Experimental Investigation of the Peel Strength of Artificial Leather and Polypropylene Specimens
Abstract
:1. Introduction
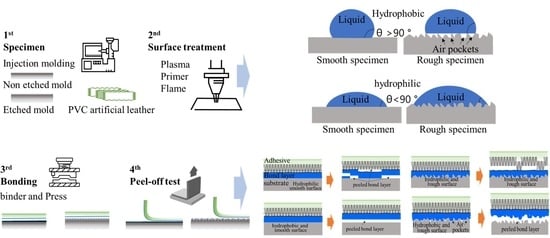
2. Surface Treatments and Characterizations
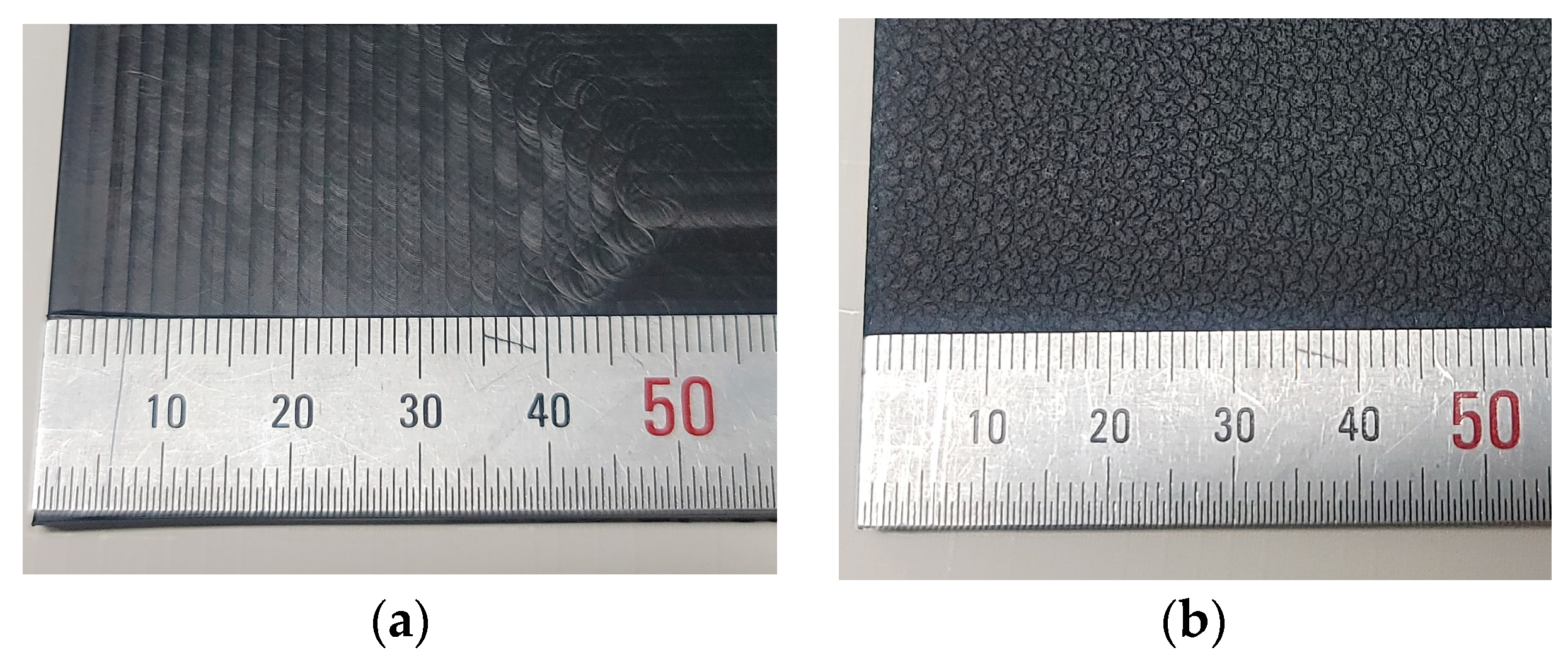
2.1. Materials
2.2. Specimens Preparation and Surface Treatment
2.3. Characterization of Samples
3. Results and Discussion
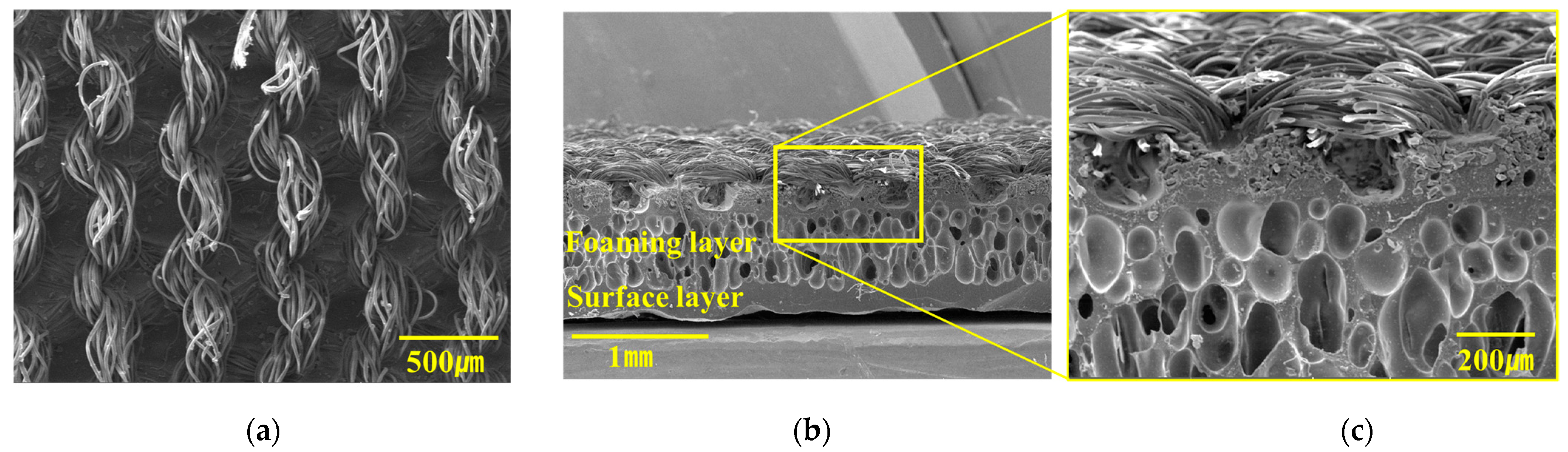
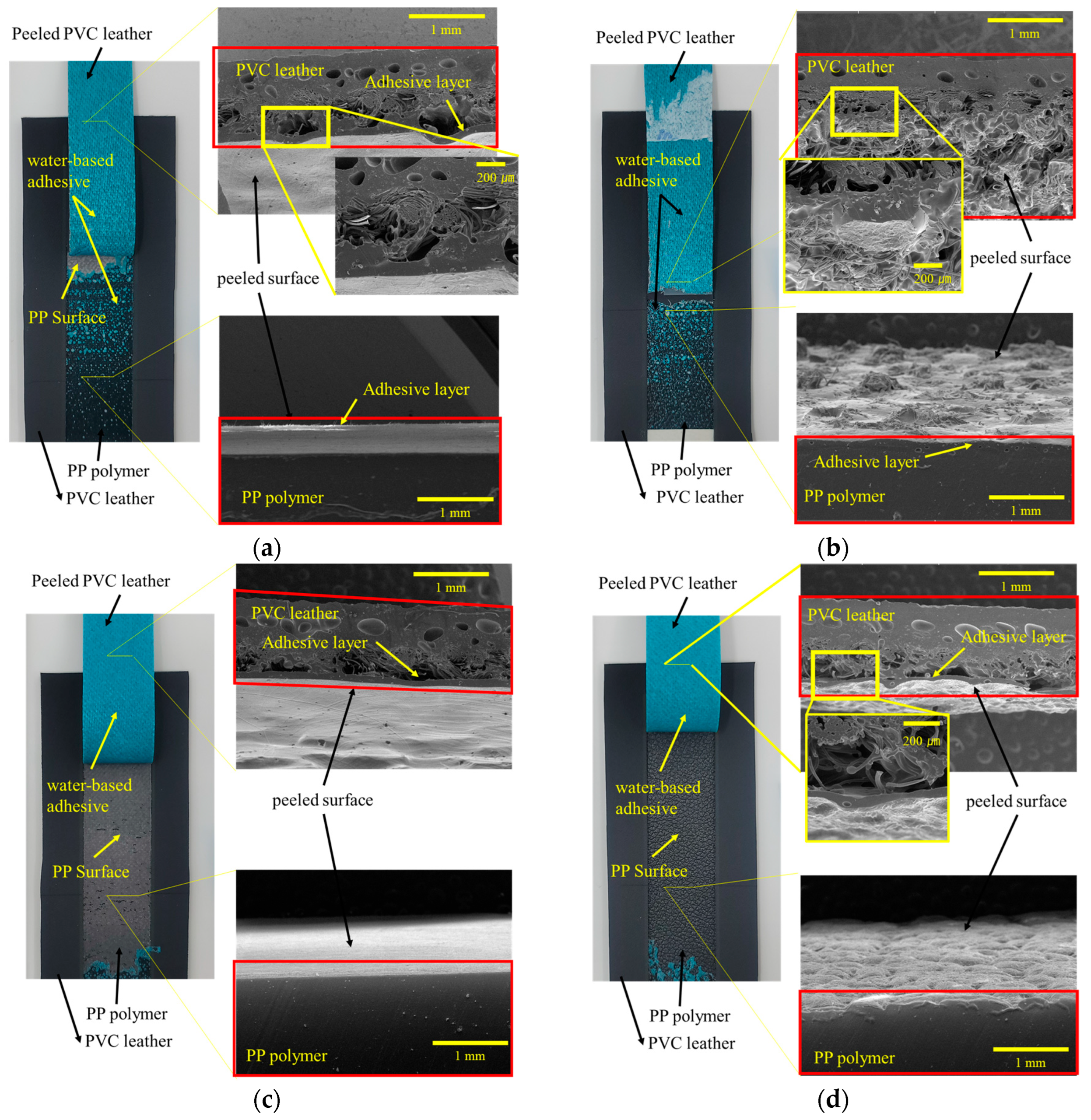
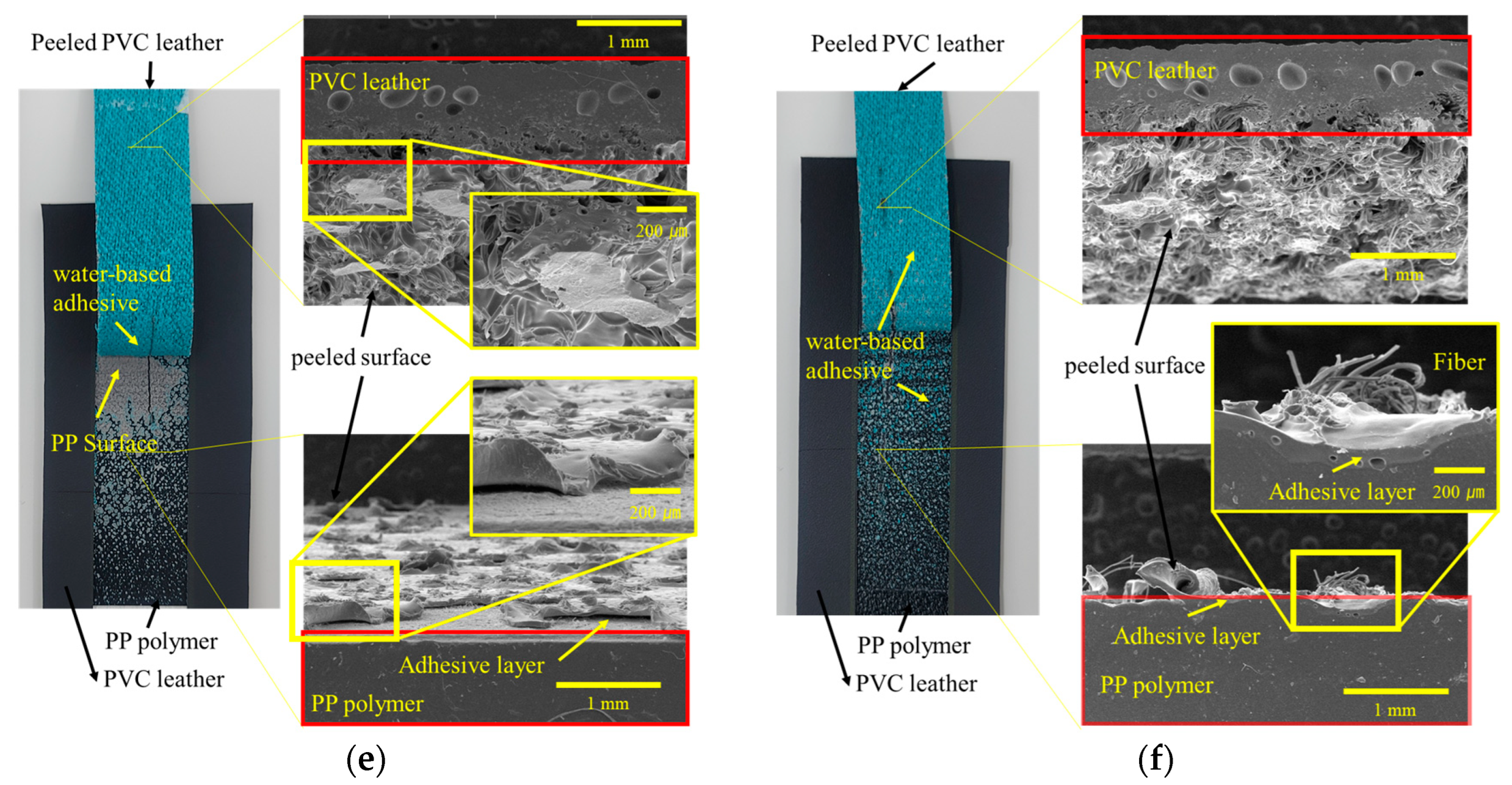
3.1. Morphologies
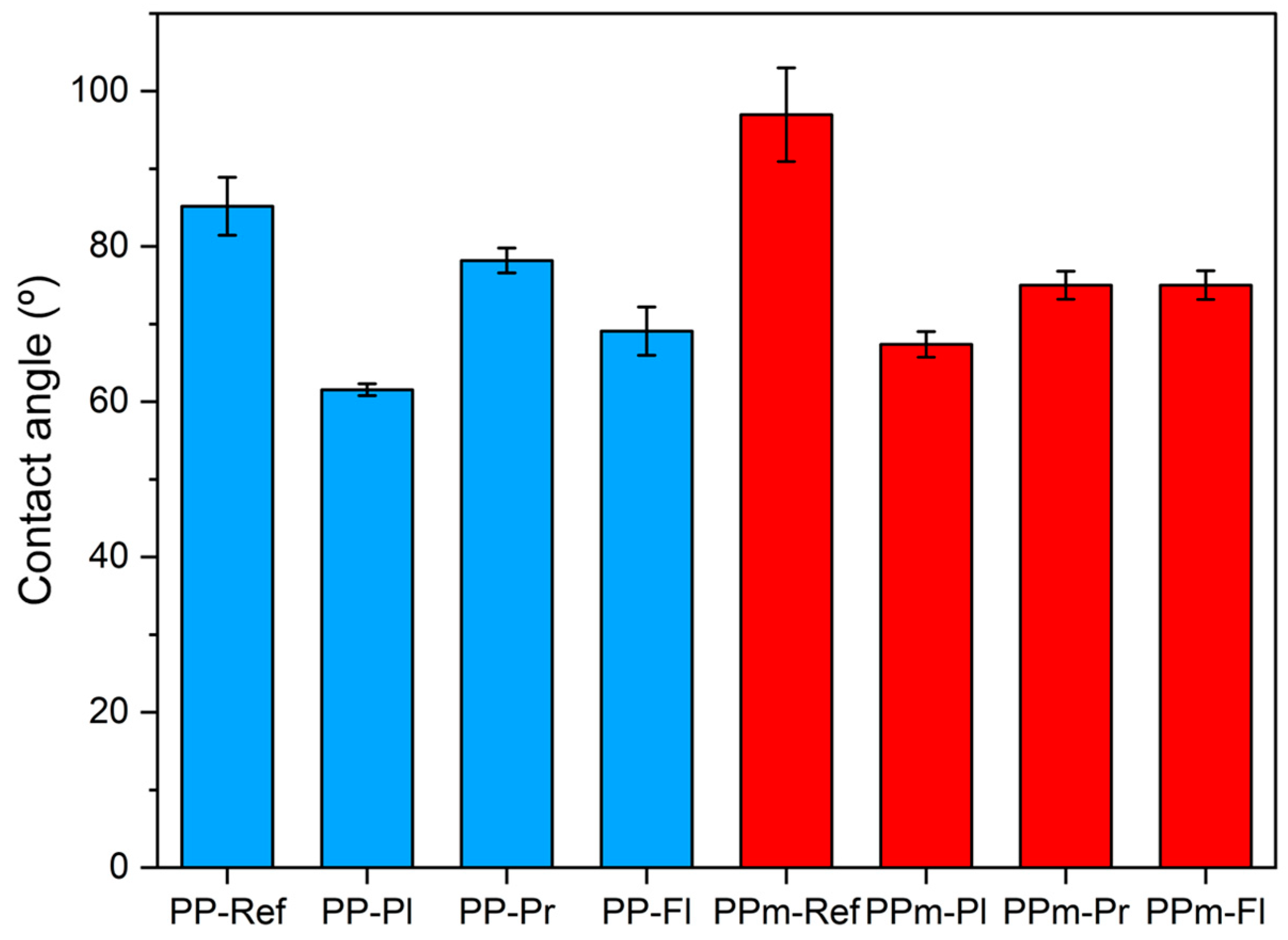
3.2. Analysis of the Contact Angle of PP Specimens
3.3. Analysis of PP Specimen Roughness
3.4. Bonding Strength of PP-Skin
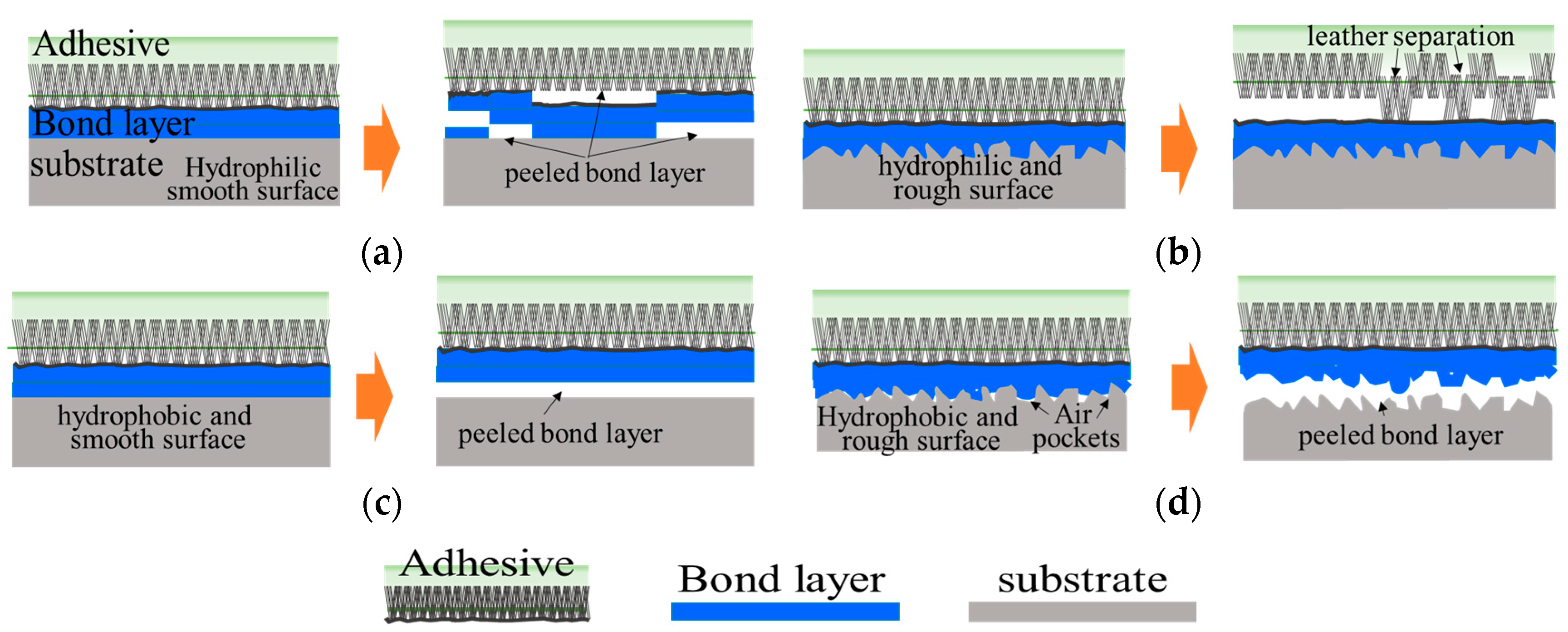
3.5. Analysis of Delamination of PP-Skin
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Conflicts of Interest
Appendix A

References
- Sergi, C.; Tirillò, J.; Iacovacci, C.; Sarasini, F. Influence of reprocessing cycles on the morphological, thermal and mechanical properties of flax/basalt hybrid polypropylene composites. Sustain. Mater. Technol. 2023, 36, e00648. [Google Scholar] [CrossRef]
