Development, Characterization, and Evaluation as Food Active Packaging of Low-Density-Polyethylene-Based Films Incorporated with Rich in Thymol Halloysite Nanohybrid for Fresh “Scaloppini” Type Pork Meat Fillets Preservation
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Preparation of Reach in Thymol TO@HNT Nanohybrids
2.3. Preparation of LDPE/xHNT and LDPE/xTO@HNT Films
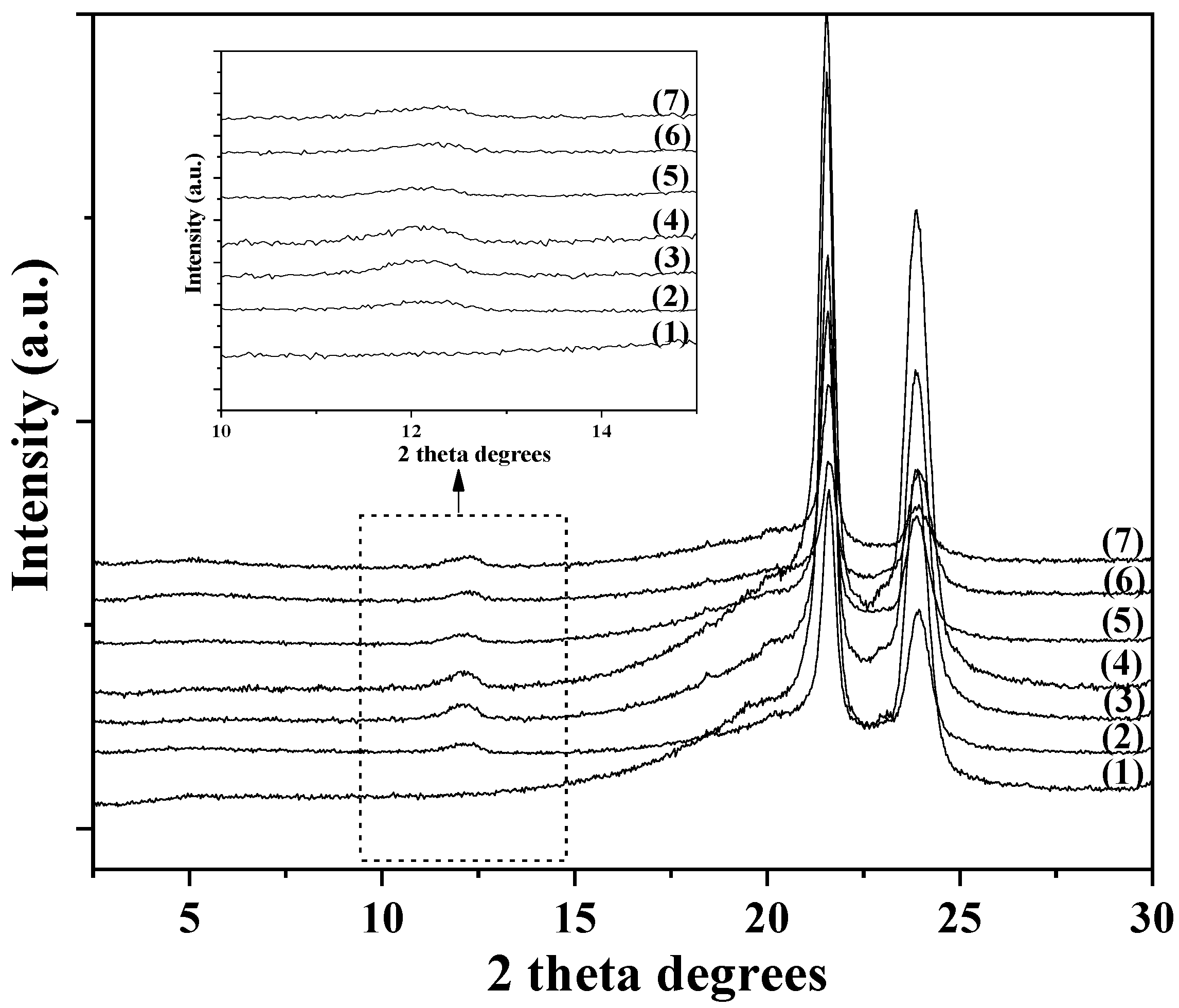
2.4. XRD Analysis
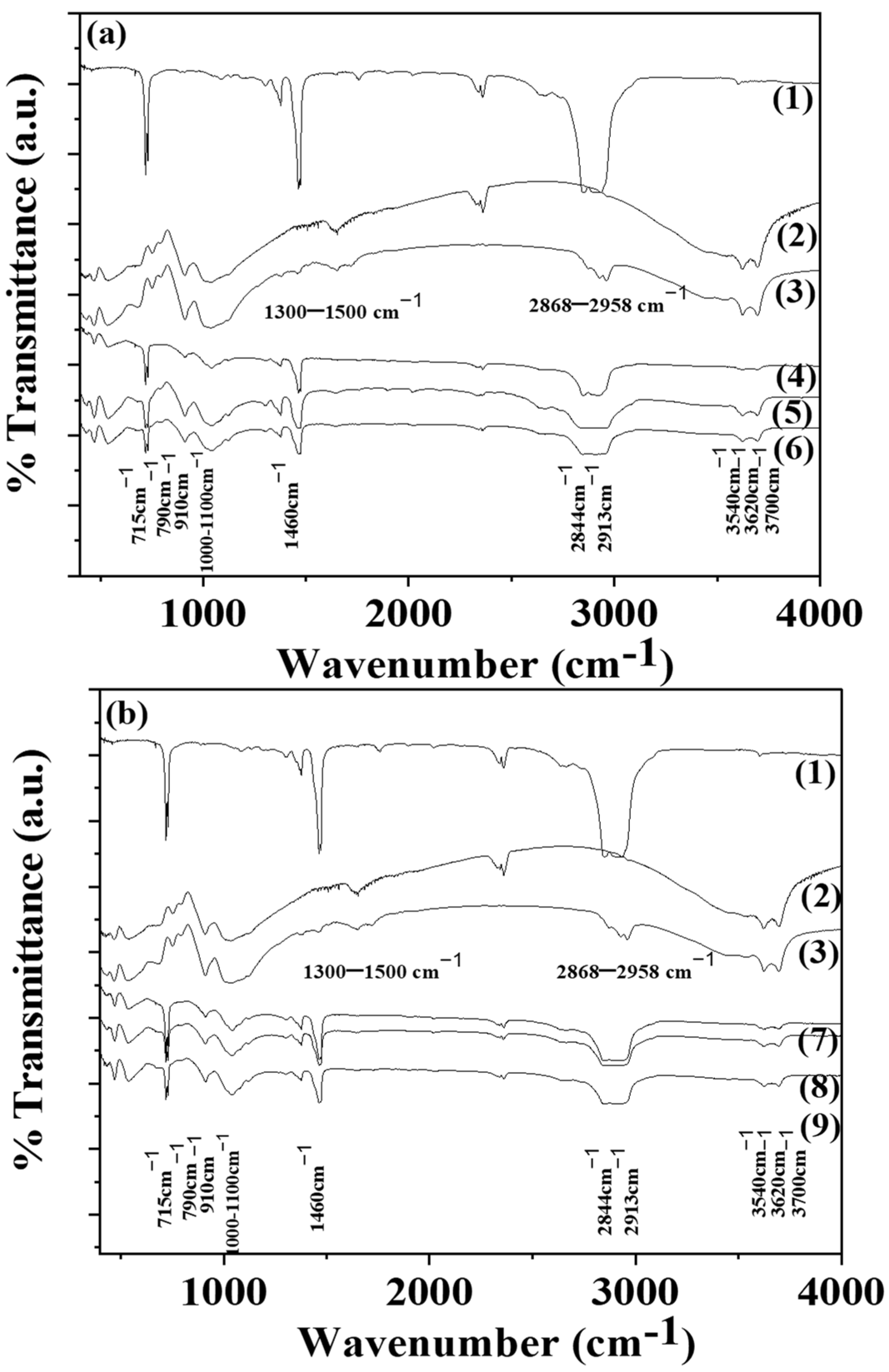
2.5. FTIR Spectroscopy
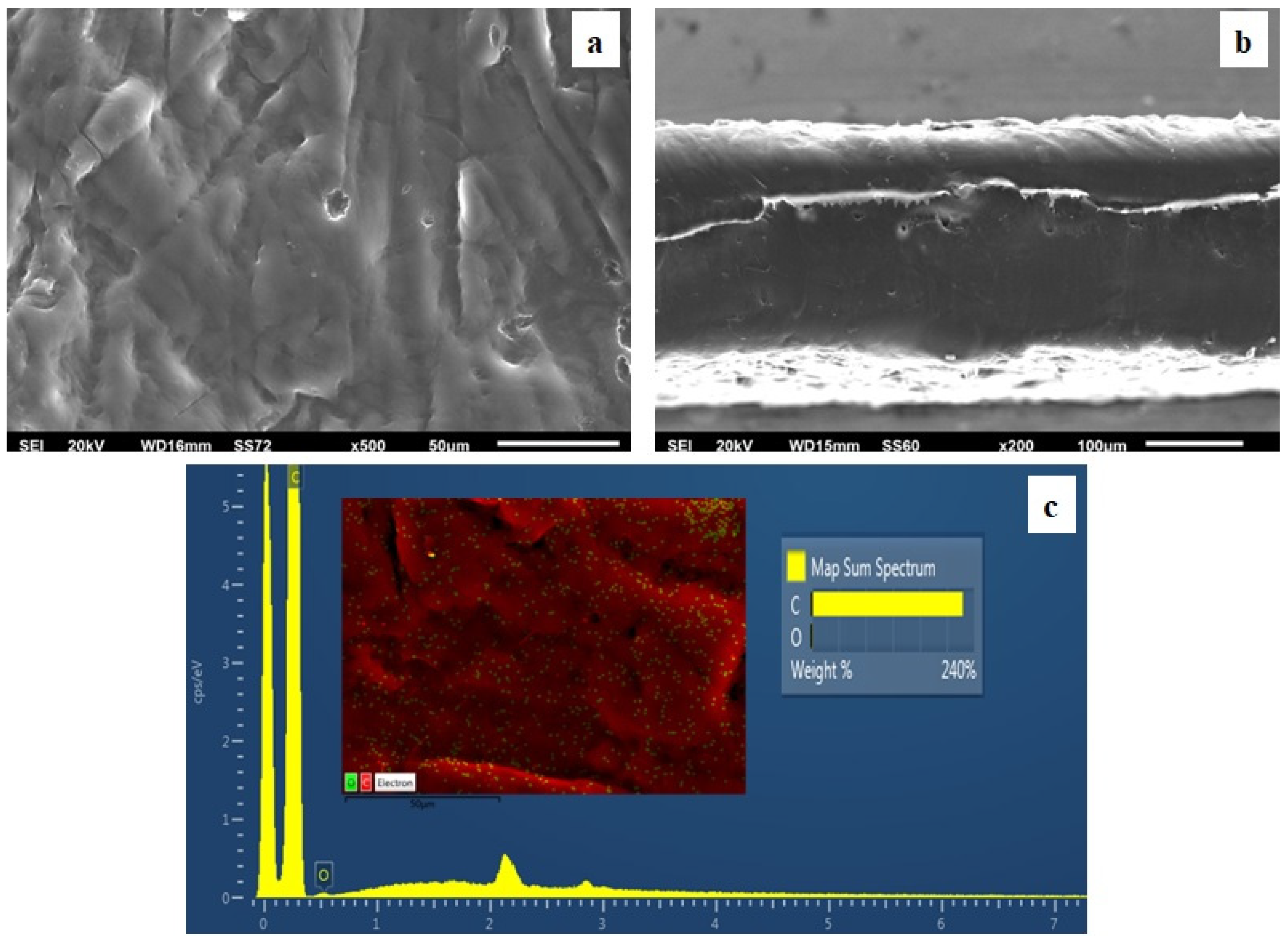
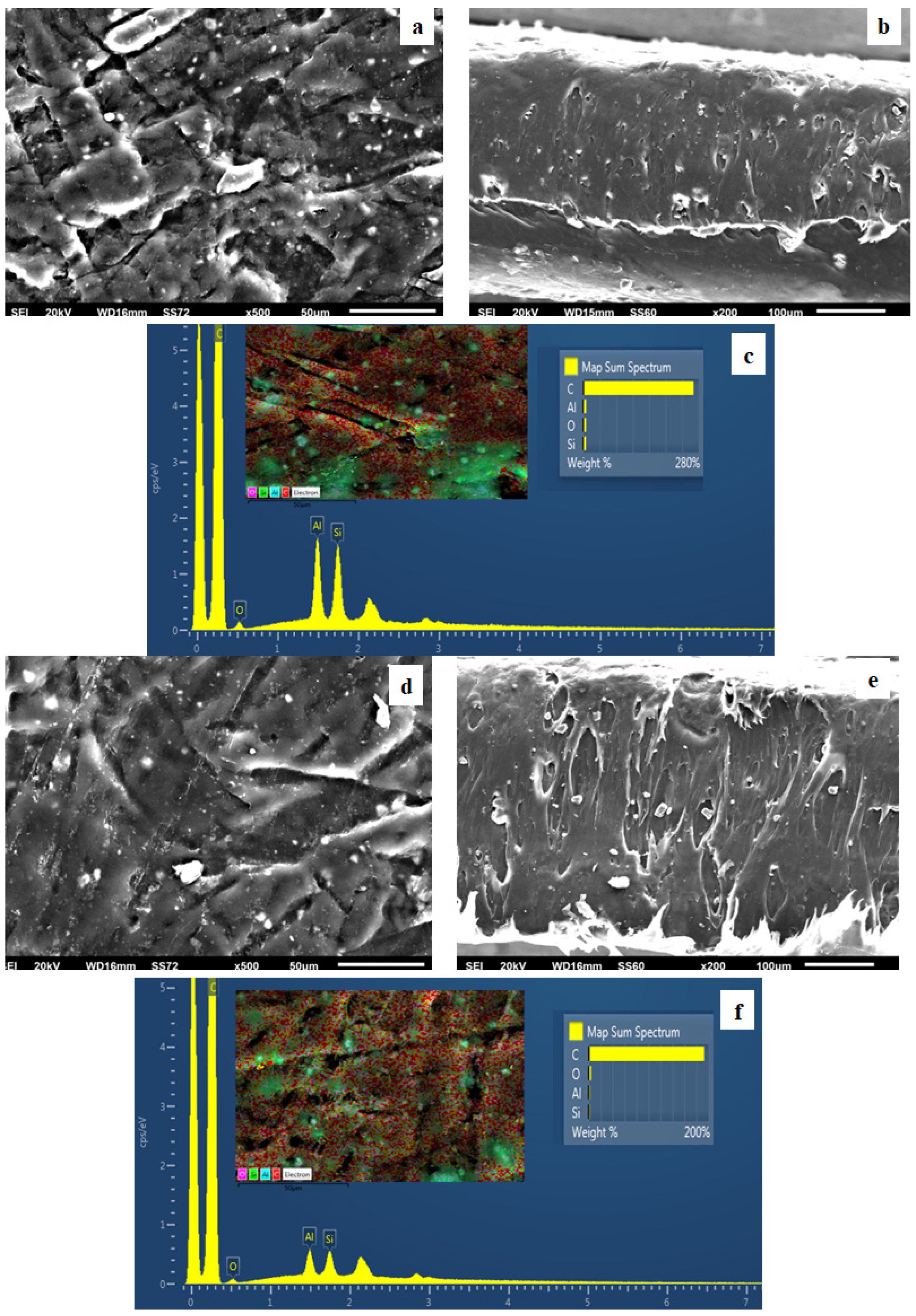
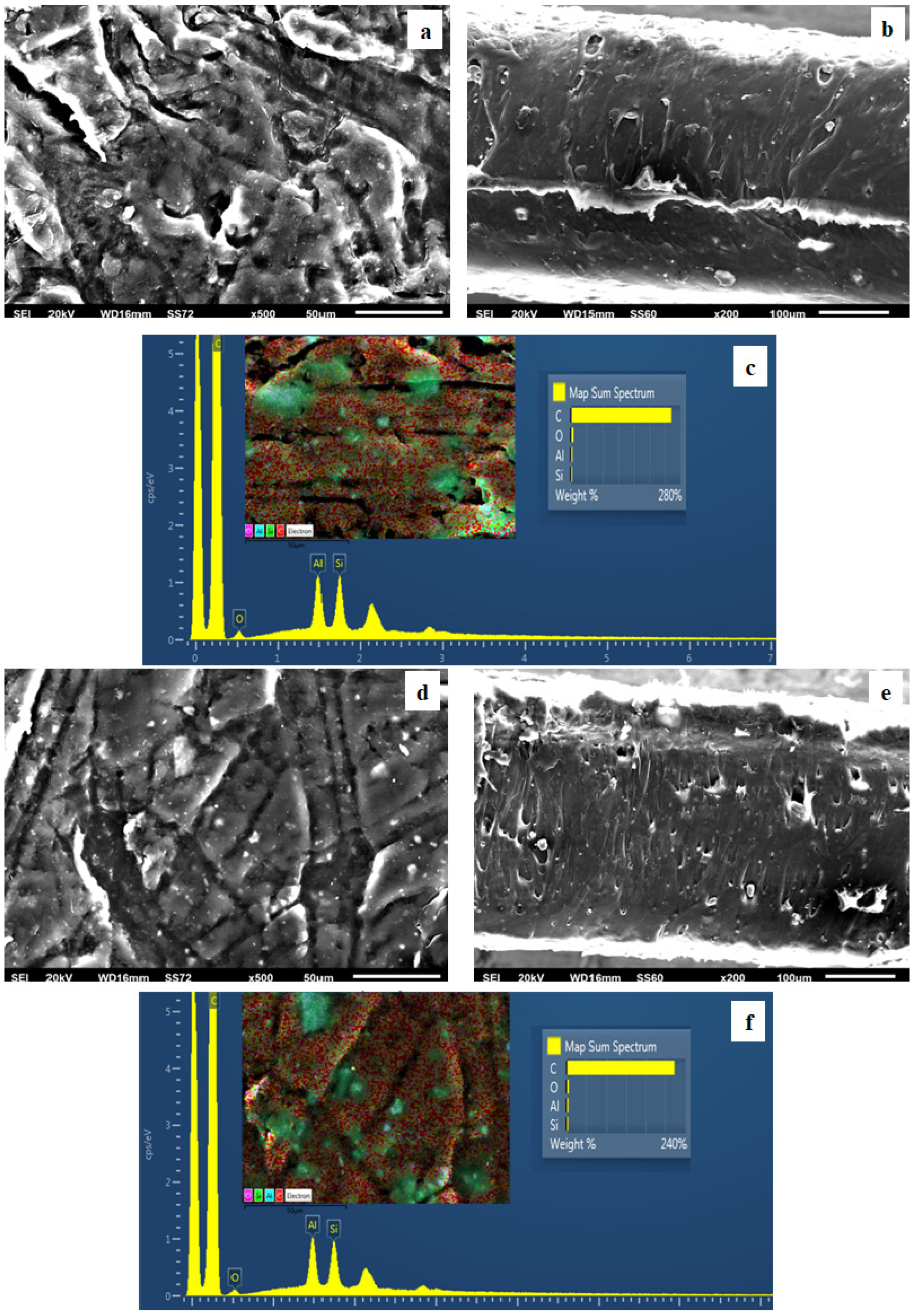
2.6. SEM Images
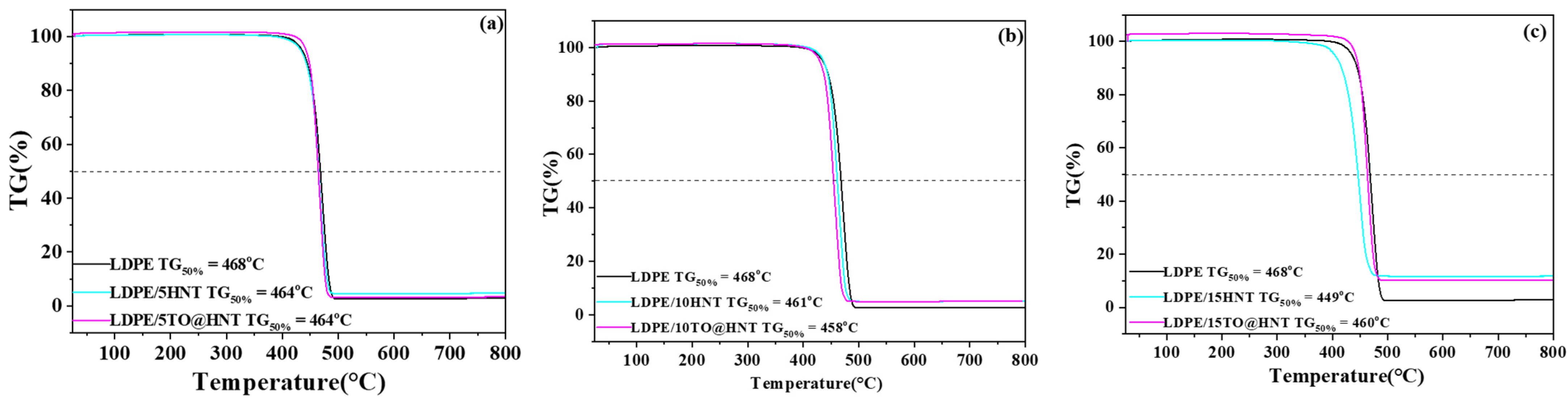
2.7. Thermogravimetric and Differential Analysis (TG-DTA)
2.8. Tensile Properties
2.9. Water Barrier Properties
2.10. Oxygen Barrier Properties
2.11. Determination of Fat Content of Scaloppini Pork Meat
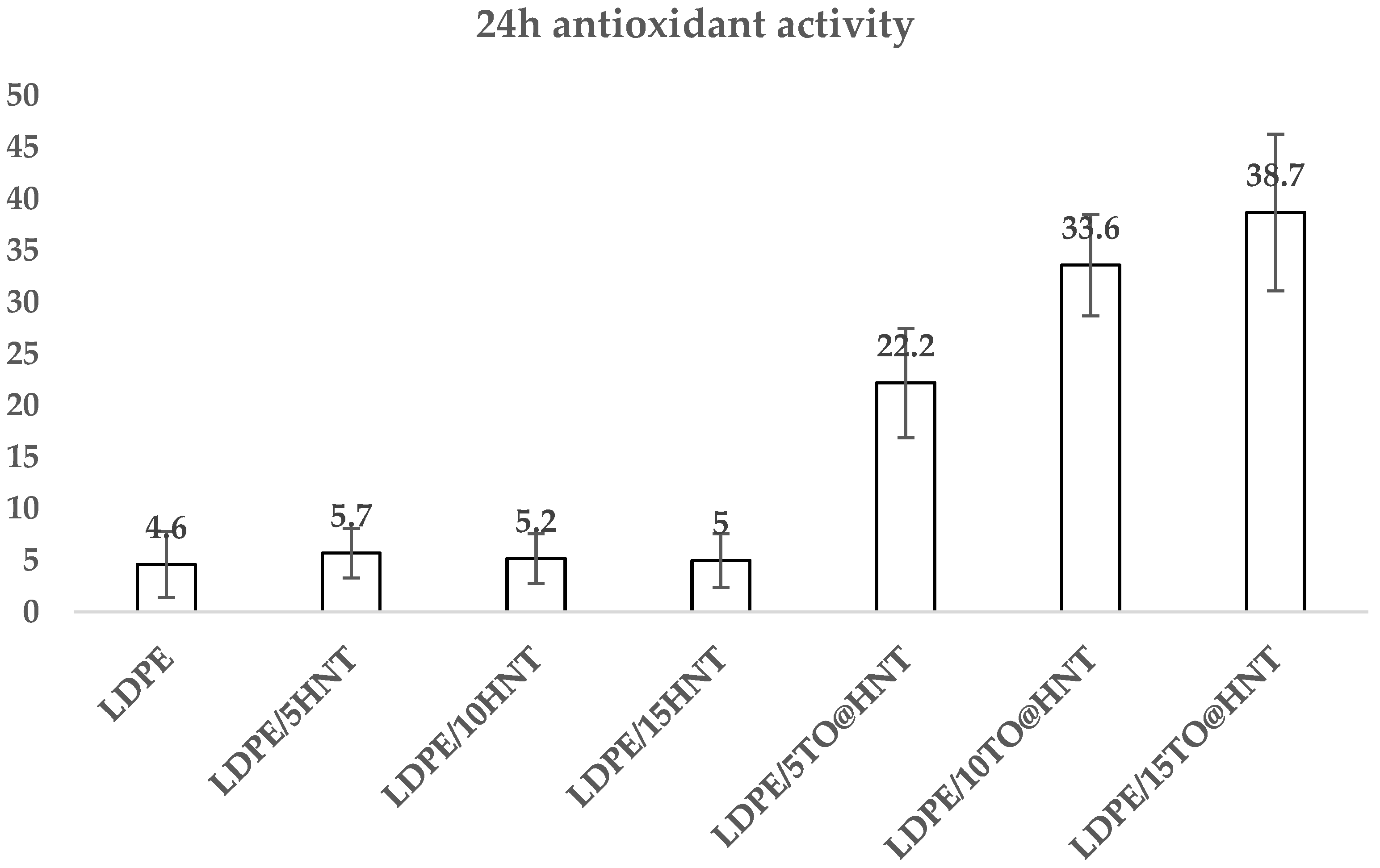
2.12. Antioxidant Activity
2.13. Packaging Preservation Test of “Scaloppini Type” Fresh Pork Meat Fillets
2.14. Lipid Oxidation of “Scaloppini” Type Fresh Pork Meat Fillets
2.14.1. Thiobarbituric Acid Reactive Substances
2.14.2. Heme Iron Content
HFe (μgHFe/gsample) = Abs640 × 680 × 0.0882
2.15. Statistical Analysis
3. Results
3.1. Characterization of Modified TO@HNT Nanohybrids
3.2. XRD Analysis of LDPE/xHNT and LDPE/xTO@HNT Films
3.3. FTIR Spectroscopy of LDPE/xHNT and LDPE/xTO@HNT Films
3.4. SEM
3.5. TG Experiments
3.6. Tensile Properties
3.7. Water Vapor Transmission Rate (WVTR) and Oxygen Transmission Rate (OTR)
3.8. Fat Content
3.9. Antioxidant Activity
3.10. Lipid Oxidation
3.10.1. TBARS
3.10.2. Heme Iron Content
