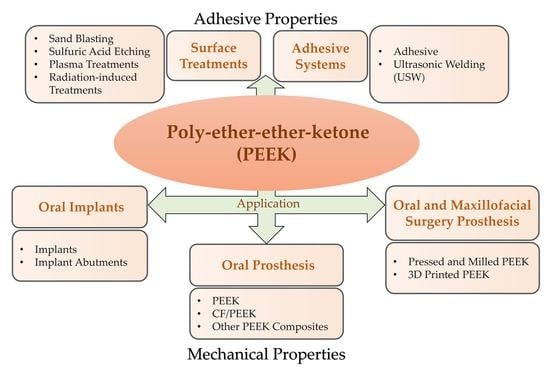
PEEK for Oral Applications: Recent Advances in Mechanical and Adhesive Properties
Abstract
1. Introduction
2. Performance Requirements for Medical Materials
3. Mechanical Properties of PEEK in Dental Applications
3.1. PEEK as an Oral Implant Material
3.1.1. Implants Made of PEEK
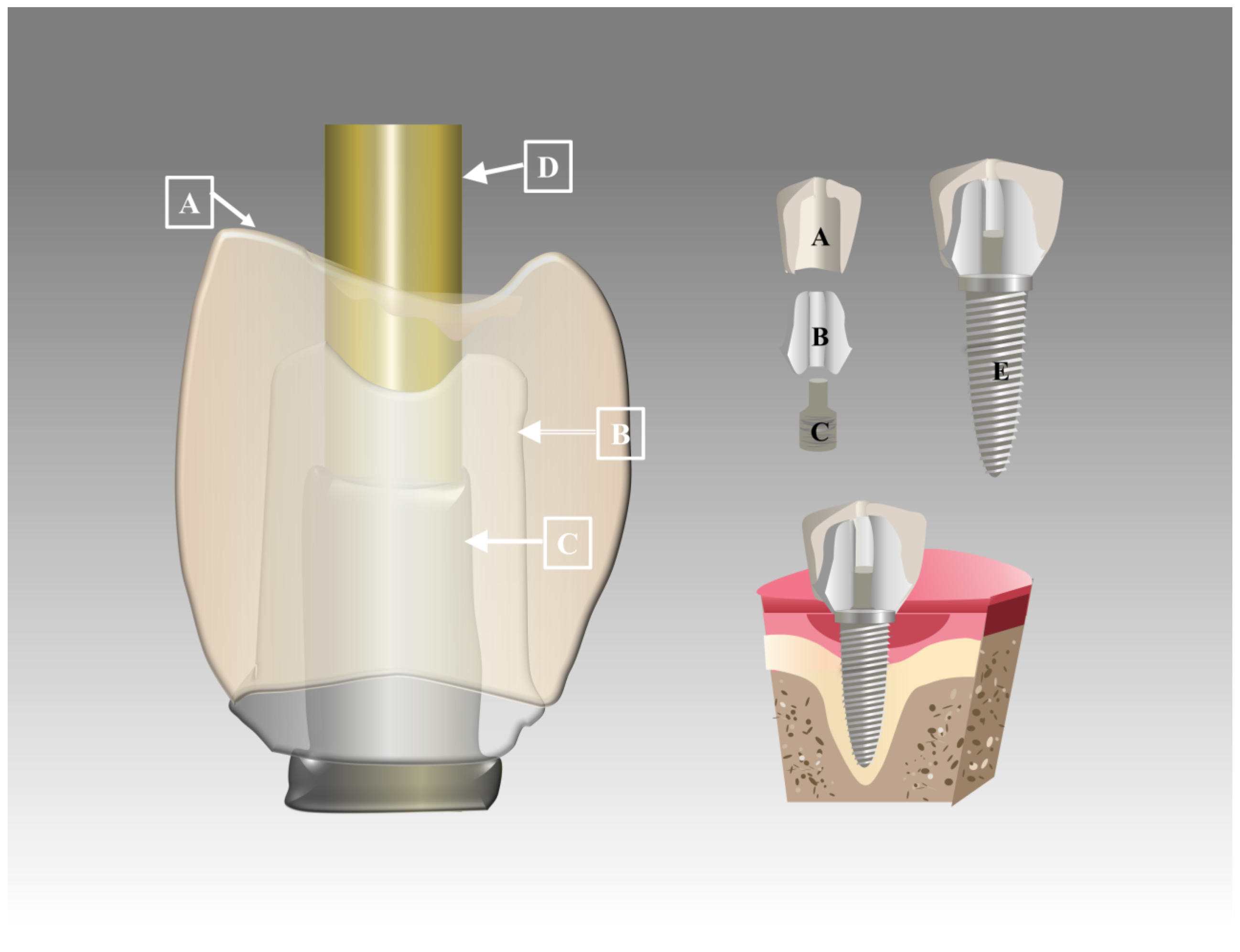
3.1.2. PEEK Implant Abutments
3.2. PEEK as an Oral Prosthesis Material
3.2.1. PEEK
3.2.2. CF/PEEK
3.2.3. Other PEEK Composites
3.3. 3D-Printed PEEK in Oral and Maxillofacial Surgery
3.4. Other Oral Applications of PEEK
4. Adhesive Properties of PEEK in Dental Applications
4.1. Surface Treatments
4.2. Adhesive Systems
5. Conclusions and Perspectives
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Uddin, M.N.; Dhanasekaran, P.S.; Asmatulu, R. Mechanical Properties of Highly Porous PEEK Bionanocomposites Incorporated with Carbon and Hydroxyapatite Nanoparticles for Scaffold Applications. Prog. Biomater. 2019, 8, 211–221. [Google Scholar] [CrossRef] [PubMed]
- Makvandi, P.; Gu, J.T.; Zare, E.N.; Ashtari, B.; Moeini, A.; Tay, F.R.; Niu, L. Polymeric and Inorganic Nanoscopical Antimicrobial Fillers in Dentistry. Acta Biomater. 2020, 101, 69–101. [Google Scholar] [CrossRef] [PubMed]
- Ammarullah, M.I.; Santoso, G.; Sugiharto, S.; Supriyono, T.; Wibowo, D.B.; Kurdi, O.; Tauviqirrahman, M.; Jamari, J. Minimizing Risk of Failure from Ceramic-on-Ceramic Total Hip Prosthesis by Selecting Ceramic Materials Based on Tresca Stress. Sustainability 2022, 14, 13413. [Google Scholar] [CrossRef]
- Jamari, J.; Ammarullah, M.I.; Santoso, G.; Sugiharto, S.; Supriyono, T.; van der Heide, E. In Silico Contact Pressure of Metal-on-Metal Total Hip Implant with Different Materials Subjected to Gait Loading. Metals 2022, 12, 1241. [Google Scholar] [CrossRef]
- Rosenblum, M.A.; Schulman, A. A Review of All-Ceramic Restorations. J. Am. Dent. Assoc. 1997, 128, 297–307. [Google Scholar] [CrossRef]
- Marie, A.; Keeling, A.; Hyde, T.P.; Nattress, B.R.; Pavitt, S.; Murphy, R.J.; Shary, T.J.; Dillon, S.; Osnes, C.; Wood, D.J. Deformation and Retentive Force Following In Vitro Cyclic Fatigue of Cobalt-Chrome and Aryl Ketone Polymer (AKP) Clasps. Dent. Mater. 2019, 35, e113–e121. [Google Scholar] [CrossRef]
- Papathanasiou, I.; Kamposiora, P.; Papavasiliou, G.; Ferrari, M. The Use of PEEK in Digital Prosthodontics: A Narrative Review. BMC Oral Health 2020, 20, 217. [Google Scholar] [CrossRef]
- Rauch, A.; Hahnel, S.; Günther, E.; Bidmon, W.; Schierz, O. Tooth-Colored CAD/CAM Materials for Application in 3-Unit Fixed Dental Prostheses in the Molar Area: An Illustrated Clinical Comparison. Materials 2020, 13, 5588. [Google Scholar] [CrossRef]
- Lommen, J.; Schorn, L.; Sproll, C.; Haussmann, J.; Kübler, N.R.; Budach, W.; Rana, M.; Tamaskovics, B. Reduction of CT Artifacts Using Polyetheretherketone (PEEK), Polyetherketoneketone (PEKK), Polyphenylsulfone (PPSU), and Polyethylene (PE) Reconstruction Plates in Oral Oncology. J. Oral Maxillofac. Surg. 2022, 80, 1272–1283. [Google Scholar] [CrossRef]
- Micheletti, C.; Hurley, A.; Gourrier, A.; Palmquist, A.; Tang, T.; Shah, F.A.; Grandfield, K. Bone Mineral Organization at the Mesoscale: A Review of Mineral Ellipsoids in Bone and at Bone Interfaces. Acta Biomater. 2022, 142, 1–13. [Google Scholar] [CrossRef]
- Skinner, H.B. Composite Technology for Total Hip Arthroplasty. Clin. Orthop. Relat. Res. 1988, 235, 224–236. [Google Scholar] [CrossRef]
- Wang, B.; Huang, M.; Dang, P.; Xie, J.; Zhang, X.; Yan, X. PEEK in Fixed Dental Prostheses: Application and Adhesion Improvement. Polymers 2022, 14, 2323. [Google Scholar] [CrossRef]
- Punia, U.; Kaushik, A.; Garg, R.K.; Chhabra, D.; Sharma, A. 3D Printable Biomaterials for Dental Restoration: A Systematic Review. Mater. Today Proc. 2022, 63, 566–572. [Google Scholar] [CrossRef]
- Liu, Y.; Fang, M.; Zhao, R.; Liu, H.; Li, K.; Tian, M.; Niu, L.; Xie, R.; Bai, S. Clinical Applications of Polyetheretherketone in Removable Dental Prostheses: Accuracy, Characteristics, and Performance. Polymers 2022, 14, 4615. [Google Scholar] [CrossRef]
- Gouveia, D.D.N.M.; Razzoog, M.E.; Sierraalta, M.; Alfaro, M.F. Effect of Surface Treatment and Manufacturing Process on the Shear Bond Strength of Veneering Composite Resin to Polyetherketoneketone (PEKK) and Polyetheretherketone (PEEK). J. Prosthet. Dent. 2021, 128, 1061–1066. [Google Scholar] [CrossRef]
- Barto, A.; Vandewalle, K.S.; Lien, W.; Whang, K. Repair of Resin-Veneered Polyetheretherketone after Veneer Fracture. J. Prosthet. Dent. 2021, 125, 704.e1–704.e8. [Google Scholar] [CrossRef]
- Xie, D.; Xu, C.; Ye, C.; Mei, S.; Wang, L.; Zhu, Q.; Chen, Q.; Zhao, Q.; Xu, Z.; Wei, J.; et al. Fabrication of Submicro-Nano Structures on Polyetheretherketone Surface by Femtosecond Laser for Exciting Cellular Responses of MC3T3-E1 Cells/Gingival Epithelial Cells. IJN 2021, 16, 3201–3216. [Google Scholar] [CrossRef]
- Yu, W.; Zhang, H.; A, L.; Yang, S.; Zhang, J.; Wang, H.; Zhou, Z.; Zhou, Y.; Zhao, J.; Jiang, Z. Enhanced Bioactivity and Osteogenic Property of Carbon Fiber Reinforced Polyetheretherketone Composites Modified with Amino Groups. Colloids Surf. B Biointerfaces 2020, 193, 111098. [Google Scholar] [CrossRef]
- Shahdad, S.A.; McCabe, J.F.; Bull, S.; Rusby, S.; Wassell, R.W. Hardness Measured with Traditional Vickers and Martens Hardness Methods. Dent. Mater. 2007, 23, 1079–1085. [Google Scholar] [CrossRef]
- Han, X.; Yang, D.; Yang, C.; Spintzyk, S.; Scheideler, L.; Li, P.; Li, D.; Geis-Gerstorfer, J.; Rupp, F. Carbon Fiber Reinforced PEEK Composites Based on 3D-Printing Technology for Orthopedic and Dental Applications. J. Clin. Med. 2019, 8, 240. [Google Scholar] [CrossRef]
- Yi, N.; Davies, R.; Chaplin, A.; Ghita, O. Novel Backbone Modified Polyetheretherketone (PEEK) Grades for Powder Bed Fusion with Enhanced Elongation at Break. Addit. Manuf. 2022, 55, 102857. [Google Scholar] [CrossRef]
- Sikder, P.; Challa, B.T.; Gummadi, S.K. A Comprehensive Analysis on the Processing-Structure-Property Relationships of FDM-Based 3-D Printed Polyetheretherketone (PEEK) Structures. Materialia 2022, 22, 101427. [Google Scholar] [CrossRef]
- Wang, P.; Zou, B. Improvement of Heat Treatment Process on Mechanical Properties of FDM 3D-Printed Short- and Continuous-Fiber-Reinforced PEEK Composites. Coatings 2022, 12, 827. [Google Scholar] [CrossRef]
- Schmeiser, F.; Arbogast, F.; Ruppel, H.; Mayinger, F.; Reymus, M.; Stawarczyk, B. Methodology Investigation: Impact of Crown Geometry, Crown, Abutment and Antagonist Material and Thermal Loading on the Two-Body Wear of Dental Materials. Dent. Mater. 2022, 38, 266–280. [Google Scholar] [CrossRef] [PubMed]
- Abdullah, A.O.; Tsitrou, E.A.; Pollington, S. Comparative in Vitro Evaluation of CAD/CAM vs Conventional Provisional Crowns. J. Appl. Oral Sci. 2016, 24, 258–263. [Google Scholar] [CrossRef] [PubMed]
