Enhanced Coating Protection of C-Steel Using Polystyrene Clay Nanocomposite Impregnated with Inhibitors
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Clay Separation, Saturation, and Organic Modification
2.3. Polystyrene-OC Nanocomposites (PCNs) Preparation
2.4. Polystyrene Microcapsules (MCs) Preparation
2.5. Coating the C-Steel Surface
2.6. Fourier Transform Infrared Spectroscopy (FT-IR)
2.7. X-ray Diffraction Analysis (XRD)
2.8. Transmission Electron Microscopy (TEM)
2.9. Electrochemical Methods
3. Results and Discussion
3.1. FT-IR Results
3.1.1. FT-IR of the Modified Clay and PCNs
3.1.2. FT-IR of IBTMS, Ce (NO3)3, W1, PS and MCs
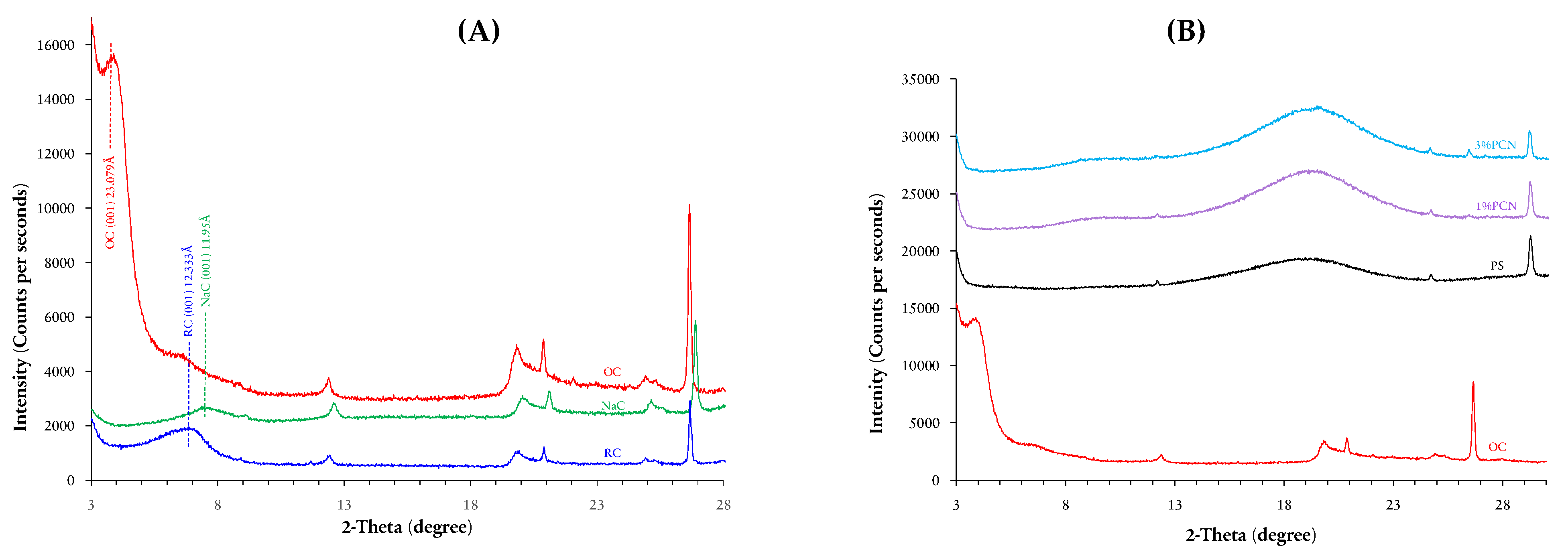
3.2. X-ray Diffraction (XRD) Analysis
3.2.1. XRD Analysis of RC, NaC and OC
3.2.2. XRD Analysis of PS, 1% PCN and 3% PCN
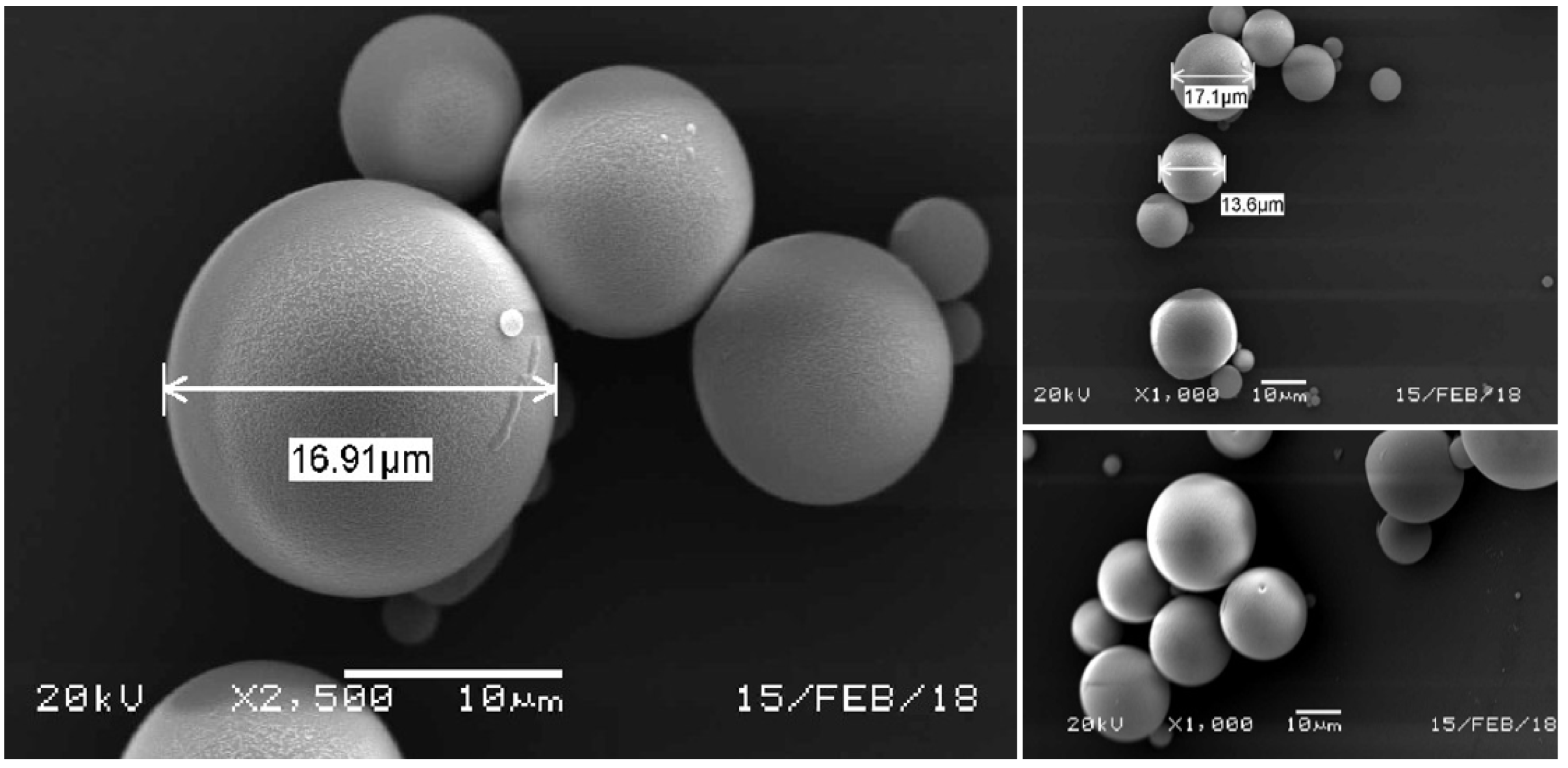
3.3. Scanning Electron Microscopy (SEM)
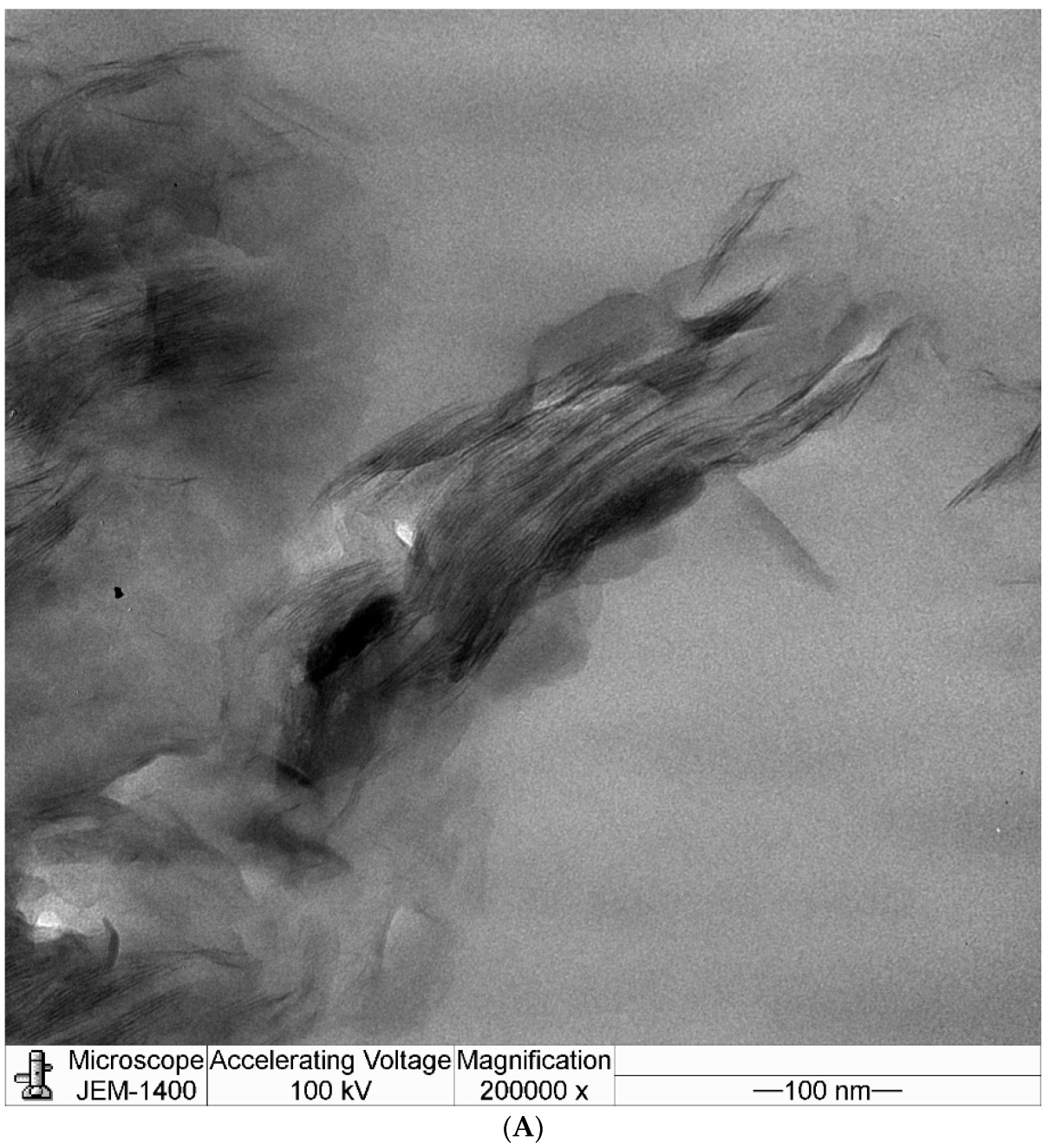
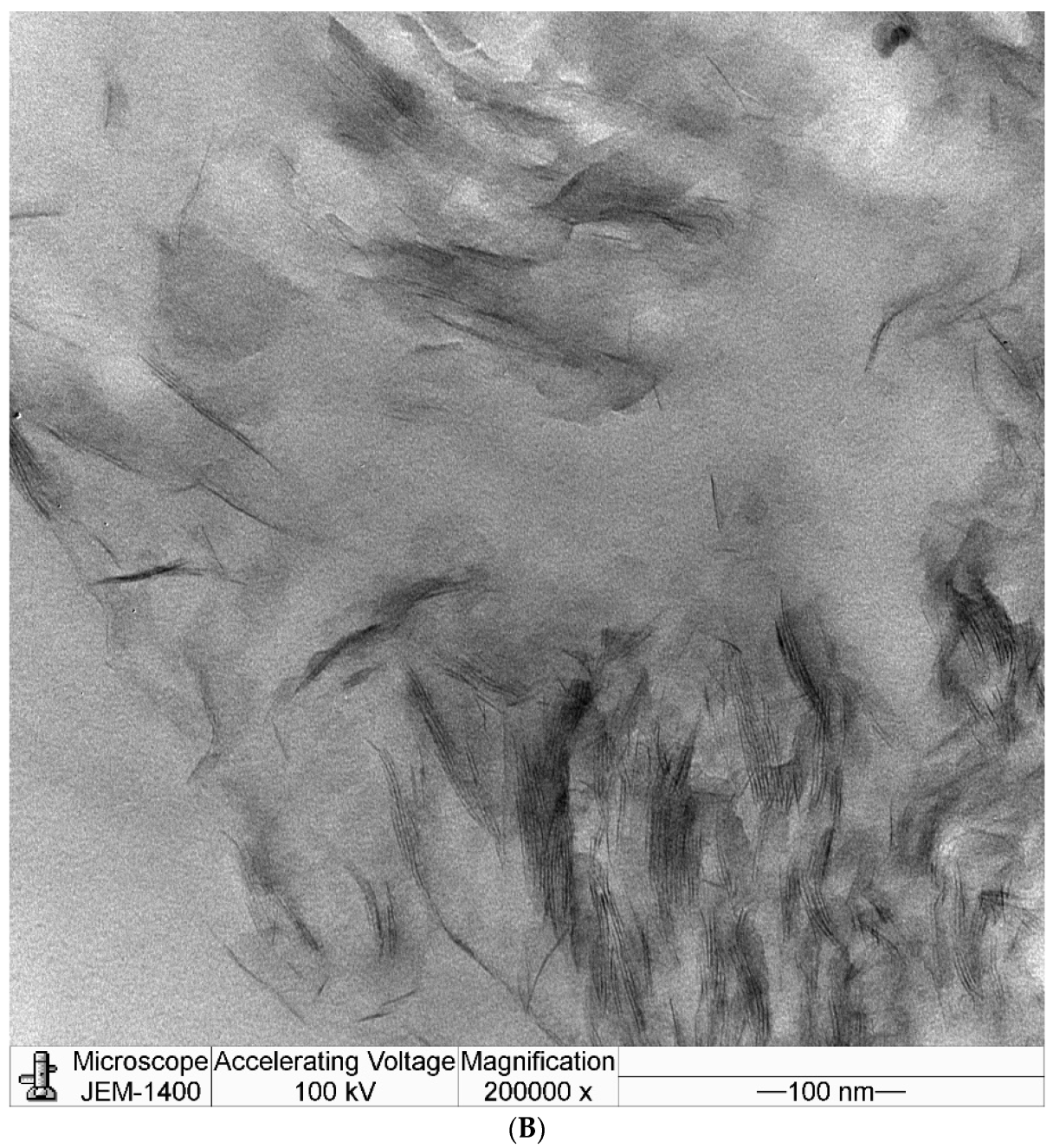
3.4. Transmission Electron Microscopy (TEM)
3.5. Electrochemical Measurements
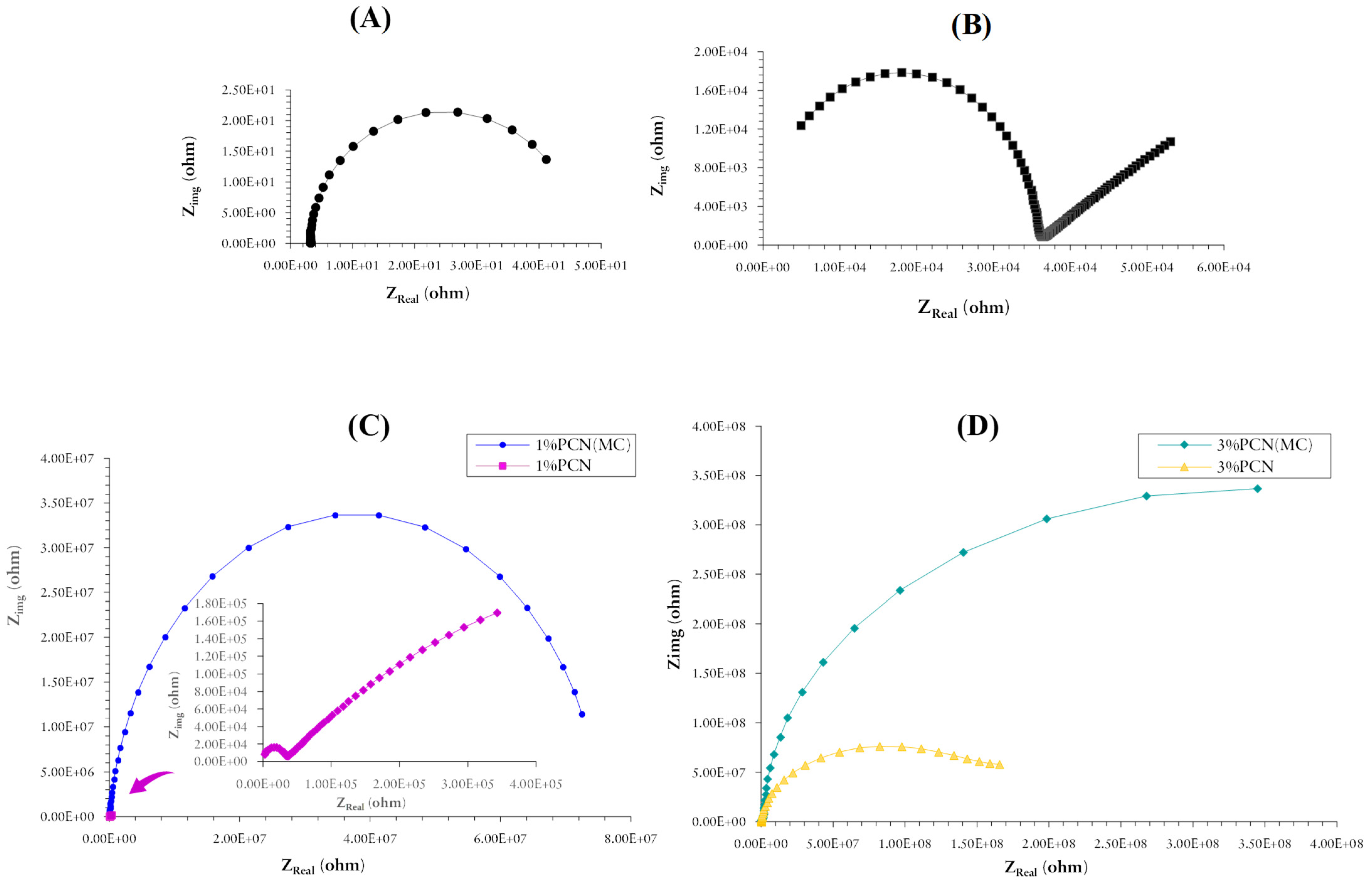
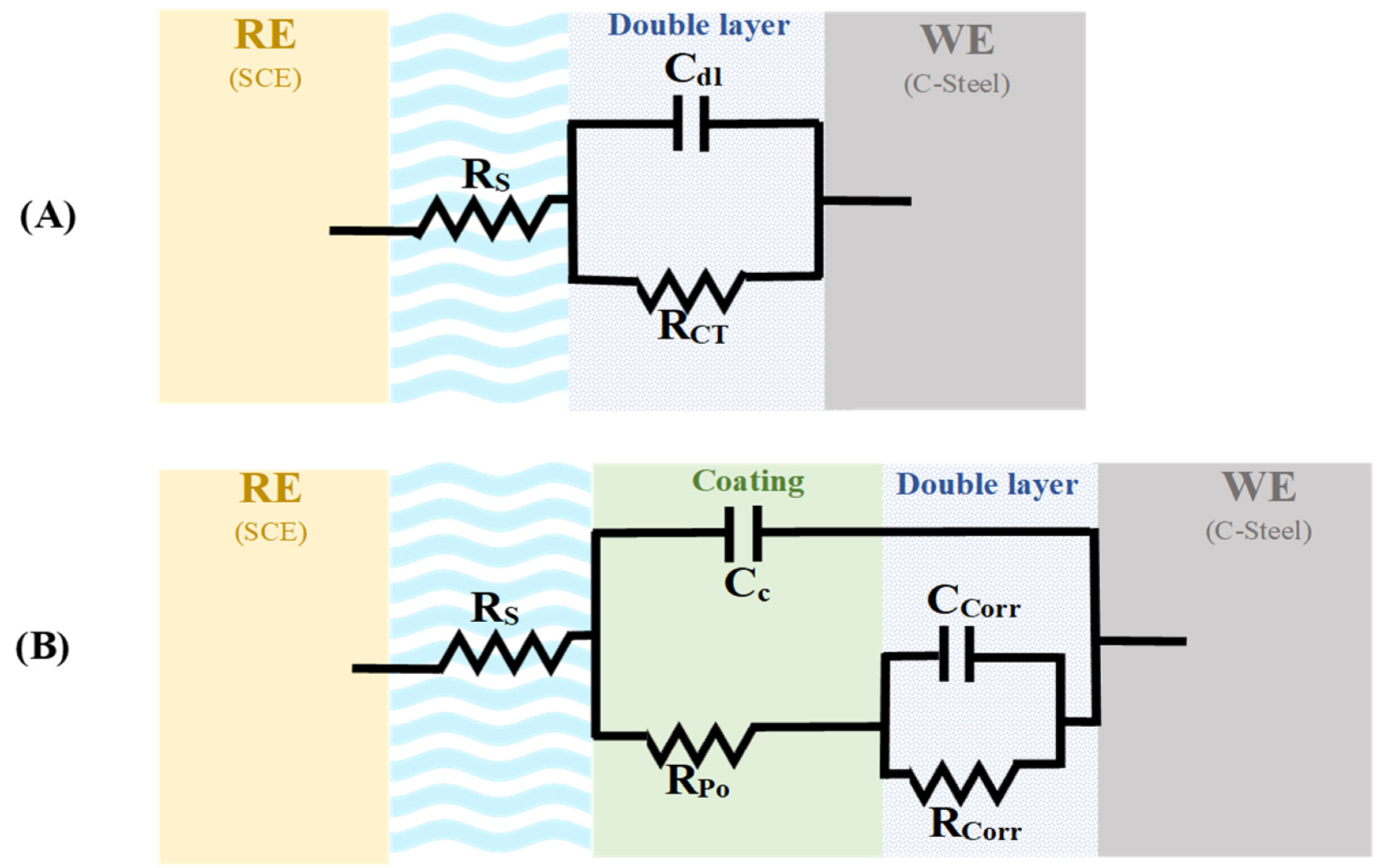
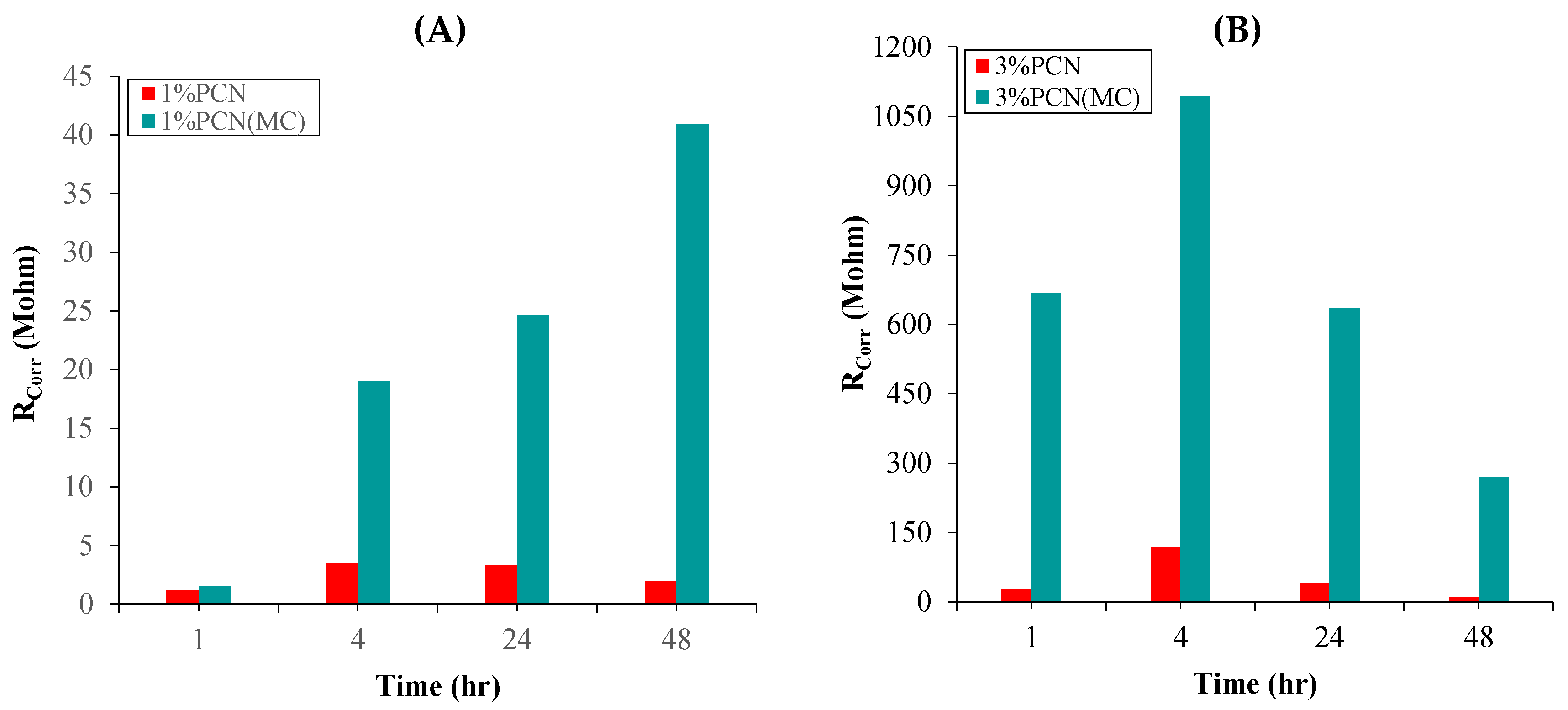
3.5.1. Electrochemical Impedance Spectroscopy (EIS)
3.5.2. Electrochemical Frequency Modulation (EFM)
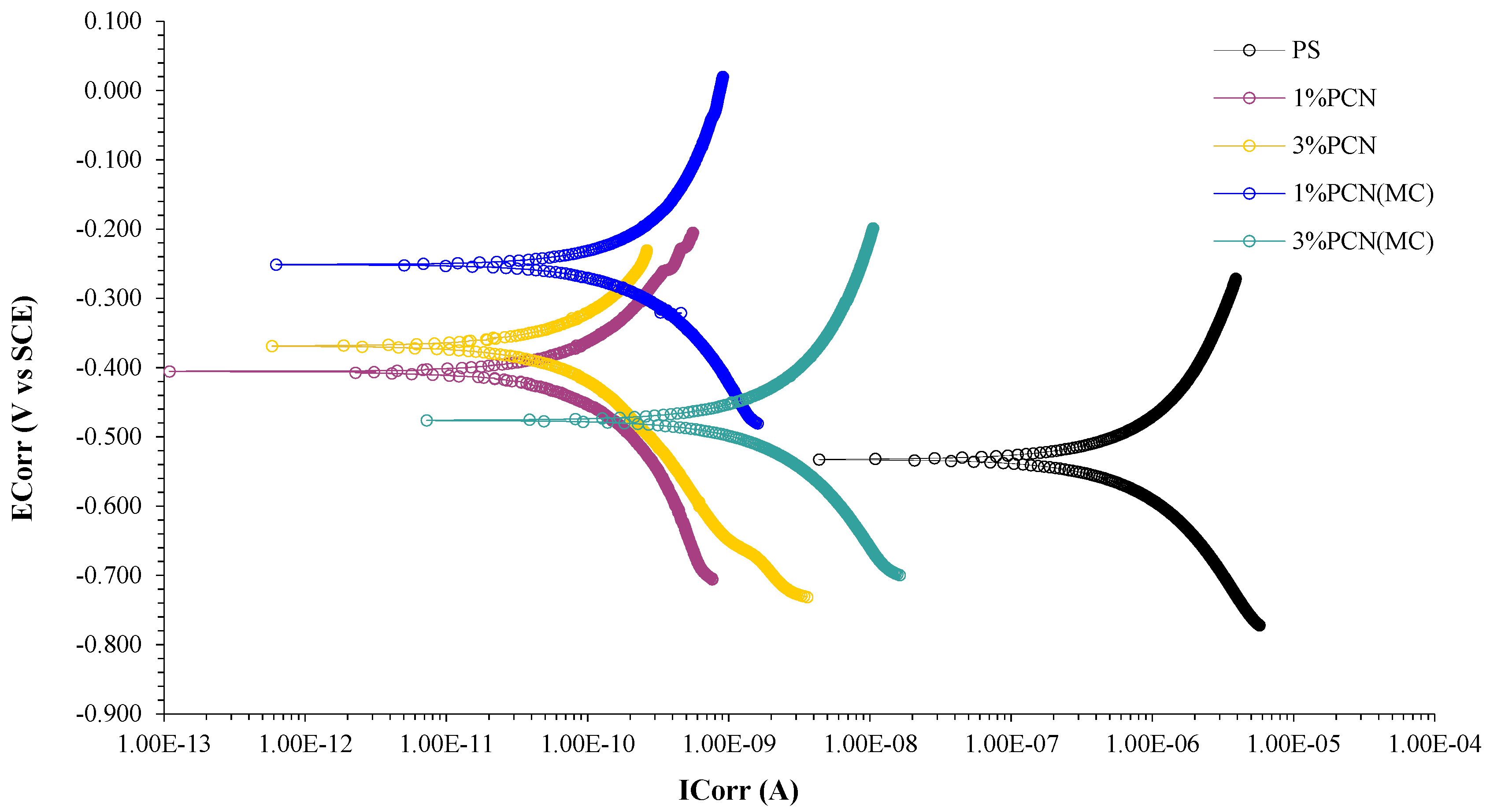
3.5.3. Potentiodynamic Polarization
3.5.4. Evaluation of the Mechanism of Enhanced Coating Protection
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Jones, D.A. Principles and Prevention of Corrosion; Macmillan Publishing Company: New York, NY, USA, 1991. [Google Scholar]
- Olad, A. Polymer/clay nanocomposites. In Advances in Diverse Industrial Applications of Nanocomposites; InTech: Nordersteds, Germany, 2011. [Google Scholar]
- Bergaya, F.; Lagaly, G. Handbook of Clay Science; Newnes: London, UK, 2013; Volume 5. [Google Scholar]
- Makhlouf, A.S.H. Handbook of Smart Coatings for Materials Protection; Elsevier: Amsterdam, The Netherlands, 2014. [Google Scholar]
- Cotting, F.; Aoki, I.V. Smart protection provided by epoxy clear coating doped with polystyrene microcapsules containing silanol and Ce (III) ions as corrosion inhibitors. Surf. Coat. Technol. 2016, 303, 310–318. [Google Scholar] [CrossRef]
