The Potential of Collagen Treatment for Comorbid Diseases
Abstract
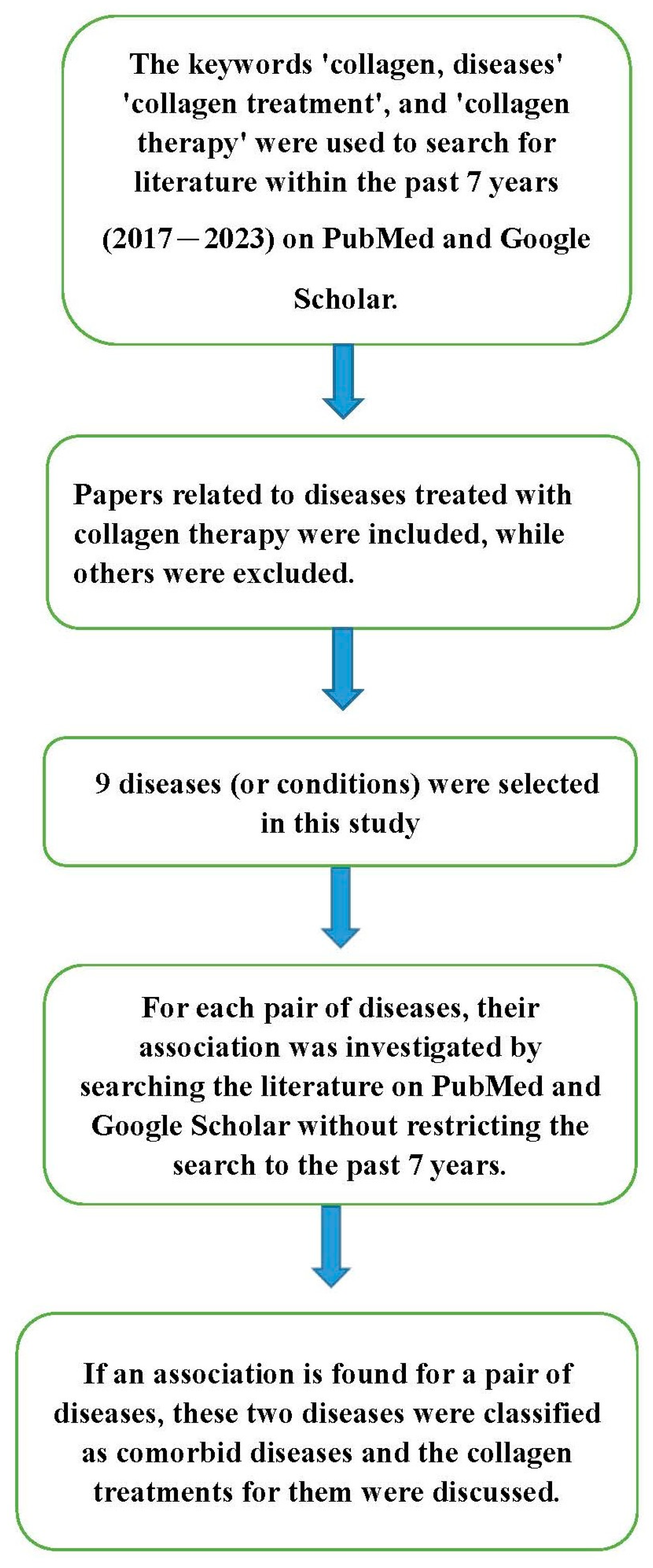
:1. Introduction
2. Types and Sources of Collagen
3. Collagen Treatment
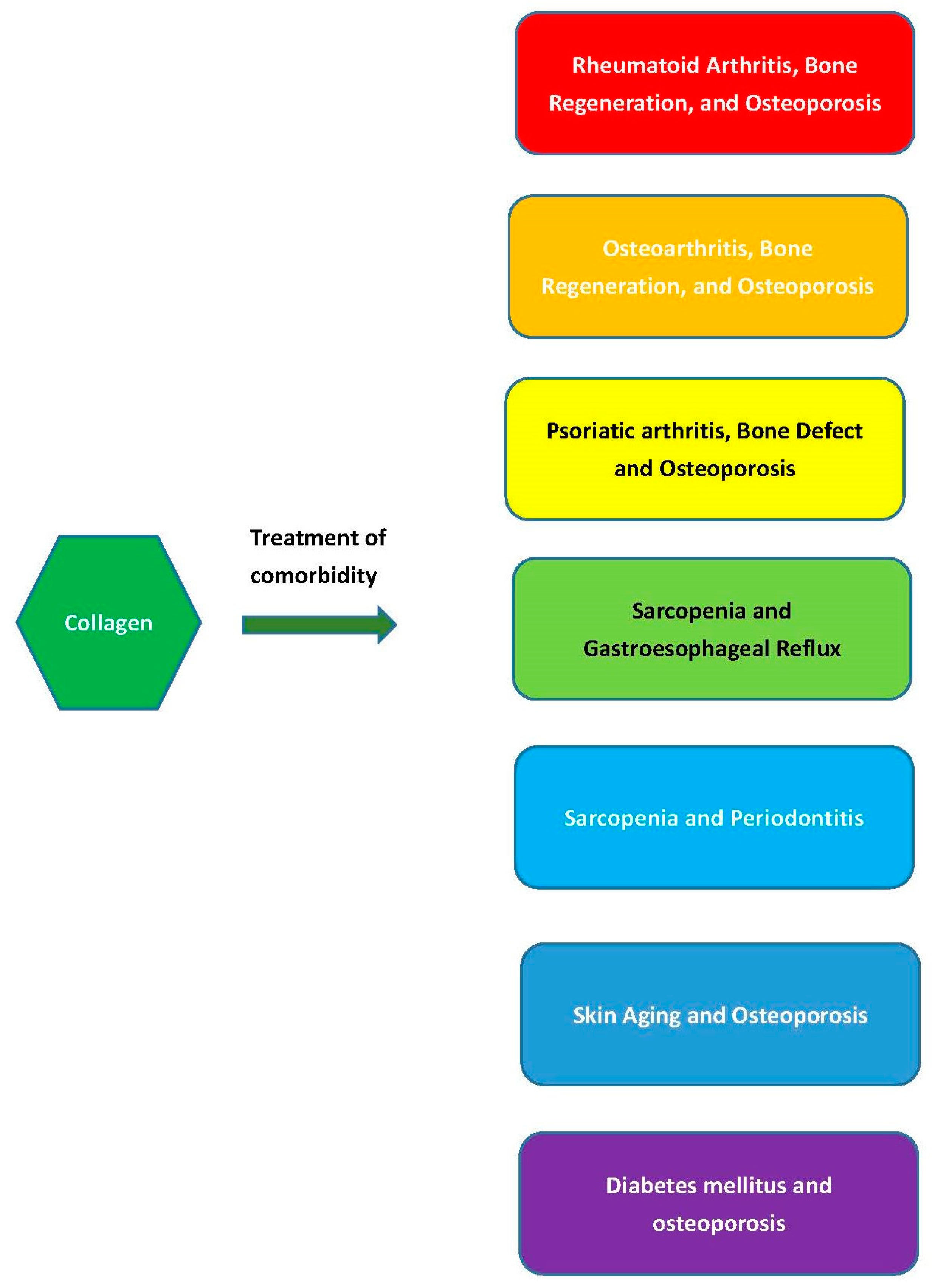
4. Comorbid Diseases’ Treatment
4.1. Rheumatoid Arthritis, Bone Regeneration, and Osteoporosis
4.2. Osteoarthritis, Bone Regeneration, and Osteoporosis
4.3. Psoriatic Arthritis, Bone Defects, and Osteoporosis
4.4. Sarcopenia and Gastroesophageal Reflux
4.5. Sarcopenia and Periodontitis
4.6. Skin Aging and Osteoporosis
4.7. Diabetes mellitus and osteoporosis
5. Discussion
6. Conclusions
Funding
Institutional Review Board Statement
Data Availability Statement
Conflicts of Interest
References
- Rodríguez, M.I.A.; Barroso, L.G.R.; Sánchez, M.L. Collagen: A review on its sources and potential cosmetic applications. J. Cosmet. Dermatol. 2017, 17, 20–26. [Google Scholar] [CrossRef] [PubMed]
- Chang, S.-W.; Shefelbine, S.J.; Buehler, M.J. Structural and Mechanical Differences between Collagen Homo- and Heterotrimers: Relevance for the Molecular Origin of Brittle Bone Disease. Biophys. J. 2012, 102, 640–648. [Google Scholar] [CrossRef] [PubMed]
- Walma, D.A.C.; Yamada, K.M. The extracellular matrix in development. Development 2020, 147, dev175596. [Google Scholar] [CrossRef] [PubMed]
- Shoulders, M.D.; Raines, R.T. Collagen structure and stability. Annu. Rev. Biochem. 2009, 78, 929–958. [Google Scholar] [CrossRef] [PubMed]
- Kirkness, M.W.; Lehmann, K.; Forde, N.R. Mechanics and structural stability of the collagen triple helix. Curr. Opin. Chem. Biol. 2019, 53, 98–105. [Google Scholar] [CrossRef]
- Wang, H. A Review of the Effects of Collagen Treatment in Clinical Studies. Polymers 2021, 13, 3868. [Google Scholar] [CrossRef]
- Sionkowska, A.; Skrzyński, S.; Śmiechowski, K.; Kołodziejczak, A. The review of versatile application of collagen. Polym. Adv. Technol. 2016, 28, 4–9. [Google Scholar] [CrossRef]
- Ghomi, E.R.; Nourbakhsh, N.; Akbari Kenari, M.; Zare, M.; Ramakrishna, S. Collagen-based biomaterials for biomedical applications. J. Biomed. Mater. Res. Part B-Appl. Biomater. 2021, 109, 1986–1999. [Google Scholar] [CrossRef]
- Reilly, D.M.; Lozano, J. Skin collagen through the lifestages: Importance for skin health and beauty. Plast. Aesthetic Res. 2021, 8. [Google Scholar] [CrossRef]
- Ioan, D.-C.; Rău, I.; Tihan, G.T.; Zgârian, R.G.; Ghica, M.V.; Kaya, M.G.A.; Dinu-Pîrvu, E.C. Piroxicam-Collagen-Based Sponges for Medical Applications. Int. J. Polym. Sci. 2019, 2019, 6062381. [Google Scholar] [CrossRef]
- Vu, Q.M.; Nguyen, T.C.; Dam, D.M.N.; Le, T.L.; Hoang, T.D.; Tran, T.K.N.; Nguyen, T.A.; Nguyen, P.H.; Thai, H. A Novel Method for Preparation of Carrageenan/Fish Scale Collagen/Allopurinol Biocomposite Film. Int. J. Polym. Sci. 2021, 2021, 9960233. [Google Scholar] [CrossRef]
- Xu, S.; Xu, H.; Wang, W.; Li, S.; Li, H.; Li, T.; Zhang, W.; Yu, X.; Liu, L. The role of collagen in cancer: From bench to bedside. J. Transl. Med. 2019, 17, 309. [Google Scholar] [CrossRef]
- Whatcott, C.J.; Diep, C.H.; Jiang, P.; Watanabe, A.; LoBello, J.; Sima, C.; Hostetter, G.; Shepard, H.M.; Von Hoff, D.D.; Han, H. Desmoplasia in Primary Tumors and Metastatic Lesions of Pancreatic Cancer. Clin. Cancer Res. 2015, 21, 3561–3568. [Google Scholar] [CrossRef]
- Naomi, R.; Ridzuan, P.M.; Bahari, H. Current Insights into Collagen Type I. Polymers 2021, 13, 2642. [Google Scholar] [CrossRef] [PubMed]
- Månsson, B.; Wenglén, C.; Mörgelin, M.; Saxne, T.; Heinegård, D. Association of Chondroadherin with Collagen Type II. J. Biol. Chem. 2001, 276, 32883–32888. [Google Scholar] [CrossRef]
