblastoma Treatment: In Vitro and In Vivo Preclinical Characterization of Si306, a Pyrazolo [3,4-d]Pyrimidine Dual Src/P-Glycoprotein Inhibitor. Cancers 2019, 11, 848. [Google Scholar] [CrossRef]
- Vignaroli, G.; Iovenitti, G.; Zamperini, C.; Coniglio, F.; Calandro, P.; Molinari, A.; Fallacara, A.L.; Sartucci, A.; Calgani, A.; Colecchia, D.; et al. Prodrugs of Pyrazolo [3,4-d]pyrimidines: From Library Synthesis to Evaluation as Potential Anticancer Agents in an Orthotopic Glioblastoma Model. J. Med. Chem. 2017, 60, 6305–6320. [Google Scholar] [CrossRef]
- Becker, C.M.; Oberoi, R.K.; McFarren, S.J.; Muldoon, D.M.; Pafundi, D.H.; Pokorny, J.L.; Brinkmann, D.H.; Ohlfest, J.R.; Sarkaria, J.N.; Largaespada, D.A.; et al. Decreased affinity for efflux transporters increases brain penetrance and molecular targeting of a PI3K/mTOR inhibitor in a mouse model of glioblastoma. Neuro Oncol. 2015, 17, 1210–1219. [Google Scholar] [CrossRef]
- Fox, E.; Widemann, B.C.; Pastakia, D.; Chen, C.C.; Yang, S.X.; Cole, D.; Balis, F.M. Pharmacokinetic and pharmacodynamic study of tariquidar (XR9576), a P-glycoprotein inhibitor, in combination with doxorubicin, vinorelbine, or docetaxel in children and adolescents with refractory solid tumors. Cancer Chemother. Pharmacol. 2015, 76, 1273–1283. [Google Scholar] [CrossRef]
- Upton, D.H.; Ung, C.; George, S.M.; Tsoli, M.; Kavallaris, M.; Ziegler, D.S. Challenges and opportunities to penetrate the blood-brain barrier for brain cancer therapy. Theranostics 2022, 12, 4734–4752. [Google Scholar] [CrossRef]
- Ruan, H.; Chen, X.; Xie, C.; Li, B.; Ying, M.; Liu, Y.; Zhang, M.; Zhang, X.; Zhan, C.; Lu, W.; et al. Stapled RGD Peptide Enables Glioma-Targeted Drug Delivery by Overcoming Multiple Barriers. ACS Appl. Mater. Interfaces 2017, 9, 17745–17756. [Google Scholar] [CrossRef] [PubMed]
- Chen, L.; Zeng, D.; Xu, N.; Li, C.; Zhang, W.; Zhu, X.; Gao, Y.; Chen, P.R.; Lin, J. Blood-Brain Barrier- and Blood-Brain Tumor Barrier-Penetrating Peptide-Derived Targeted Therapeutics for Glioma and Malignant Tumor Brain Metastases. ACS Appl. Mater. Interfaces 2019, 11, 41889–41897. [Google Scholar] [CrossRef] [PubMed]
- Pozsgai, E.; Schally, A.V.; Halmos, G.; Rick, F.; Bellyei, S. The Inhibitory Effect of a Novel Cytotoxic Somatostatin Analogue AN-162 on Experimental Glioblastoma. Horm. Metab. Res. 2010, 42, 781–786. [Google Scholar] [CrossRef] [PubMed]
- Drappatz, J.; Brenner, A.; Wong, E.T.; Eichler, A.; Schiff, D.; Groves, M.D.; Mikkelsen, T.; Rosenfeld, S.; Sarantopoulos, J.; Meyers, C.A.; et al. Phase I study of GRN1005 in recurrent malignant glioma. Clin. Cancer Res. 2013, 19, 1567–1576. [Google Scholar] [CrossRef] [PubMed]
- Zhao, M.; Broekman, L.D.; Préat, V.; Schiffelers, R.M. Nanocarrier-based drug combination therapy for glioblastoma. Theranostics 2020, 10, 1355–1372. [Google Scholar] [CrossRef]
- Kleinberg, L. Polifeprosan 20, 3.85% carmustine slow release wafer in malignant glioma: Patient selection and perspectives on a low-burden therapy. Patient Prefer. Adherence 2016, 10, 2397–2406. [Google Scholar] [CrossRef]
- Aghi, M.K.; Vogelbaum, M.A.; Bruce, J.N. Convection-enhanced drug delivery for glioblastoma: A review. J. Neurooncol. 2021, 151, 415. [Google Scholar] [CrossRef]
- Krebs-Kraft, D.L.; Frantz, K.J.; Parent, M.B. In Vivo Microdialysis: A Method for Sampling Extracellular Fluid in Discrete Brain Regions. In Handbook of Neurochemistry and Molecular Neurobiology; Lajtha, A., Baker, G., Dunn, S., Holt, A., Eds.; Springer: New York, NY, USA, 2007. [Google Scholar] [CrossRef]
- de Lange, E.C.; de Boer, B.A.; Breimer, D.D. Microdialysis for pharmacokinetic analysis of drug transport to the brain. Adv. Drug Deliv. Rev. 1999, 36, 211–227. [Google Scholar] [CrossRef]
- Dykstra, K.H.; Hsiao, J.K.; Morrison, P.F.; Bungay, P.M.; MefFord, I.N.; Scully, M.M.; Dedrick, R.L. Quantitative Examination of Tissue Concentration Profiles Associated with Microdialysis. J. Neurochem. 1992, 58, 931–940. [Google Scholar] [CrossRef]
- Theek, B.; Baues, M.; Ojha, T.; Möckel, D.; Veettil, S.K.; Steitz, J.; Van Bloois, L.; Storm, G.; Kiessling, F.; Lammers, T. Sonoporation enhances liposome accumulation and penetration in tumors with low EPR. J. Control. Release 2016, 231, 77–85. [Google Scholar] [CrossRef] [PubMed]
- Burke, C.W.; Hsiang, Y.H.J.; Alexander, E., IV; Kilbanov, A.L.; Price, R.J. Covalently linking poly(lactic-co-glycolic acid) nanoparticles to microbubbles before intravenous injection improves their ultrasound-targeted delivery to skeletal muscle. Small 2011, 7, 1227–1235. [Google Scholar] [CrossRef] [PubMed]
- De Temmerman, M.L.; Dewitte, H.; Vandenbroucke, R.E.; Lucas, B.; Libert, C.; Demeester, J.; De Smedt, S.C.; Lentacker, I.; Rejman, J. MRNA-Lipoplex loaded microbubble contrast agents for ultrasound-assisted transfection of dendritic cells. Biomaterials 2011, 32, 9128–9135. [Google Scholar] [CrossRef] [PubMed]
- Burgess, A.; Ayala-Grosso, C.A.; Ganguly, M.; Jordão, J.F.; Aubert, I.; Hynynen, K. Targeted delivery of neural stem cells to the brain using MRI-guided focused ultrasound to disrupt the blood-brain barrier. PLoS ONE 2011, 6, e27877. [Google Scholar] [CrossRef]
- Huang, Q.; Deng, J.; Xie, Z.; Wang, F.; Chen, S.; Lei, B.; Liao, P.; Huang, N.; Wang, Z.; Wang, Z.; et al. Effective Gene Transfer into Central Nervous System Following Ultrasound-Microbubbles-Induced Opening of the Blood-Brain Barrier. Ultrasound Med. Biol. 2012, 38, 1234–1243. [Google Scholar] [CrossRef]
- Chertok, B.; Langer, R. Circulating magnetic microbubbles for localized real-time control of drug delivery by ultrasonography-guided magnetic targeting and ultrasound. Theranostics 2018, 8, 341–357. [Google Scholar] [CrossRef]
- Song, H.; Harvey, B.K.; Borden, M.A. State-of-the-art of microbubble-assisted blood-brain barrier disruption. Theranostics 2018, 8, 4393–4408. [Google Scholar] [CrossRef]
- Ceña, V.; Játiva, P. Nanoparticle Crossing of Blood–Brain Barrier: A Road to New Therapeutic Approaches to Central Nervous System Diseases. Nanomedicine 2018, 13, 1513–1516. [Google Scholar] [CrossRef]
- Joshi, S.; Meyers, P.M.; Ornstein, E. Intracarotid Delivery of Drugs: The Potential and the Pitfalls. Anesthesiology 2008, 109, 543. [Google Scholar] [CrossRef]
- Eckman, W.W.; Patlak, C.S.; Fenstermacher, J.D. A critical evaluation of the principles governing the advantages of intra arterial infusions. J Pharm. Biopharm. 1974, 2, 257–285. [Google Scholar] [CrossRef]
- Fenstermacher, J.; Gazendam, J. Intra-arterial infusions of drugs and hyperosmotic solutions as ways of enhancing CNS chemotherapy. Cancer Treat Rep. 1981, 65 (Suppl. S2), 27–37. [Google Scholar] [PubMed]
- Hirano, Y.; Mineura, K.; Mizoi, K.; Tomura, N. Therapeutic results of intra-arterial chemotherapy in patients with malignant glioma. Int. J. Oncol. 1998, 13, 537–542. [Google Scholar] [CrossRef] [PubMed]
- Dropcho, E.J.; Rosenfeld, S.S.; Vitek, J.; Guthrie, B.L.; Morawetz, R.B. Phase II study of intracarotid or selective intracerebral infusion of cisplatin for treatment of recurrent anaplastic gliomas. J. Neurooncol. 1998, 36, 191–198. [Google Scholar] [CrossRef] [PubMed]
- Gundersen, S.; Lote, K.; Watne, K. A retrospective study of the value of chemotherapy as adjuvant therapy to surgery and radiotherapy in grade 3 and 4 gliomas. Eur. J. Cancer 1998, 34, 1565–1569. [Google Scholar] [CrossRef]
- Riina, H.A.; Fraser, J.F.; Fralin, S.; Knopman, J.; Scheff, R.J.; Boockvar, J.A. Superselective intraarterial cerebral infusion of bevacizumab: A revival of interventional neuro-oncology for malignant glioma. J. Exp. Oncol. 2009, 8, 145–150. [Google Scholar]
- Fowler, M.; Cotter, J.; Knight, B.; Sevick-Muraca, E.; Sandberg, D.; Sirianni, R. Intrathecal Drug Delivery in the Era of Nanomedicine. Adv. Drug Deliv. Rev. 2020, 77, 165–166. [Google Scholar] [CrossRef]
- Blasberg, R.G. Methotrexate, cytosine arabinoside, and BCNU concentration in brain after ventriculocisternal perfusion. Cancer Treat Rep. 1977, 61, 625–631. [Google Scholar]
- Jacus, M.O.; Daryani, V.M.; Harstead, K.E.; Patel, Y.T.; Throm, S.L.; Stewart, C.F. Pharmacokinetic Properties of Anticancer Agents for the Treatment of Central Nervous System Tumors: Update of the Literature. Clin. Pharmacokinet. 2016, 55, 297–311. [Google Scholar] [CrossRef]
- Groothuis, D.R.; Levy, R.M. The entry of antiviral and antiretroviral drugs into the central nervous system. J. Neurovirol. 1997, 3, 387–400. [Google Scholar] [CrossRef]
- Currie, G.M. Pharmacology, Part 2: Introduction to Pharmacokinetics. J. Nucl. Med. Technol. 2018, 46, 221–230. [Google Scholar] [CrossRef]
- Currie, G.M. Pharmacology, Part 1: Introduction to Pharmacology and Pharmacodynamics. J. Nucl. Med. Technol. 2018, 46, 81–86. [Google Scholar] [CrossRef] [PubMed]
- Wright, D.F.B.; Winter, H.R.; Duffull, S.B. Understanding the time course of pharmacological effect: A PKPD approach. Br. J. Clin. Pharmacol. 2011, 71, 815–823. [Google Scholar] [CrossRef] [PubMed]
- Loscher, W.; Schmidt, D. Experimental and clinical evidence for loss of effect (Tolerance) during prolonged treatment with antiepileptic drugs. Epilepsia 2006, 47, 1253–1284. [Google Scholar] [CrossRef] [PubMed]
- Cree, I.A.; Charlton, P. Molecular chess? Hallmarks of anticancer drug resistance. BMC Cancer 2017, 17, 10. [Google Scholar] [CrossRef]
- Valderas, J.M.; Starfield, B.; Sibbald, B.; Salisbury, C.; Roland, M. Defining Comorbidity: Implications for Understanding Health and Health Services. Ann. Fam. Med. 2009, 7, 357–363. [Google Scholar] [CrossRef]
- Caughey, G.E.; Roughead, L.; Shakib, S.; McDermott, R.A.; Vitry, A.; Gilbert, A.L. Comorbidity of chronic disease and potential treatment conflicts in older people dispensed antidepressants. Age Ageing 2010, 39, 488–494. [Google Scholar] [CrossRef]
- Bénard-Laribière, A.; Noize, P.; Pambrun, E.; Bazin, F.; Verdoux, H.; Tournier, M.; Bégaud, B.; Pariente, A. Comorbidities and concurrent medications increasing the risk of adverse drug reactions: Prevalence in French benzodiazepine users. Eur. J. Clin. Pharmacol. 2016, 72, 869–876. [Google Scholar] [CrossRef]
- Grossman, S.A.; Romo, C.G.; Rudek, M.A.; Supko, J.; Fisher, J.; Nabors, L.B.; Wen, P.Y.; Peereboom, D.M.; Ellingson, B.M.; Elmquist, W.; et al. Baseline requirements for novel agents being considered for phase II/III brain cancer efficacy trials: Conclusions from the Adult Brain Tumor Consortium’s first workshop on CNS drug delivery. Neuro Oncol. 2020, 22, 1422–1424. [Google Scholar] [CrossRef]
- Raoufinia, R.; Mota, A.; Keyhanvar, N.; Safari, F.; Shamekhi, S.; Abdolalizadeh, J. Overview of Albumin and Its Purification Methods. Adv. Pharm. Bull. 2016, 6, 495–507. [Google Scholar] [CrossRef]
- Caraceni, P.; Tufoni, M.; Bonavita, M.E. Clinical use of albumin. Blood Transfus. 2013, 11, 18–25. [Google Scholar] [CrossRef]
- Carvalho, J.R.; Machado, M.V. New Insights About Albumin and Liver Disease. Ann. Hepatol. 2018, 17, 547–560. [Google Scholar] [CrossRef] [PubMed]
- Fanali, G.; di Masi, A.; Trezza, V.; Marino, M.; Fasano, M.; Ascenzi, P. Human serum albumin: From bench to bedside. Mol. Aspects Med. 2012, 33, 209–290. [Google Scholar] [CrossRef] [PubMed]
- Lu, R.; Li, W.W.; Katzir, A.; Raichlin, Y.; Yu, H.Q.; Mizaikoff, B. Probing the secondary structure of bovine serum albumin during heat-induced denaturation using mid-infrared fiberoptic sensors. Analyst 2015, 140, 765–770. [Google Scholar] [CrossRef]
- He, X.M.; Carter, D.C. Atomic structure and chemistry of human serum albumin. Nature 1992, 358, 209–215. [Google Scholar] [CrossRef] [PubMed]
- Bujacz, A. Structures of bovine, equine and leporine serum albumin. Acta Crystallogr. D Biol. Crystallogr. 2012, 68, 1278–1289. [Google Scholar] [CrossRef] [PubMed]
- Fasano, M.; Curry, S.; Terreno, E.; Galliano, M.; Fanali, G.; Narciso, P.; Notari, S.; Ascenzi, P. The extraordinary ligand binding properties of human serum albumin. IUBMB Life 2005, 57, 787–796. [Google Scholar] [CrossRef]
- Belinskaia, D.A.; Voronina, P.A.; Batalova, A.A.; Goncharov, N.V. Serum Albumin. Encyclopedia 2021, 1, 65–75. [Google Scholar] [CrossRef]
- Sudlow, G.; Birkett, D.J.; Wade, D.N. The Characterization of Two Specific Drug Binding Sites on Human Serum Albumin. Mol. Pharmacol. 1975, 11, 824–832. [Google Scholar]
- Belinskaia, D.A.; Voronina, P.A.; Goncharov, N.V. Integrative Role of Albumin: Evolutionary, Biochemical and Pathophysiological Aspects. J. Evol. Biochem. Phys. 2021, 57, 1419–1448. [Google Scholar] [CrossRef]
- Wu, J. The Enhanced Permeability and Retention (EPR) Effect: The Significance of the Concept and Methods to Enhance Its Application. J. Pers. Med. 2021, 11, 771. [Google Scholar] [CrossRef]
- Spada, A.; Emami, J.; Tuszynski, J.A.; Lavasanifar, A. The Uniqueness of Albumin as a Carrier in Nanodrug Delivery. Mol. Pharm. 2021, 18, 1862–1894. [Google Scholar] [CrossRef] [PubMed]
- Allen, T.M.; Cullis, P.R. Drug delivery systems: Entering the mainstream. Science 2004, 303, 1818–1822. [Google Scholar] [CrossRef] [PubMed]
- Dreher, M.R.; Liu, W.; Michelich, C.R.; Dewhirst, M.W.; Yuan, F.; Chilkoti, A. Tumor vascular permeability, accumulation, and penetration of macromolecular drug carriers. J. Natl. Cancer Inst. 2006, 98, 335–344. [Google Scholar] [CrossRef] [PubMed]
- Danhier, F. To exploit the tumor microenvironment: Since the EPR effect fails in the clinic, what is the future of nanomedicine? J. Control. Release 2016, 244, 108–121. [Google Scholar] [CrossRef] [PubMed]
- Fang, J.; Nakamura, H.; Maeda, H. The EPR effect: Unique features of tumor blood vessels for drug delivery, factors involved, and limitations and augmentation of the effect. Adv. Drug Deliv. Rev. 2011, 63, 136–151. [Google Scholar] [CrossRef] [PubMed]
- Matsumura, Y.; Maeda, H. A new concept for macromolecular therapeutics in cancer chemotherapy: Mechanism of tumoritropic accumulation of proteins and the antitumor agent smancs. Cancer Res. 1986, 46, 6387–6392. [Google Scholar] [PubMed]
- Sindhwani, S.; Syed, A.M.; Ngai, J.; Kingston, B.R.; Maiorino, L.; Rothschild, J.; MacMillan, P.; Zhang, Y.; Rajesh, N.U.; Hoang, T.; et al. The entry of nanoparticles into solid tumours. Nat. Mater. 2020, 19, 566–575. [Google Scholar] [CrossRef] [PubMed]
- Langer, K.; Anhorn, M.G.; Steinhauser, I.; Dreis, S.; Celebi, D.; Schrickel, N.; Faust, S.; Vogel, V. Human serum albumin (HSA) nanoparticles: Reproducibility of preparation process and kinetics of enzymatic degradation. Int. J. Pharm. 2008, 347, 109–117. [Google Scholar] [CrossRef]
- Prabhakar, U.; Maeda, H.; Jain, R.K.; Sevick-Muraca, E.M.; Zamboni, W.; Farokhzad, O.C.; Barry, S.T.; Gabizon, A.; Grodzinski, P.; Blakey, D.C. Challenges and key considerations of the enhanced permeability and retention effect for nanomedicine drug delivery in oncology. Cancer Res. 2013, 73, 2412–2417. [Google Scholar] [CrossRef]
- Merlot, A.M.; Kalinowski, D.S.; Richardson, D.R. Unraveling the mysteries of serum albumin-more than just a serum protein. Front. Physiol. 2014, 5, 299. [Google Scholar] [CrossRef]
- Kratz, F. A clinical update of using albumin as a drug vehicle—A commentary. J. Control. Release 2014, 190, 331–336. [Google Scholar] [CrossRef] [PubMed]