- Babaier, R.; Watts, D.C.; Silikas, N. Effects of Three Food-Simulating Liquids on the Roughness and Hardness of CAD/CAM Polymer Composites. Dent. Mater. 2022, 38, 874–885. [Google Scholar] [CrossRef]
- Sun, H.; Gao, K.; Yi, Z.; Han, C.; Liu, Z.; Wang, Q.; Zhou, Q.; Zhang, Z. Cytotoxicity and Bonding Property of Bioinspired Nacre-like Ceramic-Polymer Composites. Front. Bioeng. Biotechnol. 2022, 10, 913899. [Google Scholar] [CrossRef]
- Gao, X.; Wang, H.; Luan, S.; Zhou, G. Low-Temperature Printed Hierarchically Porous Induced-Biomineralization Polyaryletherketone Scaffold for Bone Tissue Engineering. Adv. Healthc. Mater. 2022, 11, 2200977. [Google Scholar] [CrossRef]
- Kabir, H.; Munir, K.; Wen, C.; Li, Y. Recent Research and Progress of Biodegradable Zinc Alloys and Composites for Biomedical Applications: Biomechanical and Biocorrosion Perspectives. Bioact. Mater. 2021, 6, 836–879. [Google Scholar] [CrossRef]
- Wu, D.; Miao, Q.; Dai, Z.; Niu, F.; Ma, G. Effect of Voids and Crystallinity on the Interlaminar Shear Strength of In-Situ Manufactured CF/PEEK Laminates Using Repass Treatment. Compos. Sci. Technol. 2022, 224, 109448. [Google Scholar] [CrossRef]
- Ortega-Martínez, J.; Delgado, L.M.; Ortiz-Hernández, M.; Punset, M.; Cano-Batalla, J.; Cayon, M.R.; Cabratosa-Termes, J. In Vitro Assessment of PEEK and Titanium Implant Abutments: Screw Loosening and Microleakage Evaluations under Dynamic Mechanical Testing. J. Prosthet. Dent. 2022, 127, 470–476. [Google Scholar] [CrossRef]
- Khorsandi, D.; Fahimipour, A.; Abasian, P.; Saber, S.S.; Seyedi, M.; Ghanavati, S.; Ahmad, A.; De Stephanis, A.A.; Taghavinezhaddilami, F.; Leonova, A.; et al. 3D and 4D Printing in Dentistry and Maxillofacial Surgery: Printing Techniques, Materials, and Applications. Acta Biomater. 2021, 122, 26–49. [Google Scholar] [CrossRef]
- Zhao, L.; Pei, X.; Jiang, L.; Hu, C.; Sun, J.; Xing, F.; Zhou, C.; Fan, Y.; Zhang, X. Bionic Design and 3D Printing of Porous Titanium Alloy Scaffolds for Bone Tissue Repair. Compos. Part B Eng. 2019, 162, 154–161. [Google Scholar] [CrossRef]
- Nam, R.-K.; Lee, S.J.; Park, E.-J.; Kwon, H.-B.; Yoon, H.-I. Three-Dimensional Deformation and Wear of Internal Implant-Abutment Connection: A Comparative Biomechanical Study Using Titanium and Zirconia. Int. J. Oral Maxillofac. Implants 2018, 33, 1279–1286. [Google Scholar] [CrossRef]
- Barkallah, R.; Taktak, R.; Guermazi, N.; Elleuch, K.; Bouaziz, J. Mechanical Properties and Wear Behaviour of Alumina/Tricalcium Phosphate/Titania Ceramics as Coating for Orthopedic Implant. Eng. Fract. Mech. 2021, 241, 107399. [Google Scholar] [CrossRef]
- Elsayed, A.; Yazigi, C.; Kern, M.; Chaar, M.S. Mechanical Behavior of Nano-Hybrid Composite in Comparison to Lithium Disilicate as Posterior Cement-Retained Implant-Supported Crowns Restoring Different Abutments. Dent. Mater. 2021, 37, e435–e442. [Google Scholar] [CrossRef]
- Mansoor, A.; Khurshid, Z.; Khan, M.T.; Mansoor, E.; Butt, F.A.; Jamal, A.; Palma, P.J. Medical and Dental Applications of Titania Nanoparticles: An Overview. Nanomaterials 2022, 12, 3670. [Google Scholar] [CrossRef]
- Kukia, N.R.; Rasmi, Y.; Abbasi, A.; Koshoridze, N.; Shirpoor, A.; Burjanadze, G.; Saboory, E. Bio-Effects of TiO2 Nanoparticles on Human Colorectal Cancer and Umbilical Vein Endothelial Cell Lines. Asian Pac. J. Cancer Prev. 2018, 19, 2821–2829. [Google Scholar] [CrossRef]
- Caglar, I.; Ates, S.M.; Yesil Duymus, Z. An In Vitro Evaluation of the Effect of Various Adhesives and Surface Treatments on Bond Strength of Resin Cement to Polyetheretherketone. J. Prosthodont. 2019, 28, e342–e349. [Google Scholar] [CrossRef]
- Brunner, A.J. 1—Investigating the Performance of Adhesively-Bonded Composite Joints: Standards, Test Protocols, and Experimental Design. In Fatigue and Fracture of Adhesively-Bonded Composite Joints; Vassilopoulos, A.P., Ed.; Woodhead Publishing: Oxford, UK, 2015; pp. 3–42. ISBN 978-0-85709-806-1. [Google Scholar]
- Ji, S.; Yu, H.; Zhao, J.; Liang, F. Comparison of Mechanical Property and Machinability for Polyetheretherketone and Glass Fiber-Reinforced Polyetheretherketone. Adv. Mech. Eng. 2015, 7, 1687814015578357. [Google Scholar] [CrossRef]
- Tourani, H.; Molazemhosseini, A.; Khavandi, A.; Mirdamadi, S.; Shokrgozar, M.A.; Mehrjoo, M. Effects of Fibers and Nanoparticles Reinforcements on the Mechanical and Biological Properties of Hybrid Composite Polyetheretherketone/Short Carbon Fiber/Nano-SiO2. Polym. Compos. 2013, 34, 1961–1969. [Google Scholar] [CrossRef]
- Li, S.; Wang, T.; Hu, J.; Wang, B.; Li, Y.; Wang, L.; Li, G.; Zhang, J.; Zhou, Z. Effect of Hydroxyapatite Content and Particle Size on the Mechanical Behaviors and Osteogenesis In Vitro of Polyetheretherketone–Hydroxyapatite Composite. Polym. Compos. 2021, 42, 6512–6522. [Google Scholar] [CrossRef]
- Zheng, J.; Zhao, H.; Dong, E.; Kang, J.; Liu, C.; Sun, C.; Li, D.; Wang, L. Additively-Manufactured PEEK/HA Porous Scaffolds with Highly-Controllable Mechanical Properties and Excellent Biocompatibility. Mater. Sci. Eng. C Mater. Biol. Appl. 2021, 128, 112333. [Google Scholar] [CrossRef] [PubMed]
- Micovic Soldatovic, D.; Liebermann, A.; Huth, K.C.; Stawarczyk, B. Fracture Load of Different Veneered and Implant-Supported 4-UNIT Cantilever PEEK Fixed Dental Prostheses. J. Mech. Behav. Biomed. Mater. 2022, 129, 105173. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Li, Z.; Tian, L.; Li, D.; Lu, B.; Shi, C.; Niu, Q.; Liu, F.; Kong, L.; Zhang, J. Clinical Application of 3D-Printed PEEK Implants for Repairing Mandibular Defects. J. Cranio-Maxillofac. Surg. 2022, 50, 621–626. [Google Scholar] [CrossRef]
- Lümkemann, N.; Eichberger, M.; Stawarczyk, B. Different PEEK Qualities Irradiated with Light of Different Wavelengths: Impact on Martens Hardness. Dent. Mater. 2017, 33, 968–975. [Google Scholar] [CrossRef]
- Al Jabbari, Y.S. Physico-Mechanical Properties and Prosthodontic Applications of Co-Cr Dental Alloys: A Review of the Literature. J. Adv. Prosthodont. 2014, 6, 138–145. [Google Scholar] [CrossRef]
- El Magri, A.; El Mabrouk, K.; Vaudreuil, S.; Chibane, H.; Touhami, M.E. Optimization of Printing Parameters for Improvement of Mechanical and Thermal Performances of 3D Printed Poly(Ether Ether Ketone) Parts. J. Appl. Polym. Sci. 2020, 137, 49087. [Google Scholar] [CrossRef]
- Chen, P.; Wang, H.; Su, J.; Tian, Y.; Wen, S.; Su, B.; Yang, C.; Chen, B.; Zhou, K.; Yan, C.; et al. Recent Advances on High-Performance Polyaryletherketone Materials for Additive Manufacturing. Adv. Mater. 2022, 34, 2200750. [Google Scholar] [CrossRef]
