The Relationship between Microstructure and Mechanical Properties of PBST Two-Component Crystalline Random Copolymers with Different BT Contents
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
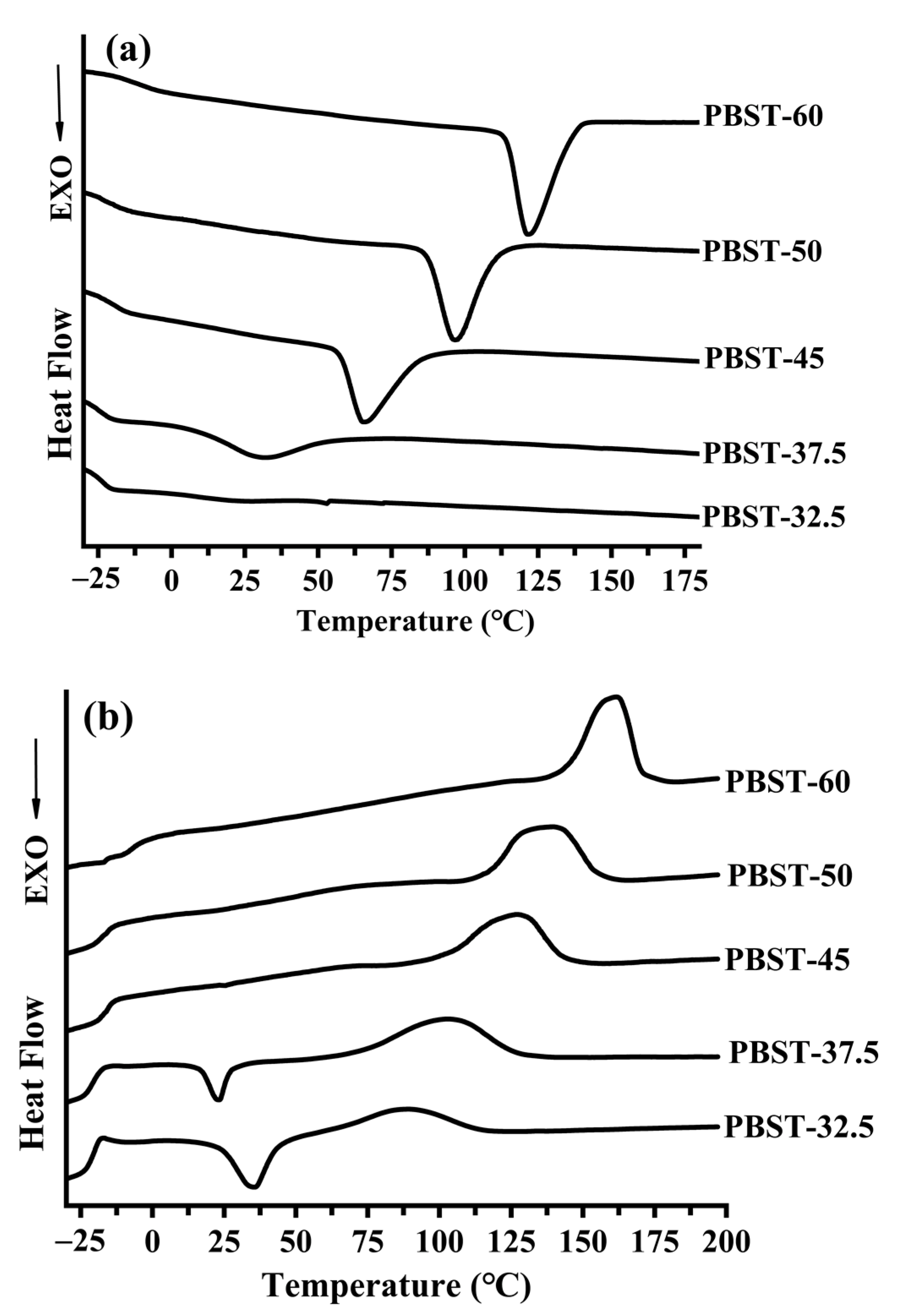
2.2. Differential Scanning Calorimetry (DSC) Analysis
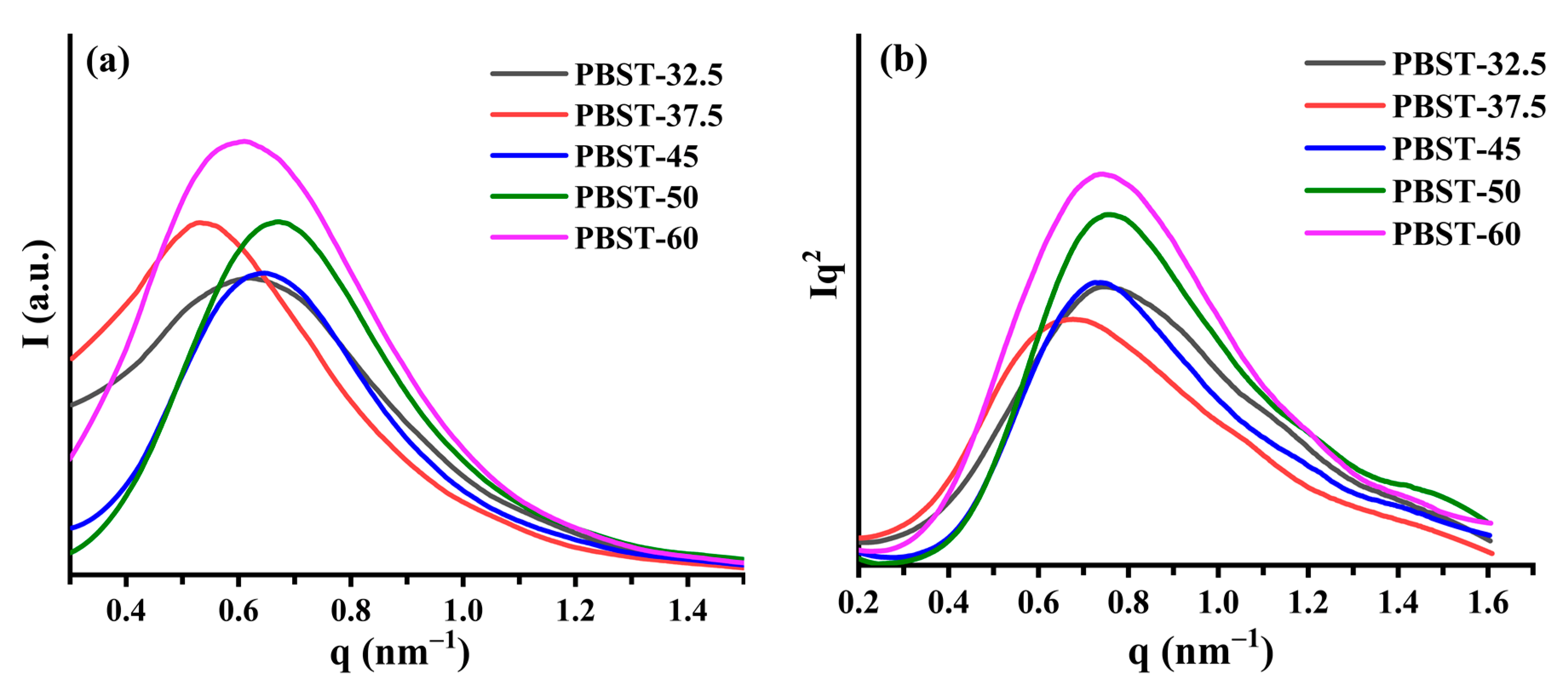
2.3. Small-Angle X-ray Scattering (SAXS)
2.4. Wide-Angle X-ray Diffraction (WAXD)
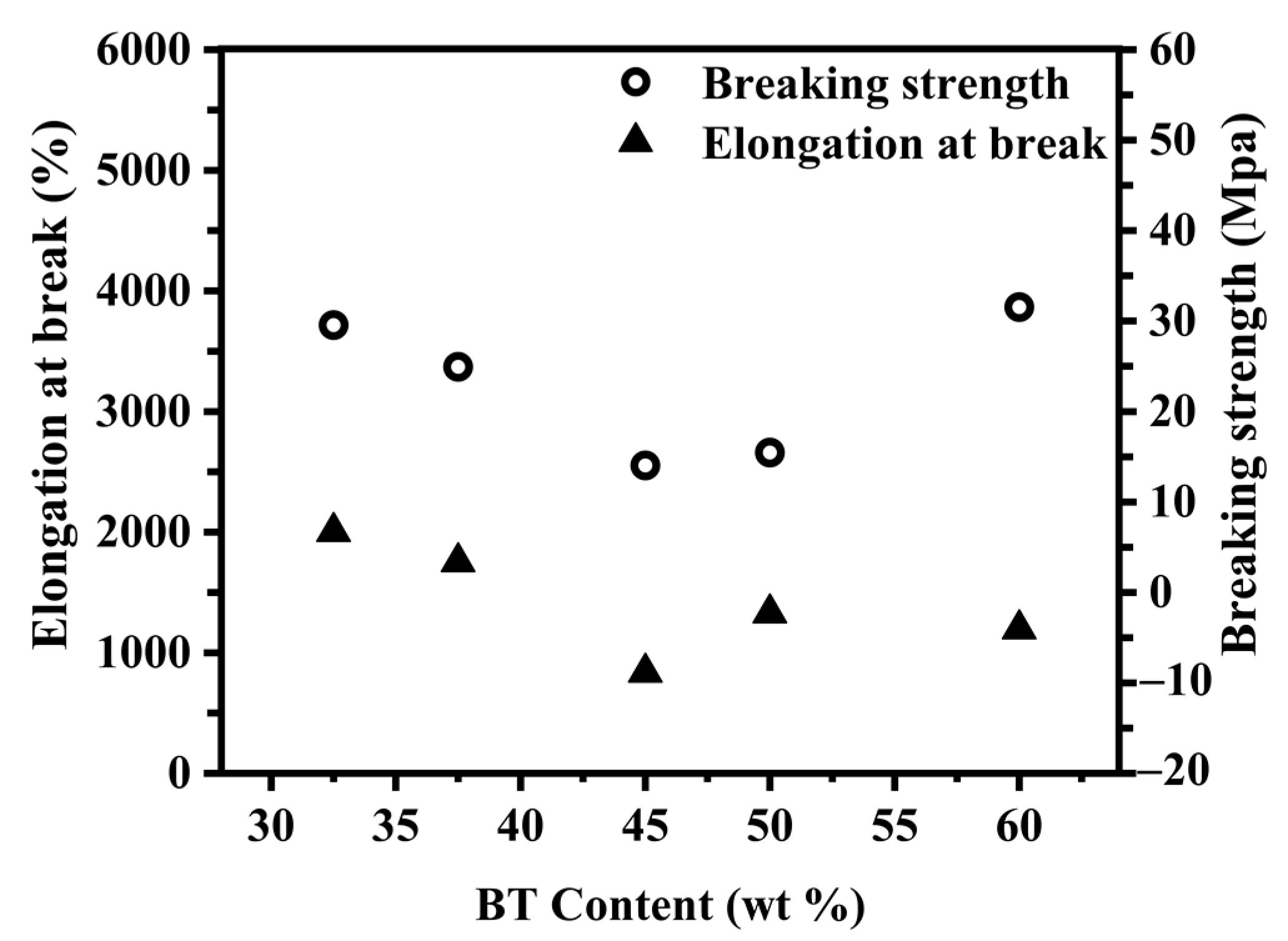
2.5. Tensile Testing
2.6. Hardness Testing
3. Results and Discussion
3.1. Study on the Microstructure of PBST by WAXD
3.2. Crystallization Behavior of the PBST Copolymers
3.3. Study on the Lamellar Structure of PBST by SAXS
3.4. Study on Mechanical Properties of PBST
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Urbanek, A.K.; Mirończuk, A.M.; García-Martín, A.; Saborido, A.; De La Mata, I.; Arroyo, M. Biochemical properties and biotechnological applications of microbial enzymes involved in the degradation of polyester-type plastics. Biochim. Biophys. Acta (BBA)-Proteins Proteom. 2019, 1868, 140315. [Google Scholar] [CrossRef]
- Luo, S.; Li, F.; Yu, J.; Cao, A. Aggregation structure development and mechanical properties of biodegradable poly(butylene succinate-co-terephthalate) fibers. J. Appl. Polym. Sci. 2012, 125, 2426–2432. [Google Scholar] [CrossRef]
- Sun, Y.; Wu, L.; Bu, Z.; Li, B.-G.; Li, N.; Dai, J. Synthesis and Thermomechanical and Rheological Properties of Biodegradable Long-Chain Branched Poly(butylene succinate-co-butylene terephthalate) Copolyesters. Ind. Eng. Chem. Res. 2014, 53, 10380–10386. [Google Scholar] [CrossRef]
- Zhang, J.; Wang, X.; Li, F.; Yu, J. Mechanical properties and crystal structure transition of biodegradable poly(butylene succinate-co-terephthalate) (PBST) fibers. Fibers Polym. 2012, 13, 1233–1238. [Google Scholar] [CrossRef]
