LIonomers-New Generation of Ionomer: Understanding of Their Interaction and Structuration as a Function of the Tunability of Cation and Anion
Abstract
:1. Introduction
2. Experimental Section
2.1. Materials
2.2. Material Preparation and Characterizations
3. Results and Discussion
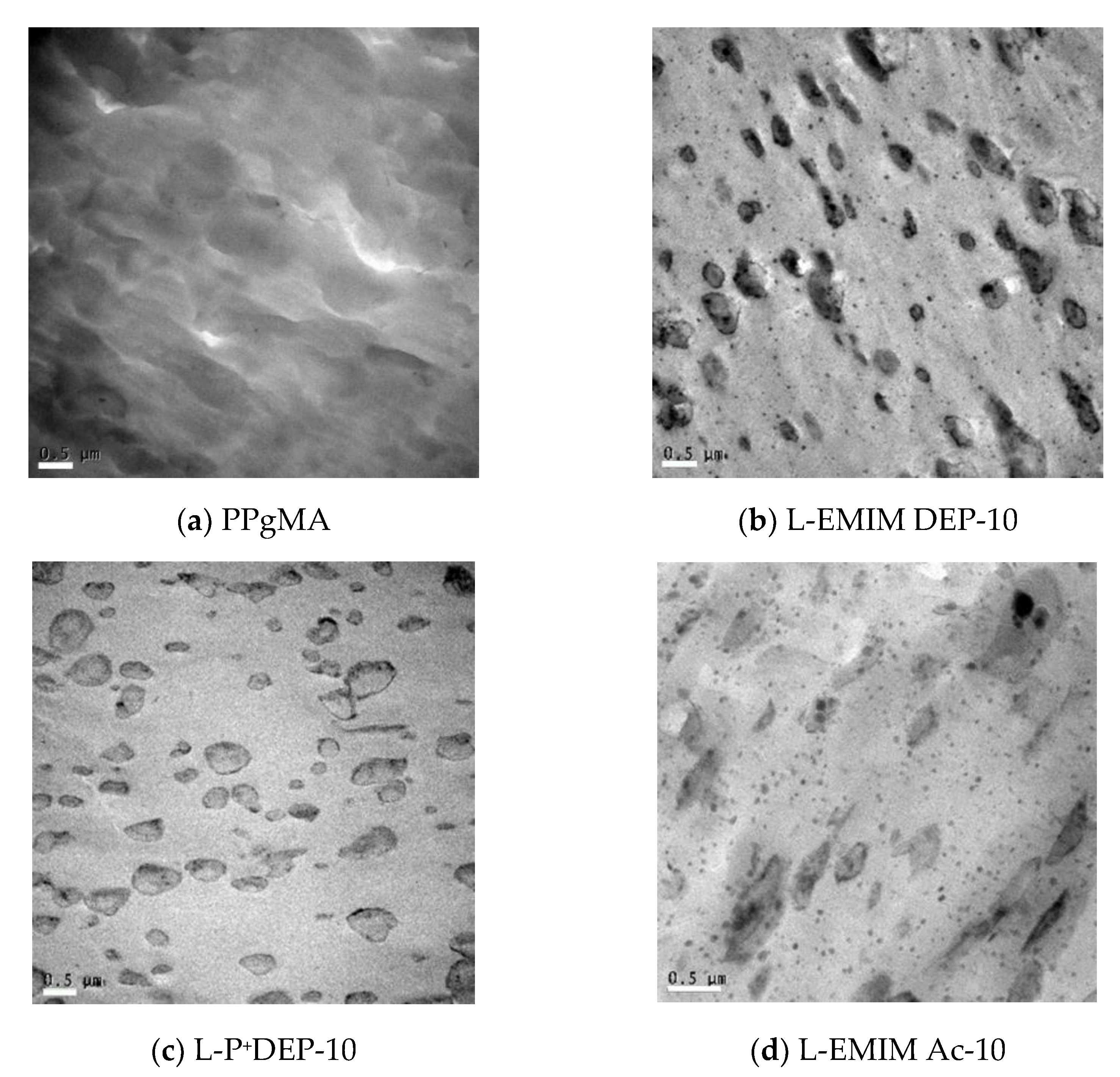
3.1. LIonomer Morphology
3.2. PPgMA–ILs Interactions
3.3. Effect of ILs on Rheological Properties: Strength of Interactions
3.4. Microstructure of LIonomers
3.5. Effect of ILs on Crystallization of PPgMA
3.5.1. Role of ILs in the Cystallization Process
3.5.2. Effect on Crystalline Phase
3.6. Effect of ILs on Mechanical Behavior of LIonomers
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Zhang, L.; Brostowitz, N.R.; Cavicchi, K.A.; Weiss, R.A. Perspective: Ionomer research and applications. Macromol. React. Eng. 2014, 8, 81–99. [Google Scholar] [CrossRef]
- Kirkmeyer, B.P.; Weiss, R.A.; Winey, K.I. Spherical and vesicular ionic aggregates in Zn-neutralized sulfonated polystyrene ionomers. J. Polym. Sci. Part B Polym. Phys. 2001, 39, 477–483. [Google Scholar] [CrossRef]
- Eisenberg, A.; Hird, B.; Moore, R.B. A new multiplet-cluster model for the morphology of random ionomers. Macromolecules 1990, 23, 4098–4107. [Google Scholar] [CrossRef]
- Datta, S.; De, P.; De, S. Blends of ionomers. J. Appl. Polym. Sci. 1996, 61, 1839–1846. [Google Scholar] [CrossRef]
- Longworth, R.; Vaughan, D.J. Physical structure of ionomers. Nature 1968, 218, 85–87. [Google Scholar] [CrossRef]
- Kim, J.-S.; Yoshikawa, K.; Eisenberg, A. Molecular Weight Dependence of the Viscoelastic Properties of Polystyrene-Based Ionomers. Macromolecules 1994, 27, 6347–6357. [Google Scholar] [CrossRef]
- Dalmas, F.; Leroy, E. New Insights into Ionic Aggregate Morphology in Zn-Neutralized Sulfonated Polystyrene Ionomers by Transmission Electron Tomography. Macromolecules 2011, 44, 8093–8099. [Google Scholar] [CrossRef]
- Aitken, B.S.; Buitrago, C.F.; Heffley, J.D.; Lee, M.; Gibson, H.W.; Winey, K.I.; Wagener, K.B. Precision ionomers: Synthesis and thermal/mechanical characterization. Macromolecules 2012, 45, 681–687. [Google Scholar] [CrossRef]
- Weiss, R.A.; Zhao, H. Rheological behavior of oligomeric ionomers. J. Rheol. 2009, 53, 191–213. [Google Scholar] [CrossRef]
- Qiao, X.; Weiss, R.A. Nonlinear Rheology of Lightly Sulfonated Polystyrene Ionomers. Macromolecules 2013, 46, 2417–2424. [Google Scholar] [CrossRef]
