Study of a Mixed Conductive Layer Fabricated by Ion Implantation and Distribution Theory
Abstract
:1. Introduction
2. Theoretical Model
2.1. Initial Model
2.2. Model Modification
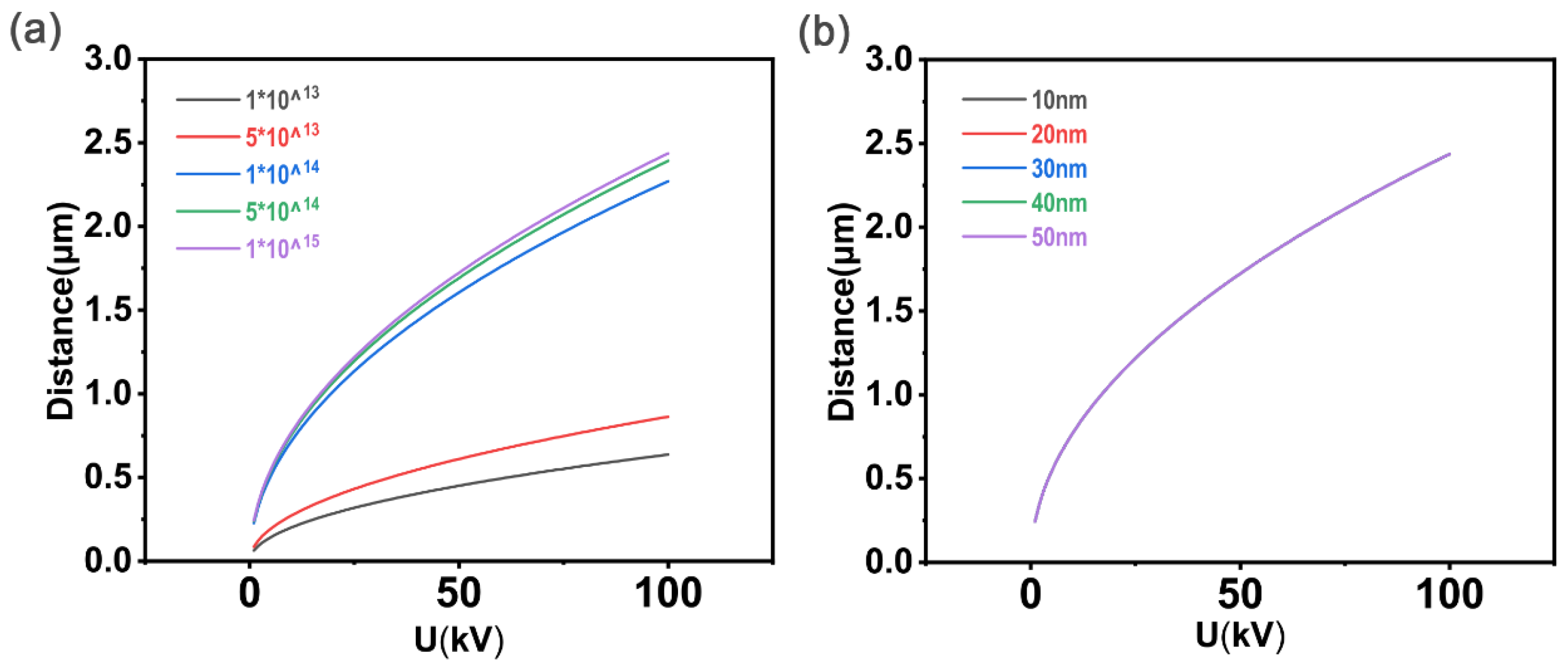
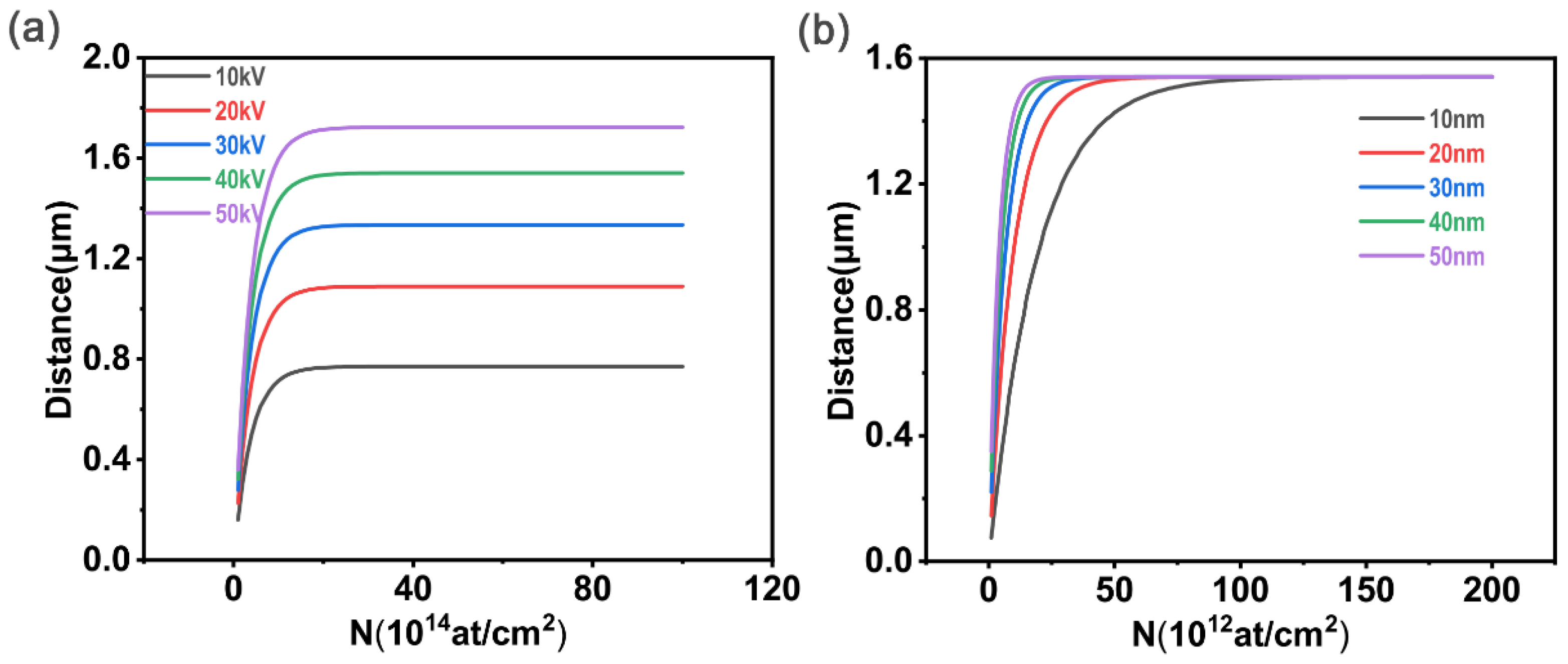
2.2.1. Amendment of N
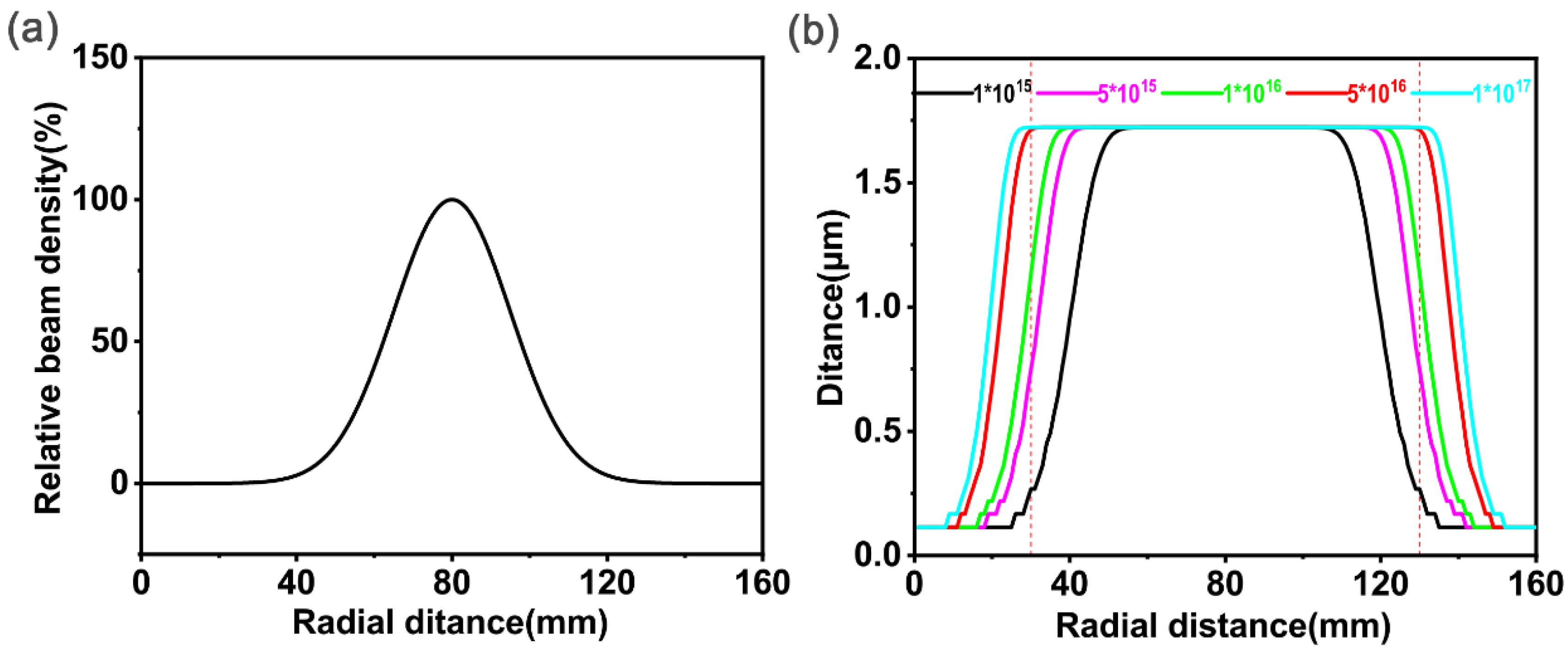
2.2.2. Amendment of U
3. Experimental Section
3.1. Experimental Materials
3.2. Preparation of Samples and Experimentation
3.3. Testing Instruments
4. Results and Discussion
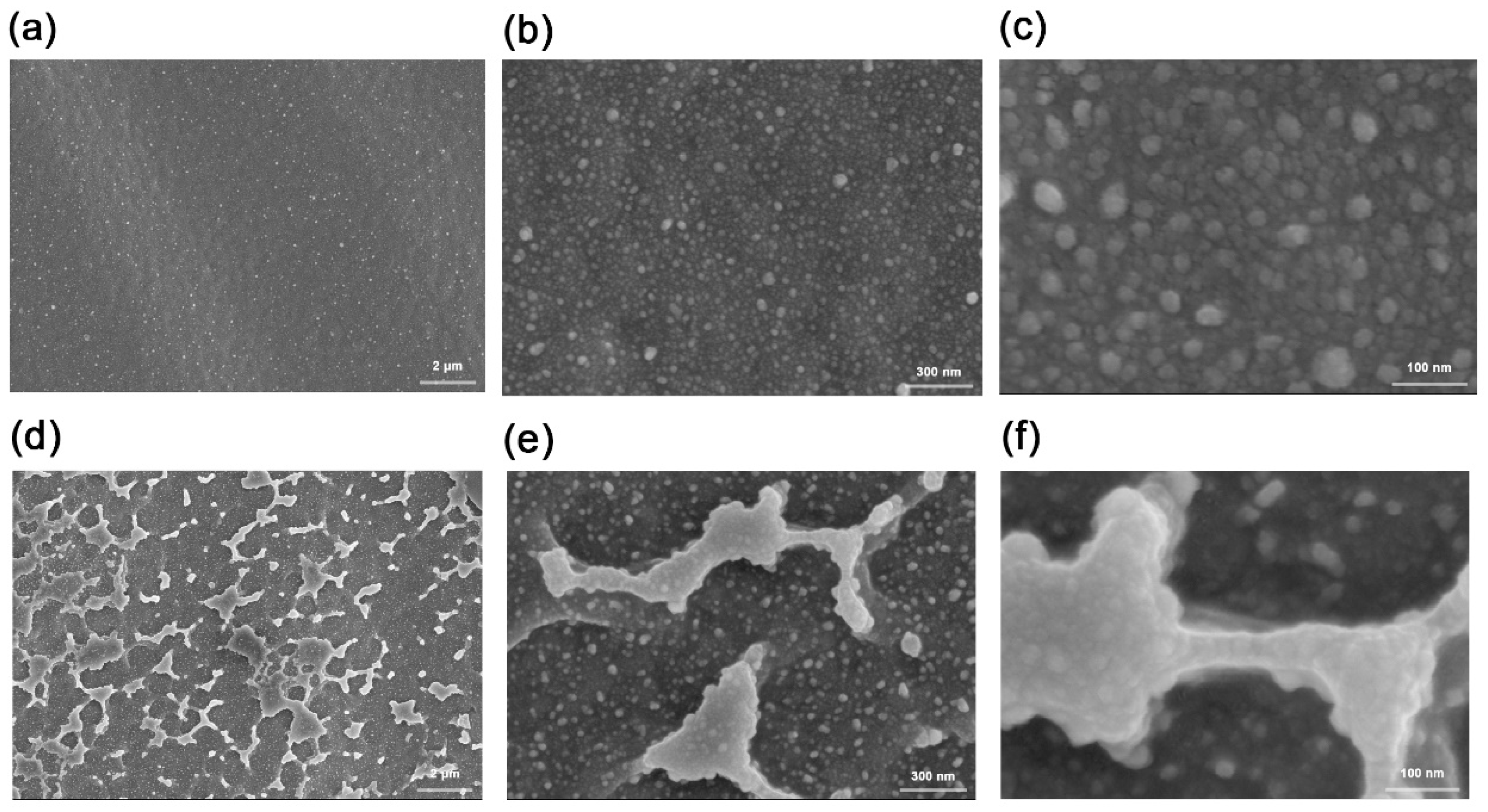
4.1. Morphologies and Electrical Performances of the Samples after Silver Ion Implantation
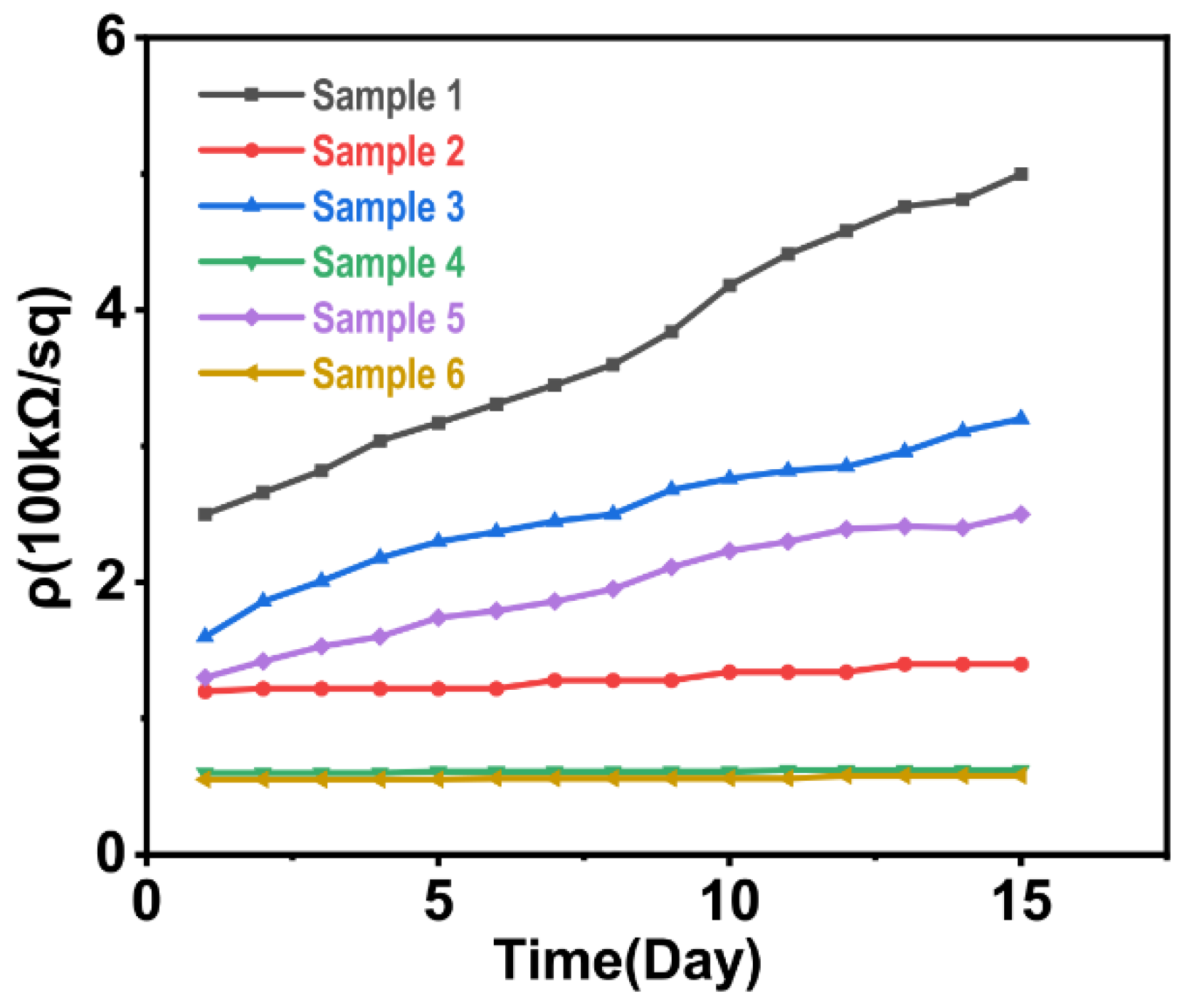
4.1.1. Changes in the Morphologies and Conductivities of the Sample Surfaces after Silver Ion Implantation
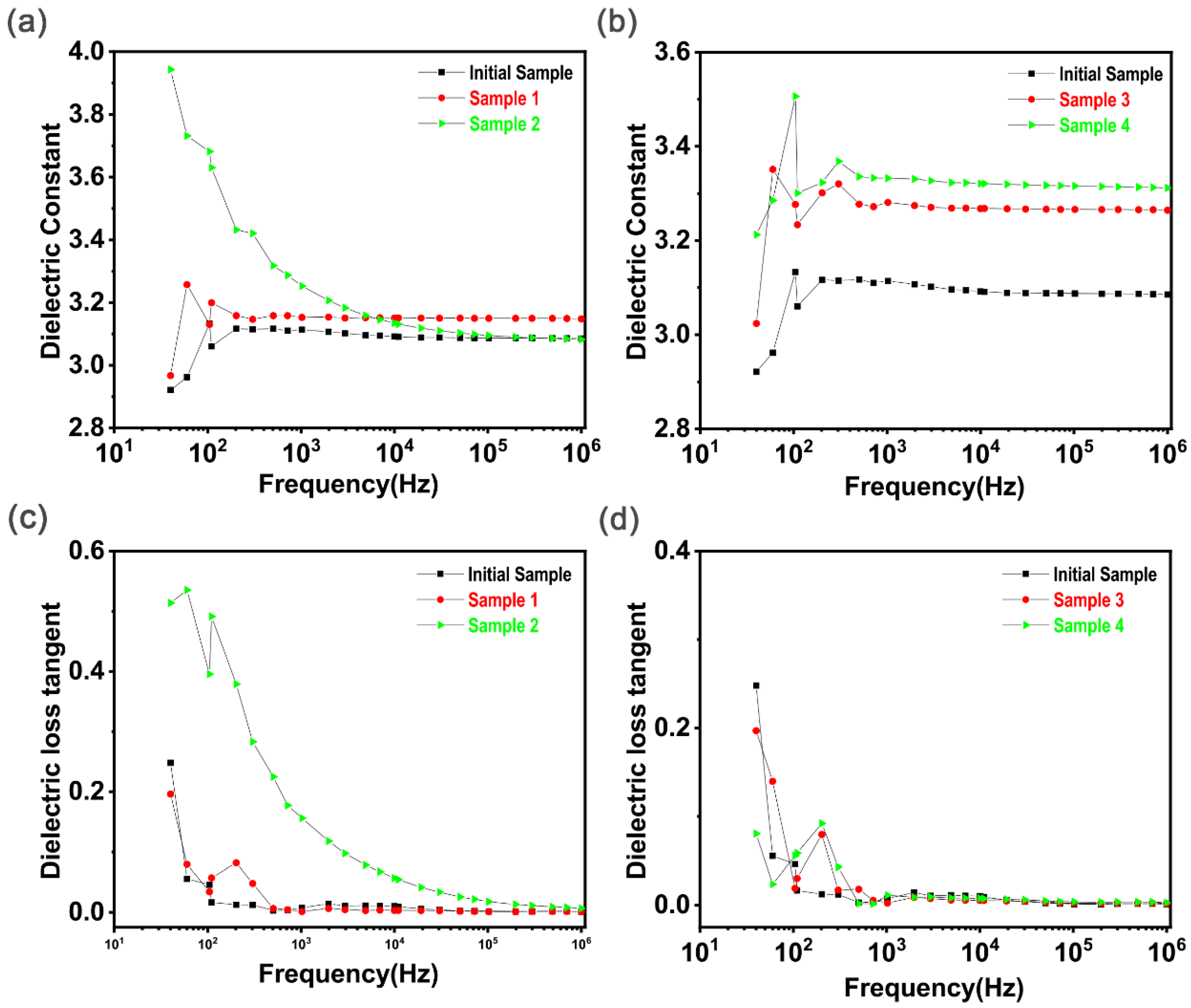
4.1.2. Dielectric Property Analysis
4.2. Structural Analysis and Material-Phase Analysis
4.2.1. Infrared Structure Analysis
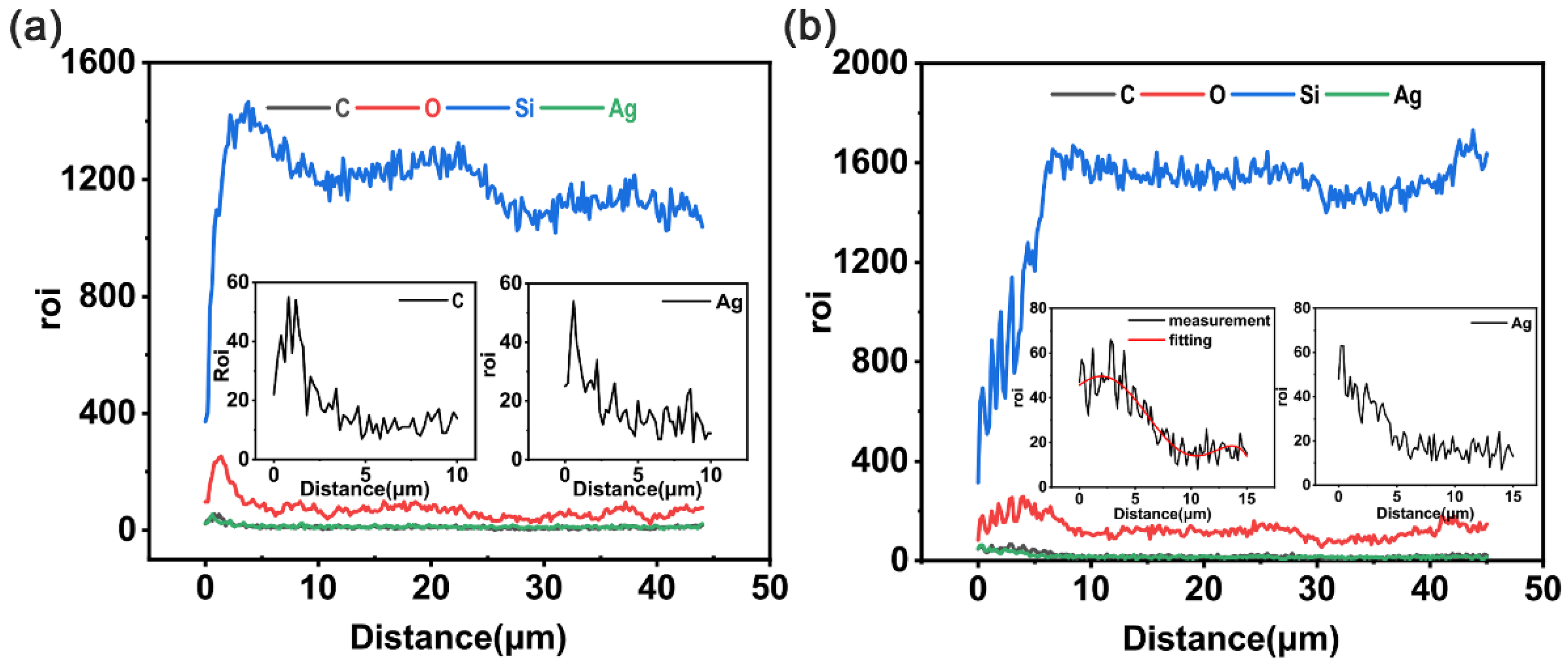
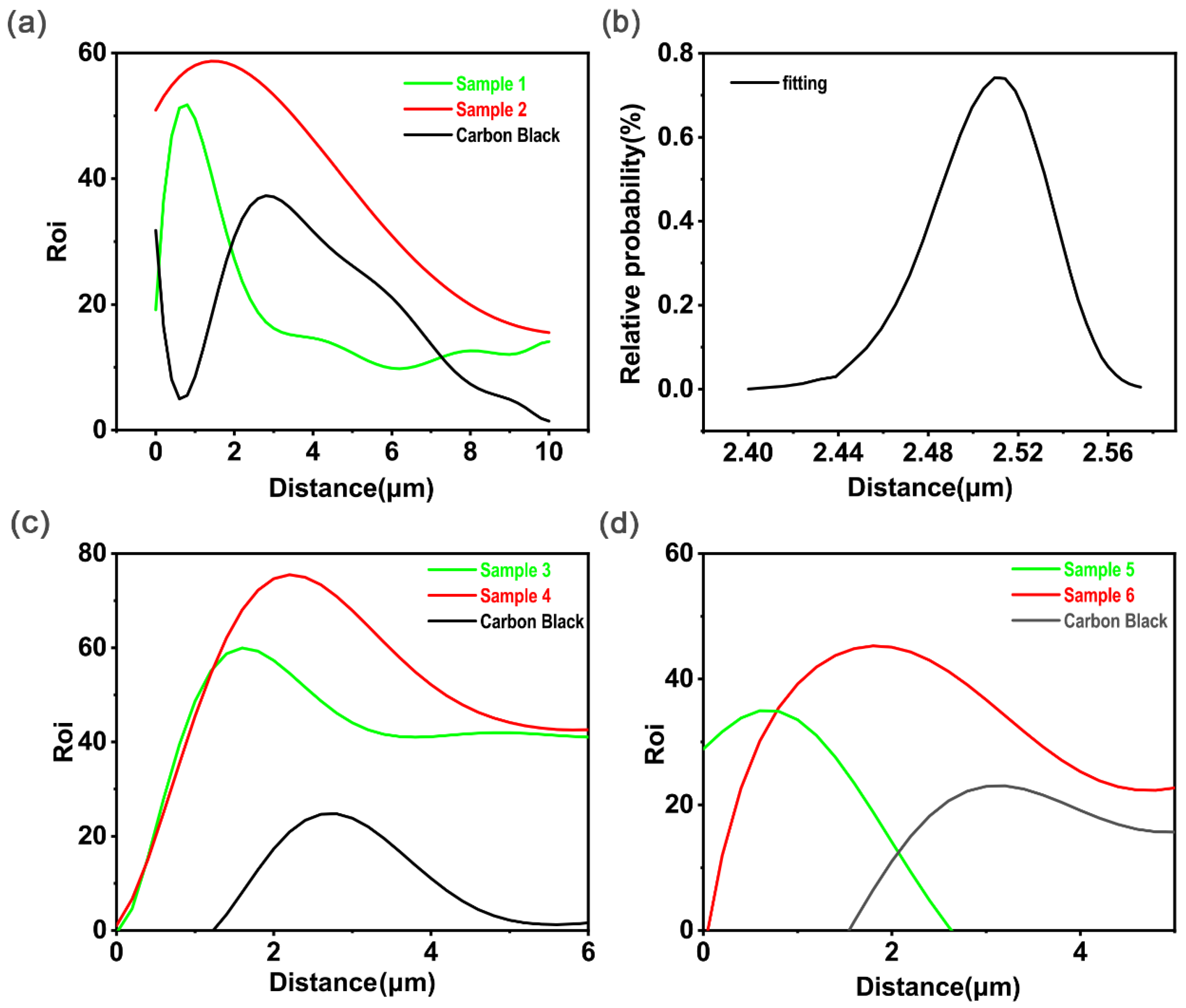
4.2.2. Energy Dispersive Spectroscopy Analysis
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Opris, D.M.; Molberg, M.; Walder, C.; Ko, Y.S.; Fischer, B.; Nüesch, F.A. New Silicone Composites for Dielectric Elastomer Actuator Applications In Competition with Acrylic Foil. Adv. Funct. Mater. 2011, 21, 3531–3539. [Google Scholar] [CrossRef]
- Carpi, F.; Gallone, G.; Galantini, F.; De Rossi, D. Silicone–Poly(hexylthiophene) Blends as Elastomers with Enhanced Electromechanical Transduction Properties. Adv. Funct. Mater. 2008, 18, 235–241. [Google Scholar] [CrossRef]
