A Novel Cationic Polymer Surfactant for Regulation of the Rheological and Biocidal Properties of the Water-Based Drilling Muds
Abstract
1. Introduction
2. Experimental
2.1. Materials
2.2. Methods
2.2.1. Spectrometric Methods
2.2.2. Determination of the Monomer Reactivity Ratios
2.2.3. Structure-Formation Test
2.2.4. Antimicrobial Action of the DADMAC–DMAPMA Copolymer against SRB Investigation
2.2.5. Synthesis
3. Results and Discussion
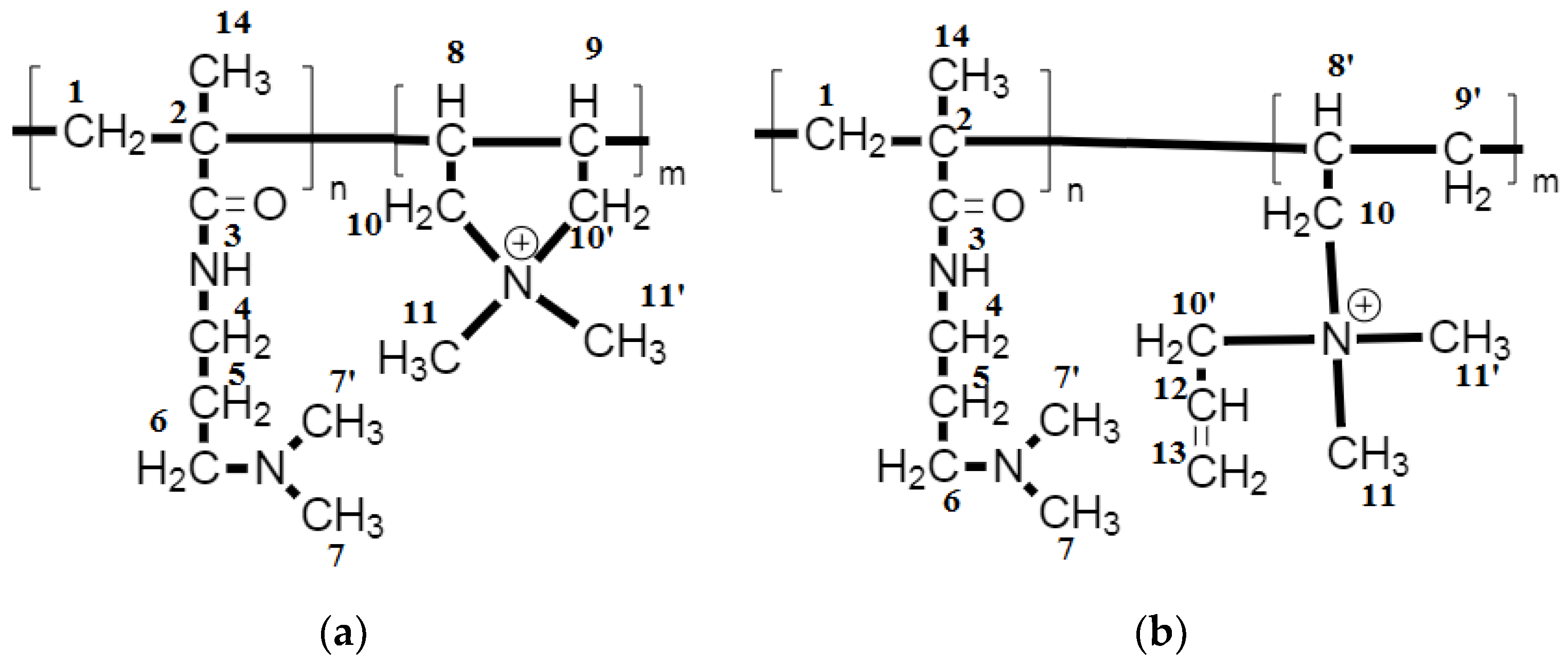
3.1. Determination of Copolymer Composition