- Gradishar, W.J. Albumin-bound paclitaxel: A next-generation taxane. Expert Opin. Pharmacother. 2006, 7, 1041–1053. [Google Scholar] [CrossRef] [PubMed]
- Gradishar, W.J.; Tjulandin, S.; Davidson, N.; Shaw, H.; Desai, N.; Bhar, P.; Hawkins, M.; O’Shaughnessy, J. Phase III trial of nanoparticle albumin-bound paclitaxel compared with polyethylated castor oil-based paclitaxel in women with breast cancer. J. Clin. Oncol. 2005, 23, 7794–7803. [Google Scholar] [CrossRef] [PubMed]
- Harries, M.; Ellis, P.; Harper, P. Nanoparticle albumin-bound paclitaxel for metastatic breast cancer. J. Clin. Oncol. 2005, 23, 7768–7771. [Google Scholar] [CrossRef]
- Socinski, M.A.; Bondarenko, I.; Karaseva, N.A.; Makhson, A.M.; Vynnychenko, I.; Okamoto, I.; Hon, J.K.; Hirsh, V.; Bhar, P.; Zhang, H.; et al. Weekly nab-paclitaxel in combination with carboplatin versus solvent-based paclitaxel plus carboplatin as first-line therapy in patients with advanced non-small-cell lung cancer: Final results of a phase III trial. J. Clin. Oncol. 2012, 30, 2055–2062. [Google Scholar] [CrossRef]
- Bobo, D.; Robinson, K.J.; Islam, J.; Thurecht, K.J.; Corrie, S.R. Nanoparticle-Based Medicines: A Review of FDA-Approved Materials and Clinical Trials to Date. Pharm. Res. 2016, 33, 2373–2387. [Google Scholar] [CrossRef]
- Saif, M.W. US Food and Drug Administration approves paclitaxel protein-bound particles (Abraxane(R)) in combination with gemcitabine as first-line treatment of patients with metastatic pancreatic cancer. JOP 2013, 14, 686–688. [Google Scholar]
- Fanciullino, R.; Ciccolini, J.; Milano, G. Challenges, expectations and limits for nanoparticles-based therapeutics in cancer: A focus on nano-albumin-bound drugs. Crit. Rev. Oncol. Hematol. 2013, 88, 504–513. [Google Scholar] [CrossRef]
- Tiruppathi, C.; Song, W.; Bergenfeldt, M.; Sass, P.; Malik, A.B. Gp60 activation mediates albumin transcytosis in endothelial cells by tyrosine kinase-dependent pathway. J. Biol. Chem. 1997, 272, 25968–25975. [Google Scholar] [CrossRef]
- Bornstein, P.; Sage, E.H. Matricellular proteins: Extracellular modulators of cell function. Curr. Opin. Cell. Biol. 2002, 14, 608–616. [Google Scholar] [CrossRef]
- Brekken, R.A.; Sage, E.H. SPARC, a matricellular protein: At the crossroads of cell-matrix communication. Matrix Biol. 2001, 19, 816–827. [Google Scholar] [CrossRef]
- Wiessler, M.; Frei, E. Isolation and identification of heterogeneous nuclear ribonucleoproteins (hnRNP) from purified plasma membranes of human tumour cell lines as albumin-binding proteins. Biochem. Pharmacol. 2004, 67, 655–665. [Google Scholar] [CrossRef]
- Desai, N.; Trieu, V.; Damascelli, B.; Soon-Shiong, P. SPARC Expression Correlates with Tumor Response to Albumin-Bound Paclitaxel in Head and Neck Cancer Patients. Transl. Oncol. 2009, 2, 59–64. [Google Scholar] [CrossRef] [PubMed]
- Desai, N.P.; Trieu, V.; Hwang, L.Y.; Wu, R.; Soon-Shiong, P.; Gradishar, W.J. Improved effectiveness of nanoparticle albumin-bound (nab) paclitaxel versus polysorbate-based docetaxel in multiple xenografts as a function of HER2 and SPARC status. Anticancer Drugs 2008, 19, 899–909. [Google Scholar] [CrossRef] [PubMed]
- Neesse, A.; Frese, K.K.; Chan, D.S.; Bapiro, T.E.; Howat, W.J.; Richards, F.M.; Ellenrieder, V.; Jodrell, D.I.; Tuveson, D.A. SPARC independent drug delivery and antitumour effects of nab-paclitaxel in genetically engineered mice. Gut 2014, 63, 974–983. [Google Scholar] [CrossRef] [PubMed]
- Bern, M.; Sand, K.M.K.; Nilsen, J.; Sandlie, I.; Andersen, J.T. The role of albumin receptors in regulation of albumin homeostasis: Implications for drug delivery. J. Control. Release 2015, 211, 144–162. [Google Scholar] [CrossRef]
- Larsen, M.T.; Kuhlmann, M.; Hvam, M.L.; Howard, K.A. Albumin-based drug delivery: Harnessing nature to cure disease. Mol. Cell 2016, 4, 3. [Google Scholar] [CrossRef]
- Schnitzer, J.E.; Oh, P. Albondin-mediated capillary permeability to albumin. Differential role of receptors in endothelial transcytosis and endocytosis of native and modified albumins. J. Biol. Chem. 1994, 269, 6072–6082. [Google Scholar] [CrossRef]
- Schnitzer, J.; Sung, A.; Horvat, R.; Bravo, J. Preferential interaction of albumin-binding proteins, gp30 and gp18, with conformationally modified albumins. Presence in many cells and tissues with a possible role in catabolism. J. Biol. Chem. 1992, 267, 24544–24553. [Google Scholar] [CrossRef]
- Schnitzer, J.E.; Bravo, J. High affinity binding, endocytosis, and degradation of conformationally modified albumins. Potential role of gp30 and gp18 as novel scavenger receptors. J. Biol. Chem. 1993, 268, 7562–7570. [Google Scholar] [CrossRef]
- Zhao, P.; Wang, Y.; Wu, A.; Rao, Y.; Huang, Y. Roles of Albumin-Binding Proteins in Cancer Progression and Biomimetic Targeted Drug Delivery. Chembiochem 2018, 19, 1796–1805. [Google Scholar] [CrossRef] [PubMed]
- Sand, K.M.K.; Bern, M.; Nilsen, J.; Noordzij, H.T.; Sandlie, I.; Andersen, J.T. Unraveling the Interaction between FcRn and Albumin: Opportunities for Design of Albumin-Based Therapeutics. Front. Immunol. 2015, 5, 682. [Google Scholar] [CrossRef] [PubMed]
- Sleep, D.; Cameron, J.; Evans, L.R. Albumin as a versatile platform for drug half-life extension. Biochim. Biophys. Acta 2013, 1830, 5526–5534. [Google Scholar] [CrossRef] [PubMed]
- Andersen, J.T.; Dalhus, B.; Cameron, J.; Daba, M.B.; Plumridge, A.; Evans, L.; Brennan, S.O.; Gunnarsen, K.S.; Bjørås, M.; Sleep, D.; et al. Structure-based mutagenesis reveals the albumin-binding site of the neonatal Fc receptor. Nat. Commun. 2012, 3, 610. [Google Scholar] [CrossRef] [PubMed]
- Kuo, T.; de Muinck, E.; Claypool, S.; Yoshida, M.; Nagaishi, T.; Aveson, V.; Lencer, W.I.; Blumberg. R.S. N-glycan moieties in neonatal Fc receptor determine steady-state membrane distribution and directional transport of IgG. J. Biol. Chem. 2009, 284, 8292–8300. [Google Scholar] [CrossRef] [PubMed]
- Schmidt, M.M.; Townson, S.A.; Andreucci, A.J.; King, B.M.; Schirmer, E.B.; Murillo, A.J.; Dombrowski, C.; Tisdale, A.W.; Lowden, P.A.; Masci, A.L.; et al. Crystal structure of an HSA/FcRn complex reveals recycling by competitive mimicry of HSA ligands at a pH-dependent hydrophobic interface. Structure 2013, 21, 1966–1978. [Google Scholar] [CrossRef]
- Wu, Z.; Simister, N.E. Tryptophan- and dileucine-based endocytosis signals in the neonatal Fc receptor. J. Biol. Chem. 2001, 276, 5240–5247. [Google Scholar] [CrossRef]
- Elsadek, B.; Kratz, F. Impact of albumin on drug delivery—New applications on the horizon. J. Control. Release 2012, 157, 4–28. [Google Scholar] [CrossRef]
- Kratz, F.; Abu Ajaj, K.; Warnecke, A. Anticancer carrier-linked prodrugs in clinical trials. Expert Opin. Investig. Drugs 2007, 16, 1037–1058. [Google Scholar] [CrossRef]
- Bolling, C.; Graefe, T.; Lübbing, C.; Jankevicius, F.; Uktveris, S.; Cesas, A.; Meyer-Moldenhauer, W.H.; Starkmann, H.; Weigel, M.; Burk, K.; et al. Phase II study of MTX-HSA in combination with cisplatin as first line treatment in patients with advanced or metastatic transitional cell carcinoma. Investig. New Drugs 2006, 24, 521–527. [Google Scholar] [CrossRef]
- Wunder, A.; Müller-Ladner, U.; Stelzer, E.H.; Funk, J.; Neumann, E.; Stehle, G.; Pap, T.; Sinn, H.; Gay, S.; Fiehn, C. Albumin-based drug delivery as novel therapeutic approach for rheumatoid arthritis. J. Immunol. 2003, 170, 4793–4801. [Google Scholar] [CrossRef]
- Stehle, G.; Wunder, A.; Sinn, H.; Schrenk, H.H.; Schütt, S.; Frei, E.; Hartung, G.; Maier-Borst, W.; Heene, D.L. Pharmacokinetics of methotrexate-albumin conjugates in tumor-bearing rats. Anticancer Drugs 1997, 8, 835–844. [Google Scholar] [CrossRef]
- Kratz, F.; Müller-Driver, R.; Hofmann, I. A novel macromolecular prodrug concept exploiting endogenous serum albumin as a drug carrier for cancer chemotherapy. J. Med. Chem. 2000, 43, 1253–1256. [Google Scholar] [CrossRef]
- Anderson, C.L.; Chaudhury, C.; Kim, J.; Bronson, C.; Wani, M.A.; Mohanty, S. Perspective–FcRn transports albumin: Relevance to immunology and medicine. Trends Immunol. 2006, 27, 343–348. [Google Scholar] [CrossRef]
- Simon, M.; Frey, R.; Zangemeister-Wittke, U.; Plückthun, A. Orthogonal assembly of a designed ankyrin repeat protein-cytotoxin conjugate with a clickable serum albumin module for half-life extension. Bioconjug. Chem. 2013, 24, 1955–1966. [Google Scholar] [CrossRef]
- Park, C.R.; Jo, J.H.; Song, M.G.; Park, J.Y.; Kim, H.; Youn, H.; Paek, S.H.; Chung, K.; Jeong, J.M.; Lee, S.; et al. Secreted protein acidic and rich in cysteine mediates active targeting of human serum albumin in U87MG xenograft mouse models. Theranostics 2019, 9, 7447–7457. [Google Scholar] [CrossRef] [PubMed]
- Chlenski, A.; Dobratic, M.; Salwen, H.R.; Applebaum, M.; Guerrero, L.J.; Miller, R.; DeWane, G.; Solomaha, E.; Marks, J.D.; Cohn, S.L. Secreted protein acidic and rich in cysteine (SPARC) induces lipotoxicity in neuroblastoma by regulating transport of albumin complexed with fatty acids. Oncotarget 2016, 7, 77696–77706. [Google Scholar] [CrossRef] [PubMed]
- Stehle, G.; Sinn, H.; Wunder, A.; Schrenk, H.H.; Stewart, J.C.; Hartung, G.; Maier-Borst, W.; Heene, D.L. Plasma protein (albumin) catabolism by the tumor itself--implications for tumor metabolism and the genesis of cachexia. Crit. Rev. Oncol. Hematol. 1997, 26, 77–100. [Google Scholar] [CrossRef] [PubMed]
- Wang, W.; Huang, Y.; Zhao, S.; Shao, T.; Cheng, Y. Human serum albumin (HSA) nanoparticles stabilized with intermolecular disulfide bonds. Chem. Commun. 2012, 49, 2234–2236. [Google Scholar] [CrossRef]
- Varga, N.; Hornok, V.; Sebok, D.; Dékány, I. Comprehensive study on the structure of the BSA from extended-to aged form in wide (2–12) pH range. Int. J. Biol. Macromol. 2016, 88, 51–58. [Google Scholar] [CrossRef]
- Tarhini, M.; Greige-Gerges, H.; Elaissari, A. Protein-based nanoparticles: From preparation to encapsulation of active molecules. Int. J. Pharm. 2017, 522, 172–197. [Google Scholar] [CrossRef] [PubMed]
- Loureiro, A.G.; Azoia, N.C.; Gomes, A.; Cavaco-Paulo, A. Albumin-Based Nanodevices as Drug Carriers. Curr. Pharm. Des. 2016, 22, 1371–1390. [Google Scholar] [CrossRef] [PubMed]
- Coester, C.J.; Langer, K.; Von Briesen, H.; Kreuter, J. Gelatin nanoparticles by two step desolvation a new preparation method, surface modifications and cell uptake. J. Microencapsul. 2000, 17, 187–193. [Google Scholar] [CrossRef] [PubMed]
- Jahanban-Esfahlan, A.; Dastmalchi, S.; Davaran, S. A simple improved desolvation method for the rapid preparation of albumin nanoparticles. Int. J. Biol. Macromol. 2016, 91, 703–709. [Google Scholar] [CrossRef] [PubMed]
- Yu, Z.; Yu, M.; Zhang, Z.; Hong, G.; Xiong, Q. Bovine serum albumin nanoparticles as controlled release carrier for local drug delivery to the inner ear. Nanoscale Res. Lett. 2014, 9, 343. [Google Scholar] [CrossRef]
- Shi, Y.; van der Meel, R.; Chen, X.; Lammers, T. The EPR effect and beyond: Strategies to improve tumor targeting and cancer nanomedicine treatment efficacy. Theranostics 2020, 10, 7921–7924. [Google Scholar] [CrossRef]
- Kalyane, D.; Raval, N.; Maheshwari, R.; Tambe, V.; Kalia, K.; Tekade, R.K. Employment of enhanced permeability and retention effect (EPR): Nanoparticle-based precision tools for targeting of therapeutic and diagnostic agent in cancer. Mater. Sci. Eng. C 2019, 98, 1252–1276. [Google Scholar] [CrossRef]
- Zhao, S.; Wang, W.; Huang, Y.; Fu, Y.; Cheng, Y. Paclitaxel loaded human serum albumin nanoparticles stabilized with intermolecular disulfide bonds. MedChemComm 2014, 5, 1658–1663. [Google Scholar] [CrossRef]
- Prajapati, R.; Garcia-Garrido, E.; Somoza, Á. Albumin-Based Nanoparticles for the Delivery of Doxorubicin in Breast Cancer. Cancers 2021, 13, 3011. [Google Scholar] [CrossRef]
- Liu, F.; Mu, J.; Xing, B. Recent advances on the development of pharmacotherapeutic agents on the basis of human serum albumin. Curr. Pharm. Des. 2015, 21, 1866–1888. [Google Scholar] [CrossRef]
- Boye, J.I.; Alli, I.; Ismail, A.A. Interactions Involved in the Gelation of Bovine Serum Albumin. J. Agric. Food Chem. 1996, 44, 996–1004. [Google Scholar] [CrossRef]
- Clark, A.H.; Judge, F.J.; Richards, J.B.; Stubbs, J.M.; Suggett, A. Electron microscopy of network structures in thermally-induced globular protein gels. Int. J. Peptide Botein Res. 1981, 17, 380–392. [Google Scholar] [CrossRef] [PubMed]
- Militello, V.; Casarino, C.; Emanuele, A.; Giostra, A.; Pullara, F.; Leone, M. Aggregation kinetics of bovine serum albumin studied by FTIR spectroscopy and light scattering. Biophys. Chem. 2004, 107, 175–187. [Google Scholar] [CrossRef]
- Lu, Y.L.; Ma, Y.B.; Feng, C.; Zhu, D.L.; Liu, J.; Chen, L.; Liang, S.J.; Dong, C.Y. Co-delivery of Cyclopamine and Doxorubicin Mediated by Bovine Serum Albumin Nanoparticles Reverses Doxorubicin Resistance in Breast Cancer by Down-regulating P-glycoprotein Expression. J. Cancer 2019, 10, 2357–2368. [Google Scholar] [CrossRef] [PubMed]
- Demirkurt, B.; Cakan-Akdogan, G.; Akdogan, Y. Preparation of albumin nanoparticles in water-in-ionic liquid microemulsions. J. Mol. Liq. 2019, 295, 111713. [Google Scholar] [CrossRef]
- Kovács, A.N.; Varga, N.; Gombár, G.; Hornok, V.; Csapó, E. Novel feasibilities for preparation of serum albumin-based core-shell nanoparticles in flow conditions. J. Flow Chem. 2020, 10, 497–505. [Google Scholar] [CrossRef]
- Yang, L.; Cui, F.; Cun, D.; Tao, A.; Shi, K.; Lin, W. Preparation, characterization and biodistribution of the lactone form of 10-hydroxycamptothecin (HCPT)-loaded bovine serum albumin (BSA) nanoparticles. Int. J. Pharm. 2007, 340, 163–172. [Google Scholar] [CrossRef]
- Crisante, F.; Francolini, I.; Bellusci, M.; Martinelli, A.; D’Ilario, L.; Piozzi, A. Antibiotic delivery polyurethanes containing albumin and polyallylamine nanoparticles. Eur. J. Pharm. Sci. 2009, 36, 555–564. [Google Scholar] [CrossRef]
- Desai, N. Nanoparticle Albumin-Bound Paclitaxel (Abraxane®). In Albumin in Medicine; Otagiri, M., Chuang, V., Eds.; Springer: Singapore, 2016; pp. 101–119. [Google Scholar] [CrossRef]
- Demirkurt, B.; Akdogan, Y. Development of an Ionic Liquid Based Method for the Preparation of Albumin Nanoparticles. Chem. Select. 2018, 3, 9940–9945. [Google Scholar] [CrossRef]
- Xu, R.; Fisher, M.; Juliano, R.L. Targeted albumin-based nanoparticles for delivery of amphipathic drugs. Bioconjugate Chem. 2011, 22, 870–878. [Google Scholar] [CrossRef]
- Lian, H.; Wu, J.; Hu, Y.; Guo, H. Self-assembled albumin nanoparticles for combination therapy in prostate cancer. Int. J. Nanomed. 2017, 12, 7777–7787. [Google Scholar] [CrossRef] [PubMed]
- Abdel-Mageed, H.M.; Fouad, S.A.; Teaima, M.H.; Abdel-Aty, A.M.; Fahmy, A.S.; Shaker, D.S.; Mohamed, S.A. Optimization of nano spray drying parameters for production of α-amylase nanopowder for biotheraputic applications using factorial design. Dry Technol. 2019, 37, 2152–2160. [Google Scholar] [CrossRef]
- Haleema Mooneerah Neerooa, B.N.; Ooi, T.; Shameli, K.; Dahlan, N.A.; Islam, M.M.; Pushpamalar, J.; Teow, Y. Development of Polymer-Assisted Nanoparticles and Nanogels for Cancer Therapy: An Update. Gels 2021, 7, 60. [Google Scholar] [CrossRef]
