Biocompatible Silica-Polyethylene Glycol-Based Composites for Immobilization of Microbial Cells by Sol-Gel Synthesis
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Microorganism Cultivation
2.3. Sol-Gel Synthesis of Organosilica Nanocomposites and Encapsulation of Yeast Cells
2.3.1. STPEG-Composites
2.3.2. SPEG-Composites
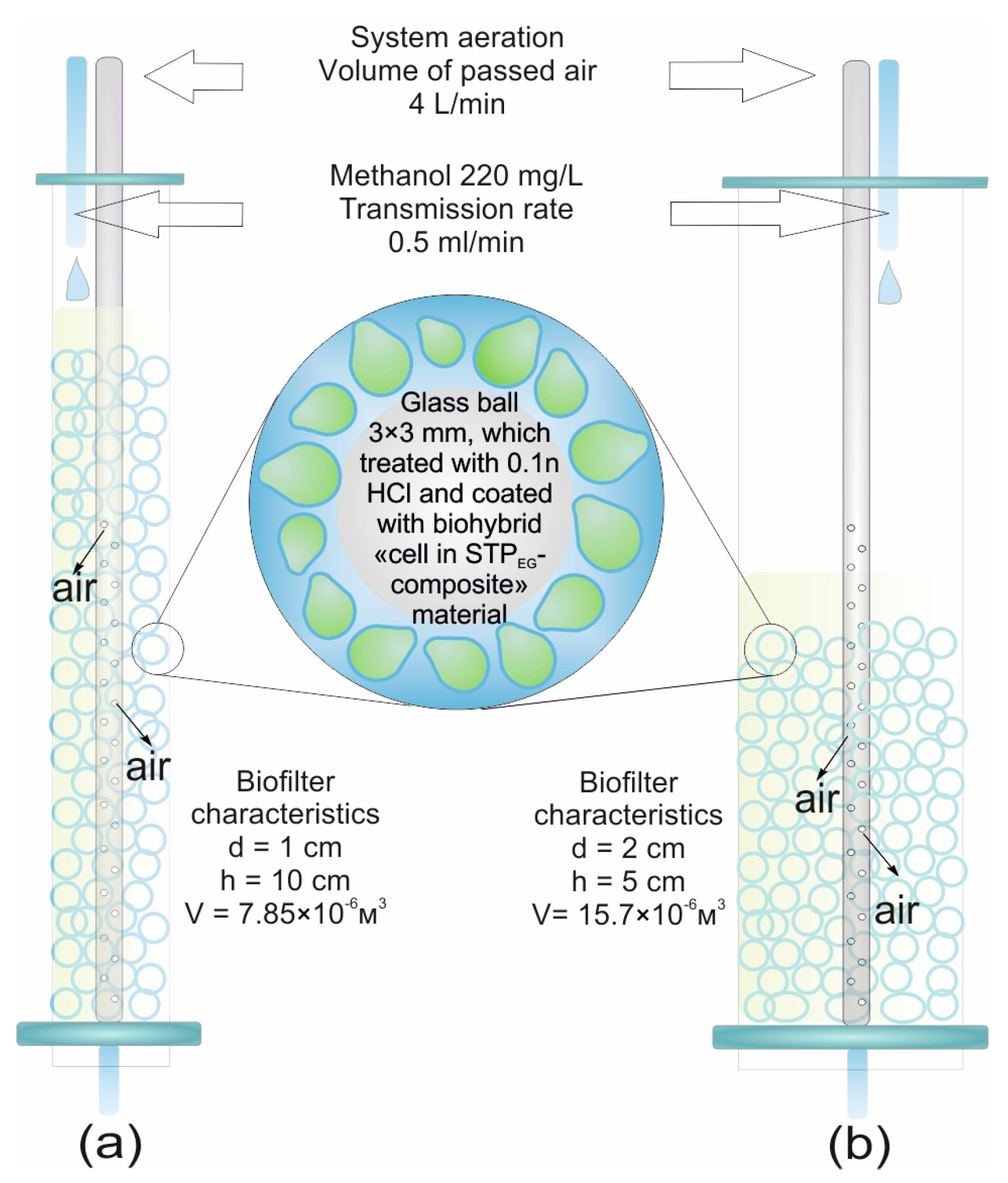
2.4. Biocatalyst Bed Preparation for Biofilter Column
2.5. Instrumental Analysis
3. Results and Discussion
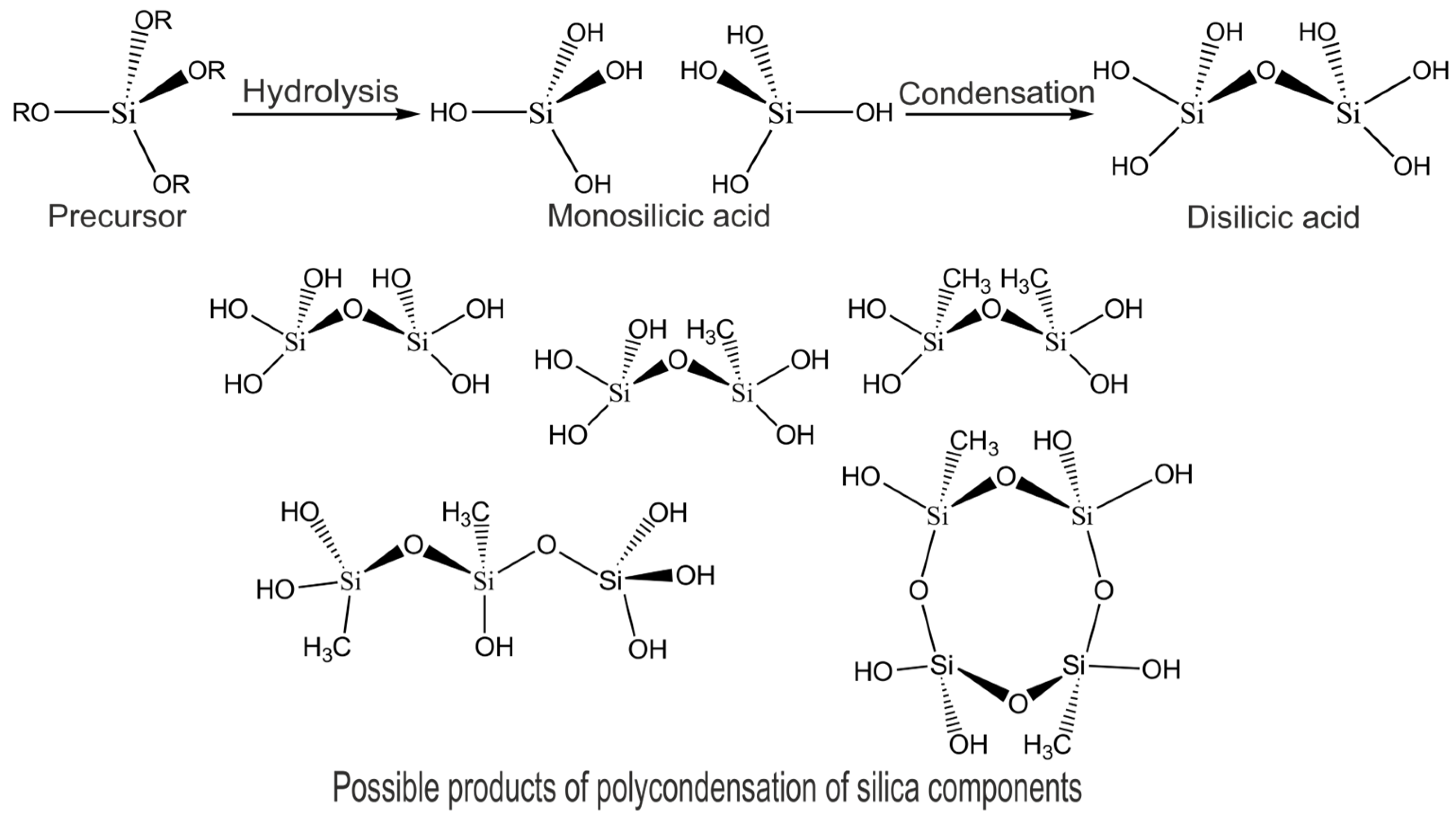
3.1. Synthesis and Properties of Organosilica Composite Materials
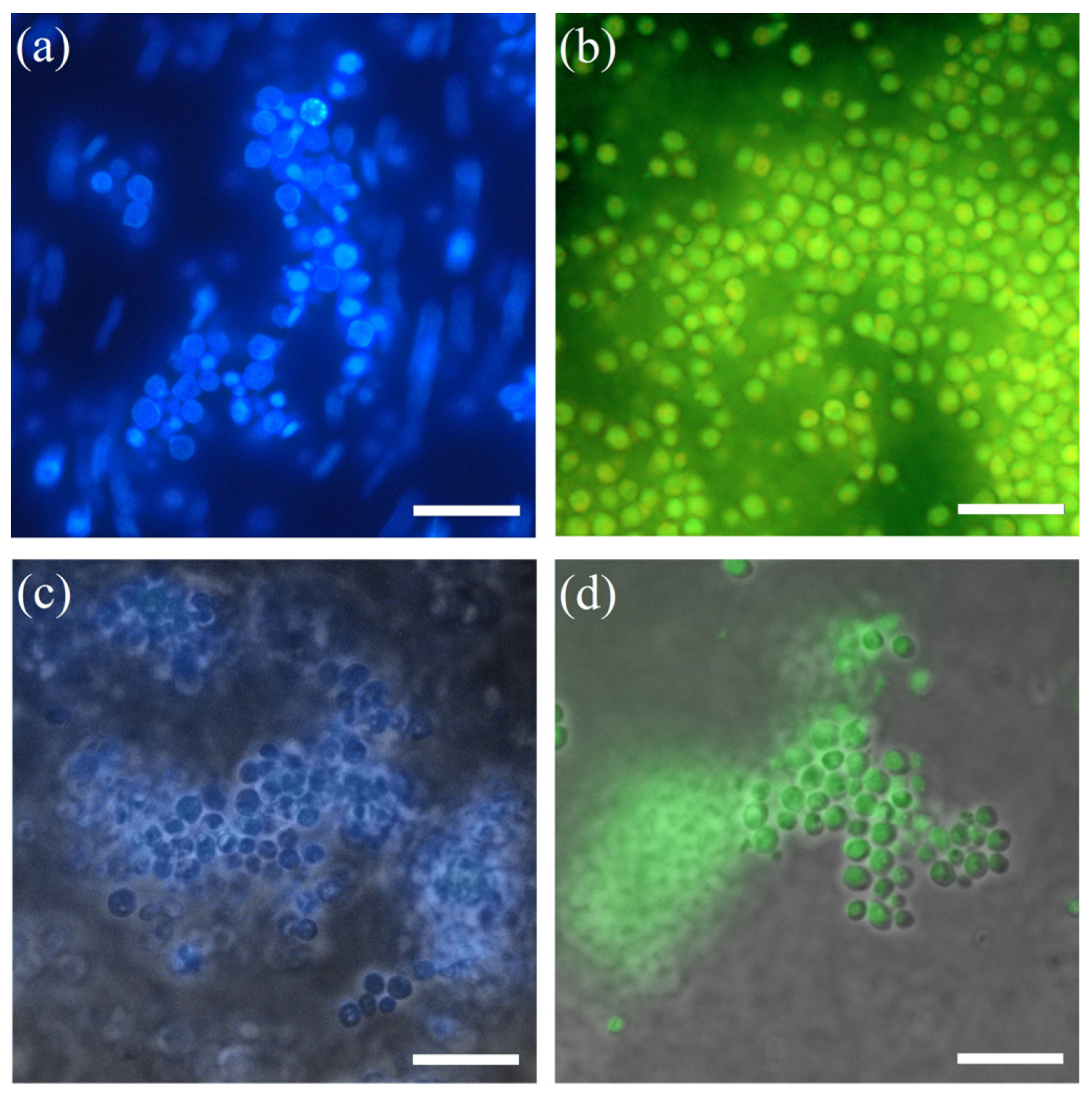
3.2. Morphology and Architecture of Biohybrid Materials on the Base of Immobilized Microorganisms in Organosilica Composites
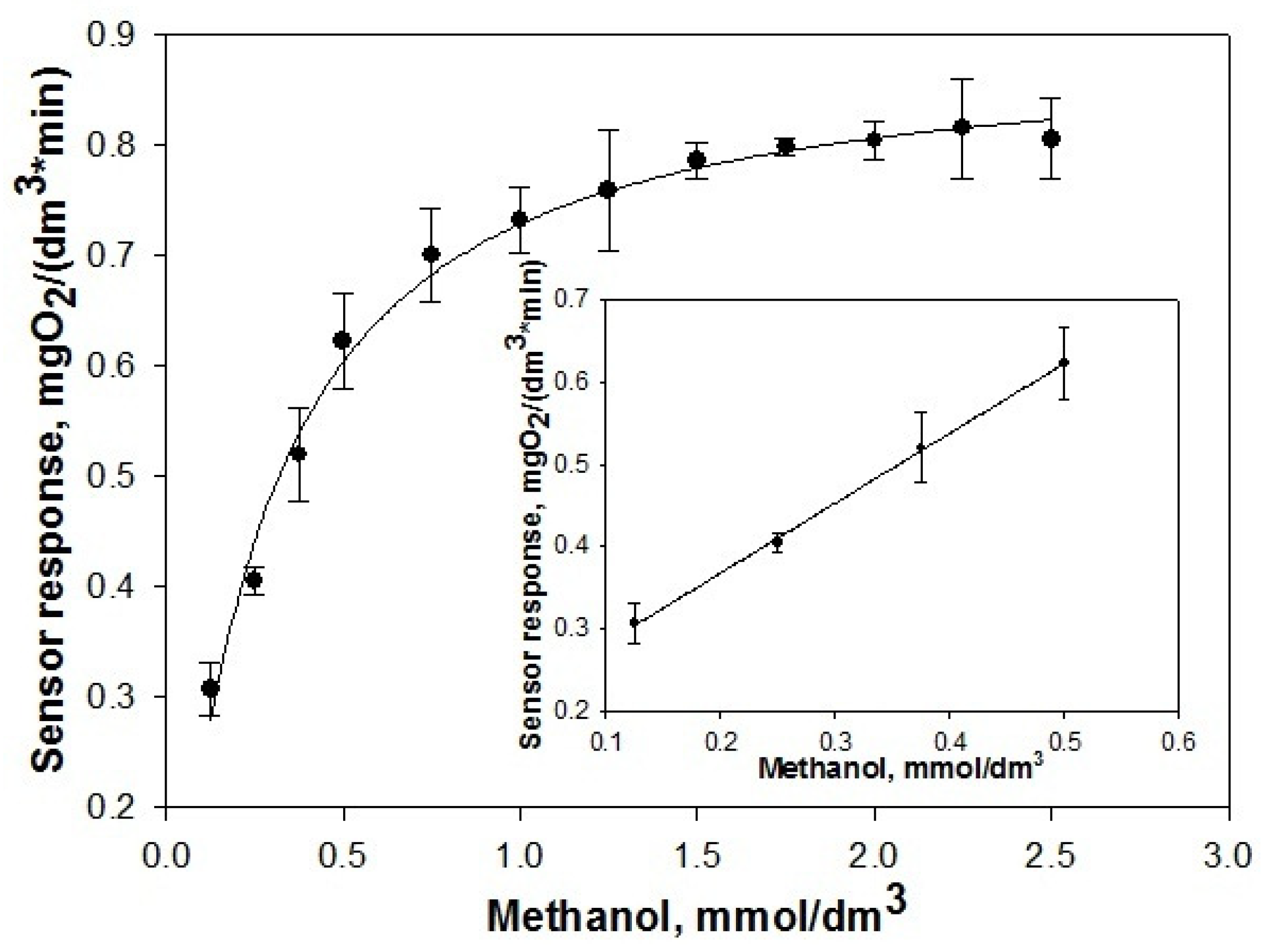
3.3. Characterization of the Encapsulated Methylotrophic Yeast as Biocatalysts
3.3.1. Characterization of the Immobilized Methylotrophic Yeast as Biocatalysts by Biosensor Assessment Technologies after UV Irradiation
3.3.2. Effect of Heavy Metal Ions on the Respiratory Activity of the Immobilized Microorganisms
3.4. Use of the Biocatalyst as a Biosystem for Methanol-Rich Wastewater Utilization
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Gon, M.; Tanaka, K.; Chujo, Y. Creative Synthesis of Organicinorganic Molecular Hybrid Materials. Bull. Chem. Soc. Jpn. 2017, 90, 463–474. [Google Scholar] [CrossRef]
- Melnikov, P.V.; Naumova, A.O.; Alexandrovskaya, A.Y.; Zaitsev, N.K. Optimizing Production Conditions for a Composite Optical Oxygen Sensor Using Mesoporous SiO2. Nanotechnologies Russ. 2018, 13, 602–608. [Google Scholar] [CrossRef]
- Antropov, A.P.; Ragutkin, A.V.; Melnikov, P.V.; Luchnikov, P.A.; Zaitsev, N.K. Composite Material for Optical Oxygen Sensor. IOP Conf. Ser. Mater. Sci. Eng. 2018, 289, 012031. [Google Scholar] [CrossRef]
- Ananikov, V.P. Organic–Inorganic Hybrid Nanomaterials. Nanomaterials 2019, 9, 1197. [Google Scholar] [CrossRef]
- Máková, V.; Holubová, B.; Krabicová, I.; Kulhánková, J.; Řezanka, M. Hybrid Organosilane Fibrous Materials and Their Contribution to Modern Science. Polymer 2021, 228, 123862. [Google Scholar] [CrossRef]
- Melnikov, P.V.; Alexandrovskaya, A.Y.; Naumova, A.O.; Popova, N.M.; Spitsyn, B.V.; Zaitsev, N.K.; Yashtulov, N.A. Modified Nanodiamonds as a Means of Polymer Surface Functionalization. From Fouling Suppression to Biosensor Design. Nanomaterials 2021, 11, 2980. [Google Scholar] [CrossRef] [PubMed]
- Postnova, I.; Shchipunov, Y. Tannic Acid as a Versatile Template for Silica Monoliths Engineering with Catalytic Gold and Silver Nanoparticles. Nanomaterials 2022, 12, 4320. [Google Scholar] [CrossRef] [PubMed]