- Sam-Daliri, O.; Ghabezi, P.; Steinbach, J.; Flanagan, T.; Finnegan, W.; Mitchell, S.; Harrison, N. Experimental study on mechanical properties of material extrusion additive manufactured parts from recycled glass fibre-reinforced polypropylene composite. Compos. Sci. Technol. 2023, 241, 110125. [Google Scholar] [CrossRef]
- Alsabri, A.; Tahir, F.; Al-Ghamdi, S.G. Environmental impacts of polypropylene (PP) production and prospects of its recycling in the GCC region. Mater. Today 2022, 56, 2245–2251. [Google Scholar] [CrossRef]
- Akonda, M.H.; Lawrence, C.A.; Weager, B.M. Recycled carbon fibre-reinforced polypropylene thermoplastic composites. Compos. Part A Appl. Sci. Manuf. 2012, 43, 79–86. [Google Scholar] [CrossRef]
- Meyer, M.; Dietrich, S.; Schulz, H.; Mondschein, A. Comparison of the technical performance of leather, artificial leather, and trendy alternatives. Coatings 2021, 11, 226. [Google Scholar] [CrossRef]
- Roh, E.K.; Oh, K.W.; Kim, S.H. Classification of synthetic polyurethane leather by mechanical properties according to consumers’ preference for fashion items. Fiber. Polym. 2013, 14, 1731–1738. [Google Scholar] [CrossRef]
- Liu, J.; Recupido, F.; Lama, G.C.; Oliviero, M.; Verdolotti, L.; Lavorgna, M. Recent advances concerning polyurethane in leather applications: An overview of conventional and greener solutions. Collagen Leather 2023, 5, 8. [Google Scholar] [CrossRef]
