Polysaccharides for Biodegradable Packaging Materials: Past, Present, and Future (Brief Review)
Abstract
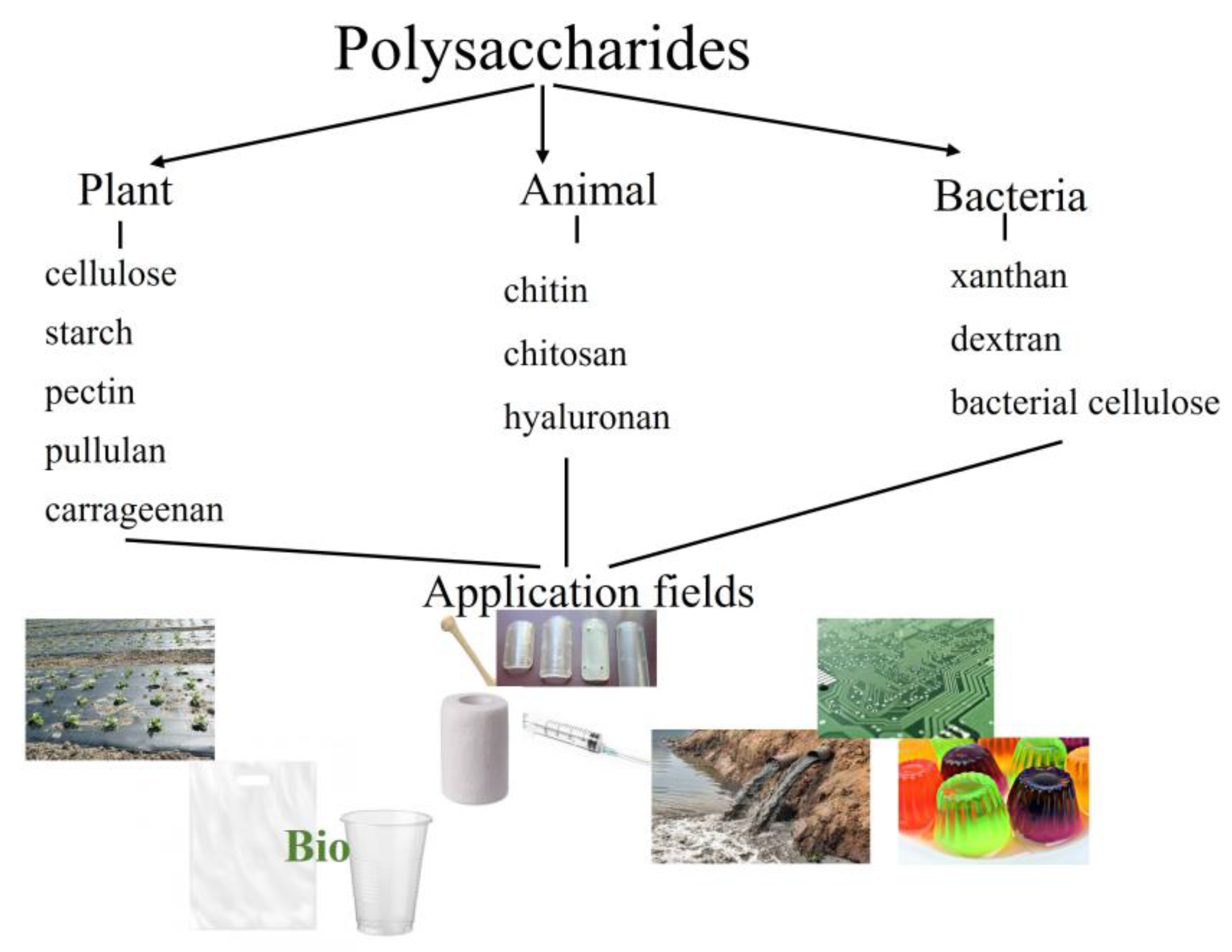
:1. Introduction
2. Polysaccharides and Their Derivatives as Components of Biodegradable Packaging Materials
2.1. Cellulose and Its Derivatives
2.2. Starch
2.3. Chitin and Chitosan
2.4. Some Other Polysaccharides
2.4.1. Pectin
2.4.2. Alginate and Carrageenan
3. Conclusions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Chawla, B.; Varghese, B.S.; Chithra, A.; Hussain, C.G.; Keçili, R.; Hussein, C.M. Environmental impacts of post-consumer plastic wastes: Treatment technologies towards eco-sustainability and circular economy. Chemosphere 2022, 308, 135867. [Google Scholar] [CrossRef] [PubMed]
- Yu, L. Biodegradable Polymer Blends and Composites from Renewable Resources; Wiley: Hoboken, NJ, USA, 2009. [Google Scholar]
- Nair, A.B.; Sivasubramanian, P.; Balakrishnan, P.; Kumar, K.A.N.A.; Sreekala, M.S. Environmental effects, biodegradation, and life cycle analysis of fully biodegradable “green” composites. In Polymer Composite: Volume 3, 1st ed.; Sabu, T., Kuruvilla, J., Malhotra, S.K., Koichi, G., Sreekala, M.S., Eds.; Wiley-VCH Verlag GmbH & Co. KGaA: Hoboken, NJ, USA, 2014; Volume 3, pp. 515–568. [Google Scholar]
- Soulestin, J.; Prashantha, K.; Lacrampe, M.F.; Krawczak, P. Bioplastics based on nanocomposites for packaging applications. In Handbook of Bioplastics and Biocomposites Engineering Applications; Pilla, S., Ed.; Scrivener Publishing LLC: Salem, MA, USA, 2011; Part 3; pp. 77–119. [Google Scholar]
- Iwata, T. Biodegradable and bio-based polymers: Future prospects of eco-friendly plastics. Angew. Chem. Int. Ed. 2015, 54, 3210–3215. [Google Scholar] [CrossRef] [PubMed]
- Dufresne, A.; Lin, N. Characterization of polysaccharide nanocrystal-based materials. In Polysaccharide-Based Nanocrystals: Chemistry and Applications, 1st ed.; Huang, J., Chang, P.R., Lin, N., Dufresne, A., Eds.; Wiley-VCH Verlag GmbH & Co. KGaA: Weinheim, Germany, 2015; Chapter 7; pp. 255–299. [Google Scholar]
- Majid, I.; Thakur, M.; Nanda, V. Biodegradable packaging materials. In Encyclopedia of Renewable and Sustainable Materials; Hashmi, S., Choudhury, I.A., Eds.; Elsevier: Amsterdam, The Netherlands, 2018; Volume 7, pp. 688–697. [Google Scholar]
- Künkel, A.; Becker, J.; Börger, L.; Hamprecht, J.; Koltzenburg, S.; Loos, R.; Bernhard Schick, M.; Schlegel, K.; Sinkel, C.; Skupin, G.; et al. Polymers, biodegradable. In Ullmann’s Encyclopedia of Industrial Chemistry; Wiley-VCH Verlag GmbH & Co. KGaA: Weinheim, Germany, 2016; pp. 1–29. [Google Scholar]
- Mastalygina, E.E.; Pantyukhov, P.V.; Popov, A.A. Biodegradation of natural reinforcing fillers for polymer composites. IOP Conf. Ser. Mater. Sci. Eng. 2018, 369, 012044. [Google Scholar] [CrossRef]
- Ponnusamy, P.G.; Mani, S. Material and environmental properties of natural polymers and their composites for packaging applications—A review. Polymers 2022, 14, 4033. [Google Scholar] [CrossRef]
- Pandey, S. Polysaccharide-based membranes for packaging applications. In Polysaccharides: Properties and Applications; Inamuddin, Ahamed, M.I., Boddula, R., Altalhi, T., Eds.; Scrivener Publishing LLC: Salem, MA, USA, 2021; pp. 477–500. [Google Scholar]
- Zhao, L.; Duan, G.; Zhang, G.; Yang, H.; He, S.; Jiang, S. Electrospun functional materials toward food packaging applications: A review. Nanomaterials 2020, 10, 150. [Google Scholar] [CrossRef] [Green Version]
- Wang, J.; Euring, M.; Ostendorf, K.; Zhang, K. Biobased materials for food packaging. J. Bioresour. Bioprod. 2022, 7, 1–13. [Google Scholar] [CrossRef]
- Tharanathan, R.N. Biodegradable films and composite coatings: Past, present and future. Trends Food Sci. Technol. 2003, 14, 71–78. [Google Scholar] [CrossRef]
- Griffin, G.J.L. Biodegradable fillers in thermoplastics. Adv. Chem. Ser. 1974, 134, 159–170. [Google Scholar]
- Griffin, G.J.L. Polypropylene/Starch Blends: Study Of Thermal. British Patent 1,586, 344, 1973. [Google Scholar]
- Rogovina, S.Z. Biodegradable polymer composites based on synthetic and natural polymers of various classes. Polym. Sci. Ser. C 2016, 58, 62–73. [Google Scholar] [CrossRef]
- Ghanbarzadeh, B.; Oleyaei, S.A.; Almasi, H. Nano-structured materials utilized in biopolymer based plastics for food packaging applications. Crit. Rev. Food Sci. Nutr. 2015, 55, 1699–1723. [Google Scholar] [CrossRef] [PubMed]
- Dierings de Souza, E.J.; Hüttner Kringel, D.; Guerra Dias, A.R.; da Rosa Zavareze, E. Polysaccharides as wall material for the encapsulation of essential oils by electrospun technique. Carbohydr. Polym. 2021, 265, 118068. [Google Scholar] [CrossRef]
- Salarbashia, D.; Tajik, S.; Ghasemlouc, M.; Shojaee-Aliabadid, S.; Noghabie, M.S.; Khaksar, R. Characterization of soluble soybean polysaccharide film incorporated essential oil intended for food packaging. Carbohydr. Polym. 2013, 98, 1127–1136. [Google Scholar] [CrossRef]
- Salarbashi, D.; Tajik, S.; Shojaee-Aliabadi, S.; Ghasemlou, M.; Moayyed, H.; Khaksar, R.; Noghabi, M.S. Development of new active packaging film made from a soluble soybean polysaccharide incorporated Zataria multiflora Boiss and Mentha pulegium essential oils. Food Chem. 2014, 146, 614–622. [Google Scholar] [CrossRef] [PubMed]
- Bhargava, N.; Sharanagat, V.S.; Mor, R.S.; Kumar, K. Active and intelligent biodegradable packaging films using food and food waste-derived bioactive compounds: A review. Trends Food Sci. Technol. 2020, 105, 385–401. [Google Scholar] [CrossRef]
- Azman, N.H.; Khairul, W.M.; Sarbon, N.M. A comprehensive review on biocompatible film sensor containing natural extract: Active/intelligent food packaging. Food Control 2022, 141, 109189. [Google Scholar] [CrossRef]
- Khanjanzadeh, H.; Park, B.-D. Covalent immobilization of bromocresol purple on cellulose nanocrystals for use in pH-responsive indicator films. Carbohydr. Polym. 2021, 273, 118550. [Google Scholar] [CrossRef]
- Khanjanzadeh, H.; Park, B.-D.; Pirayesh, H. Intelligent pH- and ammonia-sensitive indicator films using neutral red immobilized onto cellulose nanofibrils. Carbohydr. Polym. 2022, 296, 119910. [Google Scholar] [CrossRef]
- Pirayesh, H.; Park, B.-D.; Khanjanzadeh, H.; Park, H.-J.; Cho, Y.-J. Nanocellulose-based ammonia sensitive smart colorimetric hydrogels integrated with anthocyanins to monitor pork freshness. Food Control 2023, 147, 109595. [Google Scholar] [CrossRef]
- Rogovina, S.Z.; Aleksanyan, K.V.; Novikov, D.D.; Prut, E.V.; Rebrov, A.V. Synthesis and investigation of polyethylene blends with natural polysaccharides and their derivatives. Polym. Sci. Ser. A 2009, 51, 554–562. [Google Scholar] [CrossRef]
- Rogovina, S.Z.; Grachev, A.V.; Aleksanyan, K.V.; Prut, E.V. Study of the thermal stability of blends based on synthetic polymers and natural polysaccharides. Russ. J. Bioorg. Chem. 2011, 37, 791–795. [Google Scholar] [CrossRef]
- Lomakin, S.M.; Rogovina, S.Z.; Grachev, A.V.; Prut, E.V.; Alexanyan, C.V. Thermal degradation of biodegradable blends of polyethylene with cellulose and ethylcellulose. Thermochim. Acta 2011, 521, 66–73. [Google Scholar] [CrossRef]
- Rogovina, S.Z.; Lomakin, S.M.; Aleksanyan, K.V.; Prut, E.V. The Structure, properties, and thermal destruction of biodegradable blends of cellulose and ethylcellulose with synthetic polymers. Russ. J. Phys. Chem. B 2012, 6, 416–424. [Google Scholar] [CrossRef]
