Abstract
In recent years, aromatic substances have become the focus of environmental pollution-related concern due to their high stability and mutagenicity. In this regard, researchers have focused their attention on the development of photocatalytic processes to convert nitroaromatic compounds into aniline. In this work, the photocatalytic conversion of nitrobenzene (NB) to aniline (AN) was studied. The photocatalytic reaction was performed using commercial TiO2 (P25) and a photocatalytic aerogel, based on P25 embedded in syndiotactic polystyrene (sPS) aerogel (sPS/P25 aerogel) as photocatalysts. Different alcohols were used as hydrogen sources during the photocatalytic experiments. At the optimized operating conditions (photocatalysts dosage: 0.5 mg/L and 50% (v/v) EtOH%), an AN yield of over 99% was achieved. According to the results, this work could open avenues toward effective production of AN from NB using mild reaction conditions with sPS/P25 aerogel—in view of a possible scale-up of the photocatalytic process.
1. Introduction
Recently, contamination of the environment by organic contaminants has become a major global challenge. Among various organic contaminants and pollutants, nitroaromatic compounds caused concern [1,2], due to their toxicity and mutagenicity, as well as their high stability, low solubility, and intensive use as raw materials in industrial applications [3]. For this reason, different oxidation processes were employed for the complete degradation of these contaminants in nontoxic end products, such as carbon dioxide, nitrogen oxides, and water [4]. However, since these nitrated compounds are resistant to oxidative degradation, research has focused on possible alternatives to these environmental options. From this perspective, the conversion of nitroaromatic compounds into more added-value products could be a valid option. Indeed, aromatic amines, which are considered key intermediates in the synthesis of dyes, polymers, and many life-science products, including antioxidants, pharmaceuticals and agrochemicals [5,6,7,8], can be obtained by means of reduction reactions of nitroaromatic compounds.
Therefore, recent research has focused on finding suitable conversion processes for turning nitroaromatic compounds into amino derivatives, such as aniline (AN) [9]. On an industrial scale, AN is commonly synthesized by the catalytic hydrogenation of nitroaromatics, such as nitrobenzene (NB), in the liquid or vapor phase using high temperature, high H2 pressure, and the long reaction times required to achieve high selectivity for AN. Unfortunately, these synthesis processes are expensive and unsafe [10].
The most studied catalysts for the reduction of NB to AN are transition metals, such as Cu, and Ni [11,12], or noble metals, such as Pt, Pd, and Au [13]. Sn/HCl is employed commercially. However, there have been problems with waste disposal [14]. Therefore, the development of a sustainable catalytic process that could operate under mild reaction conditions by exploiting the mild reducing power of excited electrons was desirable [15]. Heterogeneous photocatalysis could be an attractive option, and has significant scientific value in ecological and green synthesis [16], as the reaction can be carried out using non-toxic metal oxides, such as TiO2, H2O, as solvents and alcohols as hydrogen source [17]. Indeed, when TiO2 is activated by light [18], the conduction band electrons reduce NB to AN [19] and, at the same time, the alcohol is oxidized to the corresponding aldehyde by the valence band positive holes [17]. Therefore, the use of H2 (as reducing agent) and toxic metals (as catalysts) could be avoided [14,15,16]. Literature studies showed that l TiO2 based photocatalysts were efficient in reducing NB to AN [20,21,22,23] under UV irradiation in slurry reactors, leading to the selective reduction of the only nitro group in the presence of suitable molecules acting as hole scavengers [22]. Although heterogeneous photocatalysis for this kind of reaction is very advantageous, one of the main drawbacks is the recovery of the powder catalyst from the reaction medium, which leads to an increase in the costs of the process—especially for a possible industrial scale-up of a photocatalytic system [24]. To overcome this limitation, the photocatalyst should be supported on materials with good chemical and mechanical stability, and be able to permanently immobilize the photocatalyst without decreasing the photocatalytic efficiency. Several papers reported that syndiotactic polystyrene-based polymer aerogels proved to be very interesting based on properties, such as their hydrophobicity, making them efficient as concentrators of organic molecules [25]. In recent years, syndiotactic polystyrene (sPS) aerogels, functionalized with TiO2 and ZnO-based photocatalysts, were effective in the degradation of water pollutants under UV and visible light irradiation [24,25,26]. However, to the best of our knowledge, these systems have yet to be studied for the photocatalytic reduction of NB to AN. For this reason this work presented a preliminary study of the photocatalytic conversion of NB to AN via photocatalysis, using an aerogel based on a commercial TiO2 powder photocatalyst (P25) embedded in sPS (sPS/P25).
2. Results and Discussion
2.1. Samples Characterization
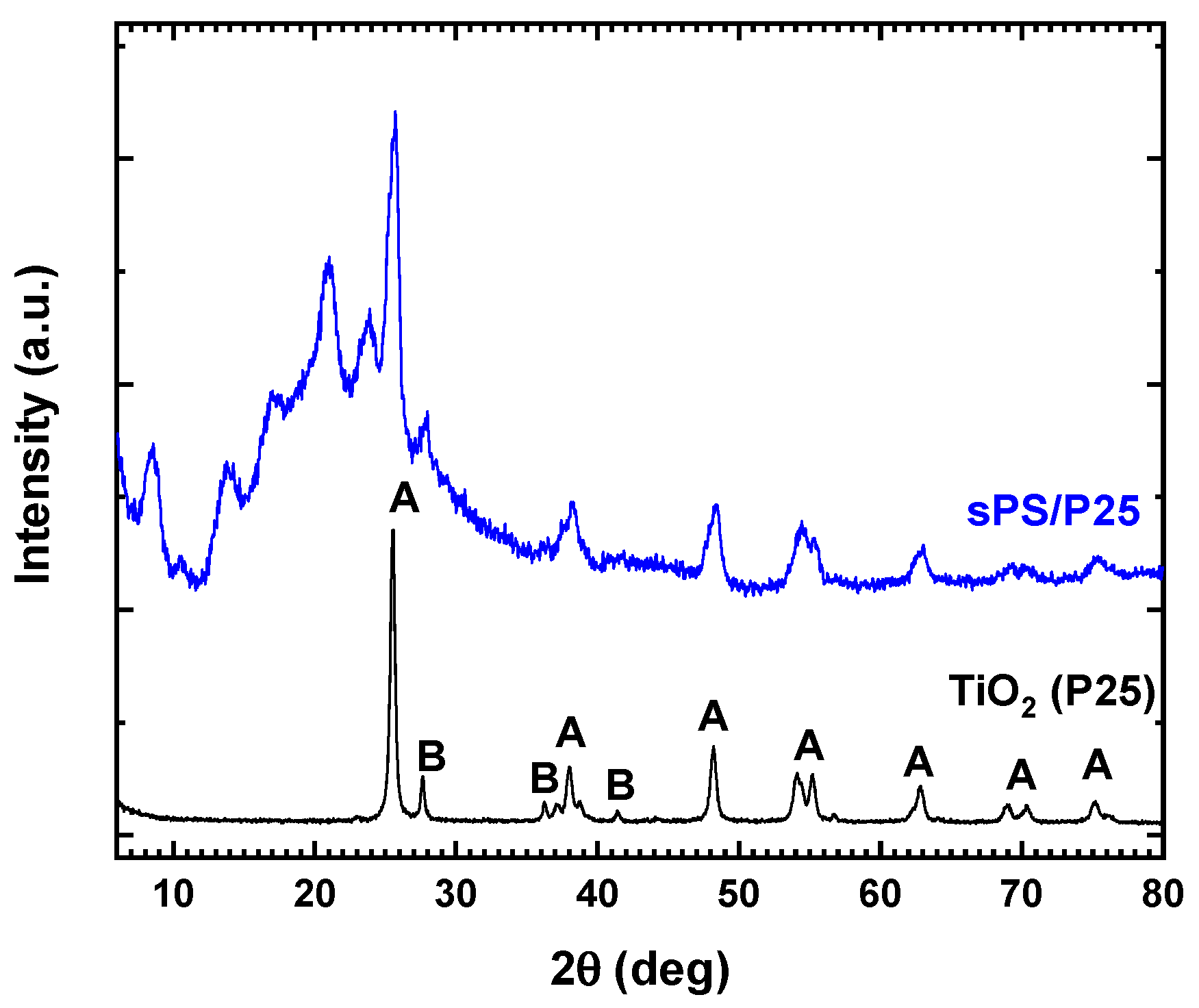
The X-ray diffraction patterns of TiO2 (P25) and sPS/P25 aerogel are reported in Figure 1. For TiO2 (P25), the typical reflexes of anatase and rutile crystalline phase were detected [27]. sPS/P25 aerogel showed peaks at 2θ = 8.3°, 13.7°, 16.7°, 20.7° and 23.6° due to the nanoporous crystalline phase of the sPS aerogel [24,25,26]. Additionally, the diffraction patterns assigned to both the anatase phase (at 25.30°, 37.17°, 37.93°, 38.68°, 47.05°, 54.05° 55.18° and 62.40°) and rutile phase (at 27.50°) of TiO2 (P25) were observed [28]. The results confirmed the successful incorporation of TiO2 (P25) particles inside the framework of sPS aerogel.
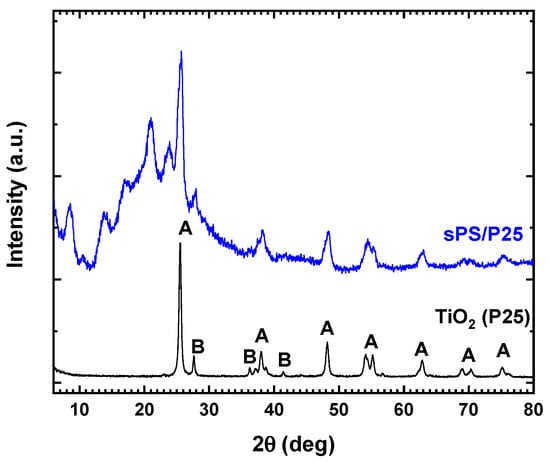
Figure 1.
X-ray diffraction patterns of commercial TiO2 (P25) and sPS/P25 aerogel. A: anatase phase; B: rutile phase.
The specific surface area (SSA) values of TiO2 powder photocatalyst and sPS/P25 aerogel were also measured. For commercial TiO2, the SSA was about 50 m2/g, the typical value found for commercial P25 [29]. In contrast, the sPS/P25 aerogel had the typical aerogel values of sPS in δ-form [24,25,26,30], 250 m2/g.
