Mechanosynthesis of Polyureas and Studies of Their Responses to Anions
Abstract
:1. Introduction
2. Materials and Methods
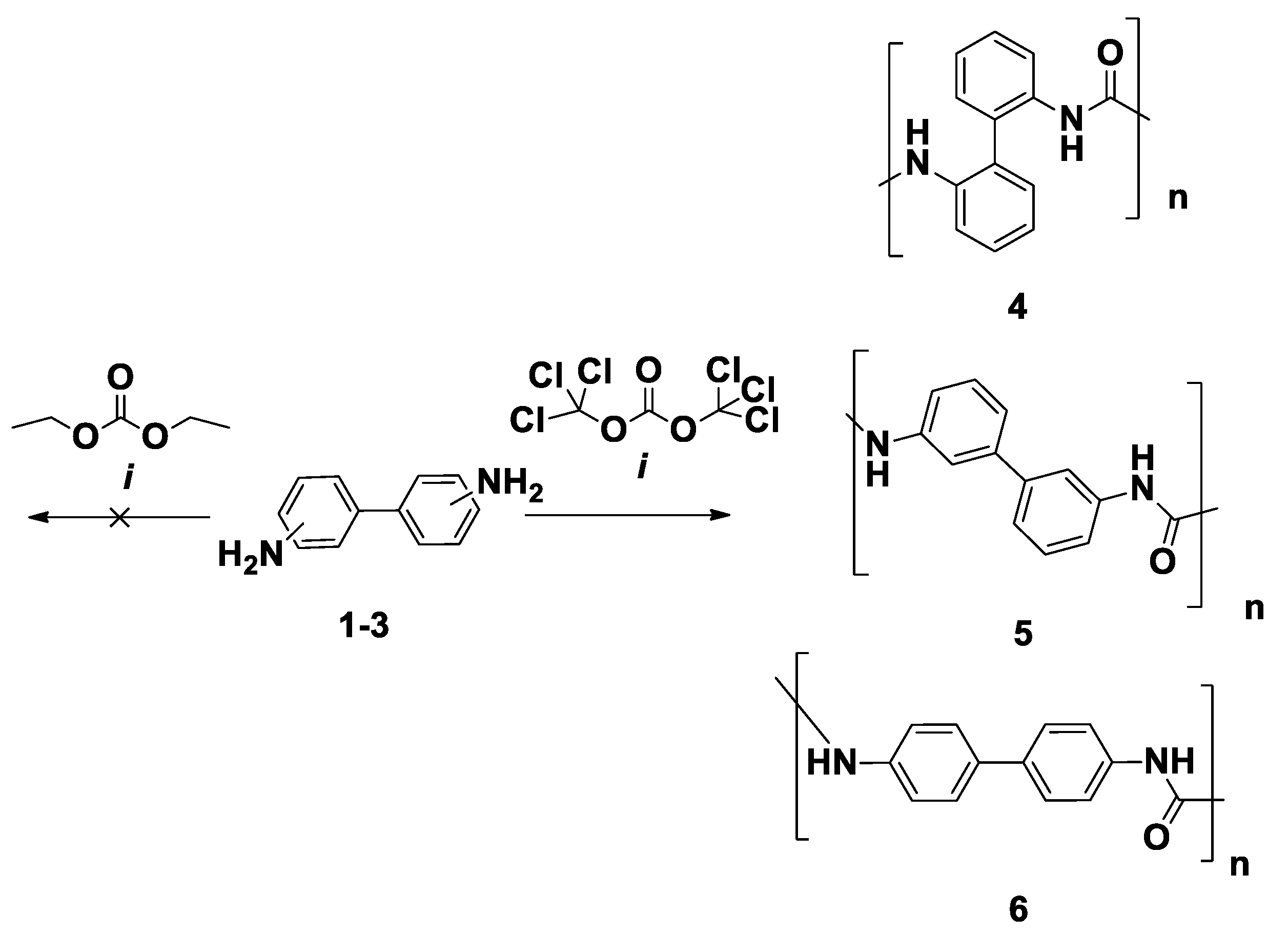
2.1. General Method for the Synthesis of Polyureas 4–6
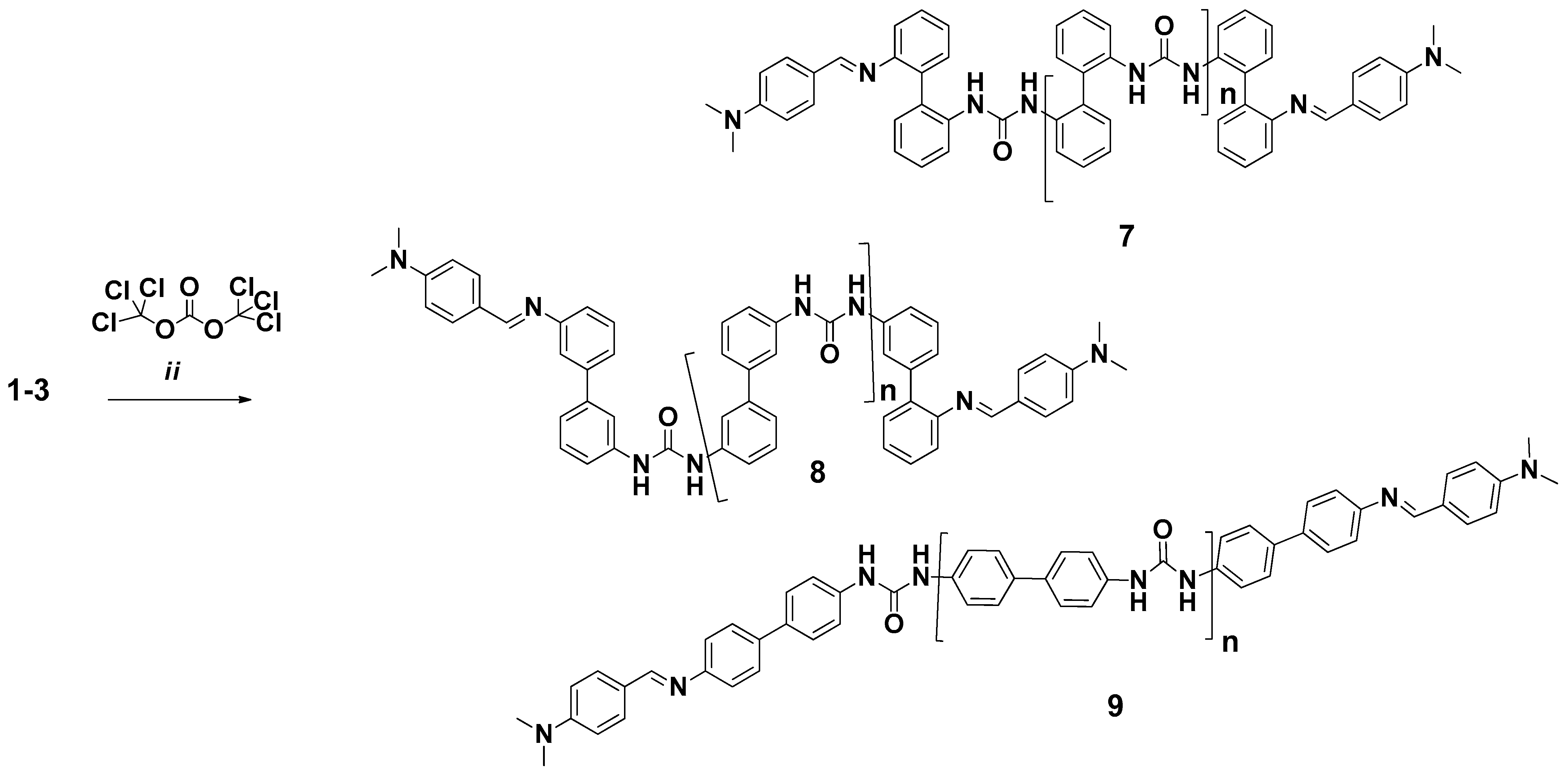
2.2. General Method for the Synthesis of Polyureas 7–9
2.3. UV/Vis and Fluorescent Titration of the PUs 4 and 7 with TBAOH and TBAF
3. Results and Discussion
3.1. Mechanosynthesis of the PUs
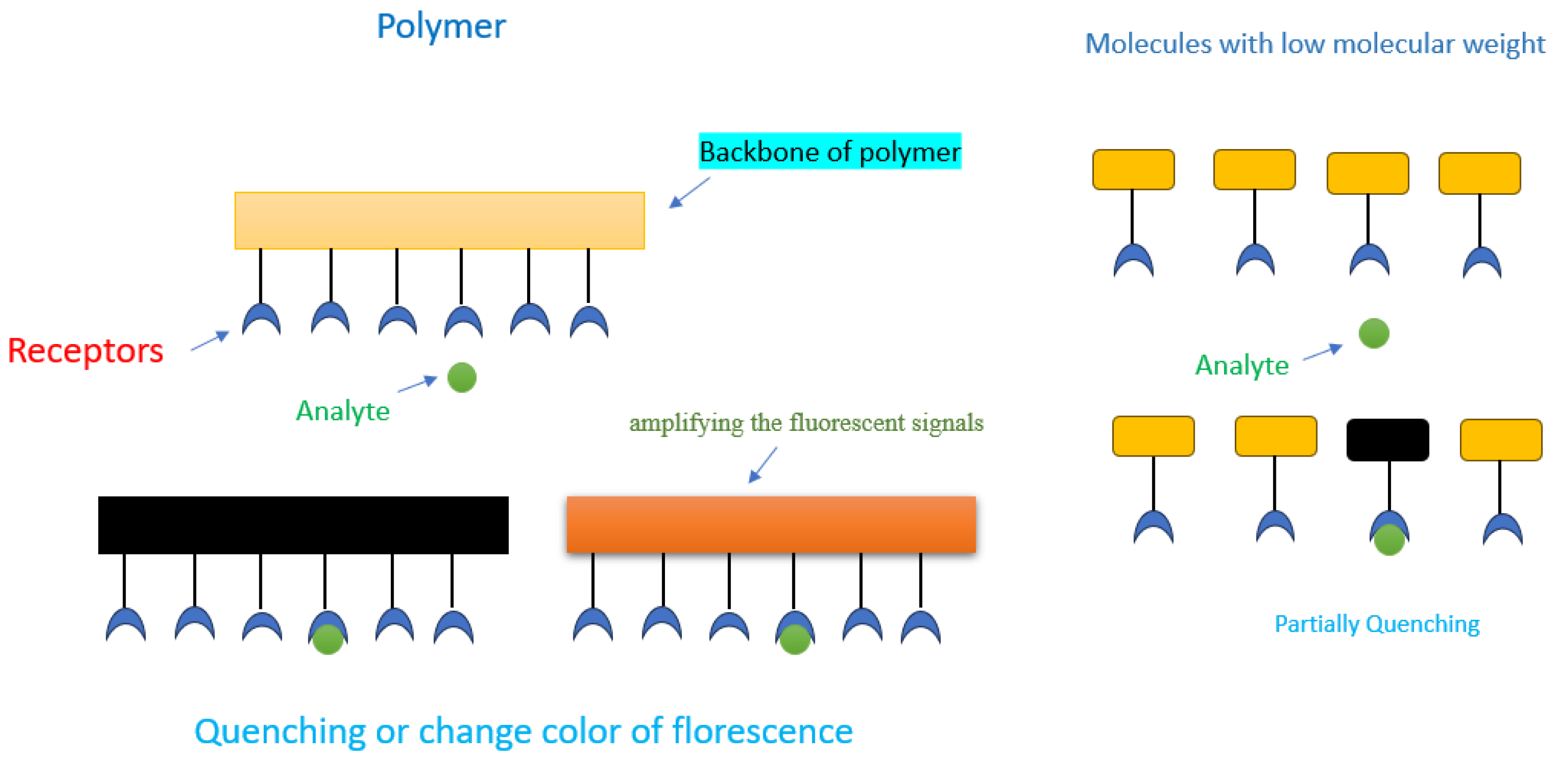
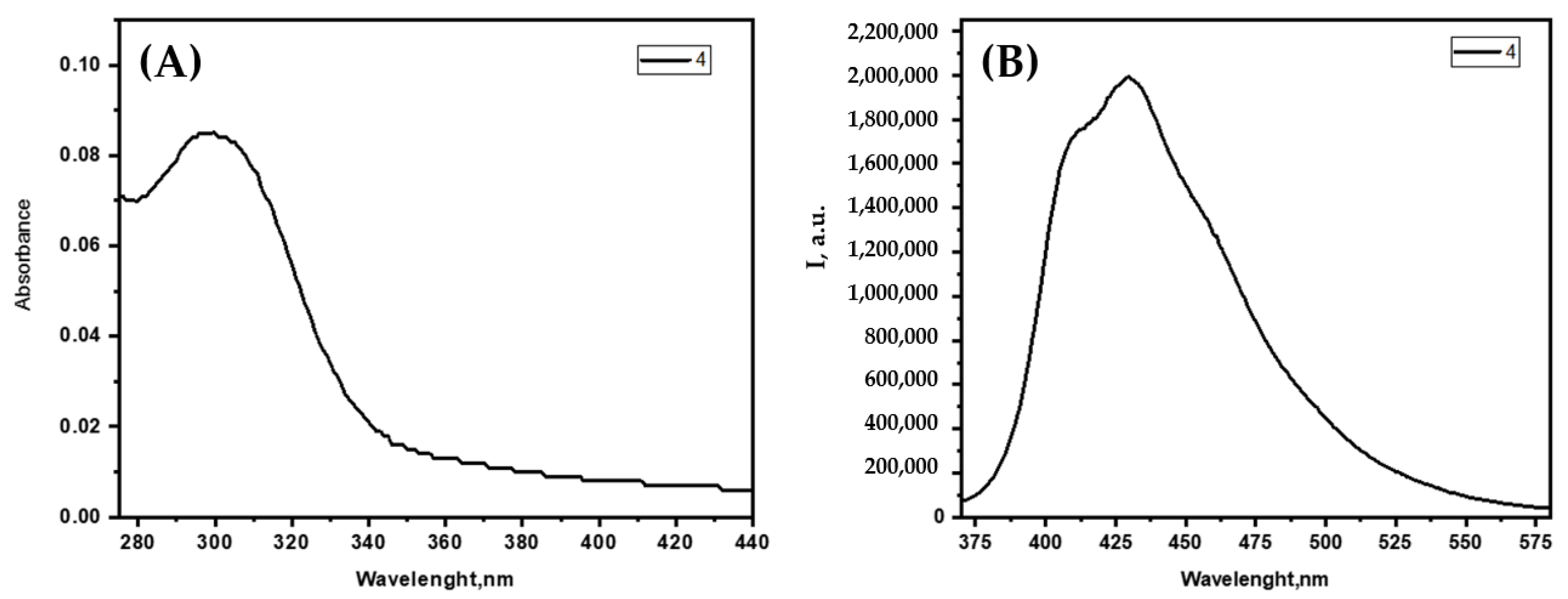
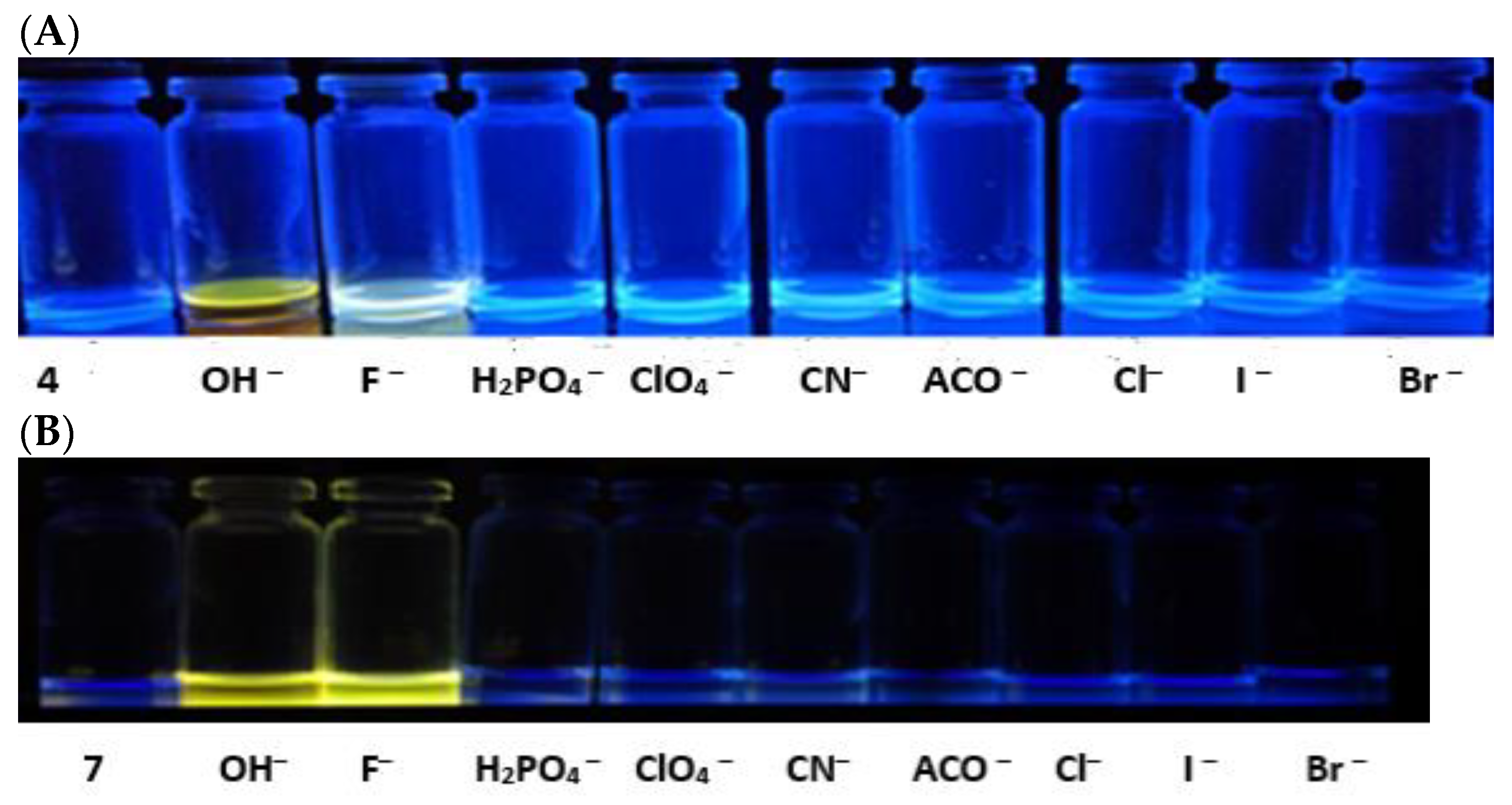
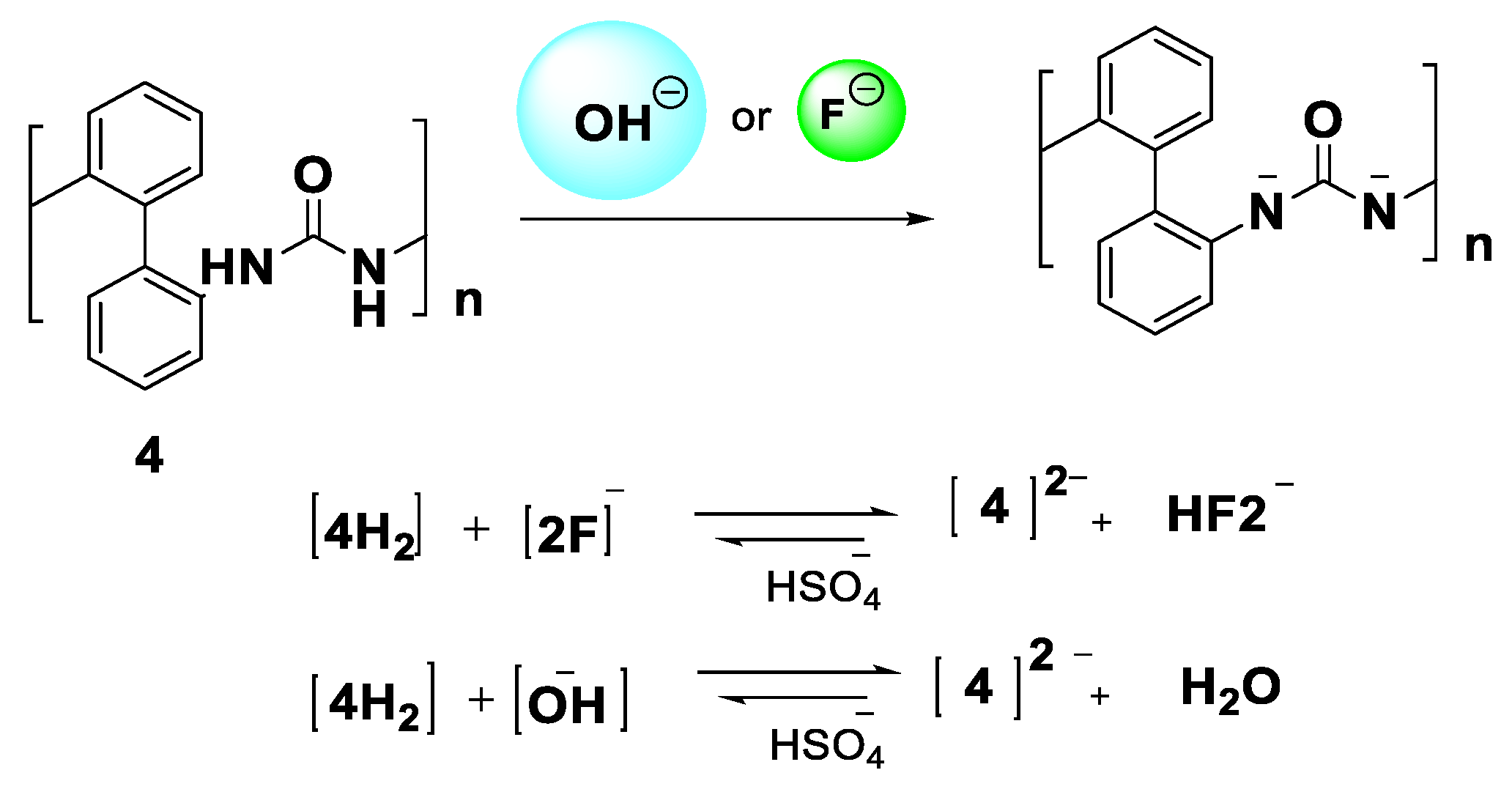
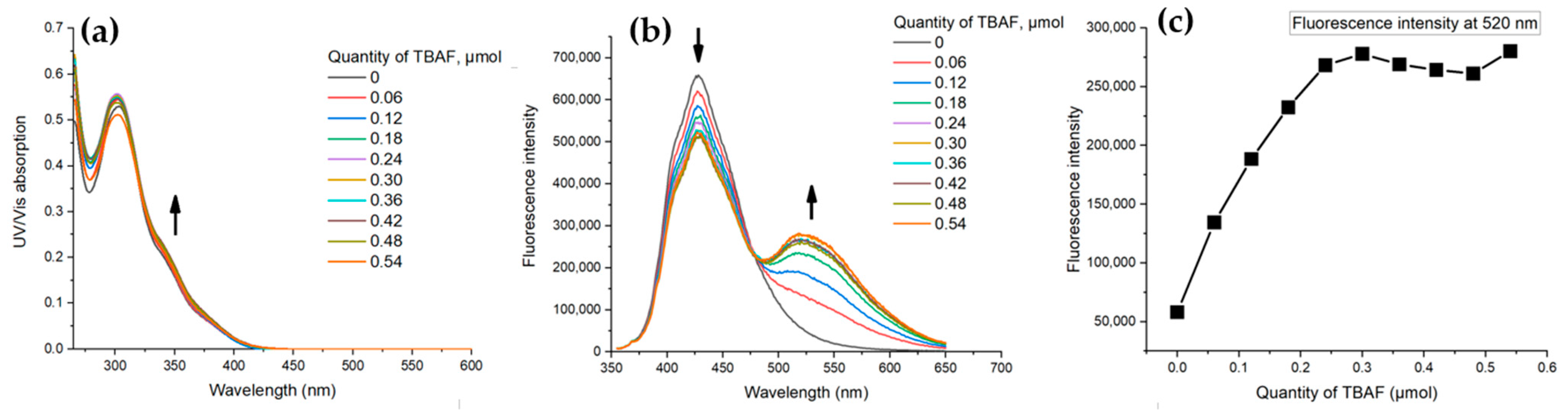
3.2. Photophysical Studies of the PUs and Their Response to Anions
3.3. DFT Studies
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Stolle, A.; Szuppa, T.; Leonhardt, S.E.S.; Ondruschka, B. Ball milling in organic synthesis: Solutions and challenges. Chem. Soc. Rev. 2011, 40, 2317–2329. [Google Scholar] [CrossRef]
- Egorov, I.N.; Santra, S.; Kopchuk, D.S.; Kovalev, I.S.; Zyryanov, G.V.; Majee, A.; Ranu, B.C.; Rusinov, V.L.; Chupakhin, O.N. Ball milling: An efficient and green approach for asymmetric organic syntheses. Green Chem. 2020, 22, 302–315. [Google Scholar] [CrossRef]
- Do, J.-L.; Friščić, T. Mechanochemistry: A Force of Synthesis. ACS Cent. Sci. 2017, 3, 13–19. [Google Scholar] [CrossRef]
- Rivas, M.E. Ball Milling Towards Green Synthesis: Applications, Projects, Challenges. Johns. Matthey Technol. Rev. 2016, 60, 148–150. [Google Scholar] [CrossRef]
- Krusenbaum, A.; Grätz, S.; Tigineh, G.T.; Borchardt, L.; Kim, J.G. The mechanochemical synthesis of polymers. Chem. Soc. Rev. 2022, 51, 2873–2905. [Google Scholar] [CrossRef]
- Al-Ithawi, W.K.A.; Khasanov, A.F.; Kovalev, I.S.; Nikonov, I.L.; Platonov, V.A.; Kopchuk, D.S.; Santra, S.; Zyryanov, G.V.; Ranu, B.C. TM-Free and TM-Catalyzed Mechanosynthesis of Functional Polymers. Polymers 2023, 15, 1853. [Google Scholar] [CrossRef]
- Mottillo, C.; Friščić, T. Advances in Solid-State Transformations of Coordination Bonds: From the Ball Mill to the Aging Chamber. Molecules 2017, 22, 144. [Google Scholar] [CrossRef]
- Park, J.; Murayama, S.; Osaki, M.; Yamaguchi, H.; Harada, A.; Matsuba, G.; Takashima, Y. Extremely Rapid Self-Healable and Recyclable Supramolecular Materials through Planetary Ball Milling and Host–Guest Interactions. Adv. Mater. 2020, 32, 2002008. [Google Scholar] [CrossRef]
- Bustamante, C.M.; Scherlis, D.A. Doping and coupling strength in molecular conductors: Polyacetylene as a case study. Phys. Chem. Chem. Phys. 2021, 23, 26974–26980. [Google Scholar] [CrossRef]
