Micro-/Nano-Carboxymethyl Cellulose as a Promising Biopolymer with Prospects in the Agriculture Sector: A Review
Abstract
:1. Introduction
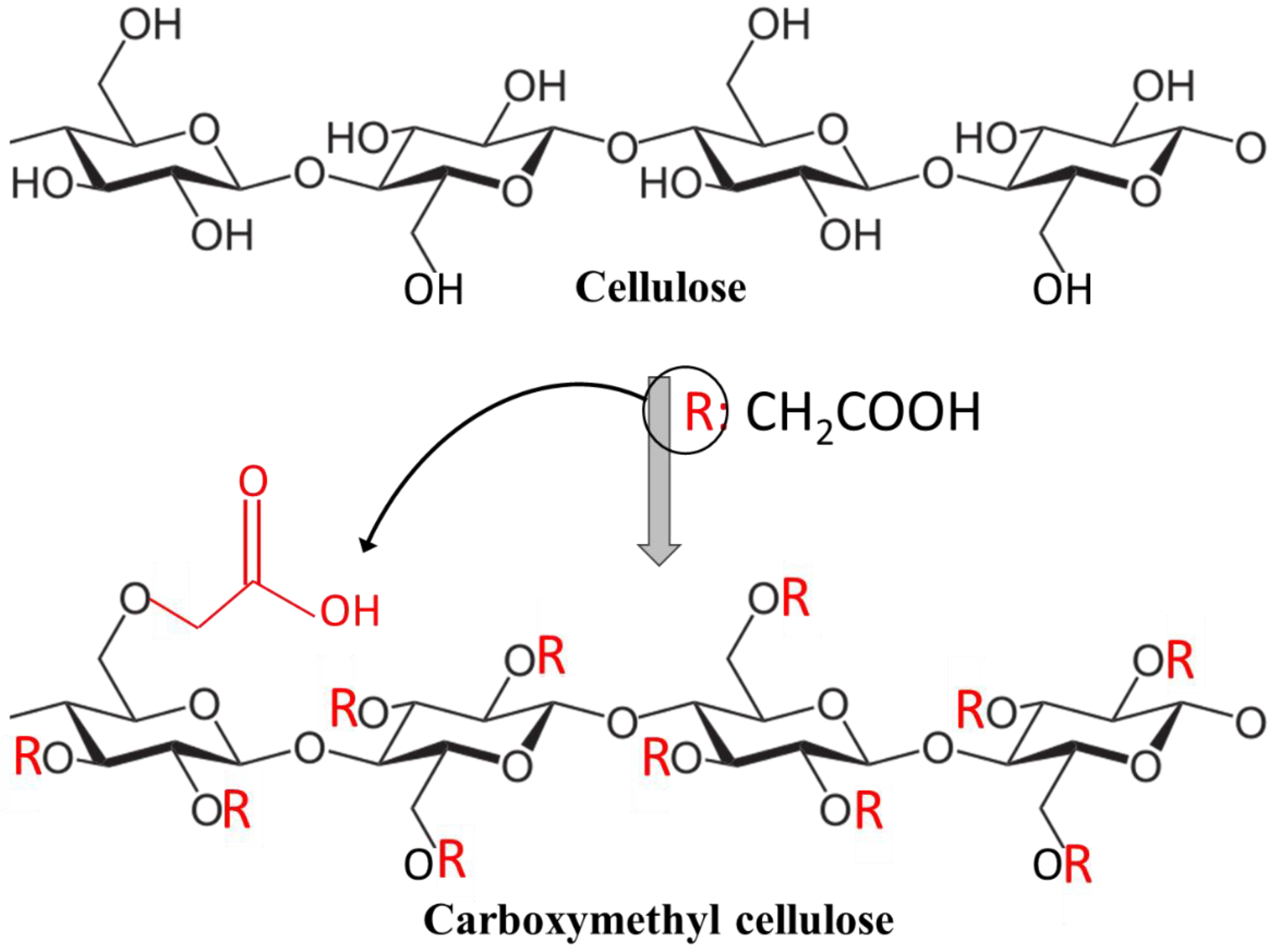
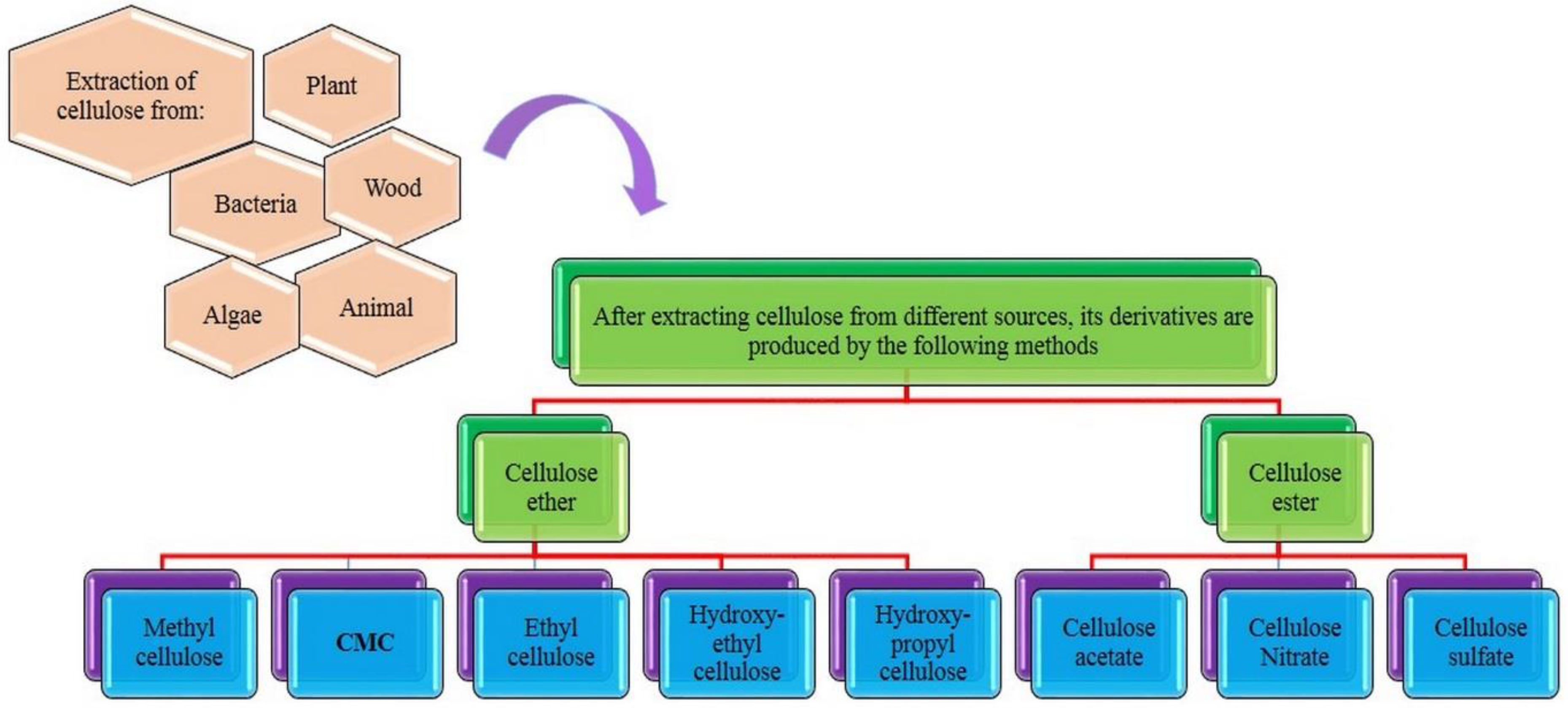
2. Cellulose and Carboxymethyl Cellulose and Their Properties
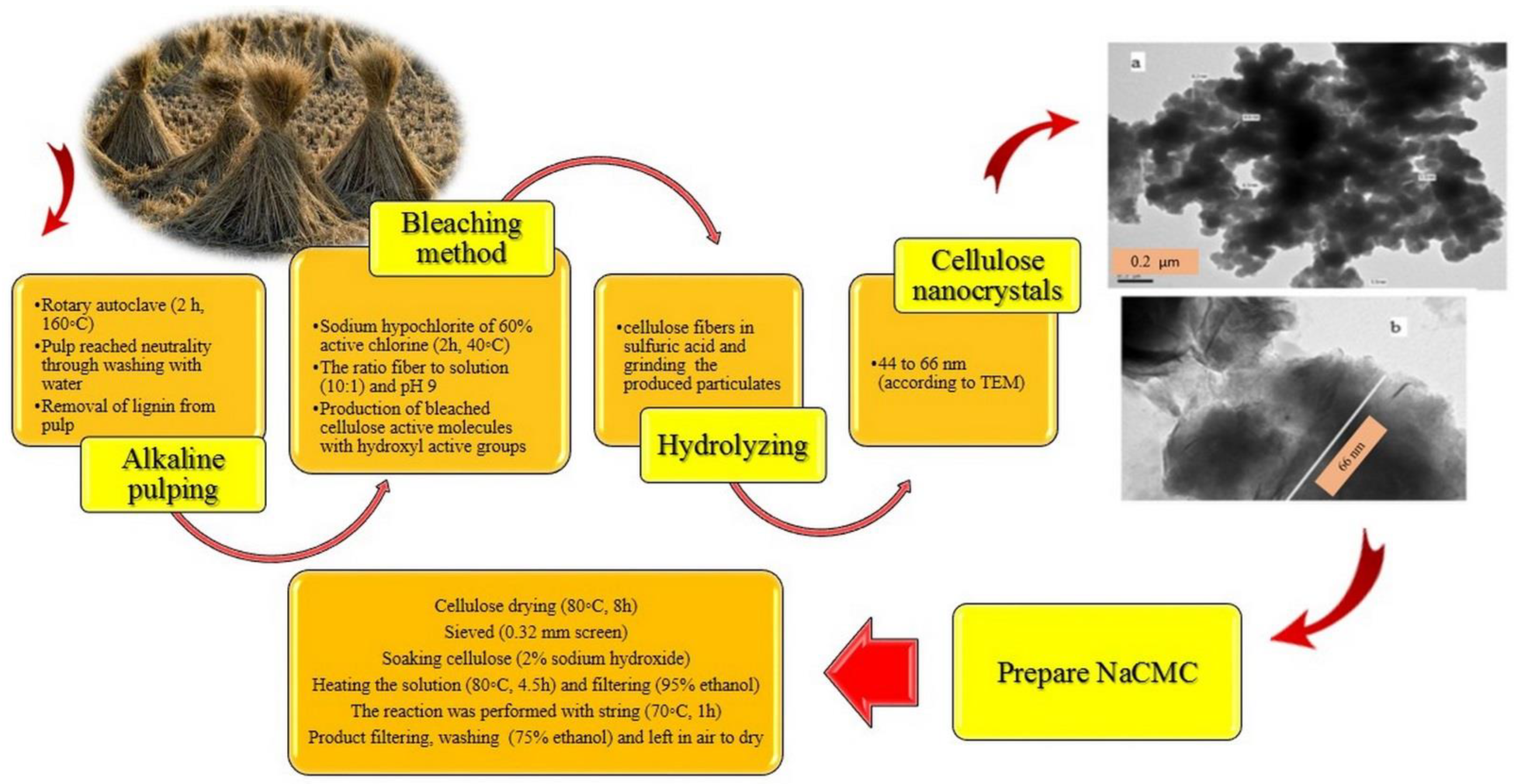
3. Synthesis and Characterization of CMC
4. CMC Applications in Agriculture
4.1. CMC in the Targeted Delivery System
- wti is the initial weight of samples before starting the degradation;
- wtf is the weight of the sample after specified time intervals of biodegradation.
- Wtf is the weight of the loading hydrogel;
- Wt0 is the weight of the unloaded hydrogel
- wtH is the final weight of dried hydrogel;
- wti is the initial weights of CMC and P4VP.