- Shao, Y.; Luo, C.; Deng, B.W.; Yin, B.; Yang, M.B. Flexible porous silicone rubber-nanofiber nanocomposites generated by supercritical carbon dioxide foaming for harvesting mechanical energy. Nano Energy 2020, 67, 104290. [Google Scholar] [CrossRef]
- Atalay, A.; Sanchez, V.; Atalay, O.; Vogt, D.M.; Haufe, F.; Wood, R.J.; Walsh, C.J. Batch Fabrication of Customizable Silicone-Textile Composite Capacitive Strain Sensors for Human Motion Tracking. Adv. Mater. Technol. 2017, 2, 1700136. [Google Scholar] [CrossRef] [Green Version]
- Gatti, D.; Haus, H.; Matysek, M.; Frohnapfel, B.; Tropea, C.; Schlaak, H.F. The dielectric breakdown limit of silicone dielectric elastomer actuators. Appl. Phys. Lett. 2014, 104, 052905. [Google Scholar] [CrossRef] [Green Version]
- Shian, S.; Huang, J.; Zhu, S.; Clarke, D.R. Optimizing the electrical energy conversion cycle of dielectric elastomer generators. Adv. Mater. 2014, 26, 6617–6621. [Google Scholar] [CrossRef]
- Huang, J.; Shian, S.; Suo, Z.; Clarke, D.R. Maximizing the Energy Density of Dielectric Elastomer Generators Using Equi-Biaxial Loading. Adv. Funct. Mater. 2013, 23, 5056–5061. [Google Scholar] [CrossRef] [Green Version]
- Wu, H.; Huang, Y.; Xu, F.; Duan, Y.; Yin, Z. Energy Harvesters for Wearable and Stretchable Electronics: From Flexibility to Stretchability. Adv. Mater. 2016, 28, 9881–9919. [Google Scholar] [CrossRef]
- De Tommasi, D.; Puglisi, G.; Zurlo, G. Electromechanical instability and oscillating deformations in electroactive polymer films. Appl. Phys. Lett. 2013, 102, 011903. [Google Scholar] [CrossRef]
