Comparison of the Degree of Acetylation of Chitin Nanocrystals Measured by Various Analysis Methods
Abstract
:1. Introduction
2. Material and Methods
2.1. Materials
2.2. Sample Preparation
2.2.1. Chitin Nanocrystal Production
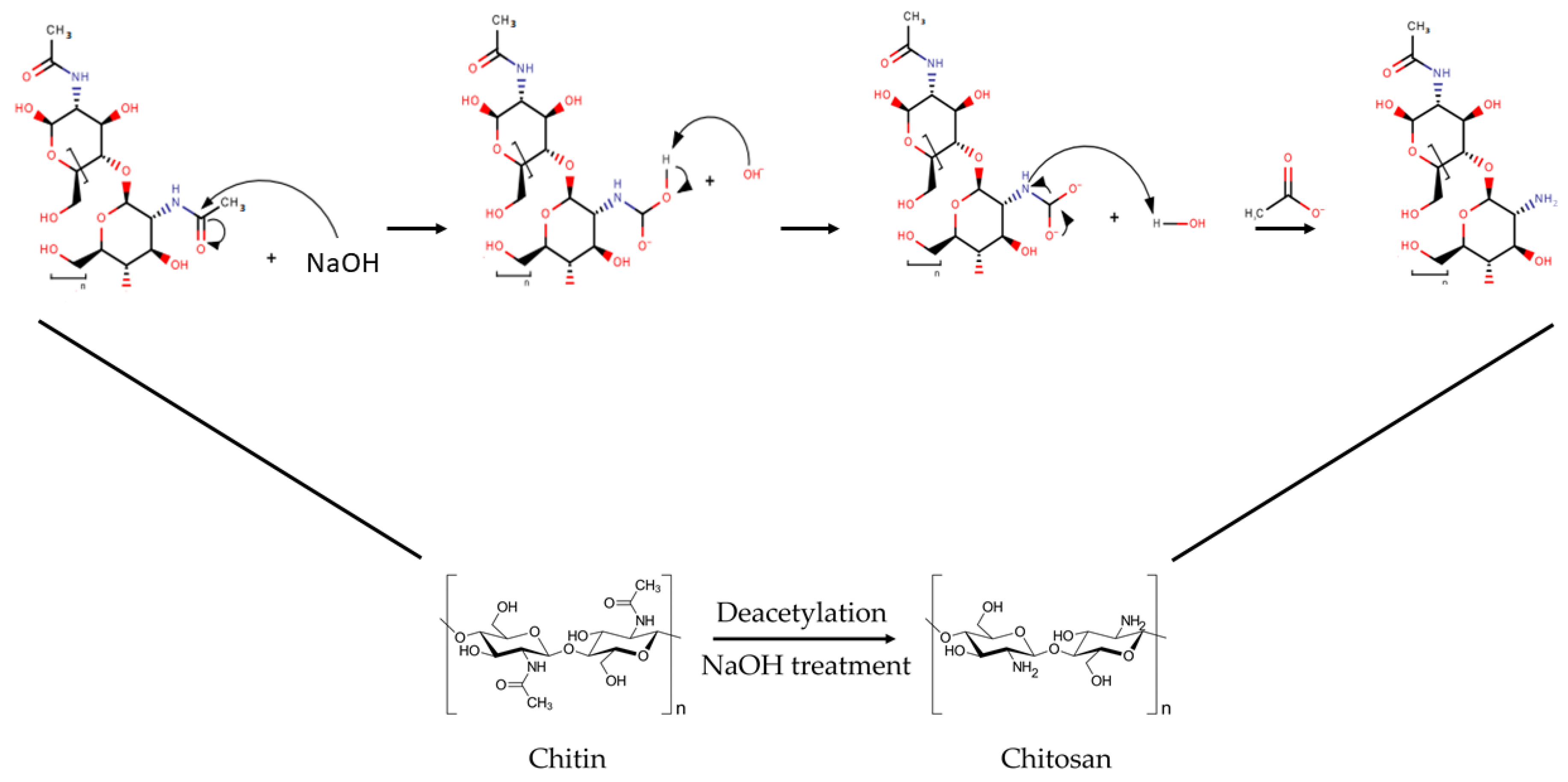
2.2.2. Deacetylation of ChNC
2.3. Quantification of the Degree of Acetylation
2.3.1. Solid-State 13C NMR
2.3.2. Liquid-State 1H NMR
2.3.3. FTIR
2.3.4. The First Derivative UV Method
3. Results and Discussion
3.1. The First Derivative UV Method Compared to 1H NMR and 13C NMR
3.2. Sample Solubility and Incubation
3.3. HMF Formation during the Solubilization Step
3.4. Other Reactions That Could Be Considered
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Appendix A
| Number | Step | Duration (h) | Temperature (°C) | Chamber Pressure (Bar) |
|---|---|---|---|---|
| 1 | Freezing | 1 | −20 | 1.03 |
| 2 | Main drying | 8 | −20 | 1.03 |
| 3 | Main drying | 8 | −15 | 0.01 |
| 4 | Main drying | 8 | −5 | 0.01 |
| 5 | Main drying | 8 | +10 | 0.01 |
| 6 | Main drying | 8 | +20 | 0.01 |
| 7 | Final drying | 7 | +20 | 1.03 |








References
- Rinaudo, M. Chitin and Chitosan: Properties and Applications. Prog. Polym. Sci. (Oxford) 2006, 31, 603–632. [Google Scholar] [CrossRef]
- Anitha, A.; Sowmya, S.; Kumar, P.T.S.; Deepthi, S.; Chennazhi, K.P.; Ehrlich, H.; Tsurkan, M.; Jayakumar, R. Chitin and Chitosan in Selected Biomedical Applications. Prog. Polym. Sci. 2014, 39, 1644–1667. [Google Scholar] [CrossRef]
- El Knidri, H.; Belaabed, R.; Addaou, A.; Laajeb, A.; Lahsini, A. Extraction, Chemical Modification and Characterization of Chitin and Chitosan. Int. J. Biol. Macromol. 2018, 120, 1181–1189. [Google Scholar] [CrossRef] [PubMed]
- Yanat, M.; Colijn, I.; Schroën, K. Chitin Nanocrystals Provide Antioxidant Activity to Polylactic Acid Films. Polymers 2022, 14, 2965. [Google Scholar] [CrossRef]
- Jayakumar, R.; Prabaharan, M.; Sudheesh Kumar, P.T.; Nair, S.V.; Tamura, H. Biomaterials Based on Chitin and Chitosan in Wound Dressing Applications. Biotechnol. Adv. 2011, 29, 322–337. [Google Scholar] [CrossRef]
- Singh, R.; Shitiz, K.; Singh, A. Chitin and Chitosan: Biopolymers for Wound Management. Int. Wound J. 2017, 14, 1276–1289. [Google Scholar] [CrossRef]
- Gassara, F.; Antzak, C.; Ajila, C.M.; Sarma, S.J.; Brar, S.K.; Verma, M. Chitin and Chitosan as Natural Flocculants for Beer Clarification. J. Food Eng. 2015, 166, 80–85. [Google Scholar] [CrossRef]
