A Comparative Analysis of Pervaporation and Membrane Distillation Techniques for Desalination Utilising the Sweeping Air Methodology with Novel and Economical Pervaporation Membranes
Abstract
:1. Introduction
2. Materials and Methods
2.1. Fabrication of PV Membranes and Alkaline Treatment
2.2. CA Membrane Characterisation
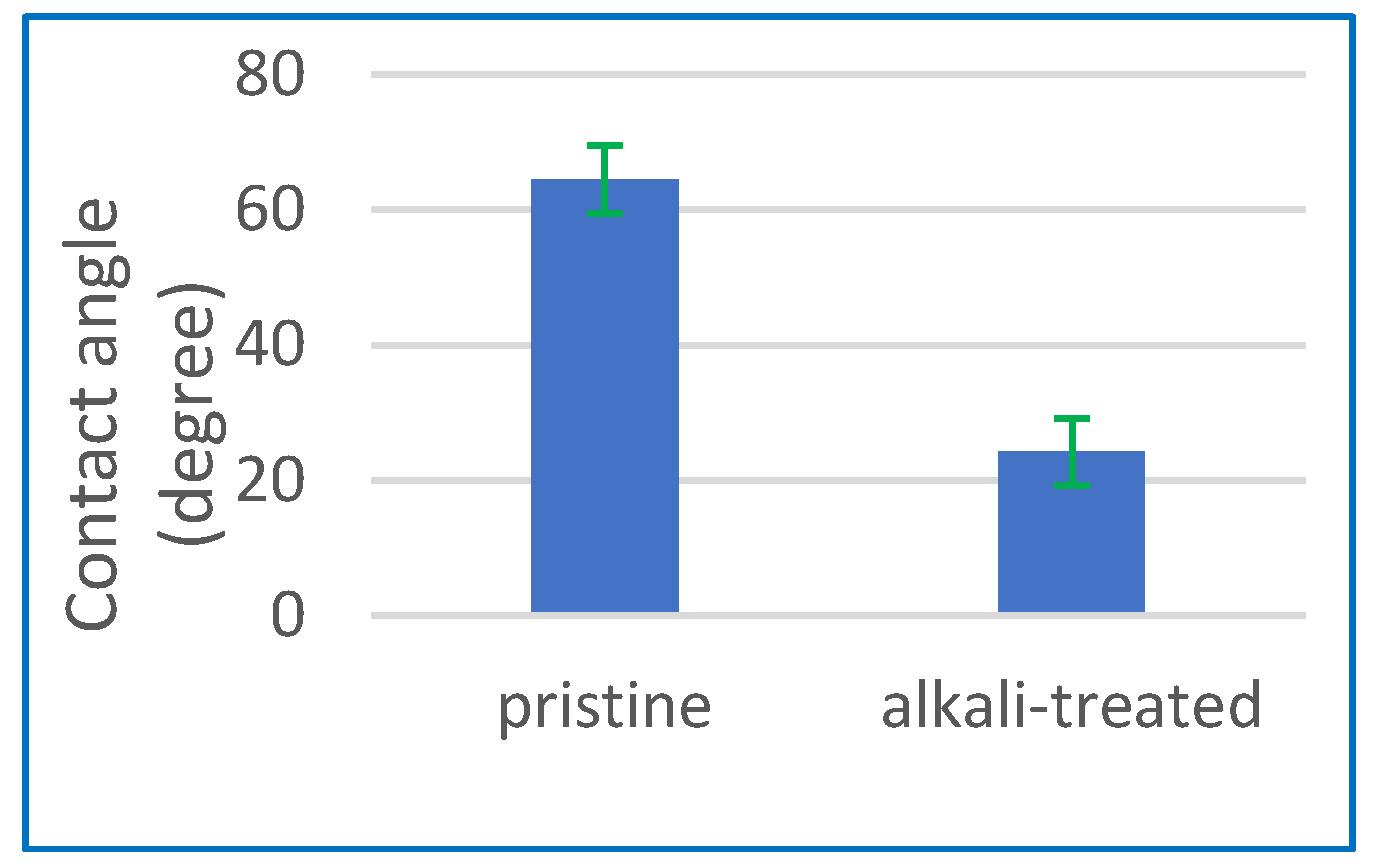
2.2.1. Analysis of Contact Angles