- Li, F.; Luo, S.; Yu, J. Mechanical, thermal properties and isothermal crystallization kinetics of biodegradable poly(butylene succinate-co-terephthalate) (PBST) fibers. J. Polym. Res. 2009, 17, 279–287. [Google Scholar] [CrossRef]
- Li, F.; Luo, S.; Ma, C.; Yu, J.; Cao, A. The crystallization and morphology of biodegradable poly(butylene succinate-co-terephthalate) copolyesters with high content of BT units. J. Appl. Polym. Sci. 2010, 118, 623–630. [Google Scholar] [CrossRef]
- Li, F.; Xu, X.; Yu, J.; Cao, A. The morphological effects upon enzymatic degradation of poly(butylene succinate-co-butylene terephthalate)s (PBST). Polym. Degrad. Stab. 2007, 92, 1053–1060. [Google Scholar] [CrossRef]
- Gu, J.; Wei, X.; Hou, X.; Wei, Z. Biodegradable Poly(butylene succinate-co-terephthalate) Fibers Incorporated with Nanoparticles under High Drawing Temperatures for Enhanced Mechanical Properties. Macromol. Mater. Eng. 2019, 304, 1900168. [Google Scholar] [CrossRef]
- Yuan, D.; Chen, Z.; Xiang, X.; Deng, S.; Liu, K.; Xiao, D.; Deng, L.; Feng, G. The establishment and biological assessment of a whole tissue-engineered intervertebral disc with PBST fibers and a chitosan hydrogel in vitro and in vivo. J. Biomed. Mater. Res. Part B Appl. Biomater. 2019, 107, 2305–2316. [Google Scholar] [CrossRef]
- Shi, Y.; Zheng, C.; Zhu, G.; Ren, Y.; Liu, L.-Z.; Zhang, W.; Han, L. A heat initiated 3D shape recovery and biodegradable thermoplastic tolerating a strain of 5. React. Funct. Polym. 2020, 154, 104680. [Google Scholar] [CrossRef]
- Zheng, C.; Zhu, G.; Shi, Y.; Liu, L.-Z.; Ren, M.; Zhang, W.; Han, L. Crystallization, structures and properties of biodegradable poly (butylene succinate-co-butylene terephthalate) with a symmetric composition. Mater. Chem. Phys. 2020, 260, 124183. [Google Scholar] [CrossRef]