3.10.3. Correlation of TBARS and Heme Iron
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Salmas, C.E.; Giannakas, A.E.; Moschovas, D.; Kollia, E.; Georgopoulos, S.; Gioti, C.; Leontiou, A.; Avgeropoulos, A.; Kopsacheili, A.; Avdylaj, L.; et al. Kiwi Fruits Preservation Using Novel Edible Active Coatings Based on Rich Thymol Halloysite Nanostructures and Chitosan/Polyvinyl Alcohol Gels. Gels 2022, 8, 823. [Google Scholar] [CrossRef]
- Kibirkštis, E.; Mayik, V.; Zatserkovna, R.; Vaitasius, K.; Stepanenko, A.; Kandrotaitė-Janutienė, R.; Venytė, I.; Danilovas, P.P. Study of physical and mechanical properties of partially biodegradable LDPE polymeric films and their application for printing and packaging. Polym. Test. 2022, 112, 107646. [Google Scholar] [CrossRef]
- Gupta, A.; Verma, J.P. Sustainable bio-ethanol production from agro-residues: A review. Renew. Sustain. Energy Rev. 2015, 41, 550–567. [Google Scholar] [CrossRef]
- Bhargava, N.; Sharanagat, V.S.; Mor, R.S.; Kumar, K. Active and intelligent biodegradable packaging films using food and food waste-derived bioactive compounds: A review. Trends Food Sci. Technol. 2020, 105, 385–401. [Google Scholar] [CrossRef]
- De Carvalho, A.P.A.; Junior, C.A.C. Green strategies for active food packagings: A systematic review on active properties of graphene-based nanomaterials and biodegradable polymers. Trends Food Sci. Technol. 2020, 103, 130–143. [Google Scholar] [CrossRef]
- Sharma, S.; Barkauskaite, S.; Jaiswal, A.K.; Jaiswal, S. Essential oils as additives in active food packaging. Food Chem. 2021, 343, 128403. [Google Scholar] [CrossRef]