- Al Juhaiman, L.; Al-Enezi, D.; Mekhamer, W. Polystyrene/Organoclay Nanocomposites as Anticorrosive Coatings of C-Steel. Int. J. Electrochem. Sci. 2016, 11, 5618–5630. [Google Scholar] [CrossRef]
- Workman, J., Jr.; Weyer, L. Practical Guide and Spectral Atlas for Interpretive Near-Infrared Spectroscopy; CRC Press: Boca Raton, FL, USA, 2012. [Google Scholar]
- Ben-Yahia, A.; El Kazzouli, S.; Essassi, E.M.; Bousmina, M.M. Synthesis and characterization of new organophilic clay. Preparation of polystyrene/clay nanocomposite. Sci. Study Res. Chem. Chem. Eng. Biotechnol. Food Ind. 2018, 19, 193–202. [Google Scholar]
- Yu, C.; Ke, Y.; Deng, Q.; Lu, S.; Ji, J.; Hu, X.; Zhao, Y. Synthesis and Characterization of Polystyrene-Montmorillonite Nanocomposite Particles Using an Anionic-Surfactant-Modified Clay and Their Friction Performance. Appl. Sci. 2018, 8, 964. [Google Scholar] [CrossRef]
- Paul, P.K.; Hussain, S.A.; Bhattacharjee, D.; Pal, M. Preparation of polystyrene–clay nanocomposite by solution intercalation technique. Bull. Mater. Sci. 2013, 36, 361–366. [Google Scholar] [CrossRef]
- Coates, J. Interpretation of infrared spectra, a practical approach. In Encyclopedia of Analytical Chemistry: Applications, Theory and Instrumentation; John Wiley & Sons Ltd.: Chichester, UK, 2006. [Google Scholar]
- Violeta, P.; Raluca, I.; Valentin, R.; Andi, N.C.; Ilie, S.C. Preparation and characterization of acrylic hybrid materials. Int. Multidiscip. Sci. GeoConf. SGEM Surv. Geol. Min. Ecol. Manag. 2017, 17, 293–300. [Google Scholar]
- Al Juhaiman, L.; Al-Enezi, D.; Mekhamer, W. Preparation and characterization of polystyrene/organoclay nanocomposites from raw clay. Dig. J. Nanomater. Biostruct. 2016, 11, 105–114. [Google Scholar]
- Zhang, Y.; Li, S.; Zhang, W.; Chen, X.; Hou, D.; Zhao, T.; Li, X. Preparation and mechanism of graphene oxide/isobutyltriethoxysilane composite emulsion and its effects on waterproof performance of concrete. Constr. Build. Mater. 2019, 208, 343–349. [Google Scholar] [CrossRef]
