Recent Advances in the Treatment of Industrial Wastewater from Different Celluloses in Continuous Systems
Abstract
:1. Introduction
2. Mechanism of Adsorptions and Characterizations of Cellulose
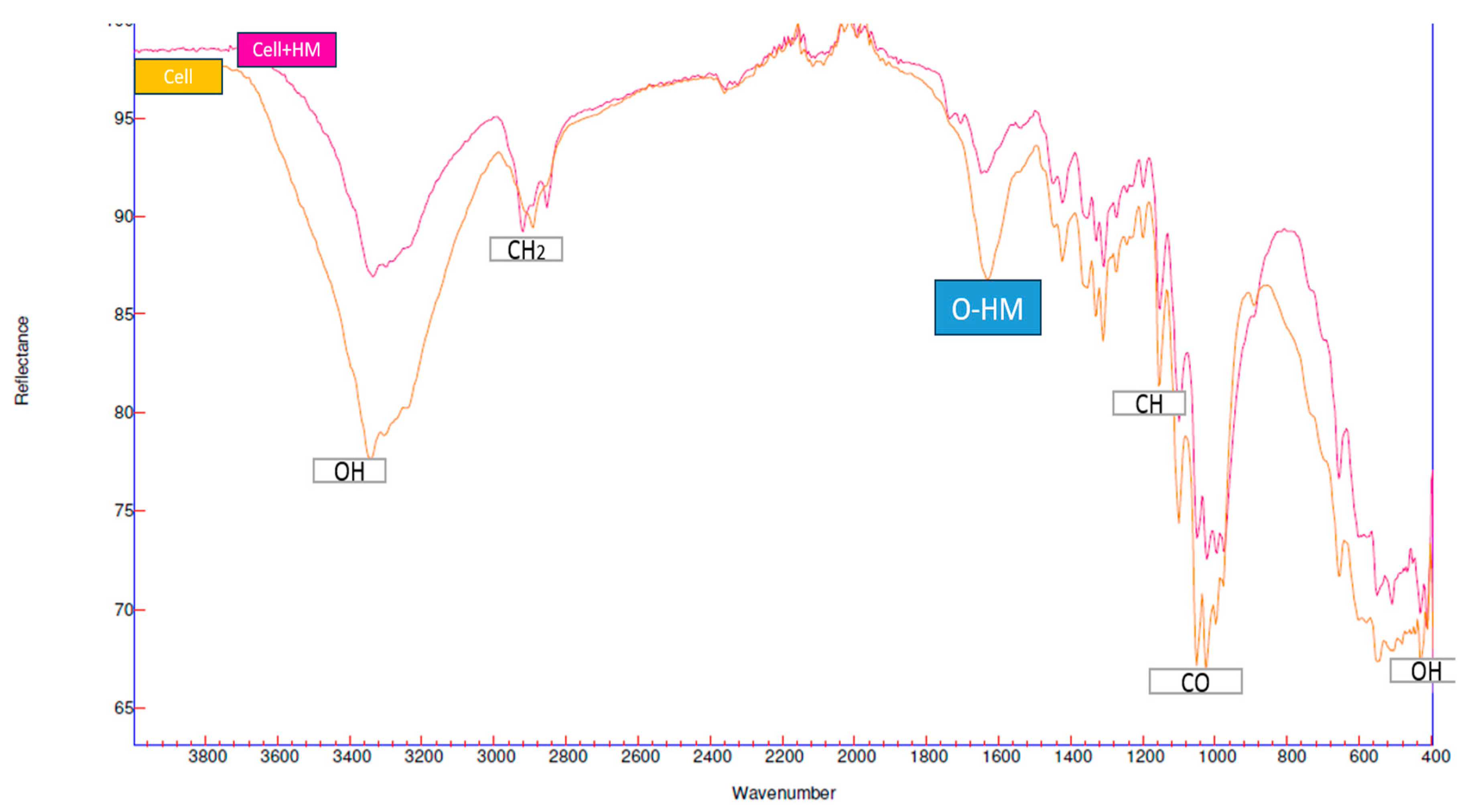
2.1. Analysis FTIR of Cellulose
2.2. Analysis of Microphotographs of Cellulose
2.3. Adsorption Mechanism
3. Design of the Process of Treatment through the Balance of Mass and Extra-Particle and Intraparticle Diffusion
3.1. Mass Balance in Treatment
- Q = design flow (mL/min);
- Tbj = break time of use number j (Min);
- Ci = initial concentration (mg/mL);
- Co = final heavy metals concentration in the treated solution (mg/mL);
- V = volume of the system (mL);
- Ε = porosity;
- M = amount of biomass used (g);
- qT = total adsorption capacity of the biomass used (mg/g).
3.2. Extra-Particle Diffusion
- Kl = Mass transfer coefficient in the liquid particle m/h;
- L: Length;
- Cs = Equilibrium concentration of pollutant.