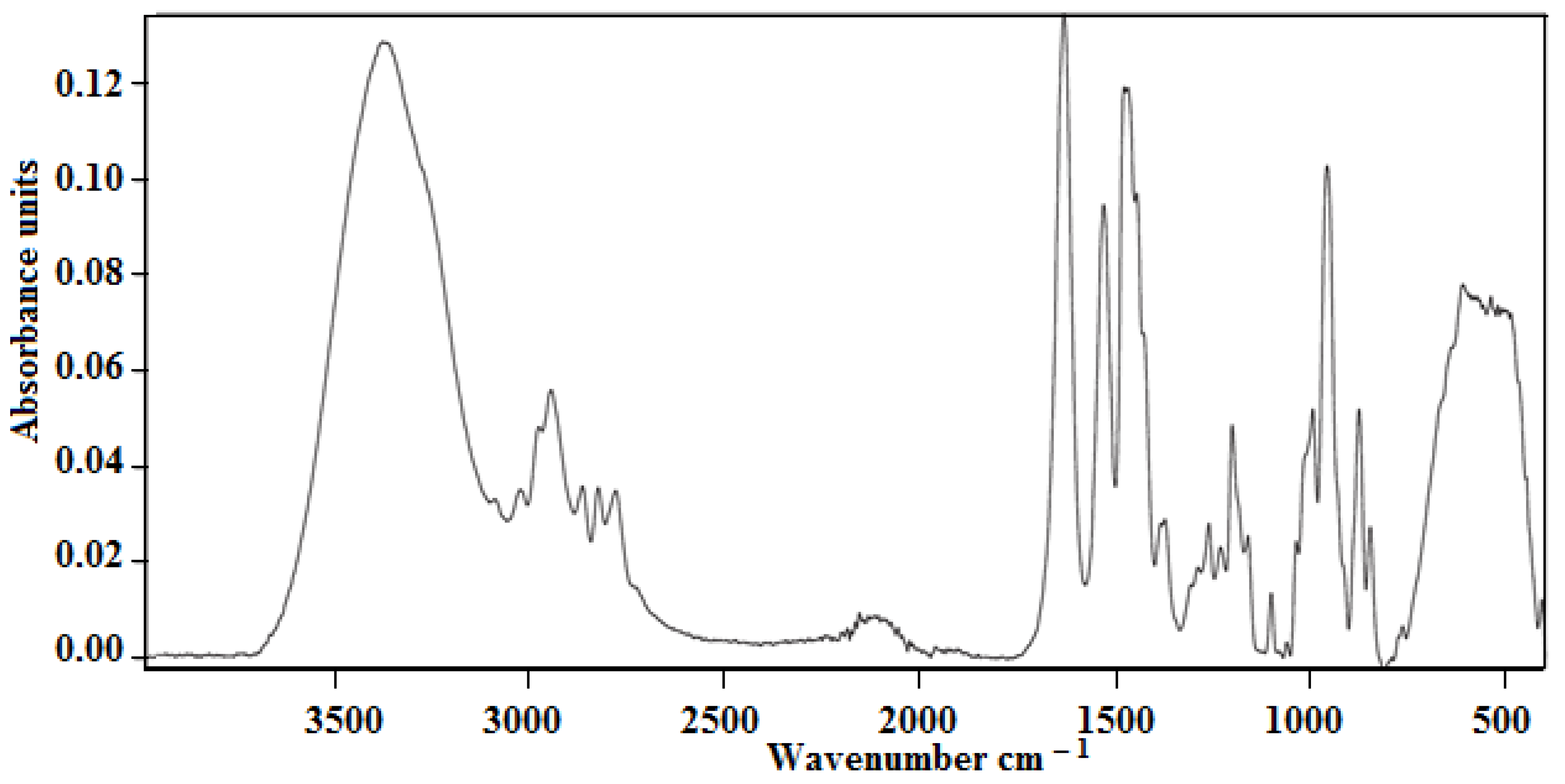
3.1.1. The Results of Infrared Spectroscopic Studies
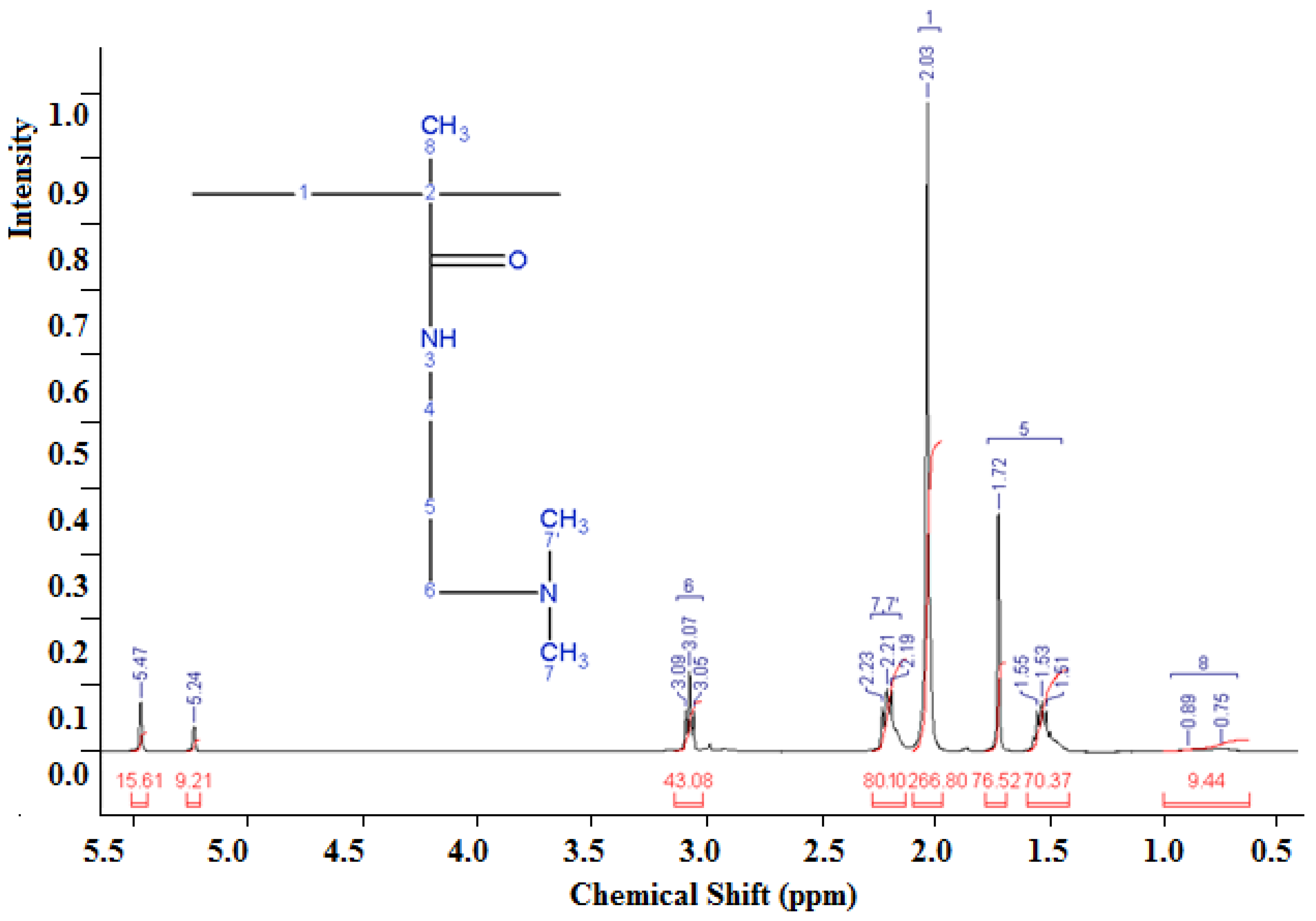
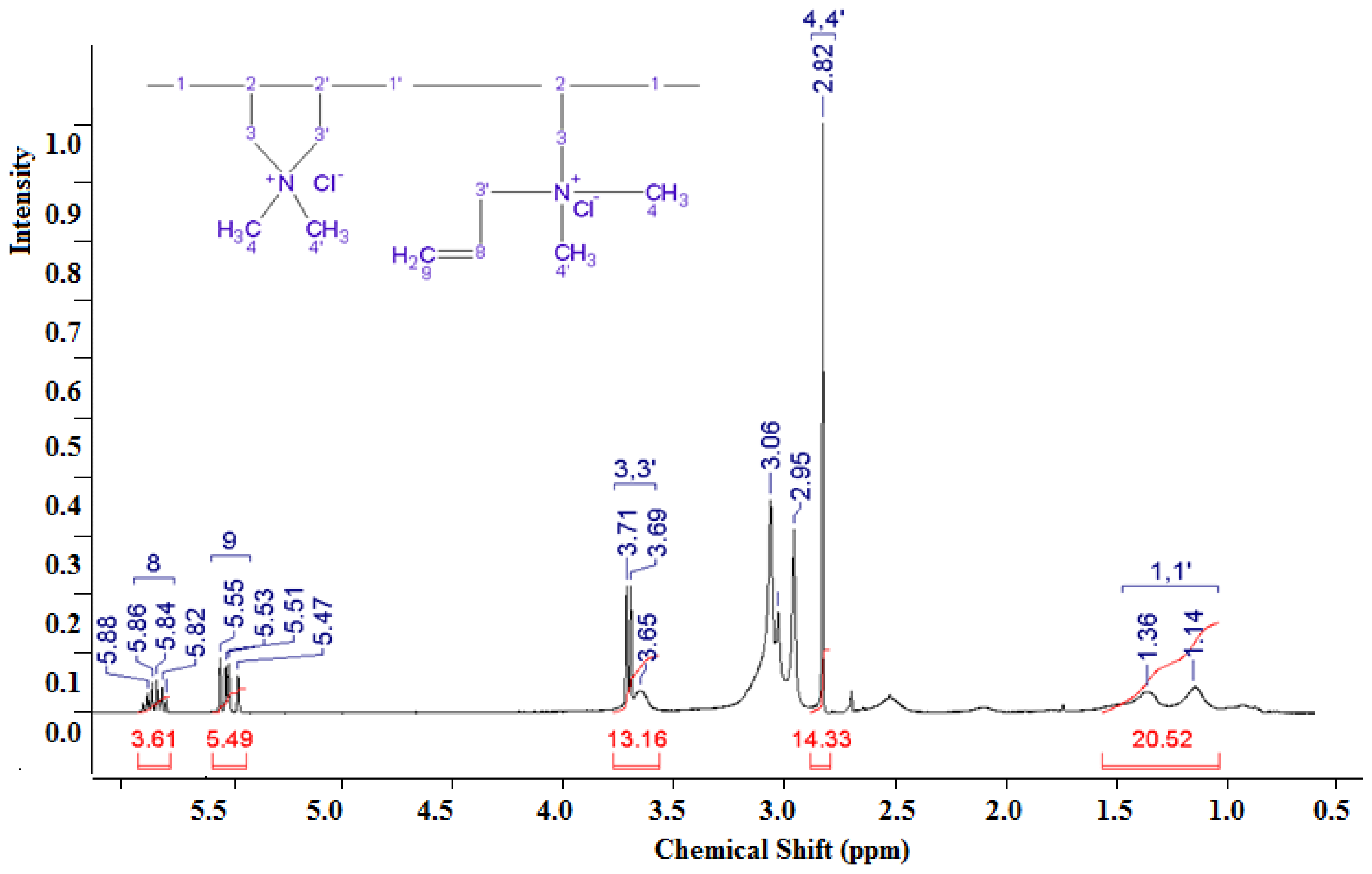
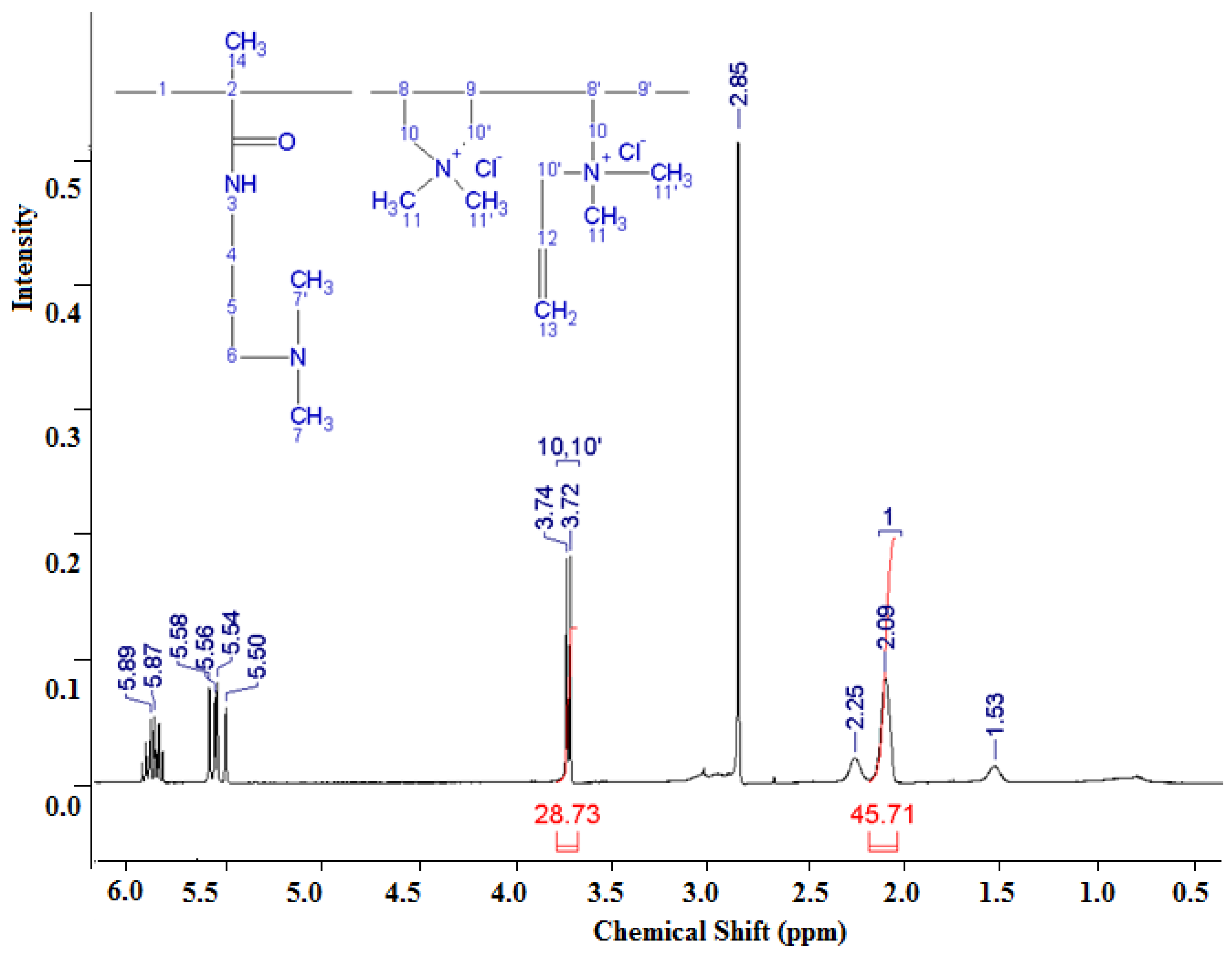
3.1.2. Analysis of the NMR Spectrum of the DADMAC–DMAPMA Copolymer
3.2. Monomer Reactivity Ratios
3.3. Results of Elemental Analysis and Conductometric Titration
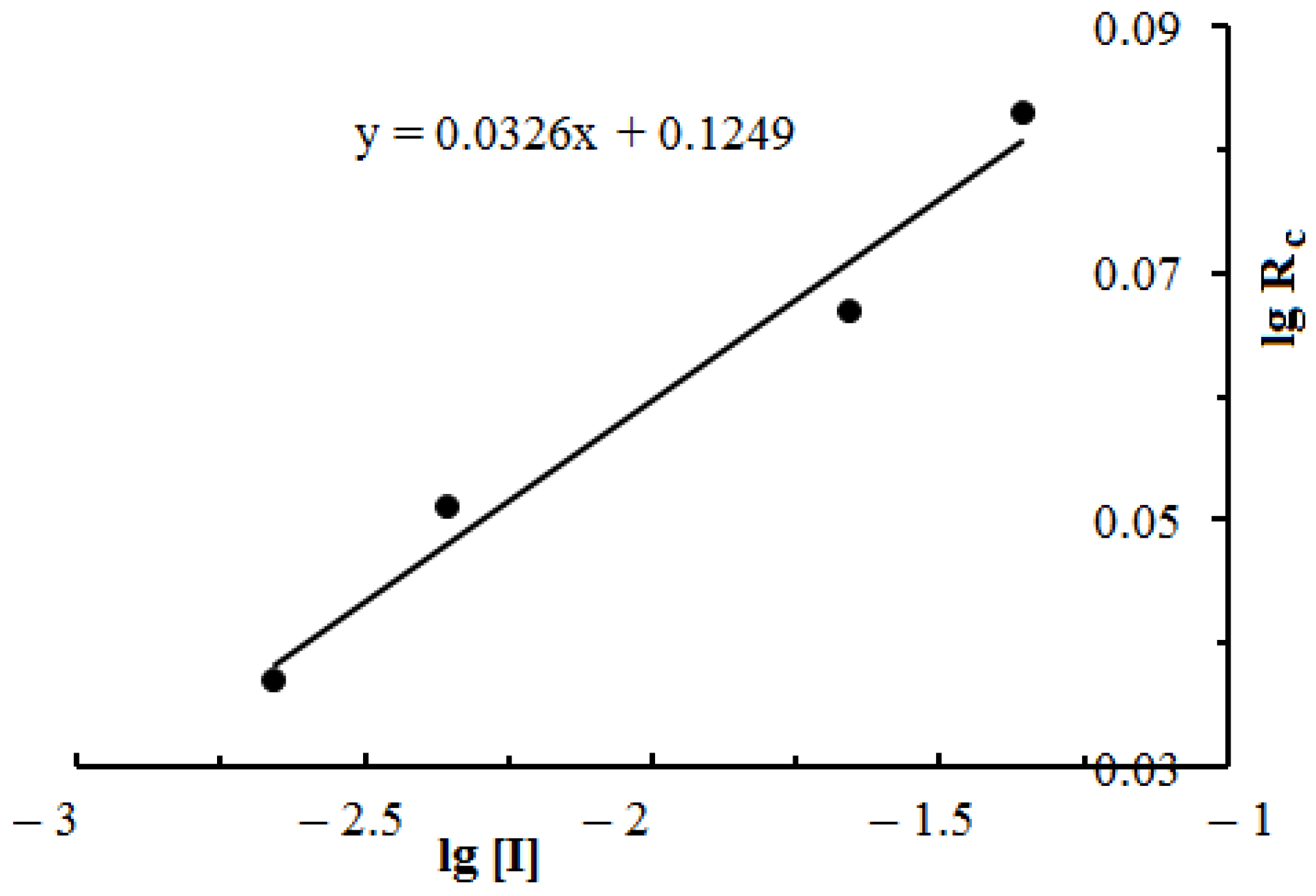
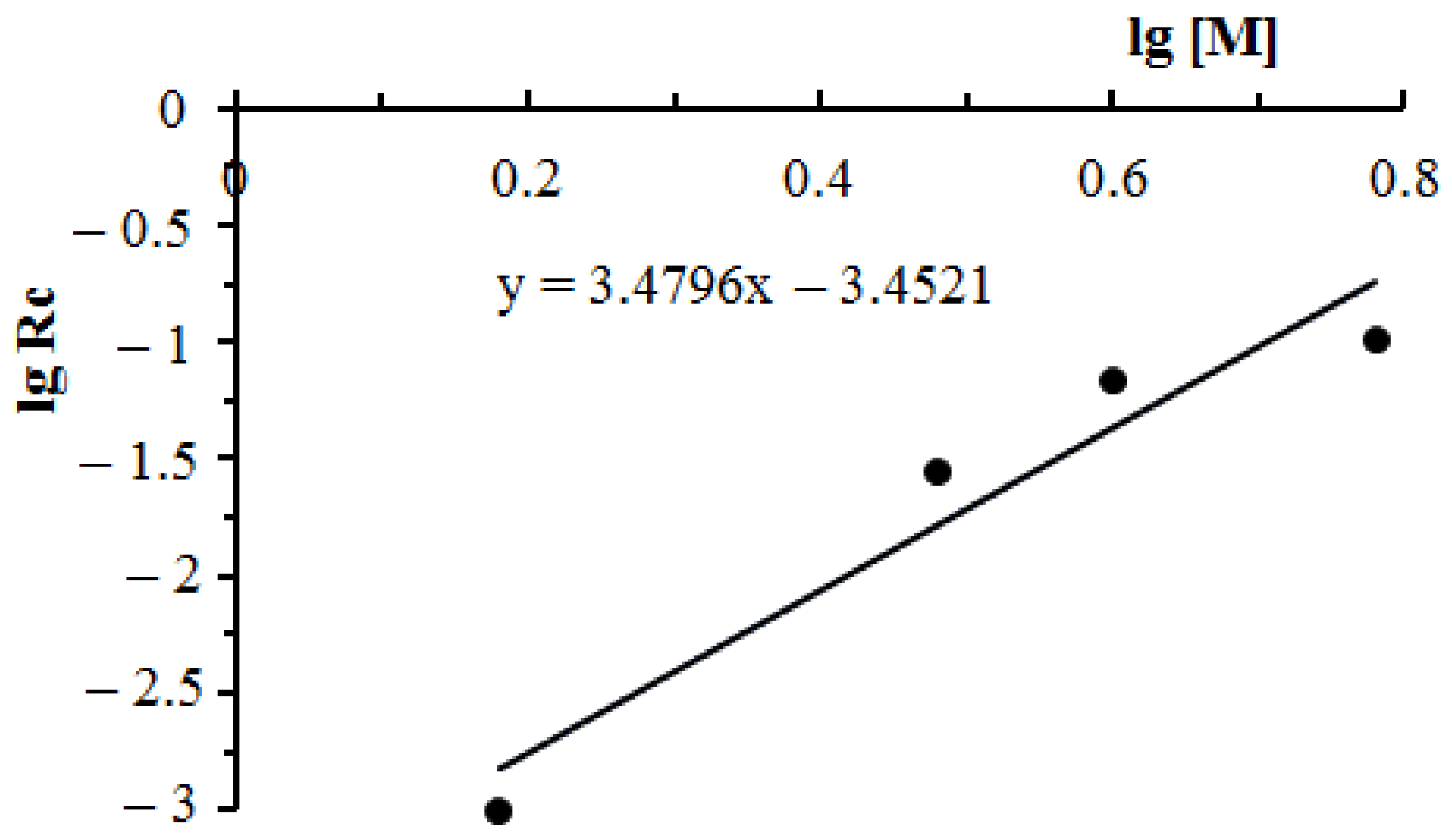