- Ling, G.H.; Wang, Y.; Weiss, R. Linear viscoelastic and uniaxial extensional rheology of alkali metal neutralized sulfonated oli-gostyrene ionomer melts. Macromolecules 2012, 45, 481–490. [Google Scholar] [CrossRef]
- Rapone, I.; Taresco, V.; Lisio, V.D.; Piozzi, A.; Francolini, I. Silver-and Zinc-Decorated Polyurethane Ionomers with Tunable Hard/Soft Phase Segregation. Int. J. Mol. Sci. 2021, 22, 6134. [Google Scholar] [CrossRef]
- Ro, A.J.; Huang, S.J.; Weiss, R. Synthesis and properties of random poly (lactic acid)-based ionomers. Polymer 2009, 50, 1134–1143. [Google Scholar] [CrossRef]
- Ro, A.J. Synthesis and Properties of Poly(lactic Acid) Ionomers. Ph.D. Thesis, University of Connecticut, Mansfield, CT, USA, 2008. [Google Scholar]
- Wu, M.-H.; Wang, C.-C.; Chen, C.-Y. Preparation of high melt strength polypropylene by addition of an ionically modified poly-propylene. Polymer 2020, 202, 122743. [Google Scholar] [CrossRef]
- Li, Y.; Yao, Z.; Chen, Z.-H.; Qiu, S.-L.; Zeng, C.; Cao, K. High melt strength polypropylene by ionic modification: Preparation, rheological properties and foaming behaviors. Polymer 2015, 70, 207–214. [Google Scholar] [CrossRef]
- Colbeaux, A.; Fenouillot, F.; Gérard, J.-F.; Taha, M.; Wautier, H. Compatibilization of a polyolefin blend through covalent and ionic coupling of grafted polypropylene and polyethylene. II. Morphology. J. Appl. Polym. Sci. 2004, 93, 2237–2244. [Google Scholar] [CrossRef]
- Colbeaux, A.; Fenouillot, F.; Gérard, J.-F.; Taha, M.; Wautier, H. Compatibilization of a polyolefin blend through covalent and ionic coupling of grafted polypropylene and polyethylene. I. Rheological, thermal, and mechanical properties. J. Appl. Polym. Sci. 2005, 95, 312–320. [Google Scholar] [CrossRef]
- Livi, S.; Duchet-Rumeau, J.; Pham, T.-N.; Gérard, J.-F. A comparative study on different ionic liquids used as surfactants: Effect on thermal and mechanical properties of high-density polyethylene nanocomposites. J. Colloid Interface Sci. 2010, 349, 424–433. [Google Scholar] [CrossRef]
- Rahman, M.; Brazel, C.S. Ionic liquids: New generation stable plasticizers for poly (vinyl chloride). Polym. Degrad. Stab. 2006, 91, 3371–3382. [Google Scholar] [CrossRef]
- Yousfi, M.; Livi, S.; Duchet-Rumeau, J. Ionic liquids: A new way for the compatibilization of thermoplastic blends. Chem. Eng. J. 2014, 255, 513–524. [Google Scholar] [CrossRef]
- Yang, J.; Pruvost, S.; Livi, S.; Duchet-Rumeau, J. Understanding of versatile and tunable nanostructuration of ionic liquids on fluorinated copolymer. Macromolecules 2015, 48, 4581–4590. [Google Scholar] [CrossRef]