- Abu Bakar, M.S.; Cheng, M.H.W.; Tang, S.M.; Yu, S.C.; Liao, K.; Tan, C.T.; Khor, K.A.; Cheang, P. Tensile Properties, Tension–Tension Fatigue and Biological Response of Polyetheretherketone–Hydroxyapatite Composites for Load-Bearing Orthopedic Implants. Biomaterials 2003, 24, 2245–2250. [Google Scholar] [CrossRef]
- Han, X.; Gao, W.; Zhou, Z.; Yang, S.; Wang, J.; Shi, R.; Li, Y.; Jiao, J.; Qi, Y.; Zhao, J. Application of Biomolecules Modification Strategies on PEEK and Its Composites for Osteogenesis and Antibacterial Properties. Colloids Surf. B Biointerfaces 2022, 215, 112492. [Google Scholar] [CrossRef]
- Zhang, H.; Shan, J.; Zhang, P.; Chen, X.; Jiang, H. Trabeculae Microstructure Parameters Serve as Effective Predictors for Marginal Bone Loss of Dental Implant in the Mandible. Sci. Rep. 2020, 10, 18437. [Google Scholar] [CrossRef]
- Ouldyerou, A.; Merdji, A.; Aminallah, L.; Roy, S.; Mehboob, H.; Özcan, M. Biomechanical Performance of Ti-PEEK Dental Implants in Bone: An in-Silico Analysis. J. Mech. Behav. Biomed. Mater. 2022, 134, 105422. [Google Scholar] [CrossRef]
- Najeeb, S.; BDS, Z.K.; BDS, S.Z.; BDS, M.S.Z. Bioactivity and Osseointegration of PEEK Are Inferior to Those of Titanium: A Systematic Review. J. Oral Implantol. 2016, 42, 512–516. [Google Scholar] [CrossRef]
- Torstrick, F.B.; Lin, A.S.P.; Potter, D.; Safranski, D.L.; Sulchek, T.A.; Gall, K.; Guldberg, R.E. Porous PEEK Improves the Bone-Implant Interface Compared to Plasma-Sprayed Titanium Coating on PEEK. Biomaterials 2018, 185, 106–116. [Google Scholar] [CrossRef]
- Wang, L.; He, S.; Wu, X.; Liang, S.; Mu, Z.; Wei, J.; Deng, F.; Deng, Y.; Wei, S. Polyetheretherketone/Nano-Fluorohydroxyapatite Composite with Antimicrobial Activity and Osseointegration Properties. Biomaterials 2014, 35, 6758–6775. [Google Scholar] [CrossRef]
- Wu, X.; Liu, X.; Wei, J.; Ma, J.; Deng, F.; Wei, S. Nano-TiO2/PEEK Bioactive Composite as a Bone Substitute Material: In Vitro and In Vivo Studies. Int. J. Nanomed. 2012, 7, 1215–1225. [Google Scholar] [CrossRef]
- Agustín-Panadero, R.; Serra-Pastor, B.; Roig-Vanaclocha, A.; Román-Rodriguez, J.-L.; Fons-Font, A. Mechanical Behavior of Provisional Implant Prosthetic Abutments. Med. Oral Patol. Oral Cir. Bucal. 2015, 20, e94–e102. [Google Scholar] [CrossRef]
- Borie, M.; Lambert, F.; Lecloux, G.; Bosshardt, D.; Barrantes, A.; Haugen, H.J.; Bacevic, M. Peri-Implant Soft Tissue Integration in Humans—Influence of Materials: A Study Protocol for a Randomised Controlled Trial and a Pilot Study Results. Contemp. Clin. Trials Commun. 2020, 19, 100643. [Google Scholar] [CrossRef]
- Bezerra, F.J.B.; Araujo, F.M.; de Oliveira, G.J.P.L.; Ghiraldini, B. Clinical Application of the Customizable PEEK Healing Abutment. A Case Report. J. Multidiscip. Dent. 2020, 10, 93–96. [Google Scholar] [CrossRef]
- Chokaree, P.; Poovarodom, P.; Chaijareenont, P.; Yavirach, A.; Rungsiyakull, P. Biomaterials and Clinical Applications of Customized Healing Abutment—A Narrative Review. J. Funct. Biomater. 2022, 13, 291. [Google Scholar] [CrossRef] [PubMed]
- Taha, D.; Cesar, P.F.; Sabet, A. Influence of Different Combinations of CAD-CAM Crown and Customized Abutment Materials on the Force Absorption Capacity in Implant Supported Restorations—In Vitro Study. Dent. Mater. 2022, 38, e10–e18. [Google Scholar] [CrossRef] [PubMed]
- Kaleli, N.; Sarac, D.; Külünk, S.; Öztürk, Ö. Effect of Different Restorative Crown and Customized Abutment Materials on Stress Distribution in Single Implants and Peripheral Bone: A Three-Dimensional Finite Element Analysis Study. J. Prosthet. Dent. 2018, 119, 437–445. [Google Scholar] [CrossRef] [PubMed]
- Mourya, A.; Nahar, R.; Mishra, S.K.; Chowdhary, R. Stress Distribution around Different Abutments on Titanium and CFR-PEEK Implant with Different Prosthetic Crowns under Parafunctional Loading: A 3D FEA Study. J. Oral Biol. Craniofacial Res. 2021, 11, 313–320. [Google Scholar] [CrossRef] [PubMed]
- Roy, M.; Kubacki, J.; Psiuk, B.; Mrozek-Wilczkiewicz, A.; Malarz, K.; Corti, A.; Pompella, A.; Szade, J. Photofunctionalization Effect and Biological Ageing of PEEK, TiO2 and ZrO2 Abutments Material. Mater. Sci. Eng. C Mater. Biol. Appl. 2021, 121, 111823. [Google Scholar] [CrossRef]
- Ghazal-Maghras, R.; Vilaplana-Vivo, J.; Camacho-Alonso, F.; Martínez-Beneyto, Y. Properties of Polyetheretheretherketone (PEEK) Implant Abutments: A Systematic Review. J. Clin. Exp. Dent. 2022, 14, e349–e358. [Google Scholar] [CrossRef]
- Rozeik, A.S.; Chaar, M.S.; Sindt, S.; Wille, S.; Selhuber-Unkel, C.; Kern, M.; El-Kholy, S.; Dörfer, C.; Fawzy El-Sayed, K.M. Cellular Properties of Human Gingival Fibroblasts on Novel and Conventional Implant-Abutment Materials. Dent. Mater. 2022, 38, 540–548. [Google Scholar] [CrossRef]
- Kimura, H.; Morita, K.; Nishio, F.; Abekura, H.; Tsuga, K. Clinical Report of Six-Month Follow-up after Cementing PEEK Crown on Molars. Sci. Rep. 2022, 12, 19070. [Google Scholar] [CrossRef]
- Rueggeberg, F.A. State-of-the-Art: Dental Photocuring--a Review. Dent. Mater. 2011, 27, 39–52. [Google Scholar] [CrossRef]
- Zafar, M.S. Prosthodontic Applications of Polymethyl Methacrylate (PMMA): An Update. Polymers 2020, 12, 2299. [Google Scholar] [CrossRef]
- Liebermann, A.; Wimmer, T.; Schmidlin, P.R.; Scherer, H.; Löffler, P.; Roos, M.; Stawarczyk, B. Physicomechanical Characterization of Polyetheretherketone and Current Esthetic Dental CAD/CAM Polymers after Aging in Different Storage Media. J. Prosthet. Dent. 2016, 115, 321–328. [Google Scholar] [CrossRef]
- Benli, M.; Eker-Gümüş, B.; Kahraman, Y.; Huck, O.; Özcan, M. Can Polylactic Acid Be a CAD/CAM Material for Provisional Crown Restorations in Terms of Fit and Fracture Strength? Dent. Mater. J. 2021, 40, 772–780. [Google Scholar] [CrossRef]
- Shetty, S.K.; Hasan, M.S.; Zahid, M.; Suhaim, K.S.; Mohammad, F.; Fayaz, T. Evaluation of Fracture Resistance and Color Stability of Crowns Obtained by Layering Composite over Zirconia and Polyetheretherketone Copings before and after Thermocycling to Simulate Oral Environment: An In Vitro Study. J. Pharm. Bioallied Sci. 2020, 12, 523. [Google Scholar] [CrossRef]
- Tasopoulos, T.; Pachiou, A.; Kouveliotis, G.; Karaiskou, G.; Ottenga, M.; Zoidis, P. An 8-Year Clinical Outcome of Posterior Inlay Retained Resin Bonded Fixed Dental Prosthesis Utilizing High Performance Polymer Materials: A Clinical Report. J. Prosthodont. 2021, 30, 19–23. [Google Scholar] [CrossRef]
- Zoidis, P. The Use of Modified Polyetheretherketone Post and Core for an Esthetic Lithium Disilicate Anterior Ceramic Restoration: A Clinical Report. Int. J. Prosthodont. 2021, 34, 120–125. [Google Scholar] [CrossRef]
- Hallak, A.G.; Caldas, R.A.; Silva, I.D.; Miranda, M.E.; Brandt, W.C.; Vitti, R.P. Stress Distribution in Restorations with Glass Fiber and Polyetheretherketone Intraradicular Posts: An In Silico Analysis. Dent. Mater. J. 2022, 41, 376–381. [Google Scholar] [CrossRef]