- Rogovina, S.; Aleksanyan, K.; Prut, E.; Gorenberg, A. Biodegradable blends of cellulose with synthetic polymers and some other polysaccharides. Eur. Polym. J. 2013, 49, 194–202. [Google Scholar] [CrossRef]
- Rogovina, S.Z.; Aleksanyan, K.V.; Vladimirov, L.V.; Prut, E.V.; Berlin, A.A. New ternary biodegradable compositions based on polyethylene and polysaccharides. Dokl. Phys. Chem. 2015, 465, 270–272. [Google Scholar] [CrossRef]
- Rogovina, S.Z.; Aleksanyan, K.V.; Berlin, A.A. Structureds and properties of biodegradable composites based on synthetic and natural polymers. Fibre Chem. 2016, 48, 191–198. [Google Scholar] [CrossRef]
- Rogovina, S.; Aleksanyan, K.; Vladimirov, L.; Prut, E.; Ivanushkina, N.; Berlin, A. Development of novel biodegradable polysaccharide-based composites and investigation of their structure and properties. J. Polym. Environ. 2018, 26, 1727–1736. [Google Scholar] [CrossRef]
- Rogovina, S.Z.; Aleksanyan, K.V.; Gorenberg, A.Y.; Ivanushkina, N.E.; Prut, E.V.; Berlin, A.A. Investigation of biodegradability of composites based on polyethylene and polysaccharides by some independent methods. Mendeleev Commun. 2018, 28, 105–107. [Google Scholar] [CrossRef]
- Shumigin, D.; Tarasova, E.; Krumme, A.; Meier, P. Rheological and mechanical properties of poly(lactic) acid/cellulose and LDPE/cellulose composites. Mater. Sci. (Medžg.) 2011, 17, 32–37. [Google Scholar] [CrossRef] [Green Version]
- Behjat, T.; Russly, A.R.; Luqman, C.A.; Yus, A.Y.; Nor Azowa, I. Effect of PEG on the biodegradability studies of Kenaf cellulose -polyethylene composites. Int. Food Res. J. 2009, 16, 243–247. [Google Scholar]
- Zykova, A.K.; Pantyukhov, P.V.; Mastalygina, E.E.; Chaverri-Ramos, C.; Nikolaeva, S.G.; Saavedra-Aria, J.J.; Popov, A.A.; Wortman, S.E.; Poletto, M. Biocomposites of low-density polyethylene plus wood flour or flax straw: Biodegradation kinetics across three environments. Polymers 2021, 13, 2138. [Google Scholar] [CrossRef] [PubMed]
- Sailaja, R.R.N.; Seetharamu, S. Mechanical and thermal Properties of LDPE-cellulose acetate phthalate blends—Effect of maleic anhydride-grafted LDPE compatibilizer. J. Appl. Polym. Sci. 2009, 112, 649–659. [Google Scholar] [CrossRef]
- Wang, J.; Han, X.; Zhang, C.; Liu, K.; Duan, G. Source of nanocellulose and its application I nnanocomposite packaging material: A review. Nanomaterials 2022, 12, 3158. [Google Scholar] [CrossRef] [PubMed]
- Saleh Ali, M.A.S.; Jimat, D.N.; Nawawi, W.M.F.W.; Sulaiman, S. Antibacterial, mechanical and thermal properties of PVA/starch composite film reinforced with cellulose nanofiber of sugarcane bagasse. Arabian J. Sci. Eng. 2022, 47, 5747–5754. [Google Scholar] [CrossRef]
- Chan, M.-K.; Tang, T.-H. The properties of starch/cellulose/polyvinyl alcohol composite as hydrodegradable film. Polym. Polym. Compos. 2022, 30, 09673911221100353. [Google Scholar] [CrossRef]
- Ulaganathan, R.K.; Senusi, N.A.M.; Amin, M.A.M.; Abdul Razab, M.K.A.; Ismardi, A.; Abdullah, N.H. Effect of cellulose nanocrystals (CNC) on PVA/CNC bio-nanocomposite film as potential food packaging application. Mater. Today Proc. 2022, 66, 3150–3153. [Google Scholar] [CrossRef]
- Zhao, Y.; Zhou, S.; Xia, X.; Tan, M.; Lv, Y.; Cheng, Y.; Tao, Y.; Lu, J.; Du, J.; Wang, H. High-performance carboxymethyl cellulose-based hydrogel film for food packaging and preservation system. Int. J. Biol. Macromol. 2022, 223, 1126–1137. [Google Scholar] [CrossRef] [PubMed]
- Dey, D.; Selvam, S.P.; Kumar, M.M.; Sadiku, E.R.; Dey, A. Mechanical and structural characterization of eco-friendly films prepared using poly(vinyl alcohol), cellulose nanocrystals and chitosan nanoparticle blend. Asian J. Chem. 2017, 29, 2254–2258. [Google Scholar] [CrossRef]
- Zhang, L.; Zhang, G.; Lu, J.; Liang, H. Preparation and characterization of carboxymethyl cellulose/polyvinyl alcohol blend film as a potential coating material. Polym.-Plast. Technol. Eng. 2013, 52, 163–167. [Google Scholar] [CrossRef]
- Oyeoka, H.C.; Ewulonu, C.M.; Nwuzor, I.C.; Obele, C.M.; Nwabanne, J.T. Packaging and degradability properties of polyvinyl alcohol/gelatin nanocomposite films filled water hyacinth cellulose nanocrystals. J. Bioresour. Bioprod. 2021, 6, 168–185. [Google Scholar] [CrossRef]
- Eichers, M.; Bajwa, D.; Shojaeiarani, J.; Bajwa, S. Biobased plasticizer and cellulose nanocrystals improve mechanical properties of polylactic acid composites. Ind. Crops Prod. 2022, 183, 114981. [Google Scholar] [CrossRef]
- Rizal, S.; Olaiya, F.G.; Saharudin, N.I.; Abdullah, C.K.; Olaiya, N.G.; Mohamad Haafiz, M.K.; Bashir Yahya, E.; Sabaruddin, F.A.; Ikramullah; Abdul Khalil, H.P.S. Isolation of textile waste cellulose nanofibrillated fibre reinforced in polylactic acid-chitin biodegradable composite for green packaging application. Polymers 2021, 13, 325. [Google Scholar] [CrossRef] [PubMed]
- Claro, P.I.C.; Neto, A.R.S.; Bibbo, A.C.C.; Mattoso, L.H.C.; Bastos, M.S.R.; Marconcini, J.M. Biodegradable blends with potential use in packaging: A comparison of PLA/chitosan and PLA/cellulose acetate films. J. Polym. Environ. 2016, 24, 363–371. [Google Scholar] [CrossRef]
- Rogovina, S.Z.; Aleksanyan, K.V.; Kosarev, A.A.; Ivanushkina, N.E.; Prut, E.V.; Berlin, A.A. Biodegradable polymer composites based on polylactide and cellulose. Polym. Sci. Ser. B 2016, 58, 38–46. [Google Scholar] [CrossRef]
- Rogovina, S.Z.; Aleksanyan, K.V.; Gorenberg, A.Y.; Deryabina, Y.I.; Isakova, E.P.; Prut, E.V.; Berlin, A.A. Investigation of mechanical properties, morphology, and biodegradability of compositions based on polylactide and polysaccharides. Russ. J. Bioorg. Chem. 2016, 42, 685–693. [Google Scholar] [CrossRef]
- Rogovina, S.Z.; Aleksanyan, K.V.; Grachev, A.V.; Berlin, A.A.; Prut, E.V. Investigation of mechanical and thermophysical properties of biodegradable compositions of polylactide with ethyl cellulose and chitosan containing poly(ethylene glycol). Mendeleev Commun. 2015, 25, 361–363. [Google Scholar] [CrossRef]
- Rogovina, S.Z.; Aleksanyan, K.V.; Vladimirov, L.V.; Berlin, A.A. Biodegradable polymer materials based on polylactide. Russ. J. Phys. Chem. B 2019, 13, 812–818. [Google Scholar] [CrossRef]
- Pracella, M.; Mura, C.; Galli, G. Polyhydroxyalkanoate nanocomposites with cellulose nanocrystals as biodegradable coating and packaging materials. ACS Appl. Nano Mater. 2021, 4, 260–270. [Google Scholar] [CrossRef]
- Usurelu, C.D.; Badila, S.; Frone, A.N.; Panaitescu, D.M. Poly(3-hydroxybutyrate) nanocomposites with cellulose nanocrystals. Polymers 2022, 14, 1974. [Google Scholar] [CrossRef]
- Rapisarda, M.; Patanè, C.; Pellegrino, A.; Malvuccio, A.; Rizzo, V.; Muratore, G.; Rizzarelli, P. Compostable polylactice and cellulose based packaging for fresh-cut cherry tomatoes: Performance evaluatio and influence of sterilization treatment. Materials 2020, 13, 3432. [Google Scholar] [CrossRef]
- Garcia-Garcia, D.; Lopez-Martinez, J.; Balart, R.; Strömberg, E.; Moriana, R. Reinforcing capability of cellulose nanocrystals obtained from pine cones in a biodegradable poly(3-hydroxybutyrate)/poly(ε-caprolactone) (PHB/PCL) thermoplastic blend. Eur. Polym. J. 2018, 104, 10–18. [Google Scholar] [CrossRef]