- Yong, W.Y.D.; Zhang, Z.; Cristobal, G.; Chin, W.S. One-pot synthesis of surface functionalized spherical silica particles. Colloids Surf. A Physicochem. Eng. Asp. 2014, 460, 151–157. [Google Scholar] [CrossRef]
- Zheludkevich, M.L.; Tedim, J.; Freire, C.S.R.; Fernandes, S.C.; Kallip, S.; Lisenkov, A.; Gandini, A.; Ferreira, M.G.S. Self-healing protective coatings with “green” chitosan based pre-layer reservoir of corrosion inhibitor. J. Mater. Chem. 2011, 21, 4805–4812. [Google Scholar] [CrossRef]
- Ozkazanc, E.; Zor, S.; Ozkazanc, H.; Guney, H.Y.; Abaci, U. Synthesis, characterization and dielectric behavior of (ES)-form polyaniline/cerium(III)-nitrate-hexahydrate composites. Mater. Chem. Phys. 2012, 133, 356–362. [Google Scholar] [CrossRef]
- Pop, O.L.; Diaconeasa, Z.; Mesaroş, A.; Vodnar, D.C.; Cuibus, L.; Ciontea, L.; Socaciu, C. FT-IR Studies of Cerium Oxide Nanoparticles and Natural Zeolite Materials. Bull. UASVM Food Sci. Technol. 2015, 72, 50–55. [Google Scholar] [CrossRef]
- Thakur, S.; Patil, P. Rapid synthesis of cerium oxide nanoparticles with superior humidity-sensing performance. Sens. Actuators B Chem. 2014, 194, 260–268. [Google Scholar] [CrossRef]
- Song, X.; Jiang, N.; Li, Y.; Xu, D.; Qiu, G. Synthesis of CeO2-coated SiO2 nanoparticle and dispersion stability of its suspension. Mater. Chem. Phys. 2008, 110, 128–135. [Google Scholar] [CrossRef]
- Chen, B.; Zhu, L.; Zhu, J.; Xing, B. Configurations of the Bentonite-Sorbed Myristylpyridinium Cation and Their Influences on the Uptake of Organic Compounds. Environ. Sci. Technol. 2005, 39, 6093–6100. [Google Scholar] [CrossRef]
- Zhu, R.; Zhou, Q.; Zhu, J.; Xi, Y.; He, H. Organo-Clays As Sorbents of Hydrophobic Organic Contaminants: Sorptive Characteristics and Approaches to Enhancing Sorption Capacity. Clays Clay Miner. 2015, 63, 199–221. [Google Scholar] [CrossRef]
- Selvaraj, M.; Kim, B.H.; Lee, T.G. FT-IR studies on selected mesoporous metallosilicate molecular sieves. Chem. Lett. 2005, 34, 1290–1291. [Google Scholar] [CrossRef]
- Li, J.; Zhu, L.; Cai, W. Characteristics of organobentonite prepared by microwave as a sorbent to organic contaminants in water. Colloids Surf. A Physicochem. Eng. Asp. 2006, 281, 177–183. [Google Scholar] [CrossRef]