2.2.2. Examination of Water Absorption
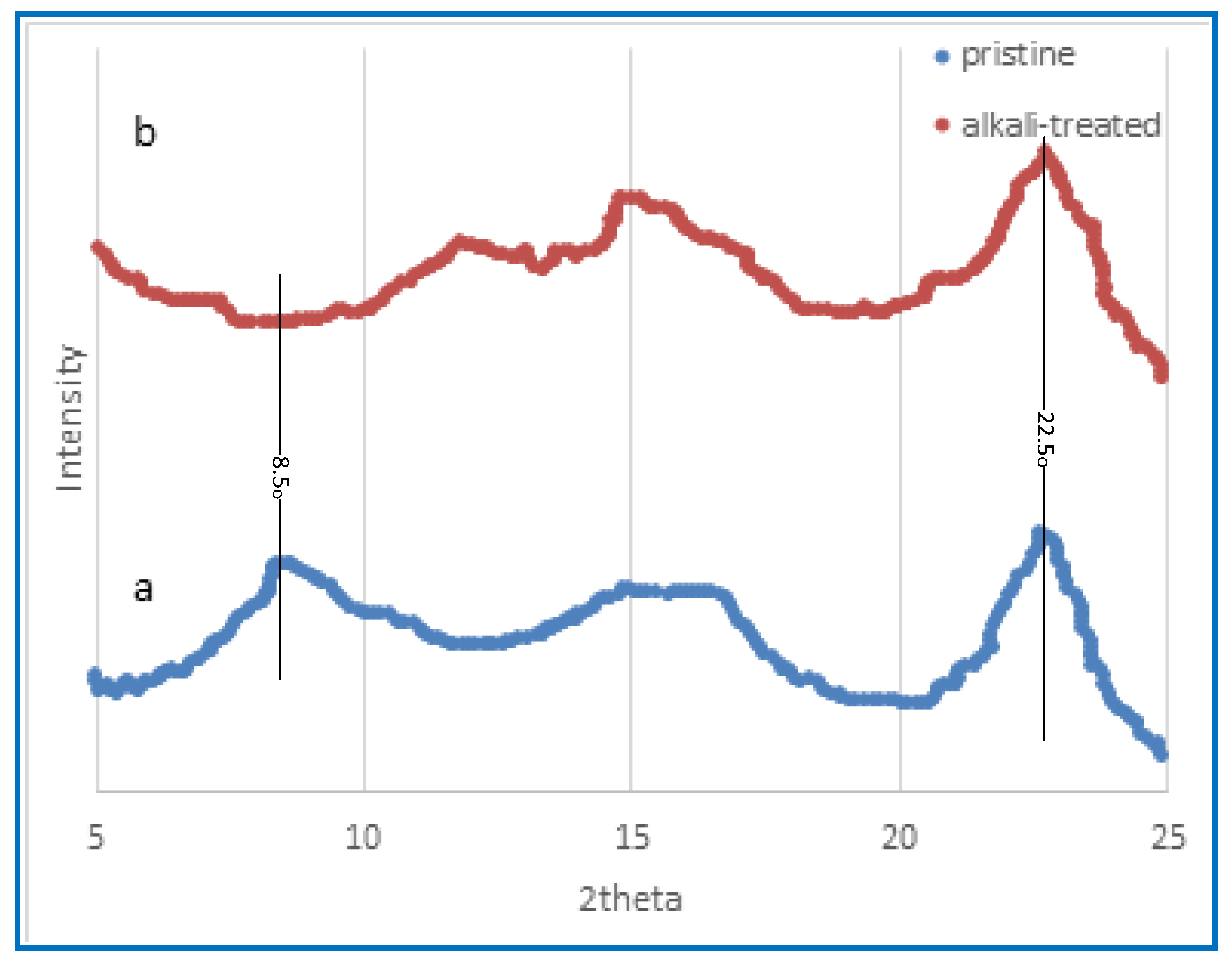
2.2.3. The Methodology of X-ray Diffraction (XRD)
2.2.4. Analysis Using Fourier-Transform Infrared (FTIR)
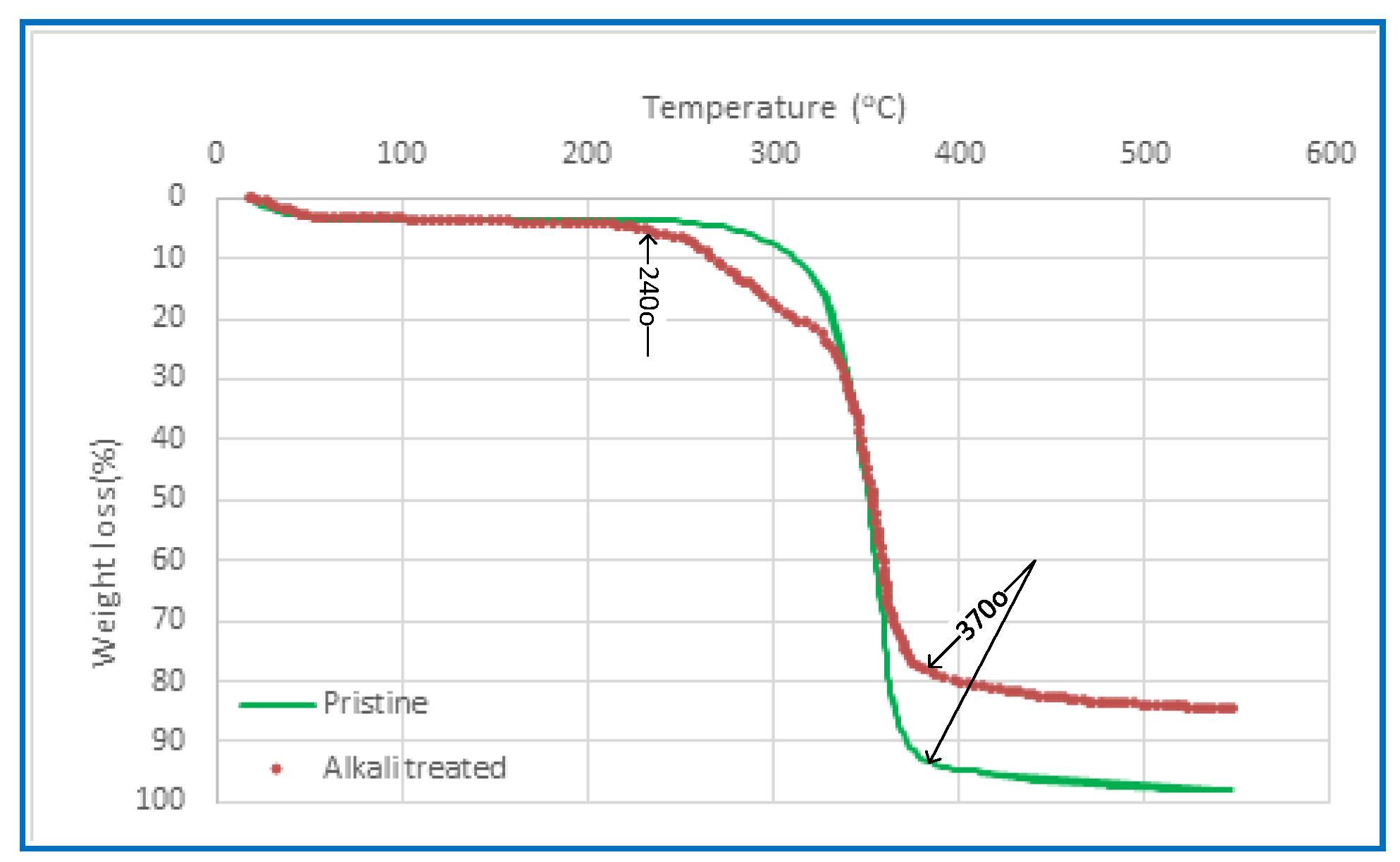