- Bakkali, F.; Averbeck, S.; Averbeck, D.; Idaomar, M. Biological effects of essential oils—A review. Food Chem. Toxicol. 2008, 46, 446–475. [Google Scholar] [CrossRef]
- Adorjan, B.; Buchbauer, G. Biological properties of essential oils: An updated review. Flavour Fragr. J. 2010, 25, 407–426. [Google Scholar] [CrossRef]
- Almeida-Souza, F.; Magalhães, I.F.B.; Guedes, A.C.; Santana, V.M.; Teles, A.M.; Mouchrek, A.N.; Calabrese, K.S.; Abreu-Silva, A.L. Safety Assessment of Essential Oil as a Food Ingredient. In Essential Oils: Applications and Trends in Food Science and Technology; Santana de Oliveira, M., Ed.; Springer International Publishing: Cham, Switzerland, 2022; pp. 123–171. ISBN 978-3-030-99476-1. [Google Scholar]
- Satyal, P.; Murray, B.L.; McFeeters, R.L.; Setzer, W.N. Essential Oil Characterization of Thymus vulgaris from Various Geographical Locations. Foods 2016, 5, 70. [Google Scholar] [CrossRef]
- Dias, M.V.; de Medeiros, H.S.; Soares, N.D.F.F.; de Melo, N.R.; Borges, S.V.; Carneiro, J.D.D.S.; Pereira, J.M.T.D.A.K. Development of low-density polyethylene films with lemon aroma. LWT. Food Sci. Technol. 2013, 50, 167–171. [Google Scholar] [CrossRef]
- Solano, A.C.V.; Gante, C.D.R. Two Different Processes to Obtain Antimicrobial Packaging Containing Natural Oils. Food Bioprocess Technol. 2012, 5, 2522–2528. [Google Scholar] [CrossRef] [Green Version]
- Coelho, L.B.; Geraldine, R.M.; Silveira, M.F.A.; De Souza, A.R.M.; Torres, M.C.L.; Gonçalves, M.Á.B. Characterization of films of low density polyethylene incorporated with oregano essential oil. Res. Soc. Dev. 2020, 9, e3849108722. [Google Scholar] [CrossRef]