- Lian, C.; Wang, X.; Qiu, X.; Wu, Z.; Gao, B.; Liu, L.; Liang, G.; Zhou, H.; Yang, X.; Peng, Y.; et al. Collagen type II suppresses articular chondrocyte hypertrophy and osteoarthritis progression by promoting integrin beta 1-SMAD1 interaction. Bone Res. 2019, 7, 8. [Google Scholar] [CrossRef]
- Wu, Z.; Korntner, S.; Mullen, A.; Zeugolis, D. Collagen type II: From biosynthesis to advanced biomaterials for cartilage engineering. Biomater. Biosyst. 2021, 4, 100030. [Google Scholar] [CrossRef] [PubMed]
- Gong, R.; Xia, Z. Collagen changes in pelvic support tissues in women with pelvic organ prolapse. Eur. J. Obstet. Gynecol. Reprod. Biol. 2019, 234, 185–189. [Google Scholar] [CrossRef]
- Sand, J.; Genovese, F.; Karsdal, M. Type IV collagen. In Biochemistry of Collagens, Laminins and Elastin; Elsevier: Amsterdam, The Netherlands, 2016; pp. 31–41. [Google Scholar]
- Xu, J.; Luo, X.; Zhang, Y.; Gao, J.; Huang, C.-C.; Bai, X.; Zhang, G. Extraction and characterization of bovine collagen Type V and its effects on cell behaviors. Regen. Biomater. 2022, 9, rbac028. [Google Scholar] [CrossRef]
- Ferraro, V.; Gaillard-Martinie, B.; Sayd, T.; Chambon, C.; Anton, M.; Santé-Lhoutellier, V. Collagen type I from bovine bone. Effect of animal age, bone anatomy and drying methodology on extraction yield, self-assembly, thermal behaviour and electrokinetic potential. Int. J. Biol. Macromol. 2016, 97, 55–66. [Google Scholar] [CrossRef] [PubMed]
- Nocera, A.D.; Comín, R.; Salvatierra, N.A.; Cid, M.P. Development of 3D printed fibrillar collagen scaffold for tissue engineering. Biomed. Microdevices 2018, 20, 26. [Google Scholar] [CrossRef] [PubMed]
- Fauzi, M.B.; Lokanathan, Y.; Aminuddin, B.S.; Ruszymah, B.H.I.; Chowdhury, S.R. Ovine tendon collagen: Extraction, characterisation and fabrication of thin films for tissue engineering applications. Mater. Sci. Eng. C 2016, 68, 163–171. [Google Scholar] [CrossRef] [PubMed]
- Coppola, D.; Oliviero, M.; Vitale, G.A.; Lauritano, C.; D’ambra, I.; Iannace, S.; de Pascale, D. Marine Collagen from Alternative and Sustainable Sources: Extraction, Processing and Applications. Mar. Drugs 2020, 18, 214. [Google Scholar] [CrossRef]
- Maher, M.; Glattauer, V.; Onofrillo, C.; Duchi, S.; Yue, Z.; Hughes, T.C.; Ramshaw, J.A.M.; Wallace, G.G. Suitability of Marine- and Porcine-Derived Collagen Type I Hydrogels for Bioprinting and Tissue Engineering Scaffolds. Mar. Drugs 2022, 20, 366. [Google Scholar] [CrossRef] [PubMed]
- Felician, F.F.; Xia, C.; Qi, W.; Xu, H. Collagen from Marine Biological Sources and Medical Applications. Chem. Biodivers. 2018, 15, e1700557. [Google Scholar] [CrossRef]
- Davison-Kotler, E.; Marshall, W.S.; García-Gareta, E. Sources of Collagen for Biomaterials in Skin Wound Healing. Bioengineering 2019, 6, 56. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.-P.; Liang, C.-H.; Wu, H.-T.; Pang, H.-Y.; Chen, C.; Wang, G.-H.; Chan, L.-P. Antioxidant and anti-inflammatory capacities of collagen peptides from milkfish (Chanos chanos) scales. J. Food Sci. Technol. 2018, 55, 2310–2317. [Google Scholar] [CrossRef]
- Mathew-Steiner, S.S.; Roy, S.; Sen, C.K. Collagen in Wound Healing. Bioengineering-Basel 2021, 8, 63. [Google Scholar] [CrossRef]
- Atiyeh, B.S.; Ghanem, O.A.; Chahine, F. Microneedling: Percutaneous Collagen Induction (PCI) Therapy for Management of Scars and Photoaged Skin—Scientific Evidence and Review of the Literature. Aesthetic Plast. Surg. 2020, 45, 296–308. [Google Scholar] [CrossRef]
- Basyoni, R.R.H.; Hassan, A.M.; Mohammed, D.A.; Radwan, N.K.; Hassan, G.F.R. Facial rejuvenation by microneedling with irradiated amniotic collagen matrix compared to platelet rich plasma. Dermatol. Ther. 2022, 35, e15739. [Google Scholar] [CrossRef] [PubMed]