2.2.5. Thermogravimetric Analysis (TGA)
2.2.6. Examination of Mechanical Characteristics
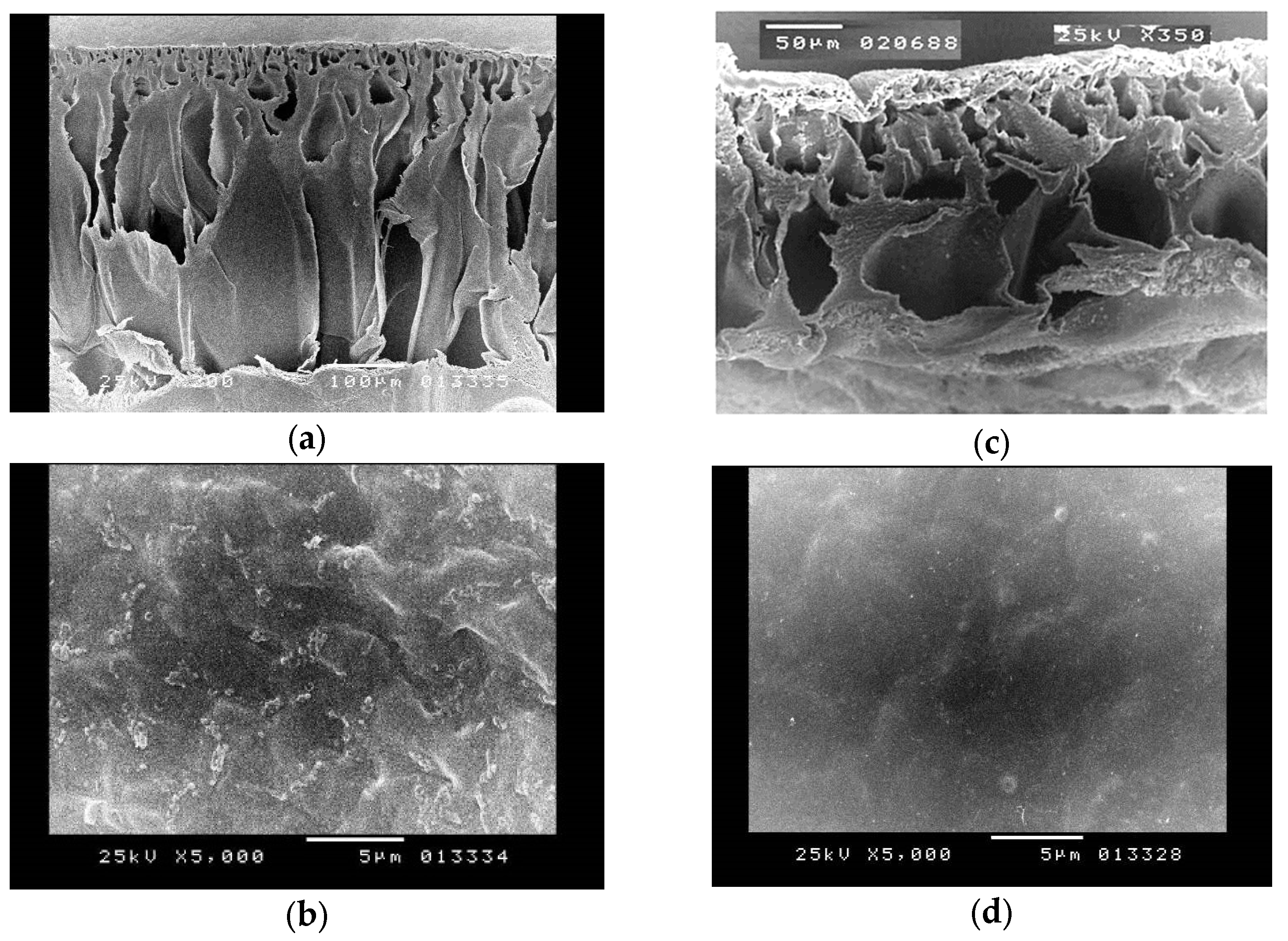
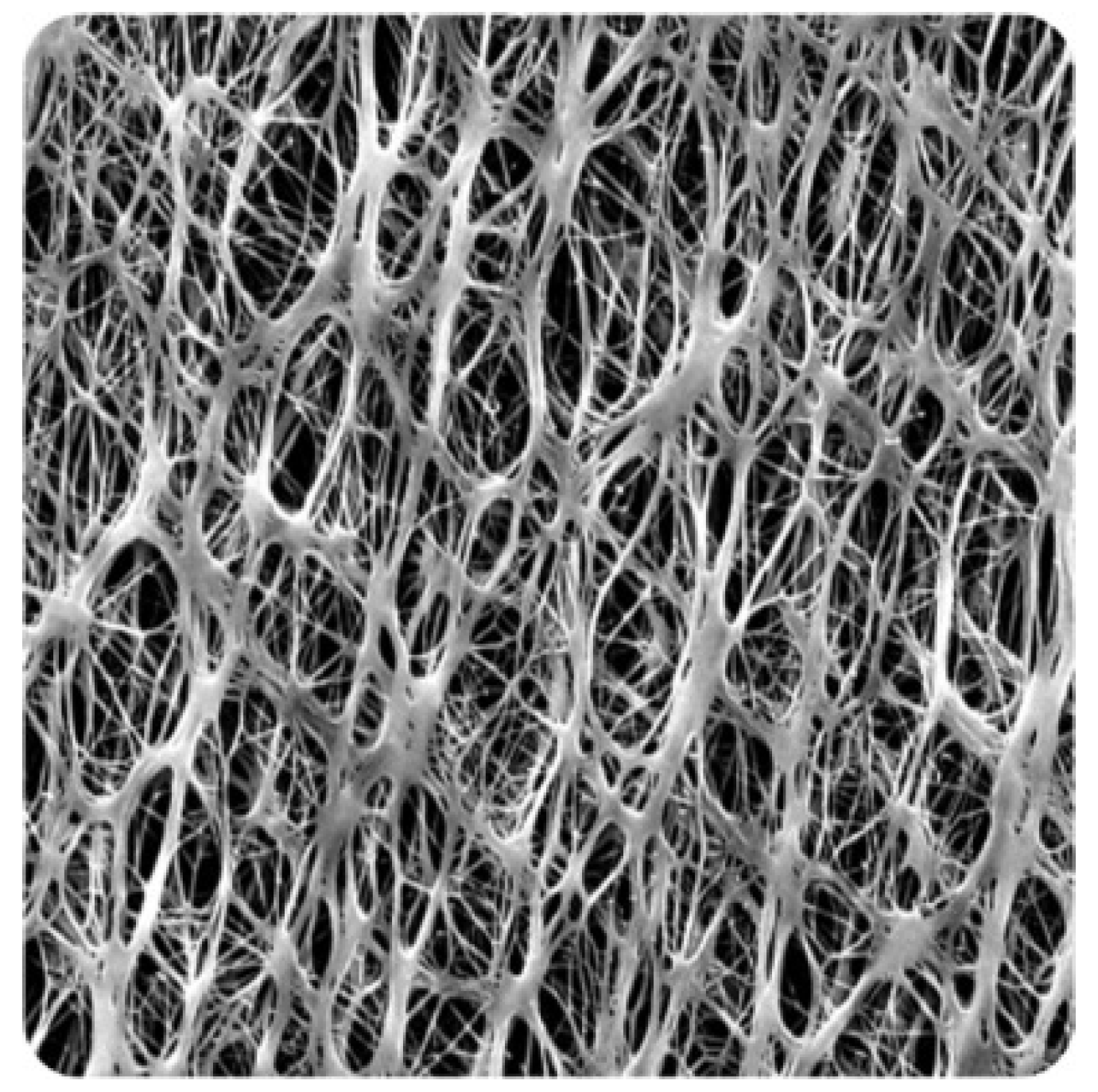
2.2.7. Morphological Characterisation