3.4. Study of the Influence of the Initiator and Monomers’ Concentration on the Kinetics and the Conversion of the Copolymerization Reaction of DADMAC with DMAPMA
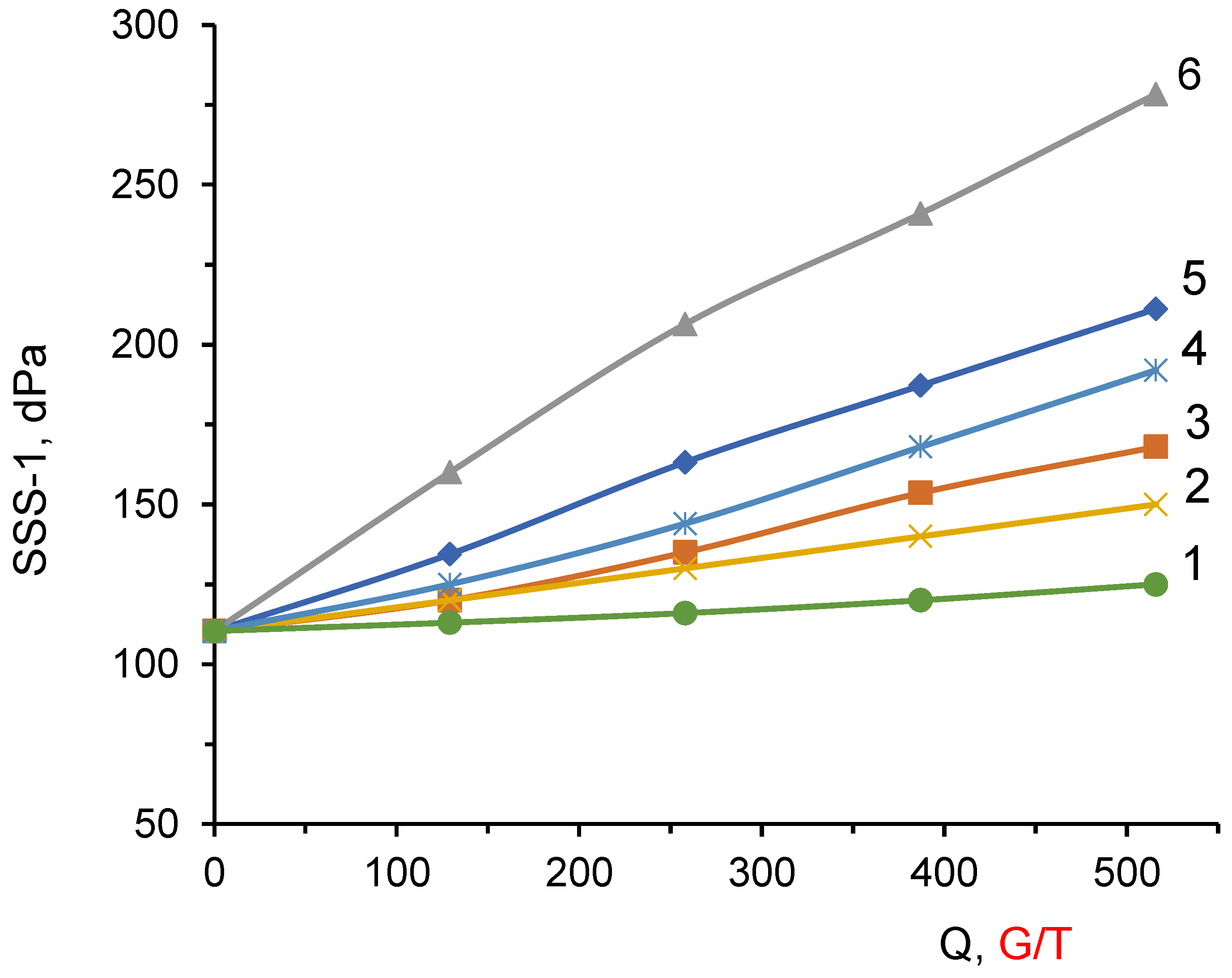
3.5. Structure-Formation Properties of the DADMAC–DMAPMA Copolymers
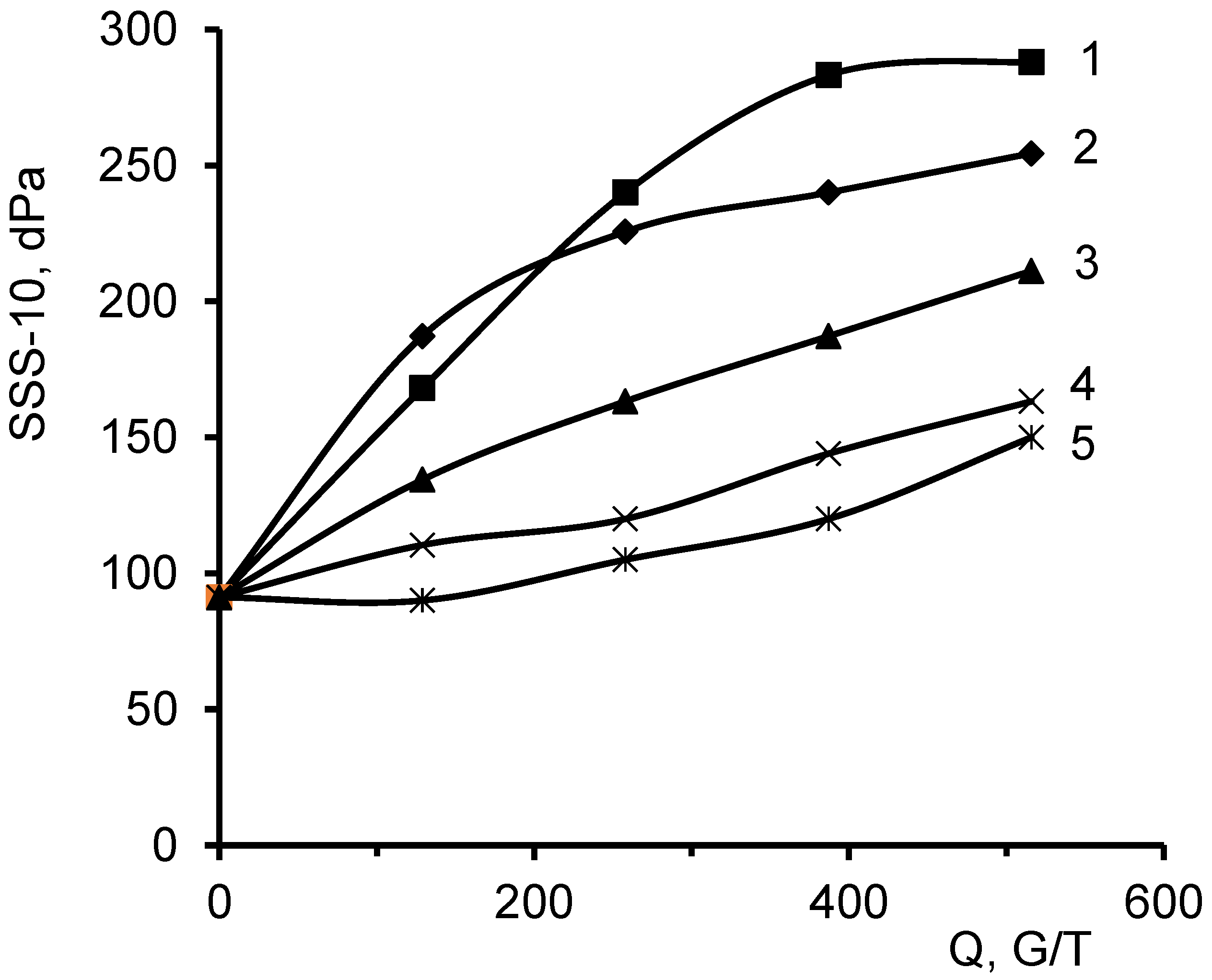
3.6. Investigations of the Antibacterial Activity of the DADMAC–DMAPMA Copolymer against the Growth of SRB
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Hamad, B.A.; He, M.; Xu, M.; Liu, W.; Mpelwa, M.; Tang, S.; Jin, L.; Song, J. A Novel Amphoteric Polymer as a Rheology Enhancer and Fluid-Loss Control Agent for Water-Based Drilling Muds at Elevated Temperatures. ACS Omega 2020, 5, 8483–8495. [Google Scholar] [CrossRef] [PubMed]
- Ghojogh, J.N. Application of smart technologies in drilling fluids. J. Neshat Ghojogh 2016, 1939, 8647. [Google Scholar]
- AVA Drilling Fluid and Services, Drilling Fluid Manual. 2002. Available online: https://vdocuments.mx/94061838-drilling-fluids-manual.html?page=1. (accessed on 9 August 2015).