- Bohn, G.; Liden, B.; Schultz, G.; Yang, Q.; Gibson, D.J. Ovine-Based Collagen Matrix Dressing: Next-Generation Collagen Dressing for Wound Care. Adv. Wound Care 2016, 5, 1–10. [Google Scholar] [CrossRef]
- Campos, L.D.; Pereira, A.T.S.d.A.; Cazarin, C.B.B. The collagen market and knowledge, attitudes, and practices of Brazilian consumers regarding collagen ingestion. Food Res. Int. 2023, 170, 112951. [Google Scholar] [CrossRef] [PubMed]
- Oesser, S. The oral intake of specific Bioactive Collagen Peptides has a positive effect on hair thickness. Int. J. Nutraceuticals Funct. Foods Nov. Foods 2020, 1, 134–138. [Google Scholar] [CrossRef]
- Lugo, J.P.; Saiyed, Z.M.; Lau, F.C.; Molina, J.P.L.; Pakdaman, M.N.; Shamie, A.N.; Udani, J.K. Undenatured type II collagen (UC-II(R)) for joint support: A randomized, double-blind, placebo-controlled study in healthy volunteers. J. Int. Soc. Sports Nutr. 2013, 10, 48. [Google Scholar] [CrossRef] [PubMed]
- Seong, H.; Kim, R.K.; Shin, Y.; Lee, H.W.; Koh, J.C. Application of purified porcine collagen in patients with chronic refractory musculoskeletal pain. Korean J. Pain 2020, 33, 395–399. [Google Scholar] [CrossRef]
- Yang, L.; Wu, H.; Lu, L.; He, Q.; Xi, B.; Yu, H.; Luo, R.; Wang, Y.; Zhang, X. A tailored extracellular matrix (ECM)—Mimetic coating for cardiovascular stents by stepwise assembly of hyaluronic acid and recombinant human type III collagen. Biomaterials 2021, 276, 121055. [Google Scholar] [CrossRef] [PubMed]
- McLaughlin, S.; McNeill, B.; Podrebarac, J.; Hosoyama, K.; Sedlakova, V.; Cron, G.; Smyth, D.; Seymour, R.; Goel, K.; Liang, W.; et al. Injectable human recombinant collagen matrices limit adverse remodeling and improve cardiac function after myocardial infarction. Nat. Commun. 2019, 10, 4866. [Google Scholar] [CrossRef]
- Ruiz-Medrano, E.; Espinosa-Ortega, H.F.; Arce-Salinas, C.A. The effect of concomitant hand osteoarthritis on pain and disease activity in patients with rheumatoid arthritis. Clin. Rheumatol. 2019, 38, 2709–2716. [Google Scholar] [CrossRef]
- Sirufo, M.M.; De Pietro, F.; Bassino, E.M.; Ginaldi, L.; De Martinis, M. Osteoporosis in Skin Diseases. Int. J. Mol. Sci. 2020, 21, 4749. [Google Scholar] [CrossRef]
- Finckh, A.; Gilbert, B.; Hodkinson, B.; Bae, S.-C.; Thomas, R.; Deane, K.D.; Alpizar-Rodriguez, D.; Lauper, K. Global epidemiology of rheumatoid arthritis. Nat. Rev. Rheumatol. 2022, 18, 591–602. [Google Scholar] [CrossRef] [PubMed]
- Batsalova, T.; Dzhambazov, B. Significance of Type II Collagen Posttranslational Modifications: From Autoantigenesis to Improved Diagnosis and Treatment of Rheumatoid Arthritis. Int. J. Mol. Sci. 2023, 24, 9884. [Google Scholar] [CrossRef] [PubMed]
- Heng, B.C.; Bai, Y.; Li, X.; Lim, L.W.; Li, W.; Ge, Z.; Zhang, X.; Deng, X. Electroactive Biomaterials for Facilitating Bone Defect Repair under Pathological Conditions. Adv. Sci. 2022, 10, 2204502. [Google Scholar] [CrossRef] [PubMed]
- Pinheiro, M.B.; Oliveira, J.; Bauman, A.; Fairhall, N.; Kwok, W.; Sherrington, C. Evidence on physical activity and osteoporosis prevention for people aged 65+ years: A systematic review to inform the WHO guidelines on physical activity and sedentary behaviour. Int. J. Behav. Nutr. Phys. Act. 2020, 17, 150. [Google Scholar] [CrossRef]
- Tanaka, Y. Managing Osteoporosis and Joint Damage in Patients with Rheumatoid Arthritis: An Overview. J. Clin. Med. 2021, 10, 1241. [Google Scholar] [CrossRef]
- Cheng, C.-H.; Chen, L.-R.; Chen, K.-H. Osteoporosis Due to Hormone Imbalance: An Overview of the Effects of Estrogen Deficiency and Glucocorticoid Overuse on Bone Turnover. Int. J. Mol. Sci. 2022, 23, 1376. [Google Scholar] [CrossRef] [PubMed]