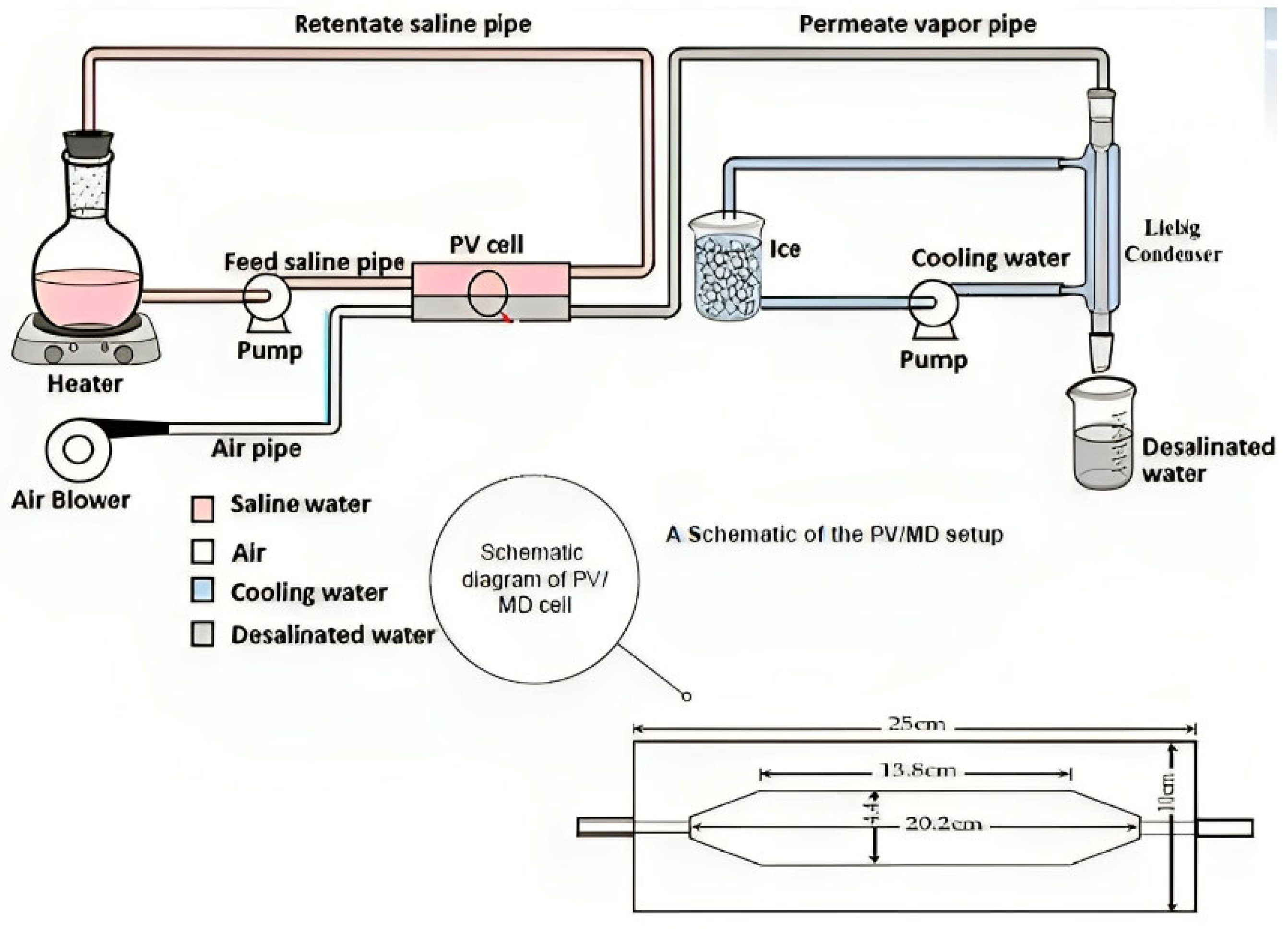
2.3. Experimental Setup
2.4. PV/MD Membranes Performance
3. Results and Discussions
3.1. CA Membrane Characterisation
3.1.1. Contact Angle
3.1.2. Measurement of Water Uptake