2.2. Photocatalytic Activity Results on P25 in Powder Form
2.2.1. Effect of the Reducing Agent
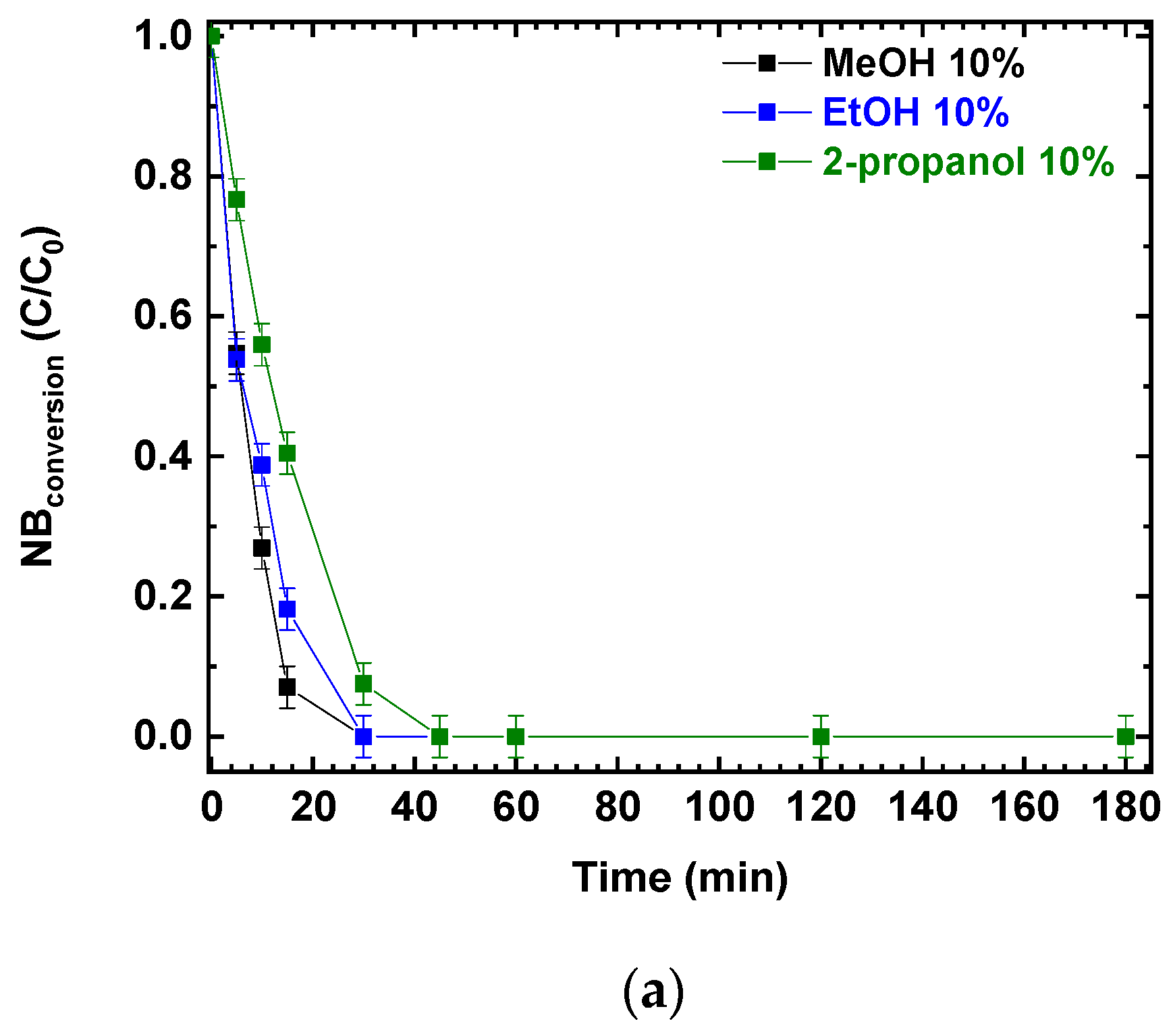
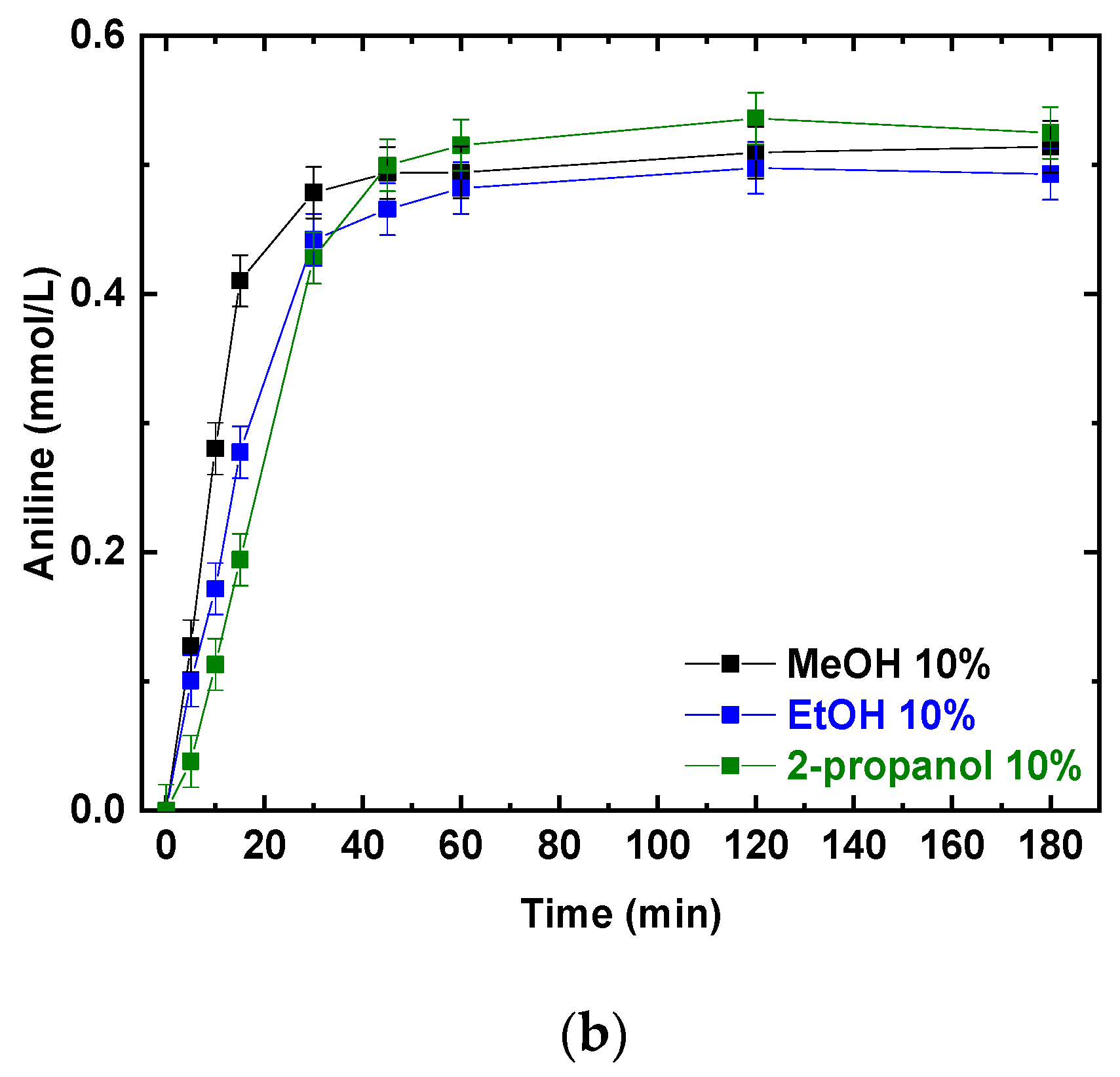
Photocatalytic experiments were performed using P25 and different alcohols as hole scavengers. The photocatalytic NB reduction was tested using the same v/v percentage of methanol, ethanol, and 2-propanol; the results are presented in Figure 2a,b. NB conversions and AN production increased with the irradiation time. After 180 min, NB was completely converted (Figure 2a) and the final conversion was not influenced by the different reducing agents (MeOH, EtOH and 2-propanol). At the same time, aniline was produced, reaching a concentration of almost 0.5 mmol/L in all three scenarios (Figure 2b).
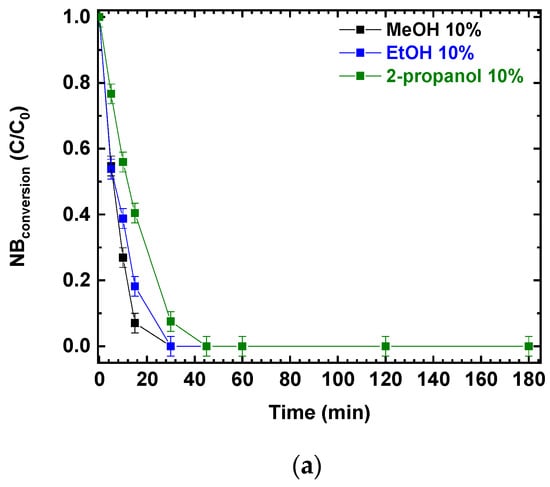
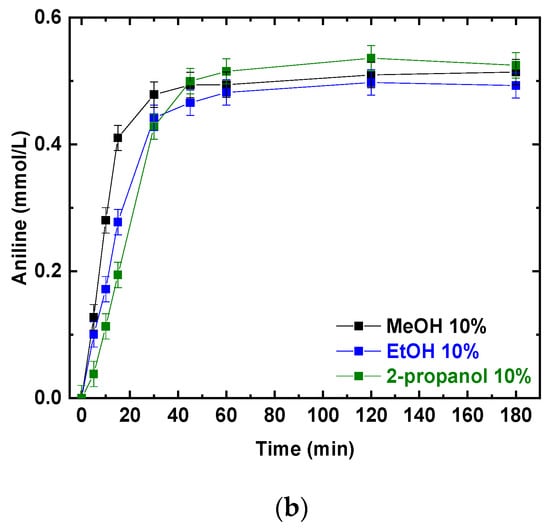
Figure 2.
Comparison of the photocatalytic reduction of NB to AN using P25, with different reducing agents (MeOH, EtOH, 2-propanol): (a) NB conversion as a function of irradiation time; (b) AN production as a function of irradiation time. Reaction conditions: NB 1 mmol/L; water 100 mL; 0.5 g/L TiO2 (P25); temperature 25 °C; reaction time 180 min. Error bar ±0.02%.
