1. Introduction
Textile dyeing is one of the most important processes in the textile industry to produce a value-added aesthetic appeal of textile products for human consumption. The conventional textile dyeing process, however, poses an undesirable threat to the environment, in which it consumes a million tons of water while it generates substantial effluent discharges containing residual dyes, chemicals, salts and alkalis with high pH, BOD and COD values [
1,
2,
3].
To address the environmental concerns from the public, different sustainable and novel dyeing approaches for cotton fabric have been found in the literature. Periyasamy [
4] and Jabar et al. [
5] used bio-mordant and natural dye from syzygium cumini fruit extracts and mangiferin for sustainable dyeing of cotton fabric, respectively. Supercritical carbon dioxide has been applied as a medium for dyeing of cotton fabric with newly synthesised dye [
6,
7]. Pei et al. [
8] reported the use of a silicon emulsion system for non-aqueous salt-free dyeing of cotton. Wei et al. [
9] investigated the use of hydrophobic deep eutectic solvent (HDES) composed of thymol and menthol for dyeing of cotton. Attempts have also been made to chemically modify cotton substrates for salt-free dyeing with anionic dyes [
10,
11]. In addition, Mamun Kabir et al. [
12] examined the use of dioctyl sodium sulfosuccinate surfactant for low-liquor-ratio dyeing of cotton.
Apart from those methods, the use of surfactant as a building block for reverse micelle formation as a reactive dye carrier in non-aqueous solvent medium is also one of the promising ways to achieve a salt-free and water-saving approach for dyeing of cotton fabric [
13]. Surfactant, also known as surface active agent, is an amphiphilic molecule comprising two distinct structural moieties in which one is polar (hydrophilic) while another is nonpolar (hydrophobic). Owing to its amphiphilic property, it may either be incorporated as micelle in aqueous phase or self-assembled as reverse micelles in nonpolar oil phase once when the surfactant concentration is above the critical micelle concentration (CMC) [
14,
15].
Reverse micelles are nano-spherical aggregates self-assembled by surfactants in non-aqueous medium with the ability to solubilise a small amount of water, forming a water pool in their interior region [
16]. They were first applied in cotton dyeing with reactive dye by the use of anionic surfactant, Aerosol-OT, as their building block [
17]. However, the uneven aqueous microenvironment caused by the ionic functional groups of anionic surfactant shifts the selection of surfactant towards non-ionic in nature. Yi et al. [
18] and Yi et al. [
19] then reported the use of non-ionic surfactant, Triton X-100, for reverse micelle formation. Nevertheless, due to the presence of the aromatic group and poor biodegradability of Triton X-100, it has been regarded as an environmentally unfriendly surfactant which is forbidden, especially in European countries [
20,
21].
Our previous works focused on the use of non-ionic polyethylene glycol (PEG)-based [
22] and alkyl polyglucoside (APG)-based [
23] surfactants and rhamnolipid biosurfactant [
24] for reverse micellar dyeing of cotton fabric in a different non-aqueous solvent medium. Compared with poly(ethylene glycol) (12) tridecyl ether (PEG-12), which is a mixture of C
11 to C
14 iso-alkyl ethers, Tergitol type 15-S-12 (T15S12) is a secondary alcohol ethoxylate (SAE) with distinctive branched hydrophobic tails [
25], and it is readily biodegradable and specially created to meet the “designed to degrade” principle [
21,
26]. To the best of our knowledge, using T15S12 SAE-based biodegradable non-ionic surfactant (HLB value of 14.5) as a dye carrier for reverse micellar dyeing of cotton fabric is unknown and has not yet been explored and found in the literature.
In this study, the feasibility of using T15S12 non-ionic surfactant as a building block for reverse micelle formation and as a reactive dye carrier for dyeing of cotton fabric is investigated. Several purposes of this work include: (a) to optimise the parameters for reverse micellar dyeing of cotton fabric; (b) to compare the colour properties of the SAE-dyed fabrics with water-dyed fabrics in terms of colour yield, levelness, reflectance and CIE L*a*b* values; (c) to examine the surface damage of the SAE-dyed fabric; (d) to observe the morphology of dye-encapsulated reverse micelle formed by T15S12 surfactant; and (e) to assess the colourfastness properties of SAE-dyed fabrics.
2. Materials and Methods
2.1. Materials and Reagents
Commercially available ready-for-dyeing 100% cotton woven fabric (density: 127 ends and picks per cm; weight: 139 g/m
2) was pre-washed with 2 g/L of home laundry detergent at 49 °C for 45 min, tumble-dried and conditioned for 24 h at standard environment (20 ± 2 °C and 65 ± 2%) before subsequent experiments. Non-ionic Tergitol type 15-S-12 (T15S12) secondary alcohol ethoxylated (SAE)-based biodegradable surfactant (CAS No.: 84133-50-6) (
Figure 1) was purchased from Sigma Aldrich, St Louis, MO, USA. Octane (98+% purity) (CAS No.: 111-65-9) and n-octanol (>99% purity) (CAS No.: 111-87-5) were purchased from Alfa Aesar. They were of reagent grade. Sodium chloride (NaCl) (CAS No.: 7647-14-5) was purchased from VWR and used in a conventional water-based dyeing system only. Sodium carbonate anhydrous (soda ash, Na
2CO
3) (CAS No.: 497-19-8) was purchased from Sigma Aldrich. Warm-type reactive dyes of Levafix Red CA (RCA), Levafix Blue CA (BCA) and Levafix Yellow CA (YCA) were purchased from Dystar, China, and used as received.