- Huang, Y.; Tang, Z.; Liu, Z.; Wei, J.; Hu, H.; Zhi, C. Toward enhancing wearability and fashion of wearable supercapacitor with modified polyurethane artificial leather electrolyte. Nanomicro Lett. 2018, 10, 38. [Google Scholar] [CrossRef]
- Qin, Y.; Liu, Y.; Kuang, G.; Zhou, Y.; Xin, B. Study on Modification and Application of Functional Polyurethane in Leather Finishing. J. Phys. Conf. Ser. 2023, 2470, 012030. [Google Scholar] [CrossRef]
- Kinge, A.P.; Landage, S.M.; Wasif, A.I. Nonwoven for artificial leather. Int. J. Adv. Res. Eng. Appl. Sci. 2013, 2, 18–33. [Google Scholar]
- Shimizu, R.N.; Demarquette, N.R. Evaluation of surface energy of solid polymers using different models. J. Appl. Polym. Sci. 2000, 76, 1831–1845. [Google Scholar] [CrossRef]
- Clint, J.H. Adhesion and components of solid surface energies. Curr. Opin. Colloid Interface Sci. 2001, 6, 28–33. [Google Scholar] [CrossRef]
- Chen, J.; Du, K.; Chen, X.; Li, Y.; Huang, J.; Wu, Y.; Yang, C.; Xia, X. Influence of surface microstructure on bonding strength of modified polypropylene/aluminum alloy direct adhesion. Appl. Surf. Sci. 2019, 489, 392–402. [Google Scholar] [CrossRef]