- Pelrine, R.; Kornbluh, R.; Pei, Q.; Joseph, J. High-Speed Electrically Actuated Elastomers with Strain Greater Than 100%. Science 2000, 287, 836–839. [Google Scholar] [CrossRef]
- Acome, E.; Mitchell, S.K.; Morrissey, T.G.; Emmett, M.B.; Benjamin, C.; King, M.; Radakovitz, M.; Keplinger, C. Hydraulically amplified self-healing electrostatic actuators with muscle-like performance. Science 2018, 359, 61–65. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Romasanta, L.J.; Lopez-Manchado, M.A.; Verdejo, R. Increasing the performance of dielectric elastomer actuators: A review from the materials perspective. Prog. Polym. Sci. 2015, 51, 188–211. [Google Scholar] [CrossRef] [Green Version]
- Ha, S.M.; Yuan, W.; Pei, Q.; Pelrine, R.; Stanford, S. Interpenetrating Polymer Networks for High-Performance Electroelastomer Artificial Muscles. Adv. Mater. 2006, 18, 887–891. [Google Scholar] [CrossRef]
- Mirvakili, S.M.; Hunter, I.W. Artificial Muscles: Mechanisms, Applications, and Challenges. Adv. Mater. 2018, 30, 1704407. [Google Scholar] [CrossRef] [PubMed]
- Brochu, P.; Pei, Q. Advances in Dielectric Elastomers for Actuators and Artificial Muscles. Macromol. Rapid Commun. 2010, 31, 10–36. [Google Scholar] [CrossRef] [PubMed]
- Gu, G.Y.; Zhu, J.; Zhu, L.M.; Zhu, X. A survey on dielectric elastomer actuators for soft robots. Bioinspir. Biomim. 2017, 12, 011003. [Google Scholar] [CrossRef]
- Bauer, S.; Bauer-Gogonea, S.; Graz, I.; Kaltenbrunner, M.; Keplinger, C.; Schwödiauer, R. 25th Anniversary Article: A Soft Future: From Robots and Sensor Skin to Energy Harvesters. Adv. Mater. 2014, 26, 149–162. [Google Scholar] [CrossRef] [Green Version]