4.2. CMC for Encapsulation of Bioactive Materials
4.3. CMC as Superabsorbent Hydrogels
4.4. CMC to Remediate Pesticides and Heavy Metals from Agricultural Water
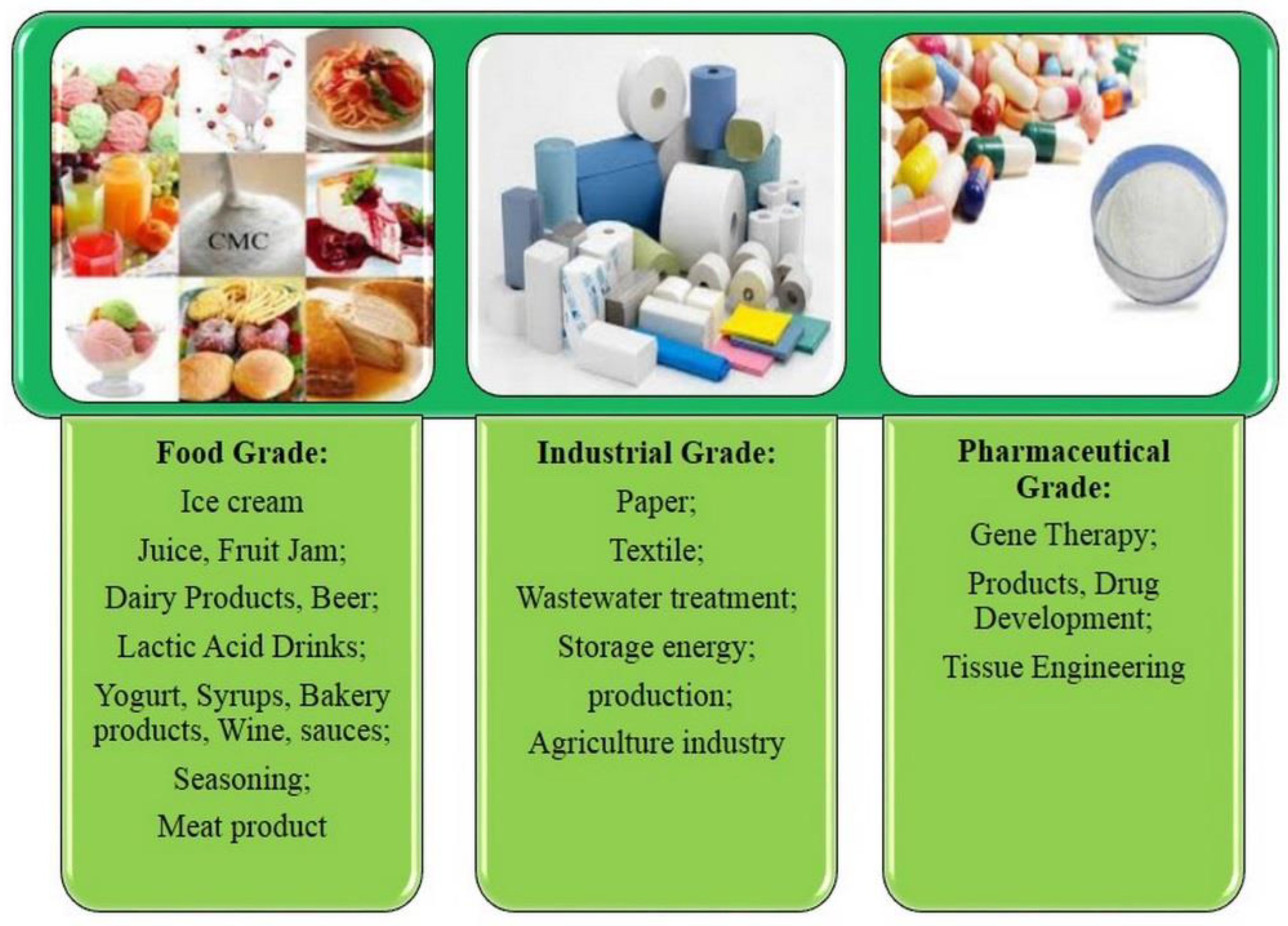
5. CMC Applications in Food Industry
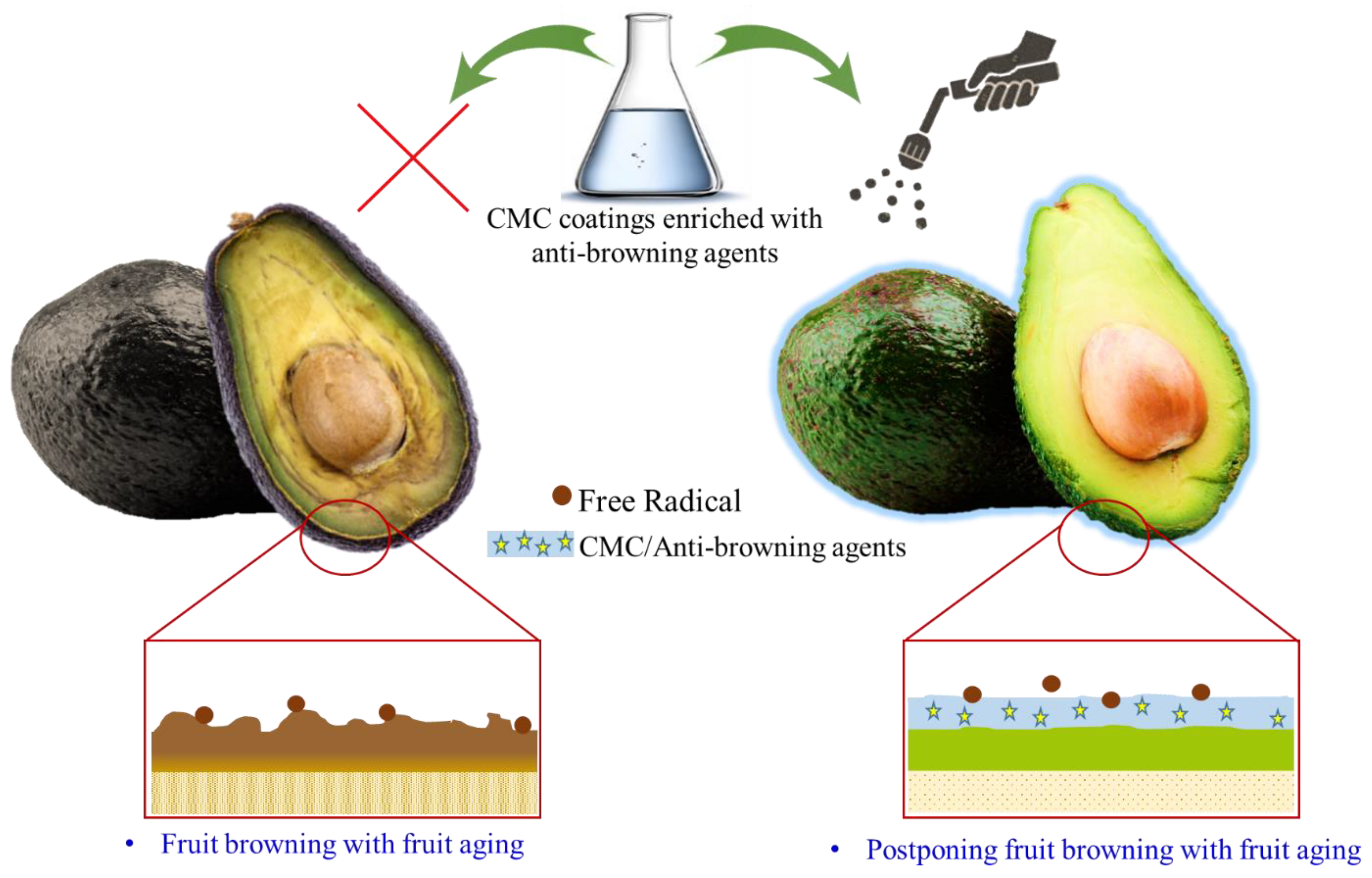
6. CMC as Edible Coating Substances in the Preservation of Agricultural Products
6.1. CMC-Based Active Coating for Physical and Physiological Protection
6.2. CMC-Based Active Coating for Microbial Protection
6.3. CMC-Based Active Coating for Biochemical Protection
7. Perspectives and Future Outlook
8. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Conflicts of Interest
References
- Desa, U. World Population Prospects: The 2008 Revision; United Nations Department of Economic and Social Affairs/Population Division. 2009. Available online: http://esa.un.org/unpp (accessed on 19 May 2017).
- Vega-Vásquez, P.; Mosier, N.S.; Irudayaraj, J. Nanoscale drug delivery systems: From medicine to agriculture. Front. Bioeng. Biotechnol. 2020, 8, 79. [Google Scholar] [CrossRef] [Green Version]
- Gowthaman, N.; Lim, H.; Sreeraj, T.; Amalraj, A.; Gopi, S. Advantages of biopolymers over synthetic polymers: Social, economic, and environmental aspects. In Biopolymers and Their Industrial Applications; Elsevier: Amsterdam, The Netherlands, 2021; pp. 351–372. [Google Scholar]
- Saberi Riseh, R.; Skorik, Y.A.; Thakur, V.K.; Moradi Pour, M.; Tamanadar, E.; Noghabi, S.S. Encapsulation of plant biocontrol bacteria with alginate as a main polymer material. Int. J. Mol. Sci. 2021, 22, 11165. [Google Scholar] [CrossRef]
- Saberi-Riseh, R.; Moradi-Pour, M.; Mohammadinejad, R.; Thakur, V.K. Biopolymers for biological control of plant pathogens: Advances in microencapsulation of beneficial microorganisms. Polymers 2021, 13, 1938. [Google Scholar] [CrossRef]
- Akter, M.; Bhattacharjee, M.; Dhar, A.K.; Rahman, F.B.A.; Haque, S.; Rashid, T.U.; Kabir, S.F. Cellulose-based hydrogels for wastewater treatment: A concise review. Gels 2021, 7, 30. [Google Scholar] [CrossRef]
- Mohammad, Z.H.; Ahmad, F.; Ibrahim, S.A.; Zaidi, S. Application of nanotechnology in different aspects of the food industry. Discov. Food 2022, 2, 12. [Google Scholar] [CrossRef]
- Singh, T.; Shukla, S.; Kumar, P.; Wahla, V.; Bajpai, V.K.; Rather, I. Application of nanotechnology in food science: Perception and overview. Front. Microbiol. 2017, 8, 1501. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yadav, S.K.; Lal, S.; Yadav, S.; Laxman, J.; Verma, B.; Sushma, M.; Choudhary, R.; Singh, P.; Singh, S.; Sharma, V. Use of nanotechnology in agri-food sectors and apprehensions: An overview. Seed Res. 2019, 47, 99–149. [Google Scholar]
- Sargazi, S.; Laraib, U.; Er, S.; Rahdar, A.; Hassanisaadi, M.; Zafar, M.N.; Díez-Pascual, A.M.; Bilal, M. Application of green gold nanoparticles in cancer therapy and diagnosis. Nanomaterials 2022, 12, 1102. [Google Scholar] [CrossRef]
- Hassanisaadi, M.; Bonjar, G.; Rahdar, A.; Pandey, S.; Hosseinipour, A.; Abdolshahi, R. Environmentally safe biosynthesis of gold nanoparticles using plant water extracts. Nanomaterials 2021, 11, 2033. [Google Scholar] [CrossRef]
- Okey-Onyesolu, C.F.; Hassanisaadi, M.; Bilal, M.; Barani, M.; Rahdar, A.; Iqbal, J.; Kyzas, G.Z. Nanomaterials as nanofertilizers and nanopesticides: An overview. ChemistrySelect 2021, 6, 8645–8663. [Google Scholar] [CrossRef]
- Islam, M.T.; Alam, M.M.; Patrucco, A.; Montarsolo, A.; Zoccola, M. Preparation of nanocellulose: A review. AATCC J. Res. 2014, 1, 17–23. [Google Scholar] [CrossRef]
- Zhao, X.; Cui, H.; Wang, Y.; Sun, C.; Cui, B.; Zeng, Z. Development strategies and prospects of nano-based smart pesticide formulation. J. Agric. Food Chem. 2017, 66, 6504–6512. [Google Scholar] [CrossRef]
- Brondi, M.; Florencio, C.; Mattoso, L.; Ribeiro, C.; Farinas, C. Encapsulation of trichoderma harzianum with nanocellulose/carboxymethyl cellulose nanocomposite. Carbohydr. Polym. 2022, 295, 119876. [Google Scholar] [CrossRef]
- Cokmus, C.; Elcin, Y.M. Stability and controlled release properties of carboxymethylcellulose-encapsulated bacillus thuringiensis var. Israelensis. Pestic. Sci. 1995, 45, 351–355. [Google Scholar] [CrossRef]
- Fathi, F.; Saberi-Riseh, R.; Khodaygan, P. Survivability and controlled release of alginate-microencapsulated pseudomonas fluorescens vupf506 and their effects on biocontrol of rhizoctonia solani on potato. Int. J. Biol. Macromol. 2021, 183, 627–634. [Google Scholar] [CrossRef]
- Saberi Riseh, R.; Hassanisaadi, M.; Vatankhah, M.; Soroush, F.; Varma, R.S. Nano/microencapsulation of plant biocontrol agents by chitosan, alginate, and other important biopolymers as a novel strategy for alleviating plant biotic stresses. Int. J. Biol. Macromol. 2022, 222, 1589–1604. [Google Scholar] [CrossRef]
- Kumar, S.; Nehra, M.; Dilbaghi, N.; Marrazza, G.; Hassan, A.A.; Kim, K.-H. Nano-based smart pesticide formulations: Emerging opportunities for agriculture. J. Control. Release 2019, 294, 131–153. [Google Scholar] [CrossRef]
- Hao, L.; Lin, G.; Lian, J.; Chen, L.; Zhou, H.; Chen, H.; Xu, H.; Zhou, X. Carboxymethyl cellulose capsulated zein as pesticide nano-delivery system for improving adhesion and anti-uv properties. Carbohydr. Polym. 2020, 231, 115725. [Google Scholar] [CrossRef] [PubMed]
- Li, J.; Jiang, M.; Wu, H.; Li, Y. Addition of modified bentonites in polymer gel formulation of 2, 4-d for its controlled release in water and soil. J. Agric. Food Chem. 2009, 57, 2868–2874. [Google Scholar] [CrossRef]
- Laftah, W.A.; Hashim, S. Preparation and Possible Agricultural Applications of Polymer Hydrogel Composite as Soil Conditioner. Adv. Mater. Res. 2013, 626, 6–10. [Google Scholar] [CrossRef]
- Wang, J.; Wang, D.; Zhang, G.; Wang, Y.; Wang, C.; Teng, Y.; Christie, F. Nitrogen and phosphorus leaching losses from intensively managed paddy fields with straw retention. Agric. Water Manag. 2014, 141, 66–73. [Google Scholar] [CrossRef]
- Kabir, S.; Sikdar, P.P.; Haque, B.; Bhuiyan, M.; Ali, A.; Islam, M. Cellulose-based hydrogel materials: Chemistry, properties and their prospective applications. Prog. Biomater. 2018, 7, 153–174. [Google Scholar] [CrossRef] [Green Version]
- Devasia, L.; John, N. Carboxymethyl cellulose-starch-gelatin based hydrogel membranes for heavy metal removal. Int. J. Innov. Sci. D Res. Technol. 2021, 6, 66–72. [Google Scholar]
- Dolatabadi, M.; Naidu, H.; Ahmadzadeh, S. Adsorption characteristics in the removal of chlorpyrifos from groundwater using magnetic graphene oxide and carboxy methyl cellulose composite. Sep. Purif. Technol. 2022, 300, 121919. [Google Scholar] [CrossRef]
- Olad, A.; Zebhi, H.; Salari, D.; Mirmohseni, A.; Tabar, A.R. Slow-release npk fertilizer encapsulated by carboxymethyl cellulose-based nanocomposite with the function of water retention in soil. Mater. Sci. Eng. C 2018, 90, 333–340. [Google Scholar] [CrossRef]
- Omer, A.M.; Tamer, T.M.; Hassan, M.E.; Khalifa, R.E.; El-Monaem, A.; Eman, M.; Eltaweil, A.S.; Mohy Eldin, M.S. Fabrication of grafted carboxymethyl cellulose superabsorbent hydrogel for water retention and sustained release of ethephon in sandy soil. Arab. J. Sci. Eng. 2022, 48, 561–572. [Google Scholar] [CrossRef]
- Bauli, C.R.; Lima, G.F.; de Souza, A.G.; Ferreira, R.R.; Rosa, D.S. Eco-friendly carboxymethyl cellulose hydrogels filled with nanocellulose or nanoclays for agriculture applications as soil conditioning and nutrient carrier and their impact on cucumber growing. Colloids Surf. A Physicochem. Eng. Asp. 2021, 623, 126771. [Google Scholar] [CrossRef]
- Davidson, D.W.; Verma, M.S.; Gu, F.X. Controlled root targeted delivery of fertilizer using an ionically crosslinked carboxymethyl cellulose hydrogel matrix. SpringerPlus 2013, 2, 318. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hou, X.; Li, Y.; Pan, Y.; Jin, Y.; Xiao, H. Controlled release of agrochemicals and heavy metal ion capture dual-functional redox-responsive hydrogel for soil remediation. Chem. Commun. 2018, 54, 13714–13717. [Google Scholar] [CrossRef]