- Tripathi, V.S.; Kandimalla, V.B.; Ju, H. Preparation of Ormosil and Its Applications in the Immobilizing Biomolecules. Sensors Actuators B Chem. 2006, 114, 1071–1082. [Google Scholar] [CrossRef]
- Avnir, D.; Coradin, T.; Lev, O.; Livage, J. Recent Bio-Applications of Sol-Gel Materials. J. Mater. Chem. 2006, 16, 1013–1030. [Google Scholar] [CrossRef]
- Lai, Y.C.; Lai, C.S.; Tai, J.T.; Nguyen, T.P.; Wang, H.L.; Lin, C.Y.; Tsai, T.Y.; Ho, H.C.; Wang, P.H.; Liao, Y.C.; et al. Understanding Ligand-Nanoparticle Interactions for Silica, Ceria, and Titania Nanopowders. Adv. Powder Technol. 2015, 26, 1676–1686. [Google Scholar] [CrossRef]
- Tański, T.; Matysiak, W.; Krzemiński, Ł.; Jarka, P.; Gołombek, K. Optical Properties of Thin Fibrous PVP/SiO 2 Composite Mats Prepared via the Sol-Gel and Electrospinning Methods. Appl. Surf. Sci. 2017, 424, 184–189. [Google Scholar] [CrossRef]
- Xu, X.; Niu, S.; Wang, X.; Li, X.; Li, H.; Su, X.; Luo, S. Fabrication and Casting Simulation of Composite Ceramic Cores with Silica Nanopowders. Ceram. Int. 2019, 45, 19283–19288. [Google Scholar] [CrossRef]
- Najafi, A.; Golestani-Fard, F.; Rezaie, H.R.; Saeb, S.P. Sol-Gel Synthesis and Characterization of SiC–B4C Nano Powder. Ceram. Int. 2021, 47, 6376–6387. [Google Scholar] [CrossRef]
- Judeinstein, P.; Sanchez, C.; Simpelkamp, J.; Lobo, R.F.; Kikuch, A.; Sakurai, Y.; Okano, T.; Landry, M.R.; Long, V.K.; Nicol, J.; et al. Hybrid Organic–Inorganic Materials: A Land of Multidisciplinarity. J. Mater. Chem. 1996, 6, 511–525. [Google Scholar] [CrossRef]
- Zarour, A.; Abu-Reziq, R. Poly(Ethylene Glycol)@Silica Hybrid Microparticles Prepared via a Non-Aqueous Sol-Gel Process: A Method for Merging Both Classes of Hybrid Materials. Materialia 2020, 9, 100526. [Google Scholar] [CrossRef]
- Pierre, A.C. Introduction to Sol-Gel Processing; Kluwer Academic Publishers: New York, NY, USA, 1998. [Google Scholar]
- Melnikov, P.; Bobrov, A.; Marfin, Y. On the Use of Polymer-Based Composites for the Creation of Optical Sensors: A Review. Polymers 2022, 14, 4448. [Google Scholar] [CrossRef]
- Postnova, I.; Silant’ev, V.; Sarin, S.; Shchipunov, Y. Chitosan Hydrogels and Bionanocomposites Formed through the Mineralization and Regulated Charging. Chem. Rec. 2018, 18, 1247–1260. [Google Scholar] [CrossRef]
- Catauro, M.; Bollino, F.; Papale, F.; Gallicchio, M.; Pacifico, S. Influence of the Polymer Amount on Bioactivity and Biocompatibility of SiO2/PEG Hybrid Materials Synthesized by Sol–Gel Technique. Mater. Sci. Eng. C 2015, 48, 548–555. [Google Scholar] [CrossRef] [PubMed]
- Landry, C.J.; Coltrain, B.K.; Wesson, J.A.; Zumbulyadis, N.; Lippert, J.L. In Situ Polymerization of Tetraethoxysilane in Polymers: Chemical Nature of the Interactions. Polymer 1992, 33, 1496–1506. [Google Scholar] [CrossRef]