- Khan, R.A.; Salmieri, S.; Dussault, D.; Sharmin, N.; Lacroix, M. Mechanical, barrier, and interfacial properties of biodegradable composite films made of methylcellulose and poly(caprolactone). J. Appl. Polym. Sci. 2012, 123, 1690–1698. [Google Scholar] [CrossRef]
- Arvanitoyannis, I.; Biliaderis, C. Physical properties of polyol-plasticized edible blends made of methyl cellulose and soluble starch. Carbohydr. Polym. 1999, 38, 47–58. [Google Scholar] [CrossRef]
- Riyajan, S.-A. Fabrication and properties of inspired green modified cellulose/cassava starch blend. Ind. Crops Prod. 2022, 187, 115339. [Google Scholar] [CrossRef]
- Yu, Z.; Ji, Y.; Bourg, V.; Bilgen, M.; Carson Meredith, J. Chitin- and cellulose-based sustainable barrier materials: A review. Emergent Mater. 2020, 3, 919–936. [Google Scholar] [CrossRef]
- de Mesquita, J.P.; Donnici, C.L.; Pereira, F.V. Biobased nanocomposites from layer-by-layer assembly of cellulose nanowhiskers with chitosan. Biomacromolecules 2010, 11, 473–480. [Google Scholar] [CrossRef]
- Chaichi, M.; Hashemi, M.; Badii, F.; Mohammadi, A. Preparation and characterization of a novel bionanocomposite ediblefilm based on pectin and crystalline nanocellulose. Carbohydr. Polym. 2017, 157, 167–175. [Google Scholar] [CrossRef]
- Reichert, A.A.; Ribas Sá, M.; Castilhos de Freitas, T.; Barbosa, R.; Soares Alves, T.; Beckes, E.H.; Alano, J.H.; Oliveira, A.D. Barrier, mechanical and morphological properties of biodegradable films based on corn starch incorporated with cellulose obtained from pineapple crowns. J. Nat. Fibers 2022, 19, 8541–8554. [Google Scholar] [CrossRef]
- Ghalehno, M.D.; Yousefi, H. Green nanocomposite made from carboxymethyl cellulose reinforced with four types of cellulose nanomaterials of wheat straw. J. Appl. Polym. Sci. 2022, 139, e52802. [Google Scholar] [CrossRef]
- Suvorova, A.I.; Tyukova, I.S. Biodegradable systems: Thermodynamics, rheological properties, and biocorrosion. Polym. Sci. Ser. A 2008, 50, 743–750. [Google Scholar] [CrossRef]
- Azeredo, H.M.C.; Mattoso, L.H.C.; Avena-Bustillos, R.J.; Filho, G.C.; Munford, M.L.; Wood, D.; McHugh, T.H. Nanocellulose reinforced chitosan composite films as affected by nanofiller loading and plasticizer content. J. Food Sci. 2009, 75, 1. [Google Scholar] [CrossRef]
- Abdul Khalil, H.P.S.; Saurabha, C.K.; Adnan, A.S.; Nurul Fazitaa, M.R.; Syakira, M.I.; Davoudpoura, Y.; Rafatullaha, M.; Abdullaha, C.K.; Haafiza, M.K.M.; Dungani, R. A review on chitosan-cellulose blends and nanocellulose reinforced chitosan biocomposites: Properties and their applications. Carbohydr. Polym. 2016, 150, 216–226. [Google Scholar]
- Coelho, C.C.S.; Cerqueira, M.A.; Pereira, R.N.; Pastrana, L.M.; Freitas-Silva, O.; Vicente, A.A.; Cabral, L.M.C.; Teixeira, J.A. Effect of moderate electric fields in the properties of starch andchitosan films reinforced with microcrystalline cellulose. Carbohydr. Polym. 2017, 147, 1181–1191. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Satam, C.C.; Irvin, C.W.; Coffey, C.J.; Geran, R.K.; Ibarra- Rivera, R.; Shofner, M.L.; Meredith, J.C. Controlling barrier and mechanical properties of cellulose nanocrystals by blending with chitin nanofibers. Biomacromolecules 2020, 21, 545–555. [Google Scholar] [CrossRef] [PubMed]
- Rajeswari, A.; Jackcina Stobel Christy, E.; Swathi, E.; Pius, A. Fabrication of improved cellulose acetate-based biodegradable films for food packaging applications. Environ. Chem. Ecotoxicol. 2020, 2, 107–114. [Google Scholar] [CrossRef]
- Huang, J.; Zhang, L.; Wang, X. Soy protein-lignosulphonate plastics strengthened with cellulose. J. Appl. Polym. Sci. 2003, 89, 1685–1689. [Google Scholar] [CrossRef]
- Zárate-Ramírez, L.S.; Romero, A.; Bengoechea, C.; Partal, P.; Guerrero, A. Thermo-mechanical and hydrophilic properties of polysaccharide/gluten-based bioplastics. Carbohydr. Polym. 2014, 112, 24–31. [Google Scholar] [CrossRef] [PubMed]
- Chan, R.; Lim, L.-T.; Barbut, S.; Marcone, M.F. Extrusion and characterization of soy protein film incorporated with soy cellulose microfibers. Int. Polym. Process. 2014, 29, 467–476. [Google Scholar] [CrossRef]
- Kumar, M.; Kumar, V.; Saran, S. Efficient production of bacterial cellulose based composites using zein protein extracted from corn gluten meal. J. Food Sci. Technol. 2022. [Google Scholar] [CrossRef]
- da Silva Filipini, g.; Romani, V.P.; Guimarães Martins, V. Blending collagen, methylcellulose, and whey protein in films as a greener alternative for food packaging: Physicochemical and biodegradable properties. Packag. Technol. Sci. 2021, 34, 91–103. [Google Scholar] [CrossRef]
- Yao, Y.; Wang, R.; Chai, Y. Preparation and characterization of homogeneous and enhanced casein protein-based composite films via incorporating cellulose microgel. Sci. Rep. 2019, 9, 1221. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sánchez-González, L.; Pastor, C.; Vargas, M.; Chiralt, A.; Conzález-Martínez, C.; Cháfer, M. Effect of hydroxypropylmethylcellulose and chitosan coatings with and without bergamot essential oil on quality and safety of cold-stored grapes. Postharvest Biol. Technol. 2011, 60, 57–63. [Google Scholar] [CrossRef] [Green Version]
- Hasheminya, S.-M.; Mokarram, R.R.; Zhanbarzadeh, B.; Hamishekar, H.; Samadi Kafil, H.; Dehghannya, J. Development and characterization of biocomposite films made from kefiran, carboxymethyl cellulose and Satureja Khuzestanica essential oil. Food Chem. 2019, 289, 443–452. [Google Scholar] [CrossRef] [PubMed]
- Bahrami, A.; Fattahi, R. Biodegradable carboxymethyl cellulose–polyvinyl alcohol composite incorporated with Glycyrrhiza Glabra L. essential oil: Physicochemical and antibacterial features. Food Sci. Nutr. 2021, 9, 4979–4985. [Google Scholar] [CrossRef] [PubMed]
- Wigati, L.P.; Wardana, A.A.; Tanaka, F.; Tanaka, F. Application of pregelatinized corn starch and basil essential oil edible coating with cellulose nanofiber as Pickering emulsion agent to prevent quality-quantity loss of mandarin orange. Food Packag. Shelf Life 2023, 35, 101010. [Google Scholar] [CrossRef]
- dos Santos Acosta, P.P.; Lattores, L.M.; Guimarães Martins, V. The influence of cinnamon and listea cubeba essential oils on methylcellulose films. J. Appl. Polym. Sci. 2022, 140, e53342. [Google Scholar] [CrossRef]
- Amjadi, S.; Nouri, S.; Ashrafi Yorghanlou, R.; Roufegarinejad, L. Development of hydroxypropyl methylcellulose/sodium alginate blend active film incorporated with Dracocephalum moldavica L. essential oil for food preservation. J. Thermoplast. Compos. Mater. 2020, 35, 0892705720962153. [Google Scholar] [CrossRef]
- Arvanitoyannis, I.; Psomiadou, E.; Biliaderis, C.G.; Ogawa, H.; Kawasaki, N.; Nakayama, A. Biodegradable films made from low density polyethylene (LDPE), ethylene acrylic acid (EAA), polycaprolactone (PCL) and wheat starch for food packaging applications: Part 3. Starch-Starke 1997, 49, 306–322. [Google Scholar] [CrossRef]
- Raj, B.; Sankar, K.U.; Siddaramaiah. Low density polyethylene/starch blend films for food packaging applications. Adv. Polym. Technol. 2004, 23, 32–45. [Google Scholar] [CrossRef]
- Kormin, S.; Kormin, F.; Beg, M.D.H.; Piah, M.B.M. Physical and mechanical properties of LDPE incorporated with different starch sources. IOP Conf. Ser. Mater. Sci. Eng. 2017, 226, 012157. [Google Scholar] [CrossRef] [Green Version]