- Kubo, M.; Komada, W.; Otake, S.; Inagaki, T.; Omori, S.; Miura, H. The Effect of Glass Fiber Posts and Ribbons on the Fracture Strength of Teeth with Flared Root Canals Restored Using Composite Resin Post and Cores. J. Prosthodont. Res. 2018, 62, 97–103. [Google Scholar] [CrossRef]
- Guo, F.; Huang, S.; Liu, N.; Hu, M.; Shi, C.; Li, D.; Liu, C. Evaluation of the Mechanical Properties and Fit of 3D-Printed Polyetheretherketone Removable Partial Dentures. Dent. Mater. J. 2022, 41, 816–823. [Google Scholar] [CrossRef]
- Priester, M.; Müller, W.-D.; Beuer, F.; Schmidt, F.; Schwitalla, A.D. Performance of PEEK Based Telescopic Crowns, a Comparative Study. Dent. Mater. 2021, 37, 1667–1675. [Google Scholar] [CrossRef]
- Muhsin, S.A.; Hatton, P.V.; Johnson, A.; Sereno, N.; Wood, D.J. Determination of Polyetheretherketone (PEEK) Mechanical Properties as a Denture Material. Saudi Dent. J. 2019, 31, 382–391. [Google Scholar] [CrossRef]
- Kim, J.J. Revisiting the Removable Partial Denture. Dent. Clin. N. Am. 2019, 63, 263–278. [Google Scholar] [CrossRef] [PubMed]
- El-Baz, R.; Fayad, M.; Abas, M.; Shoieb, A.; Gad, M.; Helal, M.A. Comparative Study of Some Mechanical Properties of Cobalt Chromium and Polyether Ether Ketone Thermoplastic Removable Partial Denture Clasps: An In-Vitro Study. Braz. Dent. Sci. 2020, 23, 6. [Google Scholar] [CrossRef]
- Zheng, J.; Aarts, J.M.; Ma, S.; Waddell, J.N.; Choi, J.J.E. Fatigue Behavior of Removable Partial Denture Cast and Laser-Sintered Cobalt-Chromium (CoCr) and Polyetheretherketone (PEEK) Clasp Materials. Clin. Exp. Dent. Res. 2022, 8, 1496–1504. [Google Scholar] [CrossRef] [PubMed]
- Peng, T.-Y.; Ogawa, Y.; Akebono, H.; Iwaguro, S.; Sugeta, A.; Shimoe, S. Finite-Element Analysis and Optimization of the Mechanical Properties of Polyetheretherketone (PEEK) Clasps for Removable Partial Dentures. J. Prosthodont. Res. 2020, 64, 250–256. [Google Scholar] [CrossRef] [PubMed]
- Torii, M.; Nakata, T.; Takahashi, K.; Kawamura, N.; Shimpo, H.; Ohkubo, C. Fitness and Retentive Force of Cobalt-Chromium Alloy Clasps Fabricated with Repeated Laser Sintering and Milling. J. Prosthodont. Res. 2018, 62, 342–346. [Google Scholar] [CrossRef]
- Tribst, J.P.M.; de Oliveira Dal Piva, A.M.; Borges, A.L.S.; Araújo, R.M.; da Silva, J.M.F.; Bottino, M.A.; Kleverlaan, C.J.; de Jager, N. Effect of Different Materials and Undercut on the Removal Force and Stress Distribution in Circumferential Clasps during Direct Retainer Action in Removable Partial Dentures. Dent. Mater. 2020, 36, 179–186. [Google Scholar] [CrossRef]
- Zhang, N.; Mao, B.; Chen, S.; Chen, X.; Yan, P.; Yu, H. Optimization of the Dimension of Computer Numerical Control-Milled Polyetheretherketone Clasps: An in Vitro Evaluation of Accuracy. J. Prosthet. Dent. 2022, in press. [Google Scholar] [CrossRef]
- Ma, H.; Suonan, A.; Zhou, J.; Yuan, Q.; Liu, L.; Zhao, X.; Lou, X.; Yang, C.; Li, D.; Zhang, Y. PEEK (Polyether-Ether-Ketone) and Its Composite Materials in Orthopedic Implantation. Arab. J. Chem. 2021, 14, 102977. [Google Scholar] [CrossRef]
- Scholes, S.C.; Unsworth, A. The Wear Properties of CFR-PEEK-OPTIMA Articulating against Ceramic Assessed on a Multidirectional Pin-on-Plate Machine. Proc. Inst. Mech. Eng. Part H 2007, 221, 281–289. [Google Scholar] [CrossRef]
- Guo, Y.; Chen, C.; Zhang, S.; Ren, L.; Zhao, Y.; Guo, W. Mediation of Mechanically Adapted TiCu/TiCuN/CFR-PEEK Implants in Vascular Regeneration to Promote Bone Repair In Vitro and In Vivo. J. Orthop. Transl. 2022, 33, 107–119. [Google Scholar] [CrossRef]
- Wang, P.; Zou, B.; Ding, S.; Li, L.; Huang, C. Effects of FDM-3D Printing Parameters on Mechanical Properties and Microstructure of CF/PEEK and GF/PEEK. Chin. J. Aeronaut. 2021, 34, 236–246. [Google Scholar] [CrossRef]
- Wang, P.; Zou, B.; Xiao, H.; Ding, S.; Huang, C. Effects of Printing Parameters of Fused Deposition Modeling on Mechanical Properties, Surface Quality, and Microstructure of PEEK. J. Mater. Process. Technol. 2019, 271, 62–74. [Google Scholar] [CrossRef]
- Blanco, I. A Brief Review of the Applications of Selected Thermal Analysis Methods to 3D Printing. Thermo 2022, 2, 74–83. [Google Scholar] [CrossRef]
- Dua, R.; Rashad, Z.; Spears, J.; Dunn, G.; Maxwell, M. Applications of 3D-Printed PEEK via Fused Filament Fabrication: A Systematic Review. Polymers 2021, 13, 4046. [Google Scholar] [CrossRef]
- Rodzeń, K.; Harkin-Jones, E.; Wegrzyn, M.; Sharma, P.K.; Zhigunov, A. Improvement of the Layer-Layer Adhesion in FFF 3D Printed PEEK/Carbon Fibre Composites. Compos. Part A: Appl. Sci. Manuf. 2021, 149, 106532. [Google Scholar] [CrossRef]
- Ai, J.-R.; Li, S.; Vogt, B.D. Increased Strength in Carbon-Poly(Ether Ether Ketone) Composites from Material Extrusion with Rapid Microwave Post Processing. Addit. Manuf. 2022, 60, 103209. [Google Scholar] [CrossRef]
- Gudadhe, A.; Bachhar, N.; Kumar, A.; Andrade, P.; Kumaraswamy, G. Three-Dimensional Printing with Waste High-Density Polyethylene. ACS Appl. Polym. Mater. 2019, 1, 3157–3164. [Google Scholar] [CrossRef]
- Das, A.; Marnot, A.E.C.; Fallon, J.J.; Martin, S.M.; Joseph, E.G.; Bortner, M.J. Material Extrusion-Based Additive Manufacturing with Blends of Polypropylene and Hydrocarbon Resins. ACS Appl. Polym. Mater. 2020, 2, 911–921. [Google Scholar] [CrossRef]
- Golbang, A.; Harkin-Jones, E.; Wegrzyn, M.; Campbell, G.; Archer, E.; McIlhagger, A. Production and Characterization of PEEK/IF-WS2 Nanocomposites for Additive Manufacturing: Simultaneous Improvement in Processing Characteristics and Material Properties. Addit. Manuf. 2020, 31, 100920. [Google Scholar] [CrossRef]
- Munir, K.S.; Wen, C.; Li, Y. Carbon Nanotubes and Graphene as Nanoreinforcements in Metallic Biomaterials: A Review. Adv. Biosyst. 2019, 3, 1800212. [Google Scholar] [CrossRef]
- Qin, W.; Li, Y.; Ma, J.; Liang, Q.; Cui, X.; Jia, H.; Tang, B. Osseointegration and Biosafety of Graphene Oxide Wrapped Porous CF/PEEK Composites as Implantable Materials: The Role of Surface Structure and Chemistry. Dent. Mater. 2020, 36, 1289–1302. [Google Scholar] [CrossRef] [PubMed]
- Jovanović, M.; Živić, M.; Milosavljević, M. A Potential Application of Materials Based on a Polymer and CAD/CAM Composite Resins in Prosthetic Dentistry. J. Prosthodont. Res. 2021, 65, 137–147. [Google Scholar] [CrossRef] [PubMed]
- Chen, S.-G.; Yang, J.; Jia, Y.-G.; Lu, B.; Ren, L. TiO2 and PEEK Reinforced 3D Printing PMMA Composite Resin for Dental Denture Base Applications. Nanomaterials 2019, 9, 1049. [Google Scholar] [CrossRef] [PubMed]
- Nabiyouni, M.; Ren, Y.; Bhaduri, S.B. Magnesium Substitution in the Structure of Orthopedic Nanoparticles: A Comparison between Amorphous Magnesium Phosphates, Calcium Magnesium Phosphates, and Hydroxyapatites. Mater. Sci. Eng. C 2015, 52, 11–17. [Google Scholar] [CrossRef] [PubMed]