- Nwabor, O.F.; Singh, S.; Marlina, D.; Voravuthikunchai, S.P. Chemical characterization, release, and bioactivity of eucalyptus camaldulensis polyphenols from freeze-dried sodium alginate and sodium carboxymethyl cellulose matrix. Food Qual. Saf. 2020, 4, 203–212. [Google Scholar] [CrossRef]
- Sarkar, D.J.; Singh, A. Base triggered release of insecticide from bentonite reinforced citric acid crosslinked carboxymethyl cellulose hydrogel composites. Carbohydr. Polym. 2017, 156, 303–311. [Google Scholar] [CrossRef]
- Sharif, S.N.M.; Hashim, N.; Isa, I.M.; Bakar, S.A.; Saidin, M.I.; Ahmad, M.S.; Mamat, M.; Hussein, M.Z.; Zainul, R.; Kamari, A. The effect of swellable carboxymethyl cellulose coating on the physicochemical stability and release profile of a zinc hydroxide nitrate–sodium dodecylsulphate–imidacloprid. Chem. Phys. Impact 2021, 2, 100017. [Google Scholar] [CrossRef]
- Das, S.K.; Vishakha, K.; Das, S.; Chakraborty, D.; Ganguli, A. Carboxymethyl cellulose and cardamom oil in a nanoemulsion edible coating inhibit the growth of foodborne pathogens and extend the shelf life of tomatoes. Biocatal. Agric. Biotechnol. 2022, 42, 102369. [Google Scholar] [CrossRef]
- Vargas-Torrico, M.F.; von Borries-Medrano, E.; Valle-Guadarrama, S.; Aguilar-Méndez, M.A. Development of gelatin-carboxymethylcellulose coatings incorporated with avocado epicarp and coconut endocarp extracts to control fungal growth in strawberries for shelf-life extension. CyTA-J. Food 2022, 20, 27–38. [Google Scholar] [CrossRef]
- Rachtanapun, P.; Luangkamin, S.; Tanprasert, K.; Suriyatem, R. Carboxymethyl cellulose film from durian rind. LWT Food Sci. Technol. 2012, 48, 52–58. [Google Scholar] [CrossRef]
- Seddiqi, H.; Oliaei, E.; Honarkar, H.; Jin, J.; Geonzon, L.C.; Bacabac, R.G.; Klein-Nulend, J.J.C. Cellulose and its derivatives: Towards biomedical applications. Cellulose 2021, 28, 1893–1931. [Google Scholar] [CrossRef]
- Zuppolini, S.; Salama, A.; Cruz-Maya, I.; Guarino, V.; Borriello, A. Cellulose amphiphilic materials: Chemistry, process and applications. Pharmaceutics 2022, 14, 386. [Google Scholar] [CrossRef]
- Bochek, A. Effect of hydrogen bonding on cellulose solubility in aqueous and nonaqueous solvents. Russ. J. Appl. Chem. 2003, 76, 1711–1719. [Google Scholar] [CrossRef]
- Lavanya, D.; Kulkarni, P.; Dixit, M.; Raavi, P.K.; Krishna, L. Sources of cellulose and their applications—A review. Int. J. Drug Formul. Res. 2011, 2, 19–38. [Google Scholar]
- Altunina, L.; Tikhonova, L.; Yarmukhametova, E. Method for deriving carboxymethyl cellulose. Eurasian Chem. J. 2001, 3, 49–53. [Google Scholar] [CrossRef] [Green Version]
- Yang, X.H.; Zhu, W.L. Viscosity properties of sodium carboxymethylcellulose solutions. Cellulose 2007, 14, 409–417. [Google Scholar] [CrossRef]
- Ibikunle, A.; Ogunneye, A.; Soga, I.; Sanyaolu, N.; Yussuf, S.; Sonde, O.; Badejo, O. Food grade carboxymethyl cellulose preparation from african star apple seed (chrysophyllum albidum) shells: Optimization and characterization. Ife J. Sci. 2019, 21, 245–255. [Google Scholar] [CrossRef] [Green Version]
- Dumitriu, C.; Voicu, S.I.; Muhulet, A.; Nechifor, G.; Popescu, S.; Ungureanu, C.; Carja, A.; Miculescu, F.; Trusca, R.; Pirvu, C. Production and characterization of cellulose acetate–titanium dioxide nanotubes membrane fraxiparinized through polydopamine for clinical applications. Carbohydr. Polym. 2018, 181, 215–223. [Google Scholar] [CrossRef]
- Tilki, T.; Yavuz, M.; Karabacak, Ç.; Çabuk, M.; Ulutürk, M. Investigation of electrorheological properties of biodegradable modified cellulose/corn oil suspensions. Carbohydr. Res. 2010, 345, 672–679. [Google Scholar] [CrossRef]
- Vilela, A.; Cosme, F.; Pinto, T. Emulsions, foams, and suspensions: The microscience of the beverage industry. Beverages 2018, 4, 25. [Google Scholar] [CrossRef] [Green Version]
- Ergun, R.; Guo, J.; Huebner-Keese, B. Cellulose. In Encyclopedia of Food and Health; Academic Press: Oxford, UK, 2016. [Google Scholar]
- Almasei, H.; Ganbarzadeh, B.; Najafabadi, P. Improvement of physical properties of starch and biodegradable films of starch and carboxymethyl cellulose. J. Food Sci. Technol. 2011, 6, 1–11. [Google Scholar]
- Dashipour, A.; Khaksar, R.; Hosseini, H.; Shojaee-Aliabadi, S.; Ghanati, K. Physical, antioxidant and antimicrobial characteristics of carboxymethyl cellulose edible film cooperated with clove essential oil. Zahedan J. Res. Med. Sci. 2014, 16, 34–42. [Google Scholar]
- Kadry, G. Comparison between gelatin/carboxymethyl cellulose and gelatin/carboxymethyl nanocellulose in tramadol drug loaded capsule. Heliyon 2019, 5, e02404. [Google Scholar] [CrossRef]
- Miljković, V.; Gajić, I.; Nikolić, L.J.P. Waste materials as a resource for production of cmc superabsorbent hydrogel for sustainable agriculture. Polymers 2021, 13, 4115. [Google Scholar] [CrossRef]
- Mondal, M.I.H.; Ahmed, F. Synthesis and grafting of carboxymethyl cellulose from environmental pollutant cellulosic wastes of textile industry. Res. J. Text. Appar. 2016, 20, 126–135. [Google Scholar] [CrossRef]
- Joshi, G.; Naithani, S.; Varshney, V.; Bisht, S.S.; Rana, V.; Gupta, P. Synthesis and characterization of carboxymethyl cellulose from office waste paper: A greener approach towards waste management. Waste Manag. 2015, 38, 33–40. [Google Scholar] [CrossRef] [PubMed]
- Sheikh, J.; Bramhecha, I.; Teli, M. Recycling of terry towel (cellulosic) waste into carboxymethyl cellulose (cmc) for textile printing. Fibers Polym. 2015, 16, 1113–1118. [Google Scholar] [CrossRef]
- Arancibia, C.; Navarro-Lisboa, R.; Zúñiga, R.; Matiacevich, S. Application of cmc as thickener on nanoemulsions based on olive oil: Physical properties and stability. Int. J. Polym. Sci. 2016, 2016, 1–10. [Google Scholar] [CrossRef] [Green Version]
- Sun, Z.; Yin, Y.; An, Y.; Deng, C.; Wei, Z.; Jiang, Z.; Duan, X.; Xu, X.; Chen, J. A novel modified carboxymethyl cellulose hydrogel adsorbent for efficient removal of poisonous metals from wastewater: Performance and mechanism. J. Environ. Chem. Eng. 2022, 10, 108179. [Google Scholar] [CrossRef]
- Khawas, P.; Deka, S.C. Isolation and characterization of cellulose nanofibers from culinary banana peel using high-intensity ultrasonication combined with chemical treatment. Carbohydr. Polym. 2016, 137, 608–616. [Google Scholar] [CrossRef] [PubMed]
- Klunklin, W.; Jantanasakulwong, K.; Phimolsiripol, Y.; Leksawasdi, N.; Seesuriyachan, P.; Chaiyaso, T.; Insomphun, C.; Phongthai, S.; Jantrawut, P.; Sommano, S.R.; et al. Synthesis, characterization, and application of carboxymethyl cellulose from asparagus stalk end. Polymers 2020, 13, 81. [Google Scholar] [CrossRef] [PubMed]
- Rachtanapun, P.; Rattanapanone, N. Synthesis and characterization of carboxymethyl cellulose powder and films from mimosa pigra. J. Appl. Polym. Sci. 2011, 122, 3218–3226. [Google Scholar] [CrossRef]
- Rasid, N.A.; Zainol, M.; Amin, N. Synthesis and characterization of carboxymethyl cellulose derived from empty fruit bunch. Sains Malays. 2021, 50, 2523–2535. [Google Scholar] [CrossRef]
- Golbaghi, L.; Khamforoush, M.; Hatami, T. Carboxymethyl cellulose production from sugarcane bagasse with steam explosion pulping: Experimental, modeling, and optimization. Carbohydr. Polym. 2017, 174, 780–788. [Google Scholar] [CrossRef]
- Tasaso, P. Optimization of reaction conditions for synthesis of carboxymethyl cellulose from oil palm fronds. Int. J. Chem. Eng. Appl. 2015, 6, 101. [Google Scholar] [CrossRef] [Green Version]
- Hebeish, A.; El-Rafie, M.; Abdel-Mohdy, F.; Abdel-Halim, E.; Emam, H. Carboxymethyl cellulose for green synthesis and stabilization of silver nanoparticles. Carbohydr. Polym. 2010, 82, 933–941. [Google Scholar] [CrossRef]
- Adinugraha, M.P.; Marseno, D.W.; Haryadi, M. Synthesis and characterization of sodium carboxymethylcellulose from cavendish banana pseudo stem (musa cavendishii lambert). Carbohydr. Polym. 2005, 62, 164–169. [Google Scholar] [CrossRef]
- Moussa, I.; Khiari, R.; Moussa, A.; Belgacem, M.N.; Mhenni, M. Preparation and characterization of carboxymethyl cellulose with a high degree of substitution from agricultural wastes. Fibers Polym. 2019, 20, 933–943. [Google Scholar] [CrossRef]