- Sabetzadeh, M.; Bagheri, R.; Masoomi, M. Study on ternary low density polyethylene/linear low density polyethylene/thermoplastic starch blend films. Carbohydr. Polym. 2015, 119, 126–133. [Google Scholar] [CrossRef]
- Jayarathna, S.; Andersson, M.; Andersson, R. Recent advances in starch-based blends and composites for bioplastics applications. Polymers 2022, 14, 4557. [Google Scholar] [CrossRef]
- Hilmi, F.F.; Wahit, M.U.; Shukri, N.A.; Ghazali, Z.; Zanuri, A.Z. Physico-chemical properties of biodegradable films of polyvinyl alcohol/sago starch for food packaging. Mater. Today. Proc. 2019, 16, 1819–1824. [Google Scholar] [CrossRef]
- Cano, A.I.; Cháfer, M.; Chiralt, A.; González-Martínez, C. Physical and microstructural properties of biodegradable films based on pea starch and PVA. J. Food. Eng. 2015, 167, 59–64. [Google Scholar] [CrossRef]
- Priya, B.; Kumar Gupta, V.; Pathania, D.; Singh Singha, A. Synthesis, characterization and antibacterial activity of biodegradable starch/PVA composite films reinforced with cellulosic fibre. Carbohydr. Polym. 2014, 109, 171–179. [Google Scholar] [CrossRef] [PubMed]
- Parvin, F.; Arifur Rahman, M.; Islam, J.M.M.; Khan, M.A.; Saadat, A.H.M. Preparation and characterization of starch/PVA blend for biodegradable packaging material. Adv. Mater. Res. 2010, 123–125, 351–354. [Google Scholar] [CrossRef]
- Listyarini, A.; Fauzia, V.; Imawan, C. Effect of glycerol on mechanical and water barrier properties of cassava starch/PVA composite films. In AIP Conference Proceedings; AIP Publishing LLC: Melville, NY, USA, 2020; Volume 2314, p. 020006. [Google Scholar] [CrossRef]
- Rogovina, S.Z.; Aleksanyan, K.V.; Loginova, A.A.; Ivanushkina, N.E.; Vladimirov, L.V.; Prut, E.V.; Berlin, A.A. Influence of PEG on mechanical properties and biodegradability of composites based on PLA and starch. Starch/Stärke 2018, 70, 1700268. [Google Scholar] [CrossRef]
- Rogovina, S.Z.; Prut, E.V.; Aleksanyan, K.V.; Krasheninnikov, V.G.; Perepelitsina, E.O.; Shashkin, D.P.; Berlin, A.A. Composites based on starch and polylactide. Polym. Sci. Ser. B 2019, 61, 334–340. [Google Scholar] [CrossRef]
- Rogovina, S.Z.; Aleksanyan, K.V. Biodegradable composites based on polylactide and starch. Fibre Chem. 2019, 51, 170–174. [Google Scholar] [CrossRef]
- Aleksanyan, K.V.; Rogovina, S.Z.; Ivanushkina, N.E. Novel biodegradable low-density polyethylene–poly(lactic acid)–starch ternary blends. Polym. Eng. Sci. 2021, 61, 802–809. [Google Scholar] [CrossRef]
- Aleksanyan, K.V.; Rogovina, S.Z.; Shakhov, A.M.; Ivanushkina, N.E. Effect of biodegradation conditions on morphology of ternary compositions of low-density polyethylene with poly(lactic acid) and starch. Mendeleev Commun. 2022, 32, 558–560. [Google Scholar] [CrossRef]
- Kozlowski, M.; Masirek, R.; Piorkowska, E.; Gazicki-Lipman, M. Biodegradable blends of poly(L-lactide) and starch. J. Appl. Polym. Sci. 2007, 105, 269–277. [Google Scholar] [CrossRef]
- Wang, N.; Yu, J.; Ma, X. Preparation and characterization of compatible thermoplastic dry starch/poly(lactic acid). Polym. Compos. 2008, 29, 551–559. [Google Scholar] [CrossRef]
- Huneault, M.A.; Li, H. Morphology and properties of compatibilized polylactide/thermoplastic starch blends. Polymer 2007, 48, 270–280. [Google Scholar] [CrossRef]
- Bastioli, C. Global status of the production for biobased packaging materials. Starch/Stärke 2001, 53, 351–355. [Google Scholar] [CrossRef]
- Li, H.; Huneault, M.A. Comparison of sorbitol and glycerol as plasticizers for thermoplastic starch in TPS/PLA blends. J. Appl. Polym. Sci. 2011, 119, 2439–2448. [Google Scholar] [CrossRef] [Green Version]
- Wang, N.; Yu, J.; Chan, P.; Ma, X. Influence of formamide and water on the properties of thermoplastic starch/poly(lactic acid) blends. Carbohydr. Polym. 2008, 71, 109–118. [Google Scholar] [CrossRef]
- Dubois, P.; Narayan, R. Biodegradable compositions by reactive processing aliphatic polyester/polysaccharide blends. Macromol. Symp. 2003, 198, 233–243. [Google Scholar] [CrossRef]
- Kulkarni, A.; Narayan, R. Effects of modified thermoplastic starch on crystallization kinetics and barrier properties of PLA. Polymers 2021, 13, 4125. [Google Scholar] [CrossRef]
- Myllymäki, O.; Myllärinen, P.; Forssell, P.; Suortti, T.; Lähteenkorva, K.; Ahvenainen, R.; Poutanen, K. Mechanical and permeability properties of biodegradable extruded starch/polycaprolactone films. Packag. Technol. Sci. 1998, 11, 265–274. [Google Scholar] [CrossRef]
- Ninago, M.D.; López, O.V.; Soledad Lencina, M.M.; García, M.A.; Andreucetti, N.A.; Ciolino, A.E.; Villar, M.A. Enhancement of thermoplastic starch final properties by blending with poly(ε-caprolactone). Carbohydr. Polym. 2015, 134, 205–212. [Google Scholar] [CrossRef] [PubMed]
- Shirai, M.A.; Grossmann, M.V.E.; Mali, S.; Yamashita, F.; Garcia, P.S.; Müller, C.M.O. Development of biodegradable flexible films of starch and poly(lactic acid) plasticized with adipate or citrate esters. Carbohydr. Polym. 2013, 92, 19–22. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Shirai, M.A.; Zanela, J.; Kunita, M.H.; Pereira, G.M.; Rubira, A.F.; Müller, C.M.O.; Grossmann, M.V.E.; Yamashita, F. Influence of carboxylic acids on poly(lactic acid)/thermoplastic starch biodegradable sheets produced by calendering–extrusion. Adv. Polym. Technol. 2018, 37, 332–338. [Google Scholar] [CrossRef]
- Muller, J.; González-Martínez, C.; Chiralt, A. Poly(lactic) acid (PLA) and starch bilayer films, containing cinnamaldehyde, obtained by compression moulding. Eur. Polym. J. 2017, 95, 56–70. [Google Scholar] [CrossRef]
- Souza Rosa, R.C.R.; Andrade, C.T. Effect of chitin addition on injection-molded thermoplastic corn starch. J. Appl. Polym. Sci. 2004, 92, 2706–2713. [Google Scholar] [CrossRef]
- Zamudio-Flores, P.B.; Vargas Torres, A.; Salgado-Delgado, R.; Bello-Pérrez, L.A. Influence of the oxidation and acetylation of banana starch on the mechanical and water barrier properties of modified starch and modified starch/chitosan blend films. J. Appl. Polym. Sci. 2010, 115, 991–998. [Google Scholar] [CrossRef]
- Wu, H.; Liu, C.; Chen, J.; Chen, y.; Anderson, D.P.; Chang, P.R. Oxidized pea starch chitosan composite films: Structural characterization and properties. J. Appl. Polym. Sci. 2010, 118, 3082–3088. [Google Scholar] [CrossRef]
- Pelissari, F.M.; Yamashita, F.; Grossmann, M.V.E. Extrusion parameters related to starch chitosan active films properties. Int. J. Food Sci. Technol. 2011, 46, 702–710. [Google Scholar] [CrossRef]
- Lopez, O.; Garcia, M.A.; Villar, M.A.; Gentili, A.; Rodriguez, M.S.; Albertengo, L. Thermo-compression of biodegradable thermoplastic corn starch films containing chitin and chitosan. LWT–Food Sci. Technol. 2014, 57, 106–115. [Google Scholar] [CrossRef]
- Narender Raju, P.; Priyanka; Ganguly, S. Starch-chitosan based composite edible antimicrobial film: Modelling the growth of selected food spoilage microbiota. Indian J. Dairy Sci. 2015, 68, 316–320. [Google Scholar]
- Dang, K.M.; Yoksan, R. Morphological characteristics and barrier properties of thermoplasticstarch/chitosan blown film. Carbohydr. Polym. 2016, 150, 40–47. [Google Scholar] [CrossRef] [PubMed]
