Unveiling the Latest Developments in Molecularly Imprinted Photocatalysts: A State-of-the-Art Review
Abstract
:1. Introduction
2. Molecularly Imprinted Photocatalysts
2.1. Surface Imprinting
2.2. Molecularly Imprinted Polymers on Photocatalysts
Monomers and Cross-Linkers
3. Applications
3.1. Photo-Oxidation of Toxic Organic Pollutants
3.2. Electrochemical Sensors
3.3. Other Applications
4. Conclusions and Challenges
- Enhancing the bond strength and binding capabilities between templates and monomers. Advanced synthesis methods, like controlled radical polymerizations, can optimize the affinity of these materials.
- Ensuring stability and structural integrity of imprinted cavities in MIPs during practical use. Balancing high affinity and cavity resilience is vital, especially in high-porosity polymers that are prone to structural changes affecting binding. Effective imprinting or elution techniques need exploration.
- Mitigating cross-selectivity, where unintended binding to template-similar molecules occurs. The interactions between templates and functional monomers significantly influence the recognition traits of the final matrix. Robust, stable connections with the template should be sought after in monomer selection.
- Despite significant progress in understanding the photodegradation of molecules on imprinted semiconductor nanocomposites, a notable oversight is the lack of differentiation between standard semiconductor nanocomposites and their imprinted versions. While tools like XPS, FTIR, and EPR spectroscopy have been employed to probe the photocatalytic mechanisms of imprinted photocatalysts, there is a pressing need for real-time or continuous monitoring techniques. Such methods are crucial to shed light on how target molecules transition and transform during the photodegradation phase.
- Investigating imprinting techniques to expand target entities from minor molecules to larger biological ones, including proteins and living cells, is vital. While TiO2 is considered biologically friendly, its genuine utilization in biological sectors or clinical tests is uncommon. Crafting TiO2 nanomaterials that are enhanced with MIPs and are biocompatible is a challenging endeavor. Nevertheless, it holds the promise of ushering in a novel era of sensor materials suited for medical uses.
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Conflicts of Interest
List of Abbreviations
| MIPCs | molecularly imprinted photocatalysts |
| AOPs | advanced oxidation processes |
| EPs | environmental pollutant |
| MIT | molecular imprinting technology |
| MIPs | molecularly imprinted polymers |
| 2,4-D | herbicide 2,4-dichlorophenoxyacetic acid |
| IMID | imidacloprid (1-(6-chloro-3-pyridinylmethyl)- nitro-2-imidazolidinimine |
| 9-AnCOOH | anthracene-9-carboxylic acid |
| EP | ethyl p-hydroxybenzoate |
| MI | molecularly imprinted |
| RhB | rhodamine B |
| TBOT | titanium(IV) butoxide |
| TTIP | titanium(IV) isopropoxide |
| TEOS | tetraethyl orthosilicate |
| OTC | catalytic oxytetracycline |
| MIF | molecularly imprinted film |
| DEP | diethyl phthalate |
| SMX | sulfamethoxazole |
| PEDOT | poly-3,4-ethylenedioxythiophene |
| 3D | three-dimensional |
| POPD | polyo-phenylenediamine |
| NOR | norfloxacin |
| NIPs | non-imprinted polymers |
| DBP | dibutyl phthalate |
| MAA | methacrylic acid |
| PPy | polypyrrole |
| APTS | 3-aminopropyltriethoxylsilane |
| MPTS | 3-methacryloxypropyl trimethoxysilane |
| AIBN | azobisisobutyronitrile |
| EGMA | ethyleneglycoldimethacrylate |
| TC | tetracycline hydrochloride |
| LPD | liquid-phase deposition |
| OPDA | oxylipin 12-oxo-phytodienoic acid |
| NTs | nanotubes |
| DIC | diclofenac |
| PFCs | perfluorinated chemicals |
| PFOA | perfluorooctanoic acid |
| PFOS | perfluorooctanesulfonate |
| 2NP | 2-nitrophenol |
| 4NP | 4-nitrophenol |
| PEC | photochemistry |
| MOF | metal organic frameworks |
| CPF | organophosphate pesticide chlorpyrifos |
| Lyz | lysozyme |
| PDA | polydopamine |
| EISA | evaporation-induced self-assembly method |
| AA | acrylic acid |
| MMA | methyl methacrylate |
| DVB | divinylbenzene |
| t-Boc | t-butoxycarbonyl |
| CAR | carbazole |
| DPP | diketopyrrolopyrrole |
References
- Fujishima, A.; Honda, K. Electrochemical Photolysis of Water at a Semiconductor Electrode. Nature 1972, 238, 37–38. [Google Scholar] [CrossRef]
- Gupta, S.M.; Tripathi, M. A Review of TiO2 Nanoparticles. Chin. Sci. Bull. 2011, 56, 1639–1657. [Google Scholar] [CrossRef]
- Qi, K.; Cheng, B.; Yu, J.; Ho, W. A Review on TiO2-Based Z-Scheme Photocatalysts. Chin. J. Catal. 2017, 38, 1936–1955. [Google Scholar] [CrossRef]
- Kubiak, A.; Siwińska-Ciesielczyk, K.; Jesionowski, T. Titania-Based Hybrid Materials with ZnO, ZrO2 and MoS2: A Review. Materials 2018, 11, 2295. [Google Scholar] [CrossRef]
- Liu, N.; Chen, X.; Zhang, J.; Schwank, J.W. A Review on TiO2-Based Nanotubes Synthesized via Hydrothermal Method: Formation Mechanism, Structure Modification, and Photocatalytic Applications. Catal. Today 2014, 225, 34–51. [Google Scholar] [CrossRef]
- Mccullagh, C.; Robertson, J.M.C.; Bahnemann, D.W.; Robertson, P.K.J. The Application of TiO2 Photocatalysis for Disinfection of Water Contaminated with Pathogenic Micro-Organisms: A Review. Res. Chem. Intermed. 2007, 33, 359–375. [Google Scholar] [CrossRef]
- Li, Z.; Wang, S.; Wu, J.; Zhou, W. Recent Progress in Defective TiO2 Photocatalysts for Energy and Environmental Applications. Renew. Sustain. Energy Rev. 2022, 156, 111980. [Google Scholar] [CrossRef]
- Nakata, K.; Fujishima, A. TiO2 Photocatalysis: Design and Applications. J. Photochem. Photobiol. C Photochem. Rev. 2012, 13, 169–189. [Google Scholar] [CrossRef]