- Banerjee, J.; Dutta, K. A short overview on the synthesis, properties and major applications of poly(p-phenylene vinylene). Chem. Pap. 2021, 75, 5139–5151. [Google Scholar] [CrossRef]
- Gnida, P.; Amin, F.; Pająk, A.K.; Jarząbek, B. Polymers in High-Efficiency Solar Cells: The Latest Reports. Polymers 2022, 14, 1946. [Google Scholar] [CrossRef] [PubMed]
- Lage-Rivera, S.; Ares-Pernas, A.; Abad, M.-J. Last developments in polymers for wearable energy storage devices. Int. J. Energy Res. 2022, 46, 10475–10498. [Google Scholar] [CrossRef]
- Cousins, K.; Zhang, R. Highly Porous Organic Polymers for Hydrogen Fuel Storage. Polymers 2019, 11, 690. [Google Scholar] [CrossRef]
- Lasseuguette, E.; Comesaña-Gándara, B. Polymer Membranes for Gas Separation. Membranes 2022, 12, 207. [Google Scholar] [CrossRef]
- Sapurina, I.Y.; Shishov, M.A.; Ivanova, V.T. Sorbents for water purification based on conjugated polymers. Russ. Chem. Rev. 2020, 89, 1115. [Google Scholar] [CrossRef]
- Zhou, H.; Chua, M.H.; Zhu, Q.; Xu, J. AIE-Active Polymers for Explosive Detection. In Aggregation-Induced Emission (AIE); Elsevier: Amsterdam, The Netherlands, 2022; pp. 555–582. [Google Scholar]
- Wu, X.; Tong, H.; Wang, L. Fluorescent Polymer Materials for Detection of Explosives. Prog. Chem. 2019, 31, 1509–1527. [Google Scholar]
- Mu, J.; He, L.; Huang, P.; Chen, X. Engineering of nanoscale coordination polymers with biomolecules for advanced applications. Coord. Chem. Rev. 2019, 399, 213039. [Google Scholar] [CrossRef]
- Ishihara, K.; Hachiya, S.; Inoue, Y.; Fukazawa, K.; Konno, T. Water-Soluble and Cytocompatible Phospholipid Polymers for Molecular Complexation to Enhance Biomolecule Transportation to Cells In Vitro. Polymers 2020, 12, 1762. [Google Scholar] [CrossRef]
- Ye, L.; Mosbach, K. Polymers Recognizing Biomolecules Based on a Combination of Molecular Imprinting and Proximity Scintillation: A New Sensor Concept. J. Am. Chem. Soc. 2001, 123, 2901–2902. [Google Scholar] [CrossRef]
- Sung, Y.K.; Kim, S.W. Recent advances in polymeric drug delivery systems. Biomater. Res. 2020, 24, 12. [Google Scholar] [CrossRef]
- Borandeh, S.; van Bochove, B.; Teotia, A.; Seppälä, J. Polymeric drug delivery systems by additive manufacturing. Adv. Drug Deliver. Rev. 2021, 173, 349–373. [Google Scholar] [CrossRef]
- Beer, P.D.; Schmitt, P. Molecular recognition of anions by synthetic receptors. Curr. Opin. Chem. Biol. 1997, 1, 475–482. [Google Scholar] [CrossRef]
- Tobey, S.L.; Anslyn, E.V. Synthetic Receptors for Anion Recognition. In Fundamentals and Applications of Anion Separations; Moyer, B.A., Singh, R.P., Eds.; Springer: Boston, MA, USA, 2004. [Google Scholar] [CrossRef]