- Rojas, A.; Torres, A.; Martínez, F.; Salazar, L.; Villegas, C.; Galotto, M.J.; Guarda, A.; Romero, J. Assessment of kinetic release of thymol from LDPE nanocomposites obtained by supercritical impregnation: Effect of depressurization rate and nanoclay content. Eur. Polym. J. 2017, 93, 294–306. [Google Scholar] [CrossRef]
- Tornuk, F.; Hancer, M.; Sagdic, O.; Yetim, H. LLDPE based food packaging incorporated with nanoclays grafted with bioactive compounds to extend shelf life of some meat products. LWT. Food Sci. Technol. 2015, 2, 540–546. [Google Scholar] [CrossRef]
- Shemesh, R.; Krepker, M.; Natan, M.; Danin-Poleg, Y.; Banin, E.; Kashi, Y.; Nitzan, N.; Vaxman, A.; Segal, E. Novel LDPE/halloysite nanotube films with sustained carvacrol release for broad-spectrum antimicrobial activity. RSC Adv. 2015, 5, 87108–87117. [Google Scholar] [CrossRef]
- Darvish, M.; Ajji, A. Synergistic Antimicrobial Activities of Limonene with Mineral Carriers in LDPE Films for Active Packaging Application. Sci. J. Chem. 2022, 10, 32. [Google Scholar] [CrossRef]
- Giannakas, A. Na-Montmorillonite Vs. Organically Modified Montmorillonite as Essential Oil Nanocarriers for Melt-Extruded Low-Density Poly-Ethylene Nanocomposite Active Packaging Films with a Controllable and Long-Life Antioxidant Activity. Nanomaterials 2020, 10, 1027. [Google Scholar] [CrossRef]
- Krepker, M.; Zhang, C.; Nitzan, N.; Prinz-Setter, O.; Massad-Ivanir, N.; Olah, A.; Baer, E.; Segal, E. Antimicrobial LDPE/EVOH Layered Films Containing Carvacrol Fabricated by Multiplication Extrusion. Polymers 2018, 10, 864. [Google Scholar] [CrossRef] [Green Version]