- Fu, Q.; Sun, J.; Zhang, W.; Sui, X.; Yan, Z.; He, Z. Nanoparticle albumin-bound (NAB) technology is a promising method for anti-cancer drug delivery. Recent Pat. Anticancer Drug Discov. 2009, 4, 262–272. [Google Scholar] [CrossRef] [PubMed]
- Cortes, J.; Saura, C. Nanoparticle albumin-bound (nab™)-paclitaxel: Improving efficacy and tolerability by targeted drug delivery in metastatic breast cancer. Eur. J. Cancer Suppl. 2010, 8, 1–10. [Google Scholar] [CrossRef]
- Gelderblom, H.; Verweij, J.; Nooter, K.; Sparreboom, A. Cremophor EL: The drawbacks and advantages of vehicle selection for drug formulation. Eur. J. Cancer Suppl. 2001, 37, 1590–1598. [Google Scholar] [CrossRef]
- Gupta, R.B.; Kompella, U.B. Drugs and the Pharmaceutical Sciences: Nanoparticle Technology for Drug Delivery, 1st ed.; Kompella, U.B., Ed.; Taylor & Francis Group: New York, NY, USA, 2006; pp. 98–101. [Google Scholar] [CrossRef]
- Khandelia, R.; Bhandari, R.S.; Pan, U.N.; Ghosh, S.S.; Chattopadhyay, A. Gold Nanocluster Embedded Albumin Nanoparticles for Two-Photon Imaging of Cancer Cells Accompanying Drug Delivery. Small 2015, 11, 4075–4081. [Google Scholar] [CrossRef]
- Kianfar, E. Protein nanoparticles in drug delivery: Animal protein, plant proteins and protein cages, albumin nanoparticles. J. Nanobiotechnol. 2021, 19, 159. [Google Scholar] [CrossRef]
- Pressacco, J.; Papas, K. Gadofosveset-enhanced magnetic resonance angiography as a means of evaluating pulmonary arteriovenous malformation: A case report. Magn. Reson. Imaging 2012, 30, 886–888. [Google Scholar] [CrossRef]
- Hoogenboezem, E.N.; Duvall, C.L. Harnessing albumin as a carrier for cancer therapies. Adv. Drug Deliv. Rev. 2018, 130, 73–89. [Google Scholar] [CrossRef]
- Langer, K.; Balthasar, S.; Vogel, V.; Dinauer, N.; von Briesen, H.; Schubert, D. Optimization of the preparation process for human serum albumin (HSA) nanoparticles. Int. J. Pharm. 2003, 257, 169–180. [Google Scholar] [CrossRef] [PubMed]
- Weecharangsan, W.; Yu, B.; Zheng, Y.; Liu, S.; Pang, J.X.; Lee, L.J.; Marcucci, G.; Lee, R.J. Efficient Delivery of Antisense Oligodeoxyribonucleotide G3139 by Human Serum Albumin-Coated Liposomes. Mol. Pharm. 2009, 6, 1848–1855. [Google Scholar] [CrossRef]
- Piao, L.; Li, H.; Teng, L.; Yung, B.C.; Sugimoto, Y.; Brueggemeier, R.W.; Lee, R.J. Human serum albumin-coated lipid nanoparticles for delivery of siRNA to breast cancer. Nanomed. Nanotechnol. Biol. Med. 2013, 9, 122–129. [Google Scholar] [CrossRef] [PubMed]
- Heuberger, J.; Schmidt, S.; Derendorf, H. When is protein binding important? J. Pharm. Sci. 2013, 102, 3458–3468. [Google Scholar] [CrossRef] [PubMed]
- Aggarwal, P.; Hall, J.B.; McLeland, C.B.; Dobrovolskaia, M.A.; McNeil, S.E. Nanoparticle interaction with plasma proteins as it relates to particle biodistribution, biocompatibility and therapeutic efficacy. Adv. Drug Deliv. Rev. 2009, 61, 428–437. [Google Scholar] [CrossRef] [PubMed]
- Nel, A.E.; Mädler, L.; Velegol, D.; Xia, T.; Hoek, E.M.V.; Somasundaran, P.; Klaessig, F.; Castranova, V.; Thompson, M. Understanding biophysicochemical interactions at the nano-bio interface. Nat. Mater. 2009, 8, 543–557. [Google Scholar] [CrossRef]
- Kopac, T. Protein corona, understanding the nanoparticle–protein interactions and future perspectives: A critical review. Int. J. Biol. Macromol. 2021, 169, 290–301. [Google Scholar] [CrossRef]
- Mariam, J.; Sivakami, S.; Dongre, P.M. Albumin corona on nanoparticles—A strategic approach in drug delivery. Drug Deliv. 2016, 23, 2668–2676. [Google Scholar] [CrossRef]
- Srivastava, A.; Prajapati, A. Albumin and functionalized albumin nanoparticles: Production strategies, characterization, and target indications. Asian Biomed. 2020, 14, 217–242. [Google Scholar] [CrossRef]
- Elzoghby, A.O.; Samy, W.M.; Elgindy, N.A. Albumin-based nanoparticles as potential controlled release drug delivery systems. J. Control. Release 2012, 157, 168–182. [Google Scholar] [CrossRef]
- Pereverzeva, E.; Treschalin, I.; Bodyagin, D.; Maksimenko, O.; Langer, K.; Dreis, S.; Asmussen, B.; Kreuter, J.; Gelperina, S. Influence of the formulation on the tolerance profile of nanoparticle-bound doxorubicin in healthy rats: Focus on cardio- and testicular toxicity. Int. J. Pharm. 2007, 337, 346–356. [Google Scholar] [CrossRef] [PubMed]
- Zucchi, R.; Danesi, R. Cardiac toxicity of antineoplastic anthracyclines. Curr. Med. Chem. Anticancer Agents 2003, 3, 151–171. [Google Scholar] [CrossRef] [PubMed]
- Zhang, S.; Wang, G.; Lin, X.; Chatzinikolaidou, M.; Jennissen, H.P.; Laub, M.; Uludağ, H. Polyethylenimine-coated albumin nanoparticles for BMP-2 delivery. Biotechnol. Prog. 2008, 24, 945–956. [Google Scholar] [CrossRef] [PubMed]
- Zhang, S.; Doschak, M.R.; Uludağ, H. Pharmacokinetics and bone formation by BMP-2 entrapped in polyethylenimine-coated albumin nanoparticles. Biomaterials 2009, 30, 5143–5155. [Google Scholar] [CrossRef]
- Zhang, S.; Kucharski, C.; Doschak, M.R.; Sebald, W.; Uludağ, H. Polyethylenimine–PEG coated albumin nanoparticles for BMP-2 delivery. Biomaterials 2010, 31, 952–963. [Google Scholar] [CrossRef]
- Singh, H.D.; Wang, G.; Uludağ, H.; Unsworth, L.D. Poly-L-lysine-coated albumin nanoparticles: Stability, mechanism for increasing. In vitro enzymatic resilience and siRNA release characteristics. Acta Biomater. 2010, 6, 4277–4284. [Google Scholar] [CrossRef]
- Wang, G.; Siggers, K.; Zhang, S.; Jiang, H.; Xu, Z.; Zernicke, R.F.; Matyas, J.; Uludağ, H. Preparation of BMP-2 containing bovine serum albumin (BSA) nanoparticles stabilized by polymer coating. Pharm. Res. 2008, 25, 2896–2909. [Google Scholar] [CrossRef]
- Shen, Z.Y.; Ma, G.H.; Dobashi, T.; Maki, Y.; Su, Z.G. Preparation and characterization of thermo-responsive albumin nanospheres. Int. J. Pharm. 2008, 346, 133–142. [Google Scholar] [CrossRef]
- Shen, Z.; Wei, W.; Zhao, Y.; Ma, G.; Dobashi, T.; Maki, Y.; Su, Z.; Wan, J. Thermosensitive polymer-conjugated albumin nanospheres as thermal targeting anti-cancer drug carrier. Eur. J. Pharm. Sci. 2008, 35, 271–282. [Google Scholar] [CrossRef]
- Kouchakzadeh, H.; Shojaosadati, S.A.; Maghsoudi, A.; Vasheghani Farahani, E. Optimization of PEGylation conditions for BSA nanoparticles using response surface methodology. AAPS PharmSciTech 2010, 11, 1206–1211. [Google Scholar] [CrossRef]
- Lin, W.; Garnett, M.C.; Davis, S.S.; Schacht, E.; Ferruti, P.; Illum, L. Preparation and characterization of Rose Bengal-loaded surface-modified albumin nanoparticles. J. Control. Release 2001, 71, 117–126. [Google Scholar] [CrossRef]
- Lin, W.; Coombes, A.G.; Garnett, M.C.; Davies, M.C.; Schacht, E.; Davis, S.S.; Illum, L. Preparation of sterically stabilized human serum albumin nanospheres using a novel Dextranox-MPEG crosslinking agent. Pharm. Res. 1994, 11, 1588–1592. [Google Scholar] [CrossRef] [PubMed]
- Oyewumi, M.O.; Mumper, R.J. Influence of formulation parameters on gadolinium entrapment and tumor cell uptake using folate-coated nanoparticles. Int. J. Pharm. 2003, 251, 85–97. [Google Scholar] [CrossRef]
- Vandervoort, J.; Ludwig, A. Biocompatible stabilizers in the preparation of PLGA nanoparticles: A factorial design study. Int. J. Pharm. 2002, 238, 77–92. [Google Scholar] [CrossRef] [PubMed]
- Ulbrich, K.; Michaelis, M.; Rothweiler, F.; Knobloch, T.; Sithisarn, P.; Cinatl, J.; Kreuter, J. Interaction of folate-conjugated human serum albumin (HSA) nanoparticles with tumor cells. Int. J. Pharm. 2011, 406, 128–134. [Google Scholar] [CrossRef] [PubMed]
- Chen, D.; Tang, Q.; Xue, W.; Xiang, J.; Zhang, L.; Wang, X. The preparation and characterization of folate-conjugated human serum albumin magnetic cisplatin nanoparticles. J. Biomed. Res. 2010, 24, 26–32. [Google Scholar] [CrossRef] [PubMed]
- Zu, Y.; Zhang, Y.; Zhao, X.; Zhang, Q.; Liu, Y.; Jiang, R. Optimization of the preparation process of vinblastine sulfate (VBLS)-loaded folate conjugated bovine serum albumin (BSA) nanoparticles for tumor-targeted drug delivery using response surface methodology (RSM). Int. J. Nanomed. 2009, 4, 321–333. [Google Scholar] [CrossRef]
- Zhang, L.K.; Hou, S.X.; Zhang, J.Q.; Hu, W.J.; Wang, C.Y. Preparation, characterization, and in vivo evaluation of mitoxantrone-loaded, folate-conjugated albumin nanoparticles. Arch. Pharm. Res. 2010, 33, 1193–1198. [Google Scholar] [CrossRef]
- Zu, Y.G.; Yuan, S.; Zhao, X.H.; Zhang, Y.; Zhang, X.N.; Jiang, R. Preparation, activity and targeting ability evaluation in vitro on folate mediated epigallocatechin-3-gallate albumin nanoparticles. Acta Pharm. Sin. B 2009, 44, 525–531. [Google Scholar]
- Dubey, P.K.; Singodia, D.; Verma, R.K.; Vyas, S.P. RGD modified albumin nanospheres for tumor vasculature targeting. J. Pharm. Pharmacol. 2011, 63, 33–40. [Google Scholar] [CrossRef]
- Karmali, P.P.; Kotamraju, V.R.; Kastantin, M.; Black, M.; Missirlis, D.; Tirrell, M.; Ruoslahti, E. Targeting of albumin-embedded paclitaxel nanoparticles to tumors. Nanotechnol. Biol. Med. 2009, 5, 73–82. [Google Scholar] [CrossRef]
- Zensi, A.; Begley, D.; Pontikis, C.; Legros, C.; Mihoreanu, L.; Wagner, S.; Büchel, C.; von Briesen, H.; Kreuter, J. Albumin nanoparticles targeted with Apo E enter the CNS by transcytosis and are delivered to neurons. J. Control. Release 2009, 137, 78–86. [Google Scholar] [CrossRef] [PubMed]
- Kreuter, J.; Hekmatara, T.; Dreis, S.; Vogel, T.; Gelperina, S.; Langer, K. Covalent attachment of apolipoprotein A-I and apolipoprotein B-100 to albumin nanoparticles enables drug transport into the brain. J. Control. Release 2007, 118, 54–58. [Google Scholar] [CrossRef] [PubMed]
- Mishra, V.; Mahor, S.; Rawat, A.; Gupta, P.N.; Dubey, P.; Khatri, K.; Vyas, S.P. Targeted brain delivery of AZT via transferrin anchored pegylated albumin nanoparticles. J. Drug Target 2006, 14, 45–53. [Google Scholar] [CrossRef] [PubMed]
- Ulbrich, K.; Hekmatara, T.; Herbert, E.; Kreuter, J. Transferrin- and transferrinreceptor-antibody-modified nanoparticles enable drug delivery across the blood–brain barrier (BBB). Eur. J. Pharm. Biopharm. 2009, 71, 251–256. [Google Scholar] [CrossRef] [PubMed]
- Wartlick, H.; Michaelis, K.; Balthasar, S.; Strebhardt, K.; Kreuter, J.; Langer, K. Highly specific HER2-mediated cellular uptake of antibody-modified nanoparticles in tumor cells. J. Drug Target. 2004, 12, 461–471. [Google Scholar] [CrossRef]
- Steinhauser, I.M.; Langer, K.; Strebhardt, K.M.; Spänkuch, B. Effect of trastuzumabmodified antisense oligonucleotide-loaded human serum albumin nanoparticles prepared by heat denaturation. Biomaterials 2008, 29, 4022–4028. [Google Scholar] [CrossRef]
- Wagner, S.; Rothweiler, F.; Anhorn, M.G.; Sauer, D.; Riemann, I.; Weiss, E.C.; Katsen-Globa, A.; Michaelis, M.; Cinatl, J., Jr.; Schwartz, D.; et al. Enhanced drug targeting by attachment of an anti αv integrin antibody to doxorubicin loaded human serum albumin nanoparticles. Biomaterials 2010, 31, 2388–2398. [Google Scholar] [CrossRef]
- Sleep, D. Albumin and its application in drug delivery. Expert Opin. Drug Deliv. 2015, 12, 793–812. [Google Scholar] [CrossRef]
- Chubarov, A.S.; Zakharova, O.D.; Koval, O.A.; Romaschenko, A.V.; Akulov, A.E.; Zavjalov, E.L.; Razumov, I.A.; Koptyug, I.V.; Knorre, D.G.; Godovikova, T.S. Design of protein homocystamides with enhanced tumor uptake properties for 19F magnetic resonance imaging. Bioorg. Med. Chem. 2015, 23, 6943–6954. [Google Scholar] [CrossRef]
- Kratz, F.; Elsadek, B. Clinical impact of serum proteins on drug delivery. J. Control. Release 2012, 161, 429–445. [Google Scholar] [CrossRef] [PubMed]
- Yhee, J.Y.; Lee, J.; Chang, H.; Lee, J.; Kwon, I.C.; Kim, K. Molecular imaging and targeted drug delivery using albumin-based nanoparticles. Curr. Pharm. Des. 2015, 21, 1889–1898. [Google Scholar] [CrossRef] [PubMed]
- Kremer, P.; Fardanesh, M.; Ding, R.; Pritsch, M.; Zoubaa, S.; Frei, E. Intraoperative fluorescence staining of malignant brain tumors using 5-aminofluorescein-labeled albumin. Neurosurgery 2009, 64, 53–60. [Google Scholar] [CrossRef] [PubMed]
- Ding, R.; Frei, E.; Fardanesh, M.; Schrenk, H.-H.; Kremer, P.; Haefeli, W.E. Pharmacokinetics of 5-Aminofluorescein-Albumin, a Novel Fluorescence Marker of Brain Tumors During Surgery. J. Clin. Pharmacol. 2011, 51, 672–678. [Google Scholar] [CrossRef] [PubMed]
- Regino, C.A.; Ogawa, M.; Alford, R.; Wong, K.J.; Kosaka, N.; Williams, M.; Field, B.J.; Takahashi, M.; Choyke, P.L.; Kobayashi, H. Two-Step Synthesis of Galactosylated Human Serum Albumin as a Targeted Optical Imaging Agent for Peritoneal Carcinomatosis. J. Med. Chem. 2010, 53, 1579–1586. [Google Scholar] [CrossRef] [PubMed]
- Haubner, R.; Vera, D.R.; Farshchi-Heydari, S.; Helbok, A.; Rangger, C.; Putzer, D.; Virgolini, I.J. Development of 68Ga-labelled DTPA galactosyl human serum albumin for liver function imaging. Eur. J. Nucl. Med. Mol. Imaging 2013, 40, 1245–1255. [Google Scholar] [CrossRef]
- Watcharin, W.; Schmithals, C.; Pleli, T.; Köberle, V.; Korkusuz, H.; Huebner, F.; Zeuzem, S.; Korf, H.W.; Vogl, T.J.; Rittmeyer, C.; et al. Biodegradable human serum albumin nanoparticles as contrast agents for the detection of hepatocellular carcinoma by magnetic resonance imaging. Eur. J. Pharm. Biopharm. 2014, 87, 132–141. [Google Scholar] [CrossRef]
- Ruiz-Cabello, J.; Barnett, B.P.; Bottomley, P.A.; Bulte, J.W.M. Fluorine (19F) MRS and MRI in biomedicine. NMR Biomed. 2011, 24, 114–129. [Google Scholar] [CrossRef]
- Yu, J.X.; Kodibagkar, V.D.; Cui, W.; Mason, R.P. 19F: A Versatile Reporter for Non-Invasive Physiology and Pharmacology Using Magnetic Resonance. Curr. Med. Chem. 2005, 12, 819–848. [Google Scholar] [CrossRef]
- Nosé, Y. Is There a Role for Blood Substitutes in Civilian Medicine: A Drug for Emergency Shock Cases? Artif. Organs. 2004, 28, 807–812. [Google Scholar] [CrossRef]
- Neubauer, A.M.; Myerson, J.; Caruthers, S.D.; Hockett, F.D.; Winter, P.M.; Chen, J.; Gaffney, P.J.; Robertson, J.D.; Lanza, G.M.; Wickline, S.A. Gadolinium-modulated 19F signals from perfluorocarbon nanoparticles as a new strategy for molecular imaging. Magn. Reson. Med. 2008, 60, 1066–1072. [Google Scholar] [CrossRef] [PubMed]
- Mehta, V.D.; Kulkarni, P.V.; Mason, R.P.; Constantinescu, A.; Antich, P.P. Fluorinated proteins as potential 19F magnetic resonance imaging and spectroscopy agents. Bioconjug. Chem. 1994, 5, 257–261. [Google Scholar] [CrossRef] [PubMed]
- Maeda, H.; Wu, J.; Sawa, T.; Matsumura, Y.; Hori, K. Tumor vascular permeability and the EPR effect in macromolecular therapeutics: A review. J. Control. Release 2000, 65, 271–284. [Google Scholar] [CrossRef] [PubMed]
- Hauser, C.A.; Stockler, M.R.; Tattersall, M.H.N. Prognostic factors in patients with recently diagnosed incurable cancer: A systematic review. Support Care Cancer 2006, 14, 999–1011. [Google Scholar] [CrossRef]
- Liu, Z.; Jiao, Y.; Wang, Y.; Zhou, C.; Zhang, Z. Polysaccharides-based nanoparticles as drug delivery systems. Adv. Drug Deliv. Rev. 2008, 60, 1650–1662. [Google Scholar] [CrossRef]
- Feng, Y.; Yu, Y.B. Synthesis and characterization of fluorinated conjugates of albumin. J. Fluor. Chem. 2013, 152, 173–181. [Google Scholar] [CrossRef]
- Marczak, Ł.; Sikora, M.; Stobiecki, M.; Jakubowski, H. Analysis of site-specific N-homocysteinylation of human serum albumin in vitro and in vivo using MALDI-ToF and LC-MS/MS mass spectrometry. J. Proteom. 2011, 74, 967–974. [Google Scholar] [CrossRef]