- Jean-Mistral, C.; Sylvestre, A.; Basrour, S.; Chaillout, J.J. Dielectric properties of polyacrylate thick films used in sensors and actuators. Smart Mater. Struct. 2010, 19, 075019. [Google Scholar] [CrossRef]
- Madsen, F.B.; Daugaard, A.E.; Hvilsted, S.; Skov, A.L. The Current State of Silicone-Based Dielectric Elastomer Transducers. Macromol. Rapid Commun. 2016, 37, 378–413. [Google Scholar] [CrossRef] [Green Version]
- Wu, W.; Zhang, S.; Wu, Z.; Qin, S.; Li, F.; Song, T.; Cao, X.; Wang, Z.L.; Zhang, L. On the understanding of dielectric elastomer and its application for all-soft artificial heart. Sci. Bull. 2021, 66, 981–990. [Google Scholar] [CrossRef]
- Wu, W.; Wang, Z.L.; Zhang, L. A New Molecular Mechanism for Understanding the Actuated Strain of Dielectric Elastomers and Their Impacts. Macromol. Rapid Commun. 2022, 2200315. [Google Scholar] [CrossRef] [PubMed]
- Li, C.H.; Wang, C.; Keplinger, C.; Zuo, J.L.; Jin, L.; Sun, Y.; Zheng, P.; Cao, Y.; Lissel, F.; Linder, C.; et al. A highly stretchable autonomous self-healing elastomer. Nat. Chem. 2016, 8, 618–624. [Google Scholar] [CrossRef] [PubMed]
- Qiu, Y.; Zhang, E.; Plamthottam, R.; Pei, Q. Dielectric Elastomer Artificial Muscle: Materials Innovations and Device Explorations. Acc. Chem. Res. 2019, 52, 316–325. [Google Scholar] [CrossRef] [PubMed]
