Fluoride Release and Rechargeability of Poly(lactic acid) Composites with Glass Ionomer Cement
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
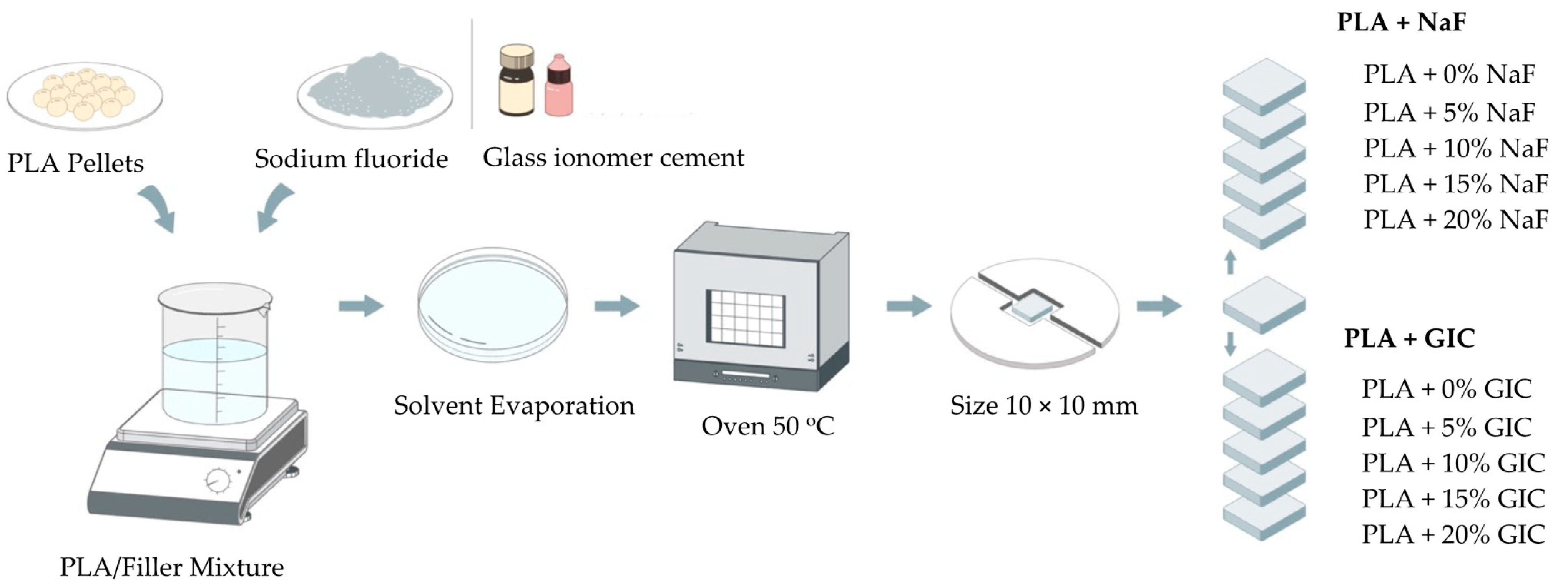
2.2. Specimen Preparation
2.3. Specimen Characterization
2.3.1. Fluoride Release
2.3.2. Fluoride Rechargeability
2.3.3. In Vitro Degradation Test and Water Absorption
2.3.4. Surface Morphology
2.4. Statistical Analysis
3. Results
3.1. Fluoride Release
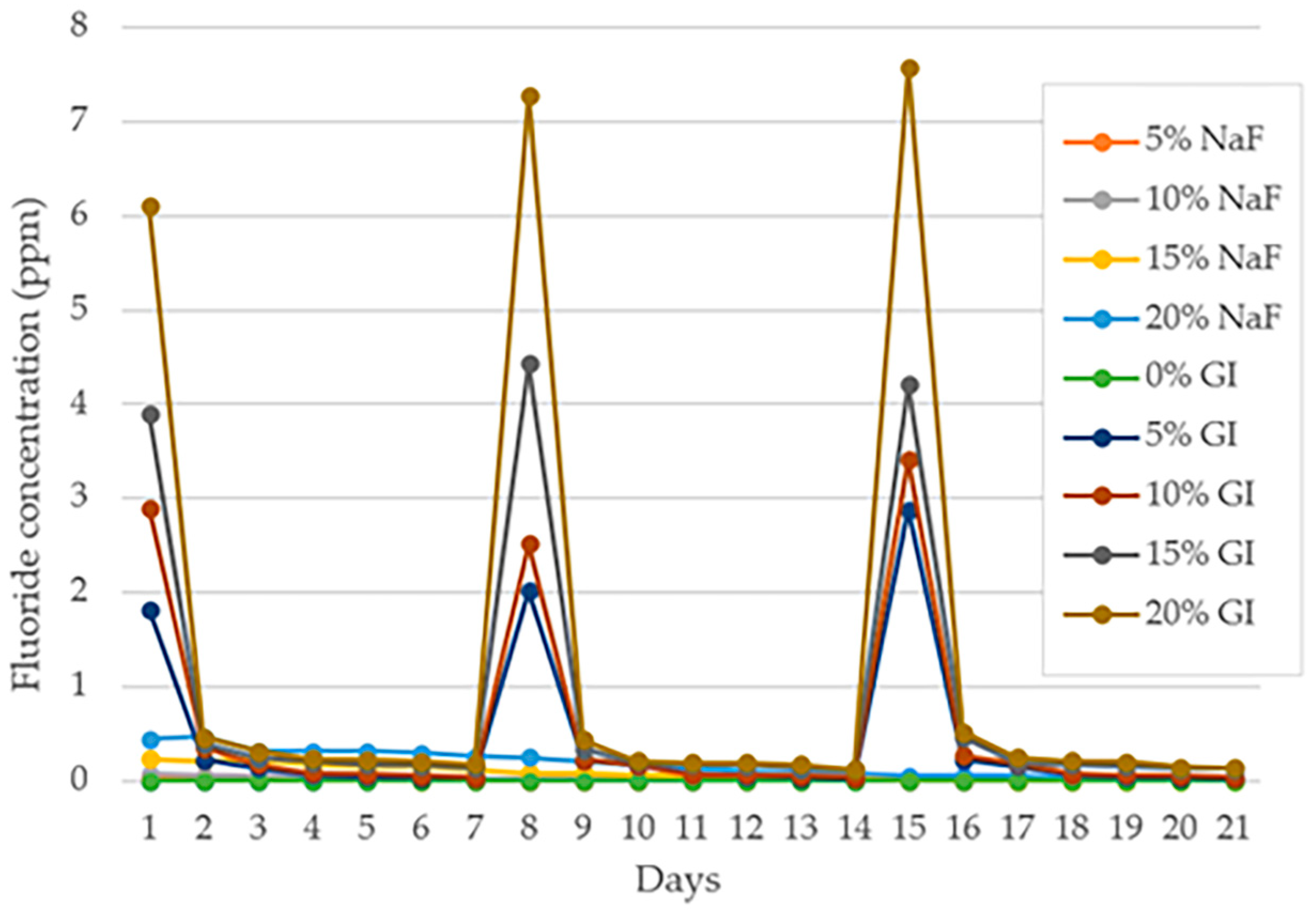
3.2. Fluoride Rechargeability
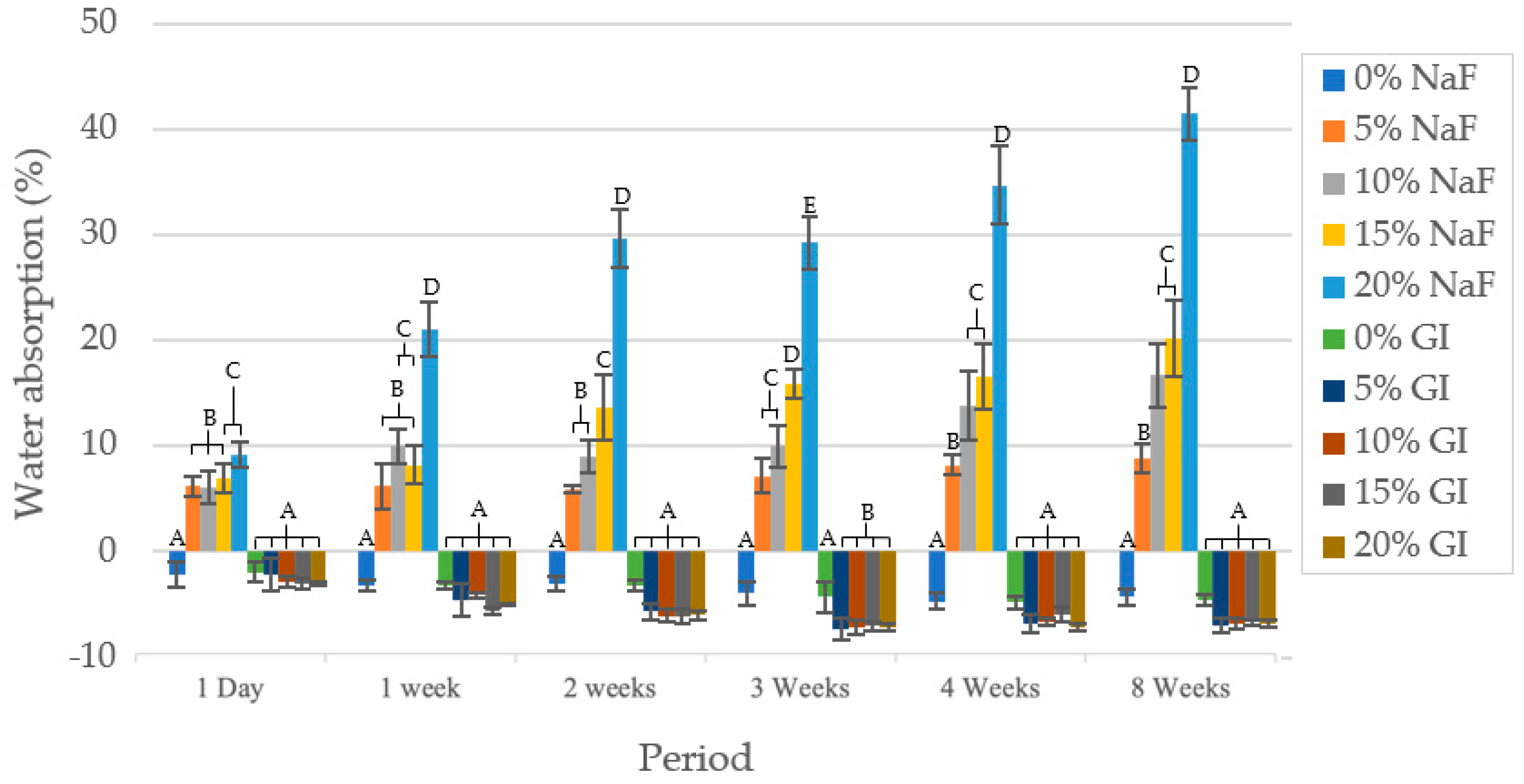
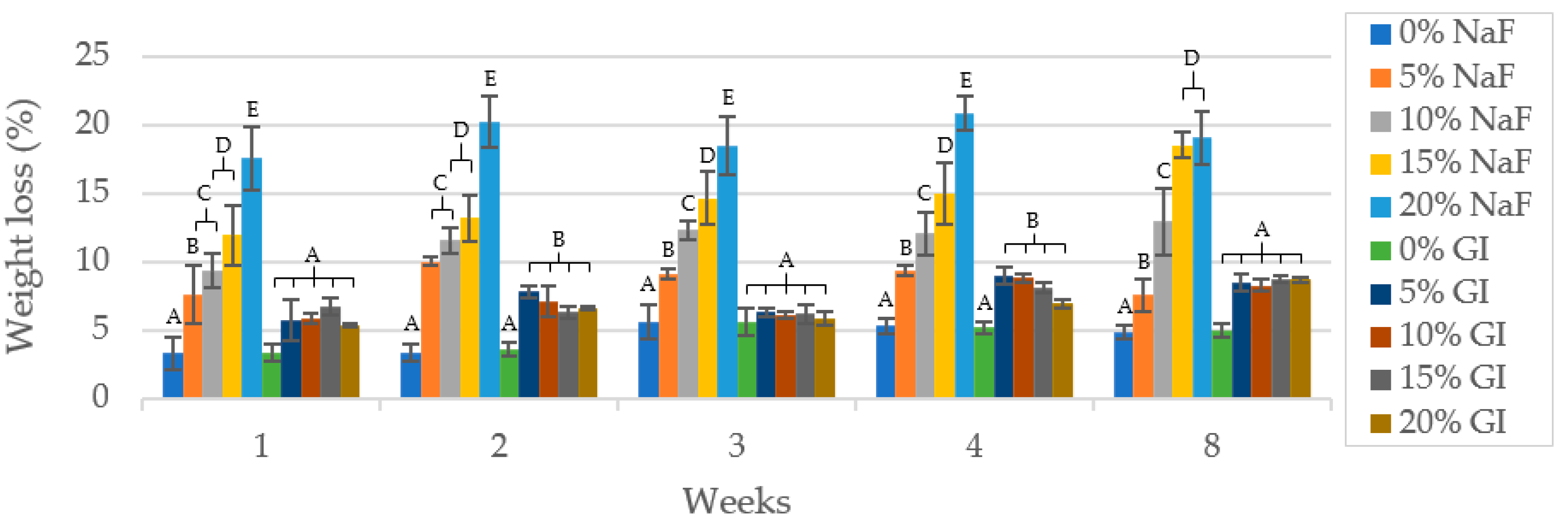
3.3. In Vitro Degradation Test and Water Absorption
3.3.1. Water Absorption
3.3.2. Weight Loss
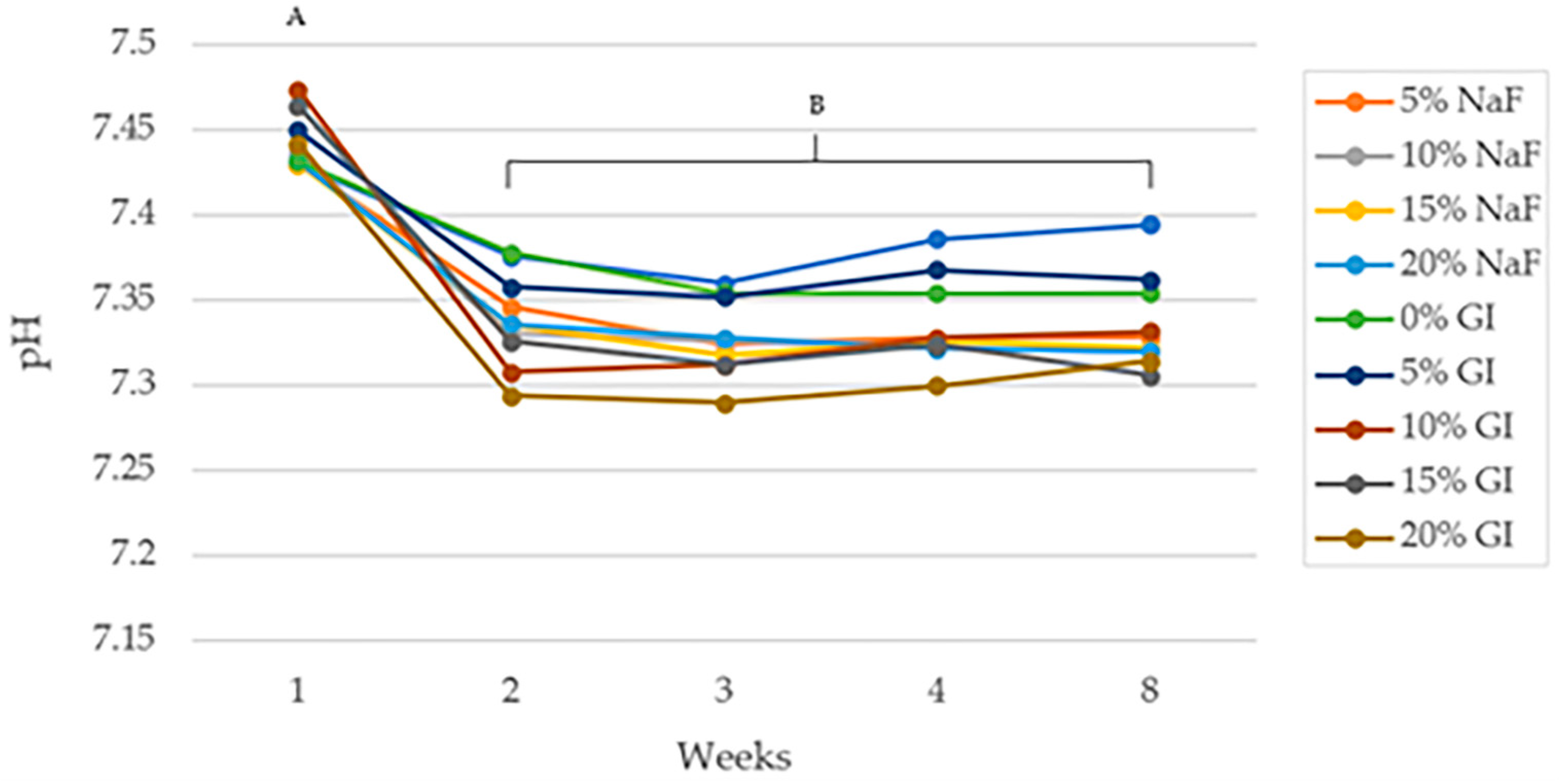
3.3.3. pH Change
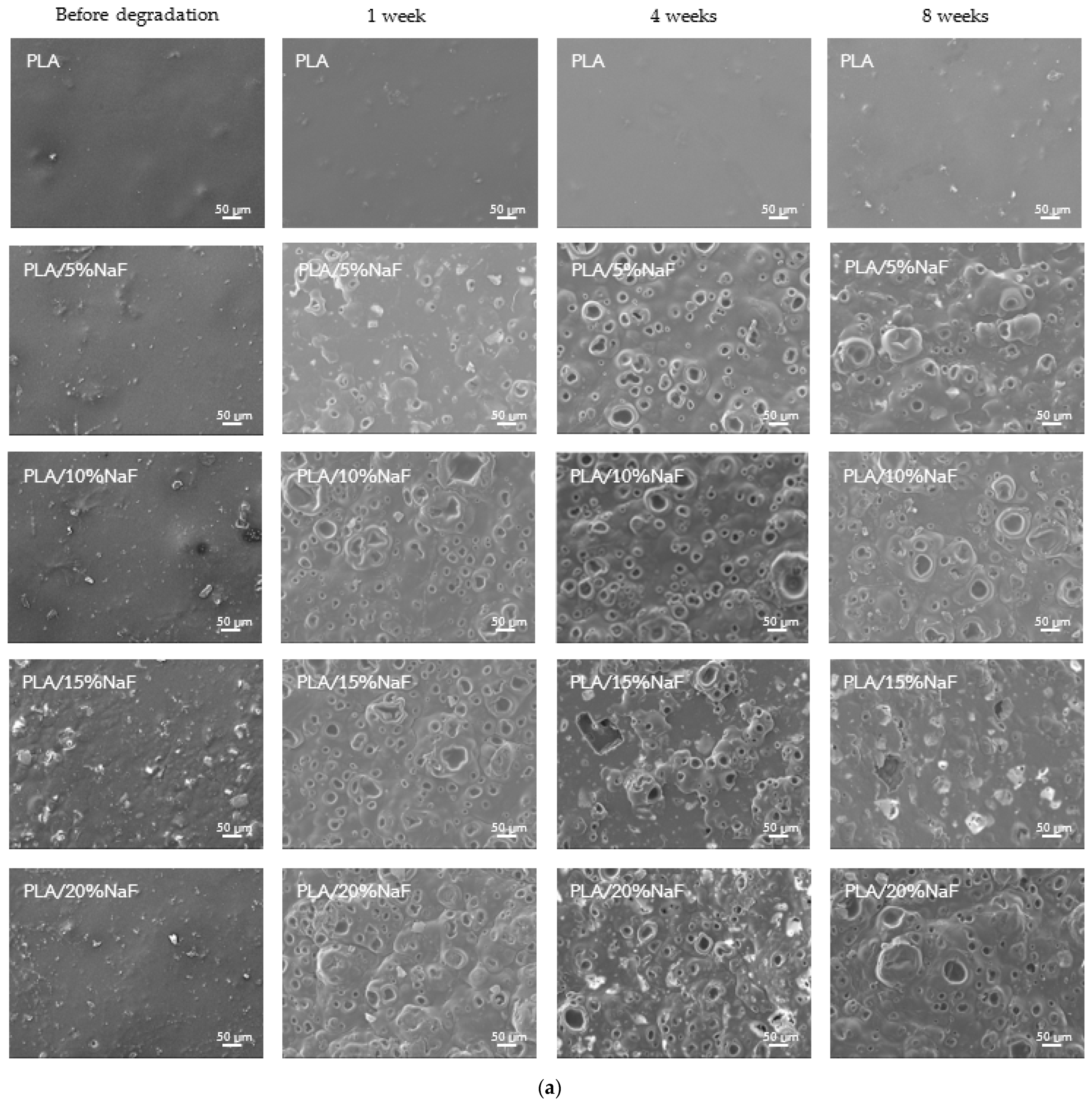
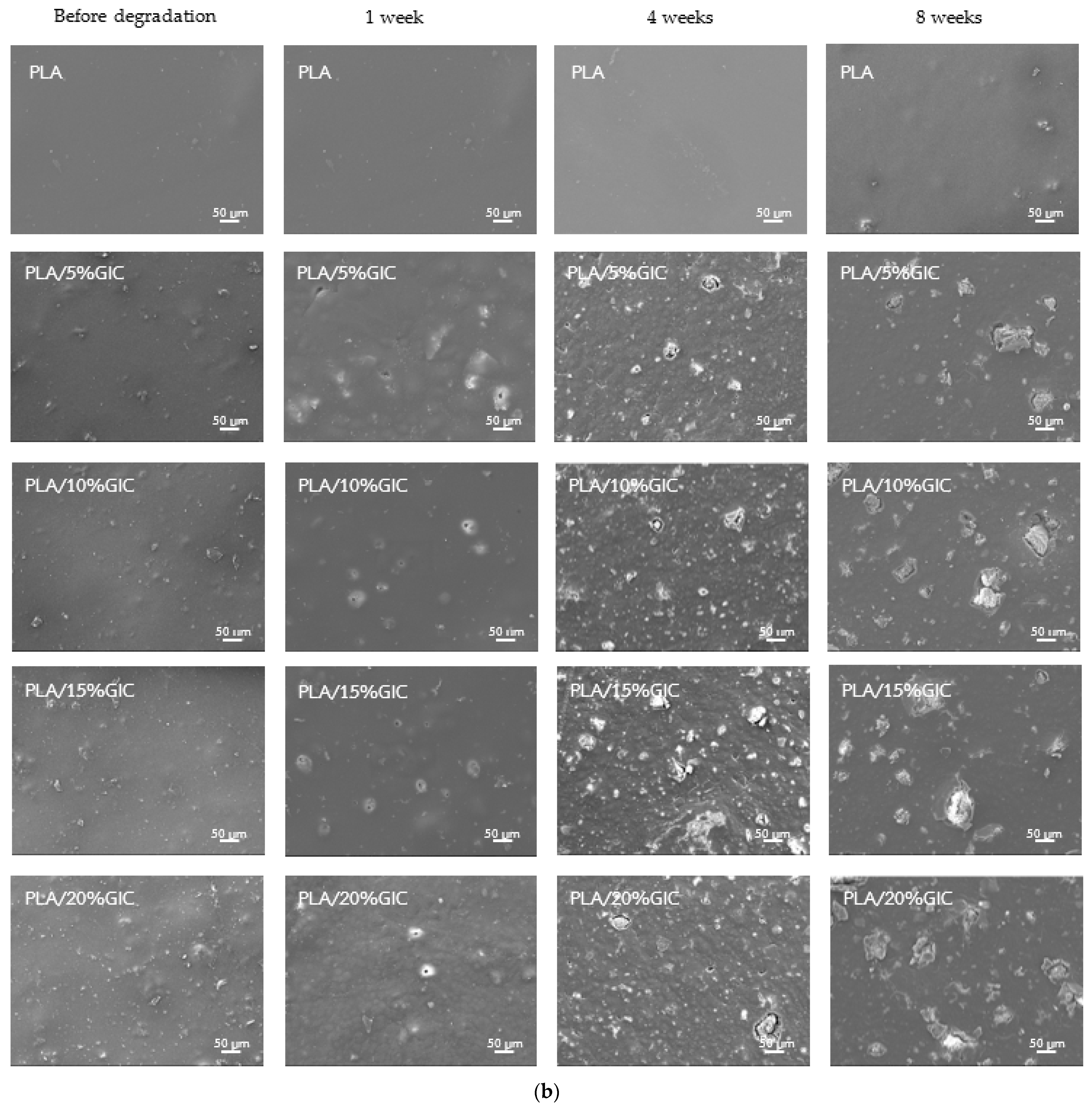
3.4. Surface Morphology
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Selwitz, R.H.; Ismail, A.I.; Pitts, N.B. Dental caries. Lancet 2007, 369, 51–59. [Google Scholar]
- Chan, A.K.Y.; Tamrakar, M.; Jiang, C.M.; Lo, E.C.M.; Leung, K.C.M.; Chu, C.H. A Systematic Review on Caries Status of Older Adults. Int. J. Environ. Res. Public Health 2021, 18, 10662. [Google Scholar] [CrossRef]
- Gati, D.; Vieira, A.R. Elderly at greater risk for root caries: A look at the multifactorial risks with emphasis on genetics susceptibility. Int. J. Dent. 2011, 2011, 647168. [Google Scholar] [CrossRef]
- Amaechi, B.T. Remineralization therapies for initial caries lesions. Curr. Oral Health Rep. 2015, 2, 95–101. [Google Scholar] [CrossRef]
- Philip, N. State of the Art Enamel Remineralization Systems: The Next Frontier in Caries Management. Caries Res. 2019, 53, 284–295. [Google Scholar] [CrossRef]
- Dogan, S.; Fong, H.; Yucesoy, D.T.; Cousin, T.; Gresswell, C.; Dag, S.; Huang, G.; Sarikaya, M. Biomimetic tooth repair: Amelogenin-derived peptide enables in vitro remineralization of human enamel. ACS Biomater. Sci. Eng. 2018, 4, 1788–1796. [Google Scholar] [CrossRef]
- Loveren, C. The antimicrobial action of fluoride and its role in caries inhibition. J. Dent. Res. 1990, 69, 676–681. [Google Scholar] [CrossRef]