- Ju, J.-H.; Cho, M.-L.; Jhun, J.-Y.; Park, M.-J.; Oh, H.-J.; Min, S.-Y.; Cho, Y.-G.; Hwang, S.-Y.; Kwok, S.-K.; Seo, S.-H.; et al. Oral administration of type-II collagen suppresses IL-17-associated RANKL expression of CD4+ T cells in collagen-induced arthritis. Immunol. Lett. 2008, 117, 16–25. [Google Scholar] [CrossRef]
- Geahchan, S.; Baharlouei, P.; Rahman, A. Marine Collagen: A Promising Biomaterial for Wound Healing, Skin Anti-Aging, and Bone Regeneration. Mar. Drugs 2022, 20, 61. [Google Scholar] [CrossRef]
- König, D.; Oesser, S.; Scharla, S.; Zdzieblik, D.; Gollhofer, A. Specific Collagen Peptides Improve Bone Mineral Density and Bone Markers in Postmenopausal Women—A Randomized Controlled Study. Nutrients 2018, 10, 97. [Google Scholar] [CrossRef]
- Wauquier, F.; Daneault, A.; Granel, H.; Prawitt, J.; Soulé, V.F.; Berger, J.; Pereira, B.; Guicheux, J.; Rochefort, G.Y.; Meunier, N.; et al. Human Enriched Serum Following Hydrolysed Collagen Absorption Modulates Bone Cell Activity: From Bedside to Bench and Vice Versa. Nutrients 2019, 11, 1249. [Google Scholar] [CrossRef]
- Rabiei, M.; Kashanian, S.; Samavati, S.S.; Derakhshankhah, H.; Jamasb, S.; McInnes, S.J. Nanotechnology application in drug delivery to osteoarthritis (OA), rheumatoid arthritis (RA), and osteoporosis (OSP). J. Drug Deliv. Sci. Technol. 2020, 61, 102011. [Google Scholar] [CrossRef]
- García-Coronado, J.M.; Martínez-Olvera, L.; Elizondo-Omaña, R.E.; Acosta-Olivo, C.A.; Vilchez-Cavazos, F.; Simental-Mendía, L.E.; Simental-Mendía, M. Effect of collagen supplementation on osteoarthritis symptoms: A meta-analysis of randomized placebo-controlled trials. Int. Orthop. 2019, 43, 531–538. [Google Scholar] [CrossRef]
- Volpi, P.; Zini, R.; Erschbaumer, F.; Beggio, M.; Busilacchi, A.; Carimati, G. Effectiveness of a novel hydrolyzed collagen formulation in treating patients with symptomatic knee osteoarthritis: A multicentric retrospective clinical study. Int. Orthop. 2020, 45, 375–380. [Google Scholar] [CrossRef] [PubMed]
- Woo, T.; Lau, L.; Cheng, N.; Chan, P.; Tan, K.; Gardner, A. Efficacy of oral collagen in joint pain-osteoarthritis and rheumatoid arthritis. J. Arthritis. 2017, 6, 2. [Google Scholar]
- Oliviero, F.; Ramonda, R.; Hoxha, A.; Scanu, A.; Galozzi, P.; Favero, M.; Frallonardo, P.; Punzi, L. Effect of an oral preparation containing hyaluronic acid, chondroitin sulfate, hydrolyzed collagen type II and hydrolyzed keratin on synovial fluid features and clinical indices in knee osteoarthritis. A pilot study. Reumatismo 2020, 72, 125–130. [Google Scholar] [CrossRef]
- Jabbari, M.; Barati, M.; Khodaei, M.; Babashahi, M.; Kalhori, A.; Tahmassian, A.H.; Mosharkesh, E.; Arzhang, P.; Eini-Zinab, H. Is collagen supplementation friend or foe in rheumatoid arthritis and osteoarthritis? A comprehensive systematic review. Int. J. Rheum. Dis. 2022, 25, 973–981. [Google Scholar] [CrossRef]
- Nielsen, S.H.; Sardar, S.; Siebuhr, A.S.; Schlemmer, A.; Schmidt, E.B.; Bay-Jensen, A.-C.; Karsdal, M.A.; Christensen, J.H.; Kristensen, S. Effect of n-3 PUFA on extracellular matrix protein turnover in patients with psoriatic arthritis: A randomized, double-blind, placebo-controlled trial. Rheumatol. Int. 2021, 41, 1065–1077. [Google Scholar] [CrossRef] [PubMed]
- Łuczaj, W.; Wroński, A.; Domingues, P.; Rosário Domingues, M.; Skrzydlewska, E. Lipidomic Analysis Reveals Specific Differences between Fibroblast and Keratinocyte Ceramide Profile of Patients with Psoriasis Vulgaris Molecules. Clin. Immunol. 2020, 214, 108397. [Google Scholar] [CrossRef]
- Ragab, G.; Elshahaly, M.; Bardin, T. Gout: An old disease in new perspective—A review. J. Adv. Res. 2017, 8, 495–511. [Google Scholar] [CrossRef]
- Lam, G.; Ross, F.L.; Chiu, E.S. Nonhealing Ulcers in Patients with Tophaceous Gout: A Systematic Review. Adv. Ski. Wound Care 2017, 30, 230–237. [Google Scholar] [CrossRef]