- Valkov, S.; Ormanova, M.; Petrov, P. Electron-beam surface treatment of metals and alloys: Techniques and trends. Metals 2020, 10, 1219. [Google Scholar] [CrossRef]
- Saxena, S.; Ray, A.R.; Kapil, A.; Pavon-Djavid, G.; Letourneur, D.; Gupta, B.; Meddahi-Pellé, A. Development of a new polypropylene-based suture: Plasma grafting, surface treatment, characterization, and biocompatibility studies. Macromol. Biosci. 2011, 11, 373–382. [Google Scholar] [CrossRef] [PubMed]
- Hall, J.R.; Westerdahl, C.A.; Devine, A.T.; Bodnar, M.J. Activated gas plasma surface treatment of polymers for adhesive bonding. J. Appl. Polym. Sci. 1969, 13, 2085–2096. [Google Scholar] [CrossRef]
- Zhao, W.; Kundu, C.K.; Li, Z.; Li, X.; Zhang, Z. Flame retardant treatments for polypropylene: Strategies and recent advances. Compos. Part A Appl. Sci. Manuf. 2021, 145, 106382. [Google Scholar] [CrossRef]
- Sutherland, I.; Brewis, D.M.; Health, R.J.; Sheng, E. Modification of polypropylene surfaces by flame treatment. Surf. Interface Anal. 1991, 17, 507–510. [Google Scholar] [CrossRef]