- Anil, A.; Ibraheem, W.I.; Meshni, A.A.; Preethanath, R.; Anil, S. Demineralization and Remineralization Dynamics and Dental Caries. In Dental Caries, The Selection of Restoration Methods and Restorative Materials; IntechOpen: London, UK, 2022. [Google Scholar]
- Sjögren, K.; Birkhed, D. Factors related to fluoride retention after toothbrushing and possible connection to caries activity. Caries Res. 1993, 27, 474–477. [Google Scholar] [CrossRef]
- Ullah, R.; Zafar, M.S. Oral and dental delivery of fluoride: A review. Fluoride 2015, 48, 195. [Google Scholar]
- Duckworth, R.M. Pharmacokinetics in the oral cavity: Fluoride and other active ingredients. Toothpastes 2013, 23, 125–139. [Google Scholar]
- Srithongsuk, S.; Anuwongnukroh, N.; Dechkunakorn, S.; Srikhirin, T.; Tua-Ngam, P. Investigation of fluoride release from orthodontic acrylic plate. Adv. Mater. Res. 2012, 378, 681–687. [Google Scholar] [CrossRef]
- Tsutsumi, C.; Takakuda, K.; Wakabayashi, N. Reduction of Candida biofilm adhesion by incorporation of prereacted glass ionomer filler in denture base resin. J. Dent. 2016, 44, 37–43. [Google Scholar]
- Kamijo, K.; Mukai, Y.; Tominaga, T.; Iwaya, I.; Fujino, F.; Hirata, Y.; Teranaka, T. Fluoride release and recharge characteristics of denture base resins containing surface pre-reacted glass-ionomer filler. Dent. Mater. J. 2009, 28, 227–233. [Google Scholar] [CrossRef]
- Ali, A. Evaluation of the effect of sodium fluoride addition on some mechanical properties of heat cure acrylic denture base materials. J. Baghdad Coll. Dent. 2014, 26, 9–13. [Google Scholar]
- Agarwal, B.; Dayal Singh, R.; Raghav, D.; Shekhar, A.; Yadav, P. Determination of Fluoride Release and Strength of a Fluoride Treated Heat Cured Acrylic Resin. EAS J. Dent. Oral Med. 2019, 1, 108–111. [Google Scholar]
- Raszewski, Z.; Nowakowska, D.; Wieckiewicz, W.; Nowakowska-Toporowska, A. Release and Recharge of Fluoride Ions from Acrylic Resin Modified with Bioactive Glass. Polymers 2021, 13, 1054. [Google Scholar] [CrossRef]
