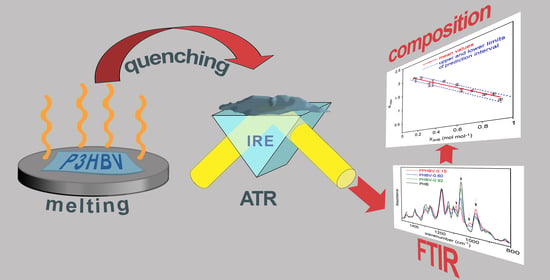
Rapid Estimation of Poly(3-hydroxybutyrate-co-3-hydroxyvalerate) Composition Using ATR-FTIR
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Sample Preparation and Characterization
2.3. Data Analysis
3. Results
3.1. ATR-FTIR Analysis
3.2. Data Analysis
4. Discussion
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Mukherjee, A.; Koller, M. Microbial PolyHydroxyAlkanoate (PHA) Biopolymers—Intrinsically Natural. Bioengineering 2023, 10, 855. [Google Scholar] [CrossRef] [PubMed]
- Haque, M.A.; Priya, A.; Hathi, Z.J.; Qin, Z.-H.; Mettu, S.; Lin, C.S.K. Advancements and Current Challenges in the Sustainable Downstream Processing of Bacterial Polyhydroxyalkanoates. Curr. Opin. Green Sustain. Chem. 2022, 36, 100631. [Google Scholar] [CrossRef]
- Pesante, G.; Frison, N. Recovery of Bio-Based Products from PHA-Rich Biomass Obtained from Biowaste: A Review. Bioresour. Technol. Rep. 2023, 21, 101345. [Google Scholar] [CrossRef]
- Alfano, S.; Lorini, L.; Majone, M.; Sciubba, F.; Valentino, F.; Martinelli, A. Ethylic Esters as Green Solvents for the Extraction of Intracellular Polyhydroxyalkanoates Produced by Mixed Microbial Culture. Polymers 2021, 13, 2789. [Google Scholar] [CrossRef] [PubMed]
- Tokiwa, Y.; Calabia, B.P. Degradation of Microbial Polyesters. Biotechnol. Lett. 2004, 26, 1181–1189. [Google Scholar] [CrossRef]
- Shogren, R. Water Vapor Permeability of Biodegradable Polymers. J. Environ. Polym. Degrad. 1997, 5, 91–95. [Google Scholar] [CrossRef]
- Shafi, H.; Bajpai, M. A Review on Importance of Biodegradable Packaging for Foods and Pharmaceuticals. Curr. Nutr. Food Sci. 2023, 19, 9–21. [Google Scholar] [CrossRef]
- Rodríguez-Cendal, A.I.; Gómez-Seoane, I.; de Toro-Santos, F.J.; Fuentes-Boquete, I.M.; Señarís-Rodríguez, J.; Díaz-Prado, S.M. Biomedical Applications of the Biopolymer Poly(3-hydroxybutyrate-co-3-hydroxyvalerate) (PHBV): Drug Encapsulation and Scaffold Fabrication. Int. J. Mol. Sci. 2023, 24, 11674. [Google Scholar] [CrossRef]
- Tareq, S.; Jeevanandam, J.; Acquah, C.; Danquah, M.K. Biomedical Applications of Bioplastics. In Handbook of Bioplastics and Biocomposites Engineering Applications; Wiley: Hoboken, NJ, USA, 2023; pp. 175–197. [Google Scholar]
- Samrot, A.V.; Samanvitha, S.K.; Shobana, N.; Renitta, E.R.; Senthilkumar, P.; Kumar, S.S.; Abirami, S.; Dhiva, S.; Bavanilatha, M.; Prakash, P.; et al. The Synthesis, Characterization and Applications of Polyhydroxyalkanoates (PHAs) and PHA-Based Nanoparticles. Polymers 2021, 13, 3302. [Google Scholar] [CrossRef]
- Ding, Z.; Kumar, V.; Sar, T.; Harirchi, S.; Dregulo, A.M.; Sirohi, R.; Sindhu, R.; Binod, P.; Liu, X.; Zhang, Z.; et al. Agro Waste as a Potential Carbon Feedstock for Poly-3-Hydroxy Alkanoates Production: Commercialization Potential and Technical Hurdles. Bioresour. Technol. 2022, 364, 128058. [Google Scholar] [CrossRef]
- Muiruri, J.K.; Yeo, J.C.C.; Loh, X.J.; Chen, G.-Q.; He, C.; Li, Z. Poly(hydroxyalkanoates) (PHAs) Based Circular Materials for a Sustainable Future. In Circularity of Plastics; Elsevier: Amsterdam, The Netherlands, 2023; pp. 273–303. [Google Scholar]
