Synthesis of Cellulose Nanoparticles from Ionic Liquid Solutions for Biomedical Applications
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Synthesis of Cellulose Nanoparticles (CNPs)
2.3. Characterisation of Cellulose Nanoparticles
2.3.1. Dynamic Light Scattering (DLS)
2.3.2. Attenuated Total Reflectance Fourier Transformed Infrared Spectroscopy (ATR-FTIR)
2.3.3. Field Emission Scanning Electron Microscopy (FESEM)
2.3.4. Thermogravimetric Analysis (TGA) and Differential Thermal Analysis (DTA)
2.3.5. In Vitro Cytotoxicity
2.4. Statistical Analysis
3. Results and Discussion
3.1. Characterisation of Cellulose Nanoparticles
3.1.1. Dynamic Light Scattering (DLS)
3.1.2. Attenuated Total Reflectance Fourier Transformed Infrared Spectroscopy (ATR-FTIR)
3.1.3. Field Emission Scanning Electron Microscopy (FESEM)
3.1.4. Thermogravimetric Analysis (TGA) and Differential Thermal Analysis (DTA)
3.1.5. In Vitro Cytotoxicity
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Conflicts of Interest
References
- Bawarski, W.E.; Chidlowsky, E.; Bharali, D.J.; Mousa, S.A. Emerging Nanopharmaceuticals. Nanomedicine 2008, 4, 273–282. [Google Scholar] [CrossRef]
- Farokhzad, O.C.; Langer, R. Impact of Nanotechnology on Drug Delivery. ACS Nano 2009, 3, 16–20. [Google Scholar] [CrossRef] [PubMed]
- Zhao, C.-Y.; Cheng, R.; Yang, Z.; Tian, Z.-M. Nanotechnology for Cancer Therapy Based on Chemotherapy. Molecules 2018, 23, 826. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Montalbán, M.G.; Carissimi, G.; Lozano-Pérez, A.A.; Cenis, J.L.; Coburn, J.M.; Kaplan, D.L.; Víllora, G. Biopolymeric Nanoparticle Synthesis in Ionic Liquids. In Recent Advances in Ionic Liquids; Rahman, M.M., Ed.; IntechOpen: London, UK, 2018; pp. 3–26. [Google Scholar]