- Hashizume, H.; Baluk, P.; Morikawa, S.; McLean, J.W.; Thurston, G.; Roberge, S.; Jain, R.K.; McDonald, D.M. Openings between Defective Endothelial Cells Explain Tumor Vessel Leakiness. Am. J. Pathol. 2000, 156, 1363–1380. [Google Scholar] [CrossRef]
- Paoli, P.; Sbrana, F.; Tiribilli, B.; Caselli, A.; Pantera, B.; Cirri, P.; De Donatis, A.; Formigli, L.; Nosi, D.; Manao, G.; et al. Protein N-Homocysteinylation Induces the Formation of Toxic Amyloid-Like Protofibrils. J. Mol. Biol. 2010, 400, 889–907. [Google Scholar] [CrossRef]
- Lee, J.J.; Chu, E. Recent Advances in the Clinical Development of Immune Checkpoint Blockade Therapy for Mismatch Repair Proficient (pMMR)/non-MSI-H Metastatic Colorectal Cancer. Clin. Color Cancer 2018, 17, 258. [Google Scholar] [CrossRef]
- Temmink, O.H.; Fukushima, M.; Kruyt, F.A. Differential activation of cell death and autophagy results in an increased cytotoxic potential for trifluorothymidine compared to 5-fluorouracil in colon cancer cells. Int. J. Cancer 2010, 126, 2457–2468. [Google Scholar] [CrossRef]
- Eckstein, J.W.; Foster, P.G.; Finer-Moore, J.; Wataya, Y.; Santi, D.V. Mechanism-Based Inhibition of Thymidylate Synthase by 5-(Trifluoromethyl)-2′-deoxyuridine 5′-Monophosphate. Biochemistry 1994, 33, 15086–15094. [Google Scholar] [CrossRef] [PubMed]
- Tanaka, N.; Sakamoto, K.; Okabe, H.; Fujioka, A.; Yamamura, K.; Nakagawa, F.; Nagase, H.; Yokogawa, T.; Oguchi, K.; Ishida, K.; et al. Repeated oral dosing of TAS-102 confers high trifluridine incorporation into DNA and sustained antitumor activity in mouse models. Oncol. Rep. 2014, 32, 2319–2326. [Google Scholar] [CrossRef]
- Suzuki, N.; Nakagawa, F.; Nukatsuka, M.; Fukushima, M. Trifluorothymidine exhibits potent antitumor activity via the induction of DNA double-strand breaks. Exp. Ther. Med. 2011, 2, 393–397. [Google Scholar] [CrossRef]
- Matsuoka, K.; Iimori, M.; Niimi, S.; Tsukihara, H.; Watanabe, S.; Kiyonari, S.; Kiniwa, M.; Ando, K.; Tokunaga, E.; Saeki, H.; et al. Trifluridine Induces p53-Dependent Sustained G2 Phase Arrest with Its Massive Misincorporation into DNA and Few DNA Strand Breaks. Mol. Cancer Ther. 2015, 14, 1004–1013. [Google Scholar] [CrossRef]
- Godovikova, T.S.; Lisitskiy, V.A.; Antonova, N.M.; Popova, T.V.; Zakharova, O.D.; Chubarov, A.S.; Koptyug, I.V.; Sagdeev, R.Z.; Kaptein, R.; Akulov, A.E.; et al. Ligand-Directed Acid-Sensitive Amidophosphate 5-Trifluoromethyl-2′-Deoxyuridine Conjugate as a Potential Theranostic Agent. Bioconjugate Chem. 2013, 24, 780–795. [Google Scholar] [CrossRef] [PubMed]
- Binauld, S.; Stenzel, M.H. Acid-degradable polymers for drug delivery: A decade of innovation. Chem. Commun. 2013, 49, 2082–2102. [Google Scholar] [CrossRef] [PubMed]
- Lisitskiy, V.A.; Khan, H.; Popova, T.V.; Chubarov, A.S.; Zakharova, O.D.; Akulov, A.E.; Shevelev, O.B.; Zavjalov, E.L.; Koptyug, I.V.; Moshkin, M.P.; et al. Multifunctional human serum albumin-therapeutic nucleotide conjugate with redox and pH-sensitive drug release mechanism for cancer theranostics. Bioorg. Med. Chem. Lett. 2017, 27, 3925–3930. [Google Scholar] [CrossRef]
- Popova, T.V.; Khan, H.; Chubarov, A.S.; Lisitskiy, V.A.; Antonova, N.M.; Akulov, A.E.; Shevelev, O.B.; Zavjalov, E.L.; Silnikov, V.N.; Ahmad, S.; et al. Biotin-decorated anti-cancer nucleotide theranostic conjugate of human serum albumin: Where the seed meets the soil? Bioorg. Med. Chem. Lett. 2018, 28, 260–264. [Google Scholar] [CrossRef]
- Andersen, J.T.; Dalhus, B.; Viuff, D.; Ravn, B.T.; Gunnarsen, K.S.; Plumridge, A.; Bunting, K.; Antunes, F.; Williamson, R.; Athwal, S.; et al. Extending Serum Half-life of Albumin by Engineering Neonatal Fc Receptor (FcRn) Binding. J. Biol. Chem. 2014, 289, 13492–13502. [Google Scholar] [CrossRef]
- Li, C.; Tan, J.; Chang, J.; Li, W.; Liu, Z.; Li, N.; Ji, Y. Radioiodine-labeled anti-epidermal growth factor receptor binding bovine serum albumin-polycaprolactone for targeting imaging of glioblastoma. Oncol. Rep. 2017, 38, 2919–2926. [Google Scholar] [CrossRef] [PubMed]
- Kao, H.; Lin, Y.; Chen, C.; Chi, K.; Tien, D.; Hsia, C.; Lin, M.; Wang, H. Evaluation of EGFR-targeted radioimmuno-gold-nanoparticles as a theranostic agent in a tumor animal model. Bioorg. Med. Chem Lett. 2013, 23, 3180–3185. [Google Scholar] [CrossRef]
- Tang, Y.; Wang, W.; Zheng, K.; Jiang, L.; Zou, Y.; Su, X.; Chen, J.; Zhang, W.; Liu, W. EGFR mutations in non-small cell lung cancer: An audit from West China University Hospital. Expert Rev. Mol. Diagn. 2016, 16, 915–919. [Google Scholar] [CrossRef]
- Verreault, M.; Weppler, S.A.; Stegeman, A.; Warburton, C.; Strutt, D.; Masin, D.; Bally, M.B. Combined RNAi-mediated suppression of Rictor and EGFR resulted in complete tumor regression in an orthotopic glioblastoma tumor model. PLoS ONE 2013, 8, e59597. [Google Scholar] [CrossRef] [PubMed]
- Israel, L.L.; Galstyan, A.; Holler, E.; Ljubimova, J.Y. Magnetic iron oxide nanoparticles for imaging, targeting and treatment of primary and metastatic tumors of the brain. J. Control. Release 2020, 320, 45–62. [Google Scholar] [CrossRef] [PubMed]
- Ahmadi, S.; Fazilati, M.; Nazem, H.; Mousavi, S.M. Green Synthesis of Magnetic Nanoparticles Using Satureja hortensis Essential Oil toward Superior Antibacterial/Fungal and Anticancer Performance. Biomed Res. Int. 2021, 2021, 8822645. [Google Scholar] [CrossRef]
- Mousavi, S.M.; Hashemi, S.A.; Gholami, A.; Omidifar, N.; Zarei, M.; Bahrani, S.; Yousefi, K.; Chiang, W.H.; Babapoor, A. Bioinorganic Synthesis of Polyrhodanine Stabilized Fe3O4/Graphene Oxide in Microbial Supernatant Media for Anticancer and Antibacterial Applications. Bioinorg. Chem. Appl. 2021, 2021, 9972664. [Google Scholar] [CrossRef]
- Ulbrich, K.; Holá, K.; Šubr, V.; Bakandritsos, A.; Tuček, J.; Zbořil, R. Targeted Drug Delivery with Polymers and Magnetic Nanoparticles: Covalent and Noncovalent Approaches, Release Control, and Clinical Studies. Chem. Rev. 2016, 116, 5338–5431. [Google Scholar] [CrossRef]
- Shabatina, T.I.; Vernaya, O.I.; Shabatin, V.P.; Melnikov, M.Y. Magnetic nanoparticles for biomedical purposes: Modern trends and prospects. Magnetochemistry 2020, 6, 30. [Google Scholar] [CrossRef]
- Ganapathe, L.S.; Mohamed, M.A.; Yunus, R.M.; Berhanuddin, D.D. Magnetite (Fe3O4) nanoparticles in biomedical application: From synthesis to surface functionalisation. Magnetochemistry 2020, 6, 68. [Google Scholar] [CrossRef]
- Stueber, D.D.; Villanova, J.; Aponte, I.; Xiao, Z. Magnetic Nanoparticles in Biology and Medicine: Past, Present, and Future Trends. Pharmaceutics 2021, 13, 943. [Google Scholar] [CrossRef] [PubMed]
- Khramtsov, P.; Barkina, I.; Kropaneva, M.; Bochkova, M.; Timganova, V.; Nechaev, A.; Byzov, I.; Zamorina, S.; Yermakov, A.; Rayev, M. Magnetic nanoclusters coated with albumin, casein, and gelatin: Size tuning, relaxivity, stability, protein corona, and application in nuclear magnetic resonance immunoassay. Nanomaterials 2019, 9, 1345. [Google Scholar] [CrossRef] [PubMed]
- Sakulkhu, U.; Mahmoudi, M.; Maurizi, L.; Salaklang, J.; Hofmann, H. Protein corona composition of superparamagnetic iron oxide nanoparticles with various physico-Chemical properties and coatings. Sci. Rep. 2014, 4, 5020. [Google Scholar] [CrossRef] [PubMed]
- Srimanee, A.; Regberg, J.; Hallbrink, M.; Kurrikoff, K.; Veiman, K.-L.; Vajragupta, O.; Langel, Ü. Peptide-Based Delivery of Oligonucleotides Across Blood–Brain Barrier Model. Int. J. Pept. Res. 2014, 20, 169–178. [Google Scholar] [CrossRef]
- Patil, R.; Ljubimov, A.V.; Gangalum, P.R.; Ding, H.; Portilla-Arias, J.; Wagner, S.; Inoue, S.; Konda, B.; Rekechenetskiy, A.; Chesnokova, A.; et al. MRI Virtual Biopsy and Treatment of Brain Metastatic Tumors with Targeted Nanobioconjugates: Nanoclinic in the Brain. ACS Nano 2015, 9, 5594–5608. [Google Scholar] [CrossRef] [PubMed]
- Kou, L.; Hou, Y.; Yao, Q.; Guo, W.; Wang, G.; Wang, M.; Fu, Q.; He, Z.; Ganapathy, V.; Sun, J. L-Carnitine-conjugated nanoparticles to promote permeation across blood–brain barrier and to target glioma cells for drug delivery via the novel organic cation/carnitine transporter OCTN2. Artif. Cells Nanomed. Biotechnol. 2018, 46, 1605–1616. [Google Scholar] [CrossRef] [PubMed]
- Sockolosky, J.T.; Szoka, F.C. The neonatal Fc receptor, FcRn, as a target for drug delivery and therapy. Adv. Drug Deliv. Rev. 2015, 91, 109–124. [Google Scholar] [CrossRef]
- Schmidt, E.G.W.; Hvam, M.L.; Antunes, F.; Cameron, J.; Viuff, D.; Andersen, B.; Kristensen, N.N.; Howard, K.A. Direct demonstration of a neonatal Fc receptor (FcRn)-driven endosomal sorting pathway for cellular recycling of albumin. J. Biol. Chem. 2017, 292, 13312–13322. [Google Scholar] [CrossRef]
- Canaparo, R.; Foglietta, F.; Limongi, T.; Serpe, L. Biomedical applications of reactive oxygen species generation by metal nanoparticles. Materials 2021, 14, 53. [Google Scholar] [CrossRef]
- Hepel, M. Magnetic nanoparticles for nanomedicine. Magnetochemistry 2020, 6, 3. [Google Scholar] [CrossRef]
- Chouhan, R.S.; Horvat, M.; Ahmed, J.; Alhokbany, N.; Alshehri, S.M.; Gandhi, S. Magnetic nanoparticles—A multifunctional potential agent for diagnosis and therapy. Cancers 2021, 13, 2213. [Google Scholar] [CrossRef] [PubMed]
- Baki, A.; Remmo, A.; Löwa, N.; Wiekhorst, F.; Bleul, R. Albumin-coated single-core iron oxide nanoparticles for enhanced molecular magnetic imaging (Mri/mpi). Int. J. Mol. Sci. 2021, 22, 6235. [Google Scholar] [CrossRef] [PubMed]
- Parashar, P.; Kumar, P.; Gautam, A.K.; Singh, N.; Bera, H.; Sarkar, S.; Saraf, S.A.; Saha, S. Albumin-based nanomaterials in drug delivery and biomedical applications. In Biopolymer-Based Nanomaterials in Drug Delivery and Biomedical Applications; Elsevier Inc.: Amsterdam, The Netherlands, 2021; pp. 407–426. [Google Scholar]
- Chen, Y.; Liu, L. Modern Methods for Delivery of Drugs across the Blood-Brain Barrier. Adv. Drug Deliv. Rev. 2012, 64, 640–665. [Google Scholar] [CrossRef]
- Roger, M.; Clavreul, A.; Venier-Julienne, M.C.; Passirani, C.; Sindji, L.; Schiller, P.; Montero-Menei, C.; Menei, P. Mesenchymal Stem Cells as Cellular Vehicles for Delivery of Nanoparticles to Brain Tumors. Biomaterials 2010, 31, 8393–8401. [Google Scholar] [CrossRef] [PubMed]
- Xue, J.; Zhao, Z.; Zhang, L.; Xue, L.; Shen, S.; Wen, Y.; Wei, Z.; Wang, L.; Kong, L.; Sun, H.; et al. Neutrophil-Mediated Anticancer Drug Delivery for Suppression of Postoperative Malignant Glioma Recurrence. Nat. Nanotechnol. 2017, 12, 692–700. [Google Scholar] [CrossRef]
- Benmelouka, A.Y.; Munir, M.; Sayed, A.; Attia, M.S.; Ali, M.M.; Negida, A.; Alghamdi, B.S.; Kamal, M.A.; Barreto, G.E.; Ashraf, G.M.; et al. Neural Stem Cell-Based Therapies and Glioblastoma Management: Current Evidence and Clinical Challenges. Int. J. Mol. Sci. 2021, 22, 2258. [Google Scholar] [CrossRef] [PubMed]
- Yi, T.; Tang, D.; Wang, F.; Zhang, J.; Zhang, J.; Wang, J.; Xu, X.; Zhang, J. Enhancing Both Oral Bioavailability and Brain Penetration of Puerarin Using Borneol in Combination with Preparation Technologies. Drug Deliv. 2017, 24, 422–429. [Google Scholar] [CrossRef]
- Fu, H.; McCarty, D.M. Crossing the Blood-Brain-Barrier with Viral Vectors. Curr. Opin. Virol. 2016, 21, 87–92. [Google Scholar] [CrossRef]
- Ahmad, F.; Varghese, R.; Panda, S.; Ramamoorthy, S.; Areeshi, M.Y.; Fagoonee, S.; Haque, S. Smart Nanoformulations for Brain Cancer Theranostics: Challenges and Promises. Cancers 2022, 14, 5389. [Google Scholar] [CrossRef]
- Moya, C.; Escudero, R.; Malaspina, D.C.; De La Mata, M.; Hernández-Saz, J.; Faraudo, J.; Roig, A. Insights into Preformed Human Serum Albumin Corona on Iron Oxide Nanoparticles: Structure, Effect of Particle Size, Impact on MRI Efficiency, and Metabolization. ACS Appl. Bio Mater. 2019, 2, 3084–3094. [Google Scholar] [CrossRef]
- Malhotra, N.; Lee, J.-S.; Liman, R.A.D.; Ruallo, J.M.S.; Villaflores, O.B.; Ger, T.-R.; Hsiao, C.-D. Potential Toxicity of Iron Oxide Magnetic Nanoparticles: A Review. Molecules 2020, 25, 3159. [Google Scholar] [CrossRef] [PubMed]
- Nelson, N.; Port, J.; Pandey, M. Use of Superparamagnetic Iron Oxide Nanoparticles (SPIONs) via Multiple Imaging Modalities and Modifications to Reduce Cytotoxicity: An Educational Review. J. Nanotheranost. 2020, 1, 105–135. [Google Scholar] [CrossRef]
- Chrishtop, V.V.; Mironov, V.A.; Prilepskii, A.Y.; Nikonorova, V.G.; Vinogradov, V.V. Organ-specific toxicity of magnetic iron oxide-based nanoparticles. Nanotoxicology 2021, 15, 167–204. [Google Scholar] [CrossRef]
- Abakumov, M.A.; Semkina, A.S.; Skorikov, A.S.; Vishnevskiy, D.A.; Ivanova, A.V.; Mironova, E.; Davydova, G.A.; Majouga, A.G.; Chekhonin, V.P. Toxicity of iron oxide nanoparticles: Size and coating effects. J. Biochem. Mol. Toxicol. 2018, 32, e22225. [Google Scholar] [CrossRef]
- Xie, J.; Wang, J.; Niu, G.; Huang, J.; Nosrati, H.; Sefidi, N.; Sharafi, A.; Danafar, H.; Kheiri Manjili, H. Bovine Serum Albumin (BSA) coated iron oxide magnetic nanoparticles as biocompatible carriers for curcumin-anticancer drug. Bioorg. Chem. 2018, 76, 501–509. [Google Scholar] [CrossRef]
- Nosrati, H.; Salehiabar, M.; Manjili, H.K.; Danafar, H.; Davaran, S. Preparation of magnetic albumin nanoparticles via a simple and one-pot desolvation and co-precipitation method for medical and pharmaceutical applications. Int. J. Biol. Macromol. 2018, 108, 909–915. [Google Scholar] [CrossRef] [PubMed]
- Balk, M.; Haus, T.; Band, J.; Unterweger, H.; Schreiber, E.; Friedrich, R.P.; Alexiou, C.; Gostian, A.O. Cellular spion uptake and toxicity in various head and neck cancer cell lines. Nanomaterials 2021, 11, 726. [Google Scholar] [CrossRef]
- Toropova, Y.G.; Motorina, D.S.; Zelinskaya, I.; Korolev, D.V.; Schulmeister, G.; Skorik, Y. Generation of Reactive Oxygen Species by Human Whole Blood Cells Exposed to Iron Oxide Magnetic Nanoparticles Coated with Different Shells. Bull. Exp. Biol. Med. 2021, 171, 77–80. [Google Scholar] [CrossRef]
- An, L.; Yan, C.; Mu, X.; Tao, C.; Tian, Q.; Lin, J.; Yang, S. Paclitaxel-Induced Ultrasmall Gallic Acid-Fe@BSA Self-Assembly with Enhanced MRI Performance and Tumor Accumulation for Cancer Theranostics. ACS Appl. Mater. Interfaces 2018, 10, 28483–28493. [Google Scholar] [CrossRef]
- Nunes, A.D.C.; Gomes-Silva, L.A.; Zufelato, N.; Prospero, A.G.; Quini, C.C.; Matos, R.V.R.; Miranda, J.R.A.; Bakuzis, A.F.; Castro, C.H. Albumin Coating Prevents Cardiac Effect of the Magnetic Nanoparticles. IEEE Trans. Nanobiosci. 2019, 18, 640–650. [Google Scholar] [CrossRef]
- Kostevšek, N. A review on the optimal design of magnetic nanoparticle-based t2 mri contrast agents. Magnetochemistry 2020, 6, 11. [Google Scholar] [CrossRef]
- Xie, J.; Chen, K.; Huang, J.; Lee, S.; Wang, J.; Gao, J.; Li, X.; Chen, X. PET/NIRF/MRI triple functional iron oxide nanoparticles. Biomaterials 2010, 31, 3016–3022. [Google Scholar] [CrossRef] [PubMed]
- Vismara, E.; Bongio, C.; Coletti, A.; Edelman, R.; Serafini, A.; Mauri, M.; Simonutti, R.; Bertini, S.; Urso, E.; Assaraf, Y.G.; et al. Albumin and hyaluronic acid-coated superparamagnetic iron oxide nanoparticles loaded with paclitaxel for biomedical applications. Molecules 2017, 22, 1030. [Google Scholar] [CrossRef] [PubMed]