- Guo, M.; Hu, Y.; Wang, R.; Yu, H.; Sun, L. Molecularly Imprinted Polymer-Based Photocatalyst for Highly Selective Degradation of Methylene Blue. Environ. Res. 2021, 194, 110684. [Google Scholar] [CrossRef]
- Poonia, K.; Raizada, P.; Singh, A.; Verma, N.; Ahamad, T.; Alshehri, S.M.; Khan, A.A.P.; Singh, P.; Hussain, C.M. Magnetic Molecularly Imprinted Polymer Photocatalysts: Synthesis, Applications and Future Perspective. J. Ind. Eng. Chem. 2022, 113, 1–14. [Google Scholar] [CrossRef]
- Chen, J.; Gao, P.; Wang, H.; Han, L.; Zhang, Y.; Wang, P.; Jia, N. A PPy/Cu2O Molecularly Imprinted Composite Film-Based Visible Light-Responsive Photoelectrochemical Sensor for Microcystin-LR. J. Mater. Chem. C Mater. 2018, 6, 3937–3944. [Google Scholar] [CrossRef]
- Xu, S.; Lu, H.; Chen, L.; Wang, X. Molecularly Imprinted TiO2 hybridized Magnetic Fe3O4 nanoparticles for Selective Photocatalytic Degradation and Removal of Estrone. RSC Adv. 2014, 4, 45266–45274. [Google Scholar] [CrossRef]
- Zeng, L.; Zhang, X.; Wang, X.; Cheng, D.; Li, R.; Han, B.; Wu, M.; Zhuang, Z.; Ren, A.; Zhou, Y. Simultaneous Fluorescence Determination of Bisphenol A and Its Halogenated Analogs Based on a Molecularly Imprinted Paper-Based Analytical Device and a Segment Detection Strategy. Biosens. Bioelectron. 2021, 180, 113106. [Google Scholar] [CrossRef]
- Galloni, M.G.; Bortolotto, V.; Falletta, E.; Bianchi, C.L. PH-Driven Selective Adsorption of Multi-Dyes Solutions by Loofah Sponge and Polyaniline-Modified Loofah Sponge. Polymers 2022, 14, 4897. [Google Scholar] [CrossRef] [PubMed]
- Mergbi, M.; Galloni, M.G.; Aboagye, D.; Elimian, E.; Su, P.; Ikram, B.M.; Nabgan, W.; Bedia, J.; Amor, H.B.; Contreras, S.; et al. Valorization of Lignocellulosic Biomass into Sustainable Materials for Adsorption and Photocatalytic Applications in Water and Air Remediation. Environ. Sci. Pollut. Res. 2023, 30, 74544–74574. [Google Scholar] [CrossRef] [PubMed]
- Galloni, M.G.; Ferrara, E.; Falletta, E.; Bianchi, C.L. Olive Mill Wastewater Remediation: From Conventional Approaches to Photocatalytic Processes by Easily Recoverable Materials. Catalysts 2022, 12, 923. [Google Scholar] [CrossRef]
- Djellabi, R.; Giannantonio, R.; Falletta, E.; Bianchi, C.L. SWOT Analysis of Photocatalytic Materials towards Large Scale Environmental Remediation. Curr. Opin. Chem. Eng. 2021, 33, 100696. [Google Scholar] [CrossRef]
- Lu, J.; Qin, Y.Y.; Wu, Y.L.; Zhu, Z.; Chen, M.N.; Yan, Y.S.; Li, C.X. Bio-Synthesis of Molecularly Imprinted Membrane with Photo-Regeneration Availability for Selective Separation Applications. Mater. Today Chem. 2022, 24, 100836. [Google Scholar] [CrossRef]
- Wang, H.T.; Wu, X.; Zhao, H.M.; Quan, X. Enhanced Photocatalytic Degradation of Tetracycline Hydrochloride by Molecular Imprinted Film Modified TiO2 Nanotubes. Chin. Sci. Bull. 2012, 57, 601–605. [Google Scholar] [CrossRef]
- Li, Q.; Huang, Y.; Pan, Z.; Ni, J.; Yang, W.; Chen, J.; Zhang, Y.; Li, J. Hollow C, N-TiO2@C Surface Molecularly Imprinted Microspheres with Visible Light Photocatalytic Regeneration Availability for Targeted Degradation of Sulfadiazine. Sep. Purif. Technol. 2022, 299, 121814. [Google Scholar] [CrossRef]
- Kaya, S.I.; Cetinkaya, A.; Ozkan, S.A. Molecularly Imprinted Polymers as Highly Selective Sorbents in Sample Preparation Techniques and Their Applications in Environmental Water Analysis. Trends Environ. Anal. Chem. 2023, 37, e00193. [Google Scholar] [CrossRef]
- Belbruno, J.J. Molecularly Imprinted Polymers. Chem. Rev. 2019, 119, 94–119. [Google Scholar] [CrossRef]
- Ding, X.; Heiden, P.A. Recent Developments in Molecularly Imprinted Nanoparticles by Surface Imprinting Techniques. Macromol. Mater. Eng. 2014, 299, 268–282. [Google Scholar] [CrossRef]
- de Escobar, C.C.; dos Santos, J.H.Z. Nanostructured Imprinted Supported Photocatalysts: Organic and Inorganic Matrixes. In Nanophotocatalysis and Environmental Applications; Inamuddin, Sharma, G., Kumar, A., Lichtfouse, E., Asiri, A., Eds.; Environmental Chemistry for a Sustainable World; Springer: Berlin/Heidelberg, Germany, 2019. [Google Scholar] [CrossRef]
- Stachowiak, M.; Cegłowski, M.; Kurczewska, J. Hybrid Chitosan/molecularly Imprinted Polymer Hydrogel Beads Doped with Iron for Selective Ibuprofen Adsorption. Int. J. Biol. Macromol. 2023, 251, 126356. [Google Scholar] [CrossRef]
- Chen, X.; Xu, X.; Jia, X.; Qian, H.; Zhu, X. Surface and Interface Engineering of Z-Scheme 1D/2D Imprinted CoZn-LDH/C3N4 Nanorods for Boosting Selective Visible-Light Photocatalytic Activity. Adv. Powder Technol. 2022, 33, 103531. [Google Scholar] [CrossRef]
- Wu, Y.; Li, Y.; Tian, A.; Mao, K.; Liu, J. Selective Removal of Perfluorooctanoic Acid Using Molecularly Imprinted Polymer-Modified TiO2 Nanotube Arrays. Int. J. Photoenergy 2016, 2016, 7368795. [Google Scholar] [CrossRef]
- Kumar, S.; Karfa, P.; Majhi, K.C.; Madhuri, R. Photocatalytic, Fluorescent BiPO4@Graphene Oxide Based Magnetic Molecularly Imprinted Polymer for Detection, Removal and Degradation of Ciprofloxacin. Mater. Sci. Eng. C 2020, 111, 110777. [Google Scholar] [CrossRef]
- Lu, Z.; Huo, P.; Luo, Y.; Liu, X.; Wu, D.; Gao, X.; Li, C.; Yan, Y. Performance of Molecularly Imprinted Photocatalysts Based on Fly-Ash Cenospheres for Selective Photodegradation of Single and Ternary Antibiotics Solution. J. Mol. Catal. A Chem. 2013, 378, 91–98. [Google Scholar] [CrossRef]
- Anirudhan, T.S.; Madanan Anju, S. Synthesis and Evaluation of TiO2 Nanotubes/Silylated Graphene Oxide-Based Molecularly Imprinted Polymer for the Selective Adsorption and Subsequent Photocatalytic Degradation of 2,4-Dichlorophenoxyacetic Acid. J. Environ. Chem. Eng. 2019, 7, 103355. [Google Scholar] [CrossRef]