- Pandiyaraj, K.N.; Selvarajan, V.; Deshmukh, R.R.; Gao, C. Adhesive properties of polypropylene (PP) and polyethylene terephthalate (PET) film surfaces treated by DC glow discharge plasma. Vacuum 2008, 83, 332–339. [Google Scholar] [CrossRef]
- Mandolfino, C. Polypropylene surface modification by low pressure plasma to increase adhesive bonding: Effect of process parameters. Surf. Coat. Technol. 2019, 366, 331–337. [Google Scholar] [CrossRef]
- Sato, T.; Ise, S.; Horiuchi, S.; Akiyama, H.; Miyamae, T. Influences of low-temperature ambient pressure N2 plasma and flame treatments on polypropylene surfaces. Int. J. Adhes. Adhes 2019, 93, 102322. [Google Scholar] [CrossRef]
- Senniangiri, N.; Velliyangiri, B.; Sakthikumar, A.; Sanjay, V.S.; Arunkumar, S.; Sanjeev, A. Investigation on the mechanical behaviour and impact of plasma treated polypropylene in carbon fibre composites. Mater. Today 2023. [Google Scholar] [CrossRef]
- Cui, H.; Gao, X. Surface Modification on Polyimide Yarn by Plasma Treatment to Enhance Adhesion with Polypropylene Resin. Polymers 2022, 14, 4232. [Google Scholar] [CrossRef]
- Feng, J.; Yang, F.; Qian, S. Improving the bond between polypropylene fiber and cement matrix by nano calcium carbonate modification. Constr. Build. Mater. 2021, 269, 121249. [Google Scholar] [CrossRef]