- Takei, T.; Ikeda, K.; Ijima, H.; Kawakami, K. Fabrication of Poly(Vinyl Alcohol) Hydrogel Beads Crosslinked Using Sodium Sulfate for Microorganism Immobilization. Process. Biochem. 2011, 46, 566–571. [Google Scholar] [CrossRef]
- Niu, Z.W.; Xu, H.; Li, Z. “Fish-in-Net”, a Novel Method for Cell Immobilization of Zymomonas Mobilis. PLoS ONE 2013, 8, e79569. [Google Scholar] [CrossRef] [PubMed]
- Liu, L.; Shang, L.; Guo, S.; Li, D.; Liu, C.; Qi, L.; Dong, S. Organic-Inorganic Hybrid Material for the Cells Immobilization: Long-Term Viability Mechanism and Application in BOD Sensors. Biosens. Bioelectron. 2009, 25, 523–526. [Google Scholar] [CrossRef]
- Benson, J.J.; Sakkos, J.K.; Radian, A.; Wackett, L.P.; Aksan, A. Enhanced Biodegradation of Atrazine by Bacteria Encapsulated in Organically Modified Silica Gels. J. Colloid Interface Sci. 2018, 510, 57–68. [Google Scholar] [CrossRef]
- Fidalgo, A.; Ciriminna, R.; Lopes, L.; Pandarus, V.; Béland, F.; Ilharco, L.M.; Pagliaro, M. The Sol-Gel Entrapment of Noble Metals in Hybrid Silicas: A Molecular Insight. Chem. Cent. J. 2013, 7, 161. [Google Scholar] [CrossRef]
- Song, J.-H.; Yoon, B.-H.; Kim, H.-E.; Kim, H.-W. Bioactive and Degradable Hybridized Nanofibers of Gelatin–Siloxane for Bone Regeneration. J. Biomed. Mater. Res. Part A 2008, 84A, 875–884. [Google Scholar] [CrossRef]
- Gorbunova, O.V.; Baklanova, O.N.; Gulyaeva, T.I.; Trenikhin, M.V.; Drozdov, V.A. Poly(Ethylene Glycol) as Structure Directing Agent in Sol-Gel Synthesis of Amorphous Silica. Microporous Mesoporous Mater. 2014, 190, 146–151. [Google Scholar] [CrossRef]
- Liu, S.; Zhang, Z.; Zhang, H.; Zhang, Y.; Wei, S.; Ren, L.; Wang, C.; He, Y.; Li, F.; Xiao, F.S. Phase Separation of Organic/Inorganic Hybrids Induced by Calcination: A Novel Route for Synthesizing Mesoporous Silica and Carbon Materials. J. Colloid Interface Sci. 2010, 345, 257–261. [Google Scholar] [CrossRef]
- Sato, S.; Murakata, T.; Suzuki, T.; Ohgawara, T. Control of Pore Size Distribution of Silica Gel through Sol-Gel Process Using Water Soluble Polymers as Additives. J. Mater. Sci. 1990, 25, 4880–4885. [Google Scholar] [CrossRef]
- Kunze, D.S. Modification of the Pore Structre of Sol-Gel-Derived Ceramic Oxide Powders by Water-Soluble Additives. Colloids Surf. B Biointerfaces 1991, 58, 327–337. [Google Scholar] [CrossRef]
- Vong, M.S.W.; Bazin, N.; Sermon, P.A. Chemical Modification of Silica Gels. J. Sol-Gel Sci. Technol. 1997, 8, 499–505. [Google Scholar] [CrossRef]
- Guo, W.; Luo, G.S.; Wang, Y.J. A New Emulsion Method to Synthesize Well-Defined Mesoporous Particles. J. Colloid Interface Sci. 2004, 271, 400–406. [Google Scholar] [CrossRef]