3.3. Intraparticle Diffusion
3.4. Modeling Process
4. Design of System with the Cellulose
5. Perspectives in Cost of Implementation
6. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Conflicts of Interest
References
- Kumar, V.; Parihar, R.D.; Sharma, A.; Bakshi, P.; Sidhu, G.P.S.; Bali, A.S.; Karaouzas, I.; Bhardwaj, R.; Thukral, A.K.; Gyasi-Agyei, Y.; et al. Global evaluation of heavy metal content in surface water bodies: A meta-analysis using heavy metal pollution indices and multivariate statistical analyses. Chemosphere 2019, 236, 124364. [Google Scholar] [CrossRef]
- Briffa, J.; Sinagra, E.; Blundell, R. Heavy metal pollution in the environment and their toxicological effects on humans. Heliyon 2020, 6, e04691. [Google Scholar] [CrossRef]
- Rahman, M.A.; Paul, M.; Bhoumik, N.; Hassan, M.; Alam, M.K.; Aktar, Z. Heavy metal pollution assessment in the groundwater of the Meghna Ghat industrial area, Bangladesh, by using water pollution indices approach. Appl. Water Sci. 2020, 10, 186. [Google Scholar] [CrossRef]
- Khadija, D.; Hicham, A.; Rida, A.; Hicham, E.; Nordine, N.; Najlaa, F. Surface water quality assessment in the semi-arid area by a combination of heavy metal pollution indices and statistical approaches for sustainable management. Environ. Chall. 2021, 5, 100230. [Google Scholar] [CrossRef]
- Mahamood, M.; Khan, F.R.; Zahir, F.; Javed, M.; Alhewairini, S.S. Bagarius bagarius, and Eichhornia crassipes are suitable bioindicators of heavy metal pollution, toxicity, and risk assessment. Sci. Rep. 2023, 13, 1824. [Google Scholar] [CrossRef]
- Khan, M.; Javed, M.; Rehman, M.T.; Urooj, M.; Ahmad, M.I. Heavy metal pollution and risk assessment by the battery of toxicity tests. Sci. Rep. 2020, 10, 16593. [Google Scholar] [CrossRef] [PubMed]
- Kapahi, M.; Sachdeva, S. Bioremediation options for heavy metal pollution. J. Health Pollut. 2019, 9, 191203. [Google Scholar] [CrossRef] [PubMed]
- Karaouzas, I.; Kapetanaki, N.; Mentzafou, A.; Kanellopoulos, T.D.; Skoulikidis, N. Heavy metal contamination status in Greek surface waters: A review with application and evaluation of pollution indices. Chemosphere 2020, 263, 128192. [Google Scholar] [CrossRef] [PubMed]
- Sayago UF, C. Design and development of a biotreatment of E. crassipes for the decontamination of water with Chromium (VI). Sci. Rep. 2021, 11, 9326. [Google Scholar] [CrossRef] [PubMed]
- Carreño-Sayago, U.F. Development of microspheres using water hyacinth (Eichhornia crassipes) for treatment of contaminated water with Cr (VI). Environ. Dev. Sustain. 2021, 23, 4735–4746. [Google Scholar] [CrossRef]
- Patel, H. Batch and continuous fixed bed adsorption of heavy metals removal using activated charcoal from neem (Azadirachta indica) leaf powder. Sci. Rep. 2020, 10, 16895. [Google Scholar] [CrossRef] [PubMed]
- Akindele, E.O.; Omisakin, O.D.; Oni, O.A.; Aliu, O.O.; Omoniyi, G.E.; Akinpelu, O.T. Heavy metal toxicity in the water column and benthic sediments of a degraded tropical stream. Ecotoxicol. Environ. Saf. 2020, 190, 110153. [Google Scholar] [CrossRef] [PubMed]
- Zia, Z.; Hartland, A.; Mucalo, M.R. Use of low-cost biopolymers and biopolymeric composite systems for heavy metal removal from water. Int. J. Environ. Sci. Technol. 2020, 17, 4389–4406. [Google Scholar] [CrossRef]
- Ma, X.; Zhao, S.; Tian, Z.; Duan, G.; Pan, H.; Yue, Y.; Li, S.; Jian, S.; Yang, W.; Liu, K.; et al. MOFs meet wood: Reusable magnetic hydrophilic composites toward efficient water treatment with super-high dye adsorption capacity at high dye concentration. Chem. Eng. J. 2022, 446, 136851. [Google Scholar] [CrossRef]
- Mohammed, Y.; Song, K.; Li, L. Fixed bed column and artificial neural network model to predict heavy metals adsorption dynamic on surfactant decorated graphene. Colloids Surf. A Physicochem. Eng. Asp. 2020, 585, 124076. [Google Scholar]
- Danish, M.; Ansari, K.B.; Aftab, R.A.; Danish, M.; Zaidi, S.; Trinh, Q.T. gPROMS-driven modeling and simulation of fixed bed adsorption of heavy metals on a biosorbent: Benchmarking and case study. Environ. Sci. Pollut. Res. 2023, 30, 71511–71526. [Google Scholar] [CrossRef]
- Fallah, N.; Taghizadeh, M. Continuous fixed-bed adsorption of Mo (VI) from aqueous solutions by Mo (VI)-IIP: Breakthrough curves analysis and mathematical modeling. J. Environ. Chem. Eng. 2020, 8, 104079. [Google Scholar] [CrossRef]
- Sayago UF, C.; Ballesteros Ballesteros, V. Development of a treatment for water contaminated with Cr (VI) using cellulose xanthogenate from E. crassipes on a pilot scale. Sci. Rep. 2023, 13, 1970. [Google Scholar]