- Sikder, P.; Ferreira, J.A.; Fakhrabadi, E.A.; Kantorski, K.Z.; Liberatore, M.W.; Bottino, M.C.; Bhaduri, S.B. Bioactive Amorphous Magnesium Phosphate-Polyetheretherketone Composite Filaments for 3D Printing. Dent. Mater. 2020, 36, 865–883. [Google Scholar] [CrossRef]
- Zimmermann, M.; Ender, A.; Attin, T.; Mehl, A. Fracture Load of Three-Unit Full-Contour Fixed Dental Prostheses Fabricated with Subtractive and Additive CAD/CAM Technology. Clin. Oral Investig. 2020, 24, 1035–1042. [Google Scholar] [CrossRef]
- Nishiyama, H.; Taniguchi, A.; Tanaka, S.; Baba, K. Novel Fully Digital Workflow for Removable Partial Denture Fabrication. J. Prosthodont. Res. 2020, 64, 98–103. [Google Scholar] [CrossRef]
- Matthews, M.J.; Guss, G.; Drachenberg, D.R.; Demuth, J.A.; Heebner, J.E.; Duoss, E.B.; Kuntz, J.D.; Spadaccini, C.M. Diode-Based Additive Manufacturing of Metals Using an Optically-Addressable Light Valve. Opt. Express 2017, 25, 11788–11800. [Google Scholar] [CrossRef]
- Zhu, S.; Yan, T.; Huang, X.; Hassan, E.A.M.; Zhou, J.; Zhang, S.; Xiong, M.; Yu, M.; Li, Z. Bioinspired Nacre-like PEEK Material with Superior Tensile Strength and Impact Toughness. RSC Adv. 2022, 12, 15584–15592. [Google Scholar] [CrossRef]
- Li, T.; Song, Z.; Yang, X.; Du, J. Influence of Processing Parameters on the Mechanical Properties of Peek Plates by Hot Compression Molding. Materials 2023, 16, 36. [Google Scholar] [CrossRef]
- Prechtel, A.; Reymus, M.; Edelhoff, D.; Hickel, R.; Stawarczyk, B. Comparison of Various 3D Printed and Milled PAEK Materials: Effect of Printing Direction and Artificial Aging on Martens Parameters. Dent. Mater. 2020, 36, 197–209. [Google Scholar] [CrossRef]
- Sharma, N.; Aghlmandi, S.; Dalcanale, F.; Seiler, D.; Zeilhofer, H.-F.; Honigmann, P.; Thieringer, F.M. Quantitative Assessment of Point-of-Care 3D-Printed Patient-Specific Polyetheretherketone (PEEK) Cranial Implants. Int. J. Mol. Sci. 2021, 22, 8521. [Google Scholar] [CrossRef]
- Hopkinson, N.; Smith, P.J. 3 D Printing/Additive Manufacturing. In Inkjet Printing in Industry; John Wiley & Sons, Ltd.: Hoboken, NJ, USA, 2022; pp. 1351–1364. ISBN 978-3-527-82807-4. [Google Scholar]
- Timoumi, M.; Barhoumi, N.; Znaidi, A.; Maazouz, A.; Lamnawar, K. Mechanical Behavior of 3D-Printed PEEK and Its Application for Personalized Orbital Implants with Various Infill Patterns and Densities. J. Mech. Behav. Biomed. Mater. 2022, 136, 105534. [Google Scholar] [CrossRef]
- Das, A.; Chatham, C.A.; Fallon, J.J.; Zawaski, C.E.; Gilmer, E.L.; Williams, C.B.; Bortner, M.J. Current Understanding and Challenges in High Temperature Additive Manufacturing of Engineering Thermoplastic Polymers. Addit. Manuf. 2020, 34, 101218. [Google Scholar] [CrossRef]
- Tan, L.J.; Zhu, W.; Zhou, K. Recent Progress on Polymer Materials for Additive Manufacturing. Adv. Funct. Mater. 2020, 30, 2003062. [Google Scholar] [CrossRef]
- Chen, P.; Cai, H.; Li, Z.; Li, M.; Wu, H.; Su, J.; Wen, S.; Zhou, Y.; Liu, J.; Wang, C.; et al. Crystallization Kinetics of Polyetheretherketone during High Temperature-Selective Laser Sintering. Addit. Manuf. 2020, 36, 101615. [Google Scholar] [CrossRef]
- Yan, M.; Tian, X.; Yao, R. Processability and Reusability of CF/PEEK Mixture for Powder Bed Fusion of High Strength Composites. Compos. Commun. 2022, 35, 101318. [Google Scholar] [CrossRef]
- Brenken, B.; Barocio, E.; Favaloro, A.; Kunc, V.; Pipes, R.B. Fused Filament Fabrication of Fiber-Reinforced Polymers: A Review. Addit. Manuf. 2018, 21, 1–16. [Google Scholar] [CrossRef]
- Ding, S.; Zou, B.; Wang, P.; Ding, H. Effects of Nozzle Temperature and Building Orientation on Mechanical Properties and Microstructure of PEEK and PEI Printed by 3D-FDM. Polym. Test. 2019, 78, 105948. [Google Scholar] [CrossRef]
- Yi, N.; Davies, R.; Chaplin, A.; McCutchion, P.; Ghita, O. Slow and Fast Crystallising Poly Aryl Ether Ketones (PAEKs) in 3D Printing: Crystallisation Kinetics, Morphology, and Mechanical Properties. Addit. Manuf. 2021, 39, 101843. [Google Scholar] [CrossRef]
- Vindokurov, I.; Pirogova, Y.; Tashkinov, M.; Silberschmidt, V.V. Effect of Heat Treatment on Elastic Properties and Fracture Toughness of Fused Filament Fabricated PEEK for Biomedical Applications. Polymers 2022, 14, 5521. [Google Scholar] [CrossRef] [PubMed]
- Steffen, C.; Sellenschloh, K.; Vollmer, M.; Morlock, M.M.; Heiland, M.; Huber, G.; Rendenbach, C. Biomechanical Comparison of Titanium Miniplates versus a Variety of CAD/CAM Plates in Mandibular Reconstruction. J. Mech. Behav. Biomed. Mater. 2020, 111, 104007. [Google Scholar] [CrossRef] [PubMed]
- Avci, T.; Omezli, M.M.; Torul, D. Investigation of the Biomechanical Stability of Cfr-PEEK in the Treatment of Mandibular Angulus Fractures by Finite Element Analysis. J. Stomatol. Oral Maxillofac. Surg. 2022, 123, 610–615. [Google Scholar] [CrossRef] [PubMed]
- Zhang, L.; Yang, G.; Johnson, B.N.; Jia, X. Three-Dimensional (3D) Printed Scaffold and Material Selection for Bone Repair. Acta Biomater. 2019, 84, 16–33. [Google Scholar] [CrossRef] [PubMed]
- Najeeb, S.; Zafar, M.S.; Khurshid, Z.; Siddiqui, F. Applications of Polyetheretherketone (PEEK) in Oral Implantology and Prosthodontics. J. Prosthodont. Res. 2016, 60, 12–19. [Google Scholar] [CrossRef]
- Tada, Y.; Hayakawa, T.; Nakamura, Y. Load-Deflection and Friction Properties of PEEK Wires as Alternative Orthodontic Wires. Materials 2017, 10, 914. [Google Scholar] [CrossRef]
- Nai, T.A.P.; Aydin, B.; Brand, H.S.; Jonkman, R.E.G. Present and Theoretical Applications of Poly-Ether-Ether-Ketone (PEEK) in Orthodontics: A Scoping Review. Materials 2022, 15, 7414. [Google Scholar] [CrossRef]
- Kadhum, A.S.; Alhuwaizi, A.F. The Efficacy of Polyether-Ether-Ketone Wire as a Retainer Following Orthodontic Treatment. Clin. Exp. Dent. Res. 2021, 7, 302–312. [Google Scholar] [CrossRef]
- Yuan, Z.; Long, T.; Zhang, J.; Lyu, Z.; Zhang, W.; Meng, X.; Qi, J.; Wang, Y. 3D Printed Porous Sulfonated Polyetheretherketone Scaffold for Cartilage Repair: Potential and Limitation. J. Orthop. Transl. 2022, 33, 90–106. [Google Scholar] [CrossRef]
- Salah, M.; Tayebi, L.; Moharamzadeh, K.; Naini, F.B. Three-Dimensional Bio-Printing and Bone Tissue Engineering: Technical Innovations and Potential Applications in Maxillofacial Reconstructive Surgery. Maxillofac. Plast. Reconstr. Surg. 2020, 42, 18. [Google Scholar] [CrossRef]
- Rocha, R.F.V.; Anami, L.C.; Campos, T.M.B.; de Melo, R.M.; Souza, R.O. de A. e; Bottino, M.A. Bonding of the Polymer Polyetheretherketone (PEEK) to Human Dentin: Effect of Surface Treatments. Braz. Dent. J. 2016, 27, 693–699. [Google Scholar] [CrossRef]
- Henriques, B.; Fabris, D.; Mesquita-Guimarães, J.; Sousa, A.C.; Hammes, N.; Souza, J.C.M.; Silva, F.S.; Fredel, M.C. Influence of Laser Structuring of PEEK, PEEK-GF30 and PEEK-CF30 Surfaces on the Shear Bond Strength to a Resin Cement. J. Mech. Behav. Biomed. Mater. 2018, 84, 225–234. [Google Scholar] [CrossRef]