- Mendes, J.F.; Paschoalin, R.T.; Carmona, V.B.; Sena Neto, A.R.; Marques, A.C.P.; Marconcini, J.M.; Mattoso, L.H.C.; Medeiros, E.S.; Oliveira, J.E. Biodegradable polymer blends based on corn starch andthermoplastic chitosan processed by extrusion. Carbohydr. Polym. 2016, 137, 452–458. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kaur, M.; Kalia, A.; Thakur, A. Effect of biodegradable chitosan—Rice-starch nanocomposite films on post-harvest quality of stored peach fruit. Starch/Stärke 2017, 69, 1600208. [Google Scholar] [CrossRef] [Green Version]
- Kasemsiri, P.; Dulsang, N.; Pongsa, U.; Hiziroglu, S.; Chindaprasirt, P. Optimization of biodegradable foam composites from cassava starch, oil palm fiber, chitosan and palm oil using Taguchi method and Grey relational analysis. J. Polym. Environ. 2017, 25, 378–390. [Google Scholar] [CrossRef]
- Bergel, B.F.; Machado da Luz, L.; Campomanes Santana, R.M. Comparative study of the influence of chitosan as coating of thermoplastic starch foam from potato, cassava and corn starch. Prog. Org. Coat. 2017, 106, 27–32. [Google Scholar] [CrossRef]
- Onyeaka, H.; Obileke, K.; Makaka, G.; Nwokolo, N. Current research and applications of starch-based biodegradable films for food packaging. Polymers 2022, 14, 1126. [Google Scholar] [CrossRef]
- Da Roz, A.L.; Veiga-Santos, P.; Ferreira, A.M.; Ribeiro Antunes, T.C.; de Lima Leite, F.; Minoru Yamaji, F.; de Carvvalho, A.J.F. Water susceptibility and mechanical properties of thermoplastic starch–pectin blends reactively extruded with edible citric acid. Mater. Res. 2016, 19, 138–142. [Google Scholar] [CrossRef] [Green Version]
- Sganzerla, W.G.; Bachega Rosa, G.; Andrade Ferreira, A.L.; Gonçalves da Rosa, C.; Beling, P.C.; Oliveira Xavier, L.; Martins Hansen, C.; Peruzzo Ferrareze, J.; Ramos Nunes, M.; Manique Barreto, P.L.; et al. Bioactive food packaging based on starch, citric pectin and functionalized with Acca sellowiana waste by-product: Characterization and application in the postharvest conservation of apple. Int. J. Biol. Macromol. 2020, 147, 295–303. [Google Scholar] [CrossRef]
- Coffin, D.R.; Fishman, M.L. Physical and mechanical properties of highly plasticized pectin/starch films. J. Appl. Polym. Sci. 1994, 54, 1311–1320. [Google Scholar] [CrossRef]
- Fishman, M.L.; Coffin, D.R.; Onwukata, C.I.; Konstance, R.P. Extrusion of pectin and glycerol with various combinations of orange albedo and starch. Carbohydr. Polym. 2004, 57, 401–413. [Google Scholar] [CrossRef]
- Sharma, M.; Beniwal, P.; Pal Toor, A. The effect of rice straw derived microfibrillated cellulose as a reinforcing agent in starch/polyvinyl alcohol/polyethylene glycol biocompatible films. Mater. Chem. Phys. 2022, 291, 126652. [Google Scholar] [CrossRef]
- Debnath, B.; Duarah, P.; Haldar, D.; Kumar Purkait, M. Improving the properties of corn starch films for application as packaging material via reinforcement with microcrystalline cellulose synthesized from elephant grass. Food Packag. Shelf Life 2022, 34, 100937. [Google Scholar] [CrossRef]
- Sueiro, A.C.; Faria-Tischer, P.C.S.; Lonni, A.A.S.G.; Mali, S. Biodegradable films of cassava starch, pullulan and bacterial cellulose. Quim. Nova 2016, 39, 1059–1064. [Google Scholar] [CrossRef]
- Fonseca Ferreira, L.; Salgadode Oliveira, A.C.; de Oliveira Begali, D.; de Sena Neto, A.R.; Martins, M.A.; Elvisde Oliveira, J.; Vilela Borges, S.; Yoshida, M.I.; Denzin Tonoli, G.H.; Vilela Dias, M. Characterization of cassava starch/soy protein isolate blends obtained by extrusion and thermocompression. Ind. Crops Prod. 2021, 160, 113092. [Google Scholar] [CrossRef]
- Aung, S.P.S.; Shein, H.H.H.; Aye, K.N.; Nwe, N. Environment-friendly biopolymers for food packaging: Starch, protein, and poly-lactic acid (PLA). In Bio-Based Materials for Food Packaging; Ahmed, S., Ed.; Springer: Singapore, 2018; pp. 173–195. [Google Scholar] [CrossRef]
- Gonzalez-Gutierrez, J.; Partal, P.; Garcia-Morales, M.; Gallegos, C. Development of highly-transparent protein/starch-based bioplastics. Bioresour. Technol. 2010, 101, 2007–2013. [Google Scholar] [CrossRef] [PubMed]
- Bergeret, A. Environment-friendly protein-/starch-based biodegradable polymers and composites. JEC Compos. Magaz. 2008, 45, 42–45. [Google Scholar]
- Hamdani, S.S.; Li, Z.; Rolland, E.; Mohiuddin, M.; Rabnawaz, M. Barrier and mechanical properties of biodegradable paper bilayer-coated with plasticized starch and zein. J. Appl. Polym. Sci. 2022, e53440. [Google Scholar] [CrossRef]
- Wang, L.; Li, F. Study on preparation and properties of starch/gelatin/polyvinyl alcohol biodegradable composite films. In Advanced Graphic Communication, Printing and Packaging Technology, Lecture Notes in Electrical Engineering; Zhao, P., Ye, Z., Xu, M., Yang, L., Eds.; Springer: Singapore, 2020; Volume 600, pp. 743–751. [Google Scholar] [CrossRef]
- Ghasemlou, M.; Aliheidari, N.; Fahmi, R.; Shojaee-Aliabadi, S.; Keshavarz, B.; Cran, M.J.; Khaksar, R. Physical, mechanical and barrier properties of corn starch films incorporated with plant essential oils. Carbohydr. Polym. 2013, 98, 1117–1126. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wigati, L.P.; Wardana, A.A.; Tanaka, F.; Tanaka, F. Strawberry preservation using combination of yam bean starch, agarwood Aetoxylon bouya essential oil, and calcium propionate edible coating during cold staorage evaluated b yTOPSIS-Shannon entropy. Prog. Org. Coat. 2023, 175, 107347. [Google Scholar] [CrossRef]
- Long, H.; Bi, Y.; Pu, L.; Xu, W.; Xue, H.; Fu, G.; Prusky, D. Preparation of chitosan/fennel seed essential oil/starch sodium octenyl succinate composite films for apple fruit preservation. LWT-Food Sci. Technol. 2022, 167, 113826. [Google Scholar] [CrossRef]
- Talón, E.; Trifkovic, K.T.; Nedovic, V.A.; Bugarski, B.M.; Vargas, M.; Chiralt, A.; González-Martínez, C. Antioxidant edible films based on chitosan and starch containingpolyphenols from thyme extracts. Carbohydr. Polym. 2017, 157, 1153–1161. [Google Scholar] [CrossRef] [PubMed]
- Piñeros-Hernandez, D.; Medina-Jaramillo, C.; López-Córdoba, A.; Goyanes, S. Edible cassava starch films carrying rosemary antioxidant extracts for potential use as active food packaging. Food Hydrocolloids 2017, 63, 488–495. [Google Scholar] [CrossRef]
- Kim, S.-K. Chitin, Chitosan, Oligosaccharides and Their Derivatives. Biological Activities and Applications; CRC Press: Boca Raton, FL, USA, 2011. [Google Scholar]
- Barikani, M.; Oliaei, E.; Seddiqi, H.; Honarkar, H. Preparation and application of chitin and its derivatives: A review. Iran. Polym. J. 2014, 23, 307–326. [Google Scholar] [CrossRef]
- Zuber, M.; Zia, K.M.; Barikani, M. Chitin and chitosan based blends, composites and nanocomposites. In Advances in Natural Polymers, Advanced Structured Magterials; Thomas, S., Visakh, P., Mathew, A., Eds.; Springer: Berlin/Heidelberg, Germany, 2012; Volume 18, pp. 55–119. [Google Scholar] [CrossRef]
- Jameela, S.R.; Misra, A.; Jayakrishnan, A. Cross-linked chitosan microspheres as carriers for prolonged delivery of macromolecular drugs. J. Biomater. Sci. Polym. Ed. 1995, 6, 621–632. [Google Scholar] [CrossRef] [PubMed]
- Koh, I.-B.; Shin, S.-C.; Lee, Y.-B. Enhanced dissolution rates of piroxicam from the ground mixtures with chitin or chitosan. Arch. Pharmacal Res. 1986, 9, 55–61. [Google Scholar] [CrossRef]
