Compatibilizing Biodegradable Poly(lactic acid)/polybutylene adipate-co-terephthalate Blends via Reactive Graphene Oxide for Screw-Based 3D Printing
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Preparation of Vinyl-Functionalized GO and Melt-Mixing with PLA/PBAT
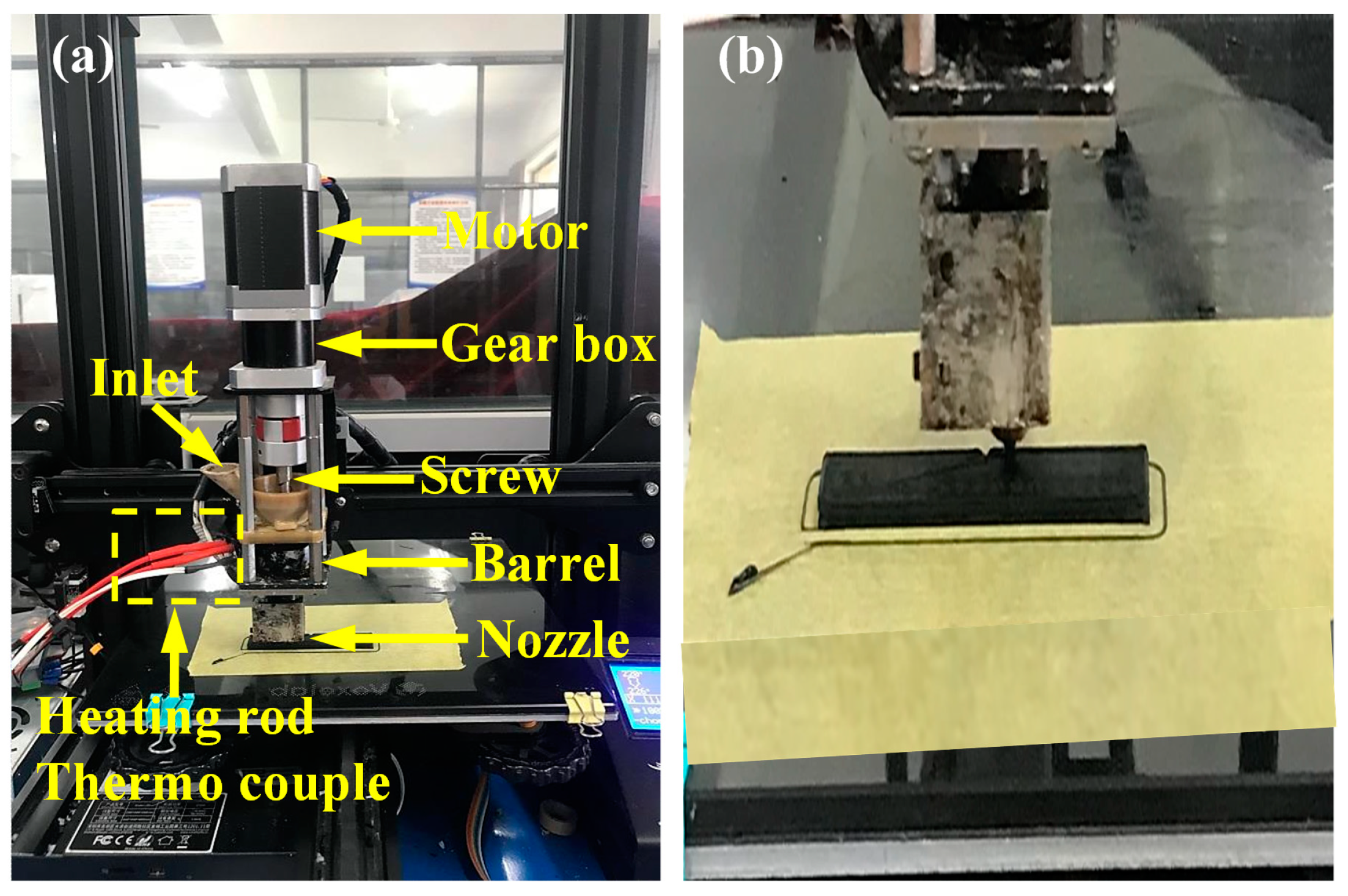
2.3. Screw-Based 3D Printing
2.4. Characterization
3. Results and Discussion
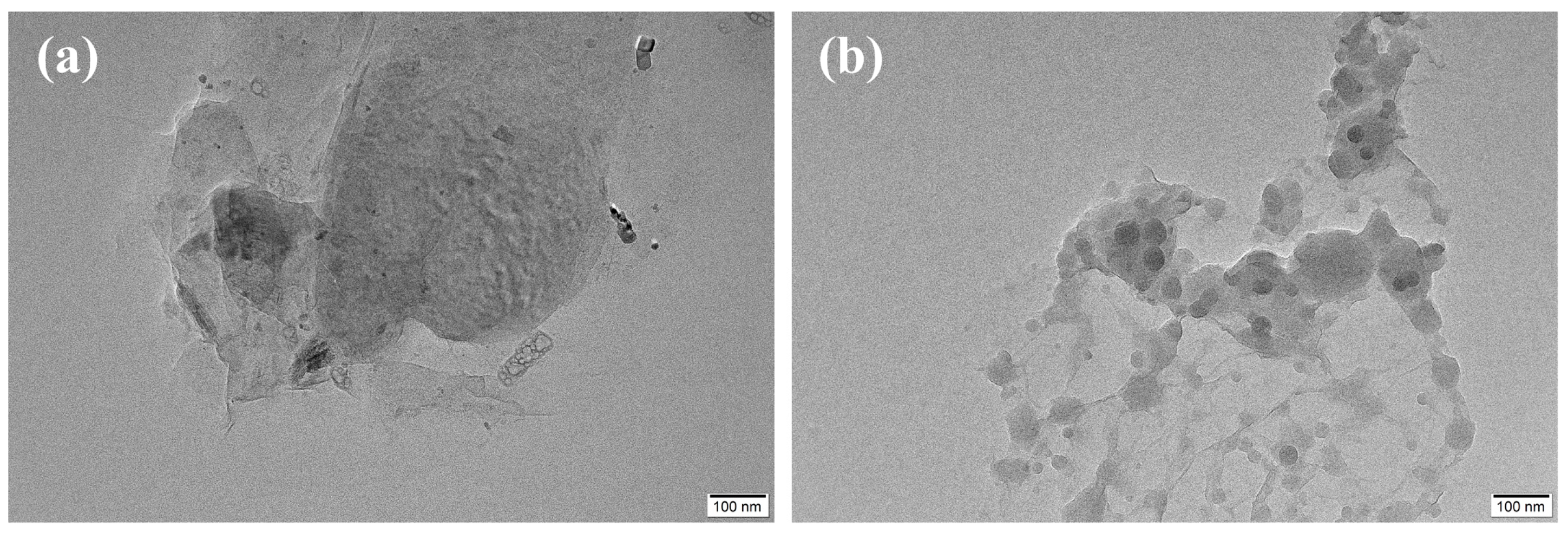
3.1. Morphologies of GO and VGO
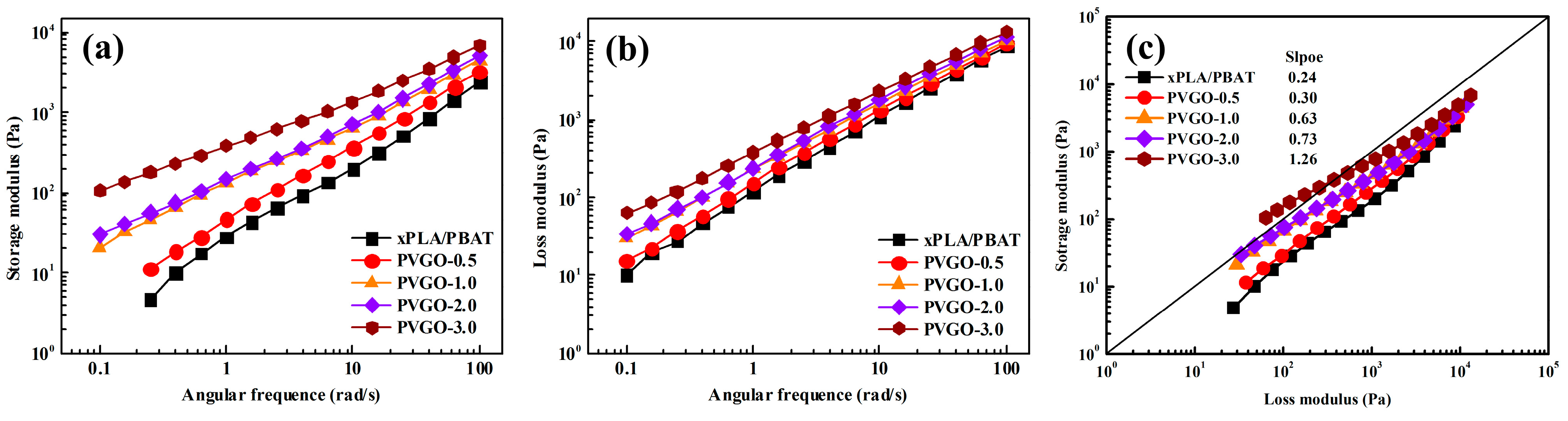
3.2. Linear Rheological Behaviors of PVGO Blend Nanocomposites
3.3. Phase Morphology of PVGO Blend Nanocomposites
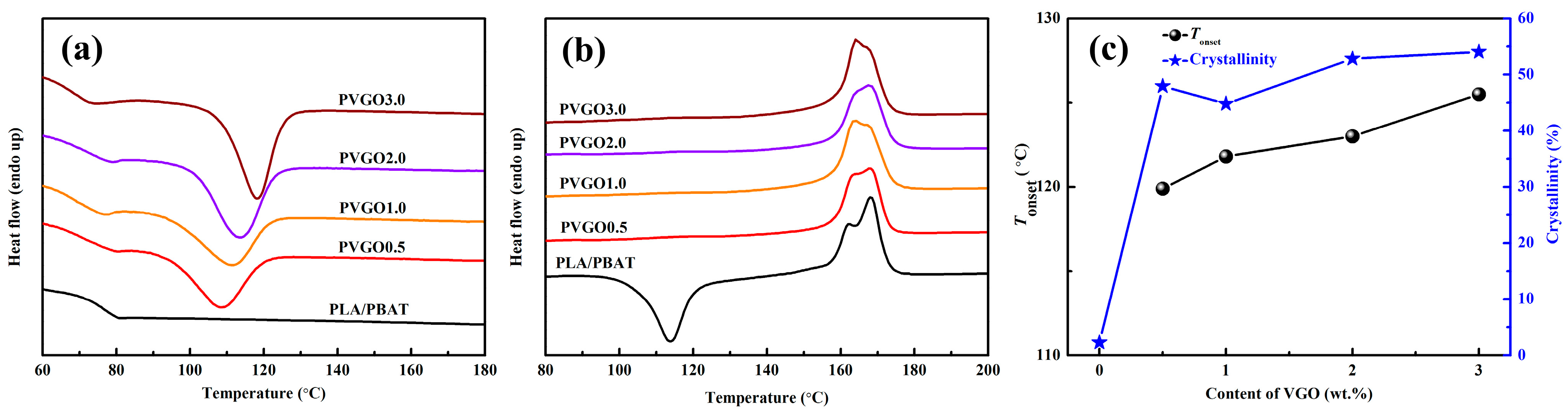
3.4. Thermal Behaviors of PVGO Blend Nanocomposites
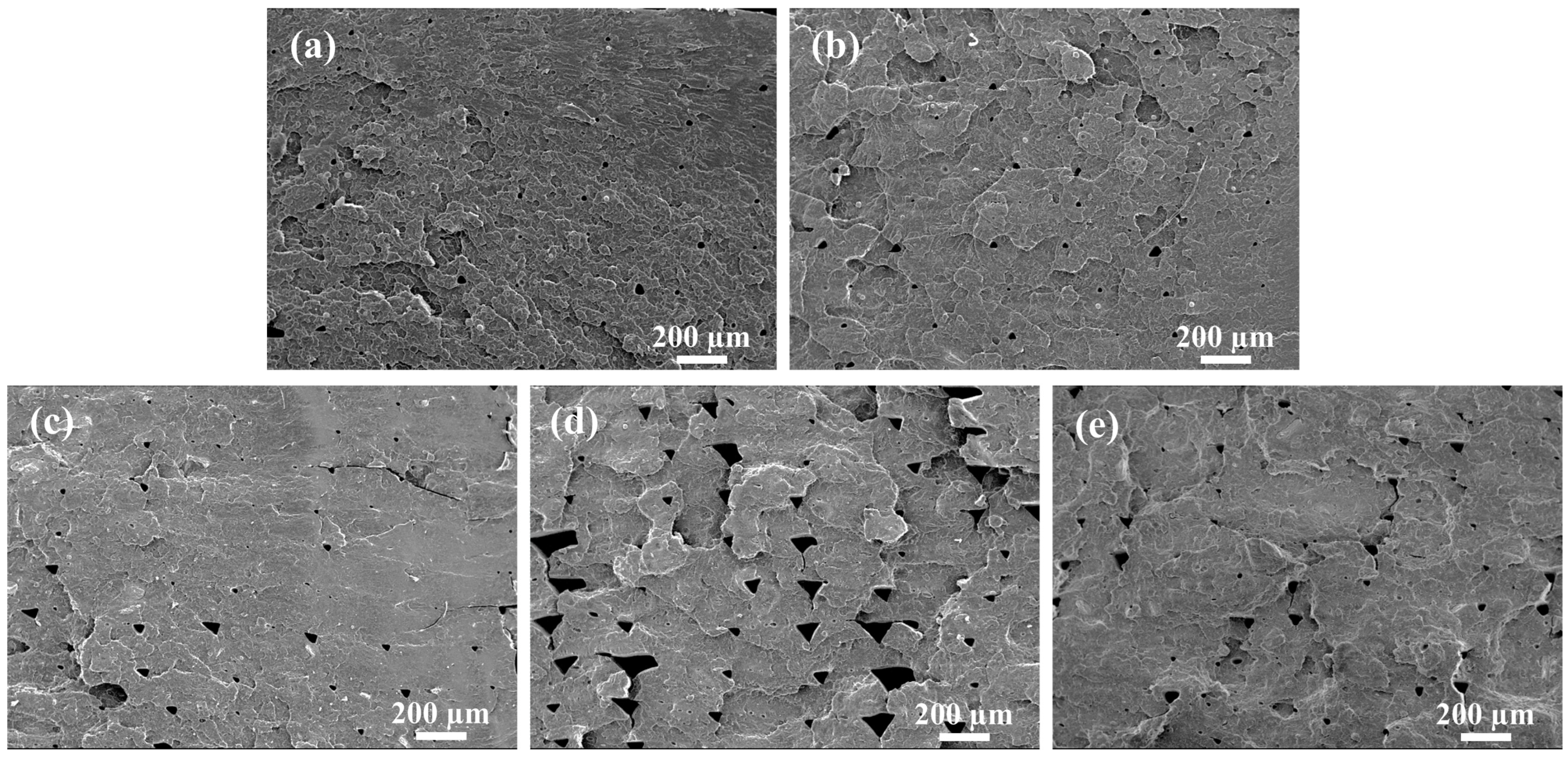
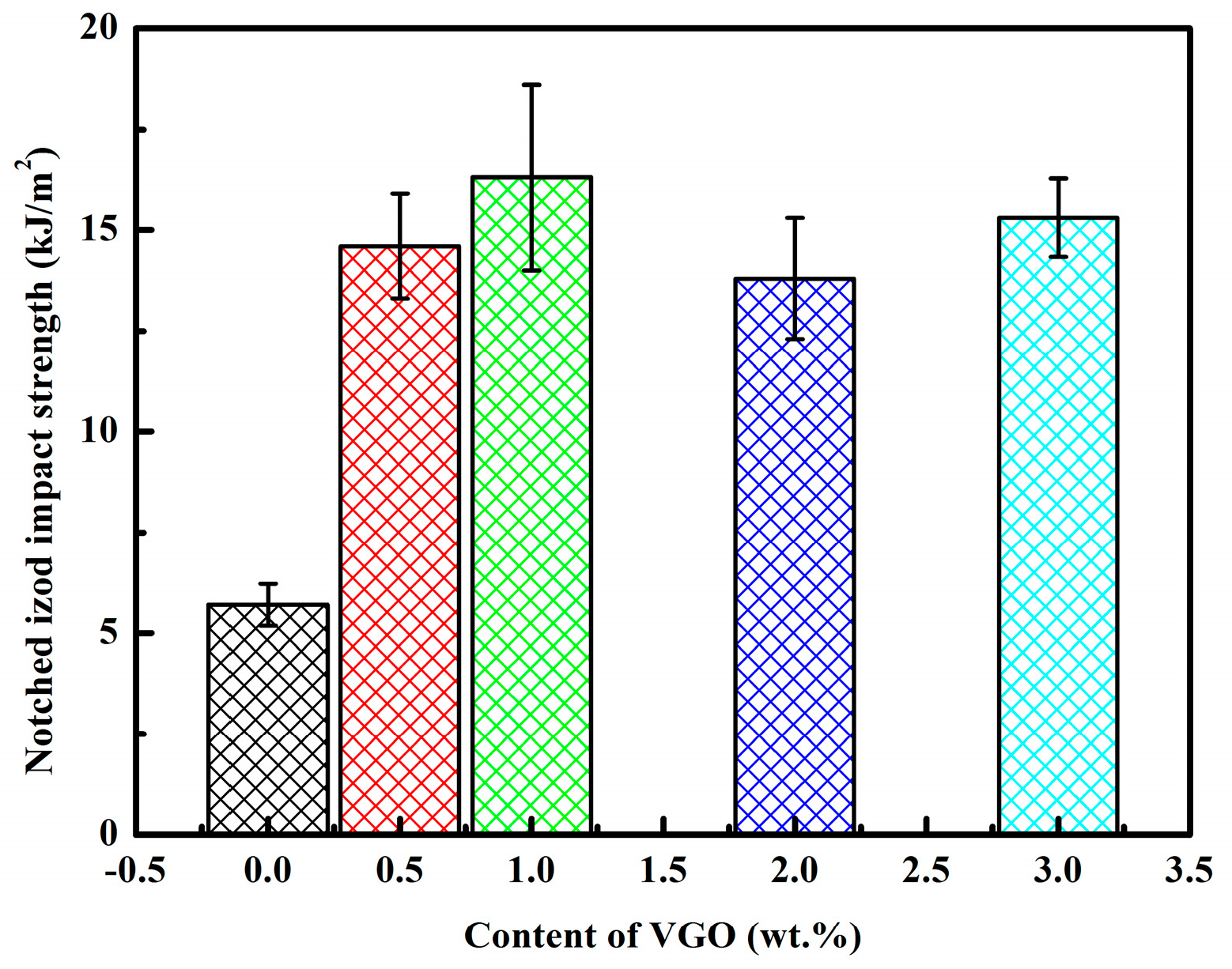
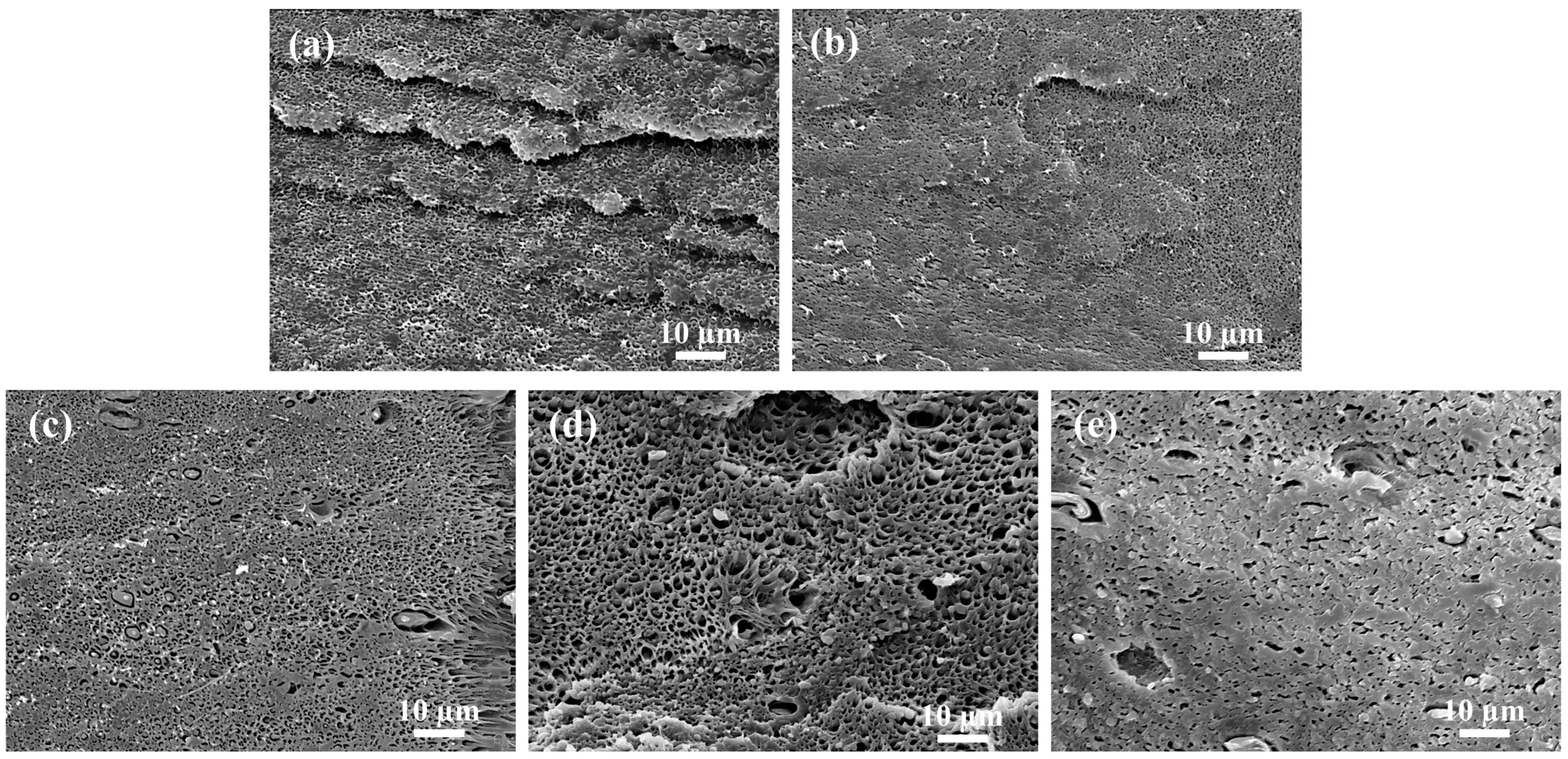
3.5. Microstructure and Impact Strength of 3D-Printed PVGO Blend Nanocomposites
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Conflicts of Interest
References
- Renjith, S.C.; Park, K.; Kremer, G.E.O. A design framework for additive manufacturing: Integration of additive manufacturing capabilities in the early design process. Int. J. Precis. Eng. Manuf. 2020, 21, 329–345. [Google Scholar] [CrossRef]