- Pushpamalar, V.; Langford, S.J.; Ahmad, M.; Lim, Y. Optimization of reaction conditions for preparing carboxymethyl cellulose from sago waste. Carbohydr. Polym. 2006, 64, 312–318. [Google Scholar] [CrossRef]
- Rachtanapun, P.; Eitssayeam, S.; Pengpat, K. Study of Carboxymethyl Cellulose from Papaya Peels as Binder in Ceramics. Adv. Mater. Res. 2010, 93–94, 17–21. [Google Scholar] [CrossRef]
- Toğrul, H.; Arslan, N. Production of carboxymethyl cellulose from sugar beet pulp cellulose and rheological behaviour of carboxymethyl cellulose. Carbohydr. Polym. 2003, 54, 73–82. [Google Scholar] [CrossRef]
- Li, H.; Zhang, H.; Xiong, L.; Chen, X.; Wang, C.; Huang, C.; Chen, X. Isolation of cellulose from wheat straw and its utilization for the preparation of carboxymethyl cellulose. Fibers Polym. 2019, 20, 975–981. [Google Scholar] [CrossRef]
- Sophonputtanaphoca, S.; Chutong, P.; Cha-Aim, K.; Nooeaid, P. Potential of thai rice straw as a raw material for the synthesis of carboxymethylcellulose. Int. Food Res. J. 2019, 26, 969–978. [Google Scholar]
- Yeasmin, M.S.; Mondal, M.I. Synthesis of highly substituted carboxymethyl cellulose depending on cellulose particle size. Int. J. Biol. Macromol. 2015, 80, 725–731. [Google Scholar] [CrossRef]
- Hokkanen, S.; Bhatnagar, A.; Sillanpää, M. A review on modification methods to cellulose-based adsorbents to improve adsorption capacity. Water Res. 2016, 91, 156–173. [Google Scholar] [CrossRef]
- Stamps, R.H.; Savage, H.M. Cellulosic water and polyacrylamide gels can delay wilting and extend watering intervals for potted poinsettias displayed in interiorscapes. Horttechnology 2012, 22, 766–770. [Google Scholar] [CrossRef]
- Contreras-Cáceres, R.; Leiva, M.C.; Ortiz, R.; Díaz, A.; Perazzoli, G.; Casado-Rodríguez, M.A.; Melguizo, C.; Baeyens, J.M.; López-Romero, J.M.; Prados, J. Paclitaxel-loaded hollow-poly (4-vinylpyridine) nanoparticles enhance drug chemotherapeutic efficacy in lung and breast cancer cell lines. Nano Res. 2017, 10, 856–875. [Google Scholar] [CrossRef]
- Mohamed, R.R.; Fahim, M.E.; Soliman, S.M.A. Development of hydrogel based on carboxymethyl cellulose/poly (4-vinylpyridine) for controlled releasing of fertilizers. BMC Chem. 2022, 16, 52. [Google Scholar] [CrossRef] [PubMed]
- Pacheco, D.; Cotas, J.; Rocha, C.P.; Araújo, G.S.; Figueirinha, A.; Gonçalves, A.M.; Bahcevandziev, K.; Pereira, L. Seaweeds’ carbohydrate polymers as plant growth promoters. Carbohydr. Polym. Technol. Appl. 2021, 2, 100097. [Google Scholar] [CrossRef]
- Axelos, M.A.; Van de Voorde, M. Nanotechnology in Agriculture and Food Science; John Wiley & Sons: Hoboken, NJ, USA, 2017. [Google Scholar]
- Schwab, F.; Zhai, G.; Kern, M.; Turner, A.; Schnoor, J.L.; Wiesner, M.R. Barriers, pathways and processes for uptake, translocation and accumulation of nanomaterials in plants–critical review. Nanotoxicology 2016, 10, 257–278. [Google Scholar] [CrossRef]
- Solanki, P.; Bhargava, A.; Chhipa, H.; Jain, N.; Panwar, J. Nano-fertilizers and their smart delivery system. In Nanotechnologies in Food and Agriculture; Springer: Cham, Switzerland, 2015; pp. 81–101. [Google Scholar]
- Raafat, A.I.; Eid, M.; El-Arnaouty, M.B. Radiation synthesis of superabsorbent cmc based hydrogels for agriculture applications. Nucl. Instrum. Methods Phys. Res. Sect. B Beam Interact. Mater. At. 2012, 283, 71–76. [Google Scholar] [CrossRef]
- Morita, A.; Takano, H.; Oota, M.; Yoneyama, T. Nitrification and denitrification in an acidic soil of tea (Camellia sinensis L.) field estimated by δ15n values of leached nitrogen from the soil columns treated with ammonium nitrate in the presence or absence of a nitrification inhibitor and with slow-release fertilizers. Soil Sci. Plant Nutr. 2002, 48, 585–593. [Google Scholar]
- Chen, D.; Suter, H.; Islam, A.; Edis, R.; Freney, J.; Walker, C.N. Prospects of improving efficiency of fertiliser nitrogen in australian agriculture: A review of enhanced efficiency fertilisers. Aust. J. Soil Res. 2008, 46, 289–301. [Google Scholar] [CrossRef]
- Zhang, W.; Liu, Y.; Xuan, Y.; Zhang, S. Synthesis and applications of carboxymethyl cellulose hydrogels. Gels 2022, 8, 529. [Google Scholar] [CrossRef]
- Rizwan, M.; Gilani, S.R.; Durani, A.I.; Naseem, S. Materials diversity of hydrogel: Synthesis, polymerization process and soil conditioning properties in agricultural field. J. Adv. Res. 2021, 33, 15–40. [Google Scholar] [CrossRef]
- Wang, W.; Hu, J.; Zhang, R.; Yan, C.; Cui, L.; Zhu, J. A ph-responsive carboxymethyl cellulose/chitosan hydrogel for adsorption and desorption of anionic and cationic dyes. Cellulose 2021, 28, 897–909. [Google Scholar] [CrossRef]
- Yi, Y.; Zhang, Y.; Mansel, B.; Wang, Y.-n.; Prabakar, S.; Shi, B. Effect of dialdehyde carboxymethyl cellulose cross-linking on the porous structure of the collagen matrix. Biomacromolecules 2022, 23, 1723–1732. [Google Scholar] [CrossRef] [PubMed]
- Guarnizo-Herrero, V.; Torrado-Salmerón, C.; Torres Pabón, N.S.; Torrado Durán, G.; Morales, J.; Torrado-Santiago, S. Study of different chitosan/sodium carboxymethyl cellulose proportions in the development of polyelectrolyte complexes for the sustained release of clarithromycin from matrix tablets. Polymers 2021, 13, 2813. [Google Scholar] [CrossRef] [PubMed]
- Kong, Q.; Wang, X.; Zhao, X.; Lou, T. Preparation of millimeter-scale hollow sphere with cationic chitosan/dimethyl diallyl ammonium chloride/carboxymethyl cellulose terpolymer and its selective removal of anionic dye. J. Clean. Prod. 2022, 331, 130017. [Google Scholar] [CrossRef]
- Tavares, K.M.; de Campos, A.; Luchesi, B.R.; Resende, A.A.; de Oliveira, J.E.; Marconcini, J.M. Effect of carboxymethyl cellulose concentration on mechanical and water vapor barrier properties of corn starch films. Carbohydr. Polym. 2020, 246, 116521. [Google Scholar] [CrossRef] [PubMed]
- Pettignano, A.; Daunay, A.l.; Moreau, C.l.; Cathala, B.; Charlot, A.l.; Fleury, E. Sustainable modification of carboxymethyl cellulose by passerini three-component reaction and subsequent adsorption onto cellulosic substrates. ACS Sustain. Chem. Eng. 2019, 7, 14685–14696. [Google Scholar] [CrossRef]
- Wang, Y.; Sun, M.; Qiao, D.; Li, J.; Wang, Y.; Liu, W.; Bunt, C.; Liu, H.; Liu, J.; Yang, X. Graft copolymer of sodium carboxymethyl cellulose and polyether polyol (cmc-g-tmn-450) improves the crosslinking degree of polyurethane for coated fertilizers with enhanced controlled release characteristics. Carbohydr. Polym. 2021, 272, 118483. [Google Scholar] [CrossRef]
- Zhu, X.; Chen, J.; Hu, Y.; Zhang, N.; Fu, Y.; Chen, X. Tuning complexation of carboxymethyl cellulose/cationic chitosan to stabilize pickering emulsion for curcumin encapsulation. Food Hydrocoll. 2021, 110, 106135. [Google Scholar] [CrossRef]
- Bugatti, V.; Vertuccio, L.; Zara, S.; Fancello, F.; Scanu, B.; Gorrasi, G. Green pesticides based on cinnamate anion incorporated in layered double hydroxides and dispersed in pectin matrix. Carbohydr. Polym. 2019, 209, 356–362. [Google Scholar] [CrossRef]
- Li, Y.; Zhou, M.; Pang, Y.; Qiu, X. Lignin-based microsphere: Preparation and performance on encapsulating the pesticide avermectin. ACS Sustain. Chem. Eng. 2017, 5, 3321–3328. [Google Scholar] [CrossRef]
- Luo, Y.; Wang, Q. Zein-based micro-and nano-particles for drug and nutrient delivery: A review. J. Appl. Polym. Sci. 2014, 131, 40696. [Google Scholar] [CrossRef]
- Lin, X.; Li, Y.; Chen, Z.; Zhang, C.; Luo, X.; Du, X.; Huang, Y. Synthesis, characterization and electrospinning of new thermoplastic carboxymethyl cellulose (TCMC). Chem. Eng. J. 2013, 215, 709–720. [Google Scholar] [CrossRef]
- Mao, C.; Song, Y.; Chen, L.; Ji, J.; Li, J.; Yuan, X.; Yang, Z.; Ayoko, G.A.; Frost, R.L.; Theiss, F. Human health risks of heavy metals in paddy rice based on transfer characteristics of heavy metals from soil to rice. Catena 2019, 175, 339–348. [Google Scholar] [CrossRef]
- Bikova, T.; Treimanis, A. Uv-absorbance of oxidized xylan and monocarboxyl cellulose in alkaline solutions. Carbohydr. Polym. 2004, 55, 315–322. [Google Scholar] [CrossRef]