- Abdulfattah, N.; Schmidt, F.; Wang, Y.; Bötticher, N.; Konzack, N.; Giuliano, M.; Müller, W.-D.; Schwitalla, A.D. Ultrasonic Welding of Polyetheretherketone for Dental Applications. J. Mech. Behav. Biomed. Mater. 2022, 130, 105225. [Google Scholar] [CrossRef]
- Schwitalla, A.D.; Bötel, F.; Zimmermann, T.; Sütel, M.; Müller, W.-D. The Impact of Argon/Oxygen Low-Pressure Plasma on Shear Bond Strength between a Veneering Composite and Different PEEK Materials. Dent. Mater. 2017, 33, 990–994. [Google Scholar] [CrossRef]
- Binhasan, M.; Alhamdan, M.M.; Al-Aali, K.A.; Vohra, F.; Abduljabbar, T. Shear Bond Characteristics and Surface Roughness of Poly-Ether-Ether-Ketone Treated with Contemporary Surface Treatment Regimes Bonded to Composite Resin. Photodiagnosis Photodyn. Ther. 2022, 38, 102765. [Google Scholar] [CrossRef]
- Shabib, S. Use of Nd:YVO4 Laser, Photodynamic Therapy, Sulfuric Acid and Sand Blasting on Improving Bond Integrity of PEEK to Resin Cement with Adhesive. Photodiagnosis Photodyn. Ther. 2022, 39, 102865. [Google Scholar] [CrossRef]
- Escobar, M.; Souza, J.C.M.; Barra, G.M.O.; Fredel, M.C.; Özcan, M.; Henriques, B. On the Synergistic Effect of Sulfonic Functionalization and Acidic Adhesive Conditioning to Enhance the Adhesion of PEEK to Resin-Matrix Composites. Dent. Mater. 2021, 37, 741–754. [Google Scholar] [CrossRef]
- Adem, N.; Bal, B.; Kazazoglu, E. Comparative Study of Chemical and Mechanical Surface Treatment Effects on Shear Bond Strength of PEEK to Veneering Ceramic. Int. J. Prosthodont. 2020, 35, 201–207. [Google Scholar] [CrossRef]
- Bötel, F.; Zimmermann, T.; Sütel, M.; Müller, W.-D.; Schwitalla, A.D. Influence of Different Low-Pressure Plasma Process Parameters on Shear Bond Strength between Veneering Composites and PEEK Materials. Dent. Mater. 2018, 34, e246–e254. [Google Scholar] [CrossRef]
- Fu, Q.; Gabriel, M.; Schmidt, F.; Müller, W.-D.; Schwitalla, A.D. The Impact of Different Low-Pressure Plasma Types on the Physical, Chemical and Biological Surface Properties of PEEK. Dent. Mater. 2021, 37, e15–e22. [Google Scholar] [CrossRef]
- Zhang, J.; Yi, Y.; Wang, C.; Ding, L.; Wang, R.; Wu, G. Effect of Acid-Etching Duration on the Adhesive Performance of Printed Polyetheretherketone to Veneering Resin. Polymers 2021, 13, 3509. [Google Scholar] [CrossRef] [PubMed]
- Chaijareenont, P.; Prakhamsai, S.; Silthampitag, P.; Takahashi, H.; Arksornnukit, M. Effects of Different Sulfuric Acid Etching Concentrations on PEEK Surface Bonding to Resin Composite. Dent. Mater. J. 2018, 37, 385–392. [Google Scholar] [CrossRef] [PubMed]
- Çulhaoğlu, A.K.; Özkır, S.E.; Şahin, V.; Yılmaz, B.; Kılıçarslan, M.A. Effect of Various Treatment Modalities on Surface Characteristics and Shear Bond Strengths of Polyetheretherketone-Based Core Materials. J. Prosthodont. 2020, 29, 136–141. [Google Scholar] [CrossRef]
- Taha, D.; Safwat, F.; Wahsh, M. Effect of Combining Different Surface Treatments on the Surface Characteristics of Polyetheretherketone-Based Core Materials and Shear Bond Strength to a Veneering Composite Resin. J. Prosthet. Dent. 2022, 127, 599.e1–599.e7. [Google Scholar] [CrossRef] [PubMed]
- Gupta, A.K.; Gupta, R.; Gill, S. Evaluation of the Failure Modes and Load-Bearing Capacity of Different Surface-Treated Polyether Ether Ketone Copings Veneered with Lithium Di-Silicate Compared to Polyether Ether Ketone Copings Veneered with Composite: An in Vitro Study. J. Indian Prosthodont. Soc. 2021, 21, 295–303. [Google Scholar] [CrossRef]
- Alkhudhairy, F.; Al-Johany, S.S.; Naseem, M.; Bin-Shuwaish, M.; Vohra, F. Dentin Bond Strength of Bioactive Cement in Comparison to Conventional Resin Cement When Photosensitized with Er,Cr:YSGG Laser. Pak. J. Med. Sci. 2019, 36, 85–90. [Google Scholar] [CrossRef]
- Parkar, U.; Dugal, R.; Madanshetty, P.; Devadiga, T.; Khan, A.S.; Godil, A. Assessment of Different Surface Treatments and Shear Bond Characteristics of Poly-Ether-Ether-Ketone: An In Vitro SEM Analysis. J. Indian Prosthodont. Soc. 2021, 21, 412–419. [Google Scholar] [CrossRef]
- Evaluation of Shear Bond Strength between PEEK and Resin-Based Luting Material. J. Oral Biosci. 2017, 59, 231–236. [CrossRef]
- Zhou, L.; Qian, Y.; Zhu, Y.; Liu, H.; Gan, K.; Guo, J. The Effect of Different Surface Treatments on the Bond Strength of PEEK Composite Materials. Dent. Mater. 2014, 30, e209–e215. [Google Scholar] [CrossRef]
- Chersoni, S.; Lorenzi, R.; Ferrieri, P.; Prati, C. Laboratory Evaluation of Compomers in Class V Restorations. Am. J. Dent. 1997, 10, 147–151. [Google Scholar]
- Benatar, A. Ultrasonic Welding of Plastics and Polymeric Composites. In Power Ultrasonics; Gallego-Juárez, J.A., Graff, K.F., Eds.; Woodhead Publishing: Oxford, UK, 2015; pp. 295–312. ISBN 978-1-78242-028-6. [Google Scholar]
- Tsuka, H.; Morita, K.; Kato, K.; Kimura, H.; Abekura, H.; Hirata, I.; Kato, K.; Tsuga, K. Effect of Laser Groove Treatment on Shear Bond Strength of Resin-Based Luting Agent to Polyetheretherketone (PEEK). J. Prosthodont. Res. 2019, 63, 52–57. [Google Scholar] [CrossRef]
- Khatri, B.; Roth, M.F.; Balle, F. Ultrasonic Welding of Additively Manufactured PEEK and Carbon-Fiber-Reinforced PEEK with Integrated Energy Directors. J. Manuf. Mater. Process. 2023, 7, 2. [Google Scholar] [CrossRef]
- Wei, X.; Zhou, W.; Tang, Z.; Wu, H.; Liu, Y.; Dong, H.; Wang, N.; Huang, H.; Bao, S.; Shi, L.; et al. Magnesium Surface-Activated 3D Printed Porous PEEK Scaffolds for In Vivo Osseointegration by Promoting Angiogenesis and Osteogenesis. Bioact. Mater. 2023, 20, 16–28. [Google Scholar] [CrossRef]


| Properties | Requirements | Brief Introduction | Test Methods | Ref. |
|---|---|---|---|---|
| Mechanical properties | Elastic modulus (i.e., Young’s modulus, stiffness; GPa) | The ability to resist elastic deformation. It is defined as the ratio of stress to strain and is determined from the initial slope of the stress–strain curve. | - Tensile test - Bending test - Compressive test | [7] |
| Tensile strength (MPa) | The ultimate strength of the material during tension. | Tensile test | [20] | |
| Elongation (%) | The ability to stretch plastically (i.e., ductility). | Tensile test | [21] | |
| Elongation at break (%) | The ratio between the length of a specimen that changes after fracture and the initial length. | Tensile test | [21] | |
| Bending strength (MPa) | The ultimate strength of the material during bending. | Bending test | [20] | |
| Compressive strength (MPa) | The ultimate strength of the material during compression. | Compressive test | [22] | |
| Shear strength (interlaminar and interfacial shear strength (ILSS, IFSS); MPa) | The resistance of the composite to delamination under shear forces parallel to the layers of the laminate, and thus, to the adhesive/adherent interface. | - Shear bonding strength test - Pull-off tensile test | [23,24] | |