- Liong, M.; Lu, J.; Kovochich, M.; Xia, T.; Ruehm, S.G.; Nel, A.E.; Tamanoi, F.; Zink, J.I. Multifunctional Inorganic Nanoparticles for Imaging, Targeting, and Drug Delivery. ACS Nano 2008, 2, 889–896. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gratton, S.E.A.; Ropp, P.A.; Pohlhaus, P.D.; Luft, J.C.; Madden, V.J.; Napier, M.E.; DeSimone, J.M. The Effect of Particle Design on Cellular Internalization Pathways. Proc. Natl. Acad. Sci. USA 2008, 105, 11613–11618. [Google Scholar] [CrossRef] [Green Version]
- Kenechukwu, F.C.; Dias, M.L.; Ricci-Júnior, E. Biodegradable Nanoparticles from Prosopisylated Cellulose as a Platform for Enhanced Oral Bioavailability of Poorly Water-Soluble Drugs. Carbohydr. Polym. 2021, 256, 117492. [Google Scholar] [CrossRef]
- Yang, X.; Jiang, X.; Yang, H.; Bian, L.; Chang, C.; Zhang, L. Biocompatible Cellulose-Based Supramolecular Nanoparticles Driven by Host–Guest Interactions for Drug Delivery. Carbohydr. Polym. 2020, 237, 116114. [Google Scholar] [CrossRef]
- Lozano-Pérez, A.A.; Montalbán, M.G.; Aznar-Cervantes, S.D.; Cragnolini, F.; Cenis, J.L.; Víllora, G. Production of Silk Fibroin Nanoparticles Using Ionic Liquids and High-Power Ultrasounds. J. Appl. Polym. Sci. 2015, 132, 1–8. [Google Scholar] [CrossRef]
- Li, S.; Bashline, L.; Lei, L.; Gu, Y. Cellulose Synthesis and Its Regulation. Arab. Book 2014, 12, e0169. [Google Scholar] [CrossRef] [Green Version]
- Brett, C.T. Cellulose Microfibrils in Plants: Biosynthesis, Deposition, and Integration into the Cell Wall. Int. Rev. Cytol. 2000, 199, 161–199. [Google Scholar] [CrossRef]
- Lee, S.H.; Miyauchi, M.; Dordick, J.S.; Linhardt, R.J. Preparation of Biopolymer-Based Materials Using Ionic Liquids for the Biomedical Application. ACS Symp. Ser. 2010, 1038, 115–134. [Google Scholar] [CrossRef]
- Al Hakkak, J.; Grigsby, W.J.; Kathirgamanathan, K.; Edmonds, N.R. Generation of Spherical Cellulose Nanoparticles from Ionic Liquid Processing via Novel Nonsolvent Addition and Drying. Adv. Mater. Sci. Eng. 2019, 2019, 2081027. [Google Scholar] [CrossRef] [Green Version]