- Kubik, S. Chapter 4. Synthetic Receptors for Small Organic and Inorganic Anions. In Synthetic Receptors for Biomolecules: Design Principles and Applications; Royal Society of Chemistry: London, UK, 2015; pp. 129–176. [Google Scholar] [CrossRef]
- Delecluse, M.; Colomban, C.; Chatelet, B.; Chevallier-Michaud, S.; Moraleda, D.; Dutasta, J.-P.; Martinez, A. Highly Selective Fluoride Recognition by a Small Tris-Urea Covalent Cage. J. Org. Chem. 2020, 85, 4706–4711. [Google Scholar] [CrossRef] [PubMed]
- Kundu, S.; Egboluche, T.K.; Hossain, M.A. Urea- and Thiourea-Based Receptors for Anion Binding. Acc. Chem. Res. 2023, 56, 1320–1329. [Google Scholar] [CrossRef] [PubMed]
- Schiller, J.; Pérez-Ruiz, R.; Sampedro, D.; Marqués-López, E.; Herrera, R.P.; Díaz, D.D. Fluoride Anion Recognition by a Multifunctional Urea Derivative: An Experimental and Theoretical Study. Sensors 2016, 16, 658. [Google Scholar] [CrossRef]
- Biswas, S.; Gangopadhyay, M.; Barman, S.; Sarkar, J. Simple and efficient coumarin-based colorimetric and fluorescent chemosensor for F− detection: An ON1–OFF–ON2 fluorescent assay. Sens. Actuators B Chem. 2016, 222, 823–828. [Google Scholar] [CrossRef]
- Kim, W.; Sahoo, S.K.; Kim, G.-D.; Choi, H.-J. Novel C3V-symmetric trindane based tripodal anion receptor with tris(coumarin-urea) extension for optical sensing of bioactive anions. Tetrahedron 2015, 71, 8111–8116. [Google Scholar] [CrossRef]
- Bregovic, V.B.; Halasz, I.; Basaric, N.; Mlinaric-Majerski, K. Anthracene adamantylbisurea receptors: Switching of anion binding by photocyclization. Tetrahedron 2015, 71, 9321–9327. [Google Scholar] [CrossRef]
- Bregović, V.B.; Basarić, N.; Mlinarić-Majerski, K. Anion binding with urea and thiourea derivatives. Coord. Chem. Rev. 2015, 295, 80–124. [Google Scholar] [CrossRef]
- Stepniak, P.; Lainer, B.; Chmurski, K.; Jurczak, J. pH-Controlled recognition of amino acids by urea derivatives of β-cyclodextrin. RSC Adv. 2017, 7, 15742–15746. [Google Scholar] [CrossRef]
- Niedbała, P.; Majdecki, M.; Dąbrowa, K.; Jurczak, J. Selective Carboxylate Recognition Using Urea-Functionalized Unclosed Cryptands: Mild Synthesis and Complexation Studies. J. Org. Chem. 2020, 85, 5058–5064. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Minami, T.; Nishiyabu, R.; Wang, Z.; Anzenbacher, P., Jr. Sensing of Carboxylate Drugs in Urine by a Supramolecular Sensor Array. J. Am. Chem. Soc. 2013, 135, 7705–7712. [Google Scholar] [CrossRef]