- Leroy, E.; Jacquet, P.; Coativy, G.; Reguerre, A.L.; Lourdin, D. Compatibilization of starch–zein melt processed blends by an ionic liquid used as plasticizer. Carbohydr. Polym. 2012, 89, 955–963. [Google Scholar] [CrossRef]
- Chen, Y.; Zhang, Y.; Ke, F.; Zhou, J.; Wang, H.; Liang, D. Solubility of neutral and charged polymers in ionic liquids studied by laser light scattering. Polymer 2011, 52, 481–488. [Google Scholar] [CrossRef]
- Hajipour, A.R.; Rafiee, F. Recent progress in ionic liquids and their applications in organic synthesis. Org. Prep. Proced. Int. 2015, 47, 249–308. [Google Scholar] [CrossRef]
- Yue, C.; Fang, D.; Liu, L.; Yi, T.-F. Synthesis and application of task-specific ionic liquids used as catalysts and/or solvents in organic unit reactions. J. Mol. Liq. 2011, 163, 99–121. [Google Scholar] [CrossRef]
- Clark, E.J.; Hoffman, J.D. Regime III crystallization in polypropylene. Macromolecules 1984, 17, 878–885. [Google Scholar] [CrossRef]
- Jeziorny, A. Parameters characterizing the kinetics of the non-isothermal crystallization of poly (ethylene terephthalate) de-termined by DSC. Polymer 1978, 19, 1142–1144. [Google Scholar] [CrossRef]
- Livi, S.; Duchet-Rumeau, J.; Gérard, J.-F. Nanostructuration of ionic liquids in fluorinated matrix: Influence on the mechanical properties. Polymer 2011, 52, 1523–1531. [Google Scholar] [CrossRef]
- Dibble, D.C.; Li, C.; Sun, L.; George, A.; Cheng, A.; Çetinkol, Ö.P.; Benke, P.; Holmes, B.M.; Singh, S.; Simmons, B.A. A facile method for the recovery of ionic liquid and lignin from biomass pretreatment. Green Chem. 2011, 13, 3255–3264. [Google Scholar] [CrossRef]
- Clough, M.T.; Geyer, K.; Hunt, P.A.; Mertes, J.; Welton, T. Thermal decomposition of carboxylate ionic liquids: Trends and mechanisms. Phys. Chem. Chem. Phys. 2013, 15, 20480–20495. [Google Scholar] [CrossRef] [PubMed]
- Dubey, S.; Bharmoria, P.; Gehlot, P.S.; Agrawal, V.; Kumar, A.; Mishra, S. 1-Ethyl-3-methylimidazolium diethylphosphate based extraction of bioplastic “Poly-hydroxyalkanoates” from bacteria: Green and sustainable approach. ACS Sustain. Chem. Eng. 2018, 6, 766–773. [Google Scholar] [CrossRef]
- Binks, F.C.; Cavalli, G.; Henningsen, M.; Howlin, B.J.; Hamerton, I. Examining the influence of anion nucleophilicity on the polymerisation initiation mechanism of phenyl glycidyl ether. Polymers 2019, 11, 657. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Weiss, R.A.; Yu, W.-C. Viscoelastic Behavior of Very Lightly Sulfonated Polystyrene Ionomers. Macromolecules 2007, 40, 3640–3643. [Google Scholar] [CrossRef]