- Onkarappa, H.S.; Prakash, G.K.; Pujar, G.H.; Rajith Kumar, C.R.; Radha; Latha, M.S.; Betageri, V.S. Synthesis and Characterization of Nanocellulose Using Renewable Resources through Ionic Liquid Medium. Adv. Nat. Sci. Nanosci. Nanotechnol. 2020, 11, 035001. [Google Scholar] [CrossRef]
- Zhao, H.; Kwak, J.H.; Wang, Y.; Franz, J.A.; White, J.M.; Holladay, J.E. Interactions between Cellulose and N-Methylmorpholine-N-Oxide. Carbohydr. Polym. 2007, 67, 97–103. [Google Scholar] [CrossRef]
- Zhao, Z.; Chen, A.; Li, Y.; Hu, J.; Liu, X.; Li, J.; Zhang, Y.; Li, G.; Zheng, Z. Fabrication of Silk Fibroin Nanoparticles for Controlled Drug Delivery. J. Nanoparticle Res. 2012, 14, 736. [Google Scholar] [CrossRef]
- Jiang, G.; Huang, W.; Li, L.; Wang, X.; Pang, F.; Zhang, Y.; Wang, H. Structure and Properties of Regenerated Cellulose Fibers from Different Technology Processes. Carbohydr. Polym. 2012, 87, 2012–2018. [Google Scholar] [CrossRef]
- Tamai, N.; Tatsumi, D.; Matsumoto, T. Rheological Properties and Molecular Structure of Tunicate Cellulose in LiCl/1,3-Dimethyl-2-Imidazolidinone. Biomacromolecules 2004, 5, 422–432. [Google Scholar] [CrossRef] [PubMed]
- Kuo, C.-H.; Lee, C.-K. Enhancement of Enzymatic Saccharification of Cellulose by Cellulose Dissolution Pretreatments. Carbohydr. Polym. 2009, 77, 41–46. [Google Scholar] [CrossRef]
- Li, Q.; He, Y.; Xian, M.; Jun, G.; Xu, X.; Yang, J.; Li, L. Improving Enzymatic Hydrolysis of Wheat Straw Using Ionic Liquid 1-Ethyl-3-Methyl Imidazolium Diethyl Phosphate Pretreatment. Bioresour. Technol. 2009, 100, 3570–3575. [Google Scholar] [CrossRef]
- Yang, Q.; Dionysiou, D.D. Photolytic Degradation of Chlorinated Phenols in Room Temperature Ionic Liquids. J. Photochem. Photobiol. A Chem. 2004, 165, 229–240. [Google Scholar] [CrossRef]
- Brennecke, J.F.; Maginn, E.J. Ionic Liquids: Innovative Fluids for Chemical Processing. Am. Inst. Chem. Eng. AIChE J. 2001, 47, 2384. [Google Scholar] [CrossRef]