- Sreedharan, V.; Sivapullaiah, P.V. Effect of Organic Modification on Adsorption Behaviour of Bentonite. Indian Geotech. J. 2012, 42, 161–168. [Google Scholar] [CrossRef]
- Li, Z.; Wang, C.-J.; Jiang, W.-T. Intercalation of Methylene Blue in a High-Charge Calcium Montmorillonite—An Indication of Surface Charge Determination. Adsorpt. Sci. Technol. 2010, 28, 297–312. [Google Scholar] [CrossRef]
- Bonczek, J.L.; Harris, W.G.; Nkedi-Kizza, P. Monolayer to Bilayer Transitional Arrangements of Hexadecyltrimethylammonium Cations on Na-montmorillonite. Clays Clay Miner. 2002, 50, 11–17. [Google Scholar] [CrossRef]
- Yeh, J.-M.; Liou, S.-J.; Lin, C.-G.; Chang, Y.-P.; Yu, Y.-H.; Cheng, C.-F. Effective enhancement of anticorrosive properties of polystyrene by polystyrene-clay nanocomposite materials. J. Appl. Polym. Sci. 2004, 92, 1970–1976. [Google Scholar] [CrossRef]
- Sasaki, A.; White, J.L. Polymer nanocomposites formation by the use of sodium montmorillonite dispersion in alcohol and a cationic surfactant. J. Appl. Polym. Sci. 2004, 91, 1951–1957. [Google Scholar] [CrossRef]
- Fu, X.; Qutubuddin, S. Polymer–clay nanocomposites: Exfoliation of organophilic montmorillonite nanolayers in polystyrene. Polymer 2001, 42, 807–813. [Google Scholar] [CrossRef]
- Blaiszik, B.; Caruso, M.; McIlroy, D.; Moore, J.; White, S.; Sottos, N. Microcapsules filled with reactive solutions for self-healing materials. Polymer 2009, 50, 990–997. [Google Scholar] [CrossRef]
- Valero-Gómez, A.; Molina, J.; Pradas, S.; López-Tendero, M.J.; Bosch, F. Microencapsulation of cerium and its application in sol–gel coatings for the corrosion protection of aluminum alloy AA2024. J. Sol-Gel Sci. Technol. 2019, 93, 36–51. [Google Scholar] [CrossRef]
- Dike, A.S.; Yilmazer, U. Mechanical, thermal and rheological characterization of polystyrene/organoclay nanocomposites containing aliphatic elastomer modifiers. Mater. Res. Express 2020, 7, 015055. [Google Scholar] [CrossRef]
- Alshabanat, M.; Al-Arrash, A.; Mekhamer, W. Polystyrene/Montmorillonite Nanocomposites: Study of the Morphology and Effects of Sonication Time on Thermal Stability. J. Nanomater. 2013, 2013, 650725. [Google Scholar] [CrossRef]