- Ford, S.; Despeisse, M. Additive manufacturing and sustainability: An exploratory study of the advantages and challenges. J. Clean. Prod. 2016, 137, 1573–1587. [Google Scholar] [CrossRef]
- Huang, S.H.; Liu, P.; Mokasdar, A.; Hou, L. Additive manufacturing and its societal impact: A literature review. Int. J. Adv. Manuf. Technol. 2013, 67, 1191–1203. [Google Scholar] [CrossRef]
- Sang, L.; Han, S.; Li, Z.; Yang, X.; Hou, W. Development of short basalt fiber reinforced polylactide composites and their feasible evaluation for 3D printing applications. Compos. Part B Eng. 2019, 164, 629–639. [Google Scholar] [CrossRef]
- Lee, C.H.; Padzil, F.N.B.M.; Lee, S.H.; Ainun, Z.M.A.; Abdullah, L.C. Potential for natural fiber reinforcement in PLA polymer filaments for fused deposition modeling (FDM) additive manufacturing: A review. Polymers 2021, 13, 1407. [Google Scholar] [CrossRef] [PubMed]
- Rahmatabadi, D.; Soltanmohammadi, K.; Pahlavani, M.; Aberoumand, M.; Soleyman, E.; Ghasemi, I.; Baniassadi, M.; Abrinia, K.; Bodaghi, M.; Baghani, M. Shape memory performance assessment of FDM 3D printed PLA-TPU composites by Box-Behnken response surface methodology. Int. J. Adv. Manuf. Technol. 2023, 127, 935–950. [Google Scholar] [CrossRef]