- Lorini, L.; Martinelli, A.; Capuani, G.; Frison, N.; Reis, M.; Sommer Ferreira, B.; Villano, M.; Majone, M.; Valentino, F. Characterization of Polyhydroxyalkanoates Produced at Pilot Scale From Different Organic Wastes. Front. Bioeng. Biotechnol. 2021, 9, 628719. [Google Scholar] [CrossRef]
- Majone, M.; Chronopoulou, L.; Lorini, L.; Martinelli, A.; Palocci, C.; Rossetti, S.; Valentino, F.; Villano, M. PHA Copolymers from Microbial Mixed Coltures: Synthesis, Extraction and Related Properties. In Current Advantages in Biopolymer Processing and Characterization; Nova Science Publisher: New York, NY, USA, 2017; pp. 223–277. [Google Scholar]
- Koller, M. Definitions and Types of Microbial Biopolyesters and Derived Biomaterials. In Functional Biomaterials; Wiley: Hoboken, NJ, USA, 2023; pp. 1–43. [Google Scholar]
- Panaksri, A.; Tanadchangsaeng, N. Fractionation of Medium-Chain-Length Polyhydroxyalkanoate Biosynthesized by Pilot-Scale Production for Improving Material Properties. Polym. Degrad. Stab. 2023, 213, 110368. [Google Scholar] [CrossRef]
- Ferre-Guell, A.; Winterburn, J. Biosynthesis and Characterization of Polyhydroxyalkanoates with Controlled Composition and Microstructure. Biomacromolecules 2018, 19, 996–1005. [Google Scholar] [CrossRef] [PubMed]
- Carvalheira, M.; Hilliou, L.; Oliveira, C.S.S.; Guarda, E.C.; Reis, M.A.M. Polyhydroxyalkanoates from Industrial Cheese Whey: Production and Characterization of Polymers with Differing Hydroxyvalerate Content. Curr. Res. Biotechnol. 2022, 4, 211–220. [Google Scholar] [CrossRef]
- Wang, Y.; Yamada, S.; Asakawa, N.; Yamane, T.; Yoshie, N.; Inoue, Y. Comonomer Compositional Distribution and Thermal and Morphological Characteristics of Bacterial Poly(3-hydroxybutyrate-co-3-hydroxyvalerate)s with High 3-Hydroxyvalerate Content. Biomacromolecules 2001, 2, 1315–1323. [Google Scholar] [CrossRef] [PubMed]
- Serafim, L.S.; Lemos, P.C.; Torres, C.; Reis, M.A.M.; Ramos, A.M. The Influence of Process Parameters on the Characteristics of Polyhydroxyalkanoates Produced by Mixed Cultures. Macromol. Biosci. 2008, 8, 355–366. [Google Scholar] [CrossRef]
- Laycock, B.; Halley, P.; Pratt, S.; Werker, A.; Lant, P. The Chemomechanical Properties of Microbial Polyhydroxyalkanoates. Prog. Polym. Sci. 2013, 38, 536–583. [Google Scholar] [CrossRef]
- Oehmen, A.; Keller-Lehmann, B.; Zeng, R.J.; Yuan, Z.; Keller, J. Optimisation of Poly-β-hydroxyalkanoate Analysis Using Gas Chromatography for Enhanced Biological Phosphorus Removal Systems. J. Chromatogr. A 2005, 1070, 131–136. [Google Scholar] [CrossRef]
- Braunegg, G.; Sonnleimer, B.; Lafferty, R.M. A Rapid Gas Chromatographic Method for the Determination of Poly-β-hydroxybutyric Acid in Microbial Biomass. Eur. J. Appl. Microbiol. Biotechnol. 1978, 6, 29–37. [Google Scholar] [CrossRef]
- Bloembergen, S.; Holden, D.A.; Hamer, G.K.; Bluhm, T.L.; Marchessault, R.H. Studies of Composition and Crystallinity of Bacterial Poly(β-hydroxybutyrate-co-β-hydroxyvalerate). Macromolecules 1986, 19, 2865–2871. [Google Scholar] [CrossRef]
- Kansiz, M.; Domínguez-Vidal, A.; McNaughton, D.; Lendl, B. Fourier-Transform Infrared (FTIR) Spectroscopy for Monitoring and Determining the Degree of Crystallisation of Polyhydroxyalkanoates (PHAs). Anal. Bioanal. Chem. 2007, 388, 1207–1213. [Google Scholar] [CrossRef]