- Tastan, O.; Baysal, T. Clarification of Pomegranate Juice with Chitosan: Changes on Quality Characteristics during Storage. Food Chem. 2015, 180, 211–218. [Google Scholar] [CrossRef]
- Liu, Y.; Liu, M.; Yang, S.; Luo, B.; Zhou, C. Liquid Crystalline Behaviors of Chitin Nanocrystals and Their Reinforcing Effect on Natural Rubber. ACS Sustain. Chem. Eng. 2018, 6, 325–336. [Google Scholar] [CrossRef]
- Broers, L.; van Dongen, S.; de Goederen, V.; Ton, M.; Spaen, J.; Boeriu, C.; Schroën, K. Addition of Chitin Nanoparticles Improves Polylactic Acid Film Properties. Nanotechnol. Adv. Mater. Sci. 2018, 1, 1–8. [Google Scholar]
- Coltelli, M.-B.; Cinelli, P.; Gigante, V.; Aliotta, L.; Morganti, P.; Panariello, L.; Lazzeri, A. Chitin Nanofibrils in Poly(Lactic Acid) (PLA) Nanocomposites: Dispersion and Thermo-Mechanical Properties. Int. J. Mol. Sci. 2019, 20, 504. [Google Scholar] [CrossRef] [PubMed]
- Colijn, I.; Yanat, M.; Terhaerdt, G.; Molenveld, K.; Boeriu, C.G.; Schroën, K. Chitin Nanocrystal Hydrophobicity Adjustment by Fatty Acid Esterification for Improved Polylactic Acid Nanocomposites. Polymers 2022, 14, 2619. [Google Scholar] [CrossRef] [PubMed]
- Yanat, M.; Schroën, K. Preparation Methods and Applications of Chitosan Nanoparticles; with an Outlook toward Reinforcement of Biodegradable Packaging. React. Funct. Polym. 2021, 161, 104849. [Google Scholar] [CrossRef]
- Salaberria, A.M.; Labidi, J.; Fernandes, S.C.M. Different Routes to Turn Chitin into Stunning Nano-Objects. Eur. Polym. J. 2015, 68, 503–515. [Google Scholar] [CrossRef]
- Czechowska-Biskup, R.; Jarosińska, D.; Rokita, B.; Ulański, P.; Rosiak, J.M. Determination of Degree of Deacetylation of Chitosan—Comparision of Methods. Prog. Chem. Appl. Chitin Its Deriv. 2012, 2012, 5–20. [Google Scholar]
- Kasaai, M.R. Various Methods for Determination of the Degree of N-Acetylation of Chitin and Chitosan: A Review. J. Agric. Food Chem. 2009, 57, 1667–1676. [Google Scholar] [CrossRef]
- Dos Santos, Z.M.; Caroni, A.L.P.F.; Pereira, M.R.; da Silva, D.R.; Fonseca, J.L.C. Determination of Deacetylation Degree of Chitosan: A Comparison between Conductometric Titration and CHN Elemental Analysis. Carbohydr. Res. 2009, 344, 2591–2595. [Google Scholar] [CrossRef]
- Kasaai, M.R. Determination of the Degree of N-Acetylation for Chitin and Chitosan by Various NMR Spectroscopy Techniques: A Review. Carbohydr. Polym. 2010, 79, 801–810. [Google Scholar] [CrossRef]