| Yield strength (MPa) | The ability to withstand stress without plastic deformation (i.e., permanent deformation). | - Tensile test - Compressive test | [21] | |
| Toughness (KJ/m2) | The energy of elastic and plastic deformation required to break a material. It increases with strength and ductility. | Impact test | [23] | |
| Fatigue strength (Sn; MPa) | The maximum alternating stress that the material can withstand for a long time. | Load-cycle fatigue test | [25] | |
| Creep | Under the condition of a constant load below the yield strength, the strain increases with time. | Martens force/depth indentation test | [26] | |
| Hardness (indentation hardness, scratch hardness, rebound hardness; MPa) | The ability of a material surface to resist plastic deformation caused by indentation and localized cracking. Rebound hardness means the magnitude of the elastic deformation work of the material. | - Vickers hardness (HV) test - Martens hardness (HM) test - Surface scratch test - Back-jump test | [19] | |
| Biological properties | Biocompatibility | The ability of a material to generate an appropriate host response in a specific application. | Cell proliferation, cytotoxicity, and adhesion test | [27] |
| Osseointegration | A phenomenon where an implant becomes fused with bone. | Microcomputed tomography (μ-CT) | [28] | |
| Chemical properties | Corrosion resistance | The ability to resist damage caused under the action of the surrounding medium. | Potentiodynamic polarization (PDP) and static immersion assay | [29] |
| Aging resistance | The ability of polymers to resist deterioration. | Cycles of thermal aging | [26] | |
| Physical characteristics | Crystallinity | A specific type of ordered structure in a solid material. Those with little crystallinity are known as amorphous polymers. | Differential scanning calorimetry (DSC) and X-ray diffraction (XRD) | [21] |
| Microscopic features | Surface, cross-sectional, and fracture surface characteristics, such as roughness. | Scanning electron microscopy (SEM) | [26,30] | |
| Porosity | The ratio of the void volume to total volume. | Water displacement method | [30] | |
| Surface tension (N/m) | The ability to cause the surface of a liquid to shrink. | Water contact angle detection | [30] |
| Material Characteristics | Materials | Advantages | Disadvantages | Dental Application | Ref. |
|---|---|---|---|---|---|
| Metal-based materials | Titanium and its alloys (Ti-6Al-4V) | - Improved strength - Biocompatible | - High Young’s moduli - Poor wear resistance - Potentially toxic | - Dental implants and abutments - Inapplicable for orthopedic use (Ti-6Al-4V) | [33] |
| Cobalt-based alloys (cobalt-chromium-molybdenum/CoCr-Mo) | - Low rigidity - High yield and tensile strength - Superior wear resistance | - Unaesthetic appearance - Potentially toxic | - Ceramic abutments - Crowns - Clasps | [34] | |
| Ceramics | Alumina (Al2O3) (α-aluminum oxide) | - Biocompatible - Wear resistant | - Less compact - Lower flexural strength | - Dental implants - Endodontic posts - Orthodontic brackets | [35] |
| Zirconia (ZrO2) | - Highly biocompatible - Good osseointegration - Good aesthetics | High Young’s moduli | - Crowns - Implant abutments | [34] | |
| Lithium disilicate | Superior aesthetics and translucency | High Young’s moduli | Crowns | [36] | |
| Titanium dioxide (TiO2) nanoparticles | Semipermanent antibacterial agent | Resulting in cytotoxicity in a dose-dependent manner | Oral antibacterial disinfectants, whitening agents, and adhesives | [37,38] | |
| Polymers | PEEK | - Good mechanical properties - Good biocompatibility | - Poor surface properties - Poor aesthetic performance | - Dental abutments - Temporary crowns | [7] |
| PMMA | - Non-biodegradable and stable aesthetic - Good flexibility | Shrink during polymerization | - Denture base - Crowns | [39] | |
| Composites | Carbon-fiber-reinforced PEEK (CF/PEEK) | Higher mechanical strength and wear resistance than PEEK | Relatively weak interlaminar strength | -Fracture fixation -Posts and cores | [40] |
| Glass-fiber-reinforced PEEK (GF/PEEK) | Higher rigidity, hardness, and deformation resistance | Poor uniformity | Posts and cores | [41] | |
| PEEK /nano-silica (PEEK/nano-SiO2) | Higher elastic modulus | Decreased toughness | Crowns | [42] | |
| Hydroxyapatite (HA)/PEEK (HA/PEEK) | Increased compressive strength and modulus with the HA content | Decreased tensile/bending strength | Bone grafting and tissue engineering scaffolds | [43,44] | |
| TiO2-reinforced PEEK | - Higher fracture and aging resistance - Improved Martens hardness (HM) | Affected by radiation | Implant-supported, 4-unit cantilever FDP | [45] | |
| Polymer-infiltrated ceramic network (PICN) | Comparable fracture toughness and better damage tolerance than glass ceramics | Significantly lower wear resistance than that of tooth enamel | Dental restorations for bruxism patients | [34] |
| Materials | Density (g/cm3) | Martens Hardness (HM, N/mm2) | Tensile Strength (MPa) | Elastic Modulus (GPa) | Bending Strength (MPa) | Ref. |
|---|---|---|---|---|---|---|
| Cortical bone | 1.92 | 104–121 | 6–30 | 225 | [46] | |
| Dentin | 3.3 | 468.2 ± 30.77 | 104 | 12–18.6 | [47] | |
| Dental enamel | 2263.6 ± 405.16 | 47.5 | 40–83 | [47] | ||
| Titanium | 4.5 | 300–400 | 954–976 | 102–110 | [47] | |
| CoCr | 6.5 | 1200 | 680 | 205 | 800–1400 | [48] |
| PMMA | 1.18 | 180 | 48–76 | 3.6 | 95–105 | [47] |
| PEEK | 1.3 | 189.55 ± 16.89 | 87.53–100 | 3–4 | 99.25–170 | [1,22] |
| Annealed FDM-printed PEEK | 97.34 | 2.6–3.45 | 104.65 | [22,49] | ||
| PEEK/CF (carbon-fiber-reinforced PEEK) | 1.3 | 330.6 ± 21.2 | 6.9–109 | 3.5–58.5 | 264.6 | [50] |
| PEEK/GF (30% glass-fiber-reinforced PEEK) | 2.61 | 295.3 ± 12.5 | 94.0 ± 2.0 | 12.38 | 167 | [26] |
| Bio-PEAK (PEEK filled with 5% TiO2) | 1.4 | 4.2–4.8 | 190–210 | |||
| Dentokeep PEEK (PEEK filled with 20% TiO2) | 1.5 | 191.45 ± 15.49 | 83.1 | 4.24 | [47] | |
| breCAM.BioHPP (PEEK filled with 20–30% TiO2) | 1.3 | 197.35 ± 19.9 | 4.2 | 160 | [45] | |
| PEEK 450G (PEEK filled with 30% TiO2) | 1.3 | 142 ± 34.7 | 95.21 ± 1.86 | 5.05 | 163 | [50] |
| PEEK composite containing 20–30% HA | 1.28 | 49–59 | 5–7 | [51] |
| Application | Subcategory | Materials Tested | Outcomes | Ref. |
|---|---|---|---|---|
| Implant therapy | Implant | - Titanium implant - Ti-PEEK composite implant | Ti-PEEK composite implant was superior in reducing bone resorption (stress shielding). | [54] |
| - 2–4 mm HA particles in PEEK - Nanosized HA particles in PEEK | Implants made from PEEK nanocomposite sites have better mechanical properties. | [57] | ||