- Kim, M.K.; Kim, M.S.; Kwon, H.B.; Jo, S.E.; Kim, Y.J. Wearable triboelectric nanogenerator using a plasma-etched PDMS–CNT composite for a physical activity sensor. RSC Adv. 2017, 7, 48368–48373. [Google Scholar] [CrossRef] [Green Version]
- Zhao, P.; Bhattacharya, G.; Fishlock, S.J.; Guy, J.G.M.; Kumar, A.; Tsonos, C.; Yu, Z.; Raj, S.; McLaughlin, J.A.; Luo, J.; et al. Replacing the metal electrodes in triboelectric nanogenerators: High-performance laser-induced graphene electrodes. Nano Energy 2020, 75, 104958. [Google Scholar] [CrossRef]
- Harnchana, V.; Ngoc, H.V.; He, W.; Rasheed, A.; Park, H.; Amornkitbamrung, V.; Kang, D.J. Enhanced Power Output of a Triboelectric Nanogenerator using Poly(dimethylsiloxane) Modified with Graphene Oxide and Sodium Dodecyl Sulfate. ACS Appl. Mater. Interfaces 2018, 10, 25263–25272. [Google Scholar] [CrossRef]
- Rosset, S.; Shea, H.R. Flexible and stretchable electrodes for dielectric elastomer actuators. Appl. Phys. A 2013, 110, 281–307. [Google Scholar] [CrossRef] [Green Version]
- Rosset, S.; Niklaus, M.; Dubois, P.; Shea, H.R. Mechanical characterization of a dielectric elastomer microactuator with ion-implanted electrodes. Sens. Actuators A Phys. 2008, 144, 185–193. [Google Scholar] [CrossRef] [Green Version]
- Gonzalez, M.; Axisa, F.; Bulcke, M.V.; Brosteaux, D.; Vandevelde, B.; Vanfleteren, J. Design of metal interconnects for stretchable electronic circuits. Microelectron. Reliab. 2008, 48, 825–832. [Google Scholar] [CrossRef]
- Lacour, S.P.; Jones, J.; Suo, Z.; Wagner, S. Design and performance of thin metal film interconnects for skin-like electronic circuits. IEEE Electron Device Lett. 2004, 25, 179–181. [Google Scholar] [CrossRef]