- Corem-Salkmon, E.; Ram, Z.; Daniels, D.; Perlstein, B.; Last, D.; Salomon, S.; Tamar, G.; Shneor, R.; Guez, D.; Margel, S.; et al. Convection-enhanced delivery of methotrexate-loaded maghemite nanoparticles. Int. J. Nanomed. 2011, 6, 1595–1602. [Google Scholar] [CrossRef]
- Babincová, M.; Vrbovská, H.; Sourivong, P.; Babinec, P.; Durdík, Š. Application of albumin-embedded magnetic nanoheaters for release of etoposide in integrated chemotherapy and hyperthermia of U87-MG glioma cells. Anticancer Res. 2018, 38, 2683–2690. [Google Scholar]
- Maier-Hauff, K.; Ulrich, F.; Nestler, D.; Niehoff, H.; Wust, P.; Thiesen, B.; Orawa, H.; Budach, V.; Jordan, A. Efficacy and safety of intratumoral thermotherapy using magnetic iron-oxide nanoparticles combined with external beam radiotherapy on patients with recurrent glioblastoma multiforme. J Neurooncol 2011, 103, 317–324. [Google Scholar] [CrossRef]
- Tabatabaei, S.N.; Girouard, H.; Carret, A.S.; Martel, S. Remote control of the permeability of the blood–brain barrier by magnetic heating of nanoparticles: A proof of concept for brain drug delivery. J. Control. Release 2015, 206, 49–57. [Google Scholar] [CrossRef]
- Xu, X.; Chen, X.; Xu, X.; Lu, T.; Wang, X.; Yang, L.; Jing, X. BCNU-loaded PEG–PLLA ultrafine fibers and their in vitro antitumor activity against Glioma C6 cells. J. Control. Release 2006, 114, 307–316. [Google Scholar] [CrossRef]
- Chae, G.S.; Lee, J.S.; Kim, S.H.; Seo, K.S.; Kim, M.S.; Lee, H.B.; Khang, G. Enhancement of the stability of BCNU using self-emulsifying drug delivery systems (SEDDS) and in vitro antitumor activity of self-emulsified BCNU-loaded PLGA wafer. Int. J. Pharm. 2005, 301, 6–14. [Google Scholar] [CrossRef]
- Li, Y.; Tyler, B.; Williams, T.; Tupper, M.; Langer, R.; Brem, H.; Cima, M.J. In vivo delivery of BCNU from a MEMS device to a tumor model. J. Control. Release 2005, 106, 138–145. [Google Scholar] [CrossRef]
- Rhines, L.D.; Sampath, P.; Dolan, M.E.; Tyler, B.M.; Brem, H.; Weingart, J. O6-benzylguanine potentiates the antitumor effect of locally delivered carmustine against an intracranial rat glioma. Cancer Res. 2000, 60, 6307–6310. [Google Scholar] [PubMed]
- Lin, F.; Xiong, X.-L.; Cui, E.-M.; Lei, Y. A novel ANG-BSA/BCNU/ICG MNPs integrated for targeting therapy of glioblastoma. Res. Sq. 2021. [Google Scholar] [CrossRef]
- Kuo, Y.C.; Cheng, S. Brain targeted delivery of carmustine using solid lipid nanoparticles modified with tamoxifen and lactoferrin for antitumor proliferation. Int. J. Pharm. 2016, 499, 10–19. [Google Scholar] [CrossRef] [PubMed]
- Shi, J.; Sun, X.; Zheng, S.; Li, J.; Fu, X.; Zhang, H. A new near-infrared persistent luminescence nanoparticle as a multifunctional nanoplatform for multimodal imaging and cancer therapy. Biomaterials 2018, 152, 15–23. [Google Scholar] [CrossRef] [PubMed]
- Deng, L.; Cai, X.; Sheng, D.; Yang, Y.; Strohm, E.M.; Wang, Z.; Ran, H.; Wang, D.; Zheng, Y.; Li, P.; et al. A Laser-Activated Biocompatible Theranostic Nanoagent for Targeted Multimodal Imaging and Photothermal Therapy. Theranostics 2017, 7, 4410–4423. [Google Scholar] [CrossRef]
- Stanescu-Segall, D.; Jackson, T.L. Vital staining with indocyanine green: A review of the clinical and experimental studies relating to safety. Eye 2009, 23, 504–518. [Google Scholar] [CrossRef]
- Abakumov, M.A.; Nukolova, N.V.; Sokolsky-Papkov, M.; Shein, S.A.; Sandalova, T.O.; Vishwasrao, H.M.; Grinenko, N.F.; Gubsky, I.L.; Abakumov, A.M.; Kabanov, A.V.; et al. VEGF-targeted magnetic nanoparticles for MRI visualization of brain tumor. Nanomedicine 2015, 11, 825–833. [Google Scholar] [CrossRef]
- Zhu, M.; Sheng, Z.; Jia, Y.; Hu, D.; Liu, X.; Xia, X.; Liu, C.; Wang, P.; Wang, X.; Zheng, H. Indocyanine Green-holo-Transferrin Nanoassemblies for Tumor-Targeted Dual-Modal Imaging and Photothermal Therapy of Glioma. ACS Appl. Mater. Interfaces 2017, 9, 39249–39258. [Google Scholar] [CrossRef]
- Gajbhiye, V.; Jain, N.K. The treatment of Glioblastoma Xenografts by surfactant conjugated dendritic nanoconjugates. Biomaterials 2011, 32, 6213–6225. [Google Scholar] [CrossRef]
- Gao, H.; Qian, J.; Yang, Z.; Pang, Z.; Xi, Z.; Cao, S.; Wang, Y.; Pan, S.; Zhang, S.; Wang, W.; et al. Whole-cell SELEX aptamer-functionalised poly(ethyleneglycol)-poly(ε-caprolactone) nanoparticles for enhanced targeted glioblastoma therapy. Biomaterials 2012, 33, 6264–6272. [Google Scholar] [CrossRef]
- Shi, K.; Zhou, J.; Zhang, Q.; Gao, H.; Liu, Y.; Zong, T.; He, Q. Arginine-glycine-aspartic acid-modified lipid-polymer hybrid nanoparticles for docetaxel delivery in glioblastoma multiforme. J. Biomed. Nanotechnol. 2015, 11, 382–391. [Google Scholar] [CrossRef] [PubMed]
- Ganipineni, L.P.; Ucakar, B.; Joudiou, N.; Riva, R.; Jérôme, C.; Gallez, B.; Danhier, F.; Préat, V. Paclitaxel-loaded multifunctional nanoparticles for the targeted treatment of glioblastoma. J. Drug Target. 2019, 27, 614–623. [Google Scholar] [CrossRef]
- Kang, T.; Jiang, M.; Jiang, D.; Feng, X.; Yao, J.; Song, Q.; Chen, H.; Gao, X.; Chen, J. Enhancing glioblastoma-specific penetration by functionalization of nanoparticles with an iron-mimic peptide targeting transferrin/transferrin receptor complex. Mol. Pharm. 2015, 12, 2947–2961. [Google Scholar] [CrossRef] [PubMed]
- Kang, T.; Zhu, Q.; Jiang, D.; Feng, X.; Feng, J.; Jiang, T.; Yao, J.; Jing, Y.; Song, Q.; Jiang, X.; et al. Synergistic targeting tenascin C and neuropilin-1 for specific penetration of nanoparticles for anti-glioblastoma treatment. Biomaterials 2016, 101, 60–75. [Google Scholar] [CrossRef] [PubMed]
- Yang, F.Y.; Wong, T.T.; Teng, M.C.; Liu, R.S.; Lu, M.; Liang, H.F.; Wei, M.C. Focused ultrasound and interleukin-4 receptor-targeted liposomal doxorubicin for enhanced targeted drug delivery and antitumor effect in glioblastoma multiforme. J. Control. Release 2012, 160, 652–658. [Google Scholar] [CrossRef] [PubMed]
- Zhou, J.; Patel, T.R.; Sirianni, R.W.; Strohbehn, G.; Zheng, M.Q.; Duong, N.; Schafbauer, T.; Huttner, A.J.; Huang, Y.; Carson, R.E.; et al. Highly penetrative, drug-loaded nanocarriers improve treatment of glioblastoma. Proc. Natl. Acad. Sci. USA 2013, 110, 11751–11756. [Google Scholar] [CrossRef] [PubMed]
- Householder, K.T.; DiPerna, D.M.; Chung, E.P.; Wohlleb, G.M.; Dhruv, H.D.; Berens, M.E.; Sirianni, R.W. Intravenous delivery of camptothecin-loaded PLGA nanoparticles for the treatment of intracranial glioma. Int. J. Pharm. 2015, 479, 374–380. [Google Scholar] [CrossRef]
- Gaudin, A.; Song, E.; King, A.R.; Saucier-Sawyer, J.K.; Bindra, R.; Desmaële, D.; Couvreur, P.; Saltzman, W.M. PEGylated squalenoyl-gemcitabine nanoparticles for the treatment of glioblastoma. Biomaterials 2016, 105, 136–144. [Google Scholar] [CrossRef] [PubMed]
- Zhang, C.X.; Zhao, W.Y.; Liu, L.; Ju, R.J.; Mu, L.M.; Zhao, Y.; Zeng, F.; Xie, H.J.; Yan, Y.; Lu, W.L. A nanostructure of functional targeting epirubicin liposomes dually modified with aminophenyl glucose and cyclic pentapeptide used for brain glioblastoma treatment. Oncotarget 2015, 6, 32681–32700. [Google Scholar] [CrossRef]
- Kaluzova, M.; Bouras, A.; Machaidze, R.; Hadjipanayis, C.G. Targeted therapy of glioblastoma stem-like cells and tumor non-stem cells using cetuximabconjugated iron-oxide nanoparticles. Oncotarget 2015, 6, 8788–8806. [Google Scholar] [CrossRef]
- Bouras, A.; Kaluzova, M.; Hadjipanayis, C.G. Radiosensitivity enhancement of radioresistant glioblastoma by epidermal growth factor receptor antibodyconjugated iron-oxide nanoparticles. J. Neurooncol. 2015, 124, 13–22. [Google Scholar] [CrossRef] [PubMed]
- Cohen, Z.R.; Ramishetti, S.; Peshes-Yaloz, N.; Goldsmith, M.; Wohl, A.; Zibly, Z.; Peer, D. Localized RNAi therapeutics of chemoresistant grade IV glioma using hyaluronan-grafted lipid-based nanoparticles. ACS Nano 2015, 9, 1581–1591. [Google Scholar] [CrossRef] [PubMed]
- Danhier, F.; Messaoudi, K.; Lemaire, L.; Benoit, J.P.; Lagarce, F. Combined anti-Galectin-1 and anti-EGFR siRNA-loaded chitosan-lipid nanocapsules decrease temozolomide resistance in glioblastoma: In vivo evaluation. Int. J. Pharm. 2015, 481, 154–161. [Google Scholar] [CrossRef] [PubMed]
- Erel-Akbaba, G.; Carvalho, L.A.; Tian, T.; Zinter, M.; Akbaba, H.; Obeid, P.J.; Chiocca, E.A.; Weissleder, R.; Kantarci, A.G.; Tannous, B.A. Radiation-induced targeted nanoparticle-based gene delivery for brain tumor therapy. ACS Nano 2019, 13, 4028–4040. [Google Scholar] [CrossRef] [PubMed]
- Agemy, L.; Friedmann-Morvinski, D.; Kotamraju, V.R.; Roth, L.; Sugahara, K.N.; Girard, O.M.; Mattrey, R.F.; Verma, I.M.; Ruoslahti, E. Targeted nanoparticle enhanced proapoptotic peptide as potential therapy for glioblastoma. Proc. Natl. Acad. Sci. USA 2011, 108, 17450–17455. [Google Scholar] [CrossRef] [PubMed]
- Spindler, K.R.; Hsu, T.H. Viral disruption of the blood-brain barrier. Trends Microbiol. 2012, 20, 282–290. [Google Scholar] [CrossRef]
- Tabatabai, G.; Weller, M.; Nabors, B.; Picard, M.; Reardon, D.; Mikkelsen, T.; Ruegg, C.; Stupp, R. Targeting integrins in malignant glioma. Target. Oncol. 2010, 5, 175–181. [Google Scholar] [CrossRef]
- Kunigal, S.; Gondi, C.S.; Gujrati, M.; Lakka, S.S.; Dinh, D.H.; Olivero, W.C.; Rao, J.S. SPARC-induced migration of glioblastoma cell lines via uPAuPAR signaling and activation of small GTPase RhoA. PLoS ONE 2017, 32, 736–740. [Google Scholar] [CrossRef]
- Golembieski, W.A.; Rempel, S.A. cDNA array analysis of SPARCmodulated changes in glioma gene expression. J. Neurooncol. 2002, 60, 213–226. [Google Scholar] [CrossRef]
- Schnitzer, J.E. Gp60 is an albumin-binding glycoprotein expressed by continuous endothelium involved in albumin transcytosis by continuous endothelium glycoprotein expressed involved in albumin transcytosis. Am. J. Physiol. 1992, 262, 246–254. [Google Scholar] [CrossRef]
- Rahmani, S.; Ashraf, S.; Hartmann, R.; Dishman, A.F.; Zyuzin, M.V.; Yu, K.J.; Parak, W.J.; Lahann, J. Engineering of nanoparticle size via electrohydrodynamic jetting. Bioeng. Transl. Med. 2016, 1, 82–93. [Google Scholar] [CrossRef] [PubMed]
- Gregory, J.V.; Kadiyala, P.; Doherty, R.; Cadena, M.; Habeel, S.; Ruoslahti, E.; Lowenstein, P.R.; Castro, M.G.; Lahann, J. Systemic brain tumor delivery of synthetic protein nanoparticles for glioblastoma therapy. Nat. Commun. 2020, 11, 5687. [Google Scholar] [CrossRef] [PubMed]
- Sugahara, K.N.; Teesalu, T.; Karmali, P.P.; Kotamraju, V.R.; Agemy, L.; Girard, O.M.; Hanahan, D.; Mattrey, R.F.; Ruoslahti, E. Tissue-penetrating delivery of compounds and nanoparticles into tumors. Cancer Cell 2009, 16, 510–520. [Google Scholar] [CrossRef] [PubMed]
- Hu, C.; Yang, X.; Liu, R.; Ruan, S.; Zhou, Y.; Xiao, W.; Yu, W.; Yang, C.; Gao, H. Coadministration of iRGD with multistage responsive nanoparticles enhanced tumor targeting and penetration abilities for breast cancer therapy. ACS Appl. Mater. Interfaces 2018, 10, 38659–38662. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.; Wang, L.; Fai Chan, H.; Xie, W.; Chen, S.; He, C.; Wang, Y.; Chen, M. Co-delivery of paclitaxel and tetrandrine via iRGD peptide conjugated lipid-polymer hybrid nanoparticles overcome multidrug resistance in cancer cells. Sci. Rep. 2017, 7, 46057. [Google Scholar] [CrossRef]
- Mittapalli, R.K.; Manda, V.K.; Adkins, C.E.; Geldenhuys, W.J.; Lockman, P.R. Exploiting Nutrient Transporters at the Blood-Brain Barrier to Improve Brain Distribution of Small Molecules. Ther. Deliv. 2010, 1, 775–784. [Google Scholar] [CrossRef] [PubMed]
- Wunder, A.; Stehle, G.; Sinn, H.; Schrenk, H.; Hoffbiederbeck, D.; Bader, F.; Friedrich, E.; Peschke, P.; Maierborst, W.; Heene, D. Enhanced Albumin Uptake by Rat Tumors. Int. J. Oncol. 1997, 11, 497–507. [Google Scholar] [CrossRef]
- He, H.; Sheng, J.; David, A.E.; Kwon, Y.M.; Zhang, J.; Huang, Y.; Wang, J.; Yang, V.C. The Use of Low Molecular Weight Protamine Chemical Chimera to Enhance Monomeric Insulin Intestinal Absorption. Biomaterials 2013, 34, 7733–7743. [Google Scholar] [CrossRef]
- Wang, H.; Zhao, Y.; Wang, H.; Gong, J.; He, H.; Shin, M.C.; Yang, V.C.; Huang, Y. Low-Molecular-Weight Protamine-Modified PLGA Nanoparticles for Overcoming Drug-Resistant Breast Cancer. J. Control. Release 2014, 192C, 47–56. [Google Scholar] [CrossRef]
- Guo, Q.Q.; Wang, H.Y.; Zhao, Y.X.; Liu, E.G.; Wang, H.X.; Hua, H.Y.; Huang, Y.Z. Cell-Penetrating Albumin Conjugates for Enhanced Doxorubicin Delivery. Polym. Chem. 2013, 4, 4584–4587. [Google Scholar] [CrossRef]
- Lin, T.; Zhao, P.; Jiang, Y.; Tang, Y.; Jin, H.; Pan, Z.; He, H.; Yang, V.C.; Huang, Y. Blood–Brain-Barrier-Penetrating Albumin Nanoparticles for Biomimetic Drug Delivery via Albumin-Binding Protein Pathways for Antiglioma Therapy. ACS Nano 2016, 10, 9999–10012. [Google Scholar] [CrossRef]
- Mahaley, M.S., Jr.; Hipp, S.W.; Dropcho, E.J.; Bertsch, L.; Cush, S.; Tirey, T.; Gillespie, G.Y. Intracarotid cisplatin chemotherapy for recurrent gliomas. J. Neurosurg. 1989, 70, 371–378. [Google Scholar] [CrossRef]
- Weiss, R.B.; Poster, D.S.; Penta, J.S. The nitrosoureas and pulmonary toxicity. Cancer Treat. Rev. 1981, 8, 111–125. [Google Scholar] [CrossRef]
- Fisher, P.G.; Buffler, P.A. Malignant gliomas in 2005: Where to go from here? JAMA 2005, 293, 615–617. [Google Scholar] [CrossRef] [PubMed]
- Milner, J. A conformation hypothesis for the suppressor and promoter functions of p53 in cell growth control and in cancer. Proc. Biol. Sci. 1991, 245, 139–145. [Google Scholar] [CrossRef] [PubMed]
- Oudard, S.; Poirson, F.; Miccoli, L.; Bourgeois, Y.; Vassault, A.; Poisson, M.; Magdelénat, H.; Dutrillaux, B.; Poupon, M.F. Mitochondria-bound hexokinase as target for therapy of malignant gliomas. Int. J. Cancer 1995, 62, 216–222. [Google Scholar] [CrossRef]
- Kirsch, W.M.; Schulz, D.; Buskirk, J.V.; Nakane, P. Anaerobic energy metabolism in brain tumors. Prog. Exp. Tumor Res. 2015, 17, 163–191. [Google Scholar] [CrossRef]
- Seyfried, T.N.; Mukherjee, P. Targeting energy metabolism in brain cancer: Review and hypothesis. Nutr. Metab. 2005, 2, 30. [Google Scholar] [CrossRef] [PubMed]
- Seyfried, T.N. Perspectives on brain tumor formation involving macrophages, glia, and neural stem cells. Perspect. Biol. Med. 2001, 44, 263–282. [Google Scholar] [CrossRef]
- Williams, Z.R.; Goodman, C.B.; Soliman, K.F. Anaerobic glycolysis protection against 1-methy-4-phenylpyridinium (mpp+) toxicity in c6 glioma cells. Neurochem. Res. 2007, 32, 1071–1080. [Google Scholar] [CrossRef]
- Lichtor, T.; Dohrmann, G.J. Respiratory patterns in human brain tumors. Neurosurgery 1986, 19, 896–899. [Google Scholar] [CrossRef] [PubMed]
- Kefas, B.; Comeau, L.; Erdle, N.; Montgomery, E.; Amos, S.; Purow, B. Pyruvate kinase m2 is a target of the tumor-suppressive microrna-326 and regulates the survival of glioma cells. Neuro-Oncol. 2010, 12, 1102–1112. [Google Scholar] [CrossRef] [PubMed]
- Hyslop, P.A.; Hinshaw, D.B.; Halsey, W.A., Jr.; Schraufstätter, I.U.; Sauerheber, R.D.; Spragg, R.G.; Jackson, J.H.; Cochrane, C.G. Mechanisms of oxidant-mediated cell injury. The glycolytic and mitochondrial pathways of adp phosphorylation are major intracellular targets inactivated by hydrogen peroxide. J. Biol. Chem. 1988, 263, 1665–1675. [Google Scholar] [CrossRef]