- Anusavice, K.J.; Shen, C.; Rawls, H.R. Phillips′ Science of Dental Materials, 12th ed.; Elsevier Health Sciences: Amsterdam, The Netherlands, 2012. [Google Scholar]
- Billington, R.W.; Williams, J.A.; Pearson, G.J. Ion processes in glass ionomer cements. J. Dent. 2006, 34, 544–555. [Google Scholar]
- Gui, Y.; Zhao, X.; Li, S.; Tang, L.; Gong, X. Fluoride release and recharge properties of six restorative materials. Chin. J. Stomatol. 2015, 50, 28–32. [Google Scholar]
- Injorhor, P.; Trongsatitkul, T.; Wittayakun, J.; Ruksakulpiwat, C.; Ruksakulpiwat, Y. Nano-Hydroxyapatite from White Seabass Scales as a Bio-Filler in Polylactic Acid Biocomposite: Preparation and Characterization. Polymers 2022, 14, 4158. [Google Scholar] [CrossRef]
- Li, H.; Ma, K.; Sun, Y.; Chen, H. Design parameters of polylactic acid custom trays manufactured by fused deposition modeling for partial edentulism: Consideration of the accuracy of the definitive cast. J. Prosthet. Dent. 2022, 127, 281–288. [Google Scholar]
- Tyler, B.; Gullotti, D.; Mangraviti, A.; Utsuki, T.; Brem, H. Polylactic acid (PLA) controlled delivery carriers for biomedical applications. Adv. Drug. Deliv. Rev. 2016, 107, 163–175. [Google Scholar] [PubMed]
- Jiang, H.; Fu, J.; Li, M.; Wang, S.; Zhuang, B.; Sun, H.; Ge, C.; Feng, B.; Jin, Y. 3D-Printed Wearable Personalized Orthodontic Retainers for Sustained Release of Clonidine Hydrochloride. AAPS PharmSciTech 2019, 20, 260. [Google Scholar] [PubMed]
- Shimizu, S.; Kotake, H.; Takagaki, T.; Shinno, K.; Miyata, S.; Burrow, M.F.; Hotta, M.; Nikaido, T. Evaluation of bonding performance and multi-ion release of S-PRG fillercontaining self-adhesive resin composite. Dent. Mater. J. 2021, 40, 1257–1263. [Google Scholar] [CrossRef] [PubMed]
- Ghajari, M.F.; Torabzadeh, H.; Safavi, N.; Sohrabi, A.; Ardakani, F.F. Fluoride release from three glass ionomers after exposure to sodium fluoride and acidulated phosphate fluoride gels. Dent. Res. J. 2014, 11, 604. [Google Scholar]
