Author Contributions
Conceptualization, E.I., A.F., E.G., P.T. and A.S.; methodology, E.I., N.M., E.M., P.T. and A.S.; formal analysis, A.F., I.D., N.S. and P.A.; investigation, E.I., A.F., I.D., E.G., M.T., E.M., P.A. and I.R.; resources, N.M., M.T., I.R., P.T. and A.S.; data curation, I.D. and N.S.; writing—original draft preparation, E.I., A.F., N.M. and I.D.; writing—review and editing, N.S., E.G., M.T., E.M., P.A., I.R., P.T. and A.S.; visualization, E.I., I.D. and E.G.; supervision, N.M., P.T. and A.S.; project administration, P.T. and A.S.; funding acquisition, A.F., N.M. and P.T. All authors have read and agreed to the published version of the manuscript.
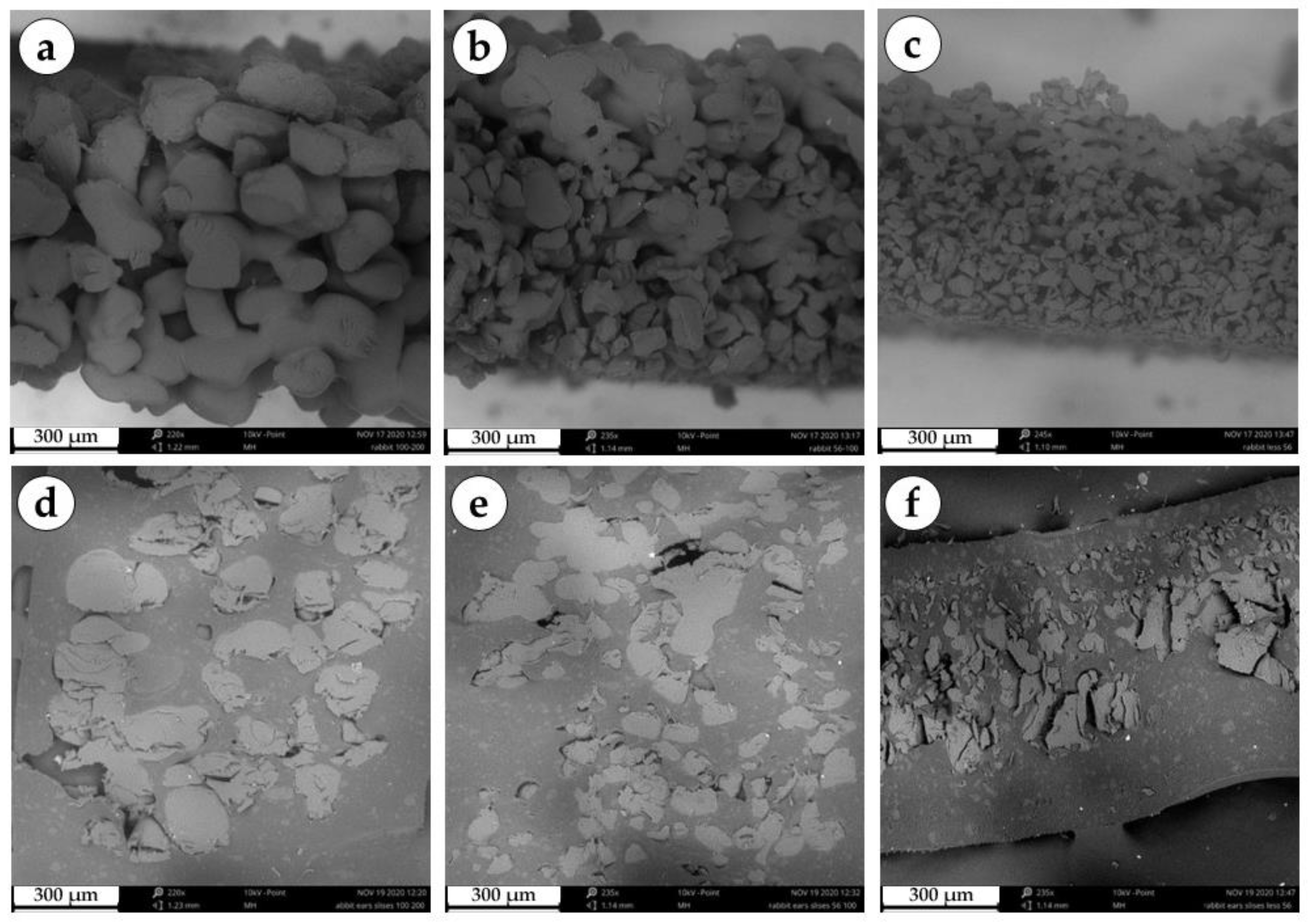
Figure 1.