- Chen, Y.; Li, S.; Li, X.; Mei, C.; Zheng, J.; E, S.; Duan, G.; Liu, K.; Jiang, S. Liquid transport and real-time dye purification via lotus petiole-inspired long-range-ordered anisotropic cellulose nanofibril aerogels. ACS Nano 2021, 15, 20666–20677. [Google Scholar] [CrossRef]
- Carreño Sayago, U.F.; Piñeros Castro, Y.; Conde Rivera, L.R. Design of a Fixed-Bed Column with Vegetal Biomass and Its Recycling for Cr (VI) Treatment. Recycling 2022, 7, 71. [Google Scholar] [CrossRef]
- Carreño Sayago, U.F. Design, scaling, and development of biofilters with E. crassipes for treatment of water contaminated with Cr (VI). Water 2021, 13, 1317. [Google Scholar] [CrossRef]
- Shi, C.; Wang, X.; Zhou, S.; Zuo, X.; Wang, C. Mechanism, application, influencing factors and environmental benefit assessment of steel slag in removing pollutants from water: A review. J. Water Process Eng. 2022, 47, 102666. [Google Scholar] [CrossRef]
- León, G.; Gómez, E.; Miguel, B.; Hidalgo, A.M.; Gómez, M.; Murcia, M.D.; Guzmán, M.A. Feasibility of adsorption kinetic models to study carrier-mediated transport of heavy metal ions in emulsion liquid membranes. Membranes 2022, 12, 66. [Google Scholar] [CrossRef] [PubMed]
- Amari, A.; Alawameleh, H.S.K.; Isam, M.; Maktoof, M.A.J.; Osman, H.; Panneerselvam, B.; Thomas, M. Thermodynamic Investigation and Study of Kinetics and Mass Transfer Mechanisms of Oily Wastewater Adsorption on UIO-66–MnFe2O4 as a Metal–Organic Framework (MOF). Sustainability 2023, 15, 2488. [Google Scholar] [CrossRef]
- Abdulrasool, M.M.; Ruaa, K.M.; Mays, A.D.; ALsailawi, H.A.; Mudhafar, M.; Bashi, A.M. Regeneration of Chitosan-Based Adsorbents Used in Heavy Metal Adsorption. J. Life Sci. 2021, 15, 11–19. [Google Scholar] [CrossRef]
- Sohrabi, N.; Mohammadi, R.; Ghassemzadeh, H.R.; Heris, S.S.S. Design and synthesis of a new magnetic molecularly imprinted polymer nanocomposite for specific adsorption and separation of diazinon insecticides from aqueous media. Microchem. J. 2021, 175, 107087. [Google Scholar] [CrossRef]
- Jiang, Q.; He, Y.; Wu, Y.; Dian, B.; Zhang, J.; Li, T.; Jiang, M. Solidification/stabilization of soil heavy metals by alkaline industrial wastes: A critical review. Environ. Pollut. 2022, 312, 120094. [Google Scholar] [CrossRef]
- Xie, X.; Zhang, L.; Luo, X.; Su, T.; Zhang, Y.; Qin, Z.; Ji, H. PEI modified magnetic porous cassava residue microspheres for adsorbing Cd (II) from aqueous solution. Eur. Polym. J. 2022, 159, 110741. [Google Scholar] [CrossRef]
- Zhao, B.; Jiang, H.; Lin, Z.; Xu, S.; Xie, J.; Zhang, A. Preparation of acrylamide/acrylic acid cellulose hydrogels for the adsorption of heavy metal ions. Carbohydr. Polym. 2019, 224, 115022. [Google Scholar] [CrossRef] [PubMed]
- Danish, M.; Ansari, K.B.; Danish, M.; Khatoon, A.; Rao, R.A.K.; Zaidi, S.; Aftab, R.A. A comprehensive investigation of external mass transfer and intraparticle diffusion for batch and continuous adsorption of heavy metals using pore volume and surface diffusion model. Sep. Purif. Technol. 2022, 292, 120996. [Google Scholar] [CrossRef]
- Zou, W.; Feng, X.; Wang, R.; Wei, W.; Luo, S.; Zheng, R.; Yang, D.; Mi, H.; Chen, H. High-efficiency core-shell magnetic heavy-metal absorbents derived from spent-LiFePO4 Battery. J. Hazard. Mater. 2021, 402, 123583. [Google Scholar] [CrossRef] [PubMed]
- Si, Y.; Li, J.; Cui, B.; Tang, D.; Yang, L.; Murugadoss, V.; Maganti, S.; Huang, M.; Guo, Z. Janus phenol–formaldehyde resin and periodic mesoporous organic silica nanoadsorbent for the removal of heavy metal ions and organic dyes from polluted water. Adv. Compos. Hybrid Mater. 2022, 5, 1180–1195. [Google Scholar] [CrossRef]
- Yu, F.; Li, Y.; Huang, G.; Yang, C.; Chen, C.; Zhou, T.; Zhao, Y.; Ma, J. Adsorption behavior of the antibiotic levofloxacin on microplastics in the presence of different heavy metals in an aqueous solution. Chemosphere 2020, 260, 127650. [Google Scholar] [CrossRef] [PubMed]
- Mittal, J.; Ahmad, R.; Mariyam, A.; Gupta, V.K.; Mittal, A. Expeditious and enhanced sequestration of heavy metal ions from aqueous environment by papaya peel carbon: A green and low-cost adsorbent. Desalin. Water Treat 2021, 210, 365–376. [Google Scholar] [CrossRef]
- Shi, T.; Ma, J.; Wu, F.; Ju, T.; Gong, Y.; Zhang, Y.; Wu, X.; Hou, H.; Zhao, L.; Shi, H. Mass balance-based inventory of heavy metals inputs to and outputs from agricultural soils in Zhejiang Province, China. Sci. Total Environ. 2019, 649, 1269–1280. [Google Scholar] [CrossRef]