| Abutments | - PEEK - Grade 5 titanium | PEEK abutments are suitable for long-term provisional restorations in the anterior part with no functional impairment. | [31] | |
| - Custom PEEK healing abutment - Standard healing caps | The custom PEEK healing abutment created a natural gingival structure and required fewer steps to create an emergency contour. | [7,60] | ||
| - Zirconia/lithium silicate/resin-based ceramic/PEEK crowns - Zirconia/PEEK abutment | High-strength rigid zirconia and lithium disilicate ceramics benefit more from a favorable stress distribution when applied on PEEK abutments. | [63] | ||
| - Straight/15° angle/25° angle abutment - Porcelain metal (PFM)/PEEK restoration crowns | Molar patients can be given straight abutments combined with PEEK crowns to reduce intraosseous stress concentration. | [65] | ||
| Prosthodontic therapy | Crown | - Milled crowns (PEEK, PMMA, and silicate ceramic) - Crown–abutment material combinations | PMMA crowns showed the highest material loss and PEEK had the lowest material loss. | [24] |
| Implant-supported, 4-unit fixed restorations | - 20%/30% TiO2-filled PEEK - Veneered resin composite/digital veneering/prefabricated veneering | The highest fracture resistance of the restorations was achieved when 30% TiO2-filled PEEK material was used and prefabricated veneers were applied. | [24,45] | |
| Partial dentures | - PMMA - PEEK | PEEK provides a higher Young’s modulus but lower flexural deformation than PMMA, which may reduce the load applied to the underlying tissue. | [81] | |
| Clasp | - Shape-optimized PEEK clasp - Standard shape CoCr clasp | -The retention forces provided by the PEEK clasp were adequate for clinical use. There was no significant difference in long-term deformation between the two materials. | [85] | |
| Oral and maxillofacial surgery | Mandibular fracture fixation | - CFR-PEEK plate/screw system - Resorbable system | CFR-PEEK plate/screw system reduces the stress on the fixation system and provides more stable fixation. | [125] |
| Bone scaffolds | - PEEK - PAEK with carboxyl groups (PAEK-COOH) | PAEK-COOH controllable porous scaffolds had better mechanical strength and are beneficial for promoting cell adhesion. | [28] | |
| Other oral applications | Orthodontic wires | - PEEK - Polyether sulfone (PES) - Polyvinylidene fluoride (PVDF) | PEEK orthodontic wires are able to deliver higher orthodontic forces, but at a similar cross-section of that of metallic wires. | [127] |
| Cartilage recovery | - PEEK - Sulfonated PEEK (SPK) | SPK favors the secretion of anti-inflammatory cytokines and promotes the recovery of cartilage functions. | [131] |
| Material | Surface Treatment | Adhesive | Shear Bonding Strength (SBS; MPa) | Surface Roughness (Ra; μm) | Ref. |
|---|---|---|---|---|---|
| PEEK | Untreated | Ultrasonic welding (USW) | 16.37 ± 1.69 | [135] | |
| Untreated | Visio.link | 3.81 ± 2.71 | 0.69 ± 0.07 | [136] | |
| 98% H2SO4 etching for 60 s | Silane coupling agent | 19.25 ± 0.68 | 2.658 ± 0.658 | [137] | |
| 98% H2SO4 etching for 60 s | Visio.link | 15.23 ± 0.6 | 0.61 ± 0.14 | [138] | |
| 98% H2SO4 etching for 60 s | Ambar Universal Adhesive | 17.84 ± 2.8 | 1.05 ± 0.59 | [139] | |
| 98% H2SO4 etching for 60 s after Al2O3 sandblasting (50 μm, 2 MPa, 10 s) | Visio.link | 11.72 ± 1.69 | - | [121] | |
| Al2O3 sandblasting (50 μm, 2 MPa, 10 s) | Visio.link | 6.43 ± 1.05 | - | [140] | |
| Al2O3 sandblasting (110 μm, 0.1 MPa, 10 s) | Silane coupling agent | 14.55 ± 1.25 | 1.552 ± 0.002 | [137] | |
| Al2O3 sandblasting (110 μm, 0.1 MPa) | Visio.link | 10.71 ± 0.52 | 0.92 ± 0.12 | [138] | |
| Al2O3 sandblasting (110 μm, 2.5 MPa) | Visio.link | 18.29 ± 1.84 | 1.64 ± 0.48 | [136] | |
| Oxygen plasma treatment for 3 min | Visio.link | 21.65 ± 5.31 | 0.69 ± 0.22 | [141] | |
| Hydrogen–oxygen, 2/1-mixed plasma treatment | - | - | 0.43 ± 0.06 | [142] | |
| Argon and oxygen 1:1 process for plasma treatment | Visio.link | 3.76 ± 2.42 | 0.06 ± 0.07 | [136] | |
| Argon and oxygen 1:1 process for plasma treatment after sandblasting (110 μm, 2.5 MPa) | Visio.link | 19.8 ± 2.46 | 1.32 ± 0.39 | [136] | |
| Photodynamic therapy (PDT) | Silane coupling agent | 11.69 ± 0.12 | 1.254 ± 0.011 | [137] | |
| Photodynamic therapy (PDT) | Visio.link | 16.21 ± 0.14 | 14.25 ± 1.21 | [138] | |
| Neodymium-doped yttrium orthovanadate (Nd: YVO4) laser treatment | Visio.link | 16.33 ± 0.71 | 15.25 ± 1.58 | [138] | |
| 3D-printed PEEK | 98% H2SO4 etching for 30 s | Visio.link | 27.90 ± 3.48 | - | [143] |
| CAD/CAM-milled PEEK | 98% H2SO4 etching for 60 s | Visio.link | 27.36 | 0.74 ± 0.25 | [144] |
| 98% H2SO4 etching for 5–120 s | Visio.link | >29 | - | [143] | |
| Vestakeep DC4420 (PEEK filled with 20% TiO2) | Argon and oxygen 1:1 process for plasma treatment after sandblasting | Visio.link | 15.86 ± 4.39 | 1.19 ± 0.4 | [136] |
| Oxygen plasma treatment for 3 min | Visio.link | 30.95 ± 6.35 | 2.0 ± 0.97 | [141] | |
| DC 4450 (filled with 20% TiO2 powder and 1% pigment) | Argon and oxygen 1:1 process for plasma treatment after sandblasting | Visio.link | 9.06 ± 3.1 | 1.83 ± 0.17 | [136] |
| Oxygen plasma treatment for 3 min | Visio.link | 34.92 ± 6.55 | 0.93 ± 0.3 | [141] | |
| breCAM.BioHPP | Silica-modified sandblasting (30 μm, 0.3 MPa, 15 s) | Visio.link | 8.07 ± 2.54 | 0.42 ± 0.03 | [145] |
| Al2O3 sandblasting (110 μm, 0.2 MPa, 15 s) | Visio.link | 10.81 ± 3.06 | 2.26 ± 0.33 | [145] | |
| Al2O3 sandblasting (50 μm, 0.25 MPa, 15 s) | Bond.lign | 17.4 ± 2.4 | 2.1 ± 0.2 | [146] | |
| Oxygen plasma treatment after sandblasting (50 μm, 0.25 MPa, 15 s) | Bond.lign | 21.2 ± 0.8 | 2.7 ± 0.1 | [146] | |
| Erbium-doped yttrium aluminum garnet (ER: YAG) laser treatment after sandblasting (50 μm, 0.25 MPa, 15 s) | Bond.lign | 22.0 ± 1.3 | 2.9 ± 0.1 | [146] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Luo, C.; Liu, Y.; Peng, B.; Chen, M.; Liu, Z.; Li, Z.; Kuang, H.; Gong, B.; Li, Z.; Sun, H. PEEK for Oral Applications: Recent Advances in Mechanical and Adhesive Properties. Polymers 2023, 15, 386. https://doi.org/10.3390/polym15020386
Luo C, Liu Y, Peng B, Chen M, Liu Z, Li Z, Kuang H, Gong B, Li Z, Sun H. PEEK for Oral Applications: Recent Advances in Mechanical and Adhesive Properties. Polymers. 2023; 15(2):386. https://doi.org/10.3390/polym15020386
Chicago/Turabian StyleLuo, Chengfeng, Ying Liu, Bo Peng, Menghao Chen, Zhaogang Liu, Zhanglong Li, Hai Kuang, Baijuan Gong, Zhimin Li, and Hongchen Sun. 2023. "PEEK for Oral Applications: Recent Advances in Mechanical and Adhesive Properties" Polymers 15, no. 2: 386. https://doi.org/10.3390/polym15020386
APA StyleLuo, C., Liu, Y., Peng, B., Chen, M., Liu, Z., Li, Z., Kuang, H., Gong, B., Li, Z., & Sun, H. (2023). PEEK for Oral Applications: Recent Advances in Mechanical and Adhesive Properties. Polymers, 15(2), 386. https://doi.org/10.3390/polym15020386