- Yan, L.; Wang, R.; Wang, H.; Sheng, K.; Liu, C.; Qu, H.; Ma, A.; Zheng, L. Formulation and characterization of chitosan hydrochloride and carboxymethyl chitosan encapsulated quercetin nanoparticles for controlled applications in foods system and simulated gastrointestinal condition. Food Hydrocoll. 2018, 84, 450–457. [Google Scholar] [CrossRef]
- Costa, A.M.; Nunes, J.; Lima, B.; Pedrosa, C.; Calado, V.; Torres, A.; Pierucci, A. Effective stabilization of cla by microencapsulation in pea protein. Food Chem. 2015, 168, 157–166. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Xu, Y.; Ma, X.-Y.; Gong, W.; Li, X.; Huang, H.-B.; Zhu, X.-M. Nanoparticles based on carboxymethylcellulose-modified rice protein for efficient delivery of lutein. Food Funct. 2020, 11, 2380–2394. [Google Scholar] [CrossRef] [PubMed]
- Hemvichian, K.; Chanthawong, A.; Suwanmala, P. Synthesis and characterization of superabsorbent polymer prepared by radiation-induced graft copolymerization of acrylamide onto carboxymethyl cellulose for controlled release of agrochemicals. Radiat. Phys. Chem. 2014, 103, 167–171. [Google Scholar] [CrossRef]
- Bortolin, A.; Aouada, F.A.; Mattoso, L.H.; Ribeiro, C. Nanocomposite paam/methyl cellulose/montmorillonite hydrogel: Evidence of synergistic effects for the slow release of fertilizers. J. Agric. Food Chem. 2013, 61, 7431–7439. [Google Scholar] [CrossRef]
- De Oliveira, J.L.; Fraceto, L.F.; Bravo, A.; Polanczyk, R.A. Encapsulation strategies for bacillus thuringiensis: From now to the future. J. Agric. Food Chem. 2021, 69, 4564–4577. [Google Scholar] [CrossRef]
- Rodrigues Sousa, H.; Lima, I.S.; Neris, L.M.L.; Silva, A.S.; Santos Nascimento, A.M.S.; Araújo, F.P.; Ratke, R.F.; Silva, D.A.; Osajima, J.A.; Bezerra, L.R. Superabsorbent hydrogels based to polyacrylamide/cashew tree gum for the controlled release of water and plant nutrients. Molecules 2021, 26, 2680. [Google Scholar] [CrossRef] [PubMed]
- Kuang, J.; Yuk, K.Y.; Huh, K.M. Polysaccharide-based superporous hydrogels with fast swelling and superabsorbent properties. Carbohydr. Polym. 2011, 83, 284–290. [Google Scholar] [CrossRef]
- Batista, R.A.; Espitia, P.J.P.; Quintans, J.d.S.S.; Freitas, M.M.; Cerqueira, M.Â.; Teixeira, J.A.; Cardoso, J.C. Hydrogel as an alternative structure for food packaging systems. Carbohydr. Polym. 2019, 205, 106–116. [Google Scholar] [CrossRef] [Green Version]
- Zhong, K.; Zheng, X.-L.; Mao, X.-Y.; Lin, Z.-T.; Jiang, G.-B. Sugarcane bagasse derivative-based superabsorbent containing phosphate rock with water–fertilizer integration. Carbohydr. Polym. 2012, 90, 820–826. [Google Scholar] [CrossRef] [PubMed]
- Li, X.; He, J.-Z.; Hughes, J.M.; Liu, Y.-R.; Zheng, Y.-M. Effects of super-absorbent polymers on a soil–wheat (triticum aestivum l.) system in the field. Appl. Soil Ecol. 2014, 73, 58–63. [Google Scholar] [CrossRef]
- Ibrahim, S.M.; El Salmawi, K.M.; Zahran, A. Synthesis of crosslinked superabsorbent carboxymethyl cellulose/acrylamide hydrogels through electron-beam irradiation. J. Appl. Polym. Sci. 2007, 104, 2003–2008. [Google Scholar] [CrossRef]
- Farzamfar, S.; Naseri-Nosar, M.; Vaez, A.; Esmaeilpour, F.; Ehterami, A.; Sahrapeyma, H.; Samadian, H.; Hamidieh, A.-A.; Ghorbani, S.; Goodarzi, A. Neural tissue regeneration by a gabapentin-loaded cellulose acetate/gelatin wet-electrospun scaffold. Cellulose 2018, 25, 1229–1238. [Google Scholar] [CrossRef]
- Fekete, T.; Borsa, J.; Takács, E.; Wojnárovits, L. Synthesis of cellulose derivative based superabsorbent hydrogels by radiation induced crosslinking. Cellulose 2014, 21, 4157–4165. [Google Scholar] [CrossRef]
- Salleh, K.M.; Zakaria, S.; Sajab, M.S.; Gan, S.; Kaco, H. Superabsorbent hydrogel from oil palm empty fruit bunch cellulose and sodium carboxymethylcellulose. Int. J. Biol. Macromol. 2019, 131, 50–59. [Google Scholar] [CrossRef] [PubMed]
- Cannazza, G.; Cataldo, A.; De Benedetto, E.; Demitri, C.; Madaghiele, M.; Sannino, A. Experimental assessment of the use of a novel superabsorbent polymer (sap) for the optimization ofwater consumption in agricultural irrigation process. Water 2014, 6, 2056–2069. [Google Scholar] [CrossRef] [Green Version]
- Li, X.; He, J.-Z.; Liu, Y.-R.; Zheng, Y.-M. Effects of super absorbent polymers on soil microbial properties and chinese cabbage (brassica chinensis) growth. J. Soils Sediments 2013, 13, 711–719. [Google Scholar] [CrossRef]
- Satriani, A.; Catalano, M.; Scalcione, E. The role of superabsorbent hydrogel in bean crop cultivation under deficit irrigation conditions: A case-study in southern italy. Agric. Water Manag. 2018, 195, 114–119. [Google Scholar] [CrossRef]
- Elbarbary, A.M.; Ghobashy, M.M. Controlled release fertilizers using superabsorbent hydrogel prepared by gamma radiation. Radiochim. Acta 2017, 105, 865–876. [Google Scholar] [CrossRef]
- El Salmawi, K.M. Application of polyvinyl alcohol (pva)/carboxymethyl cellulose (cmc) hydrogel produced by conventional crosslinking or by freezing and thawing. J. Macromol. Sci. Part A Pure Appl. Chem. 2007, 44, 619–624. [Google Scholar] [CrossRef]
- Elsaeed, S.M.; Zaki, E.G.; Abdelhafes, A.; Al-Hussaini, A.S. Response surface method based modeling and optimization of cmc-g terpolymer interpenetrating network/bentonite superabsorbent composite for enhancing water retention. ACS Omega 2022, 7, 8219–8228. [Google Scholar] [CrossRef] [PubMed]
- Watcharamul, S.; Lerddamrongchai, S.; Siripongpreda, T.; Rodtassana, C.; Nuisin, R.; Kiatkamjornwong, S. Effects of carboxymethyl cellulose/nano-calcium carbonate hydrogel amendment of loamy sand soil for maize growth. ACS Agric. Sci. Technol. 2022, 2, 1071–1080. [Google Scholar] [CrossRef]
- Rahman, M.; Hasan, M.; Nitai, A.S.; Nam, S.; Karmakar, A.K.; Ahsan, M.; Shiddiky, M.J.; Ahmed, M.B. Recent developments of carboxymethyl cellulose. Polymers 2021, 13, 1345. [Google Scholar] [CrossRef]
- Ugwu, E.I.; Agunwamba, J.C. A review on the applicability of activated carbon derived from plant biomass in adsorption of chromium, copper, and zinc from industrial wastewater. Environ. Monit. Assess. 2020, 192, 240. [Google Scholar] [CrossRef]
- Riaz, M.; Kamran, M.; Fang, Y.; Wang, Q.; Cao, H.; Yang, G.; Deng, L.; Wang, Y.; Zhou, Y.; Anastopoulos, I. Arbuscular mycorrhizal fungi-induced mitigation of heavy metal phytotoxicity in metal contaminated soils: A critical review. J. Hazard. Mater. 2021, 402, 123919. [Google Scholar] [CrossRef]
- Dhalaria, R.; Kumar, D.; Kumar, H.; Nepovimova, E.; Kuča, K.; Torequl Islam, M.; Verma, R. Arbuscular mycorrhizal fungi as potential agents in ameliorating heavy metal stress in plants. Agronomy 2020, 10, 815. [Google Scholar] [CrossRef]
- Godiya, C.B.; Cheng, X.; Li, D.; Chen, Z.; Lu, X. Carboxymethyl cellulose/polyacrylamide composite hydrogel for cascaded treatment/reuse of heavy metal ions in wastewater. J. Hazard. Mater. 2019, 364, 28–38. [Google Scholar] [CrossRef]
- Cao, J.; Xu, R.; Tang, H.; Tang, S.; Cao, M. Synthesis of monodispersed cmc-stabilized fe–cu bimetal nanoparticles for in situ reductive dechlorination of 1, 2, 4-trichlorobenzene. Sci. Total Environ. 2011, 409, 2336–2341. [Google Scholar] [CrossRef] [PubMed]
- ALOthman, Z.A.; Badjah, A.Y.; Alharbi, O.M.; Ali, I. Copper carboxymethyl cellulose nanoparticles for efficient removal of tetracycline antibiotics in water. Environ. Sci. Pollut. Res. 2020, 27, 42960–42968. [Google Scholar] [CrossRef]
- Manzoor, K.; Ahmad, M.; Ahmad, S.; Ikram, S. Removal of pb (ii) and cd (ii) from wastewater using arginine cross-linked chitosan–carboxymethyl cellulose beads as green adsorbent. RSC Adv. 2019, 9, 7890–7902, Correction in RSC Adv. 2020, 10, 2943–2943. [Google Scholar] [CrossRef] [Green Version]
- Li, J.; Tan, S.; Xu, Z. Anisotropic nanocellulose aerogel loaded with modified uio-66 as efficient adsorbent for heavy metal ions removal. Nanomaterials 2020, 10, 1114. [Google Scholar] [CrossRef]
- Wu, S.; Guo, J.; Wang, Y.; Huang, C.; Hu, Y. Facile preparation of magnetic sodium alginate/carboxymethyl cellulose composite hydrogel for removal of heavy metal ions from aqueous solution. J. Mater. Sci. 2021, 56, 13096–13107. [Google Scholar] [CrossRef]