- Whitehead, P.G.; Bussi, G.; Peters, R.; Hossain, M.A.; Softley, L.; Shawal, S.; Jin, L.; Rampley, C.P.N.; Holdship, P.; Hope, R.; et al. Modelling heavy metals in the Buriganga River System, Dhaka, Bangladesh: Impacts of tannery pollution control. Sci. Total Environ. 2019, 697, 134090. [Google Scholar] [CrossRef] [PubMed]
- Luo, H.; Wang, Q.; Guan, Q.; Ma, Y.; Ni, F.; Yang, E.; Zhang, J. Heavy metal pollution levels, source apportionment and risk assessment in dust storms in key cities in Northwest China. J. Hazard. Mater. 2022, 422, 126878. [Google Scholar] [CrossRef]
- Hong, N.; Guan, Y.; Yang, B.; Zhong, J.; Zhu, P.; Ok, Y.S.; Hou, D.; Tsang, D.C.W.; Guan, Y.; Liu, A. Quantitative source tracking of heavy metals contained in urban road deposited sediments. J. Hazard. Mater. 2020, 393, 122362. [Google Scholar] [CrossRef]
- Sayago UF, C.; Castro, Y.P.; Rivera LR, C.; Mariaca, A.G. Estimation of equilibrium times and maximum capacity of adsorption of heavy metals by E. crassipes. Environ. Monit. Assess. 2020, 192, 141. [Google Scholar] [CrossRef] [PubMed]
- Carreño Sayago, U.F. “Buchón De Agua” (Eichhornia crassipes): Impulsor De La Fitorremediación; Editorial Los Libertadores: Bogotá, Colombia, 2020. [Google Scholar]
- Jian, S.; Chen, Y.; Shi, F.; Liu, Y.; Jiang, W.; Hu, J.; Han, X.; Jiang, S.; Yang, W. Template-Free Synthesis of Magnetic La-Mn-Fe Tri-Metal Oxide Nanofibers for Efficient Fluoride Remediation: Kinetics, Isotherms, Thermodynamics and Reusability. Polymers 2022, 14, 5417. [Google Scholar] [CrossRef]
- Jin, X.; Xiang, Z.; Liu, Q.; Chen, Y.; Lu, F. Polyethyleneimine-bacterial cellulose bioadsorbent for effective removal of copper and lead ions from aqueous solution. Bioresour. Technol. 2017, 244, 844–849. [Google Scholar] [CrossRef] [PubMed]
- Stoica-Guzun, A.; Stroescu, M.; Jinga, S.I.; Mihalache, N.; Botez, A.; Matei, C.; Berger, D.; Damian, C.M.; Ionita, V. Box-Behnken experimental design for chromium (VI) ions removal by bacterial cellulose-magnetite composites. Int. J. Biol. Macromol. 2016, 91, 1062–1072. [Google Scholar] [CrossRef] [PubMed]
- Mohammed, A.B.; Omran, A.R.; Baiee, M.A.; Salman, J.M. Biosorption of Safranin-O from Aqueous Solution by Nile Rose Plant (Eichhornia crassipes). Baghdad Sci. J. 2018, 15, 26–30. [Google Scholar] [CrossRef]
- Wang, C.; Wang, H.; Gu, G. Ultrasound-assisted xanthation of cellulose from lignocellulosic biomass optimized by response surface methodology for Pb (II) sorption. Carbohydr. Polym. 2018, 182, 21–28. [Google Scholar] [CrossRef]
- Wang, J.; Lu, X.; Ng, P.F.; Lee, K.I.; Fei, B.; Xin, J.H.; Wu, J.Y. Polyethylenimine coated bacterial cellulose nanofiber membrane and application as adsorbent and catalyst. J. Colloid Interface Sci. 2015, 440, 32–38. [Google Scholar] [CrossRef]
- El-Naggar, M.E.; Radwan, E.K.; El-Wakeel, S.T.; Kafafy, H.; Gad-Allah, T.A.; El-Kalliny, A.S.; Shaheen, T.I. Synthesis, characterization and adsorption properties of microcrystalline cellulose based nanogel for dyes and heavy metals removal. Int. J. Biol. Macromol. 2018, 113, 248–258. [Google Scholar] [CrossRef] [PubMed]
- Jin, L.; Bai, R. Mechanisms of lead adsorption on chitosan/PVA hydrogel beads. Langmuir 2002, 18, 9765–9770. [Google Scholar] [CrossRef]
- Sun, S.; Wang, A. Adsorption kinetics of Cu (II) ions using N,O-carboxymethyl-chitosan. J. Hazard. Mater. 2006, 131, 103–111. [Google Scholar] [CrossRef]
- Yuwei, C.; Jianlong, W. Preparation and characterization of magnetic chitosan nanoparticles and its application for Cu (II) removal. Chem. Eng. J. 2011, 168, 286–292. [Google Scholar] [CrossRef]
- Krishnamachari, P.; Hashaikeh, R.; Tiner, M. Modified cellulose morphologies and its composites; SEM and TEM analysis. Micron 2011, 42, 751–761. [Google Scholar] [CrossRef]
- Zhou, Y.T.; Nie, H.L.; Branford-White, C.; He, Z.Y.; Zhu, L.M. Removal of Cu2+ from aqueous solution by chitosan-coated magnetic nanoparticles modified with α-ketoglutaric acid. J. Colloid Interface Sci. 2009, 330, 29–37. [Google Scholar] [CrossRef]
- Taka, A.L.; Klink, M.J.; Mbianda, X.Y.; Naidoo, E.B. Chitosan nanocomposites for water treatment by fixed-bed continuous flow column adsorption: A review. Carbohydr. Polym. 2020, 255, 117398. [Google Scholar] [CrossRef]
- Yang, X.; Liu, Z.; Jiang, Y.; Li, F.; Xue, B.; Dong, Z.; Ding, M.; Chen, R.; Yang, Q.; An, T.; et al. Micro-structure, surface properties and adsorption capacity of ball-milled cellulosic biomass derived biochar based mineral composites synthesized via carbon-bed pyrolysis. Appl. Clay Sci. 2020, 199, 105877. [Google Scholar] [CrossRef]