- Nguyen, T.B.T.; Le, N.L.; Pham, H.C.; Van Do, T.; Tran, H.N.; Nguyen, D.K. Research and development of polyurea pavement marking paint. Phy. Sci. 2017, 59, 21–25. [Google Scholar] [CrossRef] [PubMed]
- Yang, H.; Du, G.; Li, Z.; Ran, X.; Zhou, X.; Li, T.; Gao, W.; Li, J.; Lei, H.; Yang, L. Superstrong Adhesive of Isocyanate-Free Polyurea with a Branched Structure. ACS Appl. Polym. Mater. 2021, 3, 1638–1651. [Google Scholar] [CrossRef]
- Do, S.; Huynh, N.U.; Reed, N.; Shaik, A.M.; Nacy, S.; Youssef, G. Partially-Perforated Self-Reinforced Polyurea Foams. Appl. Sci. 2020, 10, 5869. [Google Scholar] [CrossRef]
- Wang, C.; Luo, Y.; Cao, X.; Li, B.; Luo, Z. Supramolecular polyurea hydrogels with anti-swelling capacity, reversible thermochromic properties, and tunable water content and mechanical performance. Polymer 2021, 233, 124213. [Google Scholar] [CrossRef]
- Sears, N.A.; Pena-Galea, G.; Cereceres, S.N.; Cosgriff-Hernandez, E. Hybrid polyurea elastomers with enzymatic degradation and tunable mechanical properties. J. Tissue Eng. 2016, 7, 2041731416679363. [Google Scholar] [CrossRef] [PubMed]
- Zhou, Y.; Fan, F.; Zhao, J.; Wang, Z.; Wang, R.; Zheng, Y.; Liu, H.; Peng, C.; Li, J.; Tan, H.; et al. Intrinsically fluorescent polyureas toward conformation-assisted metamorphosis, discoloration and intracellular drug delivery. Nat. Commun. 2022, 13, 4551. [Google Scholar] [CrossRef]
- Cho, Y.L.; Rudkevich, D.M.; Rebek, J. Expanded Calix[4]arene Tetraurea Capsules. J. Am. Chem. Soc. 2000, 122, 9868–9869. [Google Scholar] [CrossRef]
- Gaur, S.S.; Edgehouse, K.J.; Klemm, A.; Wei, P.; Gurkan, B.; Pentzer, E.B. Capsules with polyurea shells and ionic liquid cores for CO2 capture. J. Polym. Sci. 2021, 59, 2980–2989. [Google Scholar] [CrossRef]
- Rao, J.; Chandrani, A.M.; Powar, A.; Chandra, S. Design and application of polyurea microcapsules containing herbicide (oxyfluorfen). Des Monomers Polym. 2020, 23, 155–163. [Google Scholar] [CrossRef] [PubMed]
- Rao, J.; Chandrani, A.M.; Powar, A.; Chandra, S. Release behavior of oxyfluorfen polyurea capsules prepared using PVA and kraft lignin as emulsifying agents and phytotoxicity study on paddy. Green Chem. Lett. Rev. 2021, 14, 204–220. [Google Scholar] [CrossRef]
- Ramarao, C.; Ley, S.V.; Smith, S.C.; Shirley, I.M.; DeAlmeida, N. Encapsulation of palladium in polyurea microcapsules. Chem. Commun. 2002, 21, 1132–1133. [Google Scholar] [CrossRef] [PubMed]
- Ley, S.V.; Ramarao, C.; Lee, A.-L.; Østergaard, N.; Smith, S.C.; Shirley, I.M. Microencapsulation of Osmium Tetroxide in Polyurea. Org. Lett. 2003, 5, 185–187. [Google Scholar] [CrossRef]
- Custelcean, R.; Remy, P. Selective Crystallization of Urea-Functionalized Capsules with Tunable Anion-Binding Cavities. Cryst. Growth Des. 2009, 9, 1985–1989. [Google Scholar] [CrossRef]