- Abdel Ghaffar, A.; El-Arnaouty, M.; Abdel Baky, A.; Shama, S. Radiation synthesis of carboxymethyl cellulose hydrogels for removal of organic contaminants from its aqueous solution. J. Vinyl Addit. Technol. 2020, 26, 362–369. [Google Scholar] [CrossRef]
- Li, Z.; Gong, Y.; Zhao, D.; Dang, Z.; Lin, Z. Enhanced removal of zinc and cadmium from water using carboxymethyl cellulose-bridged chlorapatite nanoparticles. Chemosphere 2021, 263, 128038. [Google Scholar] [CrossRef]
- He, F.; Zhao, D.; Paul, C. Field assessment of carboxymethyl cellulose stabilized iron nanoparticles for in situ destruction of chlorinated solvents in source zones. Water Res. 2010, 44, 2360–2370. [Google Scholar] [CrossRef] [PubMed]
- Yu, Q.; Guo, J.; Muhammad, Y.; Li, Q.; Lu, Z.; Yun, J.; Liang, Y. Mechanisms of enhanced hexavalent chromium removal from groundwater by sodium carboxymethyl cellulose stabilized zerovalent iron nanoparticles. J. Environ. Manag. 2020, 276, 111245. [Google Scholar] [CrossRef] [PubMed]
- Apler, A.; Snowball, I.; Frogner-Kockum, P.; Josefsson, S. Distribution and dispersal of metals in contaminated fibrous sediments of industrial origin. Chemosphere 2019, 215, 470–481. [Google Scholar] [CrossRef] [PubMed]
- Zhang, H.; Yang, H.; Shao, J.; Chen, Y.; Zhang, S.; Chen, H. Multifunctional carboxymethyl cellulose sodium encapsulated phosphorus-enriched biochar composites: Multistage adsorption of heavy metals and controllable release of soil fertilization. Chem. Eng. J. 2023, 453, 139809. [Google Scholar] [CrossRef]
- Kontogiorgos, V. Stabilisers. In Encyclopedia of Dairy Sciences; Elsevier: Amsterdam, The Netherlands, 2022; pp. 689–694. [Google Scholar]
- Galus, S.; Kadzińska, J. Food applications of emulsion-based edible films and coatings. Trends Food Sci. Technol. 2015, 45, 273–283. [Google Scholar] [CrossRef]
- Panahirad, S.; Dadpour, M.; Peighambardoust, S.H.; Soltanzadeh, M.; Gullón, B.; Alirezalu, K.; Lorenzo, J.M. Applications of carboxymethyl cellulose-and pectin-based active edible coatings in preservation of fruits and vegetables: A review. Trends Food Sci. Technol. 2021, 110, 663–673. [Google Scholar] [CrossRef]
- Hosseini, H.; Jafari, S.M. Introducing nano/microencapsulated bioactive ingredients for extending the shelf-life of food products. Adv. Colloid Interface Sci. 2020, 282, 102210. [Google Scholar] [CrossRef] [PubMed]
- Ngamakeue, N.; Chitprasert, P. Encapsulation of holy basil essential oil in gelatin: Effects of palmitic acid in carboxymethyl cellulose emulsion coating on antioxidant and antimicrobial activities. Food Bioprocess Technol. 2016, 9, 1735–1745. [Google Scholar] [CrossRef]
- Silva-Weiss, A.; Quilaqueo, M.; Venegas, O.; Ahumada, M.; Silva, W.; Osorio, F.; Giménez, B. Design of dipalmitoyl lecithin liposomes loaded with quercetin and rutin and their release kinetics from carboxymethyl cellulose edible films. J. Food Eng. 2018, 224, 165–173. [Google Scholar] [CrossRef]
- Altam, A.A.; Zhu, L.; Babiker, D.; Yagoub, H.; Yang, S. Loading and releasing behavior of carboxymethyl cellulose and chitosan complex beads. Prog. Nat. Sci. Mater. Int. 2022; In Press. [Google Scholar] [CrossRef]
- Hassan, B.; Chatha, S.A.S.; Hussain, A.I.; Zia, K.M.; Akhtar, N. Recent advances on polysaccharides, lipids and protein based edible films and coatings: A review. Int. J. Biol. Macromol. 2018, 109, 1095–1107. [Google Scholar] [CrossRef]
- Flores-López, M.L.; Cerqueira, M.A.; de Rodríguez, D.J.; Vicente, A.A. Perspectives on utilization of edible coatings and nano-laminate coatings for extension of postharvest storage of fruits and vegetables. Food Eng. Rev. 2016, 8, 292–305. [Google Scholar] [CrossRef] [Green Version]
- Beigmohammadi, F.; Peighambardoust, S.H.; Hesari, J.; Azadmard-Damirchi, S.; Peighambardoust, S.J.; Khosrowshahi, N.K. Antibacterial properties of ldpe nanocomposite films in packaging of uf cheese. LWT-Food Sci. Technol. 2016, 65, 106–111. [Google Scholar] [CrossRef]
- Peighambardoust, S.H.; Beigmohammadi, F.; Peighambardoust, S.J. Application of organoclay nanoparticle in low-density polyethylene films for packaging of uf cheese. Packag. Technol. Sci. 2016, 29, 355–363. [Google Scholar] [CrossRef]
- Hassanisaadi, M.; Shahidi Bonjar, G.H.; Hosseinipour, A.; Abdolshahi, R.; Ait Barka, E.; Saadoun, I. Biological control of pythium aphanidermatum, the causal agent of tomato root rot by two streptomyces root symbionts. Agronomy 2021, 11, 846. [Google Scholar] [CrossRef]
- Jamali, F.; Sharifi-Tehrani, A.; Okhovvat, M.; Zakeri, Z.; Saberi-Riseh, R. Biological control of chickpea fusarium wilt by antagonistic bacteria under greenhouse condition. Commun. Agric. Appl. Biol. Sci. 2004, 69, 649–651. [Google Scholar] [PubMed]
- Zhang, L.M. New water-soluble cellulosic polymers: A review. Macromol. Mater. Eng. 2001, 286, 267–275. [Google Scholar] [CrossRef]
- McClements, D.J. Food Emulsions: Principles, Practices, and Techniques; CRC Press: Boca Raton, FL, USA, 2004. [Google Scholar]
- Bastarrachea, L.J.; Wong, D.E.; Roman, M.J.; Lin, Z.; Goddard, J.M. Active packaging coatings. Coatings 2015, 5, 771–791. [Google Scholar] [CrossRef] [Green Version]
- Zhang, X.; Zhang, X.; Liu, X.; Du, M.; Tian, Y. Effect of polysaccharide derived from osmunda japonica thunb-incorporated carboxymethyl cellulose coatings on preservation of tomatoes. J. Food Process. Preserv. 2019, 43, e14239. [Google Scholar] [CrossRef]
- Li, T.; Chi, W.; Ning, Y.; Xu, S.; Wang, L. Locust bean gum/carboxycellulose nanocrystal coating incorporating zno clusters built by the accretion of micro spindles or sheets for strawberries preservation. Int. J. Biol. Macromol. 2022, 226, 267–278. [Google Scholar] [CrossRef]
- Arnon-Rips, H.; Porat, R.; Poverenov, E. Enhancement of agricultural produce quality and storability using citral-based edible coatings; the valuable effect of nano-emulsification in a solid-state delivery on fresh-cut melons model. Food Chem. 2019, 277, 205–212. [Google Scholar] [CrossRef]
- Oztuna Taner, O.; Ekici, L.; Akyuz, L. Cmc-based edible coating composite films from brewer’s spent grain waste: A novel approach for the fresh strawberry package. Polym. Bull. 2022, 2022, 1–26. [Google Scholar] [CrossRef]
- Ballesteros, L.F.; Cerqueira, M.A.; Teixeira, J.A.; Mussatto, S.I. Production and physicochemical properties of carboxymethyl cellulose films enriched with spent coffee grounds polysaccharides. Int. J. Biol. Macromol. 2018, 106, 647–655. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tesfay, S.Z.; Magwaza, L.S.; Mbili, N.; Mditshwa, A. Carboxyl methylcellulose (cmc) containing moringa plant extracts as new postharvest organic edible coating for avocado (persea americana mill.) fruit. Sci. Hortic. 2017, 226, 201–207. [Google Scholar] [CrossRef]
- Khajeh-Ali, S.; Shahidi, F.; Sedaghat, N. Evaluation of the effect of carboxy methyl cellulose edible coating containing astragalus honey (astragalus gossypinus) on the shelf-life of pistachio kernel. Food Control 2022, 139, 109094. [Google Scholar] [CrossRef]
- Tzoumaki, M.V.; Biliaderis, C.G.; Vasilakakis, M. Impact of edible coatings and packaging on quality of white asparagus (asparagus officinalis, l.) during cold storage. Food Chem. 2009, 117, 55–63. [Google Scholar] [CrossRef]
- Monteiro Fritz, A.R.; Fonseca, J.d.M.; Trevisol, T.C.; Fagundes, C.; Valencia, G.A. Active, eco-friendly and edible coatings in the post-harvest–a critical discussion. In Polymers for Agri-Food Applications; Springer: Cham, Switzerland, 2019; pp. 433–463. [Google Scholar]
- Deepthi, V.P.; Sekhar, R.C.; Srihari, D.; Sankar, A.S. Guava fruit quality and storability as influenced by harvest maturity and postharvest application of calcium salts. Plant Arch. 2016, 16, 174–182. [Google Scholar]
- Moradinezhad, F.; Ghesmati, M.; Khayat, M. Pre-storage calcium salts treatment maintained postharvest quality and bioactive compounds of fresh jujube fruit. Fundam. Appl. Agric. 2019, 4, 890–897. [Google Scholar] [CrossRef]
- Alharaty, G.; Ramaswamy, H.S. The effect of sodium alginate-calcium chloride coating on the quality parameters and shelf life of strawberry cut fruits. J. Compos. Sci. 2020, 4, 123. [Google Scholar] [CrossRef]