- Shen, S.; Wu, W.; Guo, K.; Chen, J. Low-Cost Preparation of Mesoporous Silica with High Pore Volume. J. Univ. Sci. Technol. Beijing 2007, 14, 369–372. [Google Scholar] [CrossRef]
- Catauro, M.; Renella, R.A.; Papale, F.; Vecchio Ciprioti, S. Investigation of Bioactivity, Biocompatibility and Thermal Behavior of Sol-Gel Silica Glass Containing a High PEG Percentage. Mater. Sci. Eng. C 2016, 61, 51–55. [Google Scholar] [CrossRef] [PubMed]
- Alessi, M.L.; Norman, A.I.; Knowlton, S.E.; Ho, D.L.; Greer, S.C. Helical and Coil Conformations of Poly(Ethylene Glycol) in Isobutyric Acid and Water. Macromolecules 2005, 38, 9333–9340. [Google Scholar] [CrossRef]
- Saeki, S.; Kuwahara, N.; Nakata, M.; Kaneko, M. Upper and Lower Critical Solution Temperatures in Poly (Ethylene Glycol) Solutions. Polymer 1986, 17, 685–689. [Google Scholar] [CrossRef]
- MMilton Harris, J. Poly(Ethylene Glycol) Chemistry: Biotechnical and Biomedical Applications; Springer: New York, NY, USA, 1992. [Google Scholar]
- Oesterhelt, F.; Rief, M.; Gaub, H.E. Single Molecule Force Spectroscopy by AFM Indicates Helical Structure of Poly(Ethylene-Glycol) in Water. New J. Phys. 1999, 1, 6.1–6.11. [Google Scholar] [CrossRef]
- Jiao, J.; Li, X.; Zhang, S.; Liu, J.; Di, D.; Zhang, Y.; Zhao, Q.; Wang, S. Redox and PH Dual-Responsive PEG and Chitosan-Conjugated Hollow Mesoporous Silica for Controlled Drug Release. Mater. Sci. Eng. C 2016, 67, 26–33. [Google Scholar] [CrossRef]
- Oh, C.; Do Ki, C.; Young Chang, J.; Oh, S.G. Preparation of PEG-Grafted Silica Particles Using Emulsion Method. Mater. Lett. 2005, 59, 929–933. [Google Scholar] [CrossRef]
- Deshmukh, K.; Kovářík, T.; Křenek, T.; Docheva, D.; Stich, T.; Pola, J. Recent Advances and Future Perspectives of Sol-Gel Derived Porous Bioactive Glasses: A Review. RSC Adv. 2020, 10, 33782–33835. [Google Scholar] [CrossRef]
- Noureddini, H.; Gao, X. Characterization of Sol-Gel Immobilized Lipases. J. Sol-Gel Sci. Technol. 2007, 41, 31–41. [Google Scholar] [CrossRef]
- Muller, W.E.; Engel, S.; Wang, X.; Wolf, S.E.; Tremel, W.; Thakur, N.L.; Krasko, A.; Divekar, M.; Schroder, H.C. Bioencapsulation of Living Bacteria (Escherichia Coli) with Poly(Silicate) after Transformation with Silicatein-Alpha Gene. Biomaterials 2008, 29, 771–779. [Google Scholar] [CrossRef]
- Nassif, N.; Bouvet, O.; Noelle Rager, M.; Roux, C.; Coradin, T.; Livage, J. Living Bacteria in Silica Gels. Nat. Mater. 2002, 1, 42–44. [Google Scholar] [CrossRef]
- Fazal, Z.; Pelowitz, J.; Johnson, P.E.; Harper, J.C.; Brinker, C.J.; Jakobsson, E. Three-Dimensional Encapsulation of Saccharomyces Cerevisiae in Silicate Matrices Creates Distinct Metabolic States as Revealed by Gene Chip Analysis. ACS Nano 2017, 11, 3560–3575. [Google Scholar] [CrossRef]