- Bagheri, A.R.; Aramesh, N.; Khan, A.A.; Gul, I.; Ghotekar, S.; Bilal, M. Molecularly Imprinted Polymers-Based Adsorption and Photocatalytic Approaches for Mitigation of Environmentally-Hazardous Pollutants—A Review. J. Environ. Chem. Eng. 2021, 9, 104879. [Google Scholar] [CrossRef]
- Amiri, M.; Dashtian, K.; Ghaedi, M.; Mosleh, S. A Dual Surface Inorganic Molecularly Imprinted Bi2WO6-CuO/Ag2O Heterostructure with Enhanced Activity-Selectivity towards the Photocatalytic Degradation of Target Contaminants. Photochem. Photobiol. Sci. 2020, 19, 943–955. [Google Scholar] [CrossRef]
- Su, L.; Li, G.; Lan, Z.; Yu, T.; Chu, H.; Han, S.; Qin, S. Preparation of Molecularly Imprinted CNT/ZnO and Photocatalytic Degradation of Bisphenol A. Chin. Sci. Bull. 2020, 65, 617. [Google Scholar] [CrossRef]
- Atarodi, H.; Faghihian, H. Selective Photodegradation of Atrazine by a Novel Molecularly Imprinted Nanophotocatalyst Prepared on the Basis of Chitosan. J. Photochem. Photobiol. A Chem. 2019, 382, 111892. [Google Scholar] [CrossRef]
- Liu, S.J.; Han, S.D.; Zhao, J.P.; Xu, J.; Bu, X.H. In-Situ Synthesis of Molecular Magnetorefrigerant Materials. Coord. Chem. Rev. 2019, 394, 39–52. [Google Scholar] [CrossRef]
- Fiorenza, R.; Di Mauro, A.; Cantarella, M.; Iaria, C.; Scalisi, E.M.; Brundo, M.V.; Gulino, A.; Spitaleri, L.; Nicotra, G.; Dattilo, S. Preferential Removal of Pesticides from Water by Molecular Imprinting on TiO2 Photocatalysts. Chem. Eng. J. 2020, 379, 122309. [Google Scholar] [CrossRef]
- Sharabi, D.; Paz, Y. Preferential Photodegradation of Contaminants by Molecular Imprinting on Titanium Dioxide. Appl. Catal. B 2010, 95, 169–178. [Google Scholar] [CrossRef]
- Carboni, D.; Malfatti, L.; Pinna, A.; Lasio, B.; Tokudome, Y.; Takahashi, M.; Innocenzi, P. Molecularly Imprinted La-Doped Mesoporous Titania Films with Hydrolytic Properties toward Organophosphate Pesticides. New J. Chem. 2013, 37, 2995–3002. [Google Scholar] [CrossRef]
- Deng, F.; Liu, Y.; Luo, X.; Wu, S.; Luo, S.; Au, C.; Qi, R. Sol-Hydrothermal Synthesis of Inorganic-Framework Molecularly Imprinted TiO2/SiO2 Nanocomposite and Its Preferential Photocatalytic Degradation towards Target Contaminant. J. Hazard. Mater. 2014, 278, 108–115. [Google Scholar] [CrossRef] [PubMed]
- Luo, X.; Deng, F.; Min, L.; Luo, S.; Guo, B.; Zeng, G.; Au, C. Facile One-Step Synthesis of Inorganic-Framework Molecularly Imprinted TiO2/WO3 Nanocomposite and Its Molecular Recognitive Photocatalytic Degradation of Target Contaminant. Environ. Sci. Technol. 2013, 47, 7404–7412. [Google Scholar] [CrossRef]
- Wu, Y.; Dong, Y.; Xia, X.; Liu, X.; Li, H. Facile Synthesis of N-F Codoped and Molecularly Imprinted TiO2 for Enhancing Photocatalytic Degradation of Target Contaminants. Appl. Surf. Sci. 2016, 364, 829–836. [Google Scholar] [CrossRef]
- Fiorenza, R.; Di Mauro, A.; Cantarella, M.; Privitera, V.; Impellizzeri, G. Selective Photodegradation of 2,4-D Pesticide from Water by Molecularly Imprinted TiO2. J. Photochem. Photobiol. A Chem. 2019, 380, 111872. [Google Scholar] [CrossRef]
- Shi, H.; Wang, Y.; Tang, C.; Wang, W.; Liu, M.; Zhao, G. Mechanisim Investigation on the Enhanced and Selective Photoelectrochemical Oxidation of Atrazine on Molecular Imprinted Mesoporous TiO2. Appl. Catal. B 2019, 246, 50–60. [Google Scholar] [CrossRef]
- Zhang, C.; Yang, Z.; Zhang, C.; Sun, Y. Kinetics of Photocatalytic Degradation of Atrazine on Molecularly Imprinted Titania Film. Asia-Pac. J. Chem. Eng. 2013, 8, 318–322. [Google Scholar] [CrossRef]
- Liu, Y.; Liu, R.; Liu, C.; Luo, S.; Yang, L.; Sui, F.; Teng, Y.; Yang, R.; Cai, Q. Enhanced Photocatalysis on TiO2 Nanotube Arrays Modified with Molecularly Imprinted TiO2 Thin Film. J. Hazard. Mater. 2010, 182, 912–918. [Google Scholar] [CrossRef] [PubMed]
- Zhu, M.; Wang, S.; Li, S. Titanium Catalyst with the Molecular Imprinting of Substrate for Selective Photocatalysis. J. Chin. Adv. Mater. Soc. 2014, 2, 71–81. [Google Scholar] [CrossRef]
- Lee, S.-W.; Ichinose, I.; Kunitake, T. Molecular Imprinting of Azobenzene Carboxylic Acid on a TiO2 Ultrathin Film by the Surface Sol-Gel Process. Langmuir 1998, 10, 2857–2863. [Google Scholar] [CrossRef]
- Liu, X.; Zhu, L.; Wang, X.; Meng, X.; Zhong, L. The Photocatalytic Selectivity between Molecularly Imprinted TiO2 and Target Contaminants. J. Nanopar. Res. 2020, 22, 300. [Google Scholar] [CrossRef]
- Shen, X.; Zhu, L.; Yu, H.; Tang, H.; Liu, S.; Li, W. Selective Photocatalysis on Molecular Imprinted TiO2 Thin Films Prepared via an Improved Liquid Phase Deposition Method. New J. Chem. 2009, 33, 1673–1679. [Google Scholar] [CrossRef]
- Zhang, Y.N.; Dai, W.; Wen, Y.; Zhao, G. Efficient Enantioselective Degradation of the Inactive (S)-Herbicide Dichlorprop on Chiral Molecular-Imprinted TiO2. Appl. Catal. B 2017, 212, 185–192. [Google Scholar] [CrossRef]
- de Escobar, C.C.; Dos Santos, J.H.Z. Effect of the Sol-Gel Route on the Textural Characteristics of Silica Imprinted with Rhodamine B. J. Sep. Sci. 2014, 37, 868–875. [Google Scholar] [CrossRef]
- de Escobar, C.C.; dos Santos, F.P.; dos Santos, J.H.Z. Effect of the Amount and Time of Addition of a Dye Template on the Adsorption and Photocatalytic Performance of Molecularly Imprinted Silica. J. Environ. Chem. Eng. 2018, 6, 190–196. [Google Scholar] [CrossRef]
- Ferreira, V.R.A.; Azenha, M.A.; Pereira, C.M.; Silva, A.F. Preparation of Molecularly Imprinted Hollow TiO2 Microspheres for Selective Photocatalysis. Chem. Eng. J. Adv. 2021, 5, 100071. [Google Scholar] [CrossRef]
- Guo, J.; Li, S.; Duan, L.; Guo, P.; Li, X.; Cui, Q.; Wang, H.; Jiang, Q. Preparation of Si Doped Molecularly Imprinted TiO2 Photocatalyst and Its Degradation to Antibiotic Wastewater. Integr. Ferroelectr. 2016, 168, 170–182. [Google Scholar] [CrossRef]
- Zhu, L.; Liu, X.; Wang, X.; Meng, X. Evaluation of Photocatalytic Selectivity of Ag/Zn Modified Molecularly Imprinted TiO2 by Multiwavelength Measurement. Sci. Total Environ. 2020, 703, 134732. [Google Scholar] [CrossRef]