- Rahmatabadi, D.; Aberoumand, M.; Soltanmohammadi, K.; Soleyman, E.; Ghasemi, I.; Baniassadi, M.; Abrinia, K.; Bodaghi, M.; Baghani, M. Toughening PVC with biocompatible PCL softeners for supreme mechanical properties, morphology, shape memory effects, and FFF printability. Macromol. Mater. Eng. 2023, 2300114. [Google Scholar] [CrossRef]
- Liu, H.; Chen, N.; Shan, P.; Song, P.; Liu, X.; Chen, J. Toward fully bio-based and supertough PLA blends via in situ formation of cross-linked biopolyamide continuity network. Macromolecules 2019, 52, 8415–8429. [Google Scholar] [CrossRef]
- Colnik, M.; Hrncic, M.K.; Skerget, M.; Knez, Z. Biodegradable polymers, current trends of research and their applications, A review. Chem. Ind. Chem. Eng. Q. 2020, 26, 401–418. [Google Scholar] [CrossRef]
- Liu, Z.G.; Wang, Y.Q.; Wu, B.C.; Cui, C.Z.; Guo, Y.; Yan, C. A critical review of fused deposition modeling 3D printing technology in manufacturing polylactic acid parts. Int. J. Adv. Manuf. Technol. 2019, 102, 2877–2889. [Google Scholar] [CrossRef]