- Kamanina, O.A.; Saverina, E.A.; Rybochkin, P.V.; Arlyapov, V.A.; Vereshchagin, A.N.; Ananikov, V.P. Preparation of Hybrid Sol-Gel Materials Based on Living Cells of Microorganisms and Their Application in Nanotechnology. Nanomaterials 2022, 12, 1086. [Google Scholar] [CrossRef]
- Blondeau, M.; Coradin, T. Living Materials from Sol–Gel Chemistry: Current Challenges and Perspectives. J. Mater. Chem. 2012, 22, 22335. [Google Scholar] [CrossRef]
- Wang, S.; Guo, Z. Bio-Inspired Encapsulation and Functionalization of Living Cells with Artificial Shells. Colloids Surf. B Biointerfaces 2014, 113, 483–500. [Google Scholar] [CrossRef] [PubMed]
- Samuneva, B.; Kabaivanova, L.; Chernev, G.; Djambaski, P.; Kashchieva, E.; Emanuilova, E.; Salvado, I.M.M.; Fernandes, M.H.V.; Wu, A. Sol–Gel Synthesis and Structure of Silica Hybrid Materials. J. Sol-Gel Sci. Technol. 2008, 48, 73–79. [Google Scholar] [CrossRef]
- Chen, J.P.; Lin, W.S. Sol-Gel Powders and Supported Sol-Gel Polymers for Immobilization of Lipase in Ester Synthesis. Enzyme Microb. Technol. 2003, 32, 801–811. [Google Scholar] [CrossRef]
- Tielmann, P.; Kierkels, H.; Zonta, A.; Ilie, A.; Reetz, M.T. Increasing the Activity and Enantioselectivity of Lipases by Sol-Gel Immobilization: Further Advancements of Practical Interest. Nanoscale 2014, 6, 6220–6228. [Google Scholar] [CrossRef]
- Lee, H.; Hong, D.; Choi, J.Y.; Kim, J.Y.; Lee, S.H.; Kim, H.M.; Yang, S.H.; Choi, I.S. Layer-by-Layer-Based Silica Encapsulation of Individual Yeast with Thickness Control. Chem. An Asian J. 2015, 10, 129–132. [Google Scholar] [CrossRef]
- Homburg, S.V.; Venkanna, D.; Kraushaar, K.; Kruse, O.; Kroke, E.; Patel, A.V. Entrapment and Growth of Chlamydomonas Reinhardtii in Biocompatible Silica Hydrogels. Colloids Surf. B Biointerfaces 2019, 173, 233–241. [Google Scholar] [CrossRef] [PubMed]
- Podrazky, O.; Ripp, S.; Trogl, J.; Sayler, G.; Demnerova, K.; Vankova, R. Monitoring the Viability of Cells Immobilized by the Sol-Gel Process. J. Sol Gel Sci. Technol. 2004, 31, 335–342. [Google Scholar] [CrossRef]
- Nassif, N.; Livage, J. From Diatoms to Silica-Based Biohybrids. Chem. Soc. Rev. 2011, 40, 849–859. [Google Scholar] [CrossRef] [PubMed]
- Saeed, M.U.; Hussain, N.; Sumrin, A.; Shahbaz, A.; Noor, S.; Bilal, M.; Aleya, L.; Iqbal, H.M.N. Microbial Bioremediation Strategies with Wastewater Treatment Potentialities—A Review. Sci. Total Environ. 2022, 818, 151754. [Google Scholar] [CrossRef] [PubMed]
- Sun, J.; Sun, H.; Lv, W.; Zhang, Q.; Wan, P.; Jiang, L.; Zhong, Y. Quorum Sensing Mediates Yeast Cell Morphology to Improve Settleability: Implication for Wastewater Treatment. J. Environ. Chem. Eng. 2021, 9, 105817. [Google Scholar] [CrossRef]