- Liu, X.; Li, X.; Zhu, L.; Wang, X. Preparation of Molecularly Imprinted Ag-TiO2 for Photocatalytic Removal of Ethyl Paraben. Environ. Sci. Pollut. Res. 2022, 29, 10308–10318. [Google Scholar] [CrossRef]
- Liu, X.; Tao, X.; Xu, C.; Li, X.; Chen, R.; Chen, Y.; Zhong, L.; Zhu, L.; Wang, X. Evaluation of the Photocatalytic Performance of Molecularly Imprinted S–TiO2 by Paper Microzones. Environ. Res. 2021, 199, 111258. [Google Scholar] [CrossRef]
- Zhan, C.; Cao, X.; Xu, B.; Yan, P.; Yang, T.; Ye, Z.; Chen, X. Visible Light Induced Molecularly Imprinted Dawson-Type Heteropoly Acid Cobalt (II) Salt Modified TiO2 Composites: Enhanced Photocatalytic Activity for the Removal of Ethylparaben. Colloids Surf. A Physicochem. Eng. Asp. 2020, 586, 124244. [Google Scholar] [CrossRef]
- Fiorenza, R.; Di Mauro, A.; Cantarella, M.; Gulino, A.; Spitaleri, L.; Privitera, V.; Impellizzeri, G. Molecularly Imprinted N-Doped TiO2 Photocatalysts for the Selective Degradation of o-Phenylphenol Fungicide from Water. Mater. Sci. Semicond. Process. 2020, 112, 105019. [Google Scholar] [CrossRef]
- Zhang, C.; Chen, H.; Ma, M.; Yang, Z. Facile Synthesis of Magnetically Recoverable Fe3O4/Al2O3/Molecularly Imprinted TiO2 Nanocomposites and Its Molecular Recognitive Photocatalytic Degradation of Target Contaminant. J. Mol. Catal. A Chem. 2015, 402, 10–16. [Google Scholar] [CrossRef]
- Qi, H.P.; Wang, H.L. Facile Synthesis of Pr-Doped Molecularly Imprinted TiO2 Mesocrystals with High Preferential Photocatalytic Degradation Performance. Appl. Surf. Sci. 2020, 511, 145607. [Google Scholar] [CrossRef]
- Shen, X.; Zhu, L.; Huang, C.; Tang, H.; Yu, Z.; Deng, F. Inorganic Molecular Imprinted Titanium Dioxide Photocatalyst: Synthesis, Characterization and Its Application for Efficient and Selective Degradation of Phthalate Esters. J. Mater. Chem. 2009, 19, 4843–4851. [Google Scholar] [CrossRef]
- Han, D.M.; Dai, G.L.; Jia, W.P.; Liang, H.D. Preparation and Photocatalytic Activity of Cu2+-Doped 2, 4-Dichlorophenol Molecularly Imprinted SiO2-TiO2 Nanocomposite. Micro. Nano Lett. 2010, 5, 76–80. [Google Scholar] [CrossRef]
- Cantarella, M.; Di Mauro, A.; Gulino, A.; Spitaleri, L.; Nicotra, G.; Privitera, V.; Impellizzeri, G. Selective Photodegradation of Paracetamol by Molecularly Imprinted ZnO Nanonuts. Appl. Catal. B 2018, 238, 509–517. [Google Scholar] [CrossRef]
- de Escobar, C.C.; Lansarin, M.A.; dos Santos, J.H.Z.; Brandestini, M.D. Molecularly Imprinted Photocatalyst for Glyceraldehyde Production. J. Solgel Sci. Technol. 2018, 88, 220–226. [Google Scholar] [CrossRef]
- Xie, Y.; Wan, J.; Yan, Z.; Wang, Y.; Xiao, T.; Hou, J.; Chen, H. Targeted Degradation of Sulfamethoxazole in Wastewater by Molecularly Imprinted MOFs in Advanced Oxidation Processes: Degradation Pathways and Mechanism. Chem. Eng. J. 2022, 429, 132237. [Google Scholar] [CrossRef]
- Liu, M.; Ding, X.; Yang, Q.; Wang, Y.; Zhao, G.; Yang, N. A PM Leveled Photoelectrochemical Sensor for Microcystin-LR Based on Surface Molecularly Imprinted TiO2@CNTs Nanostructure. J. Hazard. Mater. 2017, 331, 309–320. [Google Scholar] [CrossRef] [PubMed]
- Shen, X. Molecularly Imprinted Photocatalysts. In Molecularly Imprinted Catalysts: Principles, Syntheses, and Applications; Li, S., Cao, S., Eds.; Elsevier Inc.: Amsterdam, The Netherlands, 2016; pp. 211–228. [Google Scholar] [CrossRef]
- Ali, H.R. Photocoupling of Ag3PO4-Ag2CO3 with Molecularly Imprinted Polymer for Enhanced Removal of Phenol under Solar Light: Application of Taguchi Method. Int. J. Appl. Eng. Res. 2017, 12, 3353–3359. [Google Scholar]
- Wang, T.; Korposh, S.; James, S.; Lee, S.W. Long-Period Grating Fiber-Optic Sensors Exploiting Molecularly Imprinted TiO2 Nanothin Films with Photocatalytic Self-Cleaning Ability. Microchim. Acta 2020, 187, 663. [Google Scholar] [CrossRef] [PubMed]
- Tonucci, M.C.; dos Santos Xavier, L.P.; da Silva, A.C.; Aquino, S.F.; Baeta, B.E.L. Removal of Estradiol from Water with a Hybrid MIP-TiO2 Catalytic Adsorbent. Water Air Soil Pollut. 2020, 231, 215. [Google Scholar] [CrossRef]
- Arabzadeh, N.; Mohammadi, A.; Darwish, M.; Abuzerr, S. Construction of a TiO2–Fe3O4-Decorated Molecularly Imprinted Polymer Nanocomposite for Tartrazine Degradation: Response Surface Methodology Modeling and Optimization. J. Chin. Chem. Soc. 2019, 66, 474–483. [Google Scholar] [CrossRef]
- Nussbaum, M.; Paz, Y. Ultra-Thin SiO2 Layers on TiO2: Improved Photocatalysis by Enhancing Products’ Desorption. Phys. Chem. Chem. Phys. 2012, 14, 3392–3399. [Google Scholar] [CrossRef] [PubMed]
- Zhang, H. Water-Compatible Molecularly Imprinted Polymers: Promising Synthetic Substitutes for Biological Receptors. Polymer 2014, 55, 699–714. [Google Scholar] [CrossRef]
- Tang, H.; Zhu, L.; Yu, C.; Shen, X. Selective Photocatalysis Mediated by Magnetic Molecularly Imprinted Polymers. Sep. Purif. Technol. 2012, 95, 165–171. [Google Scholar] [CrossRef]
- Sun, L.; Li, J.; Li, X.; Liu, C.; Wang, H.; Huo, P.; Yan, Y. sheng Molecularly Imprinted Ag/Ag3VO4/g-C3N4 Z-Scheme Photocatalysts for Enhanced Preferential Removal of Tetracycline. J. Colloid Interface Sci. 2019, 552, 271–286. [Google Scholar] [CrossRef]
- Tran.T, T.; Li, J.; Feng, H.; Cai, J.; Yuan, L.; Wang, N.; Cai, Q. Molecularly Imprinted Polymer Modified TiO2 Nanotube Arrays for Photoelectrochemical Determination of Perfluorooctane Sulfonate (PFOS). Sens. Actuators B Chem. 2014, 190, 745–751. [Google Scholar] [CrossRef]
- Li, X.; Chen, X.; Lv, Z.; Wang, B. Ultrahigh Ciprofloxacin Accumulation and Visible-Light Photocatalytic Degradation: Contribution of Metal Organic Frameworks Carrier in Magnetic Surface Molecularly Imprinted Polymers. J. Colloid Interface Sci. 2022, 616, 872–885. [Google Scholar] [CrossRef]