- Nguyen, C.T.; Vu, M.Q.; Phan, T.T.; Vu, T.Q.; Vo, Q.A.; Bach, G.L.; Thai, H. Novel pH-Sensitive Hydrogel Beads Based on Carrageenan and Fish Scale Collagen for Allopurinol Drug Delivery. J. Polym. Environ. 2020, 28, 1795–1810. [Google Scholar] [CrossRef]
- Cruz-Jentoft, A.J.; Sayer, A.A. Sarcopenia. Lancet 2019, 393, 2636–2646. [Google Scholar] [CrossRef]
- de Santiago, E.R.; Albéniz, E.; Estremera-Arevalo, F.; Sanchez-Vegazo, C.T.; Lorenzo-Zúñiga, V. Endoscopic anti-reflux therapy for gastroesophageal reflux disease. World J. Gastroenterol. 2021, 27, 6601. [Google Scholar] [CrossRef] [PubMed]
- Kim, Y.M.; Kim, J.H.; Baik, S.J.; Jung, D.H.; Park, J.J.; Youn, Y.H.; Park, H. Association between skeletal muscle attenuation and gastroesophageal reflux disease: A health check-up cohort study. Sci. Rep. 2019, 9, 20102. [Google Scholar] [CrossRef] [PubMed]
- Imagama, S.; Ando, K.; Kobayashi, K.; Machino, M.; Tanaka, S.; Morozumi, M.; Kanbara, S.; Ito, S.; Seki, T.; Hamada, T.; et al. Increase in lumbar kyphosis and spinal inclination, declining back muscle strength, and sarcopenia are risk factors for onset of GERD: A 5-year prospective longitudinal cohort study. Eur. Spine J. 2019, 28, 2619–2628. [Google Scholar] [CrossRef]
- Song, B.K.; Brellenthin, A.G.; Saavedra, J.M.; Lee, D.C. Associations Between Muscular Strength and Gastroesophageal Reflux Disease in Older Adults. J. Phys. Act. Health 2021, 18, 1207–1214. [Google Scholar] [CrossRef]
- Imagama, S.; Hasegawa, Y.; Wakao, N.; Hirano, K.; Hamajima, N.; Ishiguro, N. Influence of lumbar kyphosis and back muscle strength on the symptoms of gastroesophageal reflux disease in middle-aged and elderly people. Eur. Spine J. 2012, 21, 2149–2157. [Google Scholar] [CrossRef] [PubMed]
- Kim, W.J.; Shin, H.M.; Lee, J.S.; Song, D.G.; Lee, J.W.; Chang, S.H.; Park, K.Y.; Choy, W.S. Sarcopenia and Back Muscle Degeneration as Risk Factors for Degenerative Adult Spinal Deformity with Sagittal Imbalance and Degenerative Spinal Disease: A Comparative Study. World Neurosurg. 2021, 148, e547–e555. [Google Scholar] [CrossRef]
- Kim, J.-E.; Kwon, E.-Y.; Han, Y. A Collagen Hydrolysate Containing Tripeptides Ameliorates Sarcopenia in Middle-Aged Mice. Molecules 2022, 27, 2718. [Google Scholar] [CrossRef]
- Oertzen-Hagemann, V.; Kirmse, M.; Eggers, B.; Pfeiffer, K.; Marcus, K.; de Marées, M.; Platen, P. Effects of 12 Weeks of Hypertrophy Resistance Exercise Training Combined with Collagen Peptide Supplementation on the Skeletal Muscle Proteome in Recreationally Active Men. Nutrients 2019, 11, 1072. [Google Scholar] [CrossRef]
- O’connor, K.; Lehman, G. Endoscopic placement of collagen at the lower esophageal sphincter to inhibit gastroesophageal reflux: A pilot study of 10 medically intractable patients. Gastrointest. Endosc. 1988, 34, 106–112. [Google Scholar] [CrossRef]
- Kamler, J.P.; Lemperle, G.; Lemperle, S.; Lehman, G.A. Endoscopic lower esophageal sphincter bulking for the treatment of GERD: Safety evaluation of injectable polymethylmethacrylate microspheres in miniature swine. Gastrointest. Endosc. 2010, 72, 337–342. [Google Scholar] [CrossRef] [PubMed]
- Abrahams, M.; O’grady, R.; Prawitt, J. Effect of a Daily Collagen Peptide Supplement on Digestive Symptoms in Healthy Women: 2-Phase Mixed Methods Study. JMIR Form. Res. 2022, 6, e36339. [Google Scholar] [CrossRef]
- Fan, W.J.; Hou, Y.T.; Sun, X.H.; Li, X.Q.; Wang, Z.F.; Guo, M.; Zhu, L.M.; Wang, N.; Yu, K.; Li, J.N.; et al. Effect of high-fat, standard, and functional food meals on esophageal and gastric pH in patients with gastroesophageal reflux disease and healthy subjects. J. Dig. Dis. 2018, 19, 664–673. [Google Scholar] [CrossRef] [PubMed]