- Page, K.A.; Park, J.K.; Moore, R.B.; Garcia Sakai, V. Direct analysis of the ion-hopping process associated with the α-relaxation in perfluorosulfonate ionomers using quasielastic neutron scattering. Macromolecules 2009, 42, 2729–2736. [Google Scholar] [CrossRef]
- Castagna, A.M.; Wang, W.; Winey, K.I.; Runt, J. Influence of cation type on structure and dynamics in sulfonated polystyrene ionomers. Macromolecules 2011, 44, 5420–5426. [Google Scholar] [CrossRef]
- Ma, Q.; Georgiev, G.; Cebe, P. Constraints in semicrystalline polymers: Using quasi-isothermal analysis to investigate the mecha-nisms of formation and loss of the rigid amorphous fraction. Polymer 2011, 52, 4562–4570. [Google Scholar] [CrossRef]
- Arnoult, M.; Dargent, E.; Mano, J. Mobile amorphous phase fragility in semi-crystalline polymers: Comparison of PET and PLLA. Polymer 2007, 48, 1012–1019. [Google Scholar] [CrossRef]
- Bajsić, E.G.; Šmit, I.; Leskovac, M. Blends of thermoplastic polyurethane and polypropylene. I. Mechanical and phase behavior. J. Appl. Polym. Sci. 2007, 104, 3980–3985. [Google Scholar] [CrossRef]
- Xing, C.; Zhao, M.; Zhao, L.; You, J.; Cao, X.; Li, Y. Ionic liquid modified poly (vinylidene fluoride): Crystalline structures, miscibility, and physical properties. Polym. Chem. 2013, 4, 5726–5734. [Google Scholar] [CrossRef]
- Mijovic, J.; Sy, J.-W.; Kwei, T. Reorientational dynamics of dipoles in poly (vinylidene fluoride)/poly (methyl methacry-late)(PVDF/PMMA) blends by dielectric spectroscopy. Macromolecules 1997, 30, 3042–3050. [Google Scholar] [CrossRef]
- Clark, E.S. Unit cell information on some important polymers. In Physical Properties of Polymers Handbook; Springer: Berlin/Heidelberg, Germany, 2007; pp. 619–624. [Google Scholar]
- Yang, G.; Li, X.; Chen, J.; Yang, J.; Huang, T.; Liu, X.; Wang, Y. Crystallization behavior of isotactic polypropylene induced by competition action of β nucleating agent and high pressure. Colloid Polym. Sci. 2012, 290, 531–540. [Google Scholar] [CrossRef]
- Zipper, P.; Janosi, A.; Wrentschur, E. Scanning X-ray scattering of mouldings from semicrystalline polymers. J. De Phys. IV 1993, 3, C8-33–C8-36. [Google Scholar] [CrossRef]












| Polymer | IL | IL Content (wt%) | Abbreviation |
|---|---|---|---|
| PPgMA | P+DEP | 2 | L-P+DEP-2 |
| P+DEP | 10 | L-P+DEP-10 | |