- Hwu, J.M.; Ko, T.H.; Yang, W.-T.; Lin, J.C.; Jiang, G.J.; Xie, W.; Pan, W.P. Synthesis and properties of polystyrene-montmorillonite nanocomposites by suspension polymerization. J. Appl. Polym. Sci. 2004, 91, 101–109. [Google Scholar] [CrossRef]
- Fu, X.; Qutubuddin, S. Synthesis of polystyrene–clay nanocomposites. Mater. Lett. 2000, 42, 12–15. [Google Scholar] [CrossRef]
- Baldissera, A.F.; Freitas, D.B.; Ferreira, C.A. Electrochemical impedance spectroscopy investigation of chlorinated rubber-based coatings containing polyaniline as anticorrosion agent. Mater. Corros. 2010, 61, 790–801. [Google Scholar] [CrossRef]
- Abdel-Rehim, S.; Khaled, K.; Abd-Elshafi, N. Electrochemical frequency modulation as a new technique for monitoring corrosion inhibition of iron in acid media by new thiourea derivative. Electrochim. Acta 2006, 51, 3269–3277. [Google Scholar] [CrossRef]
- Fedrizzi, L.; Rodriguez, F.; Rossi, S.; Deflorian, F.; Di Maggio, R. The use of electrochemical techniques to study the corrosion behaviour of organic coatings on steel pretreated with sol–gel zirconia films. Electrochim. Acta 2001, 46, 3715–3724. [Google Scholar] [CrossRef]
- Kuriyama, N.; Sakai, T.; Miyamura, H.; Uehara, I.; Ishikawa, H.; Iwasaki, T. Electrochemical impedance and deterioration behavior of metal hydride electrodes. J. Alloys Compd. 1993, 202, 183–197. [Google Scholar] [CrossRef]
- Es-Saheb, M.; Sherif, E.S.M.; El-Zatahry, A.; El Rayes, M.M.; Khalil, A.K. Corrosion passivation in aerated 3.5% NaCl solutions of brass by nanofiber coatings of polyvinyl chloride and polystyrene. Int. J. Electrochem. Sci. 2012, 7, 10442–10455. [Google Scholar]
- Behzadnasab, M.; Mirabedini, S.; Kabiri, K.; Jamali, S. Corrosion performance of epoxy coatings containing silane treated ZrO2 nanoparticles on mild steel in 3.5% NaCl solution. Corros. Sci. 2011, 53, 89–98. [Google Scholar] [CrossRef]
- Chang, K.-C.; Chen, S.-T.; Lin, H.-F.; Lin, C.-Y.; Huang, H.-H.; Yeh, J.-M.; Yu, Y.-H. Effect of clay on the corrosion protection efficiency of PMMA/Na+-MMT clay nanocomposite coatings evaluated by electrochemical measurements. Eur. Polym. J. 2008, 44, 13–23. [Google Scholar] [CrossRef]