- Kwon, T.; Lamster, I.B.; Levin, L. Current Concepts in the Management of Periodontitis. Int. Dent. J. 2021, 71, 462–476. [Google Scholar] [CrossRef] [PubMed]
- Azzolino, D.; Passarelli, P.C.; De Angelis, P.; Piccirillo, G.B.; D’Addona, A.; Cesari, M. Poor Oral Health as a Determinant of Malnutrition and Sarcopenia. Nutrients 2019, 11, 2898. [Google Scholar] [CrossRef] [PubMed]
- Binlateh, T.; Thammanichanon, P.; Rittipakorn, P.; Thinsathid, N.; Jitprasertwong, P. Collagen-Based Biomaterials in Periodontal Regeneration: Current Applications and Future Perspectives of Plant-Based Collagen. Biomimetics 2022, 7, 34. [Google Scholar] [CrossRef]
- Zhou, T.; Chen, S.; Ding, X.; Hu, Z.; Cen, L.; Zhang, X. Fabrication and Characterization of Collagen/PVA Dual-Layer Membranes for Periodontal Bone Regeneration. Front. Bioeng. Biotechnol. 2021, 9. [Google Scholar] [CrossRef]
- Jockel-Schneider, Y.; Stoelzel, P.; Hess, J.; Haubitz, I.; Fickl, S.; Schlagenhauf, U. Impact of a Specific Collagen Peptide Food Supplement on Periodontal Inflammation in Aftercare Patients—A Randomised Controlled Trial. Nutrients 2022, 14, 4473. [Google Scholar] [CrossRef]
- Seto, H.; Toba, Y.; Takada, Y.; Kawakami, H.; Ohba, H.; Hama, H.; Horibe, M.; Nagata, T. Milk basic protein increases alveolar bone formation in rat experimental periodontitis. J. Periodontal Res. 2006, 42, 85–89. [Google Scholar] [CrossRef]
- Jayasinghe, T.N.; Harrass, S.; Erdrich, S.; King, S.; Eberhard, J. Protein Intake and Oral Health in Older Adults—A Narrative Review. Nutrients 2022, 14, 4478. [Google Scholar] [CrossRef]
- Lee, K.; Kim, J. Dairy Food Consumption is Inversely Associated with the Prevalence of Periodontal Disease in Korean Adults. Nutrients 2019, 11, 1035. [Google Scholar] [CrossRef] [PubMed]
- I Hanach, N.; McCullough, F.; Avery, A. The Impact of Dairy Protein Intake on Muscle Mass, Muscle Strength, and Physical Performance in Middle-Aged to Older Adults with or without Existing Sarcopenia: A Systematic Review and Meta-Analysis. Adv. Nutr. Int. Rev. J. 2019, 10, 59–69. [Google Scholar] [CrossRef] [PubMed]
- Gu, Y.; Han, J.; Jiang, C.; Zhang, Y. Biomarkers, oxidative stress and autophagy in skin aging. Ageing Res. Rev. 2020, 59, 101036. [Google Scholar] [CrossRef]
- Campbell, K.L.; Lichtensteiger, C.A. Structure and function of the skin. In Small Animal Dermatology Secrets; Elsevier Inc.: Amsterdam, The Netherlands, 2003; pp. 1–9. [Google Scholar]
- Zouboulis, C.C.; Ganceviciene, R.; Liakou, A.I.; Theodoridis, A.; Elewa, R.; Makrantonaki, E. Aesthetic aspects of skin aging, prevention, and local treatment. Clin. Dermatol. 2019, 37, 365–372. [Google Scholar] [CrossRef]
- Shuster, S. Osteoporosis, like skin ageing, is caused by collagen loss which is reversible. J. R. Soc. Med. 2020, 113, 158–160. [Google Scholar] [CrossRef] [PubMed]
- de Miranda, R.B.; Weimer, P.; Rossi, R.C. Effects of hydrolyzed collagen supplementation on skin aging: A systematic review and meta-analysis. Int. J. Dermatol. 2021, 60, 1449–1461. [Google Scholar] [CrossRef]
- Wongdee, K.; Charoenphandhu, N. Osteoporosis in diabetes mellitus: Possible cellular and molecular mechanisms. World J. Diabetes 2011, 2, 41–48. [Google Scholar] [CrossRef] [PubMed]
- Si, Y.; Wang, C.; Guo, Y.; Xu, G.; Ma, Y. Prevalence of Osteoporosis in Patients with Type 2 Diabetes Mellitus in the Chinese Mainland: A Systematic Review and Meta-Analysis. Iran J. Public Health 2019, 48, 1203–1214. [Google Scholar] [CrossRef]
- Saito, M.; Marumo, K. Collagen cross-links as a determinant of bone quality: A possible explanation for bone fragility in aging, osteoporosis, and diabetes mellitus. Osteoporos. Int. 2009, 21, 195–214. [Google Scholar] [CrossRef] [PubMed]