- Lu, Z.; Zhou, G.; Song, M.; Liu, X.; Tang, H.; Dong, H.; Huo, P.; Yan, F.; Du, P.; Xing, G. Development of Magnetic Imprinted PEDOT/CdS Heterojunction Photocatalytic Nanoreactors: 3-Dimensional Specific Recognition for Selectively Photocatalyzing Danofloxacin Mesylate. Appl. Catal. B 2020, 268, 118433. [Google Scholar] [CrossRef]
- He, F.; Lu, Z.; Song, M.; Liu, X.; Tang, H.; Huo, P.; Fan, W.; Dong, H.; Wu, X.; Han, S. Selective Reduction of Cu2+ with Simultaneous Degradation of Tetracycline by the Dual Channels Ion Imprinted POPD-CoFe2O4 Heterojunction Photocatalyst. Chem. Eng. J. 2019, 360, 750–761. [Google Scholar] [CrossRef]
- Huang, M.; Zhou, T.; Wu, X.; Mao, J. Adsorption and Degradation of Norfloxacin by a Novel Molecular Imprinting Magnetic Fenton-like Catalyst. Chin. J. Chem. Eng. 2015, 23, 1698–1704. [Google Scholar] [CrossRef]
- Chi, H.; Li, C.; Huang, M.; Wan, J.; Zhou, X.; Yan, B. Targeted Accumulation and Spatial Confinement Effect of Fe(II)-MOFs@MIP for Efficiently Removing Low Concentration Dibutyl Phthalate. Chem. Eng. J. 2021, 424, 130367. [Google Scholar] [CrossRef]
- Wang, C.; Li, C.; Wang, F.; Wang, C. Phosphonate Electrochemical Recognition by Molecularly Imprinted Deposited Film. Appl. Surf. Sci. 2006, 253, 2282–2288. [Google Scholar] [CrossRef]
- Tatemichi, M.; Sakamoto, M.A.; Mizuhata, M.; Deki, S.; Takeuchi, T. Protein-Templated Organic/Inorganic Hybrid Materials Prepared by Liquid-Phase Deposition. J. Am. Chem. Soc. 2007, 129, 10906–10910. [Google Scholar] [CrossRef] [PubMed]
- Zaidi, S.A.; Cheong, W.J. Preparation of an Open-Tubular Capillary Column with a Monolithic Layer of S-Ketoprofen Imprinted and 4-Styrenesulfonic Acid Incorporated Polymer and Its Enhanced Chiral Separation Performance in Capillary Electrochromatography. J. Chromatogr. A 2009, 1216, 2947–2952. [Google Scholar] [CrossRef]
- Fu, C.; Ma, Y.; Zuo, P.; Zhao, W.; Tang, W.; Yin, G.; Wang, J.; Gao, Y. In-Situ Thermal Polymerization Boosts Succinonitrile-Based Composite Solid-State Electrolyte for High Performance Li-Metal Battery. J. Power Sources 2021, 496, 229861. [Google Scholar] [CrossRef]
- Yao, X.; Zhang, W.; Huang, J.; Du, Z.; Hong, X.; Chen, X.; Hu, X.; Wang, X. Enhanced Photocatalytic Nitrogen Fixation of Ag/B-Doped g-C3N4 Nanosheets by One-Step in-Situ Decomposition-Thermal Polymerization Method. Appl. Catal. A Gen. 2020, 601, 117647. [Google Scholar] [CrossRef]
- Yang, L.; Yu, Y.; Zhang, J.; Chen, F.; Meng, X.; Qiu, Y.; Dan, Y.; Jiang, L. In-Situ Fabrication of Diketopyrrolopyrrole-Carbazole-Based Conjugated Polymer/TiO2 Heterojunction for Enhanced Visible Light Photocatalysis. Appl. Surf. Sci. 2018, 434, 796–805. [Google Scholar] [CrossRef]
- Sun, L.; Guan, J.; Xu, Q.; Yang, X.; Wang, J.; Hu, X. Synthesis and Applications of Molecularly Imprinted Polymers Modified TiO2 Nanomaterials: A Review. Polymers 2018, 10, 1248. [Google Scholar] [CrossRef] [PubMed]
- Luo, Y.; Feng, X.; Chen, Z.; Shen, X. Molecularly Imprinted Photocatalysts: Fabrication, Application and Challenges. Mater. Adv. 2022, 3, 8830–8847. [Google Scholar] [CrossRef]
- Li, L.; Zheng, X.; Chi, Y.; Wang, Y.; Sun, X.; Yue, Q.; Gao, B.; Xu, S. Molecularly Imprinted Carbon Nanosheets Supported TiO2: Strong Selectivity and Synergic Adsorption-Photocatalysis for Antibiotics Removal. J. Hazard. Mater. 2020, 383, 121211. [Google Scholar] [CrossRef]
- Zhou, G.; Cao, Y.; Jin, Y.; Wang, C.; Wang, Y.; Hua, C.; Wu, S. Novel Selective Adsorption and Photodegradation of BPA by Molecularly Imprinted Sulfur Doped Nano-Titanium Dioxide. J. Clean. Prod. 2020, 274, 122929. [Google Scholar] [CrossRef]
- Bao, L.; Meng, M.; Sun, K.; Li, W.; Zhao, D.; Li, H.; He, M. Selective Adsorption and Degradation of Rhodamine B with Modified Titanium Dioxide Photocatalyst. J. Appl. Polym. Sci. 2014, 131, 40890. [Google Scholar] [CrossRef]
- Huang, Y.; Wang, P.; Chen, F.; Zhou, G.; Song, M.; Liu, X.; Ma, C.; Han, S.; Yan, Y.; Lu, Z. Enhanced Controllable Degradation Ability of Magnetic Imprinted Photocatalyst via Photoinduced Surface Imprinted Technique for Ciprofloxacin Selectively Degradation. J. Photochem. Photobiol. A Chem. 2021, 410, 113159. [Google Scholar] [CrossRef]
- Luo, Y.; Lu, Z.; Jiang, Y.; Wang, D.; Yang, L.; Huo, P.; Da, Z.; Bai, X.; Xie, X.; Yang, P. Selective Photodegradation of 1-Methylimidazole-2-Thiol by the Magnetic and Dual Conductive Imprinted Photocatalysts Based on TiO2/Fe3O4/MWCNTs. Chem. Eng. J. 2014, 240, 244–252. [Google Scholar] [CrossRef]
- Melvin Ng, H.K.; Leo, C.P.; Abdullah, A.Z. Selective Removal of Dyes by Molecular Imprinted TiO2 Nanoparticles in Polysulfone Ultrafiltration Membrane. J. Environ. Chem. Eng. 2017, 5, 3991–3998. [Google Scholar] [CrossRef]
- He, M.Q.; Bao, L.L.; Sun, K.Y.; Zhao, D.X.; Li, W.B.; Xia, J.X.; Li, H.M. Synthesis of Molecularly Imprinted Polypyrrole/Titanium Dioxide Nanocomposites and Its Selective Photocatalytic Degradation of Rhodamine B under Visible Light Irradiation. Express. Polym. Lett. 2014, 8, 850–861. [Google Scholar] [CrossRef]
- Liu, Y.; Zhu, J.; Liu, X.; Li, H. A Convenient Approach of MIP/Co-TiO2 Nanocomposites with Highly Enhanced Photocatalytic Activity and Selectivity under Visible Light Irradiation. RSC Adv. 2016, 6, 69326–69333. [Google Scholar] [CrossRef]
- Zhang, W.; Li, Y.; Wang, Q.; Wang, C.; Wang, P.; Mao, K. Performance Evaluation and Application of Surface-Molecular-Imprinted Polymer-Modified TiO2 Nanotubes for the Removal of Estrogenic Chemicals from Secondary Effluents. Environ. Sci. Pollut. Res. 2013, 20, 1431–1440. [Google Scholar] [CrossRef]