- Kulinski, Z.; Piorkowska, E.; Gadzinowska, K.; Stasiak, M. Plasticization of poly(L-lactide) with poly(propylene glycol). Biomacromolecules 2006, 7, 2128–2135. [Google Scholar] [CrossRef]
- Alias, N.F.; Ismail, H. An overview of toughening polylactic acid by an elastomer. Polym. Plast. Technol. Mater. 2019, 58, 1399–1422. [Google Scholar] [CrossRef]
- Xia, Y.W.; Wang, G.X.; Feng, Y.L.; Hu, Y.X.; Zhao, G.Y.; Jiang, W. Highly toughened poly(lactic acid) blends prepared by reactive blending with a renewable poly(ether-block-amide) elastomer. J. Appl. Polym. Sci. 2021, 138, e50097. [Google Scholar] [CrossRef]
- Sun, J.; Luo, S.S.; Huang, A.R.; Shi, M.; Luo, H.; Wei, L.Q.; Sui, Y.M. Double yielding behavior of in situ microfibrillar polyolefin elastomer/poly(lactic acid) composites: Effect of microfibrillar morphology. Polym. Eng. Sci. 2020, 60, 1676–1685. [Google Scholar] [CrossRef]
- Chang, F.L.; Hu, B.; Huang, W.T.; Chen, L.; Yin, X.C.; Cao, X.W.; He, G.J. Improvement of rheology and mechanical properties of PLA/PBS blends by in-situ UV-induced reactive extrusion. Polymer 2022, 259, 125336. [Google Scholar] [CrossRef]