- Zhu, C.; Zhang, W.; Mu, B.; Zhang, F.; Lai, N.; Zhou, J.; Xu, A.; Liu, J.; Li, Y. Effects of marine collagen peptides on glucose metabolism and insulin resistance in type 2 diabetic rats. J. Food Sci. Technol. 2017, 54, 2260–2269. [Google Scholar] [CrossRef]
- Devasia, S.; Kumar, S.; Stephena, P.S.; Inoue, N.; Sugihara, F.; Suzuki, K. Double blind, randomized clinical study to evaluate efficacy of collagen peptide as add on nutritional supplement in Type 2 diabetes. J. Clin. Nutr. Food Sci. 2018, 1, 6–11. [Google Scholar]
- Zhu, C.; Zhang, W.; Liu, J.; Mu, B.; Zhang, F.; Lai, N.; Zhou, J.; Xu, A.; Li, Y. Marine collagen peptides reduce endothelial cell injury in diabetic rats by inhibiting apoptosis and the expression of coupling factor 6 and microparticles. Mol. Med. Rep. 2017, 16, 3947–3957. [Google Scholar] [CrossRef] [PubMed]
- Blum, A.; Adawi, M. RETRACTED: Rheumatoid arthritis (RA) and cardiovascular disease. Autoimmun. Rev. 2019, 18, 679–690. [Google Scholar] [CrossRef]
- Cho, Y.-G.; Cho, M.-L.; Min, S.-Y.; Kim, H.-Y. Type II collagen autoimmunity in a mouse model of human rheumatoid arthritis. Autoimmun. Rev. 2007, 7, 65–70. [Google Scholar] [CrossRef] [PubMed]
- Yokota, T.; McCourt, J.; Ma, F.; Ren, S.; Li, S.; Kim, T.-H.; Kurmangaliyev, Y.Z.; Nasiri, R.; Ahadian, S.; Nguyen, T.; et al. Type V Collagen in Scar Tissue Regulates the Size of Scar after Heart Injury. Cell 2020, 182, 545–562.e23. [Google Scholar] [CrossRef]
- Ahmadi, P.; Mahmoudi, M.; Kheder, R.K.; Faraj, T.A.; Mollazadeh, S.; Abdulabbas, H.S.; Esmaeili, S.-A. Impacts of Porphyromonas gingivalis periodontitis on rheumatoid arthritis autoimmunity. Int. Immunopharmacol. 2023, 118, 109936. [Google Scholar] [CrossRef]
- Nakamura, T.; Yamashita, M.; Ikegami, K.; Suzuki, M.; Yanagita, M.; Kitagaki, J.; Kitamura, M.; Murakami, S. Autophagy facilitates type I collagen synthesis in periodontal ligament cells. Sci. Rep. 2021, 11, 1291. [Google Scholar] [CrossRef] [PubMed]
- Wang, H. Tolerance Limits for Mixture-of-Normal Distributions with Application to COVID-19 Data; Wiley Interdisciplinary Reviews-Computational Statistics; Wiley: New York, NY, USA, 2023. [Google Scholar]
- Chen, Y.-H.; Wang, H. Exploring Diversity of COVID-19 Based on Substitution Distance. Infect. Drug Resist. 2020, ume 13, 3887–3894. [Google Scholar] [CrossRef]
- Wang, H. COVID−19, Anti-NMDA Receptor Encephalitis and MicroRNA. Front. Immunol. 2022, 13, 825103. [Google Scholar] [CrossRef]
- Desai, A.D.; Lavelle, M.; Boursiquot, B.C.; Wan, E.Y. Long-term complications of COVID-19. Am. J. Physiol. Physiol. 2021, 322, C1–C11. [Google Scholar] [CrossRef]
- Méndez-Flores, S.; Priego-Ranero, Á.; Azamar-Llamas, D.; Olvera-Prado, H.; Rivas-Redonda, K.I.; Ochoa-Hein, E.; Perez-Ortiz, A.; Rendón-Macías, M.E.; Rojas-Castañeda, E.; Urbina-Terán, S.; et al. Effect of polymerised type I collagen on hyperinflammation of adult outpatients with symptomatic COVID-19. Clin. Transl. Med. 2022, 12, e763. [Google Scholar] [CrossRef]
- Salvatore, L.; Natali, M.L.; Brunetti, C.; Sannino, A.; Gallo, N. An Update on the Clinical Efficacy and Safety of Collagen Injectables for Aesthetic and Regenerative Medicine Applications. Polymers 2023, 15, 1020. [Google Scholar] [CrossRef]
- Xu, E.; Xie, Y.; Al-Aly, Z. Long-term gastrointestinal outcomes of COVID-19. Nat. Commun. 2023, 14, 983. [Google Scholar] [CrossRef] [PubMed]
- Tripathy, A.; Swain, N.; Gupta, B. The COVID-19 pandemic: An increased risk of rheumatoid arthritis. Futur. Virol. 2021, 16, 431–442. [Google Scholar] [CrossRef] [PubMed]
- Gazzaz, Z.J. Diabetes and COVID-19. Open Life Sci. 2021, 16, 297–302. [Google Scholar] [CrossRef]