- Jurasic, M.M.; Gibson, G.; Orner, M.B.; Wehler, C.J.; Jones, J.A.; Cabral, H.J. Topical Fluoride Effectiveness in High Caries Risk Adults. J. Dent. Res. 2022, 101, 898–904. [Google Scholar] [CrossRef]
- ISO 15814:1999; Implants for Surgery-Copolymers and Blends Based on Polylactide-In Vitro Degradation Testing. ISO: Geneva, Switzerland, 1999.
- DeStefano, V.; Khan, S.; Tabada, A. Applications of PLA in modern medicine. Eng. Regen. 2020, 1, 76–87. [Google Scholar] [CrossRef]
- Bakola, V.; Karagkiozaki, V.; Tsiapla, A.R.; Pappa, F.; Moutsios, I.; Pavlidou, E.; Logothetidis, S. Dipyridamole-loaded biodegradable PLA nanoplatforms as coatings for cardiovascular stents. Nanotechnology 2018, 29, 275101. [Google Scholar] [CrossRef]
- Margolis, H.; Moreno, E.; Murphy, B. Effect of low levels of fluoride in solution on enamel demineralization in vitro. J. Dent. Res. 1986, 65, 23–29. [Google Scholar] [CrossRef]
- Kiatsirirote, K.; Sitthisettapong, T.; Phantumvanit, P.; Chan, D.C.N. Fluoride-Releasing Effect of a Modified Resin Denture Containing S-PRG Fillers on Salivary Fluoride Retention: A Randomized Clinical Study. Caries Res. 2019, 53, 137–144. [Google Scholar] [CrossRef]
- Reynolds, J.; Belsher, J. A Review of Sodium Fluoride Solubility in Water. J. Chem. Eng. 2017, 62, 1743–1748. [Google Scholar] [CrossRef]
- Lee, S.Y.; Dong, D.R.; Huang, H.M.; Shih, Y.H. Fluoride ion diffusion from a glass–ionomer cement. J. Oral Rehabil. 2000, 27, 576–586. [Google Scholar]
- Akerlund, E.; Diez-Escudero, A.; Grzeszczak, A.; Persson, C. The Effect of PCL Addition on 3D-Printable PLA/HA Composite Filaments for the Treatment of Bone Defects. Polymers 2022, 14, 3305. [Google Scholar] [CrossRef]
- Boonmee, C.; Kositanont, C.; Leejarkpai, T. Degradation of poly(lactic acid) under simulated landfill conditions. Environ. Nat. Resour. J. 2016, 14, 1–9. [Google Scholar]
- Joseph, A.; Karthikeyan, S.; Padmanabhan, R.G.; Rajeshkumar, S.; Dilip Kumar, B.; Rajesh, S. A review on PLA with different fillers used as a filament in 3D printing. Mater. Today Proc. 2022, 50, 2057–2064. [Google Scholar]