3.1.3. X-ray Diffraction
3.1.4. Fourier-Transform Infrared (FTIR) Characterisation
3.1.5. Thermogravimetric Analysis (TGA)
3.1.6. The Mechanical Properties
3.1.7. Morphological Characterisation
3.2. PTFE Membrane Properties
3.3. CA and PTFE Membrane Performance in PV/MD Desalination
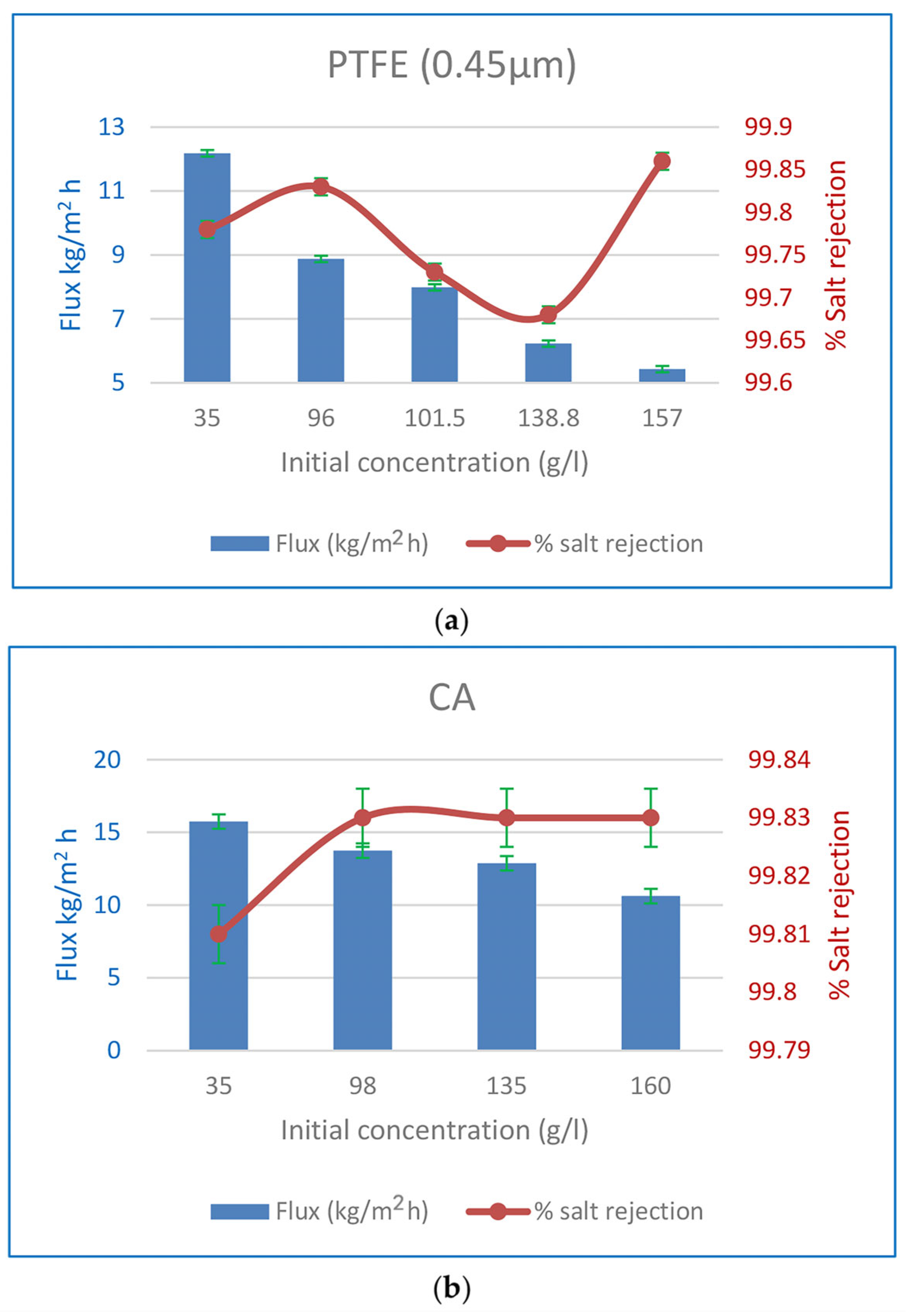
3.3.1. The Influence of Input Concentration on Flow and %Salt Rejection