- Moradi Pour, M.; Saberi-Riseh, R.; Mohammadinejad, R.; Hosseini, A. Investigating the formulation of alginate-gelatin encapsulated pseudomonas fluorescens (vupf5 and t17-4 strains) for controlling fusarium solani on potato. Int. J. Biol. Macromol. 2019, 133, 603–613. [Google Scholar] [CrossRef]
- Saberi-Riseh, R.; Moradi-Pour, M. A novel encapsulation of streptomyces fulvissimus uts22 by spray drying and its biocontrol efficiency against gaeumannomyces graminis, the causal agent of take-all disease in wheat. Pest Manag. Sci. 2021, 77, 4357–4364. [Google Scholar] [CrossRef] [PubMed]
- Zeynadini-Riseh, A.; Mahdikhani-Moghadam, E.; Rouhani, H.; Moradi, M.; Saberi-Riseh, R.; Mohammadi, A. Effect of some probiotic bacteria as biocontrol agents of meloidogyne incognita and evaluation of biochemical changes of plant defense enzymes on two cultivars of pistachio. J. Agric. Sci. Technol. 2018, 20, 179–191. [Google Scholar]
- Hassanisaadi, M.; Shahidi Bonjar, A.H.; Rahdar, A.; Varma, R.S.; Ajalli, N.; Pandey, S. Eco-friendly biosynthesis of silver nanoparticles using aloysia citrodora leaf extract and evaluations of their bioactivities. Mater. Today Commun. 2022, 33, 104183. [Google Scholar] [CrossRef]
- Hassanisaadi, M.; Barani, M.; Rahdar, A.; Heidary, M.; Thysiadou, A.; Kyzas, G.Z. Role of agrochemical-based nanomaterials in plants: Biotic and abiotic stress with germination improvement of seeds. Plant Growth Regul. 2022, 97, 375–418. [Google Scholar] [CrossRef]
- Mohammadzadeh, V.; Barani, M.; Amiri, M.S.; Yazdi, M.E.T.; Hassanisaadi, M.; Rahdar, A.; Varma, R.S. Applications of plant-based nanoparticles in nanomedicine: A review. Sustain. Chem. Pharm. 2022, 25, 100606. [Google Scholar] [CrossRef]
- Riseh, R.S.; Hassanisaadi, M.; Vatankhah, M.; Babaki, S.A.; Barka, E.A. Chitosan as potential natural compound to manage plant diseases. Int. J. Biol. Macromol. 2022, 220, 998–1009. [Google Scholar] [CrossRef]
- Oliveira, J.; Parisi, M.; Baggio, J.; Silva, P.; Paviani, B.; Spoto, M.; Gloria, E. Control of rhizopus stolonifer in strawberries by the combination of essential oil with carboxymethylcellulose. Int. J. Food Microbiol. 2019, 292, 150–158. [Google Scholar] [CrossRef] [PubMed]
- Berninger, T.; González López, Ó.; Bejarano, A.; Preininger, C.; Sessitsch, A. Maintenance and assessment of cell viability in formulation of non-sporulating bacterial inoculants. Microb. Biotechnol. 2018, 11, 277–301. [Google Scholar] [CrossRef] [Green Version]
- Nocker, A.; Fernández, P.S.; Montijn, R.; Schuren, F. Effect of air drying on bacterial viability: A multiparameter viability assessment. J. Microbiol. Methods 2012, 90, 86–95. [Google Scholar] [CrossRef]
- Cadena, M.B.; Preston, G.M.; Van der Hoorn, R.A.; Flanagan, N.A.; Townley, H.E.; Thompson, I.P. Enhancing cinnamon essential oil activity by nanoparticle encapsulation to control seed pathogens. Ind. Crops Prod. 2018, 124, 755–764. [Google Scholar] [CrossRef]
- Saekow, M.; Naradisorn, M.; Tongdeesoontorn, W.; Hamauzu, Y. Effect of carboxymethyl cellulose coating containing zno-nanoparticles for prolonging shelf life of persimmon and tomato fruit. J. Food Sci. Agric. Technol. (JFAT) 2019, 5, 41–48. [Google Scholar]
- Yinzhe, R.; Shaoying, Z. Effect of carboxymethyl cellulose and alginate coating combined with brewer yeast on postharvest grape preservation. Int. Sch. Res. Not. 2013, 2013, 1–7. [Google Scholar] [CrossRef]
- Lante, A.; Tinello, F.; Nicoletto, M. Uv-a light treatment for controlling enzymatic browning of fresh-cut fruits. Innov. Food Sci. Emerg. Technol. 2016, 34, 141–147. [Google Scholar] [CrossRef]
- Thivya, P.; Bhosale, Y.; Anandakumar, S.; Hema, V.; Sinija, V. Development of active packaging film from sodium alginate/carboxymethyl cellulose containing shallot waste extracts for anti-browning of fresh-cut produce. Int. J. Biol. Macromol. 2021, 188, 790–799. [Google Scholar] [CrossRef] [PubMed]
- Yu, K.; Zhou, L.; Xu, J.; Jiang, F.; Zhong, Z.; Zou, L.; Liu, W. Carboxymethyl cellulose-based water barrier coating regulated postharvest quality and ros metabolism of pakchoi (brassica chinensis l.). Postharvest Biol. Technol. 2022, 185, 111804. [Google Scholar] [CrossRef]

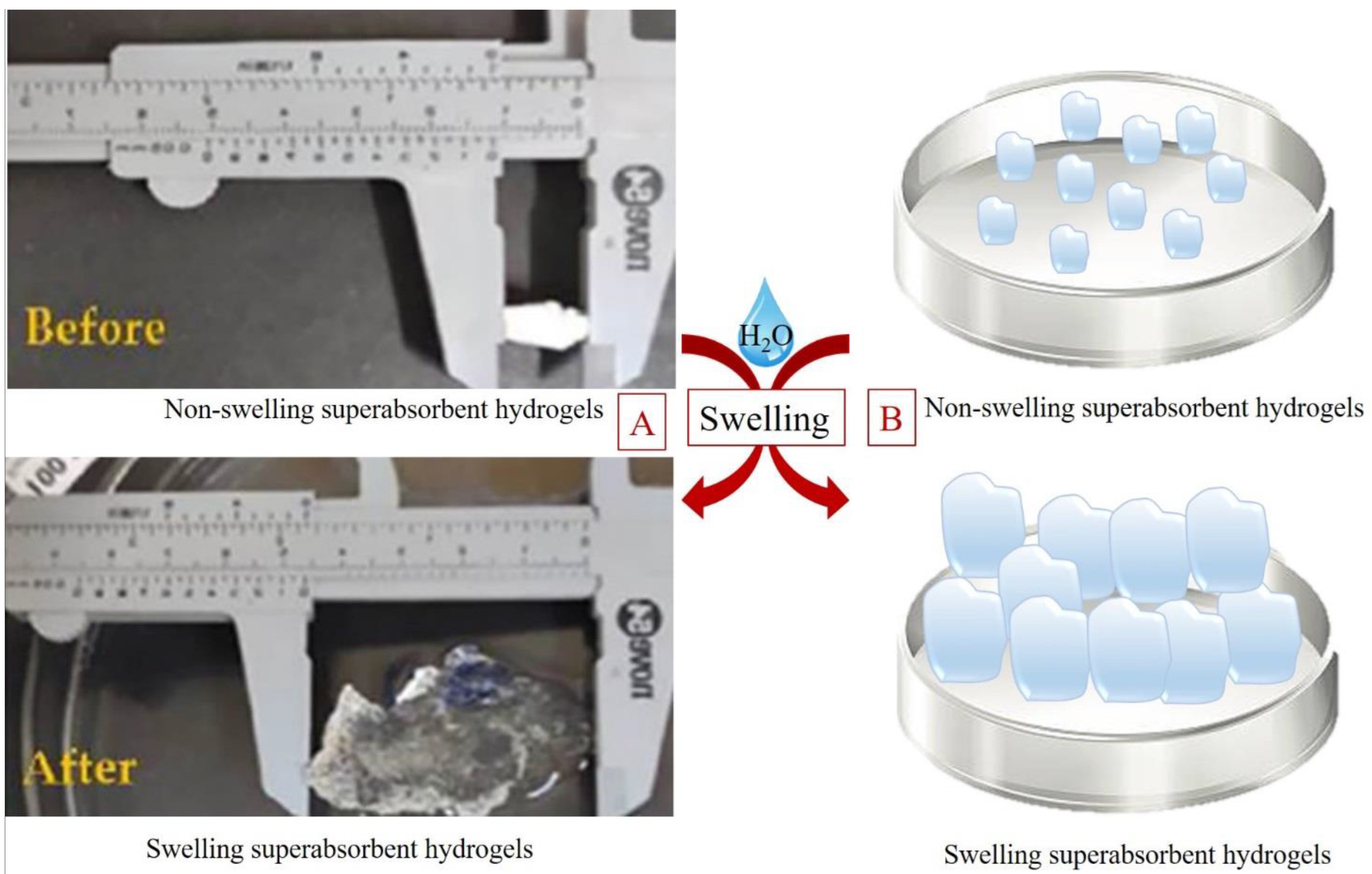

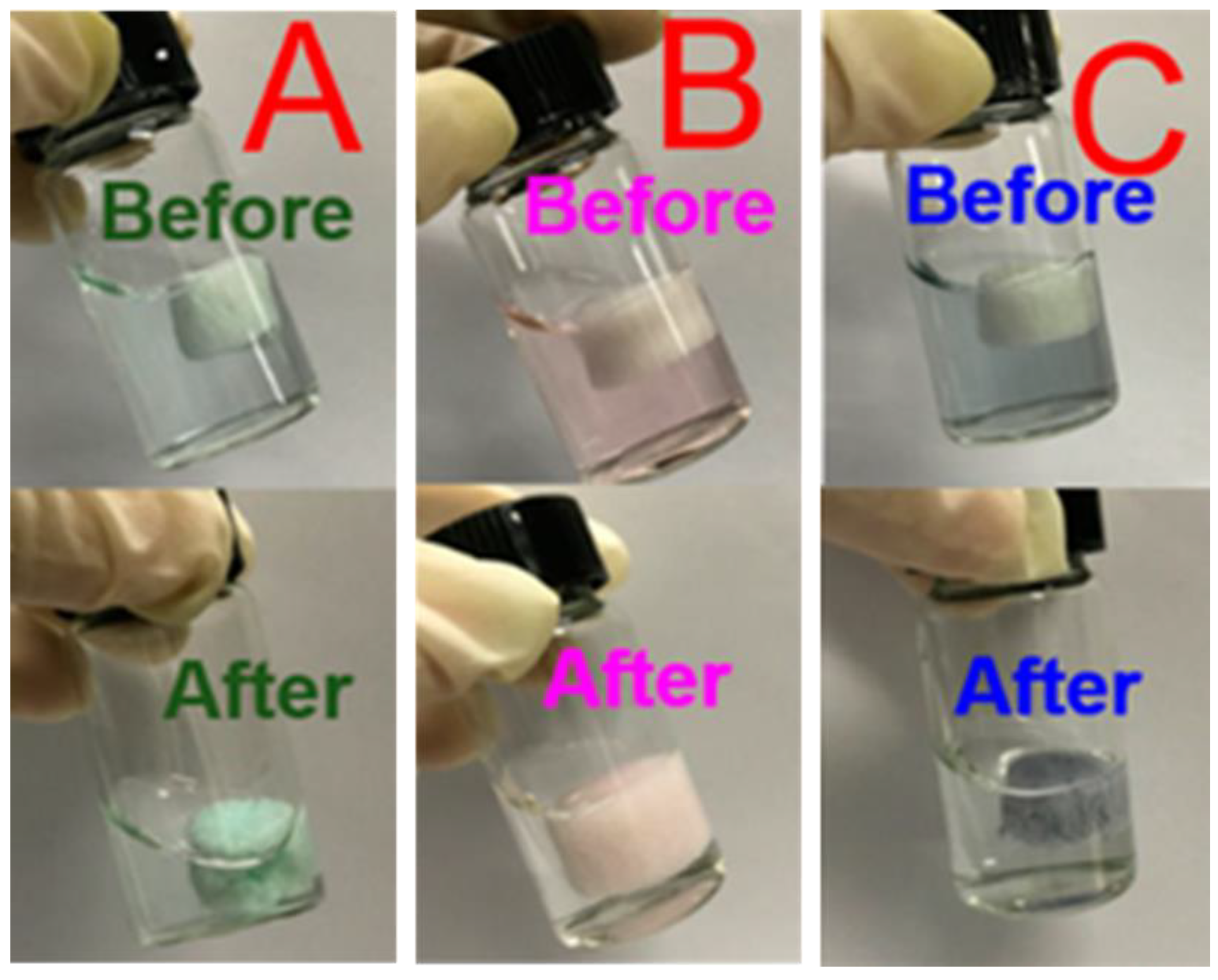
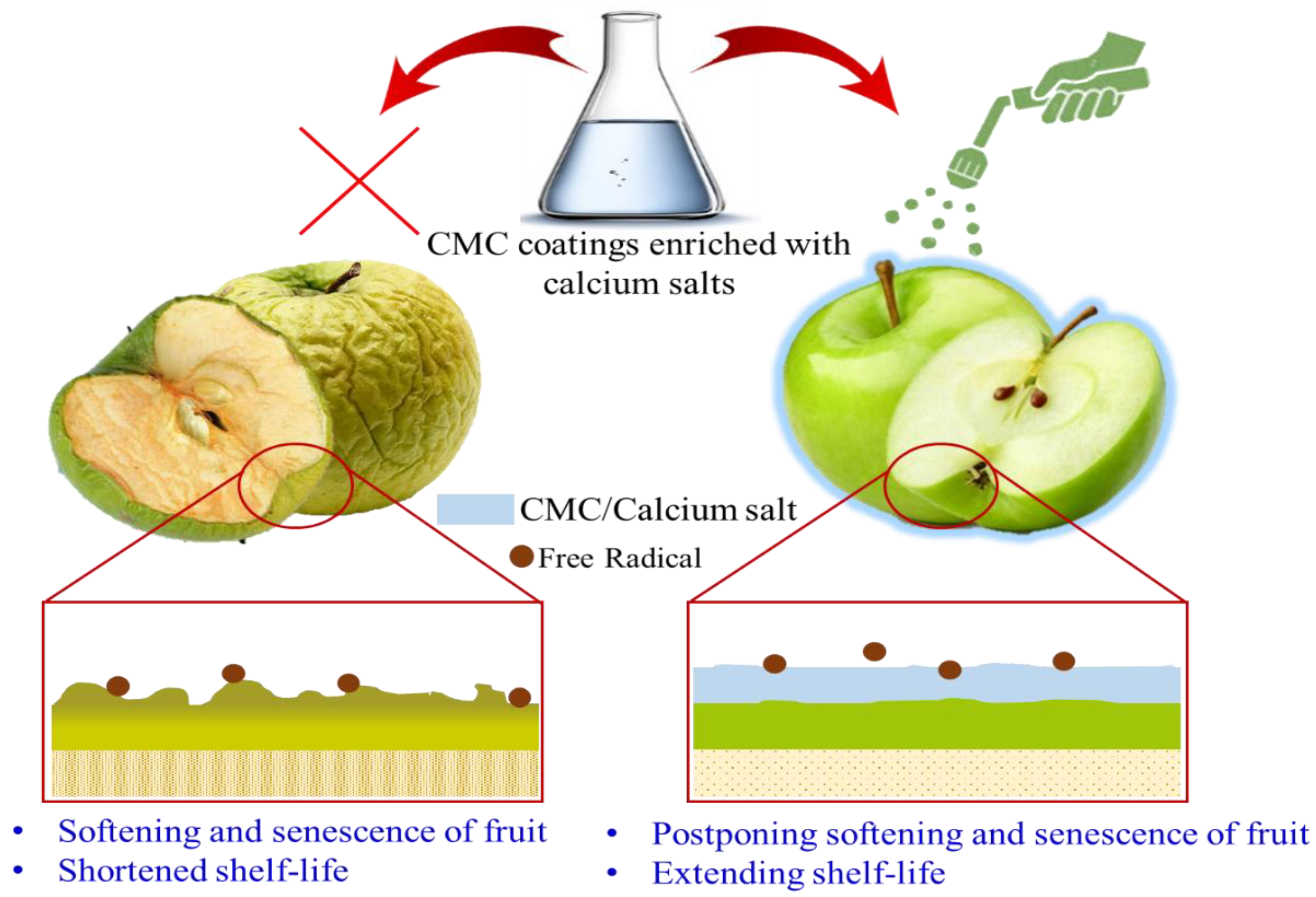
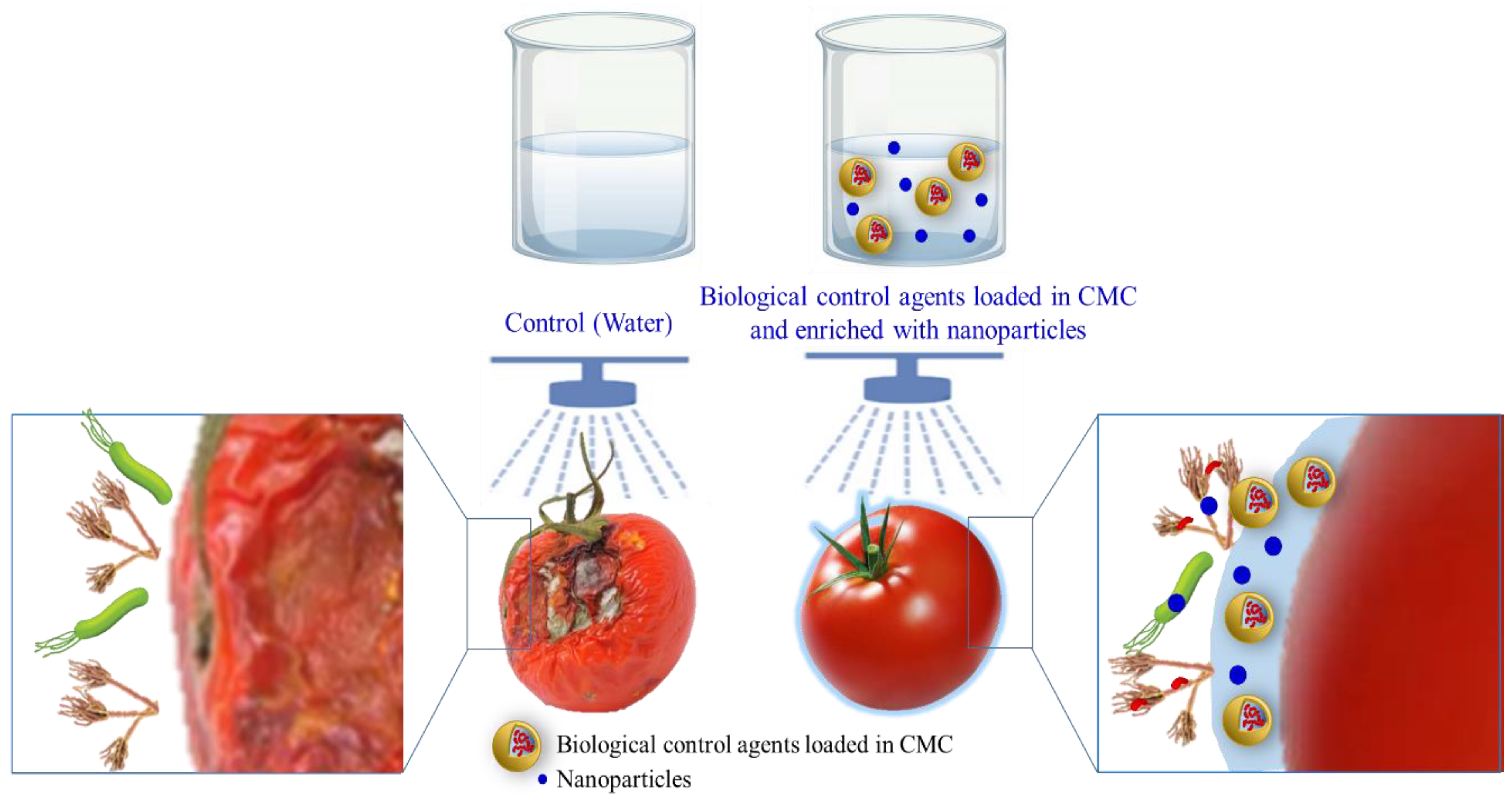
| Application | Results | Reference |
|---|---|---|
| CMC and poly 4-vinylpyridine (P4VP) hy-drogel-N, N, -methylene bis acrylamide | Enhanced the urea absorption | [28] |
| CMC-PVP hydrogel | Used as potential eco-friendly water-saving materials | [29] |
| The root targeted delivery vehicle (RTDV) with CMC in wheat | Improvement in seed yield | [30] |
| Dual-functional redox responsive hydrogel by CMC | Capture heavy metal ions in the soil | [31] |
| CMC-zein-based nanopesticide delivery system | Improve adhesion and antiultraviolet properties | [20] |
| Alginate-CMC | Highest encapsulation efficiency; a disease control agent | [32] |
| NPK fertilizer-CMC- acrylic acid | Easier and faster diffusion of water molecules into the hydrogel, and larger water absorption | [27] |
| Citric acid cross-linked CMC hydrogels | Control of insects | [33] |
| CMC is proposed as a coating agent to encapsulate zinc hydroxide nitrate–sodium dodecylsulphate–imidacloprid (ZHN–SDS–IC) for the implementation of controlled release formulation (CRF) in pesticide | Creating an external gel layer on the surface of ZHN-SDS-IC-CMC as an additional barrier that slows IC diffusion. | [34] |
| Nanoemulsion edible coating using caboxymethyl cellulose | This coating prevented aging caused by oxidative damage of tomatoes by maintaining the level of antioxidant enzymes. | [35] |
| Avocado peel-coconut-CMC in strawberries | Biopolymer coatings with plant extracts as a potential method for ecological preservation in strawberries against microbial deterioration. | [36] |
| Characteristics | Carboxymethyl Cellulose (CMC) | Cellulose |
|---|---|---|
| Solubility in water | Insoluble | Soluble |
| Mechanical strength | Moderate strength | Moderate strength |
| Availability | Abundance | Abundance |
| Sources | This is a derivative of cellulose; however, the synthesis of CMC has been reported from paper sludge, wood residue, textile wastes, mixed office waste, and terry towel waste | Cell wall of plants, algae, and oomycetes |
| Synthesis method | Alkalization or etherification of cellulose using sodium monochloroacetic acid and different sodium hydroxides | - |
| Toxicity | Nontoxic | Nontoxic |
| Applications | As hydrogel, as absorbent, in encapsulation, targeted delivery | In textiles, biomedical, industrial, electronics |
| Source of CMC | The Method of Synthesis CMC | Characterization Methods | Reference |
|---|---|---|---|
| Mixed office waste | NaOH (0.063–0.156 M); 115 mL isopropanol (30 min at 25 °C); stirring was continued (60 min); predissolved sodium monochloroacetate (0.075–0.118 M) in 10 mL isopropanol; the reaction mixture was heated (40–70 °C) for 1–4 h; alkali with acetic acid (5 M). The reaction mixture was filtered, washed with 70% methanol, and dried at 60 °C in a hot air oven. | FTIR and SEM | [54] |
| Oil palm empty fruit bunch | NaOH (10–40%); isopropanol (1.5 h to perform the alkalization reaction). The solution was heated at a reaction temperature of 45 to 75 °C for 1 to 4 h (etherification reaction). The slurry was filtered, and the solid product was washed five times with 50 mL of ethanol, followed by a one-time wash with methanol to remove sodium glycolate and chloride, and then dried in the oven at 60 °C for 3 h. | FTIR, XRD, SEM | [61] |
| Terry towel waste | An amount of 40% NaOH, isopropyl alcohol (alkalization reaction, 90 min); monochloroacetic acid was added to the mixture for 30 min (then kept at 55 °C for 3.5 h). Methanol (70%, v/v) was added to the reactor, and the mixture was neutralized with acetic acid (90% v/v). The CMC was recovered by filtration and washed six times with ethanol:water (70:30, v/v). Finally, the product was washed with methanol and oven dried at 60 °C. Two etherification treatments were performed. The synthesized CMC was then ground and filtered through a 60-mesh nylon cloth. | FTIR, TGA | [55] |