- Pérez-Camargo, R.A.; Fernández-D’Arlas, B.; Cavallo, D.; Debuissy, T.; Pollet, E.; Avérous, L.; Müller, A.J. Tailoring the Structure, Morphology, and Crystallization of Isodimorphic Poly(butylene succinate-ran-butylene adipate) Random Copolymers by Changing Composition and Thermal History. Macromolecules 2017, 50, 597–608. [Google Scholar] [CrossRef]
- Wu, T.; Wei, Z.; Ren, Y.; Yu, Y.; Leng, X.; Li, Y. Highly branched linear-comb random copolyesters of ε-caprolactone and δ-valerolactone: Isodimorphism, mechanical properties and enzymatic degradation behavior. Polym. Degrad. Stab. 2018, 155, 173–182. [Google Scholar] [CrossRef]
- Morro, A.; Catalina, F.; Sanchez-León, E.; Abrusci, C. Photodegradation and Biodegradation Under Thermophile Conditions of Mulching Films Based on Poly(Butylene Adipate-co-Terephthalate) and Its Blend with Poly(Lactic Acid). J. Polym. Environ. 2018, 27, 352–363. [Google Scholar] [CrossRef]
- Nagata, M. Synthesis and enzymatic degradation of poly(tetramethylene succinate) copolymers with terephthalic acid. Polymer 2000, 41, 4373–4376. [Google Scholar] [CrossRef]
- Ren, L.; Wang, Y.; Ge, J.; Lu, D.; Liu, Z. Enzymatic Synthesis of High-Molecular-Weight Poly(butylene succinate) and its Copolymers. Macromol. Chem. Phys. 2015, 216, 636–640. [Google Scholar] [CrossRef]
- Lu, J.; Wu, L.; Li, B.-G. Long chain branched poly(butylene succinate-co-terephthalate) copolyesters using pentaerythritol as branching agent: Synthesis, thermo-mechanical, and rheological properties. J. Appl. Polym. Sci. 2016, 134, 44544. [Google Scholar] [CrossRef]
- Li, F.; Luo, S.; Zhang, J.; Yu, J. Temperature dependences of solid structure and properties of biodegradable poly(butylene succinate-co-terephthalate) (PBST) copolyester. J. Therm. Anal. Calorim. 2012, 113, 915–921. [Google Scholar] [CrossRef]
- Wei, Z.; Liu, Y.; Wang, X.; Yu, J.; Li, F. Real-time tracking of the hierarchical structure of biodegradable poly(butylene succinate- co -terephthalate) nanocomposites with fibrous attapulgite nanoparticles. Compos. Sci. Technol. 2016, 134, 201–208. [Google Scholar] [CrossRef]