- Gottschalk, S.; Anderson, N.; Hainz, C.; Eckhardt, S.G.; Serkova, N.J. Imatinib (sti571)-mediated changes in glucose metabolism in human leukemia bcr-ablpositive cells. Clin. Cancer Res. 2004, 10, 6661–6668. [Google Scholar] [CrossRef] [PubMed]
- Pourgholami, M.H.; Cai, Z.Y.; Badar, S.; Wangoo, K.; Poruchynsky, M.S.; Morris, D.L. Potent inhibition of tumoral hypoxia-inducible factor 1α by albendazole. BMC Cancer 2010, 10, 143. [Google Scholar] [CrossRef]
- Pourgholami, M.H.; Khachigian, L.M.; Fahmy, R.G.; Badar, S.; Wang, L.; Chu, S.W.; Morris, D.L. Albendazole inhibits endothelial cell migration, tube formation, vasopermeability, vegf receptor-2 expression and suppresses retinal neovascularization in rop model of angiogenesis. Biochem. Biophys. Res. Commun. 2010, 397, 729–734. [Google Scholar] [CrossRef]
- Liang, J.; Zeng, F.; Zhang, M.; Pan, Z.; Chen, Y.; Zeng, Y.; Xu, Y.; Xu, Q.; Huang, Y. Green synthesis of hyaluronic acid-based silver nanoparticles and their enhanced delivery to cd44+ cancer cells. RSC Adv. 2015, 5, 43733–43740. [Google Scholar] [CrossRef]
- Arora, S.; Jain, J.; Rajwade, J.M.; Paknikar, K.M. Cellular responses induced by silver nanoparticles: In vitro studies. Toxicol. Lett. 2008, 179, 93–100. [Google Scholar] [CrossRef]
- Ahmadi, S.; Fazilati, M.; Mousavi, S.M.; Nazem, H. Anti-bacterial/fungal and anti-cancer performance of green synthesized Ag nanoparticles using summer savory extract. J. Exp. Nanosci. 2020, 15, 363–380. [Google Scholar] [CrossRef]
- Cai, Z.; Lei, X.; Lin, Z.; Zhao, J.; Wu, F.; Yang, Z.; Pu, J.; Liu, Z. Preparation and evaluation of sustained-release solid dispersions co-loading gastrodin with borneol as an oral brain-targeting enhancer. Acta Pharm. Sin. B 2014, 4, 86–93. [Google Scholar] [CrossRef]
- Mcginty, D.; Letizia, C.S.; Api, A.M. Fragrance material review on 3-methyl-1-cyclopentadecanone. Food Cosmet. Toxicol. 2011, 49, 120–125. [Google Scholar] [CrossRef]
- Thorup, I.; Würtzen, G.; Carstensen, J.; Olsen, P. Short term toxicity study in rats dosed with pulegone and menthol. Toxicol. Lett. 1983, 19, 207–210. [Google Scholar] [CrossRef] [PubMed]
- Zhang, L.; Du, S.; Lu, Y.; Liu, C.; Wu, H.; Yang, B.; Bai, J.; Li, P. Influence of puerarin, paeoniflorin, and menthol on structure and barrier function of tight junctions in mdck and mdck-mdr1 cells. J. Tradit. Chin. Med. Sci. 2015, 2, 111–119. [Google Scholar] [CrossRef]
- Yang, B.; Du, S.; Lu, Y.; Jia, S.; Zhao, M.; Bai, J.; Li, P.; Wu, H. Influence of paeoniflorin and menthol on puerarin transport across mdck and mdck-mdr1 cells as blood-brain barrier in vitro model. J. Pharm. Pharmacol. 2017, 70, 349–360. [Google Scholar] [CrossRef]
- Liang, J.; Zhu, Y.; Gao, C.; Ling, C.; Qin, J.; Wang, Q.; Huang, Y.; Lu, W.; Wang, J. Menthol-modified BSA nanoparticles for glioma targeting therapy using an energy restriction strategy. NPG Asia Mater. 2019, 11, 38. [Google Scholar] [CrossRef]
- Govender, T.; Stolnik, S.; Garnett, M.C.; Illum, L.; Davis, S.S. PLGA nanoparticles prepared by nanoprecipitation: Drug loading and release studies of a water soluble drug. J. Control. Release 1999, 57, 171–185. [Google Scholar] [CrossRef] [PubMed]
- Liu, L.; Bi, Y.; Zhou, M.; Chen, X.; He, X.; Zhang, Y.; Sun, T.; Ruan, C.; Chen, Q.; Wang, H.; et al. Biomimetic human serum albumin nanoparticle for efficiently targeting therapy to metastatic breast cancers. ACS Appl. Mater. Interfaces 2017, 9, 7424–7435. [Google Scholar] [CrossRef]
- Jiang, L.; Xu, Y.; Liu, Q.; Tang, Y.; Ge, L.; Zheng, C.; Zhu, J.; Liu, J. A nontoxic disulfide bond reducing method for lipophilic drug-loaded albumin nanoparticle preparation: Formation dynamics, influencing factors and formation mechanisms investigation. Int. J. Pharm. 2013, 443, 80–86. [Google Scholar] [CrossRef]
- Guo, Y.; Zhang, Y.; Li, J.; Zhang, Y.; Lu, Y.; Jiang, X.; He, X.; Ma, H.; An, S.; Jiang, C. Cell microenvironment-controlled antitumor drug releasing-nanomicelles for GLUT1-targeting hepatocellular carcinoma therapy. ACS Appl. Mater. Interfaces 2015, 7, 5444–5453. [Google Scholar] [CrossRef]
- Muñoz, M.; Coveñas, R.; Esteban, F.; Redondo, M. The substance P/NK-1 receptor system: NK-1 receptor antagonists as anti-cancer drugs. J. Biosci. 2015, 40, 441–463. [Google Scholar] [CrossRef]
- Ruan, C.; Liu, L.; Lu, Y.; Zhang, Y.; He, X.; Chen, X.; Zhang, Y.; Chen, Q.; Guo, Q.; Sun, T.; et al. Substance P-modified human serum albumin nanoparticles loaded with paclitaxel for targeted therapy of glioma. Acta Pharm. Sin. B 2018, 8, 85–96. [Google Scholar] [CrossRef] [PubMed]
- Lu, W.; Tan, Y.-Z.; Hu, K.-L.; Jiang, X.-G. Cationic albumin conjugated pegylated nanoparticle with its transcytosis ability and little toxicity against blood–brain barrier. Int. J. Pharm. 2005, 295, 247–260. [Google Scholar] [CrossRef] [PubMed]
- Kudarha, R.R.; Sawant, K.K. Hyaluronic acid conjugated albumin nanoparticles for efficient receptor mediated brain targeted delivery of temozolomide. J. Drug Deliv. Sci. Technol. 2021, 61, 102129. [Google Scholar] [CrossRef]
- Kudarha, R.R.; Sawant, K.K. Chondroitin sulfate conjugation facilitates tumor cell internalization of albumin nanoparticles for brain-targeted delivery of temozolomide via CD44 receptor-mediated targeting. Drug Deliv. Transl. Res. 2021, 11, 1994–2008. [Google Scholar] [CrossRef]
- Zhu, S.; Sun, F.; Zhao, P.; Liang, G.; Sun, X.; Zeng, L.; Huang, Y. Brain-targeting biomimetic nanoparticles for codelivery of celastrol and LY2157299 for reversing glioma immunosuppression. Int. J. Pharm. 2022, 619, 121709. [Google Scholar] [CrossRef]
- Helal, D.O.; Rouatbi, N.; Han, S.; Tzu-Wen Wang, J.; Walters, A.A.; Abdel-Mottaleb, M.M.; Kamel, A.O.; Geneidi, A.; Awad, G.A.; Al-Jamal, K.T. A natural protein based platform for the delivery of Temozolomide acid to glioma cells. Eur. J. Pharm. Biopharm. 2021, 169, 297–308. [Google Scholar] [CrossRef]
- Lu, L.; Shen, X.; Tao, B.; Lin, C.; Li, K.; Luo, Z.; Cai, K. The nanoparticle-facilitated autophagy inhibition of cancer stem cells for improved chemotherapeutic effects on glioblastomas. J. Mater. Chem. B. 2019, 7, 2054–2062. [Google Scholar] [CrossRef]
- Byeon, H.J.; Thao, L.Q.; Lee, S.; Min, S.Y.; Lee, E.S.; Shin, B.S.; Choi, H.; Youn, Y.S. Doxorubicin-loaded nanoparticles consisted of cationic- and mannose-modified-albumins for dual-targeting in brain tumors. J. Control. Release 2016, 225, 301–313. [Google Scholar] [CrossRef]
- Gao, H.; Cao, S.; Yang, Z.; Zhang, S.; Zhang, Q.; Jiang, X. Preparation, Characterization and Anti-Glioma Effects of Docetaxel-Incorporated Albumin-Lipid Nanoparticles. J. Biomed. Nanotechnol. 2015, 11, 2137–2147. [Google Scholar] [CrossRef]
- Muniswamy, V.J.; Raval, N.; Gondaliya, P.; Tambe, V.; Kalia, K.; Tekade, R.K. Dendrimer-Cationized-Albumin’ encrusted polymeric nanoparticle improves BBB penetration and anticancer activity of doxorubicin. Int. J. Pharm. 2019, 555, 77–99. [Google Scholar] [CrossRef]
- Shariatnasery, M.; Irani, S.; Soleimani, M.; Goodarzi, N.; Dinarvand, R. Synergistic effect of microRNA and albumin-bound nanoparticles for inhibition of glioblastoma cancer cell proliferation. Braz. J. Pharm. Sci. 2020, 56, e18306. [Google Scholar] [CrossRef]
- Lu, W.; Wan, J.; Zhang, Q.; She, Z.; Jiang, X. Aclarubicin-loaded cationic albumin-conjugated pegylated nanoparticle for glioma chemotherapy in rats. Int. J. Cancer 2007, 120, 420–431. [Google Scholar] [CrossRef] [PubMed]
- Kamali, M.; Dinarvand, R.; Maleki, H.; Arzani, H.; Mahdaviani, P.; Nekounam, H.; Adabi, M.; Khosravan, M. Preparation of imatinib base loaded human serum albumin for application in the treatment of glioblastoma. RSC Adv. 2015, 5, 62214–62219. [Google Scholar] [CrossRef]
- Liang, J.; Gao, C.; Zhu, Y.; Ling, C.; Wang, Q.; Huang, Y.; Qin, J.; Wang, J.; Lu, W.; Wang, J. Natural Brain Penetration Enhancer-Modified Albumin Nanoparticles for Glioma Targeting Delivery. ACS Appl. Mater. Interfaces 2018, 10, 30201–30213. [Google Scholar] [CrossRef]
- Su, Z.; Xing, L.; Chen, Y.; Xu, Y.; Yang, F.; Zhang, C.; Ping, Q.; Xiao, Y. Lactoferrin-Modified Poly(ethylene glycol)-Grafted BSA Nanoparticles as a Dual-Targeting Carrier for Treating Brain Gliomas. Mol. Pharm. 2014, 11, 1823–1834. [Google Scholar] [CrossRef]
- Wang, N.; Sun, P.; Lv, M.; Tong, G.; Jin, X.; Zhu, X. Mustard-inspired delivery shuttle for enhanced blood–brain barrier penetration and effective drug delivery in glioma therapy. Biomater. Sci. 2017, 5, 1041–1050. [Google Scholar] [CrossRef]
- Yang, Z.; Du, Y.; Sun, Q.; Peng, Y.; Wang, R.; Zhou, Y.; Wang, Y.; Zhang, C.; Qi, X. Albumin-Based Nanotheranostic Probe with Hypoxia Alleviating Potentiates Synchronous Multimodal Imaging and Phototherapy for Glioma. ACS Nano 2020, 14, 6191–6212. [Google Scholar] [CrossRef]
- Parodi, A.; Miao, J.; Soond, S.M.; Rudzińska, M.; Zamyatnin, A.A., Jr. Albumin nanovectors in cancer therapy and imaging. Biomolecules 2019, 9, 218. [Google Scholar] [CrossRef]
- Yardley, D.A.; Brufsky, A.; Coleman, R.E.; Conte, P.F.; Cortes, J.; Glück, S.; Nabholtz, J.-M.A.; O’Shaughnessy, J.; Beck, R.M.; Ko, A. Phase II/III weekly nab-paclitaxel plus gemcitabine or carboplatin versus gemcitabine/carboplatin as first-line treatment of patients with metastatic triple-negative breast cancer (the tnAcity study): Study protocol for a randomized controlled trial. Trials 2015, 16, 575. [Google Scholar] [CrossRef]
- Abraxane Therapy in Patients with Pancreatic Cancer Who Failed First-Line Gemcitabine Therapy. 2008. Available online: https://ClinicalTrials.gov/show/NCT00691054 (accessed on 12 September 2023).
- S1505: Combination Chemotherapy or Gemcitabine Hydrochloride and Paclitaxel Albumin-Stabilized Nanoparticle Formulation before Surgery in Treating Patients with Pancreatic Cancer that Can Be Removed by Surgery. 2015. Available online: https://ClinicalTrials.gov/show/NCT02562716 (accessed on 12 September 2023).
- Sohal, D.P.; Duong, M.; Ahmad, S.A.; Gandhi, N.S.; Beg, M.S.; Wang-Gillam, A.; Wade, J.L.; Chiorean, E.G.; Guthrie, K.A.; Lowy, A.M. Efficacy of perioperative chemotherapy for resectable pancreatic adenocarcinoma: A phase 2 randomized clinical trial. JAMA Oncol. 2021, 7, 421–427. [Google Scholar] [CrossRef]
- Ahmad, S.A.; Duong, M.; Sohal, D.P.; Gandhi, N.S.; Beg, M.S.; Wang-Gillam, A.; Wade, J.L., III; Chiorean, E.G.; Guthrie, K.A.; Lowy, A.M. Surgical outcome results from SWOG S1505: A randomized clinical trial of mFOLFIRINOX vs. gemcitabine/nab-paclitaxel for perioperative treatment of resectable pancreatic ductal adenocarcinoma. Ann. Surg. 2020, 272, 481. [Google Scholar] [CrossRef]
- Von Hoff, D.D.; Ervin, T.; Arena, F.P.; Chiorean, E.G.; Infante, J.; Moore, M.; Seay, T.; Tjulandin, S.A.; Ma, W.W.; Saleh, M.N. Increased survival in pancreatic cancer with nab-paclitaxel plus gemcitabine. N. Engl. J. Med. 2013, 369, 1691–1703. [Google Scholar] [CrossRef]
- Nahleh, Z.A.; Barlow, W.E.; Hayes, D.F.; Schott, A.F.; Gralow, J.R.; Sikov, W.M.; Perez, E.A.; Chennuru, S.; Mirshahidi, H.R.; Corso, S.W.; et al. SWOG S0800 (NCI CDR0000636131): Addition of bevacizumab to neoadjuvant nab-paclitaxel with dose-dense doxorubicin and cyclophosphamide improves pathologic complete response (pCR) rates in inflammatory or locally advanced breast cancer. Breast Cancer Res. Treat. 2016, 158, 485–495. [Google Scholar] [CrossRef] [PubMed]
- Jotte, R.; Cappuzzo, F.; Vynnychenko, I.; Stroyakovskiy, D.; Rodríguez-Abreu, D.; Hussein, M.; Soo, R.; Conter, H.J.; Kozuki, T.; Huang, K.C.; et al. Atezolizumab in Combination with Carboplatin and Nab-Paclitaxel in Advanced Squamous NSCLC (IMpower131): Results from a Randomized Phase III Trial. J. Thorac. Oncol. 2020, 15, 1351–1360. [Google Scholar] [CrossRef] [PubMed]
- S0800, Nab-Paclitaxel, Doxorubicin, Cyclophosphamide, and Pegfilgrastim With or Without Bevacizumab in Treating Women with Inflammatory or Locally Advanced Breast Cancer. 2017. Available online: https://ClinicalTrials.gov/show/NCT00856492 (accessed on 12 September 2023).
- Phase II NCT (Neoadjuvant Chemotherapy) w/Weekly Abraxane in Combination with Carboplatin & Bevacizumab in Breast Cancer. 2018. Available online: https://ClinicalTrials.gov/show/NCT00675259 (accessed on 13 September 2023).
- Mrózek, E.; Layman, R.; Ramaswamy, B.; Lustberg, M.; Vecchione, A.; Knopp, M.V.; Shapiro, C.L. Phase II Trial of Neoadjuvant Weekly Nanoparticle Albumin-Bound Paclitaxel, Carboplatin, and Biweekly Bevacizumab Therapy in Women with Clinical Stage II or III HER2-Negative Breast Cancer. Clin. Breast Cancer 2014, 14, 228. [Google Scholar] [CrossRef] [PubMed]
- Phase II Study with Abraxane, Bevacizumab and Carboplatin in Triple Negative Metastatic Breast Cancer (ABC). 2018. Available online: https://ClinicalTrials.gov/show/NCT00479674 (accessed on 13 September 2023).
- Carboplatin, Paclitaxel, and Bevacizumab in Treating Patients with Locally Recurrent or Metastatic Breast Cancer. 2017. Available online: https://ClinicalTrials.gov/show/NCT00654836 (accessed on 13 September 2023).
- Paclitaxel Albumin-Stabilized Nanoparticle Formulation, Gemcitabine, and Bevacizumab in Treating Patients with Metastatic Breast Cancer. 2017. Available online: https://ClinicalTrials.gov/show/NCT00662129 (accessed on 13 September 2023).
- Topical Imiquimod and Abraxane in Treating Patients with Advanced Breast Cancer. 2018. Available online: https://ClinicalTrials.gov/show/NCT00821964 (accessed on 13 September 2023).
- Salazar, L.G.; Lu, H.; Reichow, J.L.; Childs, J.S.; Coveler, A.L.; Higgins, D.M.; Waisman, J.; Allison, K.H.; Dang, Y.; Disis, M.L. Topical imiquimod plus nab-paclitaxel for breast cancer cutaneous metastases: A phase 2 clinical trial. JAMA Oncol. 2017, 3, 969–973. [Google Scholar] [CrossRef]
- Nab-Paclitaxel/Rituximab-Coated Nanoparticle AR160 in Treating Patients with Relapsed or Refractory B-Cell Non-Hodgkin Lymphoma. 2016. Available online: https://ClinicalTrials.gov/show/NCT03003546 (accessed on 13 September 2023).
- Nevala, W.K.; Butterfield, J.T.; Sutor, S.L.; Knauer, D.J.; Markovic, S.N. Antibody-targeted paclitaxel loaded nanoparticles for the treatment of CD20+ B-cell lymphoma. Sci. Rep. 2017, 7, 45682. [Google Scholar] [CrossRef] [PubMed]
- McKiernan, J.M.; Lascano, D.; Ahn, J.; Ghandour, R.; Pak, J.S.; RoyChoudhury, A.; Chang, S.; DeCastro, G.J.; Desai, N. A phase I/II multi-center study of intravesical nanoparticle albumin-bound rapamycin (ABI-009) in the treatment of BCG refractory non-muscle invasive bladder cancer. J. Clin. Oncol. 2015, 33, TPS4576. [Google Scholar] [CrossRef]
- Nab-Sirolimus and Pazopanib Hydrochloride in Treating Patients with Advanced Nonadipocytic Soft Tissue Sarcomas. 2018. Available online: https://ClinicalTrials.gov/show/NCT03660930 (accessed on 14 September 2023).
- Nanoparticle Albumin-Bound Rapamycin, Temozolomide, and Irinotecan Hydrochloride in Treating Pediatric Patients with Recurrent or Refractory Solid Tumors. 2016. Available online: https://ClinicalTrials.gov/show/NCT02975882 (accessed on 14 September 2023).
- Nab-Sirolimus in Recurrent High Grade Glioma and Newly Diagnosed Glioblastoma. 2023. Available online: https://ClinicalTrials.gov/show/NCT03463265 (accessed on 14 September 2023).