- Zhang, H.; Wu, J.; Zhang, J.; He, J. 1-Allyl-3-Methylimidazolium Chloride Room Temperature Ionic Liquid: A New and Powerful Nonderivatizing Solvent for Cellulose. Macromolecules 2005, 38, 8272–8277. [Google Scholar] [CrossRef]
- Vieira, S.R.; da Silva, J.B.A.; Druzian, J.I.; de Jesus Assis, D.; Mussagy, C.U.; Pereira, J.F.B.; Santos-Ebinuma, V.C.; Lemos, P.V.F.; Correia, P.R.; de Souza Ferreira, E.; et al. Cellulose Nanoparticles Prepared by Ionic Liquid-Assisted Method Improve the Properties of Bionanocomposite Films. J. Polym. Environ. 2022, 30, 3174–3185. [Google Scholar] [CrossRef]
- Swatloski, R.P.; Spear, S.K.; Holbrey, J.D.; Rogers, R.D. Dissolution of Cellose with Ionic Liquids. J. Am. Chem. Soc. 2002, 124, 4974–4975. [Google Scholar] [CrossRef]
- Han, J.; Zhou, C.; French, A.D.; Han, G.; Wu, Q. Characterization of Cellulose II Nanoparticles Regenerated from 1-Butyl-3-Methylimidazolium Chloride. Carbohydr. Polym. 2013, 94, 773–781. [Google Scholar] [CrossRef]
- Chin, S.F.; Jimmy, F.B.; Pang, S.C. Size Controlled Fabrication of Cellulose Nanoparticles for Drug Delivery Applications. J. Drug Deliv. Sci. Technol. 2018, 43, 262–266. [Google Scholar] [CrossRef]
- Fuster, M.G.; Carissimi, G.; Montalbán, M.G.; Víllora, G. Antitumor Activity of Rosmarinic Acid-Loaded Silk Fibroin Nanoparticles on HeLa and MCF-7 Cells. Polymers 2021, 13, 3169. [Google Scholar] [CrossRef]
- Fuster, M.G.; Montalbán, M.G.; Carissimi, G.; Lima, B.; Feresin, G.E.; Cano, M.; Giner-Casares, J.J.; López-Cascales, J.J.; Enriz, R.D.; Víllora, G. Antibacterial Effect of Chitosan-Gold Nanoparticles and Computational Modeling of the Interaction between Chitosan and a Lipid Bilayer Model. Nanomaterials 2020, 10, 2340. [Google Scholar] [CrossRef] [PubMed]
- Fuster, M.G.; Carissimi, G.; Montalbán, M.G.; Víllora, G. Improving Anticancer Therapy with Naringenin-Loaded Silk Fibroin Nanoparticles. Nanomaterials 2020, 10, 718. [Google Scholar] [CrossRef] [Green Version]