- Yu, X.; Tong, S.; Ge, M.; Wu, L.; Zuo, J.; Cao, C.; Song, W. Adsorption of heavy metal ions from aqueous solution by carboxylated cellulose nanocrystals. J. Environ. Sci. 2013, 25, 933–943. [Google Scholar] [CrossRef] [PubMed]
- Zhang, N.; Zang, G.L.; Shi, C.; Yu, H.Q.; Sheng, G.P. A novel adsorbent TEMPO-mediated oxidized cellulose nanofibrils modified with PEI: Preparation, characterization, and application for Cu (II) removal. J. Hazard. Mater. 2016, 316, 11–18. [Google Scholar] [CrossRef] [PubMed]
- Jiang, H.; Yang, Y.; Lin, Z.; Zhao, B.; Wang, J.; Xie, J.; Zhang, A. Preparation of a novel bio-adsorbent of sodium alginate grafted polyacrylamide/graphene oxide hydrogel for the adsorption of heavy metal ion. Sci. Total Environ. 2020, 744, 140653. [Google Scholar] [CrossRef] [PubMed]
- Li, H.; Wang, Y.; Ye, M.; Zhang, X.; Zhang, H.; Wang, G.; Zhang, Y. Hierarchically porous poly (amidoxime)/bacterial cellulose composite aerogel for highly efficient scavenging of heavy metals. J. Colloid Interface Sci. 2021, 600, 752–763. [Google Scholar] [CrossRef]
- Tang, C.; Brodie, P.; Li, Y.; Grishkewich, N.J.; Brunsting, M.; Tam, K.C. Shape recoverable and mechanically robust cellulose aerogel beads for efficient removal of copper ions. Chem. Eng. J. 2020, 392, 124821. [Google Scholar] [CrossRef]
- Tang, P.; Sun, Q.; Zhao, L.; Tang, Y.; Liu, Y.; Pu, H.; Gan, N.; Liu, Y.; Li, H. A simple and green method to construct cyclodextrin polymer for the effective and simultaneous estrogen pollutant and metal removal. Chem. Eng. J. 2019, 366, 598–607. [Google Scholar] [CrossRef]
- Sun, Y.; Yin, W.M.; Wang, Y.; Zhao, N.D.; Wang, X.Y.; Zhang, J.G.; Guo, Y.R.; Li, S.; Pan, Q.J. Fabrication of ultra-thin MgAl layered double oxide by cellulose templating and its immobilization effect toward heavy metal ions: Cation-exchange and deposition mechanism. Chem. Eng. J. 2022, 427, 132017. [Google Scholar] [CrossRef]
- Abere, D.V.; Ojo, S.A.; Paredes-Epinosa, M.B.; Hakami, A. Derivation of composites of chitosan-nanoparticles from crustaceans source for nanomedicine: A mini review. Biomed. Eng. Adv. 2022, 4, 100058. [Google Scholar] [CrossRef]
- Kang, X.; Cong, Z.; Pin, X.; Du, Z.; Cai, Z. Copper ion-imprinted bacterial cellulose for selectively removing heavy metal ions from aqueous solution. Cellulose 2022, 29, 4001–4019. [Google Scholar]
- Hokkanen, S.; Repo, E.; Lou, S.; Sillanpää, M. Removal of arsenic (V) by magnetic nanoparticle activated microfibrillated cellulose. Chem. Eng. J. 2011, 260, 886–894. [Google Scholar] [CrossRef]
- Chen, A.; Zeng, G.; Chen, G.; Hu, X.; Yan, M.; Guan, S.; Shang, C.; Lu, L.; Zou, Z.; Xie, G. Novel thiourea-modified magnetic ion-imprinted chitosan/TiO2 composite for simultaneous removal of cadmium and 2,4-dichlorophenol. Chem. Eng. J. 2012, 191, 85–94. [Google Scholar] [CrossRef]
- Lin, S.; Yang, H.; Na, Z.; Lin, K. A novel biodegradable arsenic adsorbent by immobilization of iron oxyhydroxide (FeOOH) on the root powder of long-root Eichhornia crassipes. Chemosphere 2018, 192, 258–266. [Google Scholar] [CrossRef]
- Huang, X.; Zhan, X.; Wen, C.; Xu, F.; Luo, L. Amino-functionalized magnetic bacterial cellulose/activated carbon composite for Pb2+ and methyl orange sorption from aqueous solution. J. Mater. Sci. Technol. 2018, 34, 855–863. [Google Scholar] [CrossRef]
- Okieimen, F.E.; Sogbaike, C.E.; Ebhoaye, J.E. Removal of cadmium and copper ions from aqueous solution with cellulose graft copolymers. Sep. Purif. Technol. 2005, 44, 85–89. [Google Scholar] [CrossRef]
- Bringas, A.; Bringas, E.; Ibañez, R.; San-Román, M.F. Fixed-bed columns mathematical modeling for selective nickel and copper recovery from industrial spent acids by chelating resins. Sep. Purif. Technol. 2023, 313, 123457. [Google Scholar] [CrossRef]
- Sounthararajah, D.P.; Loganathan, P.; Kandasamy, J.; Vigneswaran, S. Adsorptive removal of heavy metals from water using sodium titanate nanofibres loaded onto GAC in fixed-bed columns. J. Hazard. Mater. 2015, 287, 306–316. [Google Scholar] [CrossRef]
- Bhatti, H.N.; Mahmood, Z.; Kausar, A.; Yakout, S.M.; Shair, O.H.; Iqbal, M. Biocomposites of polypyrrole, polyaniline and sodium alginate with cellulosic biomass: Adsorption-desorption, kinetics and thermodynamic studies for the removal of 2,4-dichlorophenol. Int. J. Biol. Macromol. 2020, 153, 146–157. [Google Scholar] [CrossRef]
- Yang, W.; Wang, Y.; Wang, Q.; Wu, J.; Duan, G.; Xu, W.; Jian, S. Magnetically separable and recyclable Fe3O4@ PDA covalent grafted by l-cysteine core-shell nanoparticles toward efficient removal of Pb2+. Vacuum 2021, 189, 110229. [Google Scholar] [CrossRef]