- Sonabend, A.M.; Gould, A.; Amidei, C.; Ward, R.; Schmidt, K.A.; Zhang, D.Y.; Gomez, C.; Bebawy, J.F.; Liu, B.P.; Bouchoux, G.; et al. Repeated blood-brain barrier opening with an implantable ultrasound device for delivery of albumin-bound paclitaxel in patients with recurrent glioblastoma: A phase 1 trial. Lancet Oncol. 2023, 24, 509–522. [Google Scholar] [CrossRef]

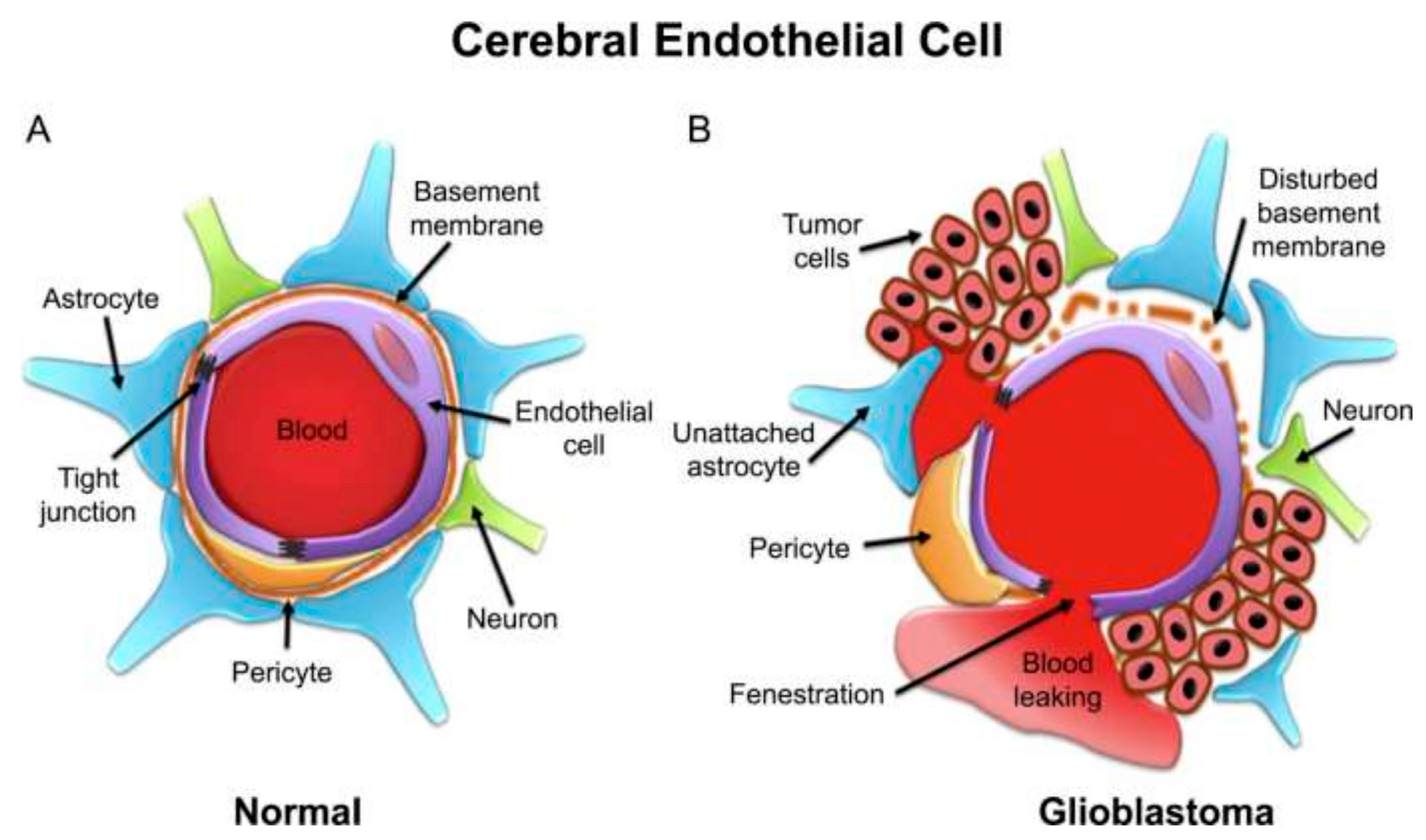
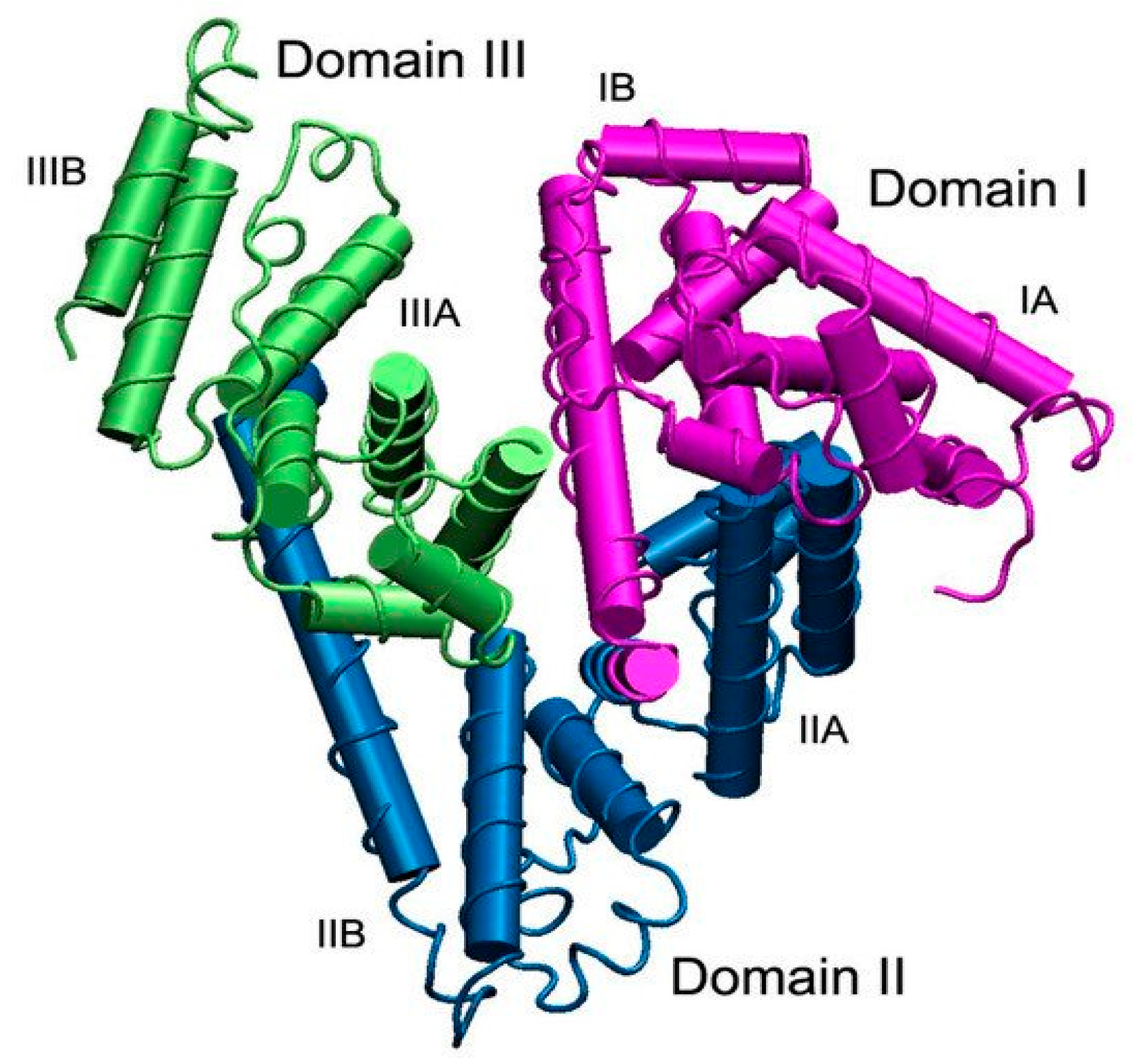
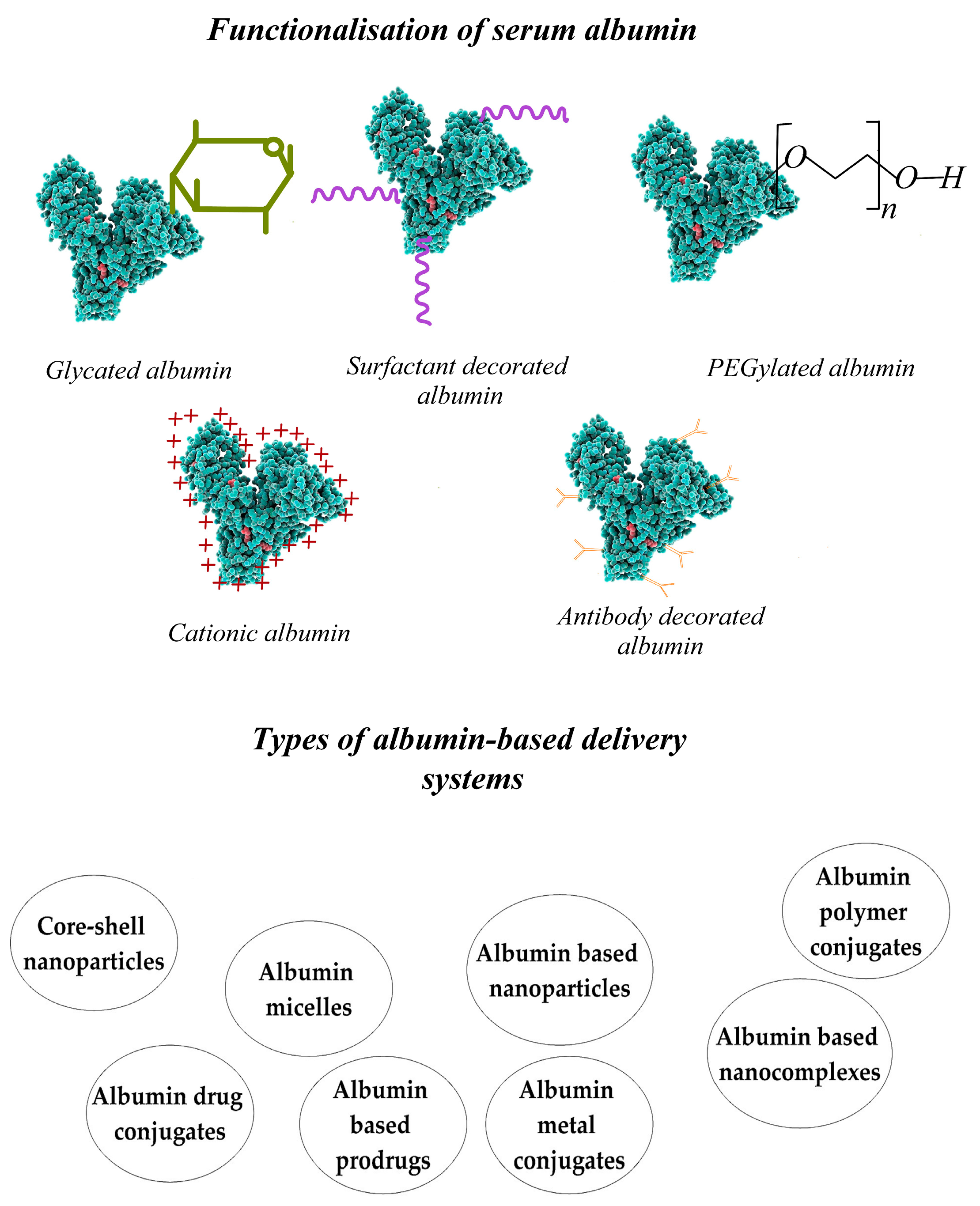
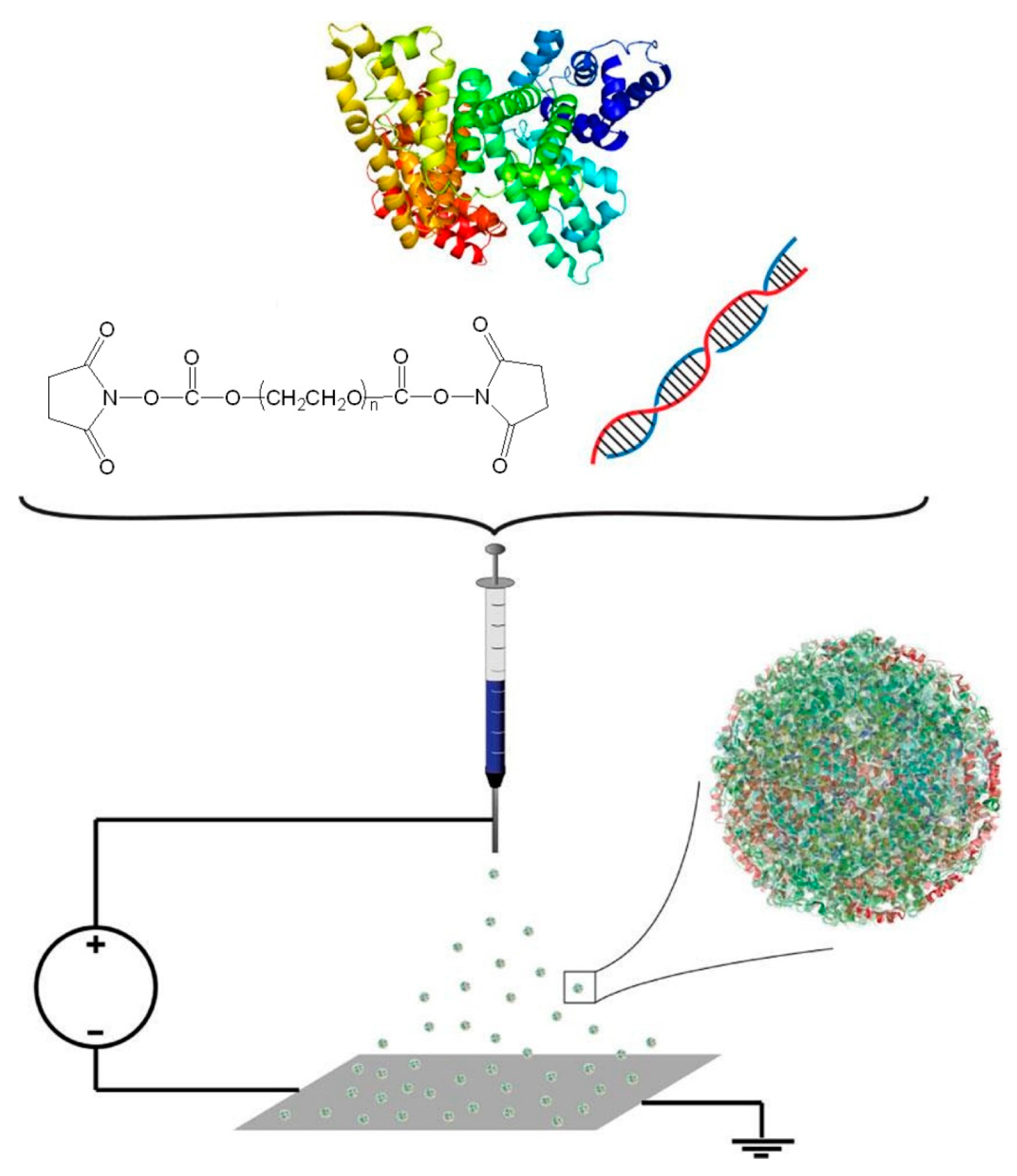
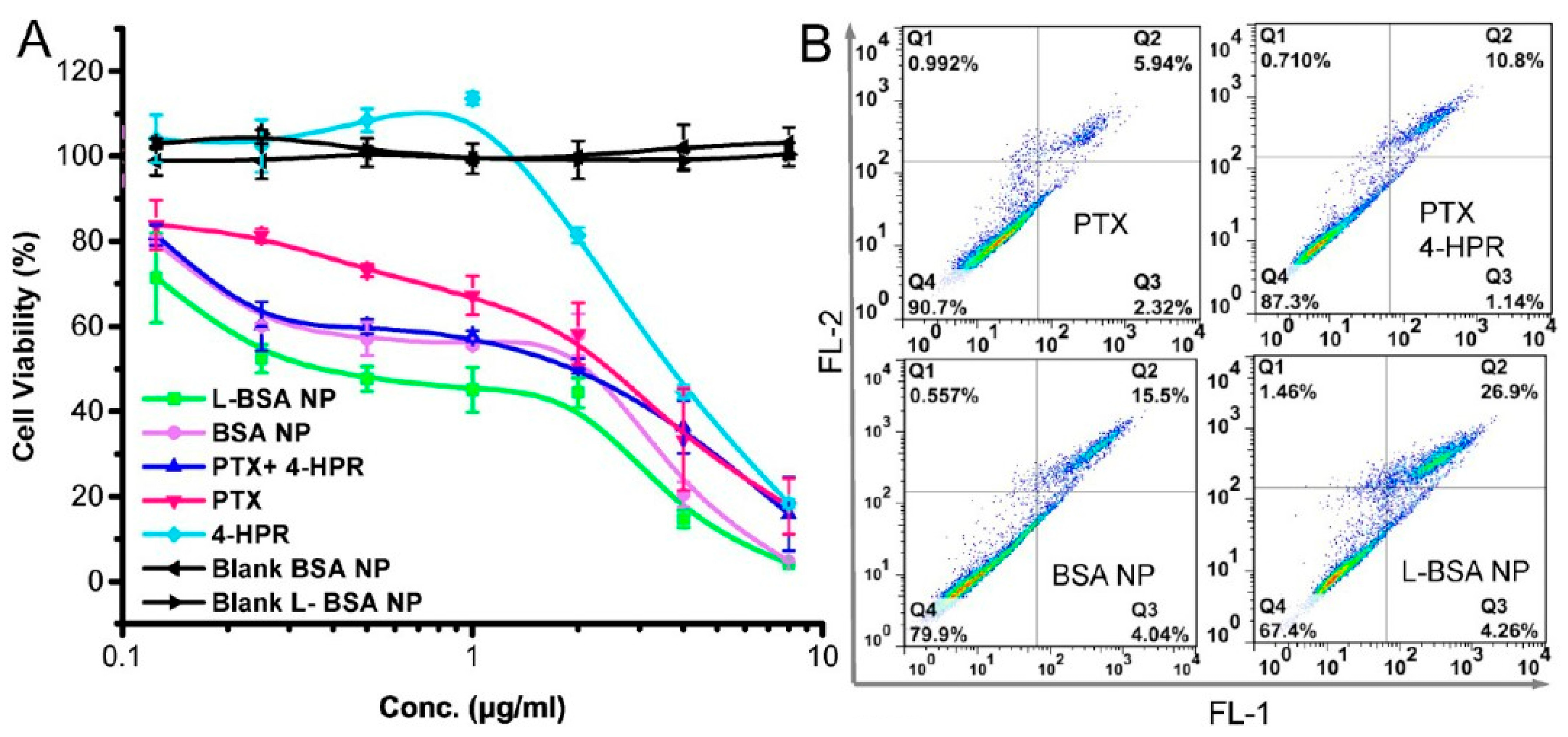
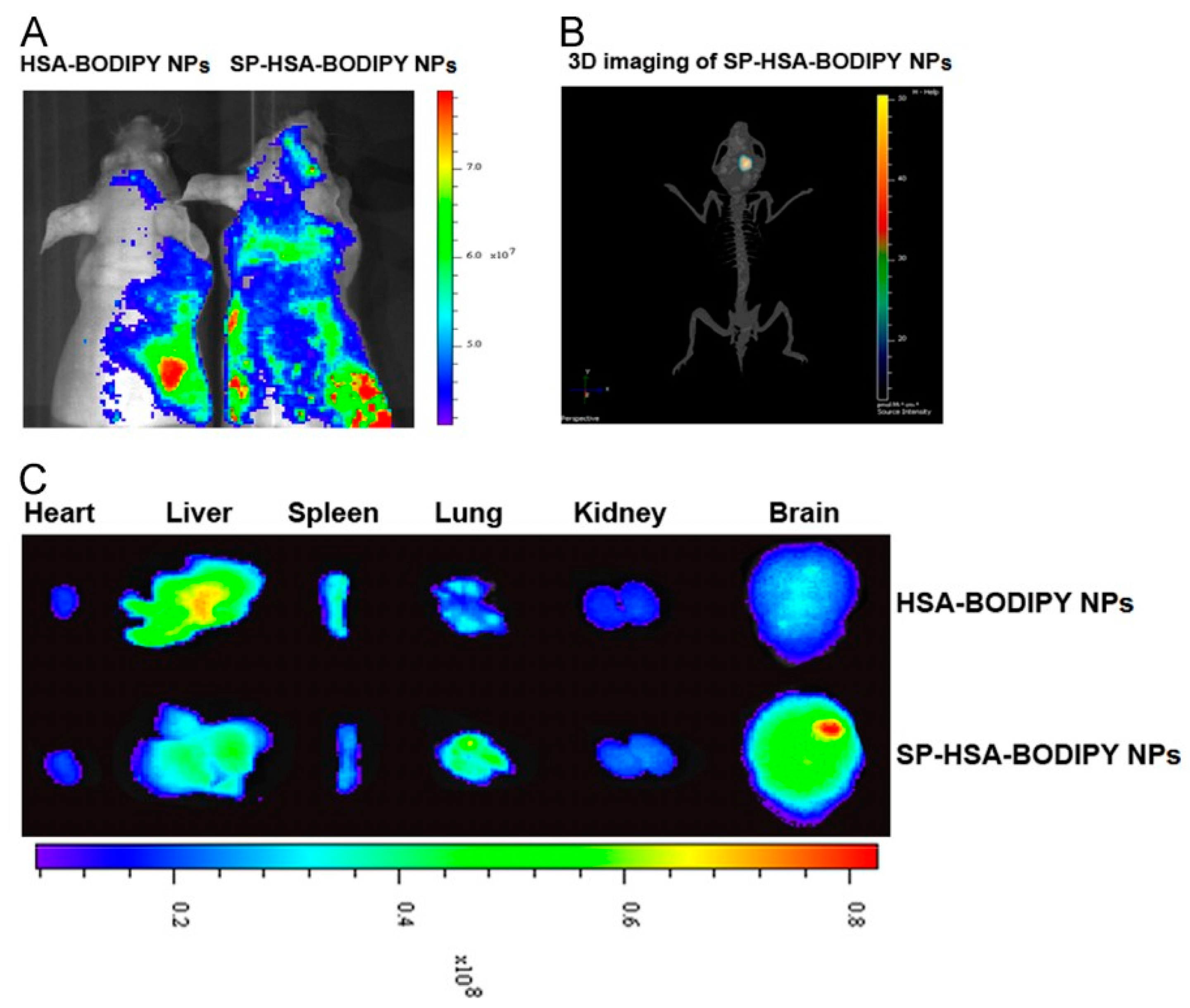
| Classes of Substances Used for Modification | The Functionalized Serum Albumin-Based Nanoparticles’ Characteristics | Active Principle | Mechanism | Refs. |
|---|---|---|---|---|
| Surfactants Polysorbate 80 | Encapsulating the drug in these delivery systems reduces its toxicity and increases its AUC while decreasing the distribution volume, clearance, and cardiotoxicity. | Doxorubicin | Cover | [328,329] |
| Cationic Polymers Poly(ethylene imine)-PEI | These functionalized albumin-based nanoparticles with PEI have numerous benefits, including protection against enzymatic degradation, the lack of a need for toxic cross-linking agents, the modification of the surface charge, reduced plasma protein adsorption, and facilitated in vivo applications. However, PEI may exhibit slight toxicity in cells. | Bone morphogenetic protein-2 (BMP-2) | Cover | [330,331] |
| PEI/Poly(ethylene glycol) or PEG | BSA-based nanoparticles, functionalized with PEI and PEG, show reduced toxicity in cells of PEI and improved biocompatibility. Coated BSA nanoparticles promote bone structure formation and exhibit improved physicochemical properties. | Bone morphogenetic protein-2 (BMP-2) | Cover | [332] |
| Poly-L-lysine (PLL) | Functionalized albumin nanoparticles with PLL have improved stability in water, which is directly proportional to the molecular weight and the concentration of PLL. | Bone morphogenetic protein-2 (BMP-2) and siRNA | Cover | [333,334] |
| Thermosensitive polymers Poly(N-isopropyl acrylamide-block-polyallylamine)(PNIPAM-AAm-b-PAA) | BSA-based nanospheres functionalized with PNIPAM-AAm-b-PAA release adriamycin less efficiently than unconjugated nanospheres at 37 °C. However, drug release efficiency increases at higher temperatures, such as the cloud temperature, due to the solubilization of the polymer, suggesting that the nanospheres can be targeted to tumors with slightly higher temperatures than the body’s physiological temperature. | Adriamycin | Conjugation of PNIPAM-Aam-b-PAA to the carboxyl groups of albumin nanospheres using the carbodiimide (EDC) reaction. | [335,336] |
| PEG PEG/mPEG succimidyl propionate | PEGylated albumin nanoparticles have numerous benefits, including prolonged systemic circulation, and increase the half-life of 5FU by 50 times. They also reduce immunogenicity and promote the nanoparticles’ accumulation in tumors through the EPR effect. Amino groups in albumin nanoparticles were PEGylated using mPEG succinimidyl propionate. | 5-Fluorouracil (5-FU) | Pegylation of BSA was performed by succinimidyl propionate-activated mPEG through their free amino groups. | [337] |
| Reduces immunogenicity. | ||||
| Polyethylene glycol)-poly (thioether amido acid)-polyethylene glycol); methoxy poly(ethylene glycol) | HSA-mPEG nanoparticles had a lower drug loading efficiency than HSA due to limited binding sites. The surface modification resulted in a slower drug release in the presence of enzymes due to a hydrophilic steric barrier on the nanoparticle’s surface. | Rose Bengal (RB) | Grafting | [338] |
| FOLATE Folic Acid | Folate receptors are often overexpressed in human cancer cells and can be used to target the nanoparticles at the tumor sites. Folic acid, used to functionalize albumin-based nanoparticles, is stable, inexpensive, and non-immunogenic compared to other options. By binding to cell surface folate receptors, it can be internalized through receptor-mediated endocytosis, making it an effective marker for directing drugs to cancer cells. Folate-conjugated albumin nanoparticles represent a drug delivery system that shows specificity for cancer cells. | Doxorubicin, PTX, cisplatin, vinblastine sulfate, mitoxantrone, and epigallocatechin-3-gallate. | The carboxylic group of folic acid was covalently conjugated to the amino groups on the surface of albumin nanoparticles using the 1-ethyl-3-(3-dimethyl aminopropyl) carbodiimide (EDC) coupling technique. | [339,340,341,342,343,344,345,346] |
| adsorption on the surface of albumin nanoparticles. | ||||
| Peptides arginine-glycine-aspartic acid (RGD) | The cyclic peptide RGD is a ligand with a high binding affinity to integrin αvβ3. The inhibition of integrin protein avb3 from binding to their specific ligands causes apoptosis in endothelial cells in newly formed blood vessels. Peptides that mimic the ligands of these integrins and anti-integrin antibodies can inhibit their ligand binding. | 5-fluorouracil | Conjugation | [347] |
| Arginine–Alanine–aspartic acid (RAD) | BSA-based nanospheres that are sterically stabilized and functionalized with RGD and RAD peptides have been developed to target tumor vasculature specifically. | 5-Fluorouracil | ||
| The functionalized BSA-based delivery systems with these two peptides have proven to be much more effective in preventing lung metastasis, angiogenesis, and tumor regression than free 5FU, unfunctionalized BSA particles, or RAD-functionalized ones. | ||||
| RGD | The drug delivery system was developed based on PEGylated HSA-based nanomicelles obtained via self-assembling and coated with cyclic RGD peptides. The delivery system was tested by incubating it with human melanoma cells (M21+) that expressed αvβ3 integrin and showed an increased drug uptake and retention in these cells. | Doxorubicin | Conjugation | [306] |
| The HSA-based delivery system also facilitated the rapid release of the drug through specific mechanisms in endosomes and lysosomes. | ||||
| CREKA (cysteine–arginine–glutamic acid–lysine alanine) | Researchers have discovered a peptide called CREKA, which can attach to clotted plasma proteins present in tumors. By utilizing this peptide to functionalize Abraxane nanoparticles, the accumulation of PTX in tumors can be enhanced, leading to better therapeutic outcomes. When antitumor treatment was carried out with CREKA-functionalized micelles, it was observed that there was no significant difference in comparison to the treatment with untargeted Abraxane. | PTX | Coupling of the peptides to Abraxane via their cysteine sulfhydryl group using a sulfo-SMCC (sulfosuccinimidyl-4-[N-maleimidomethyl] cyclohexane-1-carboxylate) crosslinker. | [348] |
| LyP-1i (Cys-Gly-Gln-Lys-Arg Thr-Arg-Gly-Cys) | The accumulation of nanoparticles occurred in the tumor’s blood vessels, resulting in the formation of aggregates that encompassed red blood cells and fibrin. | |||
| Abraxane is the albumin-based nanoparticle with an average diameter of 130 nm, in which the drug PTX has been encapsulated. | ||||
| Abraxane nanoparticles functionalized with LyP-1 peptide could be transported to extravascular sites, resulting in a significant growth inhibition of tumors when compared to untargeted and CREKA-conjugated Abraxane. | ||||
| This technique allows for the precise targeting of nanoparticles to tumor tissue, resulting in superior treatment. | ||||
| Apolipoproteins Apolipoprotein E (Apo E, A-I, B-100), | HSA nanoparticles with functionalized Apo E (covalently linked) can be uptaken into the brain endothelial cells through endocytosis after intravenous (i.v.) injection into the bloodstream. | Loperamide | Covalent linkage formed and used as a bifunctional Mal-PEGNHS cross-linker that reacts with an amino group on the surface of HSA particles as well as a thiol group introduced into Apo E | [349,350] |
| Some of these functionalized particles can penetrate the brain parenchyma, but this can only be achieved through transcytosis to overcome the BBB. TJs in brain endothelial cells are not opened or modulated, and the nanoparticles have not been observed in association with TJ complexes or in the paracellular space. | ||||
| Polysorbate-coated nanoparticles seem to deliver drugs to the CNS in a similar way as they uptake in blood circulation, as the nanoparticles functionalized with Apo E or AI after intravenous injection. | ||||
| Apolipoproteins that modified the nanoparticles in mice had significant antinociceptive effects within 15 min of injection, lasting over an hour, unlike the loperamide solution. | ||||
| Transferrin Transferrin -SPDP | The PEGylated albumin nanoparticles were functionalized with transferrin via a coupling reaction with maleimide-poly(ethylene glycol)-N-hydroxy succinimide. These nanoparticles were prepared using a nano-emulsification technique and glutaraldehyde cross-linking. | Azidothymidine, FITC-dextran | The BSA particles were coupled with thiolated transferrin at the distal end of the PEG chain. | [351] |
| The obtained nanoparticles overcome the BBB through amino acid transporters and can be used as drug delivery systems, although the drug immobilization efficiency decreases for transferrin-functionalized nanoparticles. The functionalized nanoparticle’s size is slightly larger than non-modified ones and varies from 114 nm to 124 nm. | ||||
| Transferrin receptor monoclonal antibodies (TfR-mAb) | In order to prepare the functionalized HSA nanoparticles with transferrin, a heterobifunctional cross-linker NHS-PEG-MAL-5000 was used for SH group activation, followed by adding a thiolated transferrin solution to react with the activated sulfhydryl group. The nanoparticles’ size ranged from 155 to 188 nm. | Loperamide | TfR-mAb was covalently linked to HSA nanoparticles for functionalization. | [352] |
| When HSA-based nanoparticles containing loperamide were functionalized with transferrin or TfR-mAb, the drug’s ability to cross the BBB was noticeably improved, allowing it to enter the brain. These functionalized nanoparticles loaded with loperamide demonstrated powerful antinociceptive effects. However, the nanoparticles functionalized with immunoglobulin G2a (IgG2a) could not transport this drug across the BBB. | ||||
| Monoclonal antibodies specific humanized anti-HER2 antibody, trastuzumab (Herceptin®) | The trastuzumab-conjugated HSA nanoparticles were utilized to target HER2-overexpressing cells in patients with metastatic breast cancer. The experiments demonstrated effective internalization via endocytosis, dependent on time and dosage. | Antisense oligonucleotides (ASOs) against Plk1 (Polo-like kinase 1). | The covalent binding of the monoclonal antibody took place at the sulfhydryl groups of HSA using a bifunctional compound poly(ethylene glycol)-α-maleimide-4-NHS for their activation. | [353,354] |
| The trastuzumab-conjugated HSA nanoparticles were found to attach to the surface of HER2-overexpressing cells, including BT474, MCF7, and SK BR-3. Following incubation with trastuzumab-modified HSA nanoparticles, the delivery systems significantly reduced both Plk1 mRNA and Plk1 protein expression. | ||||
| cetuximab, DI17E6 | A monoclonal antibody known as DI17E6 has shown promise in preventing the proliferation of cancer cells with the epidermal growth factor receptor (EGFR) overexpressed on their surface. It inhibits angiogenesis and can be used for cancer therapy. Encapsulating doxorubicin in DI17E6-functionalized albumin nanoparticles improves cytotoxicity. | Doxorubicin | Covalent binding to HSA nanoparticles. | [355] |
| Type of Drug Delivery System | Obtaining Methods | Diameter (nm) | Functionalization | Mechanism of Action on the Tumor and Overcoming the BBB | Specific Features of the Drug Delivery System |
|---|---|---|---|---|---|
| Nanoparticles based on BSA (BSA Nps) were cross-linked using glutaraldehyde (GA), and then the temozolomide (TMZ) was encapsulated [511,512]. | Desolvation | 167–261 | Using EDC and NHS, carbodiimide chemistry was employed to conjugate hyaluronic acid (HA) or chondroitin sulfate to BSA-based nanoparticles. | Targeting through the CD44 receptor. | In vitro tests show that BSA-based nanoparticles can overcome the BBB and inhibit U87 MG cell growth. Moreover, these nanoparticles also stimulate the production of reactive oxygen species inside the tumor cells. |
| Their uptake is facilitated through endocytosis, specifically the caveolae pathway. The CD44 receptor is responsible for directing the nanoparticles to the tumor site. | |||||
| In vivo studies show improved pharmacokinetics and brain accumulation of TMZ-loaded nanoparticles compared to the free drug. | |||||