- Dörnenburg, H.; Knorr, D. Strategies for the improvement of secondary metabolite production in plant cell cultures. Enzyme Microb. Technol. 1995, 17, 674–684. [Google Scholar] [CrossRef]
- Madni, A.; Kousar, R.; Naeem, N.; Wahid, F. Recent advancements in applications of chitosan-based biomaterials for skin tissue engineering. J. Bioresour. Bioprod. 2021, 6, 11–25. [Google Scholar] [CrossRef]
- Huq, T.; Khan, A.; Brown, D.; Dhayagude, N.; He, Z.; Ni, Y. Sources, production and commercial applications of fungal chitosan: A review. J. Bioresour. Bioprod. 2022, 7, 85–98. [Google Scholar] [CrossRef]
- Mitani, T.; Nakajima, C.; Sungkono, I.E.; Ishii, H. Effects of ionic strength on the adsorption of heavy metals by swollen chitosan beads. J. Environ. Sci. Health Part A 1995, 30, 669–674. [Google Scholar] [CrossRef]
- Jha, I.N.; Iyengar, L.; Prabhakara Rao, A.V.S. Removal of cadmium using chitosan. J. Environ. Eng. 1988, 114, 962. [Google Scholar] [CrossRef]
- Saad, E.M.; Elshaarawy, R.F.; Mahmoud, S.A.; El-Moselhy, K.M. New Ulva Lactuca algae based chitosan bio-composites for bioremediation of Cd(II) ions. J. Bioresour. Bioprod. 2021, 6, 223–242. [Google Scholar] [CrossRef]
- Makarios-Laham, I.; Lee, T.-C. Biodegradability of chitin- and chitosan-containing films in soil environment. J. Environ. Polym. Degrad. 1995, 3, 31–36. [Google Scholar] [CrossRef]
- Rogovina, S.Z.; Alexanyan, C.V.; Prut, E.V. Biodegradable blends based on chitin and chitosan: Production, structure, and properties. J. Appl. Polym. Sci. 2011, 121, 1850–1859. [Google Scholar] [CrossRef]
- Isa, S.M.; Mohamed, R. Physical and mechanical properties of chitosan and polyethylene blend for food packaging film. Int. J. Mech. Prod. Eng. 2015, 3, 51–55. [Google Scholar]
- Chi-Yan Li, S.; Sun, Y.-C.; Guan, Q.; Naguib, H. Effects of chitin nanowhiskers on the thermal, barrier, mechanical, and rheological properties of polypropylene nanocomposites. RSC Adv. 2016, 6, 72086–72095. [Google Scholar] [CrossRef]
- Huang, D.; Mu, B.; Wang, A. Preparation and properties of chitosan/poly (vinyl alcohol) nanocomposite films reinforced with rod-like sepiolite. Mater. Lett. 2012, 86, 69–72. [Google Scholar] [CrossRef]
- Hajji, S.; Chaker, A.; Jridi, M.; Maalej, H.; Jellouli, K.; Boufi, S.; Nasri, M. Structural analysis, and antioxidant and antibacterial properties of chitosan-poly (vinyl alcohol) biodegradable films. Environ. Sci. Pollut. Res. 2016, 23, 15310–15320. [Google Scholar] [CrossRef]
- Bonilla, J.; Fortunati, E.; Atarés, A.; Chiralt, A.; Kenny, J.M. Physical, structural and antimicrobial properties of poly vinyl alcohol–chitosan biodegradable films. Food Hydrocoll. 2014, 35, 463–470. [Google Scholar] [CrossRef]
- Rafique, A.; Zia, K.M.; Zuber, M.; Tabasum, S.; Rehman, S. Chitosan functionalized poly(vinyl alcohol) for prospects biomedical and industrial applications: A review. Int. J. Biol. Macromol. 2016, 87, 141–154. [Google Scholar] [CrossRef]
- Sukhlaaied, W.; Riyajan, S.-A. Preparation and properties of a novel environmentally friendly film from maleated poly(vinyl alcohol) grafted with chitosan in solution form: The effect of different production factors. Polym. Bull. 2016, 73, 791–813. [Google Scholar] [CrossRef]
- Dominguez-Martinez, B.M.; Martínez-Flores, H.E.; Berrios, J.D.J.; Otoni, C.G.; Wood, D.F.; Velazquez, G. Physical characterization of biodegradable films based on chitosan, polyvinyl alcohol and Opuntia mucilage. J. Polym. Environ. 2017, 25, 683–691. [Google Scholar] [CrossRef]
- Yu, Z.; Li, B.; Chu, J.; Zhang, P. Silica in situ enhanced PVA/chitosan biodegradable films for food packages. Carbohydr. Polym. 2018, 184, 214–220. [Google Scholar] [CrossRef] [PubMed]
- Sébastien, F.; Stéphane, G.; Copinet, A.; Coma, V. Novel biodegradable films made from chitosan and poly(lactic acid) with antifungal properties against mycotoxinogen strains. Carbohydr. Polym. 2006, 65, 185–193. [Google Scholar] [CrossRef]
- Díez-Pascual, A.M.; Díez-Vicente, A.L. Antimicrobial and sustainable food packaging based on poly(butylene adipate-co-terephthalate) and electrospun chitosan nanofibers. RSC Adv. 2015, 5, 93095–93107. [Google Scholar] [CrossRef]
- Gumienna, M.; Córna, B. Antimicrobial food packaging with biodegradable polymers and bacteriocins. Molecules 2021, 26, 3735. [Google Scholar] [CrossRef]
- Salazar, R.; Salas-Gomez, V.; Alvarado, A.A.; Baykara, H. Preparation, characterization and evaluation of antibacterial properties of polylactide-polyethylene glycol-chitosan active composite films. Polymers 2022, 14, 2266. [Google Scholar] [CrossRef]
- Lim, B.K.H.; Thian, E.S. Effects of molecular weight of chitosan in a blend with polycaprolactone and grapefruit seed extract for active packaging and biodegradation. Food Packag. Shelf Life 2022, 34, 100931. [Google Scholar] [CrossRef]
- Jayakumar, R.; Tamura, H. Synthesis, characterization and thermal properties of chitin-g-poly(ε-caprolactone) copolymers by using chitin gel. Int. J. Biol. Macromol. 2008, 43, 32–36. [Google Scholar] [CrossRef]
- Senda, T.; He, Y.; Inoue, Y. Biodegradable blends of poly(ε-caprolactone) with α-chitin and chitosan: Specific interactions, thermal properties and crystallization behavior. Polym. Int. 2011, 51, 33–39. [Google Scholar] [CrossRef]
- Wan, Y.; Lu, X.; Dalai, S.; Zhang, J. Thermophysical properties of polycaprolactone/chitosan blend membranes. Thermochim. Acta 2009, 487, 33–38. [Google Scholar] [CrossRef]
- Honma, T.; Senda, T.; Inoue, Y. Thermal properties and crystallization behaviour of blends of poly(ε-caprolactone) with chitin and chitosan. Polym. Int. 2003, 52, 1839–1846. [Google Scholar] [CrossRef]
- Zia, K.M.; Barikani, M.; Zuber, M.; Bhatti, I.A.; Barmar, M. Surface characteristics of polyurethane elastomers based on chitin/1,4-butane diol blends. Int. J. Biol. Macromol. 2009, 44, 182–185. [Google Scholar] [CrossRef]
- Iglesias-Montes, M.L.; Soccio, M.; Siracusa, V.; Gazzano, M.; Lotti, N.; Cyras, V.P.; Manfredi, L.B. Chitin nanocomposite based on plasticized poly(lactic acid)/poly(3-hydroxybutyrate) (PLA/PHB) blends as fully biodegradable packaging materials. Polymers 2022, 14, 3177. [Google Scholar] [CrossRef]
- Saad, G.R.; Salama, H.E.; Mohamed, N.A.; Sabaa, M.W. Crystallization and thermal properties of biodegradable polyurethanes based on poly[(R)-3-hydroxybutyrate] and their composites with chitin whiskers. J. Appl. Polym. Sci. 2014, 131, 40784. [Google Scholar] [CrossRef]
- Ikejima, T.; Yagi, K.; Inoue, Y. Thermal properties and crystallization behavior of poly(3-hydroxybutyric acid) in blends with chitin and chitosan. Macromol. Chem. Phys. 1999, 200, 413–421. [Google Scholar] [CrossRef]
- Zhang, Q.; Wei, S.; Huang, J.; Feng, J.; Chang, P.R. Effect of surface acetylated-chitin nanocrystals on structure and mechanical properties of poly(lactic acid). J. Appl. Polym. Sci. 2013, 131, 39809. [Google Scholar] [CrossRef]
- Herrera, N.; Singh, A.A.; Salaberria, A.M.; Labidi, J.; Mathew, A.P.; Oksman, K. Triethyl citrate (TEC) as a dispersing aid in polylactic acid/chitin nanocomposites prepared via liquid-assisted extrusion. Polymers 2017, 9, 406. [Google Scholar] [CrossRef] [Green Version]
- (Pereda, M.; Aranguren, M.I.; Marcovich, N.E. Water vapor absorption and permeability of films based on chitosan and sodium caseinate. J.Appl. Polym. Sci. 2009, 111, 2777–2784. [Google Scholar] [CrossRef]