Scanning electron microscopy of a series of granular implants. (a) Edge of a three-dimensional large granule disc. (b) Edge of a three-dimensional medium granule disc. (c) Edge of a three-dimensional small granule disc. (d) Section of a three-dimensional large granule disc. (e) Section of a three-dimensional medium granule disc. (f) Section of a three-dimensional small granule disc.
Figure 1.
Scanning electron microscopy of a series of granular implants. (a) Edge of a three-dimensional large granule disc. (b) Edge of a three-dimensional medium granule disc. (c) Edge of a three-dimensional small granule disc. (d) Section of a three-dimensional large granule disc. (e) Section of a three-dimensional medium granule disc. (f) Section of a three-dimensional small granule disc.
Figure 2.
Photograph of a drop of distilled water placed on the surface of a three-dimensional small granule disc. h is the height, and r is half the length of the line of contact between the drop and the material. θ1 = tan − 1(h/r).
Figure 2.
Photograph of a drop of distilled water placed on the surface of a three-dimensional small granule disc. h is the height, and r is half the length of the line of contact between the drop and the material. θ1 = tan − 1(h/r).
Figure 3.
Macrophotograph of a large granule disc.
Figure 3.
Macrophotograph of a large granule disc.
Figure 4.
Sites of implantation of discs made of small (a,d,g), medium (b,e,h) and large (c,f,i) granules, 7 days after surgery. (a–c) H&E stain, magnification ×50. (d–f) H&E stain, magnification ×200. (g–i) Mallory’s trichrome stain, magnification ×100. Scale bars are 100 µm. Granulation tissue is marked with green arrows.
Figure 4.
Sites of implantation of discs made of small (a,d,g), medium (b,e,h) and large (c,f,i) granules, 7 days after surgery. (a–c) H&E stain, magnification ×50. (d–f) H&E stain, magnification ×200. (g–i) Mallory’s trichrome stain, magnification ×100. Scale bars are 100 µm. Granulation tissue is marked with green arrows.
Figure 5.
Sites of implantation of discs made of small (a,d,g), medium (b,e,h) and large (c,f,i) granules, 7 days after surgery (a–c) PSR stain, magnification ×100. (d–f) PSR stain, polarized light microscopy, magnification ×200. (g–i) immunohistochemical reaction with antibodies against α-SMA, magnification ×100. Scale bars are 100 µm.
Figure 5.
Sites of implantation of discs made of small (a,d,g), medium (b,e,h) and large (c,f,i) granules, 7 days after surgery (a–c) PSR stain, magnification ×100. (d–f) PSR stain, polarized light microscopy, magnification ×200. (g–i) immunohistochemical reaction with antibodies against α-SMA, magnification ×100. Scale bars are 100 µm.
Figure 6.
Sites of implantation of discs made of small (a,d,g), medium (b,e,h) and large (c,f,i) granules, 14 days after surgery. (a–c) H&E stain, magnification ×50. (d–f) H&E stain, magnification ×200. (g–i) Mallory’s trichrome stain, magnification ×100. Scale bars are 100 µm. Immature connective tissue capsules are marked with green arrows.
Figure 6.