- Wang, J.; Sun, Y.; Zhao, X.; Chen, L.; Peng, S.; Ma, C.; Duan, G.; Liu, Z.; Wang, H.; Yuan, Y.; et al. A poly (amidoxime)-modified MOF macroporous membrane for high-efficient uranium extraction from seawater. e-Polymers 2022, 22, 399–410. [Google Scholar] [CrossRef]
- Chen, Q.; Zheng, J.; Wen, L.; Yang, C.; Zhang, L. A multi-functional-group modified cellulose for enhanced heavy metal cadmium adsorption: Performance and quantum chemical mechanism. Chemosphere 2019, 224, 509–518. [Google Scholar] [CrossRef] [PubMed]
- Ajmani, A.; Shahnaz, T.; Subbiah, S.; Narayanasamy, S. Hexavalent chromium adsorption on virgin, biochar, and chemically modifed carbons prepared from Phanera vahlii fruit biomass: Equilibrium, kinetics, and thermodynamics approach. Environ. Sci. Pollut. Res. 2019, 26, 32137–32150. [Google Scholar] [CrossRef] [PubMed]
- Gupta, S.; Jain, A.K. Biosorption of Ni (II) from aqueous solutions and real industrial wastewater using modifed A. barbadensis Miller leaves residue powder in a lab scale continuous fxed bed column. Clean. Eng. Technol. 2021, 2021, 100349. [Google Scholar] [CrossRef]
- Han, X.; Zhang, Y.; Zheng, C.; Yu, X.; Li, S.; Wei, W. Enhanced Cr (VI) removal from water using a green synthesized nanocrystalline chlorapatite: Physicochemical interpretations and fxed-bed column mathematical model study. Chemosphere 2021, 264, 128421. [Google Scholar] [CrossRef]
- Ghasemabadi, S.M.; Baghdadi, M.; Safari, E.; Ghazban, F. Investigation of continuous adsorption of Pb (II), As (III), Cd (II), and Cr (VI) using a mixture of magnetic graphite oxide and sand as a medium in a fxed-bed column. J. Environ. Chem. Eng. 2018, 6, 4840–4849. [Google Scholar] [CrossRef]
- Martín-Lara, M.A.; Blázquez, G.; Calero, M.; Almendros, A.I.; Ronda, A. Binary biosorption of copper and lead onto pine cone shell in batch reactors and in fxed bed columns. Int. J. Miner. Process. 2016, 148, 72–82. [Google Scholar] [CrossRef]
- Abdolali, A.; Ngo, H.H.; Guo, W.; Zhou, J.L.; Zhang, J.; Liang, S.; Chang, S.W.; Nguyen, D.D.; Liu, Y. Application of a breakthrough biosorbent for removing heavy metals from synthetic and real wastewaters in a lab-scale continuous fixed-bed column. Bioresour. Technol. 2017, 229, 78–87. [Google Scholar] [CrossRef]
- Xiang, T.; Zhang, Z.; Liu, H.; Yin, Z.; Li, L.; Liu, X. Characterization of cellulose-based electrospun nanofiber membrane and its adsorptive behaviours using Cu (II), Cd (II), Pb (II) as models. Sci. China Chem. 2013, 56, 567–575. [Google Scholar] [CrossRef]
- Wang, Q.; Zuo, W.; Tian, Y.; Kong, L.; Cai, G.; Zhang, H.; Li, L.; Zhang, J. An ultralight and flexible nanofibrillated cellulose/chitosan aerogel for efficient chromium removal: Adsorption-reduction process and mechanism. Chemosphere 2023, 329, 138622. [Google Scholar] [CrossRef] [PubMed]
- Abu-Danso, E.; Peräniemi, S.; Leiviskä, T.; Kim, T.; Tripathi, K.M.; Bhatnagar, A. Synthesis of clay-cellulose biocomposite for the removal of toxic metal ions from aqueous medium. J. Hazard. Mater. 2020, 381, 120871. [Google Scholar] [CrossRef] [PubMed]
- Kardam, A.; Raj, K.R.; Srivastava, S.; Srivastava, M.M. Nanocellulose fibers for biosorption of cadmium, nickel, and lead ions from aqueous solution. Clean Technol. Environ. Policy 2014, 16, 385–393. [Google Scholar] [CrossRef]
- Islam, M.S.; Rahaman, M.S.; Barbeau, B. Single and Multi Component Removal of Pb+2, Zn+2, Cu+2, and As+3 Ions from Aqueous Solution Using Kraft Pulp-Based Carboxymethylated Cellulose by Fixed-Bed Column Adsorption Process. SSRN Electron. J. 2023. [Google Scholar] [CrossRef]
- Amiri, M.; Keshtkar, A.R.; Moosavian, M.A. Th (IV) biosorption from a three-component feed solution by Ca-pretreated Cystoseria indica alga in a fixed-bed column: Experimental tests and modeling. J. Environ. Chem. Eng. 2022, 10, 108579. [Google Scholar] [CrossRef]
- Jiang, X.; An, Q.D.; Xiao, Z.Y.; Zhai, S.R.; Shi, Z. Versatile core/shell-like alginate@ polyethylenimine composites for efficient removal of multiple heavy metal ions (Pb2+, Cu2+, CrO42−): Batch and fixed-bed studies. Mater. Res. Bull. 2019, 118, 110526. [Google Scholar] [CrossRef]
- Jain, M.; Garg, V.K.; Kadirvelu, K. Cadmium (II) sorption and desorption in a fixed bed column using sunflower waste carbon calcium–alginate beads. Bioresour. Technol. 2013, 129, 242–248. [Google Scholar] [CrossRef]
- Chao, H.P.; Chang, C.C.; Nieva, A. Biosorption of heavy metals on Citrus maxima peel, passion fruit shell, and sugarcane bagasse in a fixed-bed column. J. Ind. Eng. Chem. 2014, 20, 3408–3414. [Google Scholar] [CrossRef]