- Gao, D.; Yan, H.; Yang, L.; Pang, Y.; Sun, B. Analysis of bond performance of steel bar in steel-polypropylene hybrid fiber reinforced concrete with partially recycled coarse aggregates. J. Clean. Prod. 2022, 370, 133528. [Google Scholar] [CrossRef]
- Saeki, Y.; Emura, T. Technical progresses for PVC production. Prog. Polym. Sci. 2002, 27, 2055–2131. [Google Scholar] [CrossRef]
- Li, C.; Feng, X.; Ding, E. Preparation, properties, and characterization of novel fine leather fibers/polyvinyl alcohol composites. Polym. Compos. 2015, 36, 1186–1194. [Google Scholar] [CrossRef]
- Maia, I.; Santos, J.; Abreu, M.J.; Miranda, T.; Carneiro, N.; Soares, G. PVC-based synthetic leather to provide more comfortable and sustainable vehicles. IOP Conf. Ser. Mater. Sci. Eng. 2017, 254, 122006. [Google Scholar] [CrossRef]
- Maghsoudi, K.; Jafari, R.; Momen, G.; Farzaneh, M. Micro-nanostructured polymer surfaces using injection molding: A review. Mater. Today Commun. 2017, 13, 126–143. [Google Scholar] [CrossRef]
- ASTM D5946-17; Standard Test Method for Corona-Treated Polymer Films Using Water Contact Angle Measurements. ASTM International: West Conshohocken, PA, USA, 2017.