- Collado-González, M.; Montalbán, M.G.; Peña-García, J.; Pérez-Sánchez, H.; Víllora, G.; Díaz Baños, F.G. Chitosan as Stabilizing Agent for Negatively Charged Nanoparticles. Carbohydr. Polym. 2017, 161, 63–70. [Google Scholar] [CrossRef]
- Garg, A.; Rai, G.; Lodhi, S.; Jain, A.P.; Yadav, A.K. In-Vitro and in-Vivo Assessment of Dextran-Appended Cellulose Acetate Phthalate Nanoparticles for Transdermal Delivery of 5-Fluorouracil. Drug Deliv. 2016, 23, 1525–1535. [Google Scholar] [CrossRef]
- Yih, T.C.; Al-Fandi, M. Engineered Nanoparticles as Precise Drug Delivery Systems. J. Cell. Biochem. 2006, 97, 1184–1190. [Google Scholar] [CrossRef] [PubMed]
- Dong, B.; Hadinoto, K. Carboxymethyl Cellulose Is a Superior Polyanion to Dextran Sulfate in Stabilizing and Enhancing the Solubility of Amorphous Drug-Polyelectrolyte Nanoparticle Complex. Int. J. Biol. Macromol. 2019, 139, 500–508. [Google Scholar] [CrossRef]
- Ho, B.-K.; Chin, S.-F.; Pang, S.-C. PH-Responsive Carboxylic Cellulose Acetate Nanoparticles for Controlled Release of Penicillin G. J. Sci. Adv. Mater. Devices 2020, 5, 224–232. [Google Scholar] [CrossRef]
- Dai, L.; Si, C.-L. Cellulose-Graft-Poly(Methyl Methacrylate) Nanoparticles with High Biocompatibility for Hydrophobic Anti-Cancer Drug Delivery. Mater. Lett. 2017, 207, 213–216. [Google Scholar] [CrossRef]
- Khatibi, A.; Zahedi, P.; Ghourchian, H.; Sadeghi Lari, A. Development of Microfluidic-Based Cellulose Acetate Phthalate Nanoparticles Containing Omeprazole for Antiulcer Activity: In Vitro and in Vivo Evaluations. Eur. Polym. J. 2021, 147, 110294. [Google Scholar] [CrossRef]
- Zuppolini, S.; Maya, I.C.; Diodato, L.; Guarino, V.; Borriello, A.; Ambrosio, L. Self-Associating Cellulose-Graft-Poly(ε-Caprolactone) to Design Nanoparticles for Drug Release. Mater. Sci. Eng. C 2020, 108, 110385. [Google Scholar] [CrossRef]