| EMIM DEP | 2 | L-EMIM DEP-2 | |
| EMIM DEP | 10 | L-EMIM DEP-10 | |
| EMIM Ac | 2 | L-EMIM Ac-2 | |
| EMIM Ac | 10 | L-EMIM Ac-10 |
| Materials | (°C) | (MAF, °C) | (RAF, °C) |
|---|---|---|---|
| PPgMA | −47 | 7 | 76 |
| L-P+DEP-2 | −47 | 5 | 79 |
| L-P+DEP-10 | −47 | 5 | 93 |
| L-EMIM DEP-2 | −47 | 5 | 74 |
| L-EMIM DEP-10 | −48 | 5 | 78 |
| L-EMIM Ac-2 | −47 | 7 | 78 |
| L-EMIM Ac-10 | −47 | 6 | 80 |
| Materials | (%) | , (°C) | (min) |
|---|---|---|---|
| PPgMA | 34.1 | 116.1 | 0.77 |
| L-P+DEP-2 | 37.6 | 126.8 | 0.74 |
| L-P+DEP-10 | 35.1 | 116.0 | 0.76 |
| L-EMIM DEP-2 | 36.8 | 124.6 | 0.72 |
| L-EMIM DEP-10 | 35.3 | 118.0 | 0.86 |
| L-EMIM Ac-2 | 34.2 | 118.8 | 0.77 |
| L-EMIM Ac-10 | 36.9 | 118.5 | 0.72 |
| D[110] (nm) | D[040] (nm) | D[130] (nm) | D[300] (nm) | K (%) | Average Size (Å) a | |
|---|---|---|---|---|---|---|
| PPgMA | 12.7 | 18.2 | 12.8 | - | - | 12.7 |
| L-P+DEP-2 | 13.5 | 18.2 | 14.1 | - | - | 12.3 |
| L-P+DEP-10 | 13.6 | 18.3 | 13.8 | - | - | 12.4 |
| L-EMIM DEP-2 | 13.3 | 18.0 | 13.7 | - | - | 12.5 |
| L-EMIM DEP-10 | 8.9 | 21.0 | 15.8 | 72.4 | 3.2 | 13.1 |
| L-EMIM Ac-2 | 13.0 | 18.6 | 13.0 | - | - | 12.6 |
| L-EMIM Ac-10 | 17.4 | 24.3 | 13.0 | 51.2 | 9.6 | 14.0 |
| Samples | Young’s Modulus (MPa) | Strain at Break (%) | Yield Stress (MPa) |
|---|---|---|---|
| PPgMA | 456 ± 18 | 24 ± 6 | 24 ± 1 |
| L-P+DEP-2 | 705 ± 4 | 14 ± 3 | 30 ± 1 |
| L-P+DEP-10 | 492 ± 12 | 35 ± 5 | 24 ± 1 |
| L-EMIM DEP-2 | 803 ± 10 | 23 ± 2 | 31 ± 1 |
| L-EMIM DEP-10 | 431 ± 11 | 655 ± 20 | 16 ± 1 |
| L-EMIM Ac-2 | 756 ± 9 | 65 ± 4 | 31 ± 3 |
| L-EMIM Ac-10 | 609 ± 12 | 550 ± 34 | 28 ± 1 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hou, L.; Livi, S.; Gérard, J.-F.; Duchet-Rumeau, J. LIonomers-New Generation of Ionomer: Understanding of Their Interaction and Structuration as a Function of the Tunability of Cation and Anion. Polymers 2023, 15, 370. https://doi.org/10.3390/polym15020370
Hou L, Livi S, Gérard J-F, Duchet-Rumeau J. LIonomers-New Generation of Ionomer: Understanding of Their Interaction and Structuration as a Function of the Tunability of Cation and Anion. Polymers. 2023; 15(2):370. https://doi.org/10.3390/polym15020370
Chicago/Turabian StyleHou, Liutong, Sébastien Livi, Jean-François Gérard, and Jannick Duchet-Rumeau. 2023. "LIonomers-New Generation of Ionomer: Understanding of Their Interaction and Structuration as a Function of the Tunability of Cation and Anion" Polymers 15, no. 2: 370. https://doi.org/10.3390/polym15020370