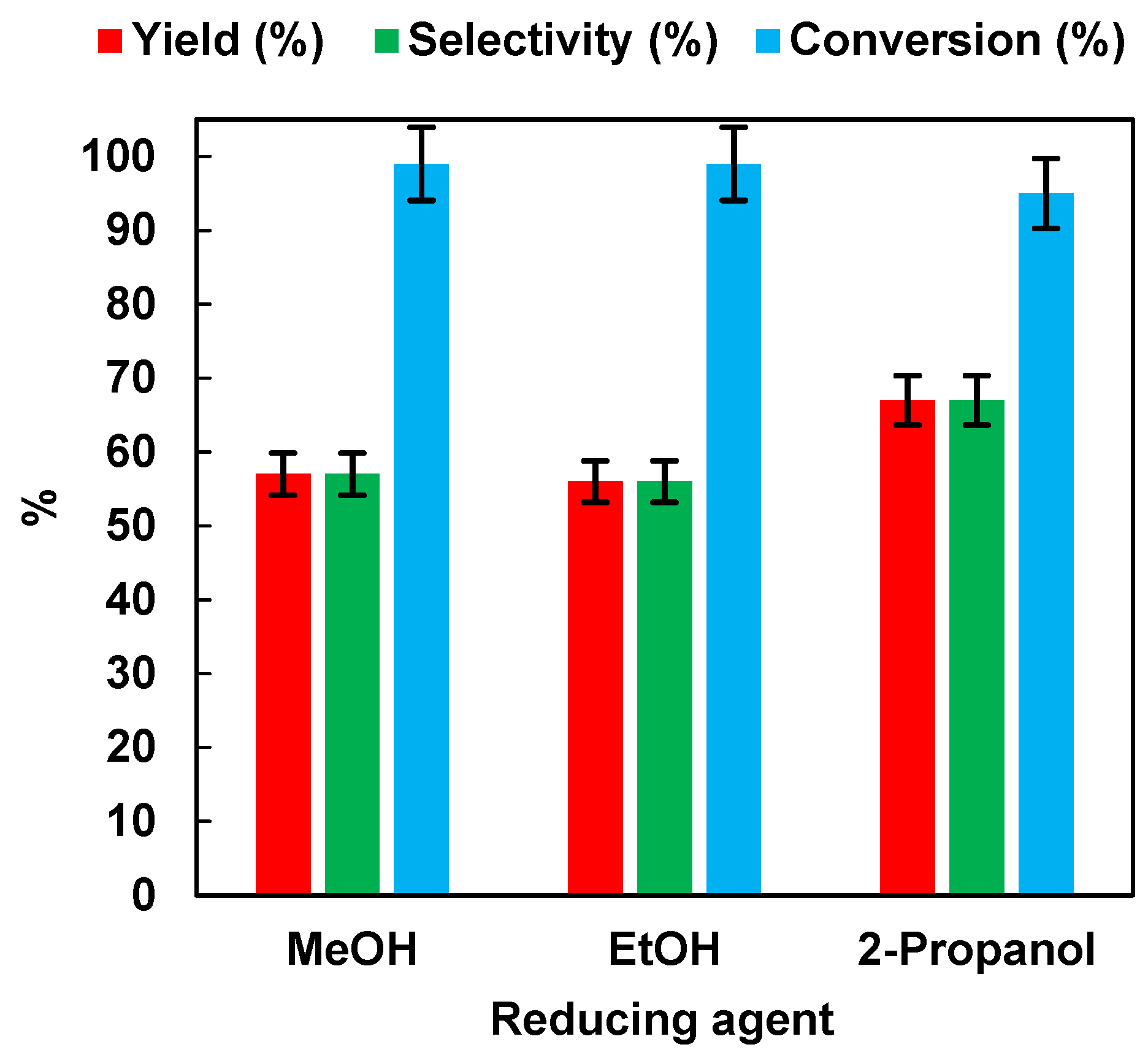
The results are summarized in Figure 3. It appeared that the reaction performance was not affected by the use of different alcohols in the aqueous solution. Indeed, after 45 min of irradiation time, the NB conversion values were 99%, 99%, and 95%, respectively, for MeOH, EtOH, and 2-propanol. AN yield and selectivity were 57% for MeOH and EtOH, and 67% for 2-propanol.
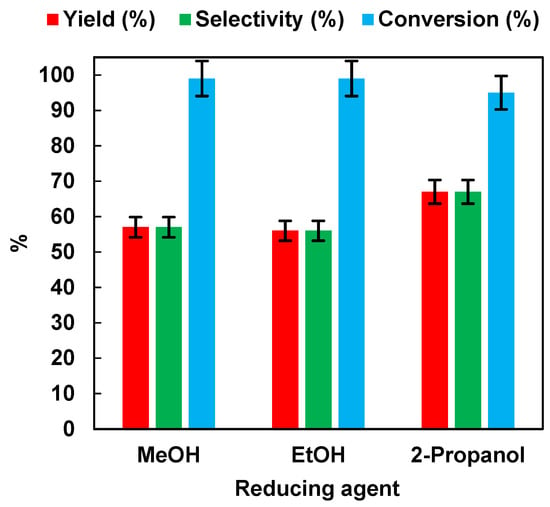
Figure 3.
Effects of different hole scavengers on AN yield, AN selectivity, and NB conversion after 45 min of irradiation time. Error bar ±5%.
Although 2-propanol led to higher yield and selectivity values than MeOH and EtOH, the latter was preferred as a hydrogen source for environmental sustainability reasons. Indeed, environmental impact studies showed that using an H2O/Ethanol solution as a solvent blend in organic reactions was recommended over an H2O/2-propanol mixture [31].
2.2.2. Effects of Initial EtOH Percentage
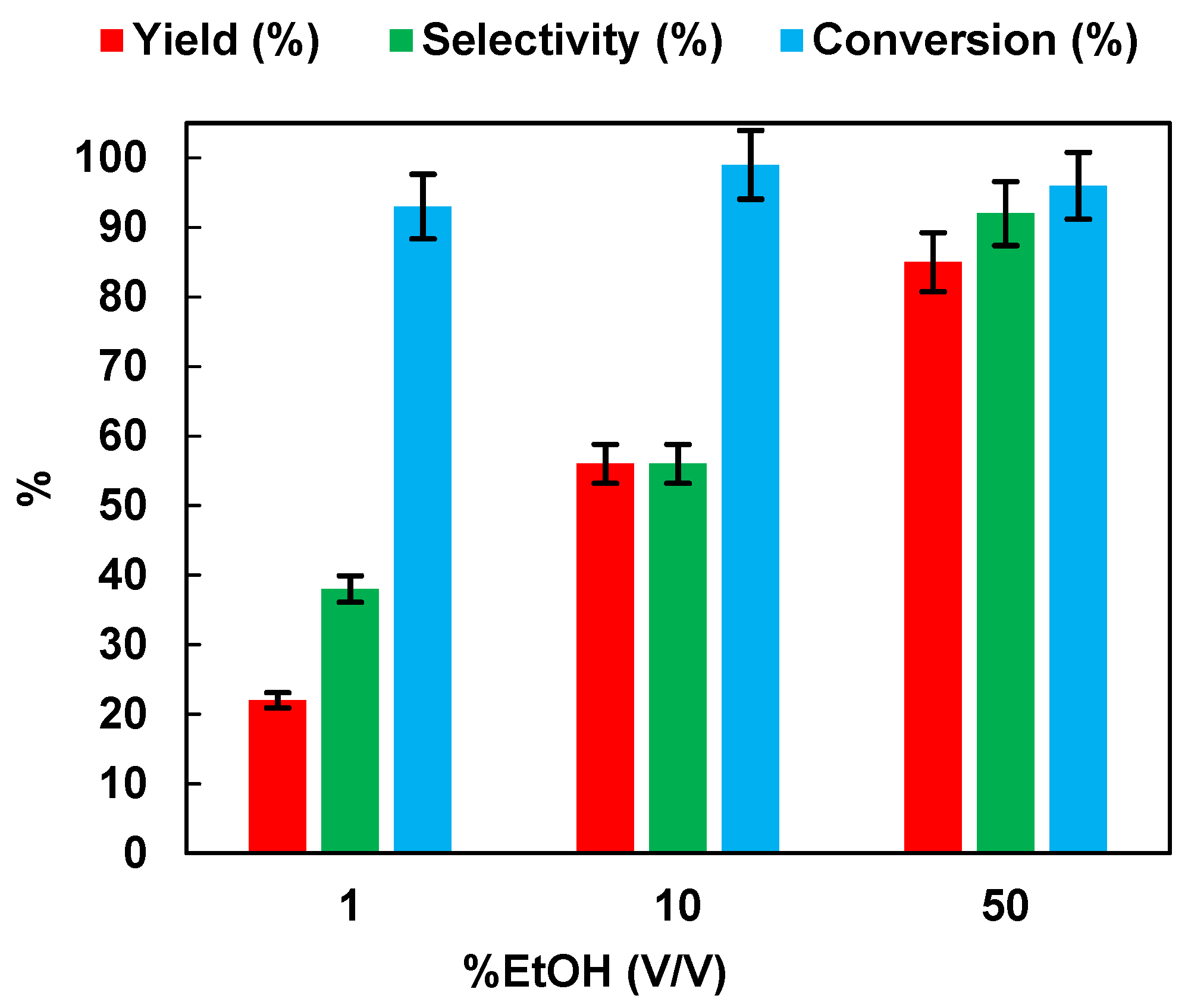
It has been documented in the literature that alcohols, in the photocatalytic conversion of NB, exert a double function; they act as a hydrogen source for the reduction and also as hole scavengers to inhibit the photoinduced electron hole-pairs [10,15,32]. Therefore, the effect of the initial ethanol percentage (v/v) on the photocatalytic reduction of NB to AN was assessed. The results are reported in Figure 4. Yield, selectivity, and conversion increased with the increase in EtOH percentage. At 50% of EtOH, 96% of NB was converted to AN, with a selectivity equal to 92%.
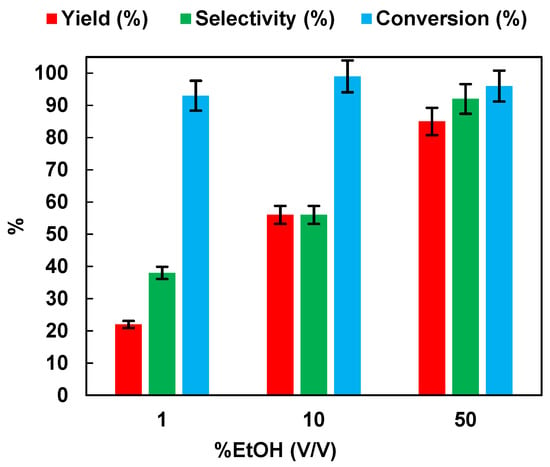
Figure 4.
Effects of the EtOH% (v/v) in aqueous solution on the photocatalytic reduction of NB to AN using P25. Reaction conditions: NB 1 mmol/L; solution volume 100 mL; 0.5 g/L TiO2 (P25); temperature 25 °C; UV irradiation time t is 45 min. Error bar ±5%.