- Drilling fluids manual, Amoco Production Company. 2000. Available online: https://www.academia.edu/27075486/Amoco_Production_Company_Drilling_Fluids_Manual (accessed on 1 March 2016).
- Bayat, A.E.; Shams, R. Appraising the impacts of SiO2, ZnO and TiO2 nanoparticles on rheological properties and shale inhibition of water-based drilling Muds. Colloids Surf. A Physicochem. Eng. Asp. 2019, 581, 123792. [Google Scholar] [CrossRef]
- Rafieefar, A.; Sharif, F.; Hashemi, A.; Bazargan, A.M. Rheological Behavior and Filtration of Water-Based Drilling Fluids Containing Graphene Oxide: Experimental Measurement, Mechanistic Understanding, and Modeling. ACS Omega 2021, 6, 29905–29920. [Google Scholar] [CrossRef] [PubMed]
- Ahmed, A.W.; Kalkan, E. Drilling Fluids; Types, Formation Choice and Environmental Impact. Int. J. Latest Technol. Eng. Manag. Appl. Sci. 2019, 8, 66–71. [Google Scholar]
- Li, M.-C.; Wu, Q.; Song, K.; French, A.D.; Mei, C.; Lei, T. pH-Responsive Water-Based Drilling Fluids Containing Bentonite and Chitin Nanocrystals. ACS Sustain. Chem. Eng. 2018, 6, 3783–3795. [Google Scholar] [CrossRef]
- Zoveidavianpoor, M.; Samsuri, A. The use of nanosized Tapioca starch as a natural water-soluble polymer for filtration control in water-based drilling muds. J. Nat. Gas Sci. Eng. 2016, 34, 832–840. [Google Scholar] [CrossRef]
- Salih, A.; Elshehabi, T.; Bilgesu, H. Impact of nanomaterials on the rheological and fil-tration properties of water-based drilling fluids. In Proceedings of the SPE Eastern Regional Meeting, Canton, OH, USA, 13–15 September 2016. [Google Scholar] [CrossRef]
- Fink, J. Petroleum Engineer’s Guide to Oil Field Chemicals and Fluids, 3rd ed.; Elsevier: Amsterdam, The Netherlands, 2021. [Google Scholar]
- Quan, H.; Li, H.; Huang, Z.; Zhang, T.; Dai, S. Copolymer SJ-1 as a Fluid Loss Additive for Drilling Fluid with High Content of Salt and Calcium. Int. J. Polym. Sci. 2014, 2014, 201301. [Google Scholar] [CrossRef]
- Sensoy, T.; Chenevert, M.E.; Sharma, M.M. Minimizing water invasion in shales using nanoparticles. In Proceedings of the SPE Annual Technical Conference and Exhibition, New Orleans, LA, USA, 4–7 October 2009. [Google Scholar] [CrossRef]
- Tian, Y.; Liu, X.; Luo, P.; Huang, J.; Xiong, J.; Liang, L.; Li, W. Study of a Polyamine Inhibitor Used for Shale Water-Based Drilling Fluid. ACS Omega 2021, 6, 15448–15459. [Google Scholar] [CrossRef]
- Wan, T.; Yao, J.; Zishun, S.; Li, W.; Juan, W. Solution and drilling fluid properties of water soluble AM-AA-SSS copolymers by inverse microemulsion. J. Petrol. Sci. Eng. 2011, 78, 334–337. [Google Scholar] [CrossRef]
- Tan, X.; Duan, L.; Han, W.; Li, Y.; Guo, M. A Zwitterionic Copolymer as Rheology Modifier and Fluid Loss Agents for Water-Based Drilling Fluids. Polymers 2021, 13, 3120. [Google Scholar] [CrossRef] [PubMed]
- Ahmad, H.M.; Kamal, M.S.; Al-Harthi, M.A. Rheological and filtration properties of clay-polymer systems: Impact of polymer structure. Appl. Clay Sci. 2018, 160, 226–237. [Google Scholar] [CrossRef]
- Chu, Q.; Lin, L. Effect of molecular flexibility on the rheological and filtration properties of synthetic polymers used as fluid loss additives in water-based drilling fluid. RSC Adv. 2019, 9, 8608–8619. [Google Scholar] [CrossRef] [PubMed]
- Ma, X.; Zhu, Z.; Shi, W.; Hu, Y. Synthesis and application of a novel betaine-type copolymer as fluid loss additive for water-based drilling fluid. Colloid Polym. Sci. 2017, 295, 53–66. [Google Scholar] [CrossRef]
- Nwosu, O.U.; Ewulonu, C.M. Rheological Behaviour of Eco-friendly Drilling Fluid from Biopolymers. J. Polym. Biopolym. Phy. Chem. 2014, 2, 50–54. [Google Scholar] [CrossRef]
- Li, M.-C.; Wu, Q.; Song, K.; De Hoop, C.F.; Lee, S.; Qing, Y.; Wu, Y. Cellulose Nano-crystals and Polyanionic Cellulose as Additives in Bentonite Water-Based Drilling Fluids: Rheological Modeling and Filtration Mechanisms. Ind. Eng. Chem. Res. 2016, 55, 133–143. [Google Scholar] [CrossRef]
- Li, M.-C.; Wu, Q.; Song, K.; Qing, Y.; Wu, Y. Cellulose Nanoparticles as Modifiers for Rheology and Fluid Loss in Bentonite Water-based Fluids. ACS Appl. Mater. Interfaces 2015, 7, 5006–5016. [Google Scholar] [CrossRef]
- Azizi, A.; Ibrahim, M.S.N.; Hamid, K.H.K.; Sauki, A.; Ghazali, N.A.; Mohd, T.A.T. Agarwood Waste as A New Fluid Loss Control Agent in Water-based Drilling Fluid. Int. J. Sci. Eng. 2013, 5, 101–105. [Google Scholar] [CrossRef]
- William, J.K.M.; Ponmani, S.; Samuel, R.; Nagarajan, R.; Sangwai, J.S. Effect of CuO and ZnO nanofluids in xanthan gum on thermal, electrical and high-pressure rheology of water-based drilling fluids. J. Petrol. Sci. Eng. 2014, 117, 15–27. [Google Scholar] [CrossRef]
- Navarrete, R.C.; Himes, R.E.; Seheult, J.M. Applications of Xanthan Gum in Fluid-Loss Control and Related Formation Damage. In Proceedings of the SPE Permian Basin Oil and Gas Recovery Conference, Midland, TX, USA, 21–23 March 2000; 21p. [Google Scholar] [CrossRef]
- Zhou, G.; Qiu, Z.; Zhong, H.; Zhao, X.; Kong, X. Study of Environmentally Friendly Wild Jujube Pit Powder as a Water-Based Drilling Fluid Additive. ACS Omega 2021, 6, 1436–1444. [Google Scholar] [CrossRef]
- Li, X.; Jiang, G.; Shen, X.; Li, G. Application of Tea Polyphenols as a Biodegradable Fluid Loss Additive and Study of the Filtration Mechanism. ACS Omega 2020, 5, 3453–3461. [Google Scholar] [CrossRef]