- Rivero, S.; García, M.A.; Pinotti, A. Composite and bi-layer films based on gelatin and chitosan. J. Food Eng. 2009, 90, 531–539. [Google Scholar] [CrossRef]
- Hosseini, S.F.; Rezaei, M.; Zandi, M.; Ghavi, F.F. Preparation and functional properties of fish gelatin–chitosan blend edible films. Food Chem. 2013, 136, 1490–1495. [Google Scholar] [CrossRef]
- Hosseini, S.F.; Rezaei, M.; Zandi, M.; Farahmandghavi, F. Fabrication of bio-nanocomposite films based on fish gelatin reinforced with chitosan nanoparticles. Food Hydrocoll. 2015, 44, 172–182. [Google Scholar] [CrossRef]
- Palma, F.; Michniak-Kohn, B.; Pérez-Correa, J.R.; Hernandez, E.; Romañach, R.J.; Valenzuela, L.M. Near-infrared chemical imaging and its correlation with themechanical properties of chitosan–gelatin edible films. Carbohydr. Polym. 2016, 136, 409–417. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sahraee, S.; Milani, J.M.; Ghanbarzadeh, B.; Hamishehkar, H. Effect of corn oil on physical, thermal, and antifungal properties of gelatin-based nanocomposite films containing nano chitin. LWT-Food Sci. Technol. 2017, 76, 33–39. [Google Scholar] [CrossRef]
- Sahraee, S.; Milani, J.M.; Ghanbarzadeh, B.; Hamishehkar, H. Physicochemical and antifungal properties of bio-nanocomposite filmbased on gelatin-chitin nanoparticle. Int. J. Biol. Macromol. 2017, 97, 373–381. [Google Scholar] [CrossRef] [PubMed]
- Haghighia, H.; De Leo, R.; Bedina, E.; Pfeifer, F.; Siesler, H.W.; Pulvirenti, A. Comparative analysis of blend and bilayer films based on chitosan and gelatin enriched with LAE (lauroyl arginate ethyl) with antimicrobial activity for food packaging applications. Food Packag. Shelf Life 2019, 19, 31–39. [Google Scholar] [CrossRef]
- Hai, L.; Choi, E.S.; Zhai, L.; Panicker, P.S.; Kim, J. Green nanocomposite made with chitin and bamboo nanofibers and its mechanical, thermal and biodegradable properties for food packaging. Int. J. Biol. Maromol. 2020, 144, 491–499. [Google Scholar] [CrossRef]
- Wu, Y.; Luo, X.; Li, W.; Song, R.; Li, J.; Li, Y.; Li, B.; Liu, Shilin. Green and biodegradable composite films with novel antimicrobial performance based on cellulose. Food Chem. 2016, 197, 250–256. [Google Scholar] [CrossRef]
- de F. Silva, M.; Lopes, P.S.; da Silva, C.F.; Yoshida, C.M.P. Active packaging material based on buriti oil—Mauritia flexuosa L.f. (Arecaceae) incorporated into chitosan films. J. Appl. Polym. Sci. 2016, 133, 43210. [Google Scholar] [CrossRef]
- Ding, X.; Zhao, L.; Khan, I.M.; Yue, L.; Zhang, Y.; Wang, Z. Emerging chitosan grafted essential oil components: A review on synthesis, characterization, and potential application. Carbohydr. Polym. 2022, 297, 120011. [Google Scholar] [CrossRef]
- Rai, M.; Wypij, M.; Ingle, A.P.; Trzcińska-Wencel, J.; Golińska, P. Emerging trends in pullulan-based antimicrobial systems for various applications. Int. J. Mol. Sci. 2021, 22, 13596. [Google Scholar] [CrossRef]
- Shanmugam, R.; Mayakrishnan, V.; Kesavan, R.; Shanmugam, K.; Veeramani, S.; Ilangovan, R. Mechanical, barrier, adhesion and antibacterial properties of pullulan/graphene bio nanocomposite coating on spray coated nanocellulose film for food packaging applications. J. Polym. Environ. 2022, 30, 1749–1757. [Google Scholar] [CrossRef]
- Bilohan, M.; Ramos, A.; Domingues, F.; Luís, Â. Production and characterization of pullulan/paper/zein laminates as active food packaging materials. J. Food Proc. Preserv. 2022, 46, e17083. [Google Scholar] [CrossRef]
- Marais, A.; Utsel, S.; Gustafsson, E.; Wågberg, L. Towards a super-strainable paper using the layer-by-layer technique. Carbohydr. Polym. 2014, 100, 218–224. [Google Scholar] [CrossRef] [PubMed]
- Oliveira, N.L.; Rodrigues, A.A.; Oliveira Neves, I.C.; Teixeira Lago, A.M.; Vilela Borges, S.; de Resende, J.V. Development and characterization of biodegradable films based on Pereskia aculeata Miller mucilage. Ind. Crops Prod. 2019, 130, 499–510. [Google Scholar] [CrossRef]
- Sganzerla, W.G.; Paes, B.B.; Azevedo, M.S.; Ferrareze, J.P.; da Rosa, C.G.; Nunes, M.R.; Veeck, A.P.L. Bioactive and biodegradable film packaging incorporated with acca sellowiana extracts: Physicochemical and antioxidant characterization. Chem. Eng. Trans. 2019, 75, 445–450. [Google Scholar]
- Liu, L.S.; Liu, C.-K.; Fishman, M.L.; Hicks, K.B. Composite films from pectin and fish skin gelation or soybean flour protein. J. Agric. Food Chem. 2007, 55, 2349–2355. [Google Scholar] [CrossRef]
- Bermúdez-Oria, A.; Rodríguez-Gutiérrez, G.; Rubio-Senent, F.; Fernández-Prior, Á.; Fernández-Bolaños, J. Effect of edible pectin-fish gelatin films containing the olive antioxidants hydroxytyrosol and 3,4-dihydroxyphenylglycol on beef meat during refrigerated storage. Meat Sci. 2019, 148, 213–218. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Pereira, D.G.M.; Vieira, J.M.; Vicente, A.A.; Cruz, R.M.S. Development and characterization of pectin films with Salicornia ramosissima: Biodegradation in soil and seawater. Polymers 2021, 13, 2632. [Google Scholar] [CrossRef]
- Nisar, T.; Wang, Z.-C.; Yang, X.; Tian, Y.; Iqbal, M.; Guo, Y. Characterization of citrus pectin films integrated with clove bud essential oil: Physical, thermal, barrier, antioxidant and antibacterial properties. Int. J. Biol. Macromol. 2018, 106, 670–680. [Google Scholar] [CrossRef]
- Almasi, H.; Azizi, S.; Amjadi, S. Development and characterization of pectin films activated by nanoemulsion and Pickering emulsion stabilized marjoram (Origanum majorana L.) essential oil. Food Hydrocoll. 2020, 99, 105338. [Google Scholar] [CrossRef]
- Akhter, R.; Masoodi, F.A.; Wani, T.A.; Rather, S.A. Functional characterization of biopolymer based composite film: Incorporation of natural essential oils and antimicrobial agents. Int. J. Biol. Macromol. 2019, 137, 1245–1255. [Google Scholar] [CrossRef] [PubMed]
- de Castro e Silva, P.; de Oliveira, A.C.S.; Pereira, L.A.S.; Valquíria, M.; Carvalho, G.R.; Miranda, K.W.E.; Marconcini, J.M.; Oliveira, J.E. Development of bionanocomposites of pectin and nanoemulsions of carnauba wax and neem oil pectin/carnauba wax/neem oil composites. Polym. Compos. 2020, 41, 858–870. [Google Scholar] [CrossRef]
- Han, H.-S.; Song, K.B. Antioxidant activities of mandarin (Citrus unshiu) peel pectin films containing sage (Salvia officinalis) leaf extract. Int. J. Food Sci. Technol. 2020, 55, 3173–3181. [Google Scholar] [CrossRef]
- Onsøyen, E. Commercial applications of alginates. Carbohydr. Eur. 1996, 14, 26–31. [Google Scholar]
- Venkatesan, J.; Bhatnagar, I.; Manivasagan, P.; Kang, K.-H.; Kim, S.-K. Alginate composites for bone tissue engineering: A review. Int. J. Biol. Macromol. 2015, 72, 269–281. [Google Scholar] [CrossRef] [PubMed]
- Sun, J.; Tan, H. Alginate-based biomaterials for regenerative medicine applications. Materials 2013, 6, 1285–1309. [Google Scholar] [CrossRef]
- Wong, M. Alginates in tissue engineering. In Biopolymer Methods in Tissue Engineering; Springer: Berlin/Heidelberg, Germany, 2004; pp. 77–86. [Google Scholar]
- Zhang, Y.; Man, J.; Li, J.; Xing, Z.; Zhao, B.; Ji, M.; Xia, H.; Li, J. Preparation of the alginate/carrageenan/shellac films reinforced with cellulose nanocrystals obtained from enteromorpha for food packaging. Int. J. Biol. Macromol. 2022, 218, 519–532. [Google Scholar] [CrossRef]