- Wang, S.; Lu, Y.; Ouyang, X.; Ling, J. Fabrication of Soy Protein Isolate/Cellulose Nanocrystal Composite Nanoparticles for Curcumin Delivery. Int. J. Biol. Macromol. 2020, 165, 1468–1474. [Google Scholar] [CrossRef]
- Gericke, M.; Trygg, J.; Fardim, P. Functional Cellulose Beads: Preparation, Characterization, and Applications. Chem. Rev. 2013, 113, 4812–4836. [Google Scholar] [CrossRef]
- Carvalho, J.P.F.; Silva, A.C.Q.; Silvestre, A.J.D.; Freire, C.S.R.; Vilela, C. Spherical Cellulose Micro and Nanoparticles: A Review of Recent Developments and Applications. Nanomaterials 2021, 11, 2744. [Google Scholar] [CrossRef]
- Montalbán, M.G.; Coburn, J.M.; Lozano-Pérez, A.A.; Cenis, J.L.; Víllora, G.; Kaplan, D.L. Production of Curcumin-Loaded Silk Fibroin Nanoparticles for Cancer Therapy. Nanomaterials 2018, 8, 126. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Carrillo, F.; Colom, X.; Suñol, J.J.; Saurina, J. Structural FTIR Analysis and Thermal Characterisation of Lyocell and Viscose-Type Fibres. Eur. Polym. J. 2004, 40, 2229–2234. [Google Scholar] [CrossRef]
- Nada, A.-A.M.A.; Kamel, S.; El-Sakhawy, M. Thermal Behaviour and Infrared Spectroscopy of Cellulose Carbamates. Polym. Degrad. Stab. 2000, 70, 347–355. [Google Scholar] [CrossRef]
- Oh, S.Y.; Yoo, D., II; Shin, Y.; Seo, G. FTIR Analysis of Cellulose Treated with Sodium Hydroxide and Carbon Dioxide. Carbohydr. Res. 2005, 340, 417–428. [Google Scholar] [CrossRef]
- Schwanninger, M.; Rodrigues, J.C.; Pereira, H.; Hinterstoisser, B. Effects of Short-Time Vibratory Ball Milling on the Shape of FT-IR Spectra of Wood and Cellulose. Vib. Spectrosc. 2004, 36, 23–40. [Google Scholar] [CrossRef]
- Široký, J.; Blackburn, R.S.; Bechtold, T.; Taylor, J.; White, P. Attenuated Total Reflectance Fourier-Transform Infrared Spectroscopy Analysis of Crystallinity Changes in Lyocell Following Continuous Treatment with Sodium Hydroxide. Cellulose 2010, 17, 103–115. [Google Scholar] [CrossRef]
- Krässig, H. Cellulose: Structure, Accessibility and Reactivity; Yverdon, S.A., Ed.; Gordon & Breach: Langhorne, PA, USA, 1993. [Google Scholar]