- Peng, J.; Yuan, H.; Ren, T.; Liu, Z.; Qiao, J.; Ma, Q.; Wu, Y. Fluorescent nanocellulose-based hydrogel incorporating titanate nanofibers for sorption and detection of Cr (VI). Int. J. Biol. Macromol. 2022, 215, 625–634. [Google Scholar] [CrossRef]
- Chatterjee, A.; Abraham, J. Desorption of heavy metals from metal loaded sorbents and e-wastes: A review. Biotechnol. Lett. 2019, 41, 319–333. [Google Scholar] [CrossRef]
- Guo, Z.; Hou, H.; Zhou, J.; Wu, X.; Li, Y.; Hu, L. Fabrication of novel 3D PEI-functionalized ZIF-8@ alginate aerogel composites for efficient elimination of Pb (II) and Cd (II) from aqueous solution. J. Environ. Chem. Eng. 2023, 11, 110446. [Google Scholar] [CrossRef]
- Júnior, W.N.; Silva MG, C.; Vieira, M.G.A. Competitive fixed-bed biosorption of Ag (I) and Cu (II) ions on Sargassum filipendula seaweed waste. J. Water Process Eng. 2020, 36, 101294. [Google Scholar] [CrossRef]
- Sarkar, S.; Bar, N.; Das, S.K. Cr (VI) and Cu (II) removal from aqueous solution in fixed bed column using rice bran; experimental, statistical and GA modelling. J. Indian Chem. Soc. 2021, 98, 100216. [Google Scholar] [CrossRef]
- Asif, U.A.; Mahmood, K.; Naqvi, S.R.; Mehran, M.T.; Noor, T. Development of high-capacity surface-engineered MXene composite for heavy metal Cr (VI) removal from industrial wastewater. Chemosphere 2023, 326, 138448. [Google Scholar] [CrossRef]
- Aslam MM, A.; Den, W.; Kuo, H.W. Removal of hexavalent chromium by encapsulated chitosan-modified magnetic carbon nanotubes: Fixed-bed column study and modelling. J. Water Process Eng. 2021, 42, 102143. [Google Scholar] [CrossRef]
- Sayago UF, C.; Castro, Y.P. Development of a composite material between bacterial cellulose and E. crassipes, for the treatment of water contaminated by chromium (VI). Int. J. Environ. Sci. Technol. 2022, 19, 6285–6298. [Google Scholar] [CrossRef]
- Ammar, N.S.; Elhaes, H.; Ibrahim, H.S.; Ibrahim, M.A. A novel structure for removal of pollutants from wastewater. Spectrochim. Acta Part A Mol. Biomol. Spectrosc. 2014, 121, 216–223. [Google Scholar] [CrossRef] [PubMed]
- Deng, L.; Geng, M.; Zhu, D.; Zhou, W.; Langdon, A.; Wu, H.; Yu, Y.; Zhu, Z.; Wang, Y. Effect of chemical and biological degumming on the adsorption of heavy metal by cellulose xanthogenates prepared from Eichhornia crassipes. Bioresour. Technol. 2012, 107, 41–45. [Google Scholar] [CrossRef] [PubMed]
- El-Zawahry, M.M.; Abdelghaffar, F.; Abdelghaffar, R.A.; Hassabo, A.G. Equilibrium and kinetic models on the adsorption of reactive black 5 from aqueous solution using Eichhornia crassipes/chitosan composite. Carbohydr. Polym. 2016, 136, 507–515. [Google Scholar] [CrossRef]
- Jian, S.; Cheng, Y.; Ma, X.; Guo, H.; Hu, J.; Zhang, K.; Jiang, S.; Yang, W.; Duan, G. Excellent fluoride removal performance by electrospun La–Mn bimetal oxide nanofibers. New J. Chem. 2022, 46, 490–497. [Google Scholar] [CrossRef]
- Feng, W.; Xiao, K.; Zhou, W.; Zhu, D.; Zhou, Y.; Yuan, Y.; Xiao, N.; Wan, X.; Hua, Y.; Zhao, J. Analysis of utilization technologies for Eichhornia crassipes biomass harvested after restoration of wastewater. Bioresour. Technol. 2017, 223, 287–295. [Google Scholar] [CrossRef] [PubMed]
- Lin, S.; Wang, G.; Na, Z.; Lu, D.; Liu, Z. Long-root Eichhornia crassipes as a biodegradable adsorbent for aqueous as (III) and as (V). Chem. Eng. J. 2012, 183, 365–371. [Google Scholar] [CrossRef]
- Liu, L.; Hu, S.; Shen, G.; Farooq, U.; Zhang, W.; Lin, S.; Lin, K. Adsorption dynamics and mechanism of aqueous sulfachloropyridazine and analogues using the root powder of recyclable long-root Eichhornia crassipes. Chemosphere 2018, 196, 409–417. [Google Scholar] [CrossRef] [PubMed]
- Man, Q.; An, Y.; Shen, H.; Wei, C.; Zhang, X.; Wang, Z.; Feng, J. MXenes and Their Derivatives for Advanced Solid-State Energy Storage Devices. Adv. Funct. Mater. 2023, 2303668. [Google Scholar] [CrossRef]
- Logar, I.; Brouwer, R.; Paillex, A. Do the societal benefits of river restoration outweigh their costs? A cost-benefit analysis. J. Environ. Manag. 2019, 232, 1075–1085. [Google Scholar] [CrossRef]
- Brown, M.; Snelling, E.; De Alba, M.; Ebrahimi, G.; Forster, B.B. Quantitative Assessment of Computed Tomography Energy Use and Cost Savings Through Overnight and Weekend Power Down in a Radiology Department. Can. Assoc. Radiol. J. 2023, 74, 298–304. [Google Scholar] [CrossRef]








| Element | Weight | % |
|---|---|---|
| Carbon | 53.86 | 60.96 |
| Oxygen | 45.62 | 38.76 |
| Silicon | 0.14 | 0.07 |