- Huhtamäki, T.; Tian, X.; Korhonen, J.T.; Ras, R.H.A. Surface-wetting characterization using contact-angle measurements. Nat. Protoc. 2018, 13, 1521–1538. [Google Scholar] [CrossRef] [PubMed]
- Marmur, A. Solid-Surface Characterization by Wetting. Annu. Rev. Mater. Res. 2009, 39, 473–489. [Google Scholar] [CrossRef]
- Ponomar, M.; Krasnyuk, E.; Butylskii, D.; Nikonenko, V.; Wang, Y.; Jiang, C.; Xu, T.; Pismenskaya, N. Sessile Drop Method: Critical Analysis and Optimization for Measuring the Contact Angle of an Ion-Exchange Membrane Surface. Membranes 2022, 12, 765. [Google Scholar] [CrossRef]
- Sur, S.; Choi, P.; Ko, J.; Lee, J.; Lee, Y.; Kim, H. Preparation and properties of DMF-based polyurethanes for wet-type polyurethane artificial leather. Int. J. Polym. Sci. 2018, 2018, 7370852. [Google Scholar] [CrossRef]
- Rayatnia, M.; Foroutan, G. Surface functionalization of polypropylene nanoparticles in a pulsed low pressure air plasma discharge. Surf. Sci. 2022, 717, 121987. [Google Scholar] [CrossRef]
- Liang, C.; Li, C.; Chen, T.; Cheng, C.; Huang, C. Surface evaluation of reactive plasma-modified microporous polypropylene membrane by static contact angle analysis. Polym. Degrad. Stab. 2019, 160, 89–95. [Google Scholar] [CrossRef]
- Vishnuvarthanan, M.; Rajeswari, N. Effect of mechanical, barrier and adhesion properties on oxygen plasma surface modified PP. Innov. Food Sci. Emerg. Technol. 2015, 30, 119–126. [Google Scholar] [CrossRef]
- Zaman, M.W.; Han, J.; Zhang, X. Evaluating wettability of geotextiles with contact angles. Geotext. Geomembr. 2022, 50, 825–833. [Google Scholar] [CrossRef]
- Carrino, L.; Polini, W.; Sorrentino, L. Ageing time of wettability on polypropylene surfaces processed by cold plasma. J. Mater. Process. Technol. 2004, 153, 519–525. [Google Scholar] [CrossRef]
- Young, T. An essay on the cohesion of fluids. Philos. Trans. R. Soc. Lond. 1805, 95, 65–87. [Google Scholar]
- White, L.R. On deviations from Young’s equation. J. Chem. Soc. Faraday Trans. 1977, 73, 390–398. [Google Scholar] [CrossRef]
- Butt, H.J.; Semprebon, C.; Papadopoulos, P.; Vollmer, D.; Brinkmann, M.; Ciccotti, M. Design principles for superamphiphobic surfaces. Soft Matter 2013, 9, 418–428. [Google Scholar] [CrossRef]
- Butt, H.J.; Liu, J.; Koynov, K.; Straub, B.; Hinduja, C.; Roismann, I.; Berger, R.; Li, X.; Vollmer, D.; Steffen, W. Contact angle hysteresis. Curr. Opin. Colloid Interface Sci. 2022, 59, 101574. [Google Scholar] [CrossRef]
- Cassie, A.; Baxter, S. Wettability of porous surfaces. Trans. Faraday Soc. 1944, 40, 546–551. [Google Scholar] [CrossRef]
- Zhang, Y.; Arfsten, J.; Pihan, S.A.; Kaule, T.; Förch, R.; Berger, R. Interface roughness of plasma deposited polymer layers. J. Colloid. Interface Sci. 2010, 351, 532–536. [Google Scholar] [CrossRef]
- Wenzel, R.N. Resistance of Solid Surfaces to Wetting by Water. Ind. Eng. Chem. 1936, 28, 988–994. [Google Scholar] [CrossRef]
- David, D.J.; Misra, A. Surface energetics characterization and relationship to adhesion using a novel contact angle measuring technique. J. Colloid Interface Sci. 1985, 108, 371–376. [Google Scholar] [CrossRef]
- Kung, C.H.; Sow, P.K.; Zahiri, B.; Mérida, W. Assessment and Interpretation of Surface Wettability Based on Sessile Droplet Contact Angle Measurement: Challenges and Opportunities. Adv. Mater. Interfaces 2019, 6, 1900839. [Google Scholar] [CrossRef]
- Eksaeva, A.; Borodin, D.; Romazanov, J.; Kirschner, A.; Kreter, A.; Eichler, M.; Rasinski, M.; Pospieszczyk, A.; Unterberg, B.; Brezinsek, S. Surface roughness effect on Mo physical sputtering and re-deposition in the linear plasma device PSI-2 predicted by ERO2. 0. Nucl. Mater. Energy 2019, 19, 13–18. [Google Scholar] [CrossRef]
- ISO 21920-2; Geometrical Product Specifications (GPS)—Surface Texture: Profile—Part 2: Terms, Definitions and Surface Texture Parameters. ISO: Geneva, Switzerland, 2021.