- Wei, Z.; Pan, Z.; Li, F.; Yu, J. Poly(butylene succinate-co-terephthalate) nanofibrous membrane composited with cyclodextrin polymer for superhydrophilic property. RSC Adv. 2018, 8, 1378–1384. [Google Scholar] [CrossRef]
- Yang, B.; Zhu, S.; Luo, Y.; Huang, J. Enhanced nonisothermal crystallization of a series of poly(butylene succinate-co-terephthalate) biopolyesters by poly(vinyl butyral). J. Polym. Sci. Part B Polym. Phys. 2017, 55, 658–672. [Google Scholar] [CrossRef]
- Luo, S.; Li, F.; Yu, J.; Cao, A. Synthesis of poly(butylene succinate-co-butylene terephthalate) (PBST) copolyesters with high molecular weights via direct esterification and polycondensation. J. Appl. Polym. Sci. 2009, 115, 2203–2211. [Google Scholar] [CrossRef]
- Wei, Z.; Lin, J.; Wang, X.; Huang, L.; Yu, J.; Li, F. In situ polymerization of biodegradable poly(butylene-co-succinate terephthlate) nanocomposites and their real-time tracking of microstructure. Compos. Sci. Technol. 2015, 117, 121–129. [Google Scholar] [CrossRef]
- Wei, Z.; Lin, J.; Tian, F.; Li, F.; Yu, J. Synchronous stimuli of biodegradable poly(butylene succinate-co-terephthalate) copolymer via uniaxial stretching at varying temperatures. J. Polym. Sci. Part B Polym. Phys. 2015, 53, 640–649. [Google Scholar] [CrossRef]
- Lin, S.-H.; Wang, H.-T.; Wang, J.-M.; Wu, T.-M. Enzymatic Degradation of Acrylic Acid-Grafted Poly(butylene succinate-co-terephthalate) Nanocomposites Fabricated Using Heat Pressing and Freeze-Drying Techniques. Materials 2020, 13, 376. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Luo, S.; Li, F.; Yu, J. The thermal, mechanical and viscoelastic properties of poly(butylene succinate-co-terephthalate) (PBST) copolyesters with high content of BT units. J. Polym. Res. 2010, 18, 393–400. [Google Scholar] [CrossRef]