| Wheat straw | Ethanol, NaOH (for alkalization treatment 1 h at 30 °C) and sodium monochloroacetate (40 °C for 0.5 h); then, the reaction mixture was heated at 70 °C for 2 h. The mixture was cooled to room temperature, added to 100 mL of 80% (v/v) ethanol, and neutralized with acetic acid. After filtration, the product was washed three times with 80% (v/v) ethanol and dried in an oven at 50 °C for 16 h. | FTIR, XRD, SEM | [70] |
| Thai rice straw | Isopropanol and NaOH (for alkalinization process overnight). The methylation process was initiated by adding sodium monochloroacetate to the suspension within 30 min; the reaction mixture was incubated at 50 °C for 3 h. The obtained CMC was purified by suspending it in 70% ethanol and neutralizing the suspension with glacial acetic acid. The CMC was washed with 70% ethanol, 80% methanol, and 95% ethanol. The CMC was dried (vacuum oven at 70 °C overnight). | FTIR, XRD | [71] |
| Corn husk | NaOH was added to a pure cellulose and ethanol solution (mechanical stirring, at room temperature, 4 h) for the alkalization reaction. The carboxymethylation reaction: monochloroacetic acid (MCA) was slowly added with constant stirring. The product was then filtered and suspended in 200 mL of methanol. The slurry was neutralized using glacial acetic acid. The sample was washed using a 70% ethanol solution and then dried at 60 °C. | FTIR, XRD | [72] |
| Microcapsule/Hydrogel | Goal | Result | Reference |
|---|---|---|---|
| QUE 1-loaded CHC-CMC nanoparticles | Food Industries | The enclosure of QUE in CDNPs improved its chemical stability and solubility, and higher biological activity. | [100] |
| Pea proteins-CMC encapsulation of linoleic acid | Food Industries | Better physico-chemical properties. | [101] |
| RPH 2–CMC nanoparticles | Food, Medical | A good biocompatible inhibitor of proliferation of breast cancer cells. | [102] |
| SAP 2-AM 3- CMC- -MBA 4- loaded with potassium nitrate | Agriculture | The swelling ratio was 190 g/g of dry gel; the amount of released KNO3 increased with an increasing loading percentage of SAP. | [103] |
| PAAm 5-MC 6-MMt 7 loaded with urea | Agriculture | For application in agriculture as a nutrient carrier vehicle. | [104] |
| Citric acid cross-linked CMC hydrogels and their bentonite composite | Agriculture | Useful for the efficient control of insects having an alkaline gut pH. | [33] |
| The encapsulation of Bti 8 in a matrix of CMC as the polymeric matrix and aluminum sulfate as the gelation agent | Agriculture | In total, 100% mosquito larval mortality, from the second day of treatment, and higher larvicidal activity of Bti at higher temperatures up to 50 °C compared to a nonencapsulated Bti spore/crystal mixture. | [105] |
| Method/Goal | Result | Reference |
|---|---|---|
| CMC-polyvinylpyrrolidone cross-linked with gamma irradiation and loading urea on hydrogel | Slow urea release, good water retention capacity, being economical, and environmentally friendly | [81] |
| Superabsorbent hydrogels polyvinylpyrrolidone-CMC of different copolymer compositions by gamma radiation and loading NPK fertilizer on hydrogel | Slow release, high swelling, and slow water retention | [118] |
| Superabsorbent hydrogels based on cross-linked CMC- acrylamide | As water-managing materials for agriculture and horticulture in drought conditions | [111] |
| Application of polyvinyl Alcohol-CMC hydrogel as a superabsorbent compound in the soil | Increase water retention in desert regions | [119] |
| CMC and poly vinyl pyrrolidone synthesized by gamma radiation and loading urea on hydrogel | Slow urea release and good water retention capacity | [81] |
| Synthesis carboxymethyl cellulose (CMC) via a free radical polymerization technique with acrylamide and 2-Acrylamido-2-methylpropanesulfonic acid (AMPS) as hydrophilic monomers | Nutrient carrier and amendment for sandy soil for advanced agricultural applications | [28] |
| The copolymer of CMC and mixtures of different comonomers | Suitable in agriculture purposes | [120] |
| Carboxymethyl cellulose/nano-CaCO3 composite amended in the loamy sand soil on maize growth | As an alternative soil amendment for agricultural applications | [121] |
| Method/Goal | Results | Reference |
|---|---|---|
| Carboxymethyl cellulose (CMC) bridged chlorapatite for removal of zinc and cadmium from water | High uptake of heavy metal from water | [133] |
| CMC-polyacrylamide for the wastewater remediation | Wastewater treatment and catalytic application | [126] |
| Nanoparticles stabilized with CMC for in situ destructions of chlorinated ethane | The biological degradation with CMC as the carbon source and hydrogen from the abiotic/biotic processes | [134] |
| Iron nanoparticles stabilized by (NaCMC) for chromium removal | CMC as an effective stabilizer in nanoparticles for the effective removal of chromium | [135] |
| A novel biochar supported nanoscale zero-valent iron stabilized by CMC for the removal of chromium | A low-cost, “green”, and effective sorbent for removal of Cr(VI) in the environment. | [136] |
| Synthesize cross-linked beads from chitosan and CMC with arginine as a cross-linker for adsorption of Pb(II) and Cd(II) | Remove Pb(II) and Cd(II) from aqueous solution with high removal efficiency | [129] |
| A novel carboxymethyl cellulose sodium (CMC-Na) encapsulated phosphorus (P)-enriched biochar for Pb(II), Cd(II), and Ni(II) removal | A low-cost and high-efficiency adsorbent | [137] |
| Method/Goal | Results | Reference |
|---|---|---|
| Polysaccharides from Osmunda japonica-CMC (0.7%) for preserve tomato | Increased quality of postharvest tomatoes and reduced weight loss and ascorbic acid | [154] |
| Locust bean gum/carboxycellulose nanocrystal (LBG/C-CNC) coating for improving properties in strawberries | Antibacterial properties and as effective preservation | [155] |
| CMC as an edible coating in fresh-cut melons | A superior antimicrobial protection and increased product storability | [156] |
| CMC extracted from Brewer’s spent grain as a new approach to coating strawberries | Protective properties in room temperature | [157] |
| Application of CMC with the aim of the development of bio-based films and with new functionalities in coffee grounds | Preservation in the physicochemical properties | [158] |
| CMC-moringa leaf and seed as a novel postharvest treatment in avocado fruit | Suppressing diseases, prolonging the shelf life, and increase in avocado quality | [159] |
| The ability of carboxymethylcellulose (CMC)-Astragalus honey (Astragalus gossypinus) to control rancidity and microbial spoilage of pistachio kernel during storage at room temperature | Increase in the shelf life of pistachio kernel | [160] |
| The effects of CMC on quality aspects of white asparagus | Increase quality of asparagus (with retarding moisture loss and reducing hardening in their basal part) | [161] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Saberi Riseh, R.; Gholizadeh Vazvani, M.; Hassanisaadi, M.; Skorik, Y.A. Micro-/Nano-Carboxymethyl Cellulose as a Promising Biopolymer with Prospects in the Agriculture Sector: A Review. Polymers 2023, 15, 440. https://doi.org/10.3390/polym15020440
Saberi Riseh R, Gholizadeh Vazvani M, Hassanisaadi M, Skorik YA. Micro-/Nano-Carboxymethyl Cellulose as a Promising Biopolymer with Prospects in the Agriculture Sector: A Review. Polymers. 2023; 15(2):440. https://doi.org/10.3390/polym15020440
Chicago/Turabian StyleSaberi Riseh, Roohallah, Mozhgan Gholizadeh Vazvani, Mohadeseh Hassanisaadi, and Yury A. Skorik. 2023. "Micro-/Nano-Carboxymethyl Cellulose as a Promising Biopolymer with Prospects in the Agriculture Sector: A Review" Polymers 15, no. 2: 440. https://doi.org/10.3390/polym15020440
APA StyleSaberi Riseh, R., Gholizadeh Vazvani, M., Hassanisaadi, M., & Skorik, Y. A. (2023). Micro-/Nano-Carboxymethyl Cellulose as a Promising Biopolymer with Prospects in the Agriculture Sector: A Review. Polymers, 15(2), 440. https://doi.org/10.3390/polym15020440