- Rosset, S.; Niklaus, M.; Dubois, P.; Shea, H.R. Metal ion implantation for the fabrication of stretchable electrodes on elastomers. Adv. Funct. Mater. 2009, 19, 470–478. [Google Scholar] [CrossRef]
- Akbari, S.; Shea, H.R. An array of 100 μm× 100 μm dielectric elastomer actuators with 80% strain for tissue engineering applications. Sens. Actuators A Phys. 2012, 186, 236–241. [Google Scholar] [CrossRef] [Green Version]
- Qin, S.; Tu, Y.; Tan, T.; Wang, S.; Wang, C. The investigation of charge transport properties of Al2O3-Epoxy composite material. In Proceedings of the 2018 12th International Conference on the Properties and Applications of Dielectric Materials (ICPADM 2018), Xi’an, China, 20–24 May 2018; IEEE: Piscataway, NJ, USA, 2018; pp. 937–940. [Google Scholar]
- Qin, S.; Tu, Y.; Chen, B.; Xu, D.; Tan, T.; Chen, G.; Yuan, Z. The effect of temperature cycles on conductivity mechanism using polyimide. J. Appl. Phys. 2020, 128, 215110. [Google Scholar] [CrossRef]
- Qin, S.; Tu, Y.; Chen, B.; Xu, D.; Li, C.; Tan, T. Dynamic conductivity of polymer dielectrics under time-varying temperature field. Appl. Phys. Express 2019, 12, 071002. [Google Scholar] [CrossRef]
- Qin, S.; Xu, Q.; Wang, Q.; Zhang, J.; Wang, C.; Shen, Z. Simulation study on the electric field of HVDC-GIL based on the dynamic conductivity model subjected to different current-carrying capacities. Jpn. J. Appl. Phys. 2021, 60, 071001. [Google Scholar] [CrossRef]