- Cheng, F.; Hu, Y.; Zhang, X.; Hu, X.; Huang, Z. Adhesive bond strength enhancing between carbon fiber reinforced polymer and aluminum substrates with different surface morphologies created by three sulfuric acid solutions. Compos. Part A Appl. Sci. Manuf. 2021, 146, 106427. [Google Scholar] [CrossRef]
- Tonietto, L.; Gonzaga, L., Jr.; Veronez, M.R.; de Souza Kazmierczak, C.; Arnold, D.C.M.; da Costa, C.A. New Method for Evaluating Surface Roughness Parameters Acquired by Laser Scanning. Sci. Rep. 2019, 9, 15038. [Google Scholar] [CrossRef]
- Wu, Y.; Lin, J.; Carlson, B.E.; Lu, P.; Balogh, M.P.; Irish, N.P.; Mei, Y. Effect of laser ablation surface treatment on performance of adhesive-bonded aluminum alloys. Surf. Coat. Technol. 2016, 304, 340–347. [Google Scholar] [CrossRef]
- Farnese, J.; Zhao, P.; Ren, C.L. Effect of Surface Roughness on Bond Strength between PCTE Membranes and PDMS towards Microfluidic Applications. Int. J. Adhes. Adhes. 2021, 106, 102800. [Google Scholar] [CrossRef]
- Shen, Y.; Wang, Y.; Yang, Y.; Sun, Q.; Luo, T.; Zhang, H. Influence of Surface Roughness and Hydrophilicity on Bonding Strength of Concrete-Rock Interface. Constr. Build. Mater. 2019, 213, 156–166. [Google Scholar] [CrossRef]
- Drelich, J.W. Contact angles: From past mistakes to new developments through liquid solid adhesion measurements. Adv. Colloid. Interface Sci. 2019, 267, 1–14. [Google Scholar] [CrossRef]








| Name of Specimen | Injection-Mold Condition | Methods of Surface Treatment |
|---|---|---|
| PP-Ref | Non-etched mold | None |
| PPm-Ref | Etched mold | |
| PP-Pl | Non-etched mold | Plasma treatment |
| PPm-Pl | Etched mold | |
| PP-Pr | Non-etched mold | Primer coating |
| PPm-Pr | Etched mold | |
| PP-Fl | Non-etched mold | Flame treatment |
| PPm-Fl | Etched mold |
| Name of Specimen | Rpv (μm) | Rq (μm) | Ra (μm) | Rz (μm) | Rsk | Rku |
|---|---|---|---|---|---|---|
| PP-Ref | 0.586 ± 0.332 | 0.053 ± 0.004 | 0.056 ± 0.023 | 0.362 ± 0.012 | −0.170 ± 0.031 | 5.618 ± 0.012 |
| PP-Pl | 0.938 ± 0.255 | 0.121 ± 0.017 | 0.133 ± 0.060 | 0.776 ± 0.158 | 0.025 ± 0.112 | 2.565 ± 0.243 |
| PP-Pr | 0.307 ± 0.158 | 0.026 ± 0.005 | 0.030 ± 0.019 | 0.096 ± 0.098 | −0.021 ± 0.072 | 3.528 ± 1.019 |
| PP-Fl | 0.653 ± 0.040 | 0.084 ± 0.006 | 0.062 ± 0.007 | 0.641 ± 0.044 | 0.223 ± 0.567 | 4.034 ± 0.011 |
| PPm-Ref | 2.824 ± 0.685 | 0.723 ± 0.220 | 0.407 ± 0.135 | 3.676 ± 0.985 | −0.193 ± 0.078 | 2.409 ± 0.035 |
| PPm-Pl | 3.467 ± 0.786 | 0.634 ± 0.108 | 0.437 ± 0.148 | 3.741 ± 0.619 | 0.040 ± 0.003 | 2.827 ± 0.221 |
| PPm-Pr | 1.482 ± 0.345 | 0.161 ± 0.003 | 0.146 ± 0.006 | 1.264 ± 0.003 | 0.381 ± 0.113 | 5.594 ± 0.365 |
| PPm-Fl | 2.754 ± 0.804 | 0.445 ± 0.219 | 0.352 ± 0.128 | 2.493 ± 0.957 | −0.012 ± 0.291 | 2.504 ± 0.121 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kim, D.; Kim, Y.; Jeon, E. Experimental Investigation of the Peel Strength of Artificial Leather and Polypropylene Specimens. Polymers 2023, 15, 4217. https://doi.org/10.3390/polym15214217
Kim D, Kim Y, Jeon E. Experimental Investigation of the Peel Strength of Artificial Leather and Polypropylene Specimens. Polymers. 2023; 15(21):4217. https://doi.org/10.3390/polym15214217
Chicago/Turabian StyleKim, Deokrae, Youngshin Kim, and Euysik Jeon. 2023. "Experimental Investigation of the Peel Strength of Artificial Leather and Polypropylene Specimens" Polymers 15, no. 21: 4217. https://doi.org/10.3390/polym15214217