- Yokoya, M.; Kimura, S.; Yamanaka, M. Urea Derivatives as Functional Molecules: Supramolecular Capsules, Supramolecular Polymers, Supramolecular Gels, Artificial Hosts, and Catalysts. Chem. Eur. J. 2020, 27, 5601–5614. [Google Scholar] [CrossRef]
- Persaud, K.C. Polymers for Chemical Sensing. Mater. Today 2005, 8, 38–44. [Google Scholar] [CrossRef]
- Sakai, R. Conjugated Polymers Applicable to Colorimetric and Fluorescent Anion Detection. Polym. J. 2016, 48, 59–65. [Google Scholar] [CrossRef]
- Sakai, R.; Sakai, N.; Satoh, T.; Li, W.; Zhang, A.; Kakuchi, T. Strict Size Specificity in Colorimetric Anion Detection Based on Poly(Phenylacetylene) Receptor Bearing Second Generation Lysine Dendrons. Macromolecules 2011, 44, 4249–4257. [Google Scholar] [CrossRef]
- McQuade, D.T.; Pullen, A.E.; Swager, T.M. Conjugated Polymer-Based Chemical Sensors. Chem. Rev. 2000, 100, 2537–2574. [Google Scholar] [CrossRef]
- Anantha-Iyengar, G.; Shanmugasundaram, K.; Nallal, M.; Lee, K.-P.; Whitcombe, M.J.; Lakshmi, D.; Sai-Anand, G. Functionalized Conjugated Polymers for Sensing and Molecular Imprinting Applications. Prog. Polym. Sci. 2019, 88, 1–129. [Google Scholar] [CrossRef]
- Wang, T.; Zhang, N.; Bai, W.; Bao, Y. Fluorescent Chemosensors Based on Conjugated Polymers with N-Heterocyclic Moieties: Two Decades of Progress. Polym. Chem. 2020, 11, 3095–3114. [Google Scholar] [CrossRef]
- Swager, T.M. The Molecular Wire Approach to Sensory Signal Amplification. Acc. Chem. Res. 1998, 31, 201–207. [Google Scholar] [CrossRef]
- Zhou, Q.; Swager, T.M. Fluorescent Chemosensors Based on Energy Migration in Conjugated Polymers: The Molecular Wire Approach to Increased Sensitivity. J. Am. Chem. Soc. 1995, 117, 12593–12602. [Google Scholar] [CrossRef]
- Goggins, S.; Frost, C.G. Approaches towards Molecular Amplification for Sensing. Analyst 2016, 141, 3157–3218. [Google Scholar] [CrossRef]
- Zhao, P.; Jiang, J.; Leng, B.; Tian, H. Polymer Fluoride Sensors Synthesized by RAFT Polymerization. Macromol. Rapid Commun. 2009, 30, 1715–1718. [Google Scholar] [CrossRef]
- Yoon, Y.; Jo, S.; Lee, D.H.; Lee, T.S. Synthesis of Fluorescent, Ortho-Azonaphthol-Containing Conjugated Polymer for Ratiometric Fluoride Ion Sensing. Polymer 2022, 261, 125421. [Google Scholar] [CrossRef]
- Qu, Y.; Wu, Y.; Gao, Y.; Qu, S.; Yang, L.; Hua, J. Diketopyrrolopyrrole-Based Fluorescent Conjugated Polymer for Application of Sensing Fluoride Ion and Bioimaging. Sens. Actuators B Chem. 2014, 197, 13–19. [Google Scholar] [CrossRef]