- de Escobar, C.C.; Moreno Ruiz, Y.P.; dos Santos, J.H.Z.; Ye, L. Molecularly Imprinted TiO2 Photocatalysts for Degradation of Diclofenac in Water. Colloids Surf. A Physicochem. Eng. Asp. 2018, 538, 729–738. [Google Scholar] [CrossRef]
- Shi, H.; Zhao, G.; Liu, M.; Zhu, Z. A Novel Photoelectrochemical Sensor Based on Molecularly Imprinted Polymer Modified TiO2 Nanotubes and Its Highly Selective Detection of 2,4-Dichlorophenoxyacetic Acid. Electrochem. Comm. 2011, 13, 1404–1407. [Google Scholar] [CrossRef]
- Wang, P.; Dai, W.; Ge, L.; Yan, M.; Ge, S.; Yu, J. Visible Light Photoelectrochemical Sensor Based on Au Nanoparticles and Molecularly Imprinted Poly(o-Phenylenediamine)-Modified TiO2 Nanotubes for Specific and Sensitive Detection Chlorpyrifos. Analyst 2013, 138, 939–945. [Google Scholar] [CrossRef]
- Sun, X.; Gao, C.; Zhang, L.; Yan, M.; Yu, J.; Ge, S. Photoelectrochemical Sensor Based on Molecularly Imprinted Film Modified Hierarchical Branched Titanium Dioxide Nanorods for Chlorpyrifos Detection. Sens. Actuators B Chem. 2017, 251, 1–8. [Google Scholar] [CrossRef]
- Geng, H.R.; Miao, S.S.; Jin, S.F.; Yang, H. A Newly Developed Molecularly Imprinted Polymer on the Surface of TiO2 for Selective Extraction of Triazine Herbicides Residues in Maize, Water, and Soil. Anal. Bioanal. Chem. 2015, 407, 8803–8812. [Google Scholar] [CrossRef] [PubMed]
- Khoddami, N.; Shemirani, F. A New Magnetic Ion-Imprinted Polymer as a Highly Selective Sorbent for Determination of Cobalt in Biological and Environmental Samples. Talanta 2016, 146, 244–252. [Google Scholar] [CrossRef]
- Zhao, Z.; Zhu, C.; Guo, Q.; Cai, Y.; Zhu, X.; Li, B. Preparation of Lysozyme-Imprinted Nanoparticles on Polydopamine-Modified Titanium Dioxide Using Ionic Liquid as a Stabilizer. RSC Adv. 2019, 9, 14974–14981. [Google Scholar] [CrossRef]
- Liu, W.; Li, Y.; Liu, F.; Jiang, W.; Zhang, D.; Liang, J. Visible-Light-Driven Photocatalytic Degradation of Diclofenac by Carbon Quantum Dots Modified Porous g-C3N4: Mechanisms, Degradation Pathway and DFT Calculation. Water. Res. 2019, 151, 8–19. [Google Scholar] [CrossRef]
- Shi, H.; Zhou, G.; Liu, Y.; Fu, Y.; Wang, H.; Wu, P. Kinetics and Pathways of Diclofenac Degradation by Heat-Activated Persulfate. RSC Adv. 2019, 9, 31370–31377. [Google Scholar] [CrossRef]
- Rao, Y.F.; Zhang, Y.; Han, F.; Guo, H.; Huang, Y.; Li, R.; Qi, F.; Ma, J. Heterogeneous Activation of Peroxymonosulfate by LaFeO3 for Diclofenac Degradation: DFT-Assisted Mechanistic Study and Degradation Pathways. Chem. Eng. J. 2018, 352, 601–611. [Google Scholar] [CrossRef]
- Zhang, W.; Zhou, L.; Shi, J.; Deng, H. Fabrication of Novel Visible-Light-Driven AgI/g-C3N4 Composites with Enhanced Visible-Light Photocatalytic Activity for Diclofenac Degradation. J. Colloid Interface Sci. 2017, 496, 167–176. [Google Scholar] [CrossRef]
- Lu, X.; Shao, Y.; Gao, N.; Chen, J.; Zhang, Y.; Xiang, H.; Guo, Y. Degradation of Diclofenac by UV-Activated Persulfate Process: Kinetic Studies, Degradation Pathways and Toxicity Assessments. Ecotoxicol. Environ. Saf. 2017, 141, 139–147. [Google Scholar] [CrossRef]
- Jian, J.M.; Guo, Y.; Zeng, L.; Liang-Ying, L.; Lu, X.; Wang, F.; Zeng, E.Y. Global Distribution of Perfluorochemicals (PFCs) in Potential Human Exposure Source–A Review. Environ. Int. 2017, 108, 51–62. [Google Scholar] [CrossRef]
- Liu, C.; Chang, V.W.C.; Gin, K.Y.H. Environmental Toxicity of PFCs: An Enhanced Integrated Biomarker Assessment and Structure-Activity Analysis. Environ. Toxicol. Chem. 2013, 32, 2226–2233. [Google Scholar] [CrossRef]
- Lai, C.; Wang, M.M.; Zeng, G.M.; Liu, Y.G.; Huang, D.L.; Zhang, C.; Wang, R.Z.; Xu, P.; Cheng, M.; Huang, C.; et al. Synthesis of Surface Molecular Imprinted TiO2/Graphene Photocatalyst and Its Highly Efficient Photocatalytic Degradation of Target Pollutant under Visible Light Irradiation. Appl. Surf. Sci. 2016, 390, 368–376. [Google Scholar] [CrossRef]
- Hirakawa, T.; Kominami, H.; Ohtani, B.; Nosaka, Y. Mechanism of Photocatalytic Production of Active Oxygens on Highly Crystalline TiO2 Particles by Means of Chemiluminescent Probing and ESR Spectroscopy. J. Phys. Chem. B 2001, 105, 6993–6999. [Google Scholar] [CrossRef]
- Ohtani, B. Photocatalysis A to Z-What We Know and What We Do Not Know in a Scientific Sense. J. Photochem. Photobiol. C Photochem. Rev. 2010, 11, 157–178. [Google Scholar] [CrossRef]
- Wang, K.; Janczarek, M.; Wei, Z.; Raja-Mogan, T.; Endo-Kimura, M.; Khedr, T.M.; Ohtani, B.; Kowalska, E. Morphology-and Crystalline Composition-Governed Activity of Titania-Based Photocatalysts: Overview and Perspective. Catalysts 2019, 9, 1054. [Google Scholar] [CrossRef]
- Wang, K.; Bielan, Z.; Endo-Kimura, M.; Janczarek, M.; Zhang, D.; Kowalski, D.; Zielińska-Jurek, A.; Markowska-Szczupak, A.; Ohtani, B.; Kowalska, E. On the Mechanism of Photocatalytic Reactions on CuxO@TiO2 core-shell Photocatalysts. J. Mater. Chem. A Mater. 2021, 9, 10135–10145. [Google Scholar] [CrossRef]
- Deng, F.; Li, Y.; Luo, X.; Yang, L.; Tu, X. Preparation of Conductive Polypyrrole/TiO2 Nanocomposite via Surface Molecular Imprinting Technique and Its Photocatalytic Activity under Simulated Solar Light Irradiation. Colloids Surf. A Physicochem. Eng. Asp. 2012, 395, 183–189. [Google Scholar] [CrossRef]
- Shen, X.; Zhu, L.; Li, J.; Tang, H. Synthesis of Molecular Imprinted Polymer Coated Photocatalysts with High Selectivity. Chem. Commun. 2007, 1163–1165. [Google Scholar] [CrossRef]
- Ma, W.; Han, D.; Gan, S.; Zhang, N.; Liu, S.; Wu, T.; Zhang, Q.; Dong, X.; Niu, L. Rapid and Specific Sensing of Gallic Acid with a Photoelectrochemical Platform Based on Polyaniline–Reduced Graphene Oxide–TiO2. Chem. Commun. 2013, 49, 7842–7844. [Google Scholar] [CrossRef] [PubMed]