- Nasiri, A.; Ameri Shahrabi, M.J.; Sharif Nik, M.A.; Heidari, H.; Valizadeh, M. Influence of monoethanolamine on thermal stability of starch in water based drilling fluid system. Pet. Explor. Dev. 2018, 45, 167–171. [Google Scholar] [CrossRef]
- Dias, F.T.G.; Souza, R.R.; Lucas, E.F. Influence of modified starches composition on their performance as fluid loss additives in invert-emulsion drilling fluids. Fuel 2015, 140, 711–716. [Google Scholar] [CrossRef]
- Zhong, H.; Kong, X.; Chen, S.; Grady, B.P.; Qiu, Z. Preparation, characterization and filtration control properties of crosslinked starch nanospheres in water-based drilling fluids. J. Mol. Liq. 2021, 325, 115221. [Google Scholar] [CrossRef]
- Jimoh, M.O.; Salawudeen, T.O.; Arinkoola, A.O.; Daramola, M.O. Rheological study of a new water-based drilling fluid using Ubakala clay in the presence of natural polymers. Chem. Eng. Commun. 2020, 208, 1335–1343. [Google Scholar] [CrossRef]
- Zhang, L.; Wu, X.; Sun, Y.; Cai, J.; Lyu, S. Experimental study of the pomelo peel powder as novel shale inhibitor in water-based drilling fluids. Energy Explor. Explor. 2020, 38, 569–588. [Google Scholar] [CrossRef]
- Minaev, K.M.; Martynova, D.O.; Knyazev, A.S.; Zakharov, A.S. Investigation of properties of drilling fluids containing glyoxal and glyoxal modified polysaccharides. Tomsk State Univ. J. 2014, 380, 225–229. (In Russian) [Google Scholar]
- Morozov, Y.D.; Molodkin, S.V. The use of bactericides and corrosion inhibitors in oil production processes. J. Ekspozitsiya Neft. 2009, 2, 23–25. (In Russian) [Google Scholar]
- Dauletov, Y.; Abdiyev, K.; Toktarbay, Z.; Nuraje, N.; Zhursumbaeva, M.; Kenzhaliyev, B. Radical Polymerization and Kinetics of N,N-diallyl-N,N-dimethylammonium Chloride and Vinyl Ether of Monoethanolamine. Fibers Polym. 2018, 19, 2023–2029. [Google Scholar] [CrossRef]
- Punyani, S.; Singh, H. Preparation of iodine containing quaternary amine methacrylate copolymers and their contact killing antimicrobial properties. J. Appl. Polym. Sci. 2006, 102, 1038–1044. [Google Scholar] [CrossRef]
- Álvarez-Paino, M.; Muñoz-Bonilla, A.; López-Fabal, F.; Gómez-Garcés, J.L.; Heuts, J.P.A.; Fernández-García, M. Effect of glycounits on the antimicrobial properties and toxicity behavior of polymers based on quaternized DMAEMA. Biomacromolecules 2015, 16, 295–303. [Google Scholar] [CrossRef] [PubMed]
- Souza, P.; Goulart, F.; Marques, J.; Bizzo, H.; Blank, A.; Groposo, C.; Seldin, L. Growth Inhibition of Sulfate-Reducing Bacteria in Produced Water from the Petroleum Industry Using Essential Oils. Molecules 2017, 22, 648. [Google Scholar] [CrossRef] [PubMed]
- Aitkeldiyeva, S.A.; Faizulina, E.R.; Tatarkina, L.G.; Auezova, O.N.; Abdiyev, K.Z.; Nurmukhanbetova, A.M.; Sadanov, A.K. Influence of biocides on growth and development of corrosive-dangerous microfloria. News Natl. Acad. Sci. Repub. Kazakhstan Ser. Biol. Med. 2018, 1, 22–28. [Google Scholar]
- Guan, J.; Xia, L.-P.; Wang, L.-Y.; Liu, J.-F.; Gu, J.-D.; Mu, B.-Z. Diversity and distribution of sulfate-reducing bacteria in four petroleum reservoirs detected by using 16S rRNA and dsrAB genes. Int. Biodeterior. Biodegrad. 2013, 76, 58–66. [Google Scholar] [CrossRef]
- Voordouw, G.; Menon, P.; Pinnock, T.; Sharma, M.; Shen, Y.; Venturelli, A.; Voordouw, J.; Sexton, A. Use of homogeneously-sized carbon steel ball bearings to study microbially-influenced corrosion in oil field samples. Front. Microbiol. 2016, 7, 351. [Google Scholar] [CrossRef]
- Turkiewicz, A.; Kapusta, P.; Kania, M. Laboratory tests on the use of Thioba-cillus and Acidihiobacillus species in the process of eliminating H2S from formation waters and process waters. Naft.-Gaz 2021, 8, 521–528. [Google Scholar] [CrossRef]
- Kenawy, E.-R.; Mahmoud, Y.A.G. Biological active polymers, 6 synthesis and antimicrobial activity of some linear copolymers with quaternary ammonium and phosphonium groups. Macromol. Biosci. 2003, 3, 107–116. [Google Scholar] [CrossRef]
- Misin, V.M.; Zezin, A.A.; Klimov, D.I.; Sybachin, A.V.; Yaroslavov, A.A. Biocidal Polymer Formulations and Coatings. Polym. Sci. Ser. B. 2021, 63, 459–469. [Google Scholar] [CrossRef]
- Ahmed, A.E.; Hay, J.N.; Bushell, M.E.; Wardell, J.N.; Cavalli, G. Biocidal polymers (I): Preparation and biocidal activity of some novel biocidal polymers based on uramil and its azodyes. React. Funct. Polym. 2008, 68, 248–260. [Google Scholar] [CrossRef]
- Kenawy, E.-R.; Worley, S.D.; Broughton, R. The Chemistry and Applications of Antimicrobial Polymers: A State-of-the-Art Review. Biomacromolecules 2007, 8, 1359–1384. [Google Scholar] [CrossRef]
- Battersby, N.S.; Stewart, D.J.; Sharma, A.P. Microbiological problems in the offshore oil and gas industries. J. Appl. Microbiol. 1985, 59, 227S–235S. [Google Scholar] [CrossRef]
- Dauletov, Y.; Nuraje, N.; Abdiyev, K.; Toktarbay, Z.; Zhursumbaeva, M. Copolymers of Diallyldimethylammonium Chloride and Vinyl Ether of Monoethanolamine: Synthesis, Flocculating, and Antimicrobial Properties. J. Surfactants Deterg. 2019, 22, 1129–1137. [Google Scholar] [CrossRef]
- Deryabina, G.I. Copolymerization; Samara University: Samara, Russia, 2013; 48p. [Google Scholar]
- Fineman, M.; Ross, S.D. Linear method for determining monomer reactivity ratios in copolymerization. J. Polym. Sci. 1950, 5, 259–262. [Google Scholar] [CrossRef]
- Bi, K.; Zhang, Y. Kinetic Study of the Polymerization of Dimethyldiallylammonium Chloride and Acrylamide. J. Appl. Polym. Sci. 2012, 125, 1636–1641. [Google Scholar] [CrossRef]