- Wei, Z.; Lun, R.; Lou, X.; Tian, F.; Lin, J.; Li, X.; Yu, J.; Li, F. Lamellae evolution of poly(butylene succinate-co-terephthalate) copolymer induced by uniaxial stretching and subsequent heating. RSC Adv. 2014, 4, 64625–64633. [Google Scholar] [CrossRef]
- Li, F.; Xu, X.; Hao, Q.; Li, Q.; Yu, J.; Cao, A. Effects of comonomer sequential structure on thermal and crystallization behaviors of biodegradable poly(butylene succinate-co-butylene terephthalate)s. J. Polym. Sci. Part B Polym. Phys. 2006, 44, 1635–1644. [Google Scholar] [CrossRef]
- Kim, Y.J.; Park, O.O. Miscibilities and rheological properties of poly (butylene succinate)–Poly (butylene terephthalate) blends. J. Appl. Polym. Sci. 1999, 72, 945–951. [Google Scholar] [CrossRef]
- Chen, W.; Zhu, G.; Chen, X.; Lu, J.; Zhang, Y.; Yan, Y.; Zhang, W. Biodegradable linear random copolyester and process for preparing it and use of the same. US Patent US7332562B2, 2008. Available online: https://kns.cnki.net/kcms/detail/detail.aspx?dbcode=SOPD&dbname=SOPD202202&filename=US07332562(B2)&unip (accessed on 15 November 2022).
- Chen, Y.-A.; Tsai, G.-S.; Chen, E.-C.; Wu, T.-M. Crystallization behaviors and microstructures of poly(butylene succinate-co-adipate)/modified layered double hydroxide nanocomposites. J. Mater. Sci. 2016, 51, 4021–4030. [Google Scholar] [CrossRef]
- Holzwarth, U.; Gibson, N. The Scherrer equation versus the ‘Debye-Scherrer equation’. Nat. Nanotechnol. 2011, 6, 534. [Google Scholar] [CrossRef] [PubMed]
- Roe, R.-J. X-ray diffraction by polymers. In Encyclopedia of Polymer Science and Technology, 4th ed.; Wiley: Cincinnati, OH, USA, 2015; pp. 1–47. [Google Scholar] [CrossRef]
- Cheng, M.-H.; Yao, I.-C.; Chiu, T.-Y.; Chen, J.-T. Block copolymer micelles confined in cylindrical nanopores: Effects of annealing solvents and hybridization. React. Funct. Polym. 2020, 150, 104534. [Google Scholar] [CrossRef]