- Bai, H.; Huang, C.; Xiu, H.; Gao, Y.; Zhang, Q.; Fu, Q. Toughening of poly(L-lactide) with poly(ε-caprolactone): Combined effects of matrix crystallization and impact modifier particle size. Polymer 2013, 54, 5257–5266. [Google Scholar] [CrossRef]
- Wang, S.; D’hooge, D.R.; Daelemans, L.; Xia, H.; De Clerck, K.; Cardon, L. The transferability and design of commercial printer settings in PLA/PBAT fused filament fabrication. Polymers 2020, 12, 2573. [Google Scholar] [CrossRef]
- Lim, L.T.; Auras, R.; Rubino, M. Processing technologies for poly(lactic acid). Prog. Polym. Sci. 2008, 33, 820–852. [Google Scholar] [CrossRef]
- Choi, N.; Kim, C.; Cho, K.; Park, J. Morphology and hydrolysis of PCL/PLLA blends compatibilized with P(LLA-co-CL) or P(LLA-b-CL). J. Appl. Polym. Sci. 2002, 86, 1892–1898. [Google Scholar] [CrossRef]
- Na, Y.H.; He, Y.; Shuai, X.; Kikkawa, Y.; Doi, Y.; Inoue, Y. Compatibilization effect of poly(ε-caprolactone)-b-poly(ethylene glycol) block copolymers and phase morphology analysis in immiscible poly(lactide)/poly(ε-caprolactone) blends. Biomacromolecules 2002, 3, 1179–1186. [Google Scholar] [CrossRef]