Sites of implantation of discs made of small (a,d,g), medium (b,e,h) and large (c,f,i) granules, 14 days after surgery. (a–c) H&E stain, magnification ×50. (d–f) H&E stain, magnification ×200. (g–i) Mallory’s trichrome stain, magnification ×100. Scale bars are 100 µm. Immature connective tissue capsules are marked with green arrows.
Figure 7.
Sites of implantation of discs made of small (a,d,g), medium (b,e,h) and large (c,f,i) granules, 14 days after surgery. (a–c) PSR stain, magnification ×100. (d–f) PSR stain, polarized light microscopy, magnification ×200. (g–i) immunohistochemical reaction with antibodies against α-SMA, magnification ×100. Scale bars are 100 µm.
Figure 7.
Sites of implantation of discs made of small (a,d,g), medium (b,e,h) and large (c,f,i) granules, 14 days after surgery. (a–c) PSR stain, magnification ×100. (d–f) PSR stain, polarized light microscopy, magnification ×200. (g–i) immunohistochemical reaction with antibodies against α-SMA, magnification ×100. Scale bars are 100 µm.
Figure 8.
Sites of implantation of discs made of small (a,d,g), medium (b,e,h) and large (c,f,i) granules, 30 days after surgery. (a–c) H&E stain, magnification ×50. (d–f) H&E stain, magnification ×200. (g–i) Mallory’s trichrome stain, magnification ×100. Scale bars are 100 µm. Connective tissue capsules are marked with green arrows.
Figure 8.
Sites of implantation of discs made of small (a,d,g), medium (b,e,h) and large (c,f,i) granules, 30 days after surgery. (a–c) H&E stain, magnification ×50. (d–f) H&E stain, magnification ×200. (g–i) Mallory’s trichrome stain, magnification ×100. Scale bars are 100 µm. Connective tissue capsules are marked with green arrows.
Figure 9.
Sites of implantation of discs made of small (a,d,g), medium (b,e,h) and large (c,f,i) granules, 30 days after surgery. (a–c) PSR stain, magnification ×100. (d–f) PSR stain, polarized light microscopy, magnification ×200. (g–i) immunohistochemical reaction with antibodies against α-SMA, magnification ×100. Scale bars are 100 µm.
Figure 9.
Sites of implantation of discs made of small (a,d,g), medium (b,e,h) and large (c,f,i) granules, 30 days after surgery. (a–c) PSR stain, magnification ×100. (d–f) PSR stain, polarized light microscopy, magnification ×200. (g–i) immunohistochemical reaction with antibodies against α-SMA, magnification ×100. Scale bars are 100 µm.
Figure 10.
Sites of implantation of discs made of small (a,d,g), medium (b,e,h) and large (c,f,i) granules, 60 days after surgery. (a–c) H&E stain, magnification ×50. (d–f) H&E stain, magnification ×200. (g–i) Mallory’s trichrome stain, magnification ×100. Scale bars are 100 µm. Connective tissue capsules are marked with green arrows.
Figure 10.
Sites of implantation of discs made of small (a,d,g), medium (b,e,h) and large (c,f,i) granules, 60 days after surgery. (a–c) H&E stain, magnification ×50. (d–f) H&E stain, magnification ×200. (g–i) Mallory’s trichrome stain, magnification ×100. Scale bars are 100 µm. Connective tissue capsules are marked with green arrows.
Figure 11.
Sites of implantation of discs made of small (a,d,g), medium (b,e,h) and large (c,f,i) granules, 60 days after surgery. (a–c) PSR stain, magnification ×100. (d–f) PSR stain, polarized light microscopy, magnification ×200. (g–i) immunohistochemical reaction with antibodies against α-SMA, magnification ×100. Scale bars are 100 µm.
Figure 11.
Sites of implantation of discs made of small (a,d,g), medium (b,e,h) and large (c,f,i) granules, 60 days after surgery. (a–c) PSR stain, magnification ×100. (d–f) PSR stain, polarized light microscopy, magnification ×200. (g–i) immunohistochemical reaction with antibodies against α-SMA, magnification ×100. Scale bars are 100 µm.
Figure 12.