- Giannakas, A.E.; Salmas, C.E.; Moschovas, D.; Zaharioudakis, K.; Georgopoulos, S.; Asimakopoulos, G.; Aktypis, A.; Proestos, C.; Karakassides, A.; Avgeropoulos, A.; et al. The Increase of Soft Cheese Shelf-Life Packaged with Edible Films Based on Novel Hybrid Nanostructures. Gels 2022, 8, 539. [Google Scholar] [CrossRef]
- Salmas, C.E.; Giannakas, A.E.; Baikousi, M.; Kollia, E.; Tsigkou, V.; Proestos, C. Effect of Copper and Titanium-Exchanged Montmorillonite Nanostructures on the Packaging Performance of Chitosan/Poly-Vinyl-Alcohol-Based Active Packaging Nanocomposite Films. Foods 2021, 10, 3038. [Google Scholar] [CrossRef]
- Tarladgis, B.G.; Watts, B.M.; Younathan, M.T.; Dugan, L. A distillation method for the quantitative determination of malonaldehyde in rancid foods. J. Am. Oil. Chem. Soc. 1960, 37, 44–48. [Google Scholar] [CrossRef]
- Karabagias, I.; Badeka, A.; Kontominas, M.G. Shelf life extension of lamb meat using thyme or oregano essential oils and.modified atmosphere packaging. Meat Sci. 2011, 88, 109–116. [Google Scholar] [CrossRef]
- Kalpalathika, P.V.M.; Clark, E.M.; Mahoney, A.W. Heme iron content in selected ready-to-serve beef products. J. Agric. Food Chem. 1991, 39, 1091–1093. [Google Scholar] [CrossRef]
- Hornsey, H.C. The colour of cooked cured pork. I.—Estimation of the Nitric oxide-Haem Pigments. J. Sci. Food Agric. 1956, 7, 534–540. [Google Scholar] [CrossRef]
- Park, S.; Marsh, K.S.; Dawson, P. Application of chitosan-incorporated LDPE film to sliced fresh red meats for shelf life extension. Meat Sci. 2010, 85, 493–499. [Google Scholar] [CrossRef]
- Martínez-Camacho, A.P.; Cortez-Rocha, M.O.; Graciano-Verdugo, A.Z.; Rodríguez-Félix, F.; Castillo-Ortega, M.M.; Burgos-Hernández, A.; Ezquerra-Brauer, J.M.; Plascencia-Jatomea, M. Extruded films of blended chitosan, low density polyethylene and ethylene acrylic acid. Carbohydr. Polym. 2013, 91, 666–674. [Google Scholar] [CrossRef]