- Ye, Z.; Ma, P.; Tang, M.; Li, X.; Zhang, W.; Hong, X.; Chen, X.; Chen, D. Interactions between calcium alginate and carrageenan enhanced mechanical property of a natural composite film for general packaging application. Polym. Bull. 2017, 74, 3421–3429. [Google Scholar] [CrossRef]
- Carneiro-da-Cunha, M.G.; Cerqueira, M.A.; Souza, B.W.S.; Carvalho, S.; Quintas, M.A.C.; Teixeira, J.A.; Vicente, A.A. Physical and thermal properties of a chitosan/alginate nanolayered PET film. Carbohydr. Polym. 2010, 82, 153–159. [Google Scholar] [CrossRef]
- Kurczewska, J.; Ratajczak, M.; Gajecka, M. Alginate and pectin films covering halloysite with encapsulated salicylic acid as food packaging components. Appl. Clay Sci. 2021, 214, 106270. [Google Scholar] [CrossRef]
- Makaremi, M.; Yousefi, H.; Cavallaro, G.; Lazzara, G.; Goh, C.B.S.; Lee, S.M.; Solouk, A.; Pasbakhsh, P. Safely dissolvable and healable active packaging films based on alginate and pectin. Polymers 2019, 11, 1594. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Benavides, S.; Villalobos-Carvajal, R.; Reyes, J.E. Physical, mechanical and antibacterial properties of alginate film: Effect of the cross linking degree and oregano essential oil concentration. J. Food Eng. 2012, 110, 232–239. [Google Scholar] [CrossRef]
- Theagarajan, R.; Dutta, S.; Moses, J.A.; Anandharamakrishnan, C. Alginates for food packaging applications. In Alginates; Ahmed, S., Ed.; Scrivener Publishing LLC: Beverly, CA, USA, 2019; Part 3; pp. 205–232. [Google Scholar] [CrossRef]
- Bico, S.L.S.; Raposo, M.F.J.; Morais, R.M.S.C.; Morais, A.M.M.B. Combined effects of chemical dip and/or carrageenan coating and/or controlled atmosphere on quality of fresh-cut banana. Food Control 2009, 20, 508–514. [Google Scholar] [CrossRef]
- Plotto, A.; Narciso, J.A.; Rattanapanone, N.; Baldwin, E.A. Surface treatments and coatings to maintain fresh-cut mango quality in storage. J. Sci. Food Agric. 2010, 90, 2333–2341. [Google Scholar] [CrossRef] [PubMed]
- Hambleton, A.; Fabra, M.J.; Debeaufort, F.; Dury-Brun, C.; Voilley, A. Interface and aroma barrier properties of iota-carrageenan emulsion–based films used for encapsulation of active food compounds. J. Food Eng. 2009, 93, 80–88. [Google Scholar] [CrossRef]
- Fabra, M.J.; Chambin, O.; Voilley, A.; Gay, J.P.; Debeaufort, F. Influence of temperature and NaCl on the release in aqueous liquid media of aroma compounds encapsulated in edible films. J. Food Eng. 2012, 108, 30–36. [Google Scholar] [CrossRef]
- Guo, J.; Dong, S.; Ye, M.; Wu, X.; Lv, X.; Xu, H.; Li, M. Effects of hydroxypropyl methylcellulose on physicochemical properties and microstcructure of κ-carrageenan film. Foods 2022, 11, 3023. [Google Scholar] [CrossRef] [PubMed]
- Adam, F.; Othman, N.A.; Yasin, N.H.M.; Cheng, C.K.; Azman, N.A.M. Evaluation of reinforced and green bioplastic from carrageenan seaweed with nanocellulose. Fibers Polym. 2022, 23, 2885–2896. [Google Scholar] [CrossRef]
- Amanda, P.; Ismadi, I.; Ningrum, R.S.; Nabila, S.; Prasetyo, K.W. Carrageenan functional film integrated with Pickering emulsion of oregano oil stabilized by cationic nanocellulose for active packaging. Food Sci. Technol. Int. 2022. [Google Scholar] [CrossRef]
- Prakaash, S.; Balasubramaniam, L.; Singh Patel, A.; Nayak, B. Fabrication of antioxidative food packaging films using cellulose nanofibers, kappa-carrageenan, and gallic acid. J. Food Proc. Preserv. 2021, 45, e15480. [Google Scholar] [CrossRef]
- Larotonda, F.D.S.; Torres, M.D.; Gonçalves, M.P.; Sereno, A.M.; Hilliou, L.J. Hybrid carrageenan-based formulations for edible film preparation: Benchmarking with kappa carrageenan. Appl. Polym. Sci. 2016, 133, 42263. [Google Scholar] [CrossRef]
- Wang, C.; An, X.; Lu, Y.; Li, Z.; Gao, Z.; Tian, S. Biodegradable active packaging material containing grape seed ethanol extract and corn starch/κ-carrageenan composite film. Polymers 2022, 14, 4857. [Google Scholar] [CrossRef] [PubMed]
- Lopes, T.A.; Bufalino, L.; Júnior, M.G.; Tonoli, G.H.D.; Mendes, L.M. Eucalyptus wood nanofibrils as reinforcement of carrageenan and starch biopolymers for improvement of physical properties. J. Trop. For. Sci. 2018, 30, 292–303. [Google Scholar] [CrossRef]
- Hartiati, A.; Harsojuwono, B.A.; Suryanto, H.; Arnata, I.W. Synthesis of starch-carrageenan bio-thermoplastic composites on the type and concentration of thermoplastic forming materials as packaging materials. IOP Conf. Ser. Earth Environ. 2021, 913, 012030. [Google Scholar] [CrossRef]
- Nur Fatin Nazurah, R.; Nur Hanani, Z.A. Physicochemical characterization of kappa-carrageenan (Euchema cottoni) based films incorporated with various plant oils. Carbohydr. Polym. 2017, 157, 1479–1487. [Google Scholar] [CrossRef]
- Castaño, J.; Guadarrama-Lezama, A.Y.; Hernández, J.; Colín-Cruz, M.; Muñoz, M.; Castillo, S. Preparation, characterization and antifungal properties of polysaccharide–polysaccharide and polysaccharide–protein films. J. Mater. Sci. 2017, 52, 353–366. [Google Scholar] [CrossRef]
| Polysaccharides | Advantages | Disadvantages | Commercial Products * | ||
|---|---|---|---|---|---|
| Common | Individual | Common | Individual | ||
| Cellulose | biodegradable; non-toxic; from renewable resources; | transparent; thermoplastic; resistance to fats and oil; | poor mechanical properties; cost; | no antimicrobial/antioxidant activity; poor water/vapor barrier; | Biograde (FKuR); Tenite (Eastman Chemical); Nature flex (Innovia films); |
| Starch | transparent; edible; thermoplastic; retrogradation good gas barrier properties; | poor water barrier properties; moisture sensitive; | Mater-Bi (Novamont); Bioplast (Biotec); Biomax (Dupotn); | ||
| Chitin/chitosan | antimicrobial; barrier to gases; biocompatible; high water/vapor permeability | production features; | ChitoClear (Primex); NorLife, Kitoflok (Norwegian Chitosan) | ||
| Pectin | edible; applicable in food industry; good gas permeability; | lack antibacterial activity; poor water barrier properties; | - | ||
| Pullulan | transparent; edible; resistance to oil and grease; good gas barrier; | production features; | - | ||
| Alginate | salts are water-soluble; applicable in food industry; | poor water resistance; brittle; | - | ||
| Carrageenan | hold texture;hold aroma; flexible; | fragile; ductile; poor water resistance; production depends on algae growth area; | - | ||
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the author. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Aleksanyan, K.V. Polysaccharides for Biodegradable Packaging Materials: Past, Present, and Future (Brief Review). Polymers 2023, 15, 451. https://doi.org/10.3390/polym15020451
Aleksanyan KV. Polysaccharides for Biodegradable Packaging Materials: Past, Present, and Future (Brief Review). Polymers. 2023; 15(2):451. https://doi.org/10.3390/polym15020451
Chicago/Turabian StyleAleksanyan, Kristine V. 2023. "Polysaccharides for Biodegradable Packaging Materials: Past, Present, and Future (Brief Review)" Polymers 15, no. 2: 451. https://doi.org/10.3390/polym15020451
APA StyleAleksanyan, K. V. (2023). Polysaccharides for Biodegradable Packaging Materials: Past, Present, and Future (Brief Review). Polymers, 15(2), 451. https://doi.org/10.3390/polym15020451