- Li, Z.; Yang, F.; Qiu, Z. Miscibility and crystallization behaviors of biodegradable poly(butylene succinate-co-butylene terephthalate)/phenoxy blends. J. Appl. Polym. Sci. 2011, 121, 720–726. [Google Scholar] [CrossRef]
- Yokohara, T.; Okamoto, K.; Yamaguchi, M. Effect of the shape of dispersed particles on the thermal and mechanical properties of biomass polymer blends composed of poly(L-lactide) and poly(butylene succinate). J. Appl. Polym. Sci. 2010, 117, 2226–2232. [Google Scholar] [CrossRef]
- Wang, X.; Zhou, J.; Li, L. Multiple melting behavior of poly(butylene succinate). Eur. Polym. J. 2007, 43, 3163–3170. [Google Scholar] [CrossRef]
- Yang, X.; Xu, H.; Odelius, K.; Hakkarainen, M. Poly(lactide)-g-poly(butylene succinate-co-adipate) with High Crystallization Capacity and Migration Resistance. Materials 2016, 9, 313. [Google Scholar] [CrossRef] [Green Version]
- Purnama, P.; Samsuri, M.; Iswaldi, I. Properties Enhancement of High Molecular Weight Polylactide Using Stereocomplex Polylactide as a Nucleating Agent. Polymers 2021, 13, 1725. [Google Scholar] [CrossRef]
- Lascano, D.; Quiles-Carrillo, L.; Balart, R.; Boronat, T.; Montanes, N. Toughened Poly (Lactic Acid)—PLA Formulations by Binary Blends with Poly(Butylene Succinate-co-Adipate)—PBSA and Their Shape Memory Behaviour. Materials 2019, 12, 622. [Google Scholar] [CrossRef] [Green Version]
- Aliotta, L.; Vannozzi, A.; Canesi, I.; Cinelli, P.; Coltelli, M.-B.; Lazzeri, A. Poly(lactic acid) (PLA)/Poly(butylene succinate-co-adipate) (PBSA) Compatibilized Binary Biobased Blends: Melt Fluidity, Morphological, Thermo-Mechanical and Micromechanical Analysis. Polymers 2021, 13, 218. [Google Scholar] [CrossRef]
- Bednarek, M.; Kubisa, P. Reversible networks of degradable polyesters containing weak covalent bonds. Polym. Chem. 2019, 10, 1848–1872. [Google Scholar] [CrossRef]