- Xu, J.; Guo, B.H.; Yang, R.; Wu, Q.; Chen, G.Q.; Zhang, Z.M. In Situ FTIR Study on Melting and Crystallization of Polyhydroxyalkanoates. Polymer 2002, 43, 6893–6899. [Google Scholar] [CrossRef]
- Zhang, J.; Sato, H.; Noda, I.; Ozaki, Y. Conformation Rearrangement and Molecular Dynamics of Poly(3-hydroxybutyrate) during the Melt-Crystallization Process Investigated by Infrared and Two-Dimensional Infrared Correlation Spectroscopy. Macromolecules 2005, 38, 4274–4281. [Google Scholar] [CrossRef]
- Jost, V.; Schwarz, M.; Langowski, H.-C. Investigation of the 3-Hydroxyvalerate Content and Degree of Crystallinity of P3HB-Co-3HV Cast Films Using Raman Spectroscopy. Polymer 2017, 133, 160–170. [Google Scholar] [CrossRef]
- Lorini, L.; Martinelli, A.; Pavan, P.; Majone, M.; Valentino, F. Downstream Processing and Characterization of Polyhydroxyalkanoates (PHAs) Produced by Mixed Microbial Culture (MMC) and Organic Urban Waste as Substrate. Biomass. Convers. Biorefin. 2021, 11, 693–703. [Google Scholar] [CrossRef]
- Montgomery Douglas, C. Design and Analysis of Experiments, 8th ed.; Wiley: Hoboken, NJ, USA, 2012; ISBN 978-1-118-14692-7. [Google Scholar]
- Danzer, K.; Currie, L.A. Currie Guidelines for Calibration in Analytical Chemistry. Part I. Fundamentals and Single Component Calibration (IUPAC Recommendations) 1998. Pure Appl. Chem. 1998, 70, 993–1014. [Google Scholar] [CrossRef]
- Scandola, M.; Ceccorulli, G.; Pizzoli, M.; Gazzano, M. Study of the Crystal Phase and Crystallization Rate of Bacterial Poly(3-hydroxybutyrate-co-3-hydroxyvalerate). Macromolecules 1992, 25, 1405–1410. [Google Scholar] [CrossRef]
- Sato, H.; Dybal, J.; Murakami, R.; Noda, I.; Ozaki, Y. Infrared and Raman Spectroscopy and Quantum Chemistry Calculation Studies of C–H⋯O Hydrogen Bondings and Thermal Behavior of Biodegradable Polyhydroxyalkanoate. J. Mol. Struct. 2005, 744–747, 35–46. [Google Scholar] [CrossRef]
- Sato, H.; Murakami, R.; Zhang, J.; Mori, K.; Takahashi, I.; Terauchi, H.; Noda, I.; Ozaki, Y. Infrared Spectroscopy and X-ray Diffraction Studies of C—H…O Hydrogen Bonding and Thermal Behavior of Biodegradable Poly(hydroxyalkanoate). Macromol. Symp. 2005, 230, 158–166. [Google Scholar] [CrossRef]
- Kann, Y.; Shurgalin, M.; Krishnaswamy, R.K. FTIR Spectroscopy for Analysis of Crystallinity of Poly(3-hydroxybutyrate-co-4 -hydroxybutyrate) Polymers and Its Utilization in Evaluation of Aging, Orientation and Composition. Polym. Test. 2014, 40, 218–224. [Google Scholar] [CrossRef]
- Izumi, C.M.S.; Temperini, M.L.A. FT-Raman Investigation of Biodegradable Polymers: Poly(3-hydroxybutyrate) and Poly(3-hydroxybutyrate-co-3-hydroxyvalerate). Vib. Spectrosc. 2010, 54, 127–132. [Google Scholar] [CrossRef]
- Gorodzha, S.N.; Muslimov, A.R.; Syromotina, D.S.; Timin, A.S.; Tcvetkov, N.Y.; Lepik, K.V.; Petrova, A.V.; Surmeneva, M.A.; Gorin, D.A.; Sukhorukov, G.B.; et al. A Comparison Study between Electrospun Polycaprolactone and Piezoelectric Poly(3-hydroxybutyrate-co-3-hydroxyvalerate) Scaffolds for Bone Tissue Engineering. Colloids Surf. B Biointerfaces 2017, 160, 48–59. [Google Scholar] [CrossRef]