- Farah, S.; Anderson, D.G.; Langer, R. Physical and mechanical properties of PLA, and their functions in widespread applications—A comprehensive review. Adv. Drug. Deliv. Rev. 2016, 107, 367–392. [Google Scholar]
- Elsawy, M.A.; Kim, K.H.; Park, J.W.; Deep, A. Hydrolytic degradation of polylactic acid (PLA) and its composites. Renew. Sustain. Energy Rev. 2017, 79, 1346–1352. [Google Scholar]
- Cifuentes, S.C.; Lieblich, M.; Saldaña, L.; González-Carrasco, J.L.; Benavente, R. In vitro degradation of biodegradable polylactic acid/Mg composites: Influence of nature and crystalline degree of the polymeric matrix. Materialia 2019, 6, 100270. [Google Scholar] [CrossRef]
- Suttiat, K.; Wattanutchariya, W.; Manaspon, C. Preparation and Characterization of Porous Poly(Lactic Acid)/Poly(Butylene Adipate-Co-Terephthalate) (PLA/PBAT) Scaffold with Polydopamine-Assisted Biomineralization for Bone Regeneration. Materials 2022, 15, 7756. [Google Scholar]
- Silva, D.; Kaduri, M.; Poley, M.; Adir, O.; Krinsky, N.; Shainsky-Roitman, J.; Schroeder, A. Biocompatibility, biodegradation and excretion of polylactic acid (PLA) in medical implants and theranostic systems. Chem. Eng. J. 2018, 340, 9–14. [Google Scholar]
- Atkins, P.; Jones, L. Chemical Principles, 7th ed.; Macmillan: New York, NY, USA, 2009; Volume 384, pp. 191–192. [Google Scholar]
- Collins, K.D. The behavior of ions in water is controlled by their water affinity. Q. Rev. Biophys. 2019, 52, 11. [Google Scholar] [CrossRef]
- Liang, K.; Carmone, S.; Brambilla, D.; Leroux, J.C. 3D printing of a wearable personalized oral delivery device: A first-in-human study. Sci. Adv. 2018, 4, 2544. [Google Scholar]
- Jingarwar, M.M.; Pathak, A.; Bajwa, N.K.; Sidhu, H.S. Quantitative assessment of fluoride release and recharge ability of different restorative materials in different media: An in vitro study. J. Clin. Diagn. Res. 2014, 8, 31–34. [Google Scholar] [CrossRef] [PubMed]
- Prapansilp, W.; Rirattanapong, P.; Surarit, R.; Vongsavan, K. Fluoride Release from Different Powder Liquid Ratios of Fuji VII. Mahidol Dent. J. 2017, 37, 217–222. [Google Scholar]