2.2. Conventional Water Dyeing of Cotton Fabric
Cotton fabric was conventionally dyed in water medium with the use of NaCl and Na
2CO
3 as auxiliaries to promote exhaustion and fixation. The recipe was used as recommended by the dye supplier (
Table 1). The liquor-to-goods ratio was 50:1 to ensure the levelness of the dyed fabric. Cotton fabric was dyed according to the profile shown in
Figure 2. Cotton fabric was immersed in dye liquor composed of a relative amount of reactive dye and placed in a water bath (30 °C) for 10 min shaking. The temperature of the bath was raised to 70 °C to allow dyeing for 40 min. Na
2CO
3 was then added to the dye liquor to allow fixation for 60 min. The dyed fabric was finally rinsed with 2 g/L of detergent twice (50 °C), cold-rinsed with tap water, air-dried and conditioned in standard environment (20 ± 2) °C and (65 ± 2) % for 24 h before further measurements.
2.3. SAE-Based Reverse Micellar Dye Carrier Formation
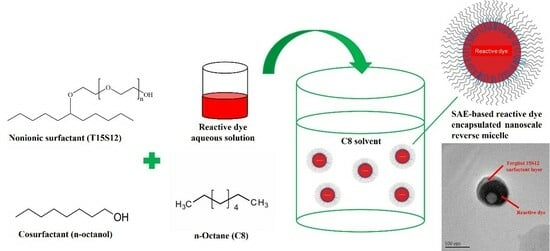
Figure 3 shows the workflow of an SAE-based dye-encapsulated reverse micelle formation. T15S12 biodegradable surfactant was mixed with n-octanol co-surfactant to form a mixture. Octane, acted as a nonpolar solvent phase, was then added to the mixture and stirred continuously to facilitate the self-assembly of SAE-based reverse micelles at room temperature. Reactive dye aqueous solution was then added dropwise into the reverse micellar solution with stirring to reinforce carrier formation by encapsulating reactive dye in the interior water-pool region of the SAE-based reverse micelles.
2.4. Optimisation of Parameters for SAE-Based Reverse Micellar Dyeing System
A dye concentration of 3.5% o.w.f. Levafix Red CA reactive dye was used to optimise several parameters of the SAE-based reverse micellar dyeing system. A one-factor-at-a-time approach was used for optimising the parameters. In this approach, in order to study the influence of one factor, all other factors are kept constant. The parameters involved: (i) effect of colour fixation agent; (ii) surfactant-to-water mole ratio (1:20, 1:25, 1:30, 1:40 and 1:50); (iii) surfactant-to-co-surfactant mole ratio (1:6, 1:8, 1:10, 1:12, 1:15 and 1:20); (iv) volume of soda ash (0, 0.3, 0.4, 0.5, 0.6 and 0.7 mL); (v) volume of dye (0.3, 0.4, 0.5, 0.6 and 0.7 mL); (vi) solvent-to-cotton ratio (v/w) (8:1, 10:1, 12:1, 15:1 and 20:1); (vii) dyeing temperature (50, 60, 70, and 80 °C); (viii) dyeing time (10, 20, 30, 40, and 50 min); (ix) fixation time (10, 20, 30, 40, 50 and 60 min); (x) soda-ash-to-cotton ratio (g/g). The optimised parameters were then applied for dyeing of cotton fabrics in five colour depths on weight of fibre (o.w.f.) (0.1, 0.5, 1.5, 2.5 and 3.5%) with the use of BCA, RCA and YCA reactive dyes.
2.5. SAE-Based Salt-Free Reverse Micellar Dyeing of Cotton Fabric
Figure 4 shows the workflow of SAE-based reverse micellar dyeing of cotton without salt. Cotton fabric was immersed in SAE reverse micellar dye solution and placed into a water bath (30 °C) for 10 min shaking. The temperature of the water bath was then raised to different temperatures (50, 60, 70, and 80 °C) for different time durations (10, 20, 30, 40, and 50 min). After that, a fixation agent, Na
2CO
3, was added into the dye solution for colour fixation with different time durations (10, 20, 30, 40, 50 and 60 min). The dyed fabric was finally rinsed with 2 g/L of detergent twice (50 °C), cold-rinsed with tap water, air-dried and conditioned in standard environment (20 ± 2 °C and 65 ± 2%) for 24 h before further measurements.