- Volova, T.G.; Zhila, N.O.; Shishatskaya, E.I.; Mironov, P.V.; Vasil’ev, A.D.; Sukovatyi, A.G.; Sinskey, A.J. The Physicochemical Properties of Polyhydroxyalkanoates with Different Chemical Structures. Polym. Sci. Ser. A 2013, 55, 427–437. [Google Scholar] [CrossRef]
- Narayanan, A.; Sajeev Kumar, V.A.; Ramana, K.V. Production and Characterization of Poly(3-hydroxybutyrate-co-3-hydroxyvalerate) from Bacillus Mycoides DFC1 Using Rice Husk Hydrolyzate. Waste Biomass Valorization 2014, 5, 109–118. [Google Scholar] [CrossRef]
- Kovalcik, A.; Obruca, S.; Kalina, M.; Machovsky, M.; Enev, V.; Jakesova, M.; Sobkova, M.; Marova, I. Enzymatic Hydrolysis of Poly(3-hydroxybutyrate-co-3-hydroxyvalerate) Scaffolds. Materials 2020, 13, 2992. [Google Scholar] [CrossRef]
- Marois, Y.; Zhang, Z.; Vert, M.; Deng, X.; Lenz, R.; Guidoin, R. Mechanism and Rate of Degradation of Polyhydroxyoctanoate Films in Aqueous Media: A Long-Termin Vitro Study. J. Biomed. Mater. Res. 2000, 49, 216–224. [Google Scholar] [CrossRef]
- Porras, M.A.; Cubitto, M.A.; Villar, M.A. A New Way of Quantifying the Production of Poly(hydroxyalkanoate)s Using FTIR. J. Chem. Technol. Biotechnol. 2016, 91, 1240–1249. [Google Scholar] [CrossRef]
- Koch, C.; Posch, A.E.; Goicoechea, H.C.; Herwig, C.; Lendl, B. Multi-Analyte Quantification in Bioprocesses by Fourier-Transform-Infrared Spectroscopy by Partial Least Squares Regression and Multivariate Curve Resolution. Anal. Chim. Acta 2014, 807, 103–110. [Google Scholar] [CrossRef]




| Intercept | Lower Limit (95%) | Upper Limit (95%) | Relative Error * (%) | Slope | Lower Limit (95%) | Upper Limit (95%) | Relative Error * (%) | R2 | |
|---|---|---|---|---|---|---|---|---|---|
| R968 | 5.11 | 4.95 | 5.26 | 6 | −2.33 | −2.58 | −2.07 | 22 | 0.930 |
| R1050 | 3.77 | 3.29 | 4.25 | 26 | 4.75 | 3.95 | 5.55 | 34 | 0.851 |
| R1084 | 5.00 | 4.82 | 5.18 | 7 | −1.75 | −2.05 | −1.45 | 34 | 0.848 |
| R1004 | 2.27 | 2.18 | 2.36 | 8 | −1.68 | −1.83 | −1.53 | 18 | 0.953 |
| MSE | MSPE | MSLOF | F Value | p Value | |
|---|---|---|---|---|---|
| R968 | 0.0284 | 0.0198 | 0.0423 | 2.1350 | 0.0850 |
| R1050 | 0.2762 | 0.1243 | 0.5191 | 4.1772 | 0.0056 |
| R1084 | 0.0380 | 0.0289 | 0.0527 | 1.8246 | 0.1367 |
| R1004 | 0.0096 | 0.0074 | 0.0131 | 1.7587 | 0.1514 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Alfano, S.; Pagnanelli, F.; Martinelli, A. Rapid Estimation of Poly(3-hydroxybutyrate-co-3-hydroxyvalerate) Composition Using ATR-FTIR. Polymers 2023, 15, 4127. https://doi.org/10.3390/polym15204127
Alfano S, Pagnanelli F, Martinelli A. Rapid Estimation of Poly(3-hydroxybutyrate-co-3-hydroxyvalerate) Composition Using ATR-FTIR. Polymers. 2023; 15(20):4127. https://doi.org/10.3390/polym15204127
Chicago/Turabian StyleAlfano, Sara, Francesca Pagnanelli, and Andrea Martinelli. 2023. "Rapid Estimation of Poly(3-hydroxybutyrate-co-3-hydroxyvalerate) Composition Using ATR-FTIR" Polymers 15, no. 20: 4127. https://doi.org/10.3390/polym15204127
APA StyleAlfano, S., Pagnanelli, F., & Martinelli, A. (2023). Rapid Estimation of Poly(3-hydroxybutyrate-co-3-hydroxyvalerate) Composition Using ATR-FTIR. Polymers, 15(20), 4127. https://doi.org/10.3390/polym15204127