- Kim, I.T.; Lee, Y.E.; Jeong, Y.; Yoo, Y.S. A Novel Method to Remove Nitrogen from Reject Water in Wastewater Treatment Plants Using a Methane- and Methanol-Dependent Bacterial Consortium. Water Res. 2020, 172, 115512. [Google Scholar] [CrossRef]
- Berillo, D.; Al-Jwaid, A.; Caplin, J. Polymeric Materials Used for Immobilisation of Bacteria for the Bioremediation of Contaminants in Water. Polymers 2021, 13, 1073. [Google Scholar] [CrossRef]
- Melnikov, P.V.; Alexandrovskaya, A.Y.; Naumova, A.O.; Arlyapov, V.A.; Kamanina, O.A.; Popova, N.M.; Zaitsev, N.K.; Yashtulov, N.A. Optical Oxygen Sensing and Clark Electrode: Face-to-Face in a Biosensor Case Study. Sensors 2022, 22, 7626. [Google Scholar] [CrossRef] [PubMed]
- Tang, H.; Wang, M.; Meng, C.; Tao, W.; Wang, C.; Yu, H. Research on Design, Fabrication, and Properties of Fe2O3@SiO2/CDs/PEG@nSiO2 Nanocomposites. Mater. Lett. 2019, 235, 39–41. [Google Scholar] [CrossRef]
- Dudás, Z.; Len, A.; Ianăși, C.; Paladini, G. Structural Modifications Caused by the Increasing MTES Amount in Hybrid MTES/TEOS-Based Silica Xerogels. Mater. Charact. 2020, 167, 33–36. [Google Scholar] [CrossRef]
- Putz, A.M.; Wang, K.; Len, A.; Plocek, J.; Bezdicka, P.; Kopitsa, G.P.; Khamova, T.V.; Ianăşi, C.; Săcărescu, L.; Mitróová, Z.; et al. Mesoporous Silica Obtained with Methyltriethoxysilane as Co-Precursor in Alkaline Medium. Appl. Surf. Sci. 2017, 424, 275–281. [Google Scholar] [CrossRef]
- Ma, Y.; Kanezashi, M.; Tsuru, T. Preparation of Organic/Inorganic Hybrid Silica Using Methyltriethoxysilane and Tetraethoxysilane as Co-Precursors. J. Sol-Gel Sci. Technol. 2010, 53, 93–99. [Google Scholar] [CrossRef]
- Ponamoreva, O.N.; Kamanina, O.A.; Alferov, V.A.; Machulin, A.V.; Rogova, T.V.; Arlyapov, V.A.; Alferov, S.V.; Suzina, N.E.; Ivanova, E.P. Yeast-Based Self-Organized Hybrid Bio-Silica Sol–Gels for the Design of Biosensors. Biosens. Bioelectron. 2015, 67, 321–326. [Google Scholar] [CrossRef] [PubMed]
- Lavrova, D.G.; Kamanina, O.A.; Alferov, V.A.; Rybochkin, P.V.; Machulin, A.V.; Sidorov, A.I.; Ponamoreva, O.N. Impact of Hydrophilic Polymers in Organosilica Matrices on Structure, Stability, and Biocatalytic Activity of Immobilized Methylotrophic Yeast Used as Biofilter Bed. Enzyme Microb. Technol. 2021, 150, 109879. [Google Scholar] [CrossRef] [PubMed]
- Ponamoreva, O.; Afonina, E.; Kamanina, O.; Lavrova, D.; Arliapov, V.; Alferov, V.; Boronin, A. Yeast Debaryomyces Hansenii within ORMOSIL Shells as a Heterogeneous Biocatalyst. Appl. Biochem. Microbiol. 2018, 54, 24–30. [Google Scholar] [CrossRef]