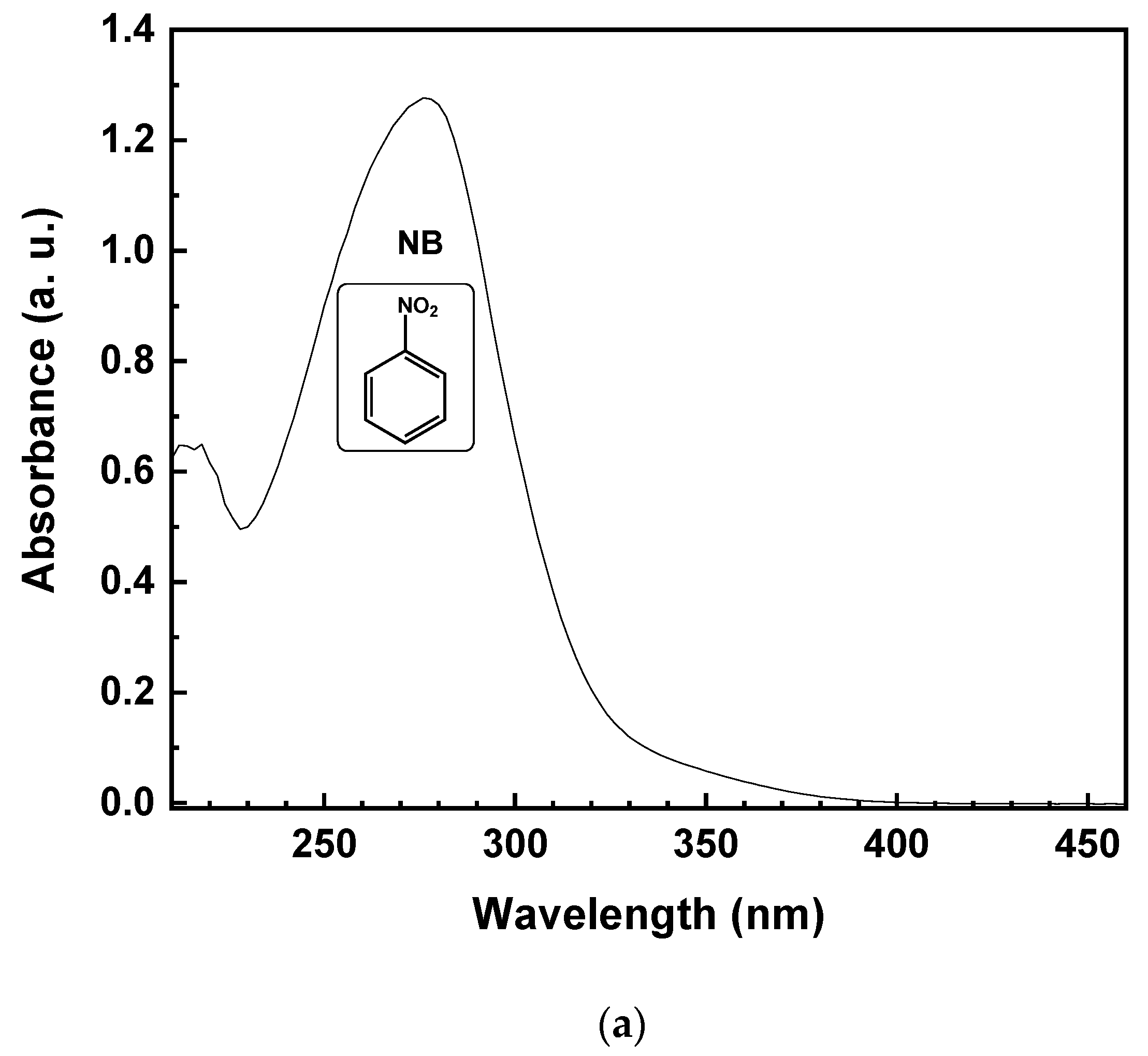
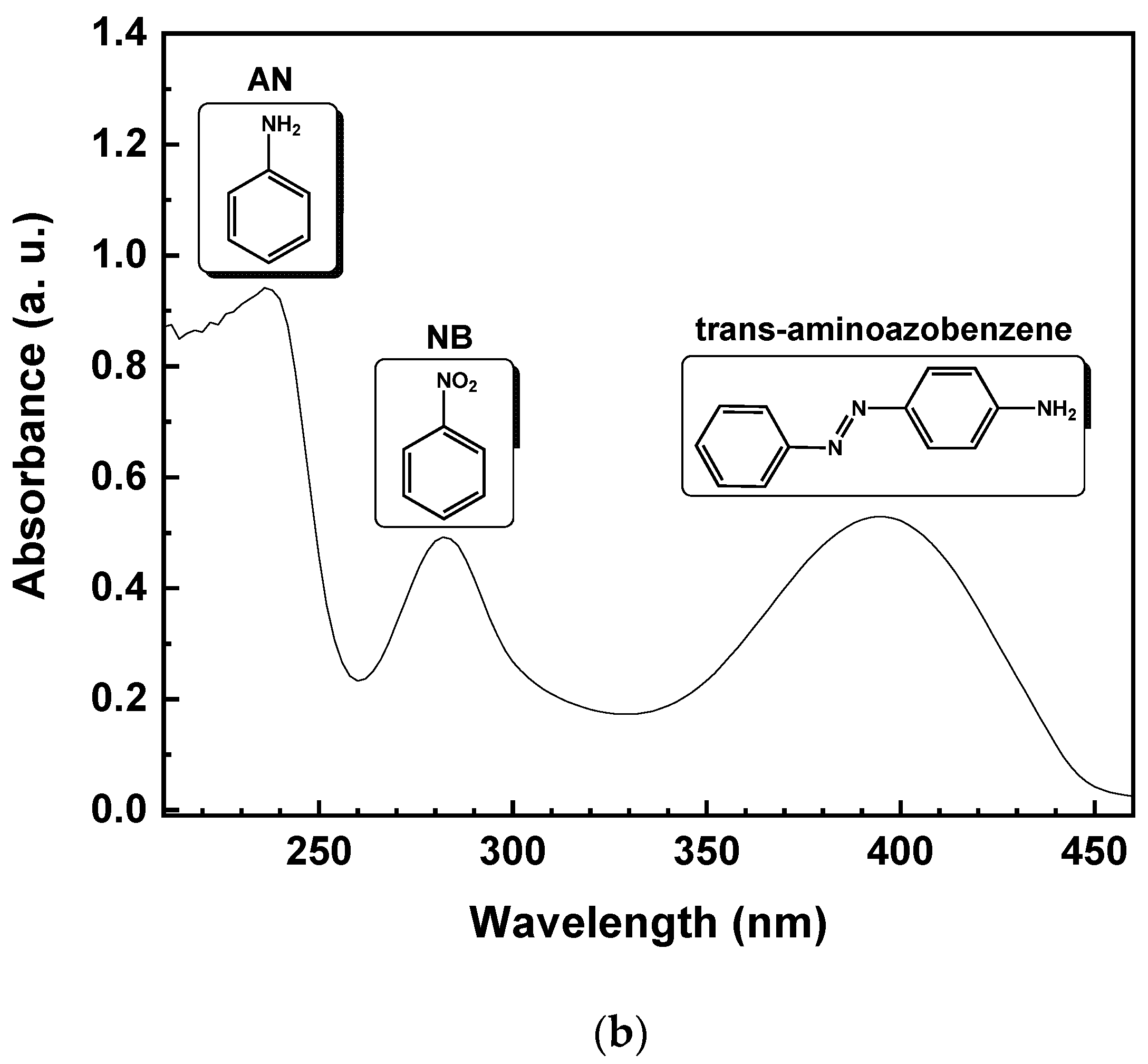
Theoretically, due to the lack of byproduct generation, the NB should be completely reduced to AN. Specifically, starting from 1 mmol/L of NB, the same amount of AN should be produced. Figure 5 reports the UV-vis spectrum of the reaction solution, with 10% of EtOH (v/v) after 15 min of irradiation time. The reduction of NB to AN was demonstrated by the absorbance peak, initially located at 277 nm (Figure 5a). After illumination with UV light, the formation of a band at 239 nm, corresponding to AN, was observed (Figure 5b). On the other hand, the lower selectivity toward AN, with the decreasing of EtOH percentage, was due to the appearance of a new band in the UV absorption spectrum at 395 nm (Figure 5b), corresponding to the formation of trans-4-aminoazobenzene (4-aminoAZ) [33].
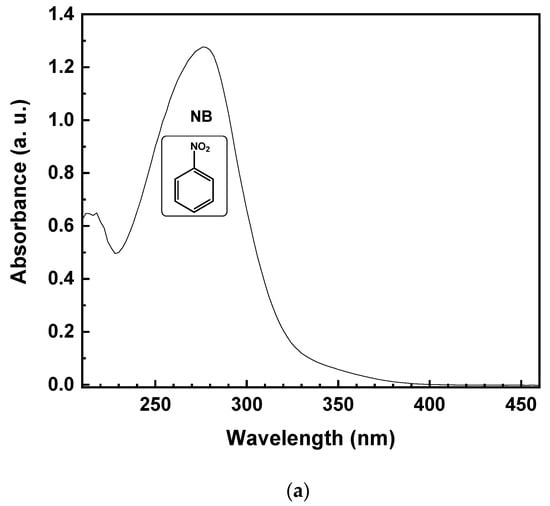
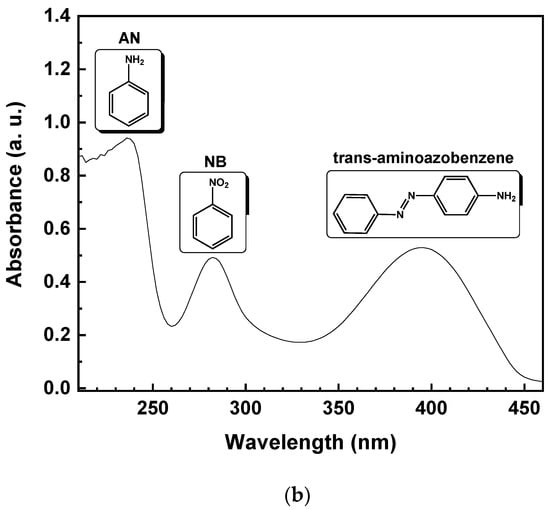
Figure 5.
UV-vis spectrum of the aqueous solution with 10% EtOH (v/v) of NB initial solution (a) and after 15 min of UV irradiation time (b).