- Mousavinasab, S.M.; Meyers, I. Fluoride release by glass ionomer cements, compomer and giomer. Dent. Res. J. 2009, 6, 75–81. [Google Scholar]
- Dionysopoulos, D.; Koliniotou-Koumpia, E.; Helvatzoglou-Antoniades, M.; Kotsanos, N. Fluoride release and recharge abilities of contemporary fluoride-containing restorative materials and dental adhesives. Dent. Mater. J. 2013, 32, 296–304. [Google Scholar] [CrossRef]
- Sabir, D.; Omer, Z. Evaluation of Fluoride release from orthodontic acrylic resin by using two different polymerizations techniques: An InVitro Study. Erbil Dent. J. 2019, 2, 149–156. [Google Scholar] [CrossRef]
- Xu, Q.; Chin, S.E.; Wang, C.H.; Pack, D.W. Mechanism of drug release from double-walled PDLLA(PLGA) microspheres. Biomaterials 2013, 34, 3902–3911. [Google Scholar] [CrossRef]
- Al-Bakri, I.A.; Swain, M.V.; Naoum, S.J.; Al-Omari, W.M.; Martin, E.; Ellakwa, A. Fluoride release, recharge and flexural properties of polymethylmethacrylate containing fluoridated glass fillers. Aust. Dent. J. 2014, 59, 208–214. [Google Scholar] [CrossRef]
- Patil, S.S.; Kontham, U.R.; Kontham, R.K.; Patil, S.S.; Kamble, S.P. Fluoride release and fluoride-recharging ability of three different sealants. J. Indian Soc. Pedod. Prev. Dent. 2020, 38, 247–252. [Google Scholar]
- Bayrak, S.; Tunc, E.S.; Aksoy, A.; Ertas, E.; Guvenc, D.; Ozer, S. Fluoride release and recharge from different materials used as fissure sealants. Eur. J. Dent. 2010, 4, 245–250. [Google Scholar] [CrossRef]
- Markovic, D.; Petrovic, B.B.; Peric, T.O. Fluoride content and recharge ability of five glassionomer dental materials. BMC Oral Health 2008, 8, 21. [Google Scholar]
- Xu, X.; Burgess, J.O. Compressive strength, fluoride release and recharge of fluoride-releasing materials. Biomaterials 2003, 24, 2451–2461. [Google Scholar]
- Zhao, X.Y.; Gui, Y.J.; Li, S.B. Fluoride Release and Recharge Ability of a Novel Fluoride Release Composite Resin. Adv. Mater. Res. 2013, 833, 355–359. [Google Scholar] [CrossRef]