- Banik, N.; Jahan, S.; Mostofa, S.; Kabir, H.; Sharmin, N.; Rahman, M.; Ahmed, S. Synthesis and characterization of organoclay modified with cetylpyridinium chloride. Bangladesh J. Sci. Ind. Res. 2015, 50, 65–70. [Google Scholar] [CrossRef]
- Lim, A.B.; Neo, W.J.; Yauw, O.; Chylak, B.; Gan, C.L.; Chen, Z. Evaluation of the corrosion performance of Cu–Al intermetallic compounds and the effect of Pd addition. Microelectron. Reliab. 2016, 56, 155–161. [Google Scholar] [CrossRef]
- ElShami, A.A.; Bonnet, S.; Makhlouf, M.H.; Khelidj, A.; Leklou, N. Novel green plants extract as corrosion inhibiting coating for steel embedded in concrete. Pigment Resin Technol. 2020, 49, 501–514. [Google Scholar] [CrossRef]
- Afsharimani, N.; Talimian, A.; Merino, E.; Durán, A.; Castro, Y.; Galusek, D. Improving corrosion protection of Mg alloys (AZ31B) using graphene-based hybrid coatings. Int. J. Appl. Glass Sci. 2021, 13, 143–150. [Google Scholar] [CrossRef]
- Onofre-Bustamante, E.; Dominguez-Crespo, M.A.; Torres, A.; Olvera-Martínez, A.; Genescá-Llongueras, J.; Rodriguez-Gomez, F. Characterization of cerium-based conversion coatings for corrosion protection of AISI-1010 commercial carbon steel. J. Solid State Electrochem. 2009, 13, 1785–1799. [Google Scholar] [CrossRef]
- Danaee, I.; Darmiani, E.; Rashed, G.R.; Zaarei, D. Self-healing and anticorrosive properties of Ce (III)/Ce (IV) in nanoclay–epoxy coatings. Iran. Polym. J. 2014, 23, 891–898. [Google Scholar] [CrossRef]
- Ubaid, F.; Radwan, A.B.; Naeem, N.; Shakoor, R.; Ahmad, Z.; Montemor, F.; Kahraman, R.; Abdullah, A.M.; Soliman, A. Multifunctional self-healing polymeric nanocomposite coatings for corrosion inhibition of steel. Surf. Coat. Technol. 2019, 372, 121–133. [Google Scholar] [CrossRef]
- Stankiewicz, A.; Szczygieł, I.; Szczygiel, B. Self-healing coatings in anti-corrosion applications. J. Mater. Sci. 2013, 48, 8041–8051. [Google Scholar] [CrossRef]
- Jackson, A.C.; Bartelt, J.A.; Marczewski, K.; Sottos, N.R.; Braun, P.V. Silica-Protected Micron and Sub-Micron Capsules and Particles for Self-Healing at the Microscale. Macromol. Rapid Commun. 2011, 32, 82–87. [Google Scholar] [CrossRef]