- Lavrova, D.G.; Kamanina, O.A.; Machulin, A.V.; Suzina, N.E.; Alferov, V.A.; Ponamoreva, O.N. Effect of Polyethylene Glycol Additives on Structure, Stability, and Biocatalytic Activity of Ormosil Sol–Gel Encapsulated Yeast Cells. J. Sol-Gel Sci. Technol. 2018, 88, 1–5. [Google Scholar] [CrossRef]
- Postnova, I.; Silant’ev, V.; Kim, M.H.; Song, G.Y.; Kim, I.; Ha, C.S.; Shchipunov, Y. Hyperbranched Polyglycerol Hydrogels Prepared through Biomimetic Mineralization. Colloids Surfaces B Biointerfaces 2013, 103, 31–37. [Google Scholar] [CrossRef]
- Khonina, T.G.; Safronov, A.P.; Ivanenko, M.V.; Shadrina, E.V.; Chupakhin, O.N. Features of Silicon– and Titanium–Polyethylene Glycol Precursors in Sol–Gel Synthesis of New Hydrogels for Medical Application. J. Mater. Chem. B 2015, 3, 5490–5500. [Google Scholar] [CrossRef] [PubMed]
- Khonina, T.G.; Safronov, A.P.; Shadrina, E.V.; Ivanenko, M.V.; Suvorova, A.I.; Chupakhin, O.N. Mechanism of Structural Networking in Hydrogels Based on Silicon and Titanium Glycerolates. J. Colloid Interface Sci. 2012, 365, 81–89. [Google Scholar] [CrossRef]
- Shkryl, Y.N.; Semiletova, I.V.; Nepomnyaschiy, A.V.; Kovalchuk, S.N.; Veremeichik, G.N.; Avramenko, T.V.; Bulgakov, V.P.; Shchipunov, Y.A.; Voznesenskiy, S.S.; Kozhemyako, V.B. Biomimetic Synthesis of Nanosized Silica Structures on a Substrate with Silicatein. Russ. J. Bioorganic Chem. 2018, 44, 469–471. [Google Scholar] [CrossRef]
- Shchipunov, Y. Bionanocomposites: Green Sustainable Materials for the near Future. Pure Appl. Chem. 2012, 84, 2579–2607. [Google Scholar] [CrossRef]
- Ivanenko, M.V.; Nikitina, E.Y.; Khonina, T.G.; Shadrina, E.V.; Novoselova, M.E.; Kuznetsov, D.K.; Karabanalov, M.S. Features of Formation and Structure of Silicon–Polysaccharide-Containing Polyolate Hydrogels Obtained by the Method of Biomimetic Mineralization. J. Sol-Gel Sci. Technol. 2019, 92, 376–385. [Google Scholar] [CrossRef]
- Kamanina, O.A.; Lavrova, D.G.; Arlyapov, V.A.; Alferov, V.A.; Ponamoreva, O.N. Silica Sol-Gel Encapsulated Methylotrophic Yeast as Filling of Biofilters for the Removal of Methanol from Industrial Wastewater. Enzyme Microb. Technol. 2016, 92, 94–98. [Google Scholar] [CrossRef] [PubMed]
- Catauro, M.; D’Angelo, A.; Fiorentino, M.; Pacifico, S.; Latini, A.; Brutti, S.; Vecchio Ciprioti, S. Thermal, Spectroscopic Characterization and Evaluation of Antibacterial and Cytotoxicity Properties of Quercetin-PEG-Silica Hybrid Materials. Ceram. Int. 2022, 1–9. [Google Scholar] [CrossRef]
- Ferkous, H.; Rouibah, K.; Hammoudi, N.E.H.; Alam, M.; Djilani, C.; Delimi, A.; Laraba, O.; Yadav, K.K.; Ahn, H.J.; Jeon, B.H.; et al. The Removal of a Textile Dye from an Aqueous Solution Using a Biocomposite Adsorbent. Polymers 2022, 14, 2396. [Google Scholar] [CrossRef]