- Piotrowicz, K.; Gąsowski, J.; Michel, J.-P.; Veronese, N. Post-COVID-19 acute sarcopenia: Physiopathology and management. Aging Clin. Exp. Res. 2021, 33, 2887–2898. [Google Scholar] [CrossRef] [PubMed]
- Marouf, N.; Cai, W.; Said, K.N.; Daas, H.; Diab, H.; Chinta, V.R.; Hssain, A.A.; Nicolau, B.; Sanz, M.; Tamimi, F. Association between periodontitis and severity of COVID-19 infection: A case–control study. J. Clin. Periodontol. 2021, 48, 483–491. [Google Scholar] [CrossRef]
- Mistry, S.K.; Ali, A.R.M.M.; Yadav, U.N.; Das Gupta, R.; Anwar, A.; Basu, S.; Huda, N.; Mitra, D.K. A tale of osteoarthritis among older adults during the COVID-19 pandemic in Bangladesh: A repeated cross-sectional study. PLoS ONE 2022, 17, e0274838. [Google Scholar] [CrossRef]
- Mei, F.; Duan, Z.; Chen, M.; Lu, J.; Zhao, M.; Li, L.; Shen, X.; Xia, G.; Chen, S. Effect of a high-collagen peptide diet on the gut microbiota and short-chain fatty acid metabolism. J. Funct. Foods 2020, 75, 104278. [Google Scholar] [CrossRef]
- Marques, C.F.; Diogo, G.S.; Pina, S.; Oliveira, J.M.; Silva, T.H.; Reis, R.L. Collagen-based bioinks for hard tissue engineering applications: A comprehensive review. J. Mater. Sci. Mater. Med. 2019, 30, 32. [Google Scholar] [CrossRef]
- Copes, F.; Pien, N.; Van Vlierberghe, S.; Boccafoschi, F.; Mantovani, D. Collagen-Based Tissue Engineering Strategies for Vascular Medicine. Front. Bioeng. Biotechnol. 2019, 7, 166. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Wang, Z.; Dong, Y. Collagen-Based Biomaterials for Tissue Engineering. ACS Biomater. Sci. Eng. 2023, 9, 1132–1150. [Google Scholar] [CrossRef] [PubMed]
- Rico-Llanos, G.A.; Borrego-González, S.; Moncayo-Donoso, M.; Becerra, J.; Visser, R. Collagen Type I Biomaterials as Scaffolds for Bone Tissue Engineering. Polymers 2021, 13, 599. [Google Scholar] [CrossRef]
- Liu, S.; Lau, C.-S.; Liang, K.; Wen, F.; Teoh, S.H. Marine collagen scaffolds in tissue engineering. Curr. Opin. Biotechnol. 2021, 74, 92–103. [Google Scholar] [CrossRef]
- Lin, C.; Ritch, R.; Lin, S.; Ni, M.-H.; Chang, Y.-C.; Lu, Y.; Lai, H.; Lin, F.-H. A new fish scale-derived scaffold for corneal regeneration. Eur. Cells Mater. 2010, 19, 50–57. [Google Scholar] [CrossRef]
- Li, Z.; Du, T.; Ruan, C.; Niu, X. Bioinspired mineralized collagen scaffolds for bone tissue engineering. Bioact. Mater. 2020, 6, 1491–1511. [Google Scholar] [CrossRef] [PubMed]
- Fan, L.; Ren, Y.; Emmert, S.; Vučković, I.; Stojanovic, S.; Najman, S.; Schnettler, R.; Barbeck, M.; Schenke-Layland, K.; Xiong, X. The Use of Collagen-Based Materials in Bone Tissue Engineering. Int. J. Mol. Sci. 2023, 24, 3744. [Google Scholar] [CrossRef] [PubMed]

| Collagen | -Chains | Tissue or Organ |
|---|---|---|
| Type I | (I) | skin, bone, teeth, tendons, ligaments, vascular ligature |
| Type II | cartilage | |
| Type III | muscle, blood vessels | |
| Type IV | (IV) (IV) (IV) | basal lamina, the epithelium-secreted layer of the basement membrane |
| Type V | hair, cell surfaces, placenta, skin, tendons, ligaments |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the author. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wang, H. The Potential of Collagen Treatment for Comorbid Diseases. Polymers 2023, 15, 3999. https://doi.org/10.3390/polym15193999
Wang H. The Potential of Collagen Treatment for Comorbid Diseases. Polymers. 2023; 15(19):3999. https://doi.org/10.3390/polym15193999
Chicago/Turabian StyleWang, Hsiuying. 2023. "The Potential of Collagen Treatment for Comorbid Diseases" Polymers 15, no. 19: 3999. https://doi.org/10.3390/polym15193999
APA StyleWang, H. (2023). The Potential of Collagen Treatment for Comorbid Diseases. Polymers, 15(19), 3999. https://doi.org/10.3390/polym15193999