| The biodistribution studies on TMZ-loaded BSA-based nanoparticles revealed a greater concentration of TMZ in the brain, while its levels in important organs like the liver and lungs were notably reduced. | |||||
| Albumin nanoparticles having encapsulated LY2157299 that inhibit the TGF-β I receptor (TGFβRI) and celastrol, an mTOR pathway inhibitor [513]. | Emulsion | 126.8 | DCDX (cgreirtgraerwsekf) mixed with albumin. | Nicotinic acetylcholine receptors. | Biomimetic nanoparticles can repolarize tumor-associated macrophages (TAMs) from the M2 to M1 phenotype by inhibiting the STAT6 pathway, decreasing TGF β1 secretion, and causing cell apoptosis. |
| It was found that the treatment effectively blocked the TGF-β/SMAD2 signaling pathway. Moreover, the use of nanoparticles significantly increased the survival rate and reduced the proportion of M2-type TAMs, TGF-β1, and lactic acid levels in glioma tissues. | |||||
| Nanoparticles based on HSA that are cross-linked with GA and contain encapsulated acidic temozolomide (TMZA) [514]. | Desolvation | 111.7–177.5 | - | Uptake/accumulation in cells. | The optimized nanoparticles contain 4 mg of TMZA with 0.05% sodium cholate, resulting in a 111.7 nm size and 5.5% loading degree. The nanoparticles did not cause a decrease in cell viability, and the drug release from them was quite rapid. The optimized nanoparticles demonstrated a remarkable cellular uptake after being incubated with glioblastoma cell line GL261 and BL6 brain cancer stem cells for 24 h. |
| BSA-based nanoparticles cross-linked with GA containing co-encapsulated two drugs, PTX, and chloroquine diphosphate salt (CQ) (autophagy inhibitor) [515]. | Desolvation | 51–53 | Folic acid conjugation on nanoparticle surfaces can be achieved through a reaction with carbodiimides DCC (N,N′-dicyclohexylcarbodiimide) and NHS (hydroxysuccinimide). | Mechanism of autophagy inhibition. | In vitro, the combination of PTX and CQ therapy resulted in a higher occurrence of cell apoptosis than treatment with PTX alone. |
| Encapsulated PTX caused the overexpression of cancer stem genes (SOX2, POU5F1, and NANOG) in glioma cells. But using nanoparticles containing chloroquine decreased their expression. Autophagy is significant in this process. | |||||
| Out of all the delivery systems, the one containing two co-encapsulated drugs was the most effective in inducing cell apoptosis. | |||||
| Drug delivery systems based on cationic HSA and HSA modified with mannose having doxorubicin encapsulated [516]. | High-pressure homogenization technique. | 90.5 ± 3.1 | In order to obtain cationic HSA, ethylenediamine was linked to HSA through an EDC reaction. Additionally, HSA was modified with mannopyranoside using a thiol-maleimide reaction. | The nanoparticle uptake mechanism uses dual cationic absorptive transcytosis through the glucose transporter pathway. | The doubly modified nanoparticles exhibited the highest efficiency level in terms of transportation through the bEnd3 mouse endothelial cell monolayer and in U87MG glioblastoma cells. |
| The c/m-HSA nanoparticles showed a higher level of localization in cerebral glioma than the native HSA-based nanoparticles. | |||||
| The enhanced effectiveness in treating glioma appeared to result from a system combining dual cationic absorptive transcytosis and glucose transportation using both c- and m-HSA. | |||||
| Album lipid nanoparticles with encapsulated docetaxel [517]. | Desolvation | 110.1± 40.2 | Enhanced permeation and retention effect (EPR). | The lethal dose of albumin lipid nanoparticles containing docetaxel was found to be 180.6 mg/kg, which is 75.3% higher than that of Taxotere®. | |
| The obtained nanoparticles have been proven to be effective in preventing the proliferation of several cell lines, such as U87, A549, Raw 264.7, and bEnd.3, and can even induce cell apoptosis. | |||||
| In addition, when used in vivo, imaging has shown that docetaxel, which is encapsulated in albumin lipid nanoparticles, can be located and accumulated at the glioma site. This delivery system can inhibit tumor growth and prolong the median survival time in mice. | |||||
| Doxorubicin was encapsulated within PLGA nanoparticles that were coated with dendrimers that contain cationized albumin [518]. | Reaction with carbodiimide (EDC). | 156 ± 10.85 | EDC reacts with BSA’s carboxyl group to generate a reactive O-acylisourea intermediate that rapidly reacts with the dendrimer’s amino group to create an amide bond. | The anticancer mechanism through caspase-mediated apoptosis. | The release of doxorubicin encapsulated in nanoparticles depends on the pH, reducing the hemolytic toxicity and increasing the drug uptake into the cells. The ex vivo test results show that the nanoparticles lead to cytotoxicity in U87MG glioblastoma cells and an increase in the expression of the caspase-3 gene (about 5.35 times), resulting in an anticancer effect. |
| HSA-based nanoparticles in which PTX was encapsulated [519]. | NAB technology | 140 | The mechanism that inhibits gene expression in glioblastoma cancer cells. | Researchers conducted a study to investigate the impact of combining miR-34a with albumin-bound PTX nanoparticles on anti-tumorigenesis in glioblastoma cell line U251. The results indicated a significant decrease in cell viability when miR-34a was combined with albumin-bound PTX nanoparticles. | |
| Furthermore, the treatment of U251 cells with miR-34a and PTX-containing nanoparticles led to a considerable inhibition in SURVIVIN gene expression compared to those treated with miR-34a alone or drug-free nanoparticles. | |||||
| Encapsulation of aclarubicin in cationic BSA-conjugated PEG nanoparticles surface [520]. | Emulsion (o/w) | 50–58 | Maleimide is used to react with thiolated PEG nanoparticles to graft cationic BSA onto their surface. | The treatment mechanism involves opening the TJs in the BBB and accumulating the nanoparticles at the tumor site. | Cationic BSA-conjugated PEG nanoparticles labeled with 6-coumarin (fluorescent probe) accumulate more in tumor mass than unconjugated ones 24 h post-intravenous injection. |
| Functionalized nanoparticles released a higher drug concentration in the tumor than non-functionalized nanoparticles or the free drug one hour and 24 h after administration. The drug concentration increased by 2.6–3.3 times after one hour and 2.7–6.6 times, respectively, after 24 h. | |||||
| After administering cationic BSA-conjugated PEG nanoparticles containing aclarubicin to rats, in vivo, tests indicated an increase in their survival rates. | |||||
| Encapsulating the drug in nanoparticles helps to reduce its toxicity. | |||||
| BSA-based nanoparticles cross-linked with GA containing the encapsulated drug imatinib [521]. | Desolvation | 80–90 | Inhibition of receptors such as c-Kit and PDGFR that are overexpressed in glioblastoma. | The study found that the HSA-based nanoparticles had a high encapsulation efficiency percentage of 98% and a drug loading degree of 6.9%. | |
| HSA nanoparticles with an encapsulated drug concentration of 40 mg/mL had 90% cytotoxicity on U87MG glioblastoma cells, while the free drug had 55%. | |||||
| BSA-based nanoparticles labeled with a fluorescent dye [522]. | Nanoprecipitation, ultrasonication. | 100–200 | BSA was conjugated with borneol using the carbodiimide reaction method with EDC and NHS. | Menthol-modified nanoparticles were internalized into cells via a temperature-dependent active mechanism and a caveolae-mediated endocytosis mechanism. | BSA-based nanoparticles modified with menthol had better brain targeting and were more efficient in overcoming the BBB than other BSA-based nanoparticles modified with different ligands, as shown in in vivo imaging tests. |
| Ketone carbonyl muscone can be linked to BSA through reductive Borch amination. | The BSA functionalized with borneol resulted in obtaining nanoparticles with increased permeability through the BBB due to improved lipophilicity, increased endocytosis, and reduced expression of proteins associated with TJs. | ||||
| Para-mentha-8-thiolone is used as a menthol analog to couple with BSA using the reaction with 2-iminothiolane hydrochloride. | Menthol-modified nanoparticles can be uptaken from the bloodstream and could enter into the pineal gland, a more efficient drug delivery pathway to the brain than the one mediated by the transferrin receptor. | ||||
| The T7 peptide was conjugated to BSA using the identical method utilized for the menthol. | |||||
| Nanoparticles based on BSA that contain encapsulated doxorubicin [523]. | Desolvation | 100–200 | The surface of BSA-based nanoparticles was modified by grafting mPEG2000 to the free amino groups within the protein. Additionally, lactoferrin was attached to the surface of the nanoparticles through electrostatic bonds. | The accumulation of BSA-based nanoparticles in tumors occurs through the EPR effect and transcytosis, which is mediated by the low-density lipoprotein receptor. Lactoferrin can interact with this receptor to facilitate the transcytosis process. | When the quantities of mPEG2000 and lactoferrin were increased, it resulted in an increase in the size of the nanoparticles while causing a decrease in the zeta potential. |
| The study conducted on healthy rats revealed that nanoparticles based on mPEG2000-modified BSA had a longer circulation time in vivo. | |||||
| Nanoparticles modified with a high amount of lactoferrin and mPEG2000 showed the strongest cytotoxicity and the highest internalization efficiency on both BCEC and C6 cell lines, improving the dual-targeting effects. | |||||
| The biodistribution of doxorubicin encapsulated in different formulations showed that lactoferrin-modified nanoparticles were able to cause a notable accumulation of doxorubicin in brain tissue, particularly 2 h after injection (with a significance level of p < 0.05). | |||||
| Albumin/poly(2-methacryloyloxyethyl phosphorylcholine)-based nanoparticles loaded with TMZ [524]. | In situ free radical polymerization method. | 8.72–32.67 | Albumin nanoparticles with synaptic acid extracted from mustard conjugated on their surface. | Internalizing nanoparticles conjugated with synaptic acid in cells requires energy and causes a temporary disruption of TJ proteins, P-gps, and claudin-5. | The functionalized nanoparticles obtained are biocompatible and are able to overcome the BBB. |
| The delivery systems that have been modified with encapsulated drugs effectively induce cell apoptosis at the tumor site. They have also been shown to increase the survival time of mice with glioma. | |||||
| Nanotheranostic probes based on albumin and catalase (catalase integrated into phototheranostic nanoprobe with biomimetic albumin) [525]. | Desolvation | 54.14 ± 5.17 × 14.36 ± 1.34 | Protein-mediated transport. | The development of a catalase-integrated biomimetic albumin phototheranostic probe used to perform multimodal imaging, amplify phototherapy, and guide surgery for glioma after overcoming the BBB, accumulating in invasive glioma by binding albumin to overexpressed proteins, was reported. | |
| The nanoprobe could effectively induce local hyperthermia and increase the level of singlet oxygen based on the attenuated hypoxic glioma microenvironment by decomposing endogenous hydrogen peroxide into oxygen to enhance phototherapy. | |||||
| Glioma growth is significantly inhibited, survival time is prolonged, tumor hypoxia is attenuated, apoptosis is enhanced, and anti-angiogenesis effects have been demonstrated in several animal models with a low toxicity for normal tissue. |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Tincu, C.-E.; Andrițoiu, C.V.; Popa, M.; Ochiuz, L. Recent Advancements and Strategies for Overcoming the Blood–Brain Barrier Using Albumin-Based Drug Delivery Systems to Treat Brain Cancer, with a Focus on Glioblastoma. Polymers 2023, 15, 3969. https://doi.org/10.3390/polym15193969
Tincu C-E, Andrițoiu CV, Popa M, Ochiuz L. Recent Advancements and Strategies for Overcoming the Blood–Brain Barrier Using Albumin-Based Drug Delivery Systems to Treat Brain Cancer, with a Focus on Glioblastoma. Polymers. 2023; 15(19):3969. https://doi.org/10.3390/polym15193969
Chicago/Turabian StyleTincu (Iurciuc), Camelia-Elena, Călin Vasile Andrițoiu, Marcel Popa, and Lăcrămioara Ochiuz. 2023. "Recent Advancements and Strategies for Overcoming the Blood–Brain Barrier Using Albumin-Based Drug Delivery Systems to Treat Brain Cancer, with a Focus on Glioblastoma" Polymers 15, no. 19: 3969. https://doi.org/10.3390/polym15193969
APA StyleTincu, C.-E., Andrițoiu, C. V., Popa, M., & Ochiuz, L. (2023). Recent Advancements and Strategies for Overcoming the Blood–Brain Barrier Using Albumin-Based Drug Delivery Systems to Treat Brain Cancer, with a Focus on Glioblastoma. Polymers, 15(19), 3969. https://doi.org/10.3390/polym15193969



.png)