- Kakuchi, R.; Nagata, S.; Sakai, R.; Otsuka, I.; Nakade, H.; Satoh, T.; Kakuchi, T. Size-Specific, Colorimetric Detection of Counteranions by Using Helical Poly(Phenylacetylene) Conjugated to L-Leucine Groups through Urea Acceptors. Chem. Eur. J. 2008, 14, 10259–10266. [Google Scholar] [CrossRef]
- Al-Ithawi, W.K.A.; Khasanov, A.F.; Kovalev, I.S.; Nikonov, I.L.; Kopchuk, D.S.; Platonov, V.A.; Santra, S.; Zyryanov, G.V.; Ranu, B.C. Mechanosynthesis of Diaminobiphenyls-Based Schiff’s Bases as Simple Probes for the Naked-Eye Detection of Cyanide Ion. Chemistry 2023, 5, 978–986. [Google Scholar] [CrossRef]
- Blood, A.; Noller, C. Notes—Preparation of 2,2′-Diaminobiphenyl by Reduction of 2,2′-Dinitrobiphenyl. J. Org. Chem. 1957, 22, 711–712. [Google Scholar] [CrossRef]
- Shrivastava, A.; Gupta, V. Methods for the Determination of Limit of Detection and Limit of Quantitation of the Analytical Methods. Chron. Young Sci. 2011, 2, 21. [Google Scholar] [CrossRef]
- Wöhler, F. “Ueber künstliche Bildung des Harnstoffs” (On the artificial formation of urea). Ann. Phys. Chem. 1828, 88, 253–256. [Google Scholar] [CrossRef]
- Nicolaou, K.C.; Montagnon, T. Molecules That Changed The World; Wiley-VCH: Hoboken, NJ, USA, 2008; p. 11. ISBN 978-3-527-30983-2. [Google Scholar]
- Gibb, B.C. Teetering towards chaos and complexity. Nat. Chem. 2009, 1, 17–18. [Google Scholar] [CrossRef] [PubMed]
- Shorter, J. The conversion of ammonium cyanate into urea—A saga in reaction mechanisms. Chem. Soc. Rev. 1978, 7, 1–14. [Google Scholar] [CrossRef]
- Hanson, D.S.; Wang, Y.; Zhou, X.; Washburn, E.; Ekmekci, M.B.; Dennis, D.; Paripati, A.; Xiao, D.; Zhou, M. Catalytic Urea Synthesis from Ammonium Carbamate Using a Copper(II) Complex: A Combined Experimental and Theoretical Study. Inorg. Chem. 2021, 60, 5573–5589. [Google Scholar] [CrossRef]
- Barzagli, F.; Mani, F.; Peruzzini, M. From Greenhouse Gas to Feedstock: Formation of Ammonium Carbamate from CO2 and NH3 in Organic Solvents and Its Catalytic Conversion into Urea under Mild Conditions. Green Chem. 2011, 13, 1267–1274. [Google Scholar] [CrossRef]
- Barzagli, F.; Mani, F.; Peruzzini, M. Carbon Dioxide Uptake as Ammonia and Amine Carbamates and Their Efficient Conversion into Urea and 1,3-Disubstituted Ureas. J. CO2 Util. 2016, 13, 81–89. [Google Scholar] [CrossRef]
- Yadav, D.K.; Yadav, A.K.; Srivastava, V.P.; Watal, G.; Yadav, L.S. Bromodimethylsulfonium bromide (BDMS)-mediated Lossen rearrangement: Synthesis of unsymmetrical ureas. Tetrahedron Lett. 2012, 53, 2890–2893. [Google Scholar] [CrossRef]