- Wang, G.L.; Jiao, H.J.; Liu, K.L.; Wu, X.M.; Dong, Y.M.; Li, Z.J.; Zhang, C. A Novel Strategy for the Construction of Photoelectrochemical Sensors Based on Quantum Dots and Electron Acceptor: The Case of Dopamine Detection. Electrochem. Commun. 2014, 41, 47–50. [Google Scholar] [CrossRef]
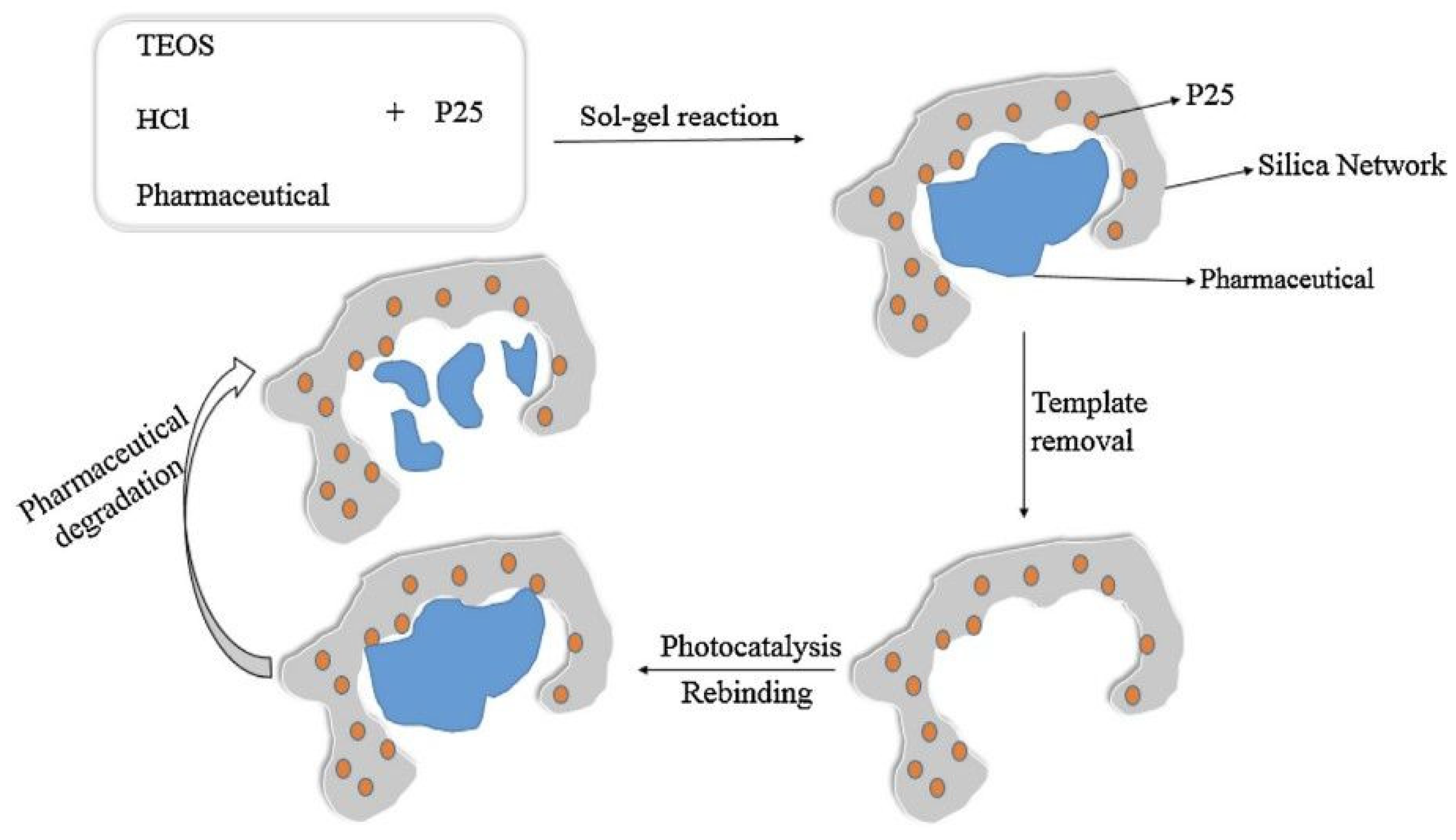
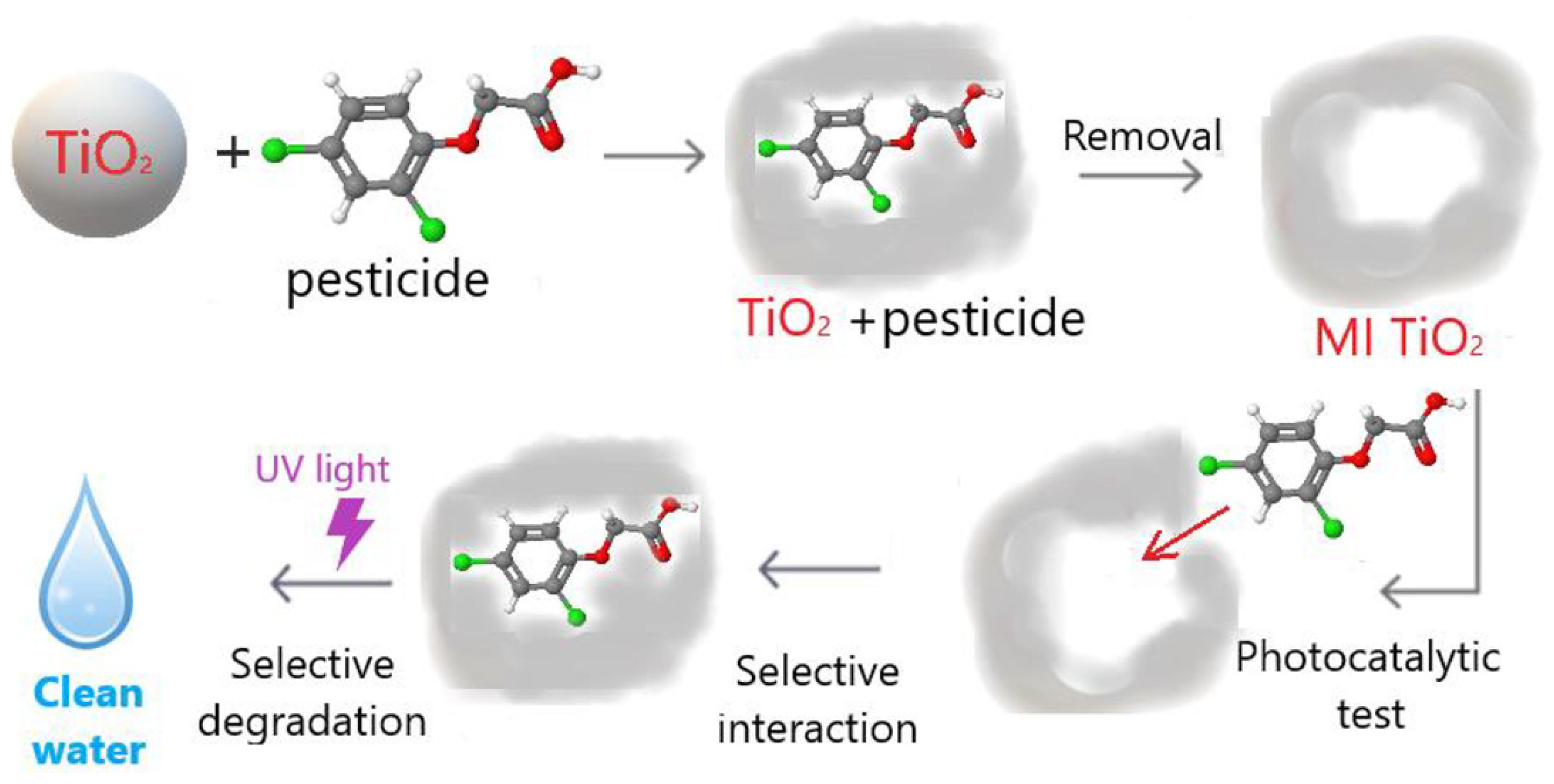

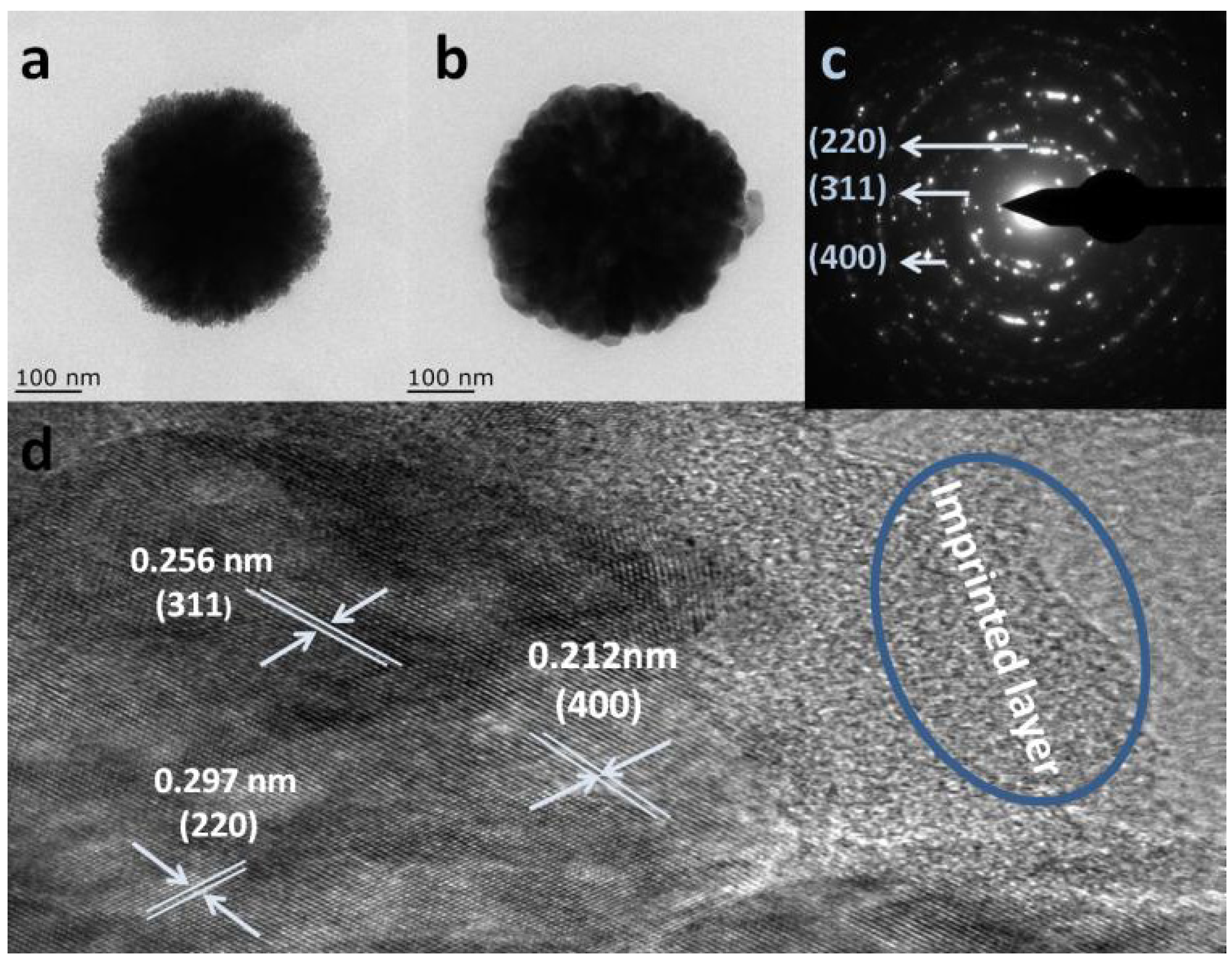
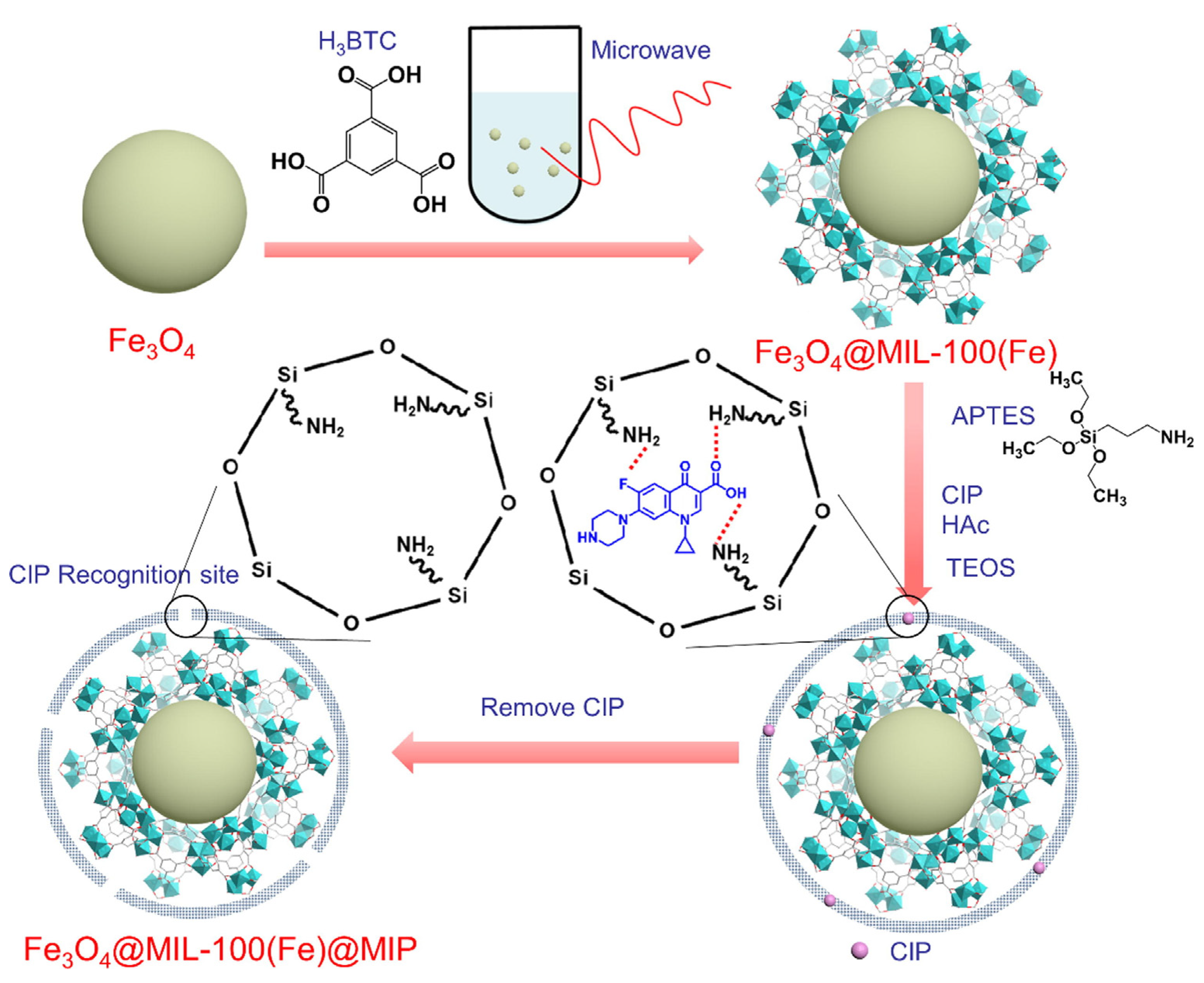
| Materials | Template | Synthesis Method | Application | Ref. |
|---|---|---|---|---|
| TiO2-Fe3O4 | estrone | liquid-phase deposition | The kapp value of the target estrone over MIPs was 0.069 min−1, being 363% of that over NIPs (0.019 min−1) and 238% of that over P25 (0.029 min−1). | [12] |
| TiO2 nanotubes | tetracycline hydrochloride (TC) | liquid-phase deposition | The rate constant for TC photodegradation by MIP-TiO2 was about 1.9 times higher than that for the TiO2 nanotubes. MIP-TiO2 photocatalyst gives the kinetics k value of degradation of TC about 0.218 h−1 | [19] |
| TiO2 | 2,4-D IMID | sol–gel | TiO2 MI/2,4D, photodegradation of template about ~47% TiO2 MI/IMID, photodegradation of template about ~35% | [36] |
| La-doped mesoporous titania films | bis-4-nitro-phenyl-phosphate | sol–gel | The reaction catalyzed by the MIF shows a constant rate 4.2171 × 10−10 M s−1, which is 27% faster than the background hydrolysis and 8% faster than the corresponding NIF. | [38] |
| TiO2/SiO2 | 4-nitrophenol | hydrothermal | The removal efficiency was about 90% at pH 4. | [39] |
| TiO2/WO3 | 2NP | sol–gel | 2NP-TiO2/WO3 k constant rate 0.00372 min−1 TiO2/WO3 k constant rate 0.0000293 min−1 | [40] |
| N-F co-doped TiO2 | 2NP 4NP | ethanol–water solvothermal | 2NP-MIP-TiO2 k constant rate 0.05233 min−1 NIP-TiO2 k constant rate 0.01962 min−1 | [41] |
| TiO2 | 2,4-dichlorophenoxyacetic acid | chemical precipitation | When the light is switched on, degradation rate of 2,4D was obtained with the MI TiO2/2,4D sample which, after 240 min, degrades ≈ 75% of initial concentration of the herbicide, whereas the bare TiO2 degrades ≈ 45% of the pesticide. | [42] |
| mesoporous TiO2 | atrazine | evaporation induced self-assembly method (EISA) | Selective photoelectrochemical oxidation of atrazine in complex polluted water samples was successfully achieved on MI-meso-TiO2 with removal rate of 91.7%. | [43] |
| quartz crystal molecularly imprinted TiO2 | atrazine | sol–gel | The degradation rate constants (K) estimated from the in situ frequency measurement is about 0.62 × 10−3s−1. | [44] |
| TiO2 nanotubes | 9-AnCOOH anthracene-9-carboxylic acid | sol–gel | Thin MIF-TiO2 NT 100% Thick MIF-TiO2 NT 70% | [45] |
| TiO2 | salicylic acid | molecular imprinting technology | Oxidation potentials of salicylic acid: 917 4-methylsalicylic acid: 887 | [46] |
| quartz crystal coated with gold and TiO2 | 4-(4-propyloxyphenylazo)benzoic acid C3AzoCO2H | sol–gel | The removal efficiency was in the range of 80–90%. | [40] |
| TiO2 | ethyl p-hydroxybenzoate | sol–gel | The MIP-TiO2 degradation rate of ethyl p-hydroxybenzoate in 2 h was 81%. | [48] |
| TiO2 | salicylic acid | (LPD) | When the concentration of SA is 25 mg L−1, the rate constant of SA decomposed over MIF is 0.01189 min−1. | [49] |
| TiO2-SiO2 | RhB | sol–gel | The maximum removal RhB 36.9% was obtained with the EA1TiRhB system (acid catalyzed route with HCl). | [51] |
| TiO2 | RhB | sol–gel | The highest obtained adsorption was 28.81 (%) ± 1.22. The highest rate of degradation was 50.72 (%) ± 1.89. | [52] |
| TiO2 microspheres | bilirubin | sol–gel | The rate of photodegradation under UV irradiation: bilirubin (0.0081–0.0118 min−1); protoporphyrin (0.0037–0.0051 min−1). | [53] |
| Si doped TiO2 | catalytic oxytetracycline (OTC) | liquid-phase deposition | The maximum photocatalytic degradation of oxytetracycline wastewater was 80.79% in 120 min. | [54] |
| Ag/Zn/TiO2 | ethyl p-hydroxybenzoate | sol–gel | The photocatalytic degradation of ethyl p-hydroxybenzoate was 99% for 2 h. | [55] |
| Ag-TiO2 | ethylparaben | sol–gel | The photocatalytic degradation of ethylparaben was 93.4% in 2 h. | [56] |
| S–TiO2 | ethylparaben | sol–gel | The S-EP-TiO2 degradation efficiency of methyl orange was 98.58% and of methylene blue 99.81% within 120 min. | [57] |
| TiO2 | ethylparaben | modified stepwise acidification | EP photo-degradation efficiency of 87.32%, 89.82% irradiation under UV for 70 min and visible light for 40 h, respectively. | [58] |
| Fe3O4/SiO2/ hydroxyapatite | simazine | solvothermal | The photodegradation remained as high as 97.2% after eight cycles. | [60] |
| Pr- TiO2 | 2-sec-butyl-4,6-dinitrophenol (DNBP) | solvothermal | MIP-TMCs and Pr-MIP-TMCs could achieve at least 90% DNBP removal for 300 min of irradiation, while pristine TMCs only achieved about 40% DNBP removal for the same irradiation time. | [61] |
| TiO2-Al2O3 | diethyl phthalate (DEP) | sol–gel | The constant rate for the photodegradation of DEP was 53.0 kDEP/10−3 min−1. | [62] |
| SiO2 –TiO2 | 2,4-dichlorophenol (DCP) | sol–gel method | The degradation efficiency of 2,4-DCP in a single Cu2+-doped 2,4-DCP imprinted TiO2-SiO2 system after 2 h of irradiation was approximately 80%. | [63] |
| ZnO | acetaminophen | co-precipitation | The degradation efficiency reached 100% for paracetamol (5 × 10−5 M) after 3 h. The kinetic constant for the photocatalytic degradation of paracetamol was approximately 1.32 × 10−2 min−1. | [64] |
| ZnO doping with Al3+ ions | glycerol | sol–gel | The maximum adsorption of glycerol (ca. 9%) is reached at 60 min from the beginning of the adsorption process. At the end of 1 h reaction, the molar fraction of degradation product was 4.5% and the rest was still unconverted glycerol. | [65] |
| TiO2@CNTs | microcystin-LR (MC-LR) a | sol–gel | The detection limit was calculated to be 0.4 pM. | [67] |