- O’Connor, R.T.; DuPré, E.F.; McCall, E.R. Applications of Infrared Absorption Spectroscopy to Investigations of Cotton and Modified Cottons: Part II: Chemical Modifications. Text. Res. J. 1958, 28, 542–554. [Google Scholar] [CrossRef]
- Nelson, M.L.; O’Connor, R.T. Relation of Certain Infrared Bands to Cellulose Crystallinity and Crystal Latticed Type. Part I. Spectra of Lattice Types I, II, III and of Amorphous Cellulose. J. Appl. Polym. Sci. 1964, 8, 1311–1324. [Google Scholar] [CrossRef]
- De Mello, V.A.; Ricci-Júnior, E. Encapsulation of Naproxen in Nanostructured System: Structural Characterization and in Vitro Release Studies. Quim. Nova 2011, 34, 933–939. [Google Scholar] [CrossRef] [Green Version]
- Ventura, C.; Pinto, F.; Lourenço, A.F.; Ferreira, P.J.T.; Louro, H.; Silva, M.J. On the Toxicity of Cellulose Nanocrystals and Nanofibrils in Animal and Cellular Models. Cellulose 2020, 27, 5509–5544. [Google Scholar] [CrossRef]



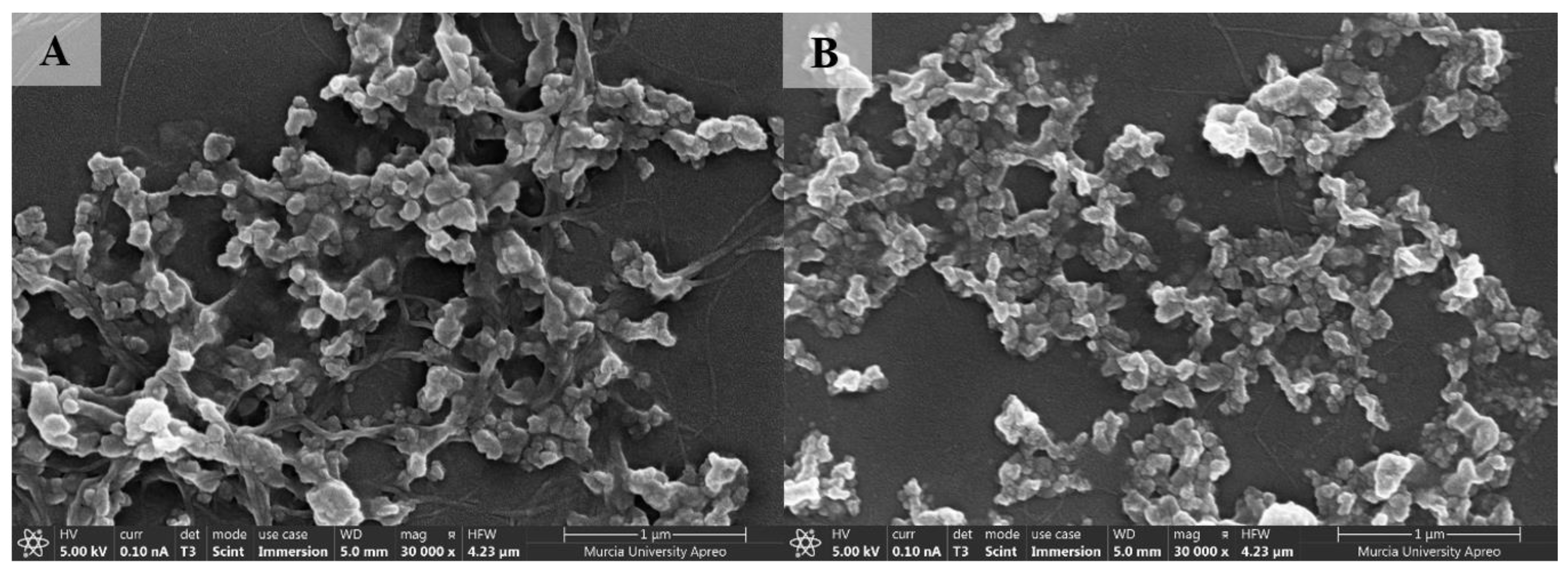

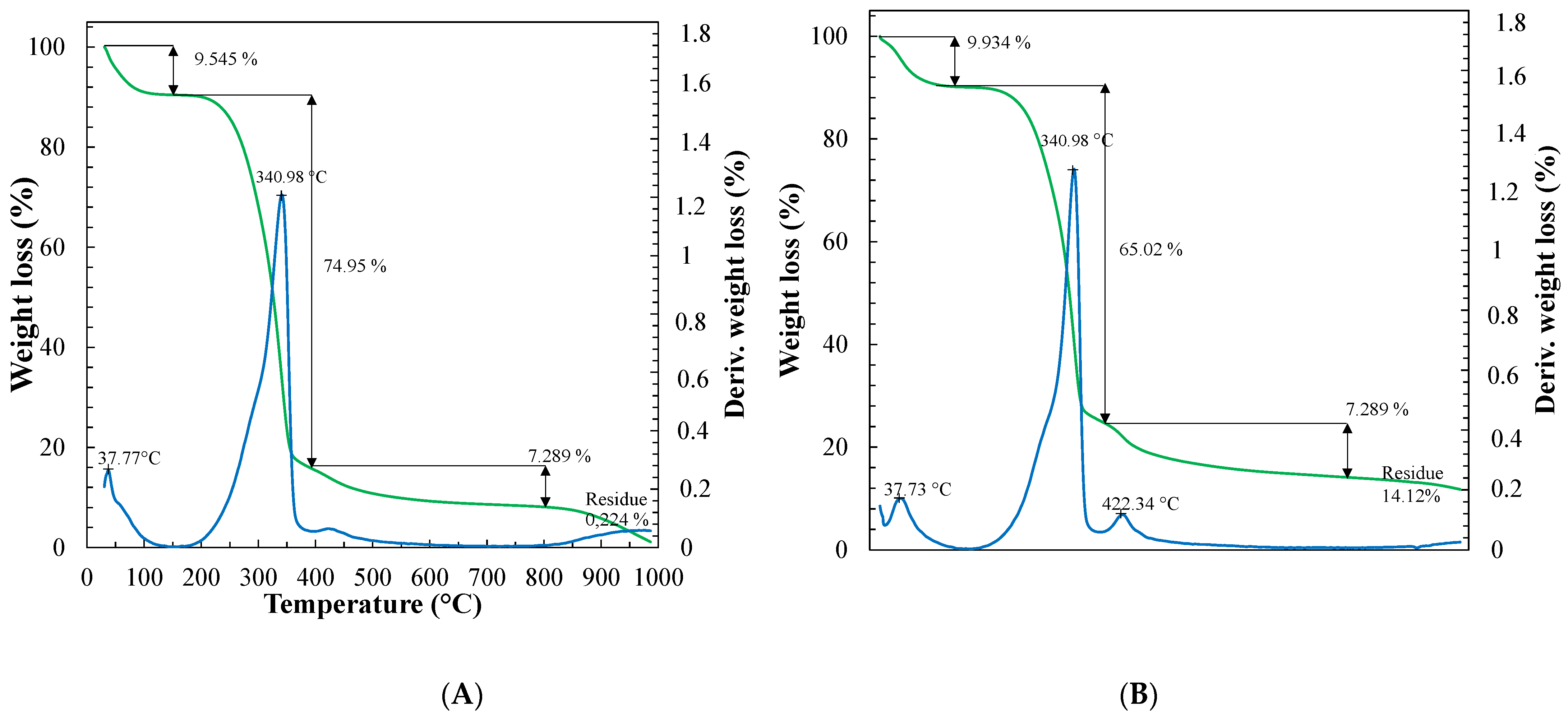
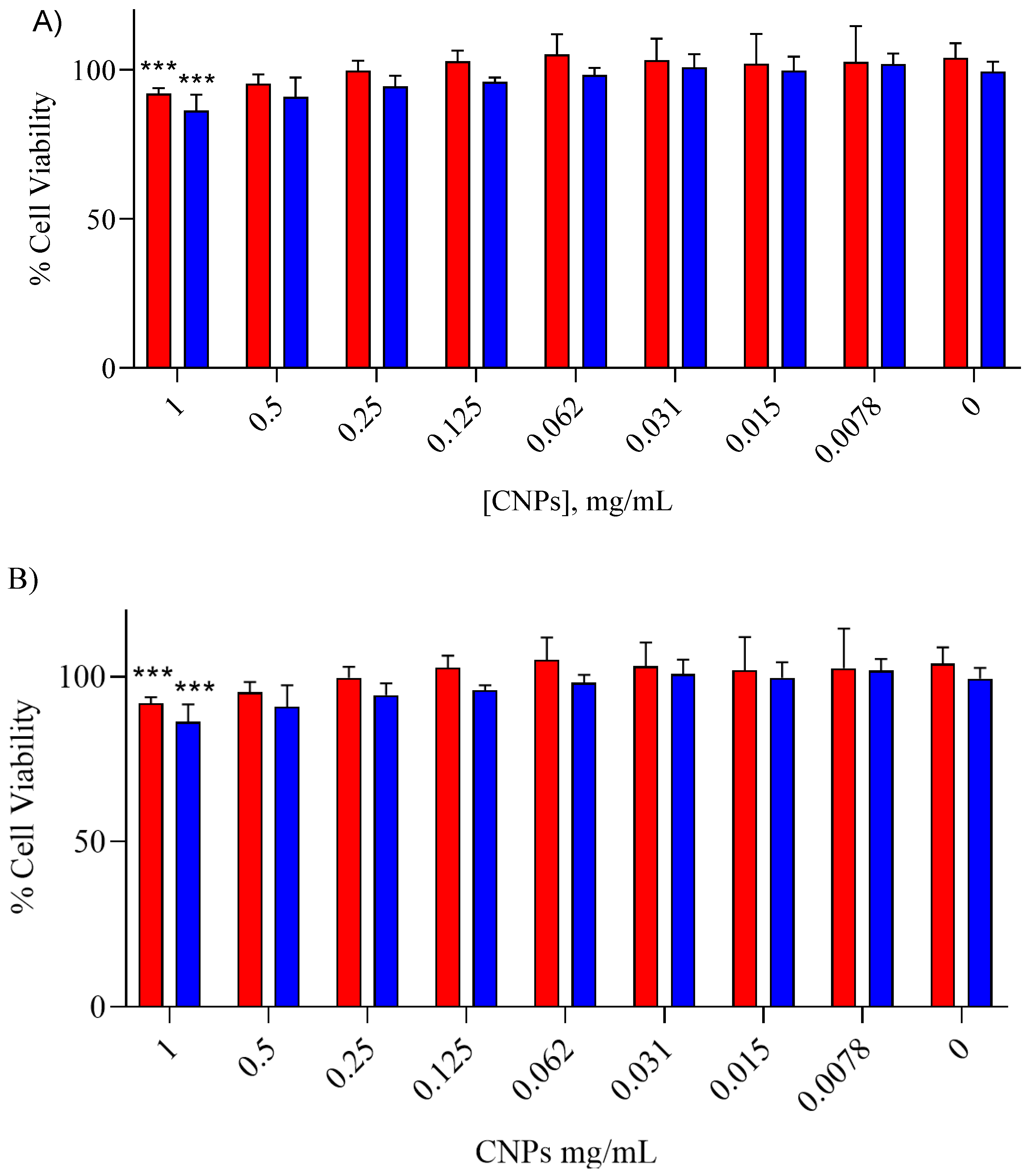
| Nanoparticle Identifier Code | Cellulose Type | Precipitation Temperature (°C) | Antisolvent Volume (mL) |
|---|---|---|---|
| CNP-1A | Cellulose Thermo | 60 | 50 |
| CNP-1B | Cellulose Thermo | 60 | 100 |
| CNP-1C | Cellulose Thermo | 60 | 150 |
| CNP-1D | Cellulose Thermo | 60 | 200 |
| CNP-1E | Cellulose Thermo | 70 | 200 |
| CNP-1F | Cellulose Thermo | 80 | 200 |
| CNP-2A | Cellulose Redwells | 80 | 200 |
| Nanoparticle Identifier Code | Z-Average (nm) | PDI | Peak Intensity (nm) | Peak Number (nm) | Peak Volume (nm) | Z-Potential (mV) |
|---|---|---|---|---|---|---|
| CNP-1A | 537.4 ± 11.9 | 0.383 ± 0.006 | 830.5 ± 104.9 | 223.2 ± 60.4 | 897.5 ± 34.8 | 25.6 ± 0.5 |
| CNP-1B | 324.1 ± 7.8 | 0.255 ± 0.030 | 522.3 ± 27.6 | 239.6 ± 6.3 | 526.6 ± 18.0 | 34.0 ± 0.3 |