- Giannakas, A.; Salmas, C.; Leontiou, A.; Tsimogiannis, D.; Oreopoulou, A.; Braouhli, J. Novel LDPE/Chitosan Rosemary and Melissa Extract Nanostructured Active Packaging Films. Nanomaterials 2019, 9, 1105. [Google Scholar] [CrossRef] [Green Version]
- Giannakas, A.E.; Salmas, C.E.; Leontiou, A.; Baikousi, M.; Moschovas, D.; Asimakopoulos, G.; Zafeiropoulos, N.E.; Avgeropoulos, A. Synthesis of a Novel Chitosan/Basil Oil Blend and Development of Novel Low Density Poly Ethylene/Chitosan/Basil Oil Active Packaging Films Following a Melt-Extrusion Process for Enhancing Chicken Breast Fillets Shelf-Life. Molecules 2021, 26, 1585. [Google Scholar] [CrossRef]
- Saucedo-Zuñiga, J.N.; Sánchez-Valdes, S.; Ramírez-Vargas, E.; Guillen, L.; Ramos-deValle, L.F.; Graciano-Verdugo, A.; Uribe-Calderón, J.A.; Valera-Zaragoza, M.; Lozano-Ramírez, T.; Rodríguez-González, J.A.; et al. Controlled release of essential oils using laminar nanoclay and porous halloysite/essential oil composites in a multilayer film reservoir. Microporous Mesoporous Mater. 2021, 316, 110882. [Google Scholar] [CrossRef]
- Jang, S.-H.; Jang, S.-R.; Lee, G.-M.; Ryu, J.-H.; Park, S.-I.; Park, N.-H. Halloysite Nanocapsules Containing Thyme Essential Oil: Preparation, Characterization, and Application in Packaging Materials. J. Food Sci. 2017, 82, 2113–2120. [Google Scholar] [CrossRef]
- Giannakas, A.; Tsagkalias, I.; Achilias, D.S.; Ladavos, A. A novel method for the preparation of inorganic and organo-modified montmorillonite essential oil hybrids. Appl. Clay Sci. 2017, 146, 362–370. [Google Scholar] [CrossRef]