- Wiegand, A.; Buchalla, W.; Attin, T. Review on fluoride-releasing restorative materials—Fluoride release and uptake characteristics, antibacterial activity and influence on caries formation. Dent. Mater. 2007, 23, 343–362. [Google Scholar]
- Naoum, S.; Ellakwa, A.; Martin, F.; Swain, M. Fluoride release, recharge and mechanical property stability of various fluoride-containing resin composites. Oper. Dent. 2011, 36, 422–432. [Google Scholar]
- Liu, X.; Wang, T.; Chow, L.C.; Yang, M.; Mitchell, J.W. Effects of Inorganic Fillers on the Thermal and Mechanical Properties of Poly(lactic acid). Int. J. Polym. Sci. 2014, 2014, 827028. [Google Scholar] [CrossRef]

| Time | |||||
|---|---|---|---|---|---|
| Group | 1 Day | 7 Days | 14 Days | 21 Days | 28 Days |
| 0% NaF | nd A | nd A | nd A | nd A | nd A |
| 5% NaF | 76.12 ± 6.45 B d | 0.16 ± 0.03 AB c | 0.13 ± 0.02 AB b | 0.14 ± 0.03 BC bc | 0.03 ± 0.01 AB a |
| 10% NaF | 147.66 ± 4.81 C e | 0.77 ± 0.16 D d | 0.37 ± 0.05 D c | 0.17 ± 0.01 C b | 0.09 ± 0.02 D a |
| 15% NaF | 202.73 ± 37.61 D e | 2.15 ± 0.33 E d | 0.90 ± 0.12 E c | 0.56 ± 0.09 D b | 0.27 ± 0.05 E a |
| 20% NaF | 310.39 ± 34.61 E e | 2.49 ± 0.34 F d | 1.39 ± 0.29 F c | 1.01 ± 0.18 E b | 0.53 ± 0.06 F a |
| 0% GI | nd A | nd A | nd A | nd A | nd A |
| 5% GI | 1.86 ± 0.18 A e | 0.11 ± 0.02 AB d | 0.08 ± 0.01 AB c | 0.06 ± 0.01 AB b | 0.03 ± 0.01 AB a |
| 10% GI | 2.19 ± 0.08 A e | 0.23 ± 0.04 AB d | 0.09 ± 0.01 AB c | 0.07 ± 0.01 AB b | 0.05 ± 0.01 BC a |
| 15% GI | 3.75 ± 0.48 A e | 0.34 ± 0.03 BC d | 0.20 ± 0.04 BC c | 0.15 ± 0.02 BC b | 0.07 ± 0.00 CD a |
| 20% GI | 5.52 ± 0.44 A e | 0.52 ± 0.06 C d | 0.29 ± 0.03 CD c | 0.20 ± 0.03 C b | 0.11 ± 0.02 D a |
| Time | |||||||||
|---|---|---|---|---|---|---|---|---|---|
| Group | 1 Day | 2 Days | 7 Days | 8 Days | 9 Days | 14 Days | 15 Days | 16 Days | 21 Days |
| 0% NaF | nd A | nd A | nd A | nd A | nd A | nd A | nd A | nd A | nd A |
| 5% NaF | 0.04 ± 0.01 AB b | 0.02 ± 0.00 A a | nd A | nd A | nd A | nd A | nd A | nd A | nd A |
| 10% NaF | 0.07 ± 0.01 AB d | 0.06 ± 0.01 A c | 0.03 ± 0.00 B c | 0.02 ± 0.00 A b | 0.02 ± 0.00 A a | nd A | nd A | nd A | nd A |