- Catauro, M.; Šiler, P.; Másilko, J.; Risoluti, R.; Ciprioti, S.V. Synthesis, Structural, Morphological and Thermal Characterization of Five Different Silica-Polyethylene Glycol-Chlorogenic Acid Hybrid Materials. Polymers 2021, 13, 1586. [Google Scholar] [CrossRef]
- Skountzos, E.N.; Karadima, K.S.; Mavrantzas, V.G. Structure and Dynamics of Highly Attractive Polymer Nanocomposites in the Semi-Dilute Regime: The Role of Interfacial Domains and Bridging Chains. Polymers 2021, 13, 2749. [Google Scholar] [CrossRef]






| Composites | Mass Ratio of Reagents in the Sol-Gel Synthesis |
|---|---|
| PEG400:TEOS (STPEG) | 1:0.125 (~90% PEG) |
| PEG3000:TEOS/MTES (15/85) (SPEG) | 1:4 (~20% PEG) |
| Parameter | STPEG | SPEG [66,68] |
|---|---|---|
| Sensitivity coefficient, mgO2 × min−1 × mmol−1 | 0.85 ± 0.08 * | 0.87 ± 0.05 * |
| Relative standard deviation, % | 10 | 3 |
| Long-term stability, days | 10 | 15 |
| Parameter | Before UV Irradiation | After UV Irradiation |
|---|---|---|
| Sensitivity coefficient, mgO2 × min−1 × mmol−1 | 0.85 ± 0.07 | 0.73 ± 0.08 |
| Relative standard deviation, % | 10 | 8 |
| Long-term stability, days | 10 | 9 |
| Column Configuration | Purification Degree, % | Oxidative Power, gO2/(m3 × Cycle) |
|---|---|---|
| Passive aeration | ||
| Long column (d = 1 cm; h = 10 cm) | 13 ± 1 * | 268 ± 1 * |
| Short column (d = 2 cm; h = 5 cm) | 10 ± 1 | 75 ± 1 |
| Active aeration | ||
| Long column (d = 1 cm; h = 10 cm) | 58 ± 1 | 898 ± 1 |
| Short column (d = 2 cm; h = 5 cm) | 31 ± 1 | 248 ± 1 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lavrova, D.G.; Zvonarev, A.N.; Alferov, V.A.; Khonina, T.G.; Shadrina, E.V.; Alferov, S.V.; Ponamoreva, O.N. Biocompatible Silica-Polyethylene Glycol-Based Composites for Immobilization of Microbial Cells by Sol-Gel Synthesis. Polymers 2023, 15, 458. https://doi.org/10.3390/polym15020458
Lavrova DG, Zvonarev AN, Alferov VA, Khonina TG, Shadrina EV, Alferov SV, Ponamoreva ON. Biocompatible Silica-Polyethylene Glycol-Based Composites for Immobilization of Microbial Cells by Sol-Gel Synthesis. Polymers. 2023; 15(2):458. https://doi.org/10.3390/polym15020458
Chicago/Turabian StyleLavrova, Daria G., Anton N. Zvonarev, Valery A. Alferov, Tat’yana G. Khonina, Elena V. Shadrina, Sergey V. Alferov, and Olga N. Ponamoreva. 2023. "Biocompatible Silica-Polyethylene Glycol-Based Composites for Immobilization of Microbial Cells by Sol-Gel Synthesis" Polymers 15, no. 2: 458. https://doi.org/10.3390/polym15020458
APA StyleLavrova, D. G., Zvonarev, A. N., Alferov, V. A., Khonina, T. G., Shadrina, E. V., Alferov, S. V., & Ponamoreva, O. N. (2023). Biocompatible Silica-Polyethylene Glycol-Based Composites for Immobilization of Microbial Cells by Sol-Gel Synthesis. Polymers, 15(2), 458. https://doi.org/10.3390/polym15020458