Morphometric graphs. Thickness of the peri-implant complex, µm at different time points. Density of vascularity in the peri-implant capsule, number of vessels per 1 mm2. Relative area of implants, % of the initial area. Mean values ± SD.
Figure 12.
Morphometric graphs. Thickness of the peri-implant complex, µm at different time points. Density of vascularity in the peri-implant capsule, number of vessels per 1 mm2. Relative area of implants, % of the initial area. Mean values ± SD.
Figure 13.
Semi-quantitative analysis of morphological features. Median values ± interquartile range.
Figure 13.
Semi-quantitative analysis of morphological features. Median values ± interquartile range.
Figure 14.
OCT scans of tissue samples in vivo. (a) Tissue without implant. (b) Tissue with implant made of small granules. (c) Tissue with implant made of medium granules. (d) Tissue with implant made of large granules.
Figure 14.
OCT scans of tissue samples in vivo. (a) Tissue without implant. (b) Tissue with implant made of small granules. (c) Tissue with implant made of medium granules. (d) Tissue with implant made of large granules.
Figure 15.
Representative examples of OCT scans of tissue samples in vivo (implant made of small granules). (a,e) 7 days after implantation. (b,f) 14 days after implantation. (c,g) 30 days after implantation. (d,h) 60 days after implantation. Layer of dense connective tissue was detected at day 60 (yellow arrows).
Figure 15.
Representative examples of OCT scans of tissue samples in vivo (implant made of small granules). (a,e) 7 days after implantation. (b,f) 14 days after implantation. (c,g) 30 days after implantation. (d,h) 60 days after implantation. Layer of dense connective tissue was detected at day 60 (yellow arrows).
Figure 16.
Representative examples of OCT scans of tissue samples in vivo (implant made of medium granules). (a,e) 7 days after implantation. (b,f) 14 days after implantation. (c,g) 30 days after implantation. (d,h) 60 days after implantation. Areas of edema at days 7 and 14 (yellow arrows). Layer of connective tissue was detected at day 30 and 60 (yellow arrows).
Figure 16.
Representative examples of OCT scans of tissue samples in vivo (implant made of medium granules). (a,e) 7 days after implantation. (b,f) 14 days after implantation. (c,g) 30 days after implantation. (d,h) 60 days after implantation. Areas of edema at days 7 and 14 (yellow arrows). Layer of connective tissue was detected at day 30 and 60 (yellow arrows).
Figure 17.
Representative examples of OCT scans of tissue samples in vivo (implant made of large granules). (a,e) 7 days after implantation. (b,f) 14 days after implantation. (c,g) 30 days after implantation. (d,h) 60 days after implantation. 60 days after implantation. Areas of edema at day 7 (yellow arrows). Signs of granular material were detected at day 30 and 60 (yellow arrows).
Figure 17.
Representative examples of OCT scans of tissue samples in vivo (implant made of large granules). (a,e) 7 days after implantation. (b,f) 14 days after implantation. (c,g) 30 days after implantation. (d,h) 60 days after implantation. 60 days after implantation. Areas of edema at day 7 (yellow arrows). Signs of granular material were detected at day 30 and 60 (yellow arrows).
Figure 18.
Analysis of the scattering coefficient of selected tissue layers on OCT images. (a–c) Changes over time of the scattering coefficient of three tissue layers during implantation of implants of the small, medium and large granules. (d,e) Comparison of scattering coefficients of the first and second layers, respectively, for implants of the small granules (marked 1), medium granules (2) and large granules (3). (f) An example of the original OCT B-scan.
Figure 18.
Analysis of the scattering coefficient of selected tissue layers on OCT images. (a–c) Changes over time of the scattering coefficient of three tissue layers during implantation of implants of the small, medium and large granules. (d,e) Comparison of scattering coefficients of the first and second layers, respectively, for implants of the small granules (marked 1), medium granules (2) and large granules (3). (f) An example of the original OCT B-scan.
Figure 19.
Graphical scheme of the impact of the granule size on the tissue reaction.
Figure 19.
Graphical scheme of the impact of the granule size on the tissue reaction.