| 15% NaF | 0.24 ± 0.03 BC h | 0.21 ± 0.06 B h | 0.11 ± 0.01 C g | 0.09 ± 0.01 A f | 0.08 ± 0.01 B e | 0.03 ± 0.00 B d | 0.02 ± 0.01 A c | 0.01 ± 0.00 A b | nd A |
| 20% NaF | 0.44 ± 0.07 C g | 0.47 ± 0.09 D g | 0.26 ± 0.02 F f | 0.25 ± 0.03 A f | 0.21 ± 0.02 C e | 0.07 ± 0.01 C d | 0.06 ± 0.00 A c | 0.05 ± 0.00 A b | 0.01 ± 0.00 B a |
| 0% GI | nd A | nd A | nd A | nd A | nd A | nd A | nd A | nd A | nd A |
| 5% GI | 1.82 ± 0.15 D d | 0.22 ± 0.04 B c | 0.03 ± 0.00 B b | 2.02 ± 0.25 B d | 0.22 ± 0.04 C c | 0.03 ± 0.00 B a | 2.88 ± 0.28 B d | 0.22 ± 0.09 B c | 0.02 ± 0.00 C a |
| 10% GI | 2.90 ± 0.06 E g | 0.35 ± 0.04 C e | 0.04 ± 0.00 B b | 2.52 ± 0.12 C f | 0.22 ± 0.02 C c | 0.03 ± 0.00 B a | 3.41 ± 0.66 C h | 0.27 ± 0.06 B d | 0.03 ± 0.01 D ab |
| 15% GI | 3.90 ± 0.28 F e | 0.38 ± 0.03 C c | 0.14 ± 0.00 D b | 4.44 ± 0.39 D f | 0.33 ± 0.06 D c | 0.12 ± 0.02 D a | 4.21 ± 0.76 D ef | 0.46 ± 0.04 C d | 0.13 ± 0.01 E a |
| 20% GI | 6.10 ± 0.37 G f | 0.46 ± 0.06 D de | 0.17 ± 0.02 E c | 7.28 ± 0.40 E g | 0.43 ± 0.06 E d | 0.12 ± 0.01 D a | 7.58 ± 0.37 E g | 0.52 ± 0.05 C e | 0.14 ± 0.01 F b |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wongphattarakul, S.; Kuson, R.; Sastraruji, T.; Suttiat, K. Fluoride Release and Rechargeability of Poly(lactic acid) Composites with Glass Ionomer Cement. Polymers 2023, 15, 4041. https://doi.org/10.3390/polym15204041
Wongphattarakul S, Kuson R, Sastraruji T, Suttiat K. Fluoride Release and Rechargeability of Poly(lactic acid) Composites with Glass Ionomer Cement. Polymers. 2023; 15(20):4041. https://doi.org/10.3390/polym15204041
Chicago/Turabian StyleWongphattarakul, Sudarat, Rungroj Kuson, Thanapat Sastraruji, and Kullapop Suttiat. 2023. "Fluoride Release and Rechargeability of Poly(lactic acid) Composites with Glass Ionomer Cement" Polymers 15, no. 20: 4041. https://doi.org/10.3390/polym15204041
APA StyleWongphattarakul, S., Kuson, R., Sastraruji, T., & Suttiat, K. (2023). Fluoride Release and Rechargeability of Poly(lactic acid) Composites with Glass Ionomer Cement. Polymers, 15(20), 4041. https://doi.org/10.3390/polym15204041