- Qin, S.; Tu, Y.; Wang, S.; Cheng, Y.; Chen, B.; Wang, C.; Zhang, Y. Accelerated Aging of Fast Thermal Cycle Effects on the behavior of Space Charge in Polyimide. IEEE Trans. Dielectr. Electr. Insul. 2017, 24, 3182–3190. [Google Scholar] [CrossRef]
- Qin, S.; Tu, Y.; Tan, T.; Wang, S.; Yuan, Z.; Wang, C.; Li, L.; Wu, Z. The effects of gamma-ray on charging behaviour using polyimide. J. Phys. D Appl. Phys. 2018, 51, 245302. [Google Scholar] [CrossRef]
- Winton, B.R.; Ionescu, M.; Dou, S.X.; Wexler, D.; Alvarez, G.A. Structural and morphological modification of PDMS thick film surfaces by ion implantation with the formation of strain-induced buckling domains. Acta Mater. 2010, 58, 1861–1867. [Google Scholar] [CrossRef]
- Niklaus, M.; Shea, H.R. Electrical conductivity and Young’s modulus of flexible nanocomposites made by metal-ion implantation of polydimethylsiloxane: The relationship between nanostructure and macroscopic properties. Acta Mater. 2011, 59, 830–840. [Google Scholar] [CrossRef]
- Lindhard, J.; Scharff, M.; Schiøtt, H.E. Range Concepts and Heavy Ion Ranges. Mat. Fys. Medd. Dan. Vid. Selsk. 1963, 33, 1–44. [Google Scholar]
- Brown, I.G.; Feinberg, B.; Galvin, J.E. Multiply stripped ion generation in the metal vapor vacuum arc. J. Appl. Phys. 1988, 63, 4889–4898. [Google Scholar] [CrossRef]
- Brown, I.G. The metal vapor vacuum arc (MEVVA) high current ion source. IEEE Trans. Nucl. Sci. 1985, 32, 1723–1727. [Google Scholar] [CrossRef] [Green Version]
- Lang, S.B. Guide to the Literature of Piezoelectricity and Pyroelectricity. 29. Ferroelectrics 2008, 366, 122–237. [Google Scholar] [CrossRef]