| CNP-1C | 332.9 ± 4.8 | 0.233 ± 0.005 | 480.1 ± 75.6 | 197 ± 58.1 | 507.1 ± 56.5 | 27.8 ± 1.4 |
| CNP-1D | 274.9 ± 5.3 | 0.212 ± 0.044 | 409.2 ± 93.9 | 194.8 ± 7.69 | 414.8 ± 87.0 | 32.1 ± 1.0 |
| CNP-1E | 271.5 ± 3.8 | 0.249 ± 0.017 | 482.2 ± 48.8 | 186.7 ± 4.2 | 480.7 ± 39.0 | 28.8 ± 0.6 |
| CNP-1F | 233.4 ± 6.0 | 0.215 ± 0.025 | 381.7 ± 110.6 | 157 ± 7.4 | 383 ± 83.0 | 28.5 ± 0.6 |
| CNP-2A | 310.0 ± 6.5 | 0.218 ± 0.026 | 496.5 ± 107.3 | 230.2 ± 7.6 | 492.6 ± 78.2 | 32.9 ± 0.3 |
| Cellulose Sample | TCI | LOI | HBI |
|---|---|---|---|
| Cellulose 1 | 1.188 ± 0.0061 (1372/2900) | 0.518 ± 0.0051 (1429/897) | 1.223 ± 0.0093 (3338/1334) |
| Cellulose 2 | 1.155 ± 0.0050 (1372/2900) | 0.527 ± 0.0060 (1429/897) | 1.282 ± 0.0095 (3338/1334) |
| CNP-1F | 0.617 ± 0.0108 (1364/2892) | 0.413 ± 0.0048 (1418/894) | 3.279 ± 0.0512 (3336/1336) |
| CNP-2A | 0.958 ± 0.0018 (1364/2892) | 1.003 ± 0.0062 (1418/894) | 3.769 ± 0.0257 (3336/1336) |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Fuster, M.G.; Moulefera, I.; Muñoz, M.N.; Montalbán, M.G.; Víllora, G. Synthesis of Cellulose Nanoparticles from Ionic Liquid Solutions for Biomedical Applications. Polymers 2023, 15, 382. https://doi.org/10.3390/polym15020382
Fuster MG, Moulefera I, Muñoz MN, Montalbán MG, Víllora G. Synthesis of Cellulose Nanoparticles from Ionic Liquid Solutions for Biomedical Applications. Polymers. 2023; 15(2):382. https://doi.org/10.3390/polym15020382
Chicago/Turabian StyleFuster, Marta G., Imane Moulefera, M. Noelia Muñoz, Mercedes G. Montalbán, and Gloria Víllora. 2023. "Synthesis of Cellulose Nanoparticles from Ionic Liquid Solutions for Biomedical Applications" Polymers 15, no. 2: 382. https://doi.org/10.3390/polym15020382
APA StyleFuster, M. G., Moulefera, I., Muñoz, M. N., Montalbán, M. G., & Víllora, G. (2023). Synthesis of Cellulose Nanoparticles from Ionic Liquid Solutions for Biomedical Applications. Polymers, 15(2), 382. https://doi.org/10.3390/polym15020382