- Timofeeva, L.M.; Vasilieva, Y.A.; Kleshcheva, N.A.; Gromova, G.L.; Topchiev, D.A. Radical polymerization of diallylamine compounds: From quantum chemical modeling to controllable synthesis of high-molecular-weight polymers. Int. J. Quantum Chem. 2002, 88, 531–541. [Google Scholar] [CrossRef]
- Vorob’Eva, A.I.; Spirikhin, L.V.; Lobov, A.N.; Galimova, S.R.; Kraikin, V.A. Radical Copolymerization of N,N-Diallyl-N,N-dimethylammonium Chloride and Fumaric Acid. Polym. Sci. Ser. B 2014, 56, 263–268. [Google Scholar] [CrossRef]
- Abdiyev, K.Z.; Toktarbay, Z.; Zhenissova, A.Z.; Zhursumbayeva, M.B.; Kainazarova, R.N. Copolymerization of N,N-dimethyl-N,N-diallylammonium chloride with N,N-dimethyl-acrylamide. Polym. Sci. Ser. B 2015, 57, 217–223. [Google Scholar] [CrossRef]
- Wandrey, C.; Jaeger, W.; Reinisch, G.; Hahn, M.; Engelhardt, G.; Jancke, H.; Ballscuh, D. Zur chemichen structur von poly(dimethyldiallylammonium chloride). Acta Polym. 1981, 32, 177–179. [Google Scholar] [CrossRef]
- Kabanov, V.A.; Topchiev, D.A.; Nazhmetdinova, G.T. On reason for the lack acts of degradative chain transfer to monomer in free radical polymerization of N,N-diallyl-N,N-dimethylammonium halides. Izv. Akad. Nauk SSSR Ser Khim. 1983, 9, 2146–2149. [Google Scholar]
- Jaeger, W.; Hahn, M.; Wandrey, C.; Seehaus, F.; Reinisch, G. Cyclopolymerization Kinetics of Dimethyl Diallyl Ammonium Chloride. J. Macromol. Sci. Part A Pure Appl. Chem. 1984, 21, 615–638. [Google Scholar] [CrossRef]
- Moore, E. Fourier Transform Infrared Spectroscopy (FTIR). Methods, Analysis and Research Insights; Nova Science Publishers, Inc.: Hauppauge, NY, USA, 2016. [Google Scholar]
- Wang, X.; Yue, Q.; Gao, B.; Si, X.; Sun, X.; Zhang, S. Kinetics of dispersion polymerization of dimethyl diallyl ammonium chloride and acrylamide. J. Polym. Res. 2011, 18, 1067–1107. [Google Scholar] [CrossRef]
- GaisinaKh, A. Radical copolymerization of N,N-dimethyl-N,N-diallylammoniumchloride with vinyl monomers, sulfur dioxide and maleic acid. Ph.D. Thesis, Ufa Scientific Center of the Russian Academy of Science, Ufa, Bashkortostan Republic, Russia, 1998; p. 118. [Google Scholar]
- Wang, X.; Yue, Q.; Gao, B.; Si, X.; Sun, X.; Zhang, S. Dispersion copolymerization of acrylamide and dimethyl diallylammonium chloride in ethanol-water solution. J. App. Polym. Sci. 2010, 120, 1496–1502. [Google Scholar] [CrossRef]
- Ayatzhan, A.; Tashenov, A.; Nurgeldi, A.; Zhanar, O.; Zhexenbek, T.; Kaldibek, A.; Nuraje, N. P(DADMAAC-co-DMAA): Synthesis, thermal stability, and kinetics. Polym. Adv. Technol. 2021, 32, 2669–2675. [Google Scholar] [CrossRef]
- Liu, L.H.; Liu, H.W.; Gong, Z.Q. Kinetics studies on polymerization of Diethyl-diallylammonium chloride and its copolymerization with acrylamide and acrylic acid. Acta Polym. Sin. 2007, 2, 183–189. [Google Scholar] [CrossRef]
- Kalyazina, O.V.; Murzabekova, T.G.; Lelyukh, T.F.; Gritskova, I.A. Copolymerization of the poly(N,N-dimethyl-N,N-diallylammonium chloride) macromonomer with acrylamide. Rus. Chem. Bull. 2007, 56, 535–539. [Google Scholar] [CrossRef]
- Longinos, S.N.; Longinou, D.D.; Myrzakhmetova, N.; Akimbayeva, N.; Zhursumbaeva, M.; Abdiyev, K.; Toktarbay, Z.; Parlaktuna, M. Kinetic Analysis of Methane Hydrate Formation with Butterfly Turbine Impellers. Molecules 2022, 27, 4388. [Google Scholar] [CrossRef]
- Abdiyev, K.Z.; Toktarbay, Z.; Zhenissova, A.Z.; Zhursumbaeva, M.B.; Kainazarova, R.N.; Nuraje, N. The new effective flocculants—Copolymers of N,N-dimethyl-N,N-diallyl-ammonium chloride and N,N-dimethylacrylamide. Colloids Surf. A Phys.-Chem. Eng. Asp. 2015, 480, 228–235. [Google Scholar] [CrossRef]
- Abdel-Khalek, M.A.; Ayman, E.M. Stabilizing of Na-bentonite by poly(diallyl dimethyl ammonium chloride) Adsorption. Inżynieria Mineralna 2021, 1, 81–87. [Google Scholar] [CrossRef]
- Abdiyev, K.Z.; Maric, M.; Orynbayev, B.Y.; Toktarbay, Z.; Zhursumbaeva, M.B.; Seitka-liyeva, N.Z. Flocculating properties of 2-acrylamido-2-methyl-1-propane sulfonic acid-co-allylamine polyampholytic copolymers. Polym. Bull. 2022, 79, 10741–10756. [Google Scholar] [CrossRef]
- Shaikhutdinov, E.M.; Khussain, S.K.; Abdiyev, K.Z.; Seitkaliyeva, N.Z. Complexation of sodium 2-acrylamido-2-methylpropanesulfonate-monoethanolamine vinyl ether copolymer with polyelectrolytes in aqueous medium. Polym. Sci. Ser. A 2007, 49, 584–592. [Google Scholar] [CrossRef]
- Abdiyev, K.Z.; Shaikhutdinov, E.M.; Zhursumbaeva, M.B.; Khussain, S.K. Effect of Polymer Concentration on the Surface Properties of Polyacid-Poly(N-vinylpyrrolidone) Complexes. Colloid J. Kolloidn. Zhurnal 2003, 65, 399–402. [Google Scholar] [CrossRef]
- Timofeeva, L.; Kleshcheva, N. Antimicrobial Polymers: Mechanism of Action, Factors of Activity, and Applications. Appl. Microbiol. Biotechnol 2011, 89, 475–492. [Google Scholar] [CrossRef] [PubMed]
- Siedenbiedel, F.; Tiller, J.C. Antimicrobial Polymers in Solution and on Surfaces: Overview and Functional Principles. Polymers 2012, 4, 46–71. [Google Scholar] [CrossRef]
- Qiu, H.; Si, Z.; Luo, Y.; Feng, P.; Wu, X.; Hou, W.; Huang, D. The Mechanisms and the Applications of Antibacterial Polymers in Surface Modification on Medical Devices. Front. Bioeng. Biotechnol. 2020, 8, 910. [Google Scholar] [CrossRef] [PubMed]
- Liu, L.; Huang, Y.; Riduan, S.N.; Gao, S.; Yang, Y.; Fan, W. Main-chain imidazolium oligomer material as a selective biomimetic antimicrobial agent. Biomaterials 2012, 33, 8625–8631. [Google Scholar] [CrossRef] [PubMed]