- Ma, P.; Cai, X.; Zhang, Y.; Wang, S.; Dong, W.; Chen, M.; Lemstra, P.J. In-situ compatibilization of poly(lactic acid) and poly(butylene adipate-co-terephthalate) blends by using dicumyl peroxide as a free-radical initiator. Polym. Degrad. Stab. 2014, 102, 145–151. [Google Scholar] [CrossRef]
- Semba, T.; Kitagawa, K.; Ishiaku, U.S.; Hamada, H. The effect of crosslinking on the mechanical properties of polylactic acid/polycaprolactone blends. J. Appl. Polym. Sci. 2006, 0101, 01816–01825. [Google Scholar] [CrossRef]
- Semba, T.; Kitagawa, K.; Ishiaku, U.S.; Kotaki, M.; Hiroyuki, H. Effect of compounding procedure on mechanical properties and dispersed phase morphology of poly(lactic acid)/polycaprolactone blends containing peroxide. J. Appl. Polym. Sci. 2007, 103, 1066–1074. [Google Scholar] [CrossRef]
- Wang, B.; Ye, X.; Wang, B.; Li, X.; Xiao, S.; Liu, H. Reactive graphene as highly effcient compatibilizer for cocontinuous poly(lactic acid)/poly(ε-caprolactone) blends toward robust biodegradable nanocomposites. Compos. Sci. Technol. 2022, 221, 109326. [Google Scholar] [CrossRef]
- Wang, B.; Zheng, Q.; Li, M.; Wang, S.; Xiao, S.; Li, X.; Liu, H. Enhancing interfacial interactions of cocontinuous poly(lactic acid)/polyethylene blends using vinylsilane grafted carbon nanotubes as generic reactive compatibilizers. Express Polym. Lett. 2022, 16, 524–539. [Google Scholar] [CrossRef]