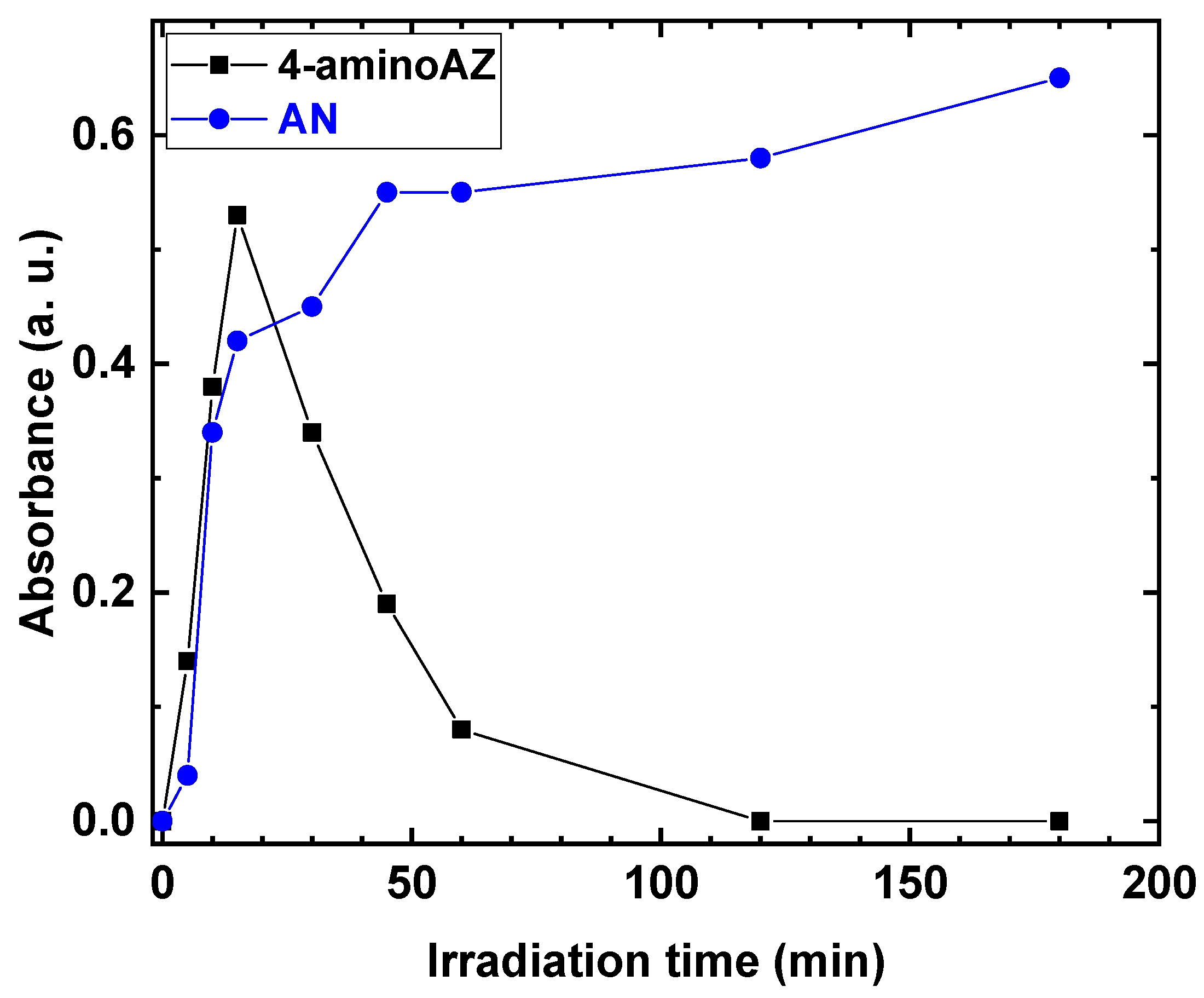
The behavior of the 4-amino AZ and AN formation, as a function of the irradiation time, was investigated. The results are shown in Figure 6.
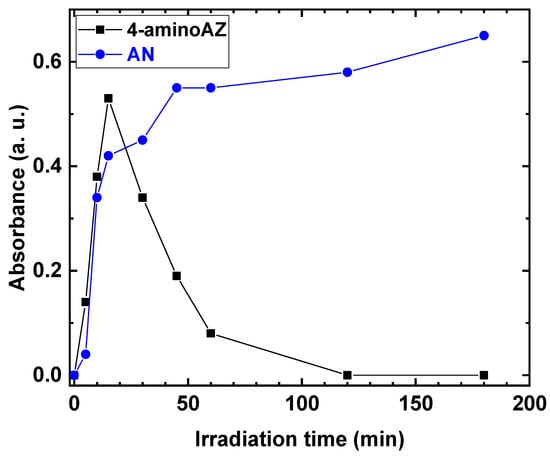
Figure 6.
Absorbance of 4-aminoAZ and AN as a function of irradiation time.
The production of 4-amino AZ increased as the irradiation time increased, reaching a maximum after 15 min, and then gradually decreased during the reaction. In contrast, for AN, an increase in absorbance values was observed as the irradiation time increased, with a maximum absorbance of 0.6 at the end of the reaction.
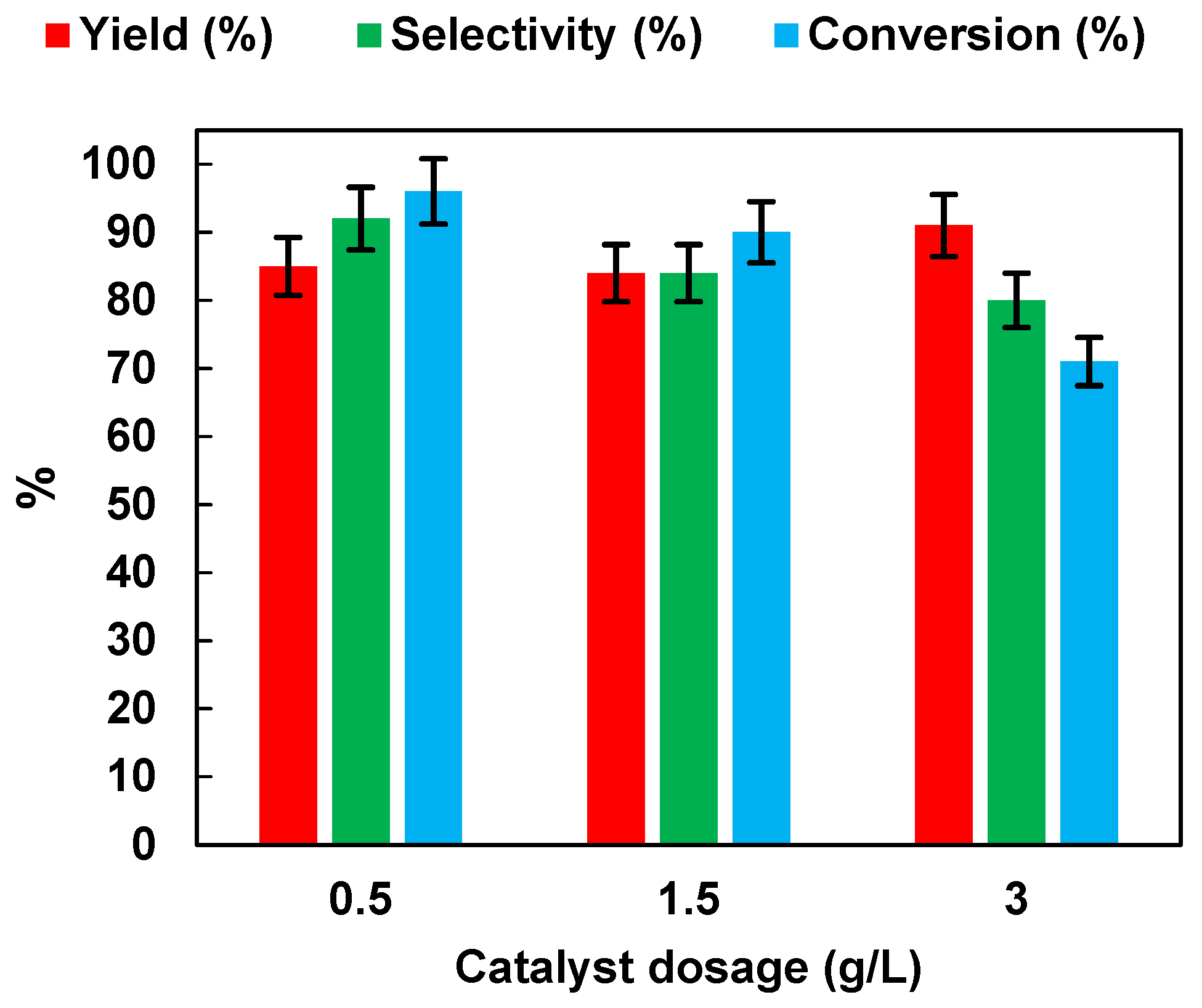
2.2.3. Effect of Photocatalyst Dosage
In order to choose the optimal photocatalyst concentration for NB reduction (initial NB concentration: 1 mmol/L), the effects of photocatalyst dosages were studied by performing experiments at different TiO2 (P25) dosages (in the range of 0.5–3 g/L) in the presence of EtOH (50% v/v). The results, in terms of yield, selectivity and conversion, are presented in Figure 7.
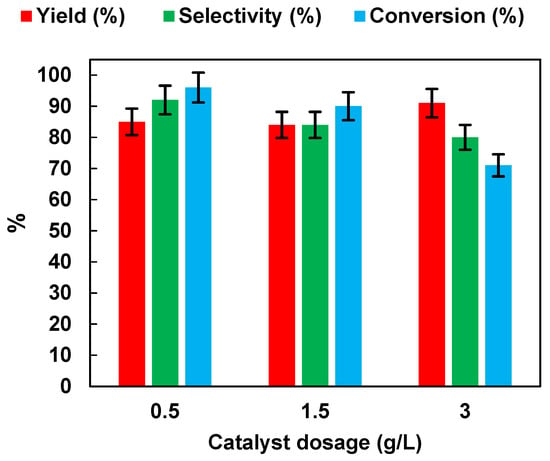
Figure 7.
Effects of TiO2 (P25) dosage on AN yield, AN selectivity, and NB conversion. UV irradiation time t is 45 min. Error bar ±5%.
As the dosage of TiO2 (P25) increased from 0.5 g/L to 3 g/L, the NB yield remained unchanged, while selectivity to AN and NB conversion decreased from 92% to 80%, and from 96% to 71%, respectively. The results may have been related to the light-scattering and screening effects, due to the opacity of the suspension, which prevented proper illumination of the catalyst in solution [34]. The results may also have been related to the increase of aggregation phenomena causing a reduction in photocatalytic activity [35].