- USDA National Nutrient Database for Standard Reference, Legacy Release|Ag Data Commons. Available online: https://data.nal.usda.gov/dataset/usda-national-nutrient-database-standard-reference-legacy-release (accessed on 1 November 2022).
- Zhang, X.; Ma, L.; Yu, Y.; Zhou, H.; Guo, T.; Dai, H.; Zhang, Y. Physico-mechanical and antioxidant properties of gelatin film from rabbit skin incorporated with rosemary acid. Food Packag. Shelf Life 2019, 19, 121–130. [Google Scholar] [CrossRef]
- Moudache, M.; Nerín, C.; Colon, M.; Zaidi, F. Antioxidant effect of an innovative active plastic film containing olive leaves extract on fresh pork meat and its evaluation by Raman spectroscopy. Food Chem. 2017, 229, 98–103. [Google Scholar] [CrossRef]
- Boeira, C.P.; Alves, J.d.S.; Flores, D.C.B.; de Moura, M.R.; Melo, P.T.S.; da Rosa, C.S. Antioxidant and antimicrobial effect of an innovative active film containing corn stigma residue extract for refrigerated meat conservation. J. Food Process. Preserv. 2021, 45, e15721. [Google Scholar] [CrossRef]
- Pretorius, B.; Schönfeldt, H.C.; Hall, N. Total and haem iron content lean meat cuts and the contribution to the diet. Food Chem. 2016, 193, 97–101. [Google Scholar] [CrossRef] [Green Version]
- Lombardi-Boccia, G.; Martinez-Dominguez, B.; Aguzzi, A. Total Heme and Non-heme Iron in Raw and Cooked Meats. J. Food Sci. 2002, 67, 1738–1741. [Google Scholar] [CrossRef]
- Antioxidant Effects of Carnosine and Phytic Acid in a Model Beef System—LEE—199—Journal of Food Science—Wiley Online Library. Available online: https://ift.onlinelibrary.wiley.com/doi/abs/10.1111/j.1365-2621.1998.tb15750.x (accessed on 15 July 2022).