- ASTM Standard F2260-18; Determining Degree of Deacetylation in Chitosan Salts by Proton Nuclear Magnetic Resonance (1H NMR) Spectroscopy. ASTM International: West Conshohocken, PA, USA, 2003.
- Kasaai, M.R. A Review of Several Reported Procedures to Determine the Degree of N-Acetylation for Chitin and Chitosan Using Infrared Spectroscopy. Carbohydr. Polym. 2008, 71, 497–508. [Google Scholar] [CrossRef]
- Han, Z.; Zeng, Y.; Lu, H.; Zhang, L. Determination of the Degree of Acetylation and the Distribution of Acetyl Groups in Chitosan by HPLC Analysis of Nitrous Acid Degraded and PMP Labeled Products. Carbohydr. Res. 2015, 413, 75–84. [Google Scholar] [CrossRef]
- Wu, T.; Zivanovic, S. Determination of the Degree of Acetylation (DA) of Chitin and Chitosan by an Improved First Derivative UV Method. Carbohydr. Polym. 2008, 73, 248–253. [Google Scholar] [CrossRef]
- Hsiao, H.Y.; Tsai, C.C.; Chen, S.; Hsieh, B.C.; Chen, R.L. Spectrophotometric Determination of Acetylation Degree of Chitinous Materials Dissolved in Phosphoric Acid. Macromol. Biosci. 2004, 4, 919–921. [Google Scholar] [CrossRef] [PubMed]
- Bosso, C.; Defaye, J.; Domard, A.; Gadelle, A.; Pedersen, C. The Behavior of Chitin towards Anhydrous Hydrogen Fluoride. Preparation of β-(1→4)-Linked 2-Acetamido-2-Deoxy-d-Glucopyranosyl Oligosaccharides. Carbohydr. Res. 1986, 156, 57–68. [Google Scholar] [CrossRef]
- Colijn, I.; Fokkink, R.; Schroën, K. Quantification of Energy Input Required for Chitin Nanocrystal Aggregate Size Reduction through Ultrasound. Sci. Rep. 2021, 11, 17217. [Google Scholar] [CrossRef] [PubMed]
- Scaffaro, R.; Botta, L.; Lopresti, F.; Maio, A.; Sutera, F. Polysaccharide Nanocrystals as Fillers for PLA Based Nanocomposites. Cellulose 2017, 24, 447–478. [Google Scholar] [CrossRef]
- Herrera, N.; Singh, A.A.; Salaberria, A.M.; Labidi, J.; Mathew, A.P.; Oksman, K. Triethyl Citrate (TEC) as a Dispersing Aid in Polylactic Acid/Chitin Nanocomposites Prepared via Liquid-Assisted Extrusion. Polymers 2017, 9, 406. [Google Scholar] [CrossRef] [Green Version]
- Heux, L.; Brugnerotto, J.; Desbrières, J.; Versali, M.F.; Rinaudo, M. Solid State NMR for Determination of Degree of Acetylation of Chitin and Chitosan. Biomacromolecules 2000, 1, 746–751. [Google Scholar] [CrossRef]
- Hirai, A.; Odani, H.; Nakajima, A. Determination of Degree of Deacetylation of Chitosan by 1H NMR Spectroscopy. Polym. Bull. 1991, 26, 87–94. [Google Scholar] [CrossRef]