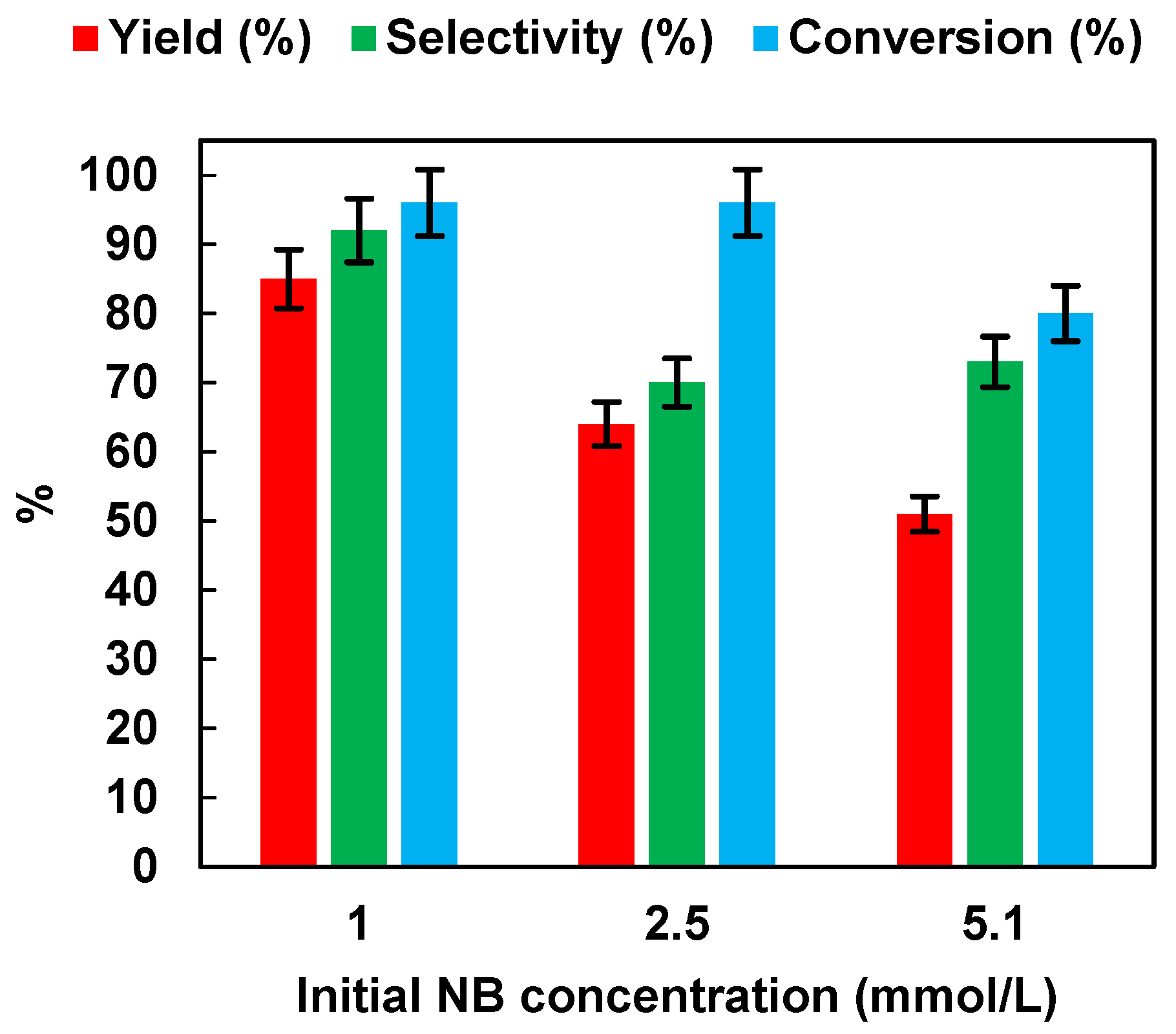
2.2.4. Effect of Initial NB Concentration
We investigated the effects of the initial NB concentration (1, 2.5 and 5.1 mmol/L) with the optimized catalyst dosage (0.5 g/L) on the photocatalytic performances. The results are reported in Figure 8. It was possible to observe that yield, selectivity, and conversion decreased with the increase of NB initial concentration from 1 up to 5.1 mmol/L. It is possible that the increase in concentration saturated the catalyst surface with NB molecules by preventing the adsorption of photons on the semiconductor surface to initiate photoreduction [36]. The above results proved that, in our study, for the reduction of the nitro group to the amino group using an NB concentration of 1 mmol/L, a photocatalyst dosage of 0.5 g/L and 50% EtOH as hydrogen source created suitable conditions for the reduction of nitro to the amino group using P25 as photocatalyst. Under these conditions, AN yield was higher than 99% after 3 h of irradiation. In Table 1, the photocatalytic NB reduction performances of different photocatalysts, as reported in the literature, are compared. It is worth noting that our optimized condition with P25 as catalyst showed a superior photocatalytic activity in comparison to other photocatalysts such as Pt-TiO2 [10], TiO2 [37], Ce2S3 [16], or even P25 itself [16], using a lower catalyst dosage and a lower irradiation time.
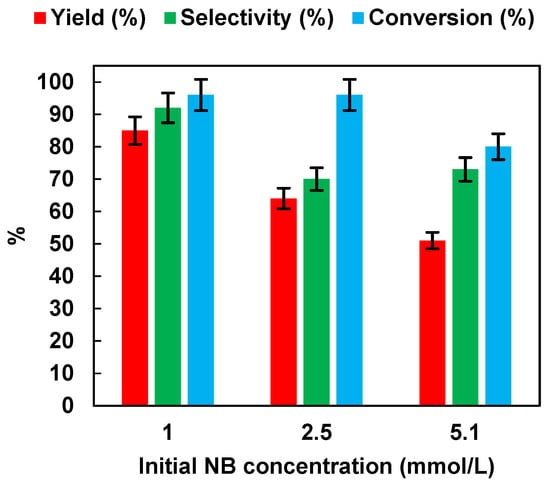
Figure 8.
Effects of the initial NB concentration on AN yield, AN selectivity, and NB conversion. UV irradiation time t is 45 min. Error bar ±5%.

Table 1.
Comparison with the available literature for photocatalytic conversion of NB.
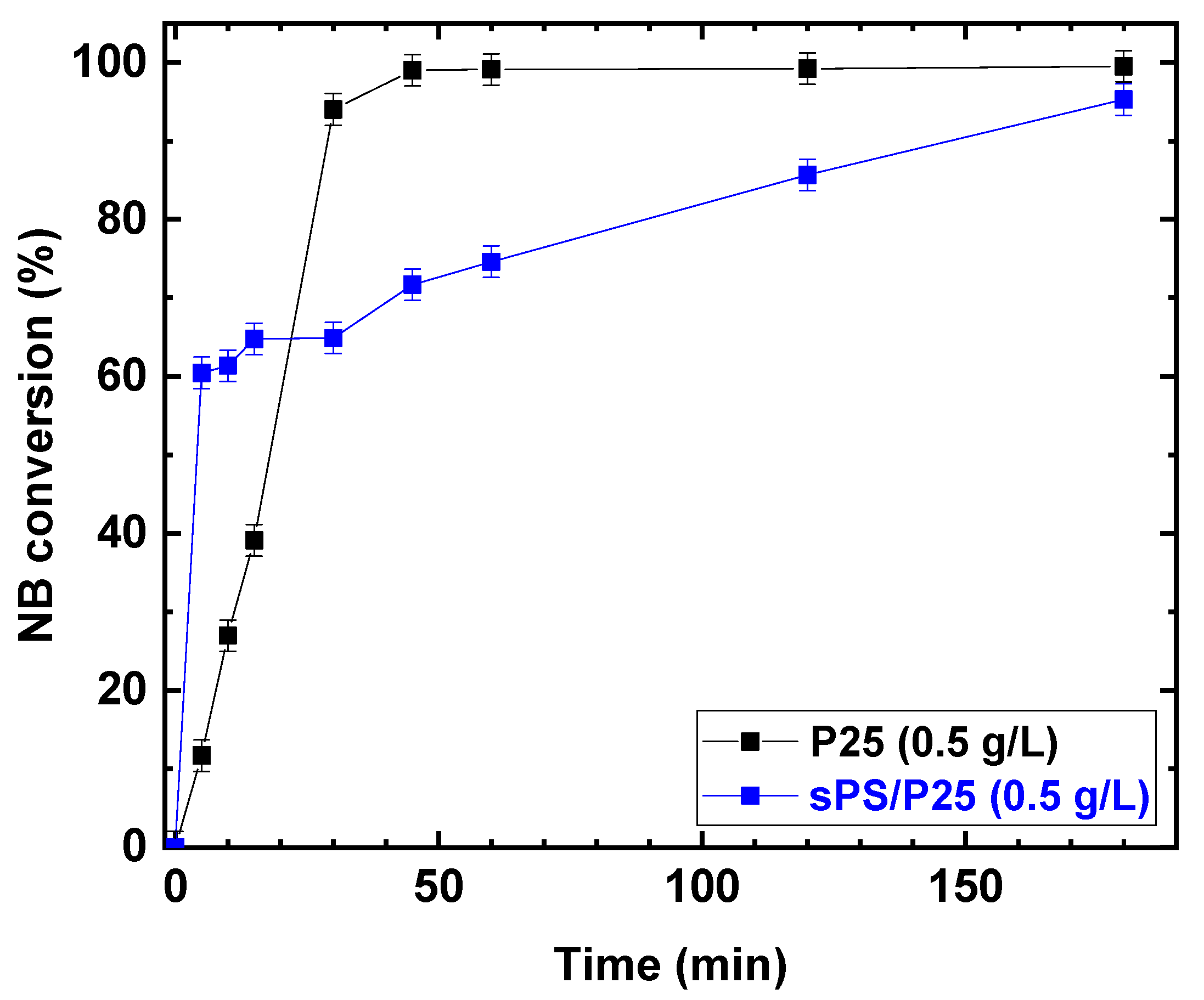
2.3. Photocatalytic Activity Results on sPS/P25 Aerogel
Once the parameters of the photocatalytic reaction were optimized, P25 was embedded in the sPS aerogel and the photocatalytic reduction was carried out. The photocatalytic performances under UV irradiation for P25 and sPS/P25 aerogel are reported in Figure 9.
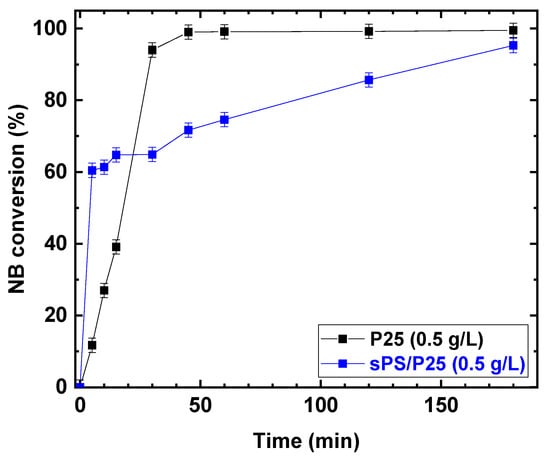
Figure 9.
Comparison of the photocatalytic reduction of NB to AN with P25 and sPS/P25 aerogel. Reaction conditions: NB 1 mmol/L; water 100 mL; temperature 25 °C. Error bar ±2%.