| Materials | Template | Synthesis Method | Monomer/Cross-Linker | Application | Ref. |
|---|---|---|---|---|---|
| TiO2 nanotube arrays | perfluorooctanoic acid | precipitation polymerization | 2,2′-azobis (2-methylpropionitrile), ethylene glycol dimethacrylate, 3-methacryloxypropyl trimethoxysilane (MPTS), and 3-aminopropyltriethoxylsilane (APTS), acrylamide | The amount of PFOA adsorbed by the MIP-TiO2 NTs was as high as 0.8125 μg/cm2. PFOA decomposition and defluorination by the MIP-TiO2 NTs reached 84% and 30.2% after 8 h reaction, respectively. The MIP-TiO2 NTs could also selectively and rapidly remove PFOA from a secondary effluent, exhibiting a decomposition of 81.1%, almost as high as that observed in pure water. | [27] |
| NH2-MIL-53(Fe) (MOF) | sulfamethoxazole | solvothermal | acrylic acid, methacrylic acid, methyl methacrylate, divinylbenzene, azobisisobutyronitrile | Removal rate of 38.04 mg/g | [66] |
| TiO2 nanotube arrays | perfluorooctanesulfonate | imprinting polymerization | 2,2-azobis (2- methylpropionitrile), 3-aminopropyltriethoxylsilane, 3-methacryloxypropyl trimethoxysilane, acrylamide | The limit of detection (LOD)of PFOS (S/N = 3) was calculated to be 86 ng mL−1. | [77] |
| MIL-100 with Fe3O4 | ciprofloxacin | liquid-phase deposition | (3–aminopropyl) triethoxysilanen humic acid | Selectivity (α(QMIP/QNIP)) = 3.54 Uptake capacity calculated by the Langmuir equation (273.65 mg/g) | [78] |
| PEDOT/CdS | danofloxacin mesylate | microwave | poly-3,4-ethylenedioxythiophene | Degradation rate of around 84.84% | [79] |
| POPD-CoFe2O4 | Cu2+ ions | microwave | polyo-phenylenediamine, ethyleneglycoldimethacrylate, azobisisobutyronitrile, P123 | The highest reduction rate of Cu2+ was 45.98%. | [80] |
| Fe2O3/chitosan | norfloxacin | microemulsion | chitosan, glutaraldehyde | The system follows the pseudo-first-order kinetic with a kobs value of 0.0012 min−1. | [81] |
| Fe(II)-MOFs@MIP | dibutyl phthalate (DBP) | microemulsion | MAA, AIBN, EGDMA | The recognition level was 169.25 μg × g−1. Degradation of DBP (0.071 min−1) The selectivity coefficient towards DBP was 7.28 for adsorption and 4.46 for photocatalytic degradation. | [82] |
| TiO2-Pep-poly-L-lysine | pepsin | liquid-phase deposition | poly-L-lysine | The binding constant of pepsin was approximately 7.3 × 105 M−1. | [84] |
| MIPsRhB–PPy/TiO2 | RhB | in situ polymerization | polypyrrole (PPy) | The rate constant (k) for the photodegradation of RhB over MIPRhB-PPy/TiO2 is 0.0158 min–1. | [97] |
| MIPs/Co-TiO2 nanocomposites. | RhB | solvothermal | p-phenylenediamine, APS ammonium persulfate | The k value for the photodegradation of RhB over MIP/Co-TiO2 was 0.03606 min−1, being 215.7% of RhB over NIP/Co-TiO2 nanocomposites (0.01672 min−1) and 337.3% of RhB over Co-TiO2 nanoparticles (0.01069 min−1). | [98] |
| S-MIP-TiO2 NTs | 17-β-estradiol | precipitation polymerization | methacrylic acid, trimethylolpropane trimethacrylate, initiator 4,40-azobis(4-cyanovaleric acid) | The removal efficiency was slightly reduced after each use and finally stable at about 84% after six cycles. | [99] |
| Cu2O-doped TiO2 | diclofenac | precipitation polymerization | MAA, acrylamide | The highest obtained selectivity parameters for adsorption stage (dark-stage) after 1 h was 70,07%. The highest obtained selectivity parameters for photodegradation stage (UV-light) after 2 h was 45,72%. | [100] |
| TiO2 nanotubes | 2,4-D | electropolymerization | pyrrole | The detection limit was calculated to be 10 nM (2.2 ng/mL) (S/N = 3). | [101] |
| TiO2 nanotubes | chlorpyrifos | potentiostatic electrodeposition | o-phenylenediamine (o-PD) monomer | The detection limit was calculated to be 0.96 nmol L−1. | [102] |
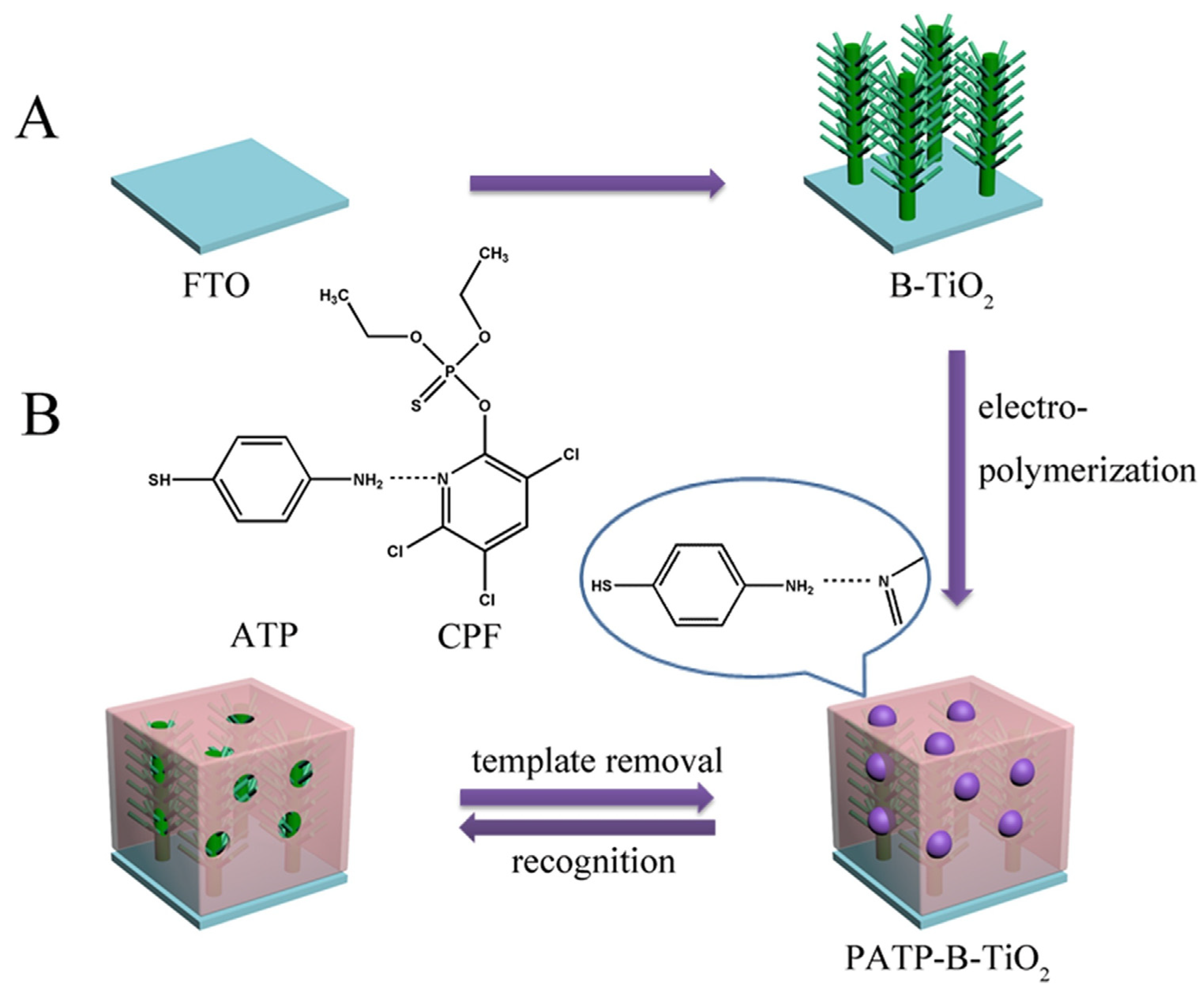
| B-TiO2 NRs | organophosphate pesticide chlorpyrifos CPF | hydrothermal | p-aminothiophenol (functional monomer) | The detection limit was calculated to be 7.4 pg·mL−1. | [103] |
| TiO2 | propazine (Pro) | precipitation polymerization | ethyleneglycol dimethacrylate, methacrylic acid, 2,2′-azobis (isobutyronitrile) | The adsorption amount of MIP (6.8076 mg g−1) when the propazine concentration was 11 mg L−1. | [104] |
| Fe3O4@TiO2@SiO2-IIP | Co(II) ions | surface imprinting technique combined with sol–gel process | 3-(2-aminoethylamino) propyltrimethoxysilane, tetraethyl orthosilicate | Fe3O4@TiO2@SiO2-IIP showed an adsorption capacity for Co(II) of 35.21 mg g−1. | [105] |
| TiO2@Lyz | lysozyme (Lyz) | free radical polymerization method | acrylamide/methylene bisacrylamide system hydroxyethyl acrylate/poly(ethylene glycol) dimethacrylate system | The best conditions resulted in an imprinting value of 4.40 and a peak adsorption limit of 120 mg g−1 for the TiO2@Lyz-MIPs, which was considerably higher than non-imprinted alternatives. | [106] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kubiak, A.; Stachowiak, M.; Cegłowski, M. Unveiling the Latest Developments in Molecularly Imprinted Photocatalysts: A State-of-the-Art Review. Polymers 2023, 15, 4152. https://doi.org/10.3390/polym15204152
Kubiak A, Stachowiak M, Cegłowski M. Unveiling the Latest Developments in Molecularly Imprinted Photocatalysts: A State-of-the-Art Review. Polymers. 2023; 15(20):4152. https://doi.org/10.3390/polym15204152
Chicago/Turabian StyleKubiak, Adam, Maria Stachowiak, and Michał Cegłowski. 2023. "Unveiling the Latest Developments in Molecularly Imprinted Photocatalysts: A State-of-the-Art Review" Polymers 15, no. 20: 4152. https://doi.org/10.3390/polym15204152
APA StyleKubiak, A., Stachowiak, M., & Cegłowski, M. (2023). Unveiling the Latest Developments in Molecularly Imprinted Photocatalysts: A State-of-the-Art Review. Polymers, 15(20), 4152. https://doi.org/10.3390/polym15204152