2.6. Colour Strength (K/Ssum Value)
A DataColor SF650 Spectrophotometer (DataColor International, USA) was used to measure the colour strength (K/S
sum value) of water-dyed and SAE-dyed fabric samples throughout the visible spectrum of 400 to 700 nm. The parameters for measurement were stated as follows: (a) aperture of 20 mm diameters; (b) illuminant D
65; (c) 10° standard observer; (d) opacity of fabric sample guaranteed by folding the fabric twice; € measurement only on the face side of fabric; (f) data collected at every 10 nm interval throughout the visible spectrum of 400 to 700 nm; and (g) average value of four measurements per sample. The K/S value of each sample was then calculated by Equation (1).
where K: absorption coefficient; S: scattering coefficient; and R: reflectance.
2.7. CIE L*a*b* Value Measurement
The CIE L*a*b* value of water-dyed and SAE-dyed fabric samples was measured using the same apparatus and parameters as stated in the colour strength section.
2.8. Levelness Evaluation
The colour levelness of water-dyed and SAE-dyed fabric samples was evaluated by using the Relative Unlevelness Indices suggested by Chong et al. [
27]. Four spots of each sample were randomly selected and evaluated by using the same apparatus and parameters as mentioned in the colour strength section. Equation (2) was then used to calculate the RUI value of each sample. Generally speaking, the lower the RUI value of the sample, the better the colour levelness of the fabric sample.
where
: standard deviation of reflectance value (specified wavelength);
: reflectance value (specific wavelength);
: photopic relative luminous efficiency function.
2.9. Scanning Electron Microscopy (SEM)
An Hitachi VP-SEM SU1510 scanning electron microscope (Hitachi, Tokyo, Japan) was used to assess the fibre surface properties and damage of water-dyed and SAE-dyed cotton samples.
2.10. Transmission Electron Microscopy (TEM)
A JEM 2010 transmission electron microscope (JEOL Co. Tokyo, Japan) with 120 kV accelerating voltage and 69 mA beam current was used to examine the dye assembly morphology in the reverse micelles.
2.11. Fastness Properties Tests
AATCC Test Method 61-2013, Test No. 2A, was performed to examine the washing fastness (colour change and colour staining) of the dyed cotton samples and the attached multifibre adjacent fabrics. AATCC Test Method 8-2013 was conducted to assess the crocking fastness (colour staining) of the dyed cotton samples and the white cloth. The colourfastness to perspiration of dyed fabrics was evaluated according to AATCC Test Method 15-2013 (Colorfastness to Perspiration). The colourfastness to light of dyed fabrics was assessed according to AATCC Test Method 16-2013 (Colorfastness to Light: Xenon Arc) by using light fastness tester (Xenotest 440, ATLAS, Hamburg, Germany). The rating of these fastness tests was given by using the grey scale.
2.12. Tensile Properties
The ASTM D5034 standard (Breaking Strength and Elongation of Textile Fabrics: Grab Test) was performed to evaluate the breaking strength and extension of both water-dyed and SAE-dyed samples prepared at 3.5% o.w.f. dye concentration.
4. Conclusions
This study aims at investigating the feasibility of using biodegradable Tergitol type 15-S-12 (T15S12) secondary alcohol ethoxylate (SAE) non-ionic surfactant as a building block for the formation of reverse micelles, functioning as reactive dye carriers for the dyeing of cotton fabric in non-aqueous octane medium. Ten dyeing parameters were optimised with the use of red reactive dye in terms of colour strength (K/Ssum value) of the dyed samples and directly applied to blue and yellow reactive dyes. The colour properties, fastness properties and physical properties of SAE-dyed samples were then compared with the conventional water-dyed samples.
Experimental results show that SAE-dyed samples have better colour strength and lower reflectance percentage than that of water-dyed samples. Reflectance curves are identical in shape without peak shifting and chromatic change between SAE-dyed samples and water-dyed samples. The CIE L*a*b* value was also measured. SAE-dyed samples generally obtain a darker shade with a lower L* value than that of water-dyed samples. The colour levelness of the dyed samples was evaluated. SAE-dyed samples can achieve good-to-excellent levelness comparable to water-dyed samples. These findings confirm that an SAE-based reverse micellar dyeing system is superior to a conventional water-based dyeing system.
Washing and crocking fastness of the dyed samples were evaluated. Both water-dyed and SAE-dyed samples can achieve excellent washing and crocking fastness, guaranteeing thorough removal of unfixed dye and auxiliaries and the accuracy of the obtained experimental results. The physical properties, in terms of breaking strength and extension, of the dyed samples were assessed. SAE-dyed samples can obtain similar breaking strength and extension to water-dyed samples. These findings ensure that SAE-dyed samples can achieve fastness and physical properties comparable to water-dyed samples.
SEM and TEM characterisation was conducted to observe the surface morphology of the dyed cotton fibre and the morphology of the reactive-dye-encapsulated reverse micelle, respectively. SEM images reveal that the dyed cotton fibres have no severe surface damage caused by an SAE-based reverse micellar dyeing system. The TEM image depicts that the reverse micelle is of nanoscale, spherical-shaped and has a core–shell structure, validating the presence of reverse micelle as a reactive dye carrier in an SAE-based reverse micellar dyeing system.