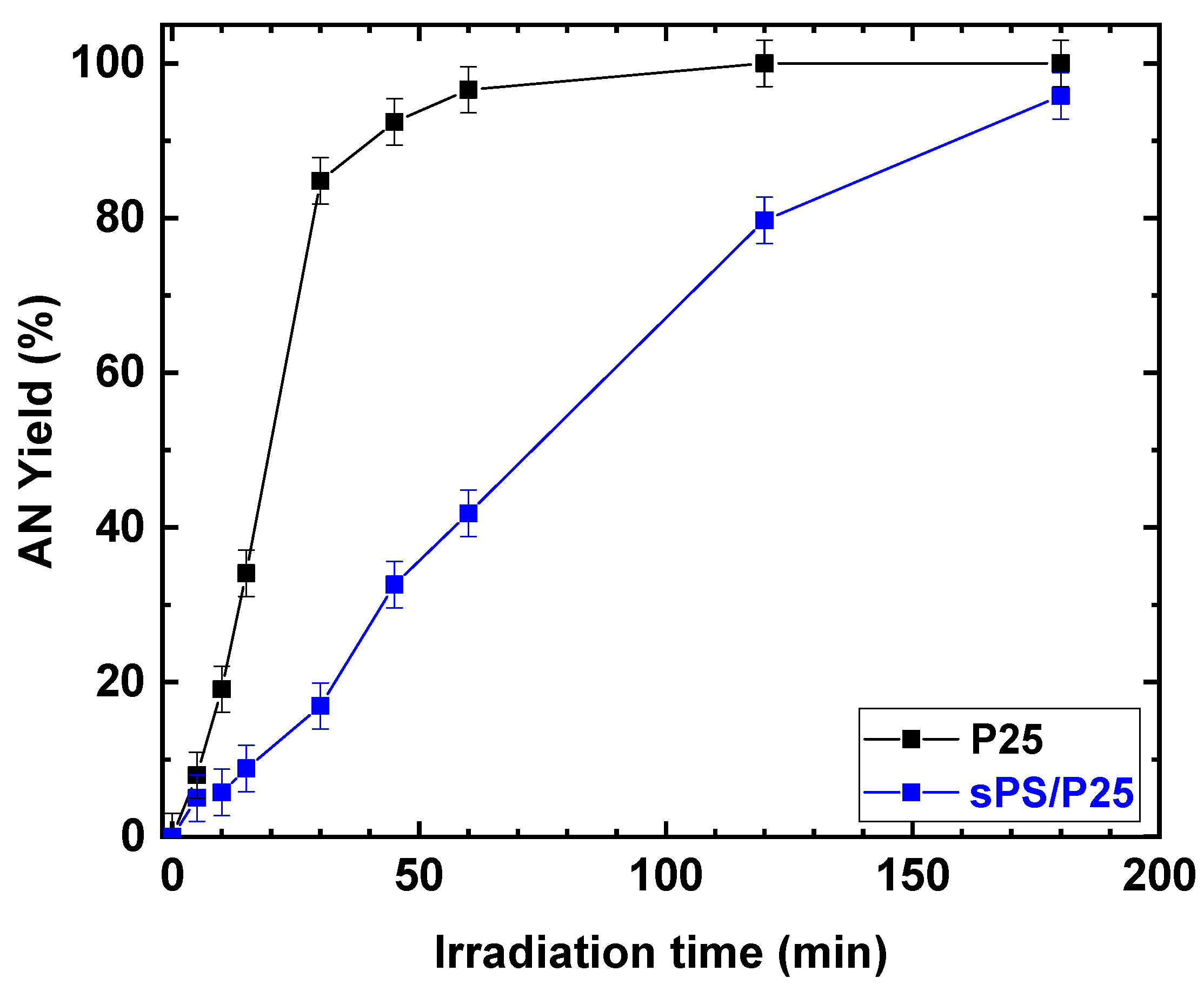
Despite the fact that the rates of increasing NB conversion for TiO2 (P25) powder and sPS/P25 aerogel were different, after 3 h of UV irradiation, similar conversion values were achieved for the P25 catalyst and sPS/P25 aerogel (99% and 95%, respectively). This resulted in the formation of AN as the only detectable reaction product with selectivity greater than 99% in both cases. In addition, the dependence of AN yield, as a function of irradiation time, was examined at an NB concentration of 1 mmol/L and EtOH percentage of 50% for P25 powder catalyst and sPS/P25 aerogel. The results are depicted in Figure 10. AN yield increased with reaction time within 180 min and, for both the tested samples, the AN yield was higher than 99%.
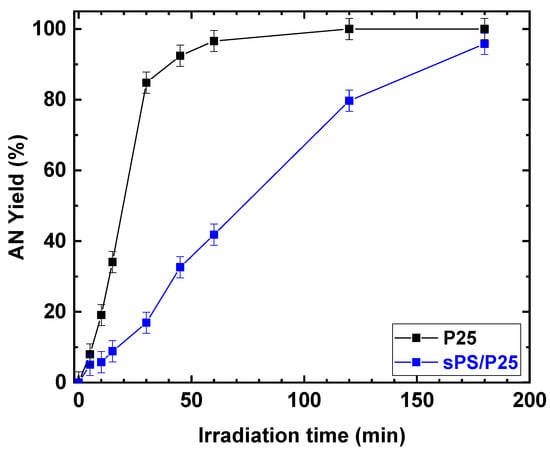
Figure 10.
Comparison of the photocatalytic reduction of NB to AN with P25 and sPS/P25 aerogel. Reaction conditions: NB, 1 mmol/L; water, 100 mL; temperature, 25 °C. Error bar ±3%.
3. Materials and Methods
3.1. Chemicals and Reagents
TiO2 (P25) particles were provided by Sigma-Aldrich. The syndiotactic polystyrene used for aerogel preparation was manufactured by Idemitsu Kosan Co., Ltd. (Chiyoda, Japan), under the trademark XAREC© 90ZC. The polymer was highly stereoregular with a content of syndiotactic triads over 98% (13C nuclear magnetic resonance data). Methanol (MeOH), Etanol (EtOH), and 2-propanol were purchased from Sigma-Aldrich, Milano, Italy.
3.2. Aerogel Preparation
A composite aerogel, based on sPS and P25 (sPS/P25 aerogel), was prepared according to the procedure reported by Sacco et al. [26]. In detail, syndiotactic polystyrene polymer and P25 photocatalyst with 95/5 weight ratio were dispersed in CHCl3 (solvent/sPS weight ratio in aerogel samples was 90/10), in a hermetically sealed test tube, and heated at 100 °C. The suspension was subsequently cooled to room temperature, forming a gel. The obtained gel was treated with supercritical carbon dioxide (using an ISCO SFX 220 extractor) for 4 h at T = 40 °C and P = 20 MPa to extract the solvent and obtain the relative monolithic composite aerogel. The sPS/P25 aerogel was a coherent phase in a cylindrical shape (diameter = 5.6 mm; height = 3 cm).
3.3. Samples Characterization
The BET method was used to evaluate the specific surface area by making dynamic N2 adsorption measurements at −196 °C using a Nova Quantachrome 4200e analyzer (Rivoli, Italy). X-ray diffraction (XRD) patterns were obtained with an automatic Bruker D2 Advance diffractometer, with reflection geometry and nickel-filtered Cu-Kα radiation. The intensities of XRD patterns were not corrected for polarization or Lorentz factors to allow easier comparison with most literature data. The acquisition interval ranged between 2θ = 5° and 90°, scanning with a step size of 0.0303° and an acquisition time of 0.200 s per point.
3.4. Photocatalytic Activity Tests
All the photocatalytic tests were performed using 100 mL of nitrobenzene (NB) aqueous solution. The reactor used for all the tests was a Pyrex cylindric photoreactor connected to a peristaltic pump (Watson-Marlow, Mazzano, Italy) to recirculate the solution. A UV-A LEDs strip, with emission at 365 nm and irradiance of 13 W/m2, was wrapped around the external surface of the reactor. During the photocatalytic experiment, N2 was bubbled inside the system, which was kept in the dark for 120 min to reach NB adsorption equilibrium on the catalyst surface and then irradiated for 180 min. At regular times, 2 mL of solution was collected and filtered. The products of the reaction were identified and quantified via gas chromatography-flame ionization detector (GC-FID) using an Agilent 7820A (Cernusco Sul Naviglio, Italy) equipped with a DB Heavy Wax capillary column (30 m × 320 µm × 0.25µm) under the following conditions: detector (FID) temperature = 300 °C; Oven = 60 °C hold 1 min; rate 25 up to 120 °C; equilibrate 3 min; rate 2 up to 210 °C; rate 25 up to 250 °C; injection volume 1 µL operated at 10:1 split mode. For the UV absorption spectrum of the intermediate (4-aminoAZ, Sigma-Aldrich, Milano, Italy), a fluorescence spectrometer, Duetta (Horiba Scientific, Torino, Italy), was used.
AN yield was calculated using the following formula:
Yield (%) = Pf/Ci × 100
NB conversion was calculated by the following formula
where:
Conversion (%) = (Ci − Cf)/Ci × 100
- Pf is AN concentration measured at the generic irradiation time t;
- Ci is the initial concentration of NB;
- Cf is NB concentration measured at the generic irradiation time t.
The selectivity to AN in the reaction process was evaluated by the following formula:
Selectivity (%) = Pf/(Ci − Cf) × 100
4. Conclusions
A selective process for the photocatalytic reduction of NB under UV irradiation for AN production, in the presence of sacrificial electron donors, was developed. We investigated the effects of various experimental conditions—such as reducing agents, initial EtOH composition, photocatalyst dosage, initial NB concentration, and immobilization of the photocatalyst within sPS aerogel—on the reaction performances. Our study showed that aniline could be selectively obtained in the presence of P25 as photocatalysts, with yields greater than 99% with NB initial concentration of 1 mmol/L, photocatalyst dosage of 0.5 g/L, and 50% EtOH as the hydrogen source. Furthermore, it was shown that dispersing P25 within sPS aerogels after three hours of irradiation resulted in similar NB conversions of 99% and 95% for P25 and sPS/P25 aerogel, respectively, as well as AN production greater than 99%. The latter could be an optimal solution for a scale-up of the process since sPS/P25 aerogel can be easily removed from the solution, thereby avoiding a post-treatment step that would be necessary if a photocatalyst were used in powder form for the photoreaction.