- Yang, X.; Wei, F.; Wang, Z.; Li, G.; Yang, S.; Feng, J. High-reactive silica nanosheets as compatibilizers for immiscible PLLA/PBAT polymer blends. Compos. Sci. Technol. 2023, 236, 109979. [Google Scholar] [CrossRef]
- Lyu, Y.; Chen, Y.; Lin, Z.; Zhang, J.; Shi, X. Manipulating phase structure of biodegradable PLA/PBAT system: Effects on dynamic rheological responses and 3D printing. Compos. Sci. Technol. 2020, 200, 108399. [Google Scholar] [CrossRef]
- Li, X.; He, J.; Hu, Z.; Ye, X.; Wang, S.; Zhao, Y.; Wang, B.; Ou, Y.; Zhang, J. High strength carbon-fber reinforced polyamide 6 composites additively manufactured by screw-based extrusion. Compos. Sci. Technol. 2022, 229, 109707. [Google Scholar] [CrossRef]
- Wang, Y.; Song, C.; Yu, X.; Li, L.; Han, Y.; Chen, J.; Fu, J. Thermo-responsive hydrogels with tunable transition temperature crosslinked by multifunctional graphene oxide nanosheets. Compos. Sci. Technol. 2017, 151, 139–146. [Google Scholar] [CrossRef]
- ASTM D256:2023; Standard Test Methods for Determining the Izod Pendulum Impact Resistance of Plastics. ASTM: West Conshohocken, PA, USA, 2023.