- Heidarzadeh, N.; Rafizadeh, M.; Taromi, F.A.; Puiggalí, J.; del Valle, L.J. Nucleating and retarding effects of nanohydroxyapatite on the crystallization of poly(butylene terephthalate-co-alkylene dicarboxylate)s with different lengths. J. Therm. Anal. Calorim. 2018, 137, 421–435. [Google Scholar] [CrossRef]



| Sample | BT (mol %) | BS (mol %) |
|---|---|---|
| PBST-32.5 | 32.5 | 67.5 |
| PBST-37.5 | 37.5 | 62.5 |
| PBST-45 | 45 | 55 |
| PBST-50 | 50 | 50 |
| PBST-60 | 60 | 40 |
| PBST-37.5 | PBST-45 | PBST-50 | PBST-60 | |
|---|---|---|---|---|
| Crystal size(nm) | 10.3 | 10.6 | 12.5 | 13.5 |
| Crystal plane spacing (nm) | 0.514 | 0.512 | 0.511 | 0.511 |
| PBST-37.5 | PBST-45 | PBST-50 | PBST-60 | |
|---|---|---|---|---|
| Xc (%) 1 | 9.33 | 10.3 | 12.1 | 12.6 |
| PBST-32.5 | PBST-37.5 | PBST-45 | PBST-50 | PBST-60 | |
|---|---|---|---|---|---|
| Lp (nm) 1 | 8.39 | 9.38 | 8.64 | 8.34 | 8.44 |
| Lamellar thickness (nm) | 1.89 | 2.78 | 2.04 | 2.04 | 2.14 |
| Amorphous layer thickness (nm) | 6.5 | 6.6 | 6.6 | 6.3 | 6.3 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Gang, M.; Wang, Y.; Zhang, Y.; Liu, L.; Shi, Y. The Relationship between Microstructure and Mechanical Properties of PBST Two-Component Crystalline Random Copolymers with Different BT Contents. Polymers 2023, 15, 383. https://doi.org/10.3390/polym15020383
Gang M, Wang Y, Zhang Y, Liu L, Shi Y. The Relationship between Microstructure and Mechanical Properties of PBST Two-Component Crystalline Random Copolymers with Different BT Contents. Polymers. 2023; 15(2):383. https://doi.org/10.3390/polym15020383
Chicago/Turabian StyleGang, Mingjun, Yuanxia Wang, Yu Zhang, Lizhi Liu, and Ying Shi. 2023. "The Relationship between Microstructure and Mechanical Properties of PBST Two-Component Crystalline Random Copolymers with Different BT Contents" Polymers 15, no. 2: 383. https://doi.org/10.3390/polym15020383