- Stocks, S.; Marin, N.G. A simple and efficient synthesis of N-benzoyl ureas. Tetrahedron Lett. 2012, 53, 4802–4804. [Google Scholar] [CrossRef]
- Guan, Z.H.; Lei, H.; Chen, M.; Ren, Z.H.; Bai, Y.J.; Wang, Y.Y. Palladium-Catalyzed Carbonylation of Amines: Switchable Approaches to Carbamates and N,N′-Disubstituted Ureas. Adv. Synth. Catal. 2012, 354, 489–496. [Google Scholar] [CrossRef]
- Vinogradova, E.V.; Fors, B.P.; Buchwald, S.L. Palladium-catalyzed cross-coupling of aryl chlorides and triflates with sodium cyanate: A practical synthesis of unsymmetrical ureas. J. Am. Chem. Soc. 2012, 134, 11132–11135. [Google Scholar] [CrossRef]
- Wang, P.; Ma, X.; Li, Q.; Yang, B.; Shang, J.; Deng, Y. Green synthesis of polyureas from CO2 and diamines with a functional ionic liquid as the catalyst. RSC Adv. 2016, 6, 54013–54019. [Google Scholar] [CrossRef]
- Chakraborty, S. Detection of Fluoride Ion by Chemosensing and Fluorosensing Technology. Results Chem. 2023, 6, 100994. [Google Scholar] [CrossRef]
- Esteban-Gómez, D.; Fabbrizzi, L.; Licchelli, M. Why, on interaction of urea-based receptors with fluoride, beautiful colors develop. J. Org. Chem. 2005, 70, 5717–5720. [Google Scholar] [CrossRef]
- Bridges, J.W.; Creaven, P.J.; Williamsin, R.T. The fluorescence of some biphenyl derivatives. Biochem. J. 1965, 96, 872–878. [Google Scholar] [CrossRef]
- Grguric, T.; Cetina, M.; Petroselli, M.; Bacchiocchi, C.; Dzolić, Z.; Camett, M. Anion binding with biphenyl-bis-urea derivatives: Solution and solid-state studies. New J. Chem. 2020, 44, 16294. [Google Scholar] [CrossRef]
- Long, J.; Shan, J.; Zhao, Y.; Ji, Y.; Tan, H.; Wan, H. Dramatically enhanced and red-shifted photoluminescence achieved by introducing an electron-withdrawing group into a non-traditional luminescent small organic compound. Chem. Asian J. 2021, 16, 2370–2567. [Google Scholar] [CrossRef] [PubMed]
- Albrecht, M.; Zauner, J.; Burgert, R.; Röttele, H.; Fröhlich, R. Synthesis of tweezer-type receptors for the recognition of anions: Observation of an additive effect of hydrogen bonds on nitrate binding. Mater. Sci. Eng. C 2001, 18, 185–190. [Google Scholar] [CrossRef]
- Otal, E.H.; Kim, M.L.; Kimura, M. Fluoride Detection and Quantification, an Overview from Traditional to Innovative Material-Based Methods. In Fluoride; IntechOpen: London, UK, 2022. [Google Scholar] [CrossRef]
- Meng, W.-Q.; Sedgwick, A.C.; Kwon, N.; Sun, M.; Xiao, K.; He, X.-P.; Anslyn, E.V.; James, T.D.; Yoon, J. Fluorescent probes for the detection of chemical warfare agents. Chem. Soc. Rev. 2023, 52, 601–662. [Google Scholar] [CrossRef]