Author Contributions
Conceptualization, V.V. (Vincenzo Vaiano) and O.S.; methodology, V.V. (Vincenzo Venditto); investigation, W.N.; data curation, O.S. and W.N.; writing—original draft preparation, W.N.; writing—review and editing, V.V. (Vincenzo Vaiano), V.V. (Vincenzo Venditto) and O.S.; supervision, V.V. (Vincenzo Vaiano) and O.S. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
Not applicable here.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Padda, R.S.; Wang, C.; Hughes, J.B.; Kutty, R.; Bennett, G.N. Mutagenicity of Nitroaromatic Degradation Compounds. Environ. Toxicol. Chem. 2003, 22, 2293–2297. [Google Scholar] [CrossRef] [PubMed]
- Purohit, V.; Basu, A.K. Mutagenicity of Nitroaromatic Compounds. Chem. Res. Toxicol. 2000, 13, 673–692. [Google Scholar] [CrossRef] [PubMed]
- Sriram, P.; Su, D.-S.; Periasamy, A.P.; Manikandan, A.; Wang, S.-W.; Chang, H.-T.; Chueh, Y.-L.; Yen, T.-J. Quadrupole Gap Plasmons: Hybridizing Strong Quadrupole Gap Plasmons Using Optimized Nanoantennas with Bilayer MoS2 for Excellent Photo-Electrochemical Hydrogen Evolution (Adv. Energy Mater. 29/2018). Adv. Energy Mater. 2018, 8, 1870127. [Google Scholar] [CrossRef]
- Bose, P.; Glaze, W.H.; Maddox, D.S. Degradation of RDX by Various Advanced Oxidation Processes: I. Reaction Rates. Water Res. 1998, 32, 997–1004. [Google Scholar] [CrossRef]
- Blaser, H.-U.; Malan, C.; Pugin, B.; Spindler, F.; Steiner, H.; Studer, M. Selective Hydrogenation for Fine Chemicals: Recent Trends and New Developments. Adv. Synth. Catal. 2003, 345, 103–151. [Google Scholar] [CrossRef]
- Mu, Q.; Zhang, Q.; Yu, W.; Su, M.; Cai, Z.; Cui, K.; Ye, Y.; Liu, X.; Deng, L.; Chen, B.; et al. Robust Multiscale-Oriented Thermoresponsive Fibrous Hydrogels with Rapid Self-Recovery and Ultrafast Response Underwater. ACS Appl. Mater. Interfaces 2020, 12, 33152–33162. [Google Scholar] [CrossRef] [PubMed]
- Mu, Q.; Cui, K.; Wang, Z.J.; Matsuda, T.; Cui, W.; Kato, H.; Namiki, S.; Yamazaki, T.; Frauenlob, M.; Nonoyama, T.; et al. Force-Triggered Rapid Microstructure Growth on Hydrogel Surface for on-Demand Functions. Nat. Commun. 2022, 13, 6213. [Google Scholar] [CrossRef] [PubMed]
- Fleischmann, C.; Lievenbrück, M.; Ritter, H. Polymers and Dyes: Developments and Applications. Polymers 2015, 7, 717–746. [Google Scholar] [CrossRef]
- Aljahdali, M.S.; Amin, M.S.; Mohamed, R.M. Gd-Cobalt Selenite as an Efficient Nanocomposite for Aniline Synthesis from Photocatalytic Reduction of Nitrobenzene. Mater. Res. Bull. 2018, 99, 161–167. [Google Scholar] [CrossRef]
- Zhou, B.; Song, J.; Zhou, H.; Wu, L.; Wu, T.; Liu, Z.; Han, B. Light-Driven Integration of the Reduction of Nitrobenzene to Aniline and the Transformation of Glycerol into Valuable Chemicals in Water. RSC Adv. 2015, 5, 36347–36352. [Google Scholar] [CrossRef]
- Wang, J.; Yuan, Z.; Nie, R.; Hou, Z.; Zheng, X. Hydrogenation of Nitrobenzene to Aniline over Silica Gel Supported Nickel Catalysts. Ind. Eng. Chem. Res. 2010, 49, 4664–4669. [Google Scholar] [CrossRef]
- Lee, S.-P.; Chen, Y.-W. Nitrobenzene Hydrogenation on Ni–P, Ni–B and Ni–P–B Ultrafine Materials. J. Mol. Catal. A: Chem. 2000, 152, 213–223. [Google Scholar] [CrossRef]
- Corma, A.; Concepción, P.; Serna, P. A Different Reaction Pathway for the Reduction of Aromatic Nitro Compounds on Gold Catalysts. Angew. Chem. Int. Ed. 2007, 46, 7266–7269. [Google Scholar] [CrossRef] [PubMed]
- Tegge, G. Ullmann’s Encyclopedia of Industrial Chemistry. Fifth, Completely Revised Edition. Volumes B2 and B3. Unit Operations I and II. VCH Verlagsgesellschaft MbH, Weinheim/Basel/Cambridge/New York 1988. ISBN 3-527-20132-7 (Weinheim …) Pp., 0-89573-537-7 (Cambridge …) Pp. Executive Editor: Wolfgang Gerhartz. Editors: Barbara Elvers, Michael Ravenscroft, James, F. Rounsaville, and Gail Schulz. Each Volume 634 Pages, with Numerous Figures and Tables. Hardcover, Each DM 490,–. Starch Stärke 1991, 43, 79. [Google Scholar] [CrossRef]
- Huang, H.; Zhou, J.; Liu, H.; Zhou, Y.; Feng, Y. Selective Photoreduction of Nitrobenzene to Aniline on TiO2 Nanoparticles Modified with Amino Acid. J. Hazard. Mater. 2010, 178, 994–998. [Google Scholar] [CrossRef] [PubMed]
- Chen, S.; Zhang, H.; Fu, X.; Hu, Y. Preparation, Characterization, and Photocatalytic Performance of Ce2S3 for Nitrobenzene Reduction. Appl. Surf. Sci. 2013, 275, 335–341. [Google Scholar] [CrossRef]
- Patzsch, J.; Berg, B.; Bloh, J.Z. Kinetics and Optimization of the Photocatalytic Reduction of Nitrobenzene. Front. Chem. 2019, 7, 289. [Google Scholar] [CrossRef]
- Bakardjieva, S.; Šubrt, J.; Štengl, V.; Dianez, M.J.; Sayagues, M.J. Photoactivity of Anatase–Rutile TiO2 Nanocrystalline Mixtures Obtained by Heat Treatment of Homogeneously Precipitated Anatase. Appl. Catal. B: Environ. 2005, 58, 193–202. [Google Scholar] [CrossRef]
- Flores, S.; Rios-Bernij, O.; Valenzuela, M.; Córdova, I.; Gómez, R.; Gutiérrez, R. Photocatalytic Reduction of Nitrobenzene over Titanium Dioxide: By-Product Identification and Possible Pathways. Top. Catal. 2007, 44, 507–511. [Google Scholar] [CrossRef]
- Ferry, J.L.; Glaze, W.H. Photocatalytic Reduction of Nitro Organics over Illuminated Titanium Dioxide: Role of the TiO2 Surface. Langmuir 1998, 14, 3551–3555. [Google Scholar] [CrossRef]
- Ferry, J.L.; Glaze, W.H. Photocatalytic Reduction of Nitroorganics over Illuminated Titanium Dioxide: Electron Transfer between Excited-State TiO2 and Nitroaromatics. J. Phys. Chem. B 1998, 102, 2239–2244. [Google Scholar] [CrossRef]
- Fukui, M.; Koshida, W.; Tanaka, A.; Hashimoto, K.; Kominami, H. Photocatalytic Hydrogenation of Nitrobenzenes to Anilines over Noble Metal-Free TiO2 Utilizing Methylamine as a Hydrogen Donor. Appl. Catal. B Environ. 2020, 268, 118446. [Google Scholar] [CrossRef]
- Wang, H.; Partch, R.E.; Li, Y. Synthesis of 2-Alkylbenzimidazoles via TiO2-Mediated Photocatalysis. J. Org. Chem. 1997, 62, 5222–5225. [Google Scholar] [CrossRef]
- Vaiano, V.; Sacco, O.; Sannino, D.; Ciambelli, P.; Longo, S.; Venditto, V.; Guerra, G. N-Doped TiO2/s-PS Aerogels for Photocatalytic Degradation of Organic Dyes in Wastewater under Visible Light Irradiation. J. Chem. Technol. Biotechnol. 2014, 89, 1175–1181. [Google Scholar] [CrossRef]
- Sacco, O.; Vaiano, V.; Daniel, C.; Navarra, W.; Venditto, V. Highly Robust and Selective System for Water Pollutants Removal: How to Transform a Traditional Photocatalyst into a Highly Robust and Selective System for Water Pollutants Removal. Nanomaterials 2019, 9, 1509. [Google Scholar] [CrossRef] [PubMed]
- Sacco, O.; Vaiano, V.; Daniel, C.; Navarra, W.; Venditto, V. Removal of Phenol in Aqueous Media by N-Doped TiO2 Based Photocatalytic Aerogels. Mater. Sci. Semicond. Process. 2018, 80, 104–110. [Google Scholar] [CrossRef]
- Machado, N.R.C.F.; Santana, V.S. Influence of Thermal Treatment on the Structure and Photocatalytic Activity of TiO2 P25. Catal. Today 2005, 107, 595–601. [Google Scholar] [CrossRef]
- Zouzelka, R.; Rathousky, J. Photocatalytic Abatement of NOx Pollutants in the Air Using Commercial Functional Coating with Porous Morphology. Appl. Catal. B Environ. 2017, 217, 466–476. [Google Scholar] [CrossRef]
- Raj, K.; Viswanathan, B. Effect of Surface Area, Pore Volume and Particle Size of P25 Titania on the Phase Transformation of Anatase to Rutile. Indian J. Chem. Sect. A Inorg. Phys. Theor. Anal. Chem. 2009, 48, 1378–1382. [Google Scholar]
- Navarra, W.; Sacco, O.; Daniel, C.; Venditto, V.; Vaiano, V.; Vignati, D.A.L.; Bojic, C.; Libralato, G.; Lofrano, G.; Carotenuto, M. Photocatalytic Degradation of Atrazine by an N-Doped TiO2/Polymer Composite: Catalytic Efficiency and Toxicity Evaluation. J. Environ. Chem. Eng. 2022, 10, 108167. [Google Scholar] [CrossRef]
- Capello, C.; Fischer, U.; Hungerbühler, K. What Is a Green Solvent? A Comprehensive Framework for the Environmental Assessment of Solvents. Green Chem. 2007, 9, 927–934. [Google Scholar] [CrossRef]
- Lin-Vien, D.; Colthup, N.B.; Fateley, W.G.; Grasselli, J.G. CHAPTER 18—Selected Infrared and Raman Spectra from the Sadtler Research Laboratories, Division of Bio-Rad Laboratories, Inc. for Compounds with Structures Discussed in Chapters 2–17. In The Handbook of Infrared and Raman Characteristic Frequencies of Organic Molecules; Lin-Vien, D., Colthup, N.B., Fateley, W.G., Grasselli, J.G., Eds.; Academic Press: San Diego, CA, USA, 1991; pp. 307–422. ISBN 978-0-12-451160-6. [Google Scholar]
- Shin, D.; Kang, J.H.; Min, K.-A.; Hong, S.; Hee Hong, B. Graphene Oxide Catalyzed Cis-Trans Isomerization of Azobenzene. APL Mater. 2014, 2, 092501. [Google Scholar] [CrossRef]
- Rahman, M.A.; Muneer, M. Photocatalysed Degradation of Two Selected Pesticide Derivatives, Dichlorvos and Phosphamidon, in Aqueous Suspensions of Titanium Dioxide. Desalination 2005, 181, 161–172. [Google Scholar] [CrossRef]
- Garcia, J.; Takashima, K. Photocatalytic Degradation of Imazaquin in an Aqueous Suspension of Titanium Dioxide. J. Photochem. Photobiol. A Chem. 2003, 155, 215–222. [Google Scholar] [CrossRef]
- Roy, P.; Periasamy, A.P.; Liang, C.-T.; Chang, H.-T. Synthesis of Graphene-ZnO-Au Nanocomposites for Efficient Photocatalytic Reduction of Nitrobenzene. Environ. Sci. Technol. 2013, 47, 6688–6695. [Google Scholar] [CrossRef]
- Chen, S.; Zhang, H.; Yu, X.; Liu, W. Photocatalytic Reduction of Nitrobenzene by Titanium Dioxide Powder. Chin. J. Chem. 2010, 28, 21–26. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).