- Jridi, M.; Mora, L.; Souissi, N.; Aristoy, M.-C.; Nasri, M.; Toldrá, F. Effects of active gelatin coated with henna (L. inermis) extract on beef meat quality during chilled storage. Food Control 2018, 84, 238–245. [Google Scholar] [CrossRef]


| Sample Name | E | σuts | ε% |
|---|---|---|---|
| LDPE | 183.3 ± 4.8 | 12.6 ± 0.5 | 29.3 ± 1.1 |
| LDPE/5HNT | 210.0 ± 3.9 | 11.9 ± 0.5 | 15.0 ± 1.5 |
| LDPE/10HNT | 266.7 ± 1.3 | 12.0 ± 1.1 | 11.2 ± 0.8 |
| LDPE/15HNT | 301.7 ± 2.7 | 10.5 ± 3.4 | 16.9 ± 1.0 |
| LDPE/5TO@HNT | 298.3 ± 8.4 | 12.1 ± 1.3 | 39.5 ± 1.2 |
| LDPE/10TO@HNT | 280.3 ± 3.8 | 12.9 ± 1.4 | 57.1 ± 2.8 |
| LDPE/15TO@HNT | 250.3 ± 4.6 | 11.2 ± 0.9 | 21.4 ± 1.5 |
| Sample Name | Film Thickness (mm) | Water Vapor Transmission Rate (10−7 g × cm−2 × day−1) | Water Vapor Diffusion Coefficient Dwv (10−4 cm2 × s−1) | Oxygen Transmission Rate (mL × m−2 × day−1) | Oxygen Permeability PeO2 (10−8 cm2 × s−1) |
|---|---|---|---|---|---|
| LDPE | 0.241 ± 0.004 | 3.67 ± 0.74 | 3.05 ± 0.40 | 6407 ± 320 | 17.9 ± 0.84 |
| LDPE/5HNT | 0.413 ± 0.015 | 6.90 ± 0.64 | 6.57 ± 0.56 | 3866.0 ± 413 | 18.4 ± 0.92 |
| LDPE/10HNT | 0.306 ± 0.011 | 4.70 ± 0.14 | 3.29 ± 0.84 | 2822 ± 141 | 9.9 ± 0.45 |
| LDPE/15HNT | 0.313 ± 0.013 | 3.95 ± 1.73 | 2.85 ± 0.12 | 3035 ± 151.0 | 11.1 ± 0.55 |
| LDPE/5TO@HNT | 0.160 ± 0.038 | 3.29 ± 0.42 | 1.20 ± 0.23 | 8670 ± 433.5 | 16.1 ± 0.81 |
| LDPE/10TO@HNT | 0.281 ± 0.010 | 3.00 ± 0.80 | 1.94 ± 0.48 | 938.7 ± 47 | 3.1 ± 0.15 |
| LDPE/15TO@HNT | 0.207 ± 0.012 | 5.69 ± 0.22 | 2.67 ± 0.91 | 3616 ± 180 | 8.7 ± 0.43 |
| TBARS | Day 0 | Day 2 | Day 4 | Day 6 | Day 8 | Day 10 | Day 12 |
|---|---|---|---|---|---|---|---|
| AVG ± SD | |||||||
| (mg/kg) | |||||||
| Control | 0.20 ± 0.01 | 0.29 ± 0.01 | 0.50 ± 0.01 | 0.75 ± 0.02 | 1.03 ± 0.02 | 1.21 ± 0.02 | 1.34 ± 0.02 |
| LDPE/10HNT | - | 0.25 ± 0.01 | 0.40 ± 0.00 | 0.60 ± 0.01 | 0.89 ± 0.03 | 1.08 ± 0.01 | 1.27 ± 0.01 |
| LDPE/10TO@HNT | - | 0.22 ± 0.00 | 0.36 ± 0.01 | 0.49 ± 0.01 | 0.80 ± 0.01 | 1.00 ± 0.00 | 1.20 ± 0.03 |
| ANOVA | |||||||
| F | - | 45.750 | 141.867 | 264.704 | 82.616 | 301.412 | 31.826 |
| p | - | 0.000 | 0.000 | 0.000 | 0.000 | 0.000 | 0.001 |
| Fe | Day 0 | Day 2 | Day 4 | Day 6 | Day 8 | Day 10 | Day 12 |
| AVG ± SD | |||||||
| (μg/g) | |||||||
| Control | 9.38 ± 0.09 | 7.46 ± 0.12 | 5.92 ± 0.21 | 4.26 ± 0.22 | 2.56 ± 0.07 | 0.92 ± 0.09 | 0.70 ± 0.03 |
| LDPE/10HNT | - | 7.86 ± 0.06 | 6.72 ± 0.12 | 4.92 ± 0.10 | 3.42 ± 0.12 | 1.50 ± 0.16 | 1.04 ± 0.03 |
| LDPE/10TO@HNT | - | 8.32 ± 0.03 | 7.50 ± 0.18 | 5.90 ± 0.24 | 4.72 ± 0.21 | 2.16 ± 0.16 | 1.80 ± 0.06 |
| ANOVA | |||||||
| F | - | 81.723 | 61.590 | 52.643 | 167.368 | 58.918 | 475.449 |
| p | - | 0.000 | 0.000 | 0.000 | 0.000 | 0.000 | 0.000 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Giannakas, A.E.; Salmas, C.E.; Moschovas, D.; Karabagias, V.K.; Karabagias, I.K.; Baikousi, M.; Georgopoulos, S.; Leontiou, A.; Katerinopoulou, K.; Zafeiropoulos, N.E.; et al. Development, Characterization, and Evaluation as Food Active Packaging of Low-Density-Polyethylene-Based Films Incorporated with Rich in Thymol Halloysite Nanohybrid for Fresh “Scaloppini” Type Pork Meat Fillets Preservation. Polymers 2023, 15, 282. https://doi.org/10.3390/polym15020282
Giannakas AE, Salmas CE, Moschovas D, Karabagias VK, Karabagias IK, Baikousi M, Georgopoulos S, Leontiou A, Katerinopoulou K, Zafeiropoulos NE, et al. Development, Characterization, and Evaluation as Food Active Packaging of Low-Density-Polyethylene-Based Films Incorporated with Rich in Thymol Halloysite Nanohybrid for Fresh “Scaloppini” Type Pork Meat Fillets Preservation. Polymers. 2023; 15(2):282. https://doi.org/10.3390/polym15020282
Chicago/Turabian StyleGiannakas, Aris E., Constantinos E. Salmas, Dimitrios Moschovas, Vassilios K. Karabagias, Ioannis K. Karabagias, Maria Baikousi, Stavros Georgopoulos, Areti Leontiou, Katerina Katerinopoulou, Nikolaos E. Zafeiropoulos, and et al. 2023. "Development, Characterization, and Evaluation as Food Active Packaging of Low-Density-Polyethylene-Based Films Incorporated with Rich in Thymol Halloysite Nanohybrid for Fresh “Scaloppini” Type Pork Meat Fillets Preservation" Polymers 15, no. 2: 282. https://doi.org/10.3390/polym15020282
APA StyleGiannakas, A. E., Salmas, C. E., Moschovas, D., Karabagias, V. K., Karabagias, I. K., Baikousi, M., Georgopoulos, S., Leontiou, A., Katerinopoulou, K., Zafeiropoulos, N. E., & Avgeropoulos, A. (2023). Development, Characterization, and Evaluation as Food Active Packaging of Low-Density-Polyethylene-Based Films Incorporated with Rich in Thymol Halloysite Nanohybrid for Fresh “Scaloppini” Type Pork Meat Fillets Preservation. Polymers, 15(2), 282. https://doi.org/10.3390/polym15020282