- Kawabata, N.; Nishiguchi, M. Antibacterial Activity of Soluble Pyridinium-Type Polymers. Appl. Environ. Microbiol. 1988, 54, 2532–2535. [Google Scholar] [CrossRef]
- Kanazawa, A.; Ikeda, T.; Endo, T. Polymeric Phosphonium Salts as a Novel Class of Cationic Biocides. IV. Synthesis and Antibacterial Activity of Polymers with Phosphonium Salts in the Main Chain. J. Polym. Sci. Part A Polym. Chem. 1993, 31, 3031–3038. [Google Scholar] [CrossRef]
- Kanazawa, A.; Ikeda, T.; Endo, T. Novel Polycationic Biocides: Synthesis and Antibacterial Activity of Polymeric Phosphonium Salts. J. Polym. Sci. Part A Polym. Chem. 1993, 31, 335–343. [Google Scholar] [CrossRef]
- Yessimova, O.; Kumargaliyeva, S.; Kerimkulova, M.; Mussabekov, K.; Toktarbay, Z. Wetting ability of a phytopreparation and their associates with polyelectrolytes. Rasayan J. Chem. 2020, 13, 481–487. [Google Scholar] [CrossRef]
- Ikeda, T.; Ledwith, A.; Bamford, C.H.; Hann, R.A. Interaction of a Polymeric Biguanide Biocide with Phospholipid Membranes. Biochim. Biophys. Acta 1984, 769, 57–66. [Google Scholar] [CrossRef]
- Abdiyev, K.; Toktarbay, Z.; Orynbayev, B.; Zhursumbaeva, M.; Seitkaliyeva, N.; Nakan, U. Structure formation in suspensions and biocidal properties of the copolymer of 2-acrylamido-2-methylpropanesulfonic acid and allylamine. Mater. Today Proc. 2022, 71, 13–17. [Google Scholar] [CrossRef]
- Chin, W.; Yang, C.; Ng, V.W.L.; Huang, Y.; Cheng, J.; Tong, Y.W. Biodegradable broad-spectrum antimicrobial polycarbonates: Investigating the role of chemical structure on activity and selectivity. Macromolecules 2013, 46, 8797–8807. [Google Scholar] [CrossRef]
- Hoque, J.; Akkapeddi, P.; Yadav, V.; Manjunath, G.B.; Uppu, D.S.S.M.; Konai, M.M. Broad spectrum antibacterial and antifungal polymeric paint materials: Synthesis, structure–activity relationship, and membrane-active mode of action. ACS Appl. Mater. Interfaces 2015, 7, 1804–1815. [Google Scholar] [CrossRef] [PubMed]

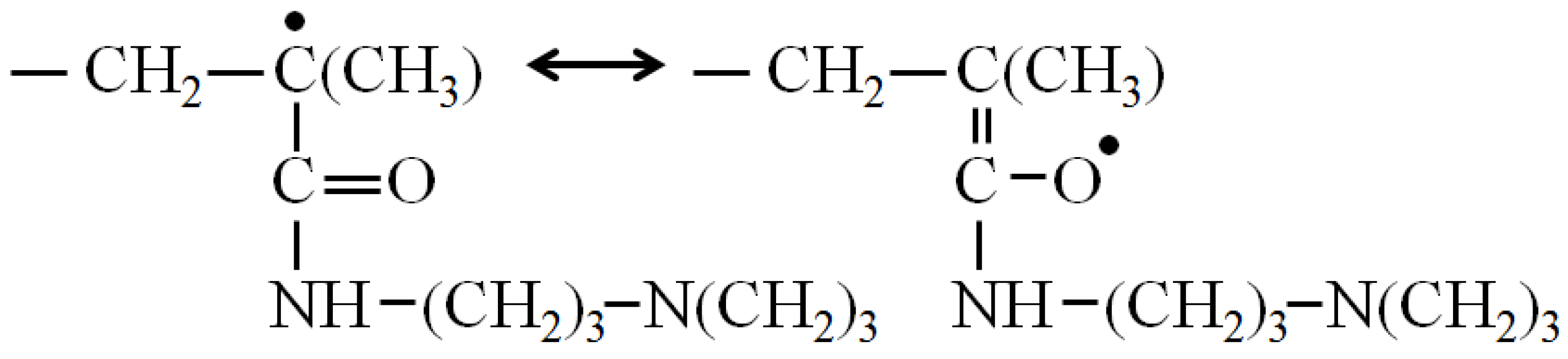



| Molar Fractions of Monomers in the Feed, % | Molar Fractions of Monomers in the Copolymer, % | Monomers Conversion, wt.% | r1 | r2 | |||||
|---|---|---|---|---|---|---|---|---|---|
| M1 | M2 | m1 | m2 | ||||||
| 80 | 20 | 88 | 12 | 8.9 | 4.0 | 7.3 | r2 = 2.19⋅r1 − 3.45 | 1.75 | 0.70 |
| 65 | 35 | 75 | 25 | 8.2 | 1.9 | 3.0 | r2 = 1.20⋅r1 − 1.27 | ||
| 50 | 50 | 63 | 37 | 7.5 | 1.0 | 1.7 | r2 = 0.59⋅r1 − 0.41 | ||
| 30 | 70 | 44 | 56 | 6.1 | 0.4 | 0.8 | r2 = 0.2⋅r1 + 0.1 | ||
| Molar Fractions of Monomers in the Feed, % | Molar Fractions of Monomers in the Copolymer, % | Monomers Conversion, wt.% | r1 | r2 | ||||||
|---|---|---|---|---|---|---|---|---|---|---|
| M1 | M2 | m1 | m2 | |||||||
| 80 | 20 | 88 | 12 | 8.9 | 4.0 | 7.3 | 2.2 | 3.5 | 1.84 | 0.66 |
| 65 | 35 | 75 | 25 | 8.2 | 1.9 | 3.0 | 1.1 | 1.2 | ||
| 50 | 50 | 63 | 37 | 7.5 | 1.0 | 1.7 | 0.6 | 0.4 | ||
| 30 | 70 | 44 | 56 | 6.0 | 0.4 | 0.8 | 0.2 | −0.1 | ||
| Method | r1 | r2 | 1/r1 | 1/r2 | r1⋅ r2 |
|---|---|---|---|---|---|
| Mayo–Lewis | 1.80 | 0.80 | 0.56 | 1.25 | 1.44 |
| Fineman–Ross | 1.84 | 0.66 | 0.54 | 1.52 | 1.21 |
| Content of Monomers in the Initial Mixture mol. % [DMAPMA]:[ DADMAC] | Composition of Copolymers from the Results of Elemental Analysis mol. % [DMAPMA]:[DADMAC] | Composition of Copolymers from the Results of Conductometric Titration, mol. %, [DMAPMA]:[DADMAC] | ||
|---|---|---|---|---|
| N | C | H | ||
| 30:70 | 42:58 | 43:57 | 41:59 | 45:55 |
| 50:50 | 64:36 | 63:37 | 64:36 | 64:36 |
| 65:35 | 77:23 | 75:25 | 76:24 | 76:24 |
| 80:20 | 93:07 | 94:06 | 93:07 | 95:05 |
| [I], wt% | 0.05 | 0.1 | 0.5 | 1.0 |
|---|---|---|---|---|
| [I], mol × L−1 | 0.002 | 0.004 | 0.022 | 0.044 |
| , mol × L−1 × min−1 | 0.037 | 0.051 | 0.067 | 0.083 |
| y (max, %) | 35.5 | 46.8 | 61.0 | 70.9 |
| log Rc | −1.432 | −1.292 | −1.174 | −1.060 |
| log [I] | −2.658 | −2,357 | −1.658 | −1.357 |
| [M], mol × L−1 | 1.5 | 3.0 | 4.0 | 6.0 |
|---|---|---|---|---|
| , mol×L−1×min−1 | 0.001 | 0.028 | 0.068 | 0.103 |
| y (max), % | 4 | 44.4 | 61.5 | 62.0 |
| log Rc | −3.00 | −1.55 | −1.17 | −0.99 |
| log [M] | 0.18 | 0.48 | 0.60 | 0.78 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Abdiyev, K.; Marić, M.; Orynbaev, B.; Zhursumbaeva, M.; Seitkaliyeva, N.; Toktarbay, Z. A Novel Cationic Polymer Surfactant for Regulation of the Rheological and Biocidal Properties of the Water-Based Drilling Muds. Polymers 2023, 15, 330. https://doi.org/10.3390/polym15020330
Abdiyev K, Marić M, Orynbaev B, Zhursumbaeva M, Seitkaliyeva N, Toktarbay Z. A Novel Cationic Polymer Surfactant for Regulation of the Rheological and Biocidal Properties of the Water-Based Drilling Muds. Polymers. 2023; 15(2):330. https://doi.org/10.3390/polym15020330
Chicago/Turabian StyleAbdiyev, Kaldibek, Milan Marić, Baurzhan Orynbaev, Mariamkul Zhursumbaeva, Nurgul Seitkaliyeva, and Zhexenbek Toktarbay. 2023. "A Novel Cationic Polymer Surfactant for Regulation of the Rheological and Biocidal Properties of the Water-Based Drilling Muds" Polymers 15, no. 2: 330. https://doi.org/10.3390/polym15020330
APA StyleAbdiyev, K., Marić, M., Orynbaev, B., Zhursumbaeva, M., Seitkaliyeva, N., & Toktarbay, Z. (2023). A Novel Cationic Polymer Surfactant for Regulation of the Rheological and Biocidal Properties of the Water-Based Drilling Muds. Polymers, 15(2), 330. https://doi.org/10.3390/polym15020330