- Huang, Y.; Liu, T.; Ma, L.; Wang, J.; Zhang, D.; Li, X. Saline-responsive triple-action self-healing coating for intelligent corrosion control. Mater. Des. 2022, 214, 110381. [Google Scholar] [CrossRef]



| Element | C | Mn | Cr | Si | Ni | Cu | Al | P | S |
|---|---|---|---|---|---|---|---|---|---|
| Weight (%) | 0.46 | 0.6 | 0.18 | 0.18 | 0.04 | 0.03 | 0.023 | 0.013 | 0.006 |
| Product Name | Source |
|---|---|
| RC | Khulays clay—Saudi Arabia |
| C-steel (Grade 1046) | ODS Co.—Berlin, Germany |
| Polystyrene (PS125) | SABIC—Riyadh, Saudi Arabia |
| Sodium Chloride (AR grade) | Win lab Company, Queensland, Australia |
| Ethanol 96%—AR grade | Avonchem—Waterloo, UK |
| Cetyl pyridinium chloride (CPC) | BDH Co., Istanbul, Turkey |
| Polyvinyl Alcohol (PVA) | LOBAChemie Co., Mumbai, India |
| Glacial acetic acid 99.5%—Extra Pure | Sigma-Aldrich Co., Saint Louis, MI, USA |
| Dichloromethane 99.5%—AR\ACS grade | Sigma-Aldrich Co. |
| Isobutyl Trimethoxy Silane 97% (IBTMS) | Alfa Aeasar Co., Ward Hill, MA, USA |
| Ce(NO3)3.6H2O—AR | LOBAChemie Co. |
| Sample | ESS (mv) | RCorr (MΩ) | RPo (MΩ) | CCorr (nF/cm2) | CC (nF/cm2) | % PE |
|---|---|---|---|---|---|---|
| Bare (C-steel) | −601 | 4.30 × 10−5 | - | 1.332 × 106 | - | - |
| PS | −549 | 0.235 | 0.0555 | 5.337 × 104 | 0.1108 | - |
| 1% PCN | −455 | 1.181 | 0.311 | 2.589 × 103 | 0.1857 | 80.127 |
| 1% PCN (MC) | −215 | 1.556 | 74.10 | 1.022 | 0.3885 | 84.916 |
| 3% PCN | −480 | 27.52 | 6.16 | 0.298 | 0.1430 | 99.146 |
| 3% PCN (MC) | −297 | 667.8 | 8.191 | 0.1142 | 0.1988 | 99.964 |
| Sample | ICorr (µA) | CR (mpy) | CF(2) | CF(3) | PE% |
|---|---|---|---|---|---|
| Bare (C-steel) | 5.577 × 102 | 5.365 × 101 | 2.132 | 2.694 | - |
| PS | 1.645 | 1.583 × 10−1 | 1.095 | 2.416 | - |
| 1% PCN | 3.697 × 10−4 | 3.556 × 10−5 | 1.469 | 1.455 | 99.977 |
| 1% PCN (MC) | 5.553 × 10−4 | 5.341 × 10−5 | 2.101 | 3.331 | 99.966 |
| 3% PCN | 1.081 × 10−4 | 1.040 × 10−5 | 1.627 | 1.043 | 99.993 |
| 3% PCN (MC) | 1.579 × 10−4 | 1.519 × 10−5 | 1.743 | 2.689 | 99.990 |
| Sample | ECorr (mV) | ICorr (µA) | CR (mpy) | Chi.Sq. | PE% |
|---|---|---|---|---|---|
| Bare (C-steel) | −634 | 5.61× 101 | 5.397 | 6.359 | - |