- Garlotta, D. A literature review of poly(Lactic Acid). J. Polym. Envorin. 2002, 9, 63–84. [Google Scholar] [CrossRef]
- Cote, L.J.; Kim, J.; Zhang, Z.; Sun, C.; Huang, J. Tunable assembly of graphene oxide surfactant sheets: Wrinkles, overlaps and impacts on thin film properties. Soft Matter 2010, 6, 6096–6101. [Google Scholar] [CrossRef]
- Sailer, C.; Handge, U.A. Reactive blending of polyamide 6 and styrene-acrylonitrile copolymer: Influence of blend composition and compatibilizer concentration on morphology and rheology. Macromolecules 2008, 41, 4258–4267. [Google Scholar] [CrossRef]
- Wang, H.; Yang, X.; Fu, Z.; Zhao, X.; Li, Y.; Li, J. Rheology of nanosilica-compatibilized immiscible polymer blends: Formation of a “heterogeneous nnetwork” facilitated by interfacially anchored hybrid nanosilica. Macromolecules 2017, 50, 9494–9506. [Google Scholar] [CrossRef]
- Bazli, L.; Khavandi, A.; Boutorabi, M.A.; Karrabi, M. Correlation between viscoelastic behavior and morphology of nanocomposites based on SR/EPDM blends compatibilized by maleic anhydride. Polymer 2017, 113, 156–166. [Google Scholar] [CrossRef]
- Zhao, T.; Yu, J.; Pan, H.; Zhao, Y.; Zhang, Q.; Yu, X.; Bian, J.; Han, L.; Zhang, H. Super-tough polylactic acid (PLA)/poly(butylene succinate) (PBS) materials prepared through reactive blending with epoxy-functionalized PMMA-GMA copolymer. Int. J. Biol. Macromol. 2023, 251, 126150. [Google Scholar] [CrossRef]
- Kulinski, Z.; Piorkowska, E. Crystallization, structure and properties of plasticized poly(L-lactide). Polymer 2005, 46, 10290–10300. [Google Scholar] [CrossRef]
- Li, H.; Huneault, M.A. Effect of nucleation and plasticization on the crystallization of poly(lactic acid). Polymer 2007, 48, 6855–6866. [Google Scholar] [CrossRef]
- Wu, D.F.; Cheng, Y.X.; Feng, S.H.; Yao, Z.; Zhang, M. Crystallization behavior of polylactide/graphene composites. Ind. Eng. Chem. Res. 2013, 52, 6731–6739. [Google Scholar] [CrossRef]
- Ahmed, J.; Luciano, G.; Maggiore, S. Nonisothermal crystallization behavior of polylactide/polyethylene glycol/graphene oxide nanosheets composite films. Polym. Compos. 2020, 41, 2108–2119. [Google Scholar] [CrossRef]
- Costa, S.F.; Duarte, F.M.; Covas, J.A. Estimation of filament temperature and adhesion development infused deposition techniques. J. Mater. Process. Technol. 2017, 245, 167–179. [Google Scholar] [CrossRef]
- Levenhagen, N.P.; Dadmun, M.D. Improving interlayer adhesion in 3D printing with surface segregating additives: Improving the isotropy of acrylonitrile–butadiene–styrene parts. ACS Appl. Polym. Mater. 2019, 1, 876–884. [Google Scholar] [CrossRef]
- Sui, X.Y.; Zhao, X.Y.; Wang, Z.C.; Sun, S.L. Super-ductile and stiff PBAT/PLA biodegradable composites balanced with random PMMA-co-GMA copolymer as compatibilizer. Polym. Int. 2023, 72, 333–341. [Google Scholar] [CrossRef]

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yu, W.; Hu, Z.; Zhang, Y.; Zhang, Y.; Dong, W.; Li, X.; Wang, S. Compatibilizing Biodegradable Poly(lactic acid)/polybutylene adipate-co-terephthalate Blends via Reactive Graphene Oxide for Screw-Based 3D Printing. Polymers 2023, 15, 3992. https://doi.org/10.3390/polym15193992
Yu W, Hu Z, Zhang Y, Zhang Y, Dong W, Li X, Wang S. Compatibilizing Biodegradable Poly(lactic acid)/polybutylene adipate-co-terephthalate Blends via Reactive Graphene Oxide for Screw-Based 3D Printing. Polymers. 2023; 15(19):3992. https://doi.org/10.3390/polym15193992
Chicago/Turabian StyleYu, Wei, Zhonglue Hu, Ye Zhang, Yakuang Zhang, Weiping Dong, Xiping Li, and Sisi Wang. 2023. "Compatibilizing Biodegradable Poly(lactic acid)/polybutylene adipate-co-terephthalate Blends via Reactive Graphene Oxide for Screw-Based 3D Printing" Polymers 15, no. 19: 3992. https://doi.org/10.3390/polym15193992