- Frisch, M.J.; Trucks, G.W.; Schlegel, H.B.; Scuseria, G.E.; Robb, M.A.; Cheeseman, J.R.; Scalmani, G.; Barone, V.; Petersson, G.A.; Nakatsuji, H.; et al. Gaussian 09, Revision C.01; Gaussian, Inc.: Wallingford, CT, USA, 2010. [Google Scholar]


| PUs | 7 | 8 | 9 |
|---|---|---|---|
| Mn, Da | 15,448 | 7453 | 12,192 |
| Entry | Model Supramolecular Association Process | ΔG, kcal/mol |
|---|---|---|
| PU 4 | ||
| 1 | ACO− + PU4 → PU4⋯AcO− | −29 |
| 2 | Br− + PU4 → PU4⋯Br− | −19 |
| 3 | Cl− + PU4 → PU4⋯Cl− | −25 |
| 4 | ClO4− + PU4 → PU4⋯ClO4− | −10 |
| 5 | CN− + PU4 → PU4⋯CN− | −25 |
| 6 | F− + PU4 → PU4⋯F− | −56 |
| 7 | H2PO4− + PU4 → PU4⋯H2PO4− | −21 |
| 8 | I− + PU4 → PU4⋯I− | −15 |
| 9 | OH− + PU4 → PU4⋯OH− | −61 |
| PU 5 | ||
| 10 | ACO− + PU5 → PU5⋯AcO− | −35 |
| 11 | Br− + PU5 → PU5⋯Br− | −25 |
| 12 | Cl− + PU5 → PU5⋯Cl− | −31 |
| 13 | ClO4− + PU5 → PU5⋯ClO4− | −13 |
| 14 | CN− + PU5 → PU5⋯CN− | −31 |
| 15 | F− + PU5 → PU5⋯F− | −61 |
| 16 | H2PO4− + PU5 → PU5⋯H2PO4− | −25 |
| 17 | I− + PU5 → PU5⋯I− | −21 |
| 18 | OH− + PU5 → PU5⋯OH− | −67 |
| PU 6 | ||
| 19 | ACO− + PU6 → PU6⋯AcO− | −36 |
| 20 | Br− + PU6 → PU6⋯Br− | −25 |
| 21 | Cl− + PU6 → PU6⋯Cl− | −31 |
| 22 | ClO4− + PU6 → PU6⋯ClO4− | −14 |
| 23 | CN− + PU6 → PU6⋯CN− | −32 |
| 24 | F− + PU6 → PU6⋯F− | −61 |
| 25 | H2PO4− + PU6 → PU6⋯H2PO4− | −28 |
| 26 | I− + PU6 → PU6⋯I− | −20 |
| 27 | OH− + PU6 → PU6⋯OH− | −67 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Al-Ithawi, W.K.A.; Aluru, R.; Baklykov, A.V.; Khasanov, A.F.; Kovalev, I.S.; Nikonov, I.L.; Kopchuk, D.S.; Novikov, A.S.; Santra, S.; Zyryanov, G.V.; et al. Mechanosynthesis of Polyureas and Studies of Their Responses to Anions. Polymers 2023, 15, 4160. https://doi.org/10.3390/polym15204160
Al-Ithawi WKA, Aluru R, Baklykov AV, Khasanov AF, Kovalev IS, Nikonov IL, Kopchuk DS, Novikov AS, Santra S, Zyryanov GV, et al. Mechanosynthesis of Polyureas and Studies of Their Responses to Anions. Polymers. 2023; 15(20):4160. https://doi.org/10.3390/polym15204160
Chicago/Turabian StyleAl-Ithawi, Wahab K. A., Rammohan Aluru, Artem V. Baklykov, Albert F. Khasanov, Igor S. Kovalev, Igor L. Nikonov, Dmitry S. Kopchuk, Alexander S. Novikov, Sougata Santra, Grigory V. Zyryanov, and et al. 2023. "Mechanosynthesis of Polyureas and Studies of Their Responses to Anions" Polymers 15, no. 20: 4160. https://doi.org/10.3390/polym15204160
APA StyleAl-Ithawi, W. K. A., Aluru, R., Baklykov, A. V., Khasanov, A. F., Kovalev, I. S., Nikonov, I. L., Kopchuk, D. S., Novikov, A. S., Santra, S., Zyryanov, G. V., & Ranu, B. C. (2023). Mechanosynthesis of Polyureas and Studies of Their Responses to Anions. Polymers, 15(20), 4160. https://doi.org/10.3390/polym15204160