- Amjadi, M.; Yoon, Y.J.; Park, I. Ultra-stretchable and skin-mountable strain sensors using carbon nanotubes–Ecoflex nanocomposites. Nanotechnology 2015, 26, 375501. [Google Scholar] [CrossRef] [PubMed]




| Dh | E/keV | Or/% |
|---|---|---|
| 1+ | 40 | 13 |
| 2+ | 80 | 61 |
| 3+ | 120 | 25 |
| 4+ | 160 | 1 |
| Event | Penetration Range/m | Probability/P | |||
|---|---|---|---|---|---|
| 1 | 0 | 0 | 150 | 2.6516 × 10−6 | 4.9091 × 10−91 |
| 2 | 0 | 1 | 149 | 2.6515 × 10−6 | 1.8262 × 10−88 |
| … | … | … | … | … | … |
| 11,475 | 149 | 1 | 0 | 1.5519 × 10−6 | 8.8317 × 10−131 |
| 11,476 | 150 | 0 | 0 | 1.5309 × 10−6 | 1.2345 × 10−133 |
| PDMS | Elongation at break | Dielectric strength | Volume resistance |
| 400% | 12 kV/mm | 1014 Ω × cm | |
| EC-600JD | Density | Specific surface area | Moisture |
| 0.12 g/cm3 | 1400 m2/g | <0.5% |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Fan, X.; Zhang, H.; Wei, Y.; Huang, Y.; He, H.; Wang, Y.; Meng, Q.; Wu, W. Study of a Mixed Conductive Layer Fabricated by Ion Implantation and Distribution Theory. Polymers 2023, 15, 270. https://doi.org/10.3390/polym15020270
Fan X, Zhang H, Wei Y, Huang Y, He H, Wang Y, Meng Q, Wu W. Study of a Mixed Conductive Layer Fabricated by Ion Implantation and Distribution Theory. Polymers. 2023; 15(2):270. https://doi.org/10.3390/polym15020270
Chicago/Turabian StyleFan, Xuerui, Huiyan Zhang, Yi Wei, Yao Huang, Huimei He, Yun Wang, Qingyun Meng, and Wenjie Wu. 2023. "Study of a Mixed Conductive Layer Fabricated by Ion Implantation and Distribution Theory" Polymers 15, no. 2: 270. https://doi.org/10.3390/polym15020270