| PS | −532 | 2.660 | 2.557 × 10−1 | 1.8 × 10−1 | - |
| 1% PCN | −404 | 2.670 × 10−4 | 2.569 × 10−5 | 6.61 × 10−4 | 99.989 |
| 1% PCN (MC) | −251 | 1.820 × 10−3 | 1.754 × 10−4 | 1.1 × 10−3 | 99.931 |
| 3% PCN | −369 | 4.290 × 10−5 | 4.128 × 10−6 | 2.48 × 10−2 | 99.998 |
| 3% PCN (MC) | −478 | 5.820 × 10−3 | 5.602 × 10−4 | 1.52 × 10−3 | 99.781 |
| Sample | Time (Hour) | RCorr (MΩ) | RPo (MΩ) | CCorr (nF.cm−2) | CC (nF.cm−2) |
|---|---|---|---|---|---|
| 1% PCN | 1 | 1.181 | 0.3108 | 2.584 × 103 | 0.1857 |
| 4 | 3.536 | 0.5156 | 3.725 × 103 | 0.1399 | |
| 24 | 3.354 | 3.122 | 172.2 | 0.1493 | |
| 48 | 1.936 | 3.295 | 285.1 | 0.1624 | |
| 1% PCN(MC) | 1 | 1.556 | 74.10 | 1.022 | 0.3885 |
| 4 | 18.97 | 8.839 | 0.4099 | 0.1623 | |
| 24 | 24.64 | 11.53 | 0.3899 | 0.1467 | |
| 48 | 40.91 | 7.013 | 0.1520 | 0.1562 | |
| 3% PCN | 1 | 27.52 | 6.16 | 0.298 | 0.1430 |
| 4 | 118.6 | 42.65 | 0.1067 | 0.1152 | |
| 24 | 42.06 | 18.58 | 0.1791 | 0.1526 | |
| 48 | 11.41 | 3.514 | 24.05 | 0.1497 | |
| 3% PCN(MC) | 1 | 667.8 | 8.191 | 0.1142 | 0.1988 |
| 4 | 1092 | 17.03 | 0.0113 | 0.3412 | |
| 24 | 636.1 | 8.951 | 0.1682 | 0.2586 | |
| 48 | 270.5 | 7.109 | 0.1635 | 0.1221 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Alangari, A.M.; Al Juhaiman, L.A.; Mekhamer, W.K. Enhanced Coating Protection of C-Steel Using Polystyrene Clay Nanocomposite Impregnated with Inhibitors. Polymers 2023, 15, 372. https://doi.org/10.3390/polym15020372
Alangari AM, Al Juhaiman LA, Mekhamer WK. Enhanced Coating Protection of C-Steel Using Polystyrene Clay Nanocomposite Impregnated with Inhibitors. Polymers. 2023; 15(2):372. https://doi.org/10.3390/polym15020372
Chicago/Turabian StyleAlangari, Aljawharah M., Layla A. Al Juhaiman, and Waffa K. Mekhamer. 2023. "Enhanced Coating Protection of C-Steel Using Polystyrene Clay Nanocomposite Impregnated with Inhibitors" Polymers 15, no. 2: 372. https://doi.org/10.3390/polym15020372
APA StyleAlangari, A. M., Al Juhaiman, L. A., & Mekhamer, W. K. (2023). Enhanced Coating Protection of C-Steel Using Polystyrene Clay Nanocomposite Impregnated with Inhibitors. Polymers, 15(2), 372. https://doi.org/10.3390/polym15020372