- Shigemasa, Y.; Matsuura, H.; Sashiwa, H.; Saimoto, H. Evaluation of Different Absorbance Ratios from Infrared Spectroscopy for Analyzing the Degree of Deacetylation in Chitin. Int. J. Biol. Macromol. 1996, 18, 237–242. [Google Scholar] [CrossRef]
- Hein, S.; Ng, C.H.; Stevens, W.F.; Wang, K. Selection of a Practical Assay for the Determination of the Entire Range of Acetyl Content in Chitin and Chitosan: UV Spectrophotometry with Phosphoric Acid as Solvent. J. Biomed. Mater. Res.—Part B Appl. Biomater. 2008, 86, 558–568. [Google Scholar] [CrossRef]
- Lee, S.B.; Jeong, G.T. Catalytic Conversion of Chitosan to 5-Hydroxymethylfurfural Under Low Temperature Hydrothermal Process. Appl. Biochem. Biotechnol. 2015, 176, 1151–1161. [Google Scholar] [CrossRef]
- Feng, J.X.; Zang, H.J.; Yan, Q.; Li, M.G.; Cheng, B.W. Conversion of Chitosan into 5-Hydroxymethylfurfural via Hydrothermal Synthesis. Adv. Mater. Res. 2015, 1095, 411–414. [Google Scholar] [CrossRef]
- Zhou, D.; Shen, D.; Lu, W.; Song, T.; Wang, M.; Feng, H.; Shentu, J.; Long, Y. Production of 5-Hydroxymethylfurfural from Chitin Biomass: A Review. Molecules 2020, 25, 541. [Google Scholar] [CrossRef]



| 1H NMR | 13C NMR | FTIR b | First Derivative UV Method c | |
|---|---|---|---|---|
| Chitosan powder a | 21% | 24% | 24% | 25% |
| Chitin powder | (-) | 102% | 98% | 92% |
| ChNC | (-) | 94% d | 75% d | 94% |
| D-ChNC | (-) | 84% | 60% | 43% |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yanat, M.; Colijn, I.; de Boer, K.; Schroën, K. Comparison of the Degree of Acetylation of Chitin Nanocrystals Measured by Various Analysis Methods. Polymers 2023, 15, 294. https://doi.org/10.3390/polym15020294
Yanat M, Colijn I, de Boer K, Schroën K. Comparison of the Degree of Acetylation of Chitin Nanocrystals Measured by Various Analysis Methods. Polymers. 2023; 15(2):294. https://doi.org/10.3390/polym15020294
Chicago/Turabian StyleYanat, Murat, Ivanna Colijn, Kieke de Boer, and Karin Schroën. 2023. "Comparison of the Degree of Acetylation of Chitin Nanocrystals Measured by Various Analysis Methods" Polymers 15, no. 2: 294. https://doi.org/10.3390/polym15020294
APA StyleYanat, M., Colijn, I., de Boer, K., & Schroën, K. (2023). Comparison of the Degree of Acetylation of Chitin Nanocrystals Measured by Various Analysis Methods. Polymers, 15(2), 294. https://doi.org/10.3390/polym15020294