| Element | Weight | % |
|---|---|---|
| Carbon | 52.2.86 | 56.96 |
| Oxygen | 45.62 | 33.76 |
| Heavy metals | 12.1 | 10.3 |
| Reference | Biomass | Contaminate Treated | Recycling | Capacity (mg/g) | Capacity (mg/g) with the Equation (1) |
|---|---|---|---|---|---|
| [20] | E. crassipes + Fe | Cr (VI) | EDTA | 16 | 46 |
| [20] | E. crassipes | Cr (VI) | EDTA | 11 | 29 |
| [75] | Phanera vahlii | Cr (VI) | NaOH | 30 | 62 |
| [76] | A. barbadensis Miller | Ni (II) | HCl | 14 | 20 |
| [77] | Green synthesized nanocrystalline chlorapatite | Cr (VI) | NaOH | 20 | 35 |
| [78] | Graphite | Cr (VI) | HNO3 | 20 | 52 |
| [79] | Pine cone shell | Pb (II) | HCl | 22 | 30 |
| [18] | Xantate of cellulose | Cr (VI) | EDTA | 16 | 51 |
| [18] | Cellulose alkaline | Cr (VI) | EDTA | 11 | 32 |
| [80] | Biochar | Cd (II) | HNO3 | 15 | 40 |
| [80] | Biomass | Cd (II) | HCl | 11 | 31 |
| [81] | Carboxymethylated Cellulose | Pb (II) | NaOH | 20 | 48 |
| [82] | nanofibrillated cellulose/chitosan aerogel | Cr (VI) | EDTA | 50 | 87 |
| [83] | Clay–cellulose biocomposite | Cd (II) | NaOH | 20 | 115 |
| [84] | Cellulose aerogels | Cu (II) | EDTA | 40 | 300 |
| Reference | Biomass | Contaminate Treated | Constant Kf | Volumes of Water |
|---|---|---|---|---|
| [9] | E. crassipes | Cr (VI) | 0.20 | 3.5 |
| [86] | Cystoseria + Ca + Fe | Th (IV) | 0.35 | 4.2 |
| [87] | Cellulose quelant | Ni (II) | 0.77 | 8.2 |
| [88] | Alginate | Pb (II) | 0.66 | 7.2 |
| [89] | Citrus maxima peel | Cr (VI) | 0.55 | 5.3 |
| [20] | E. crassipes + Fe | Cr (VI) | 0.99 | 11 |
| [18] | Xantate of cellulose | Cr (VI) | 0.79 | 10 |
| [90] | Sodium TiO2 nanofibers | Pb (II) | 0.99 | 10 |
| [91] | Rice bran | Cr (VI) | 0.76 | 7.3 |
| [92] | MXene and chitosan | Cr (VI) | 0.66 | 5.9 |
| [89] | Chitosan-modified magnetic carbon | Cr (VI) | 0.3 | 3.8 |
| [85] | Carboxymethylated Cellulose | Pb (II) | 1 | 14 |
| [82] | Cellulose/chitosan aerogel | Cr (VI) | 0.9 | 11 |
| [83] | Cellulose aerogels | Cu (II) | 1.2 | 12 |
| Reference | Biomass | Contaminate Treated | Constant ks (s) | Isotherm |
|---|---|---|---|---|
| [9] | E.crassipes | Cr (VI) | 0.0198 | Lagmuir |
| [86] | Cystoseria + Ca | Th (IV) | 0.025 | Temkin |
| [87] | Cellulose quelant | Ni (II) | 0.077 | Freundlinch |
| Langmuir | ||||
| [88] | Alginate | Pb (II) | 0.033 | Langmuir |
| [89] | Citrus maxima peel | Cr (VI) | 0.22 | Freundlinch |
| [20] | E. crassipes + Fe | Cr (VI) | 0.045 | Langmuir |
| [18] | Xantate of cellose | Cr (VI) | 0.04 | Freundlinch |
| [90] | Sodium titanate nanofibers | Pb (II) | 0.04 | Temkin |
| [94] | Rice bran | Cr (VI) | 0.035 | Langmuir |
| [95] | MXene and chitosan | Cr (VI) | 0.02 | Freunlinhchd |
| [96] | Chitosan-modified magnetic carbon | Cr (VI) | 0.03 | Langmuir |
| [85] | Carboxymethylate Cellulose | Pb (II) | 0.08 | Langmuir |
| [82] | Cellulose/chitosan aerogel | Cr (VI) | 0.075 | Langmuir |
| [84] | Cellulose aerogels | Cu (II) | 0.08 | Langmuir |
| Reference | Biomass | Recycling | Cost (USD) 1 kg Material | Capacity (mg/g) | g HM+/(USD) |
|---|---|---|---|---|---|
| [20] | E. crassipes + Fe | EDTA | 4 | 46 | 11.5 |
| [20] | E. crassipes | EDTA | 3 | 29 | 9.6 |
| [75] | Phanera vahlii | NaOH | 6 | 62 | 10 |
| [79] | Pine cone shell | HCl | 4 | 30 | 7.5 |
| [18] | Xantate of cellulose | EDTA | 6 | 51 | 9 |
| [18] | Cellulose alkaline | EDTA | 4 | 32 | 8 |
| [80] | Biochar | HNO3 | 8 | 40 | 5 |
| [82] | Cellulose/chitosan aerogel | EDTA | 10 | 87 | 8.7 |
| [83] | Clay-cellulose biocomposite | NaOH | 20 | 115 | 12.7 |
| [84] | Cellulose aerogels | EDTA | 25 | 300 | 15 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sayago, U.F.C.; Ballesteros Ballesteros, V. Recent Advances in the Treatment of Industrial Wastewater from Different Celluloses in Continuous Systems. Polymers 2023, 15, 3996. https://doi.org/10.3390/polym15193996
Sayago UFC, Ballesteros Ballesteros V. Recent Advances in the Treatment of Industrial Wastewater from Different Celluloses in Continuous Systems. Polymers. 2023; 15(19):3996. https://doi.org/10.3390/polym15193996
Chicago/Turabian StyleSayago, Uriel Fernando Carreño, and Vladimir Ballesteros Ballesteros. 2023. "Recent Advances in the Treatment of Industrial Wastewater from Different Celluloses in Continuous Systems" Polymers 15, no. 19: 3996. https://doi.org/10.3390/polym15193996
APA StyleSayago, U. F. C., & Ballesteros Ballesteros, V. (2023). Recent Advances in the Treatment of Industrial Wastewater from Different Celluloses in Continuous Systems. Polymers, 15(19), 3996. https://doi.org/10.3390/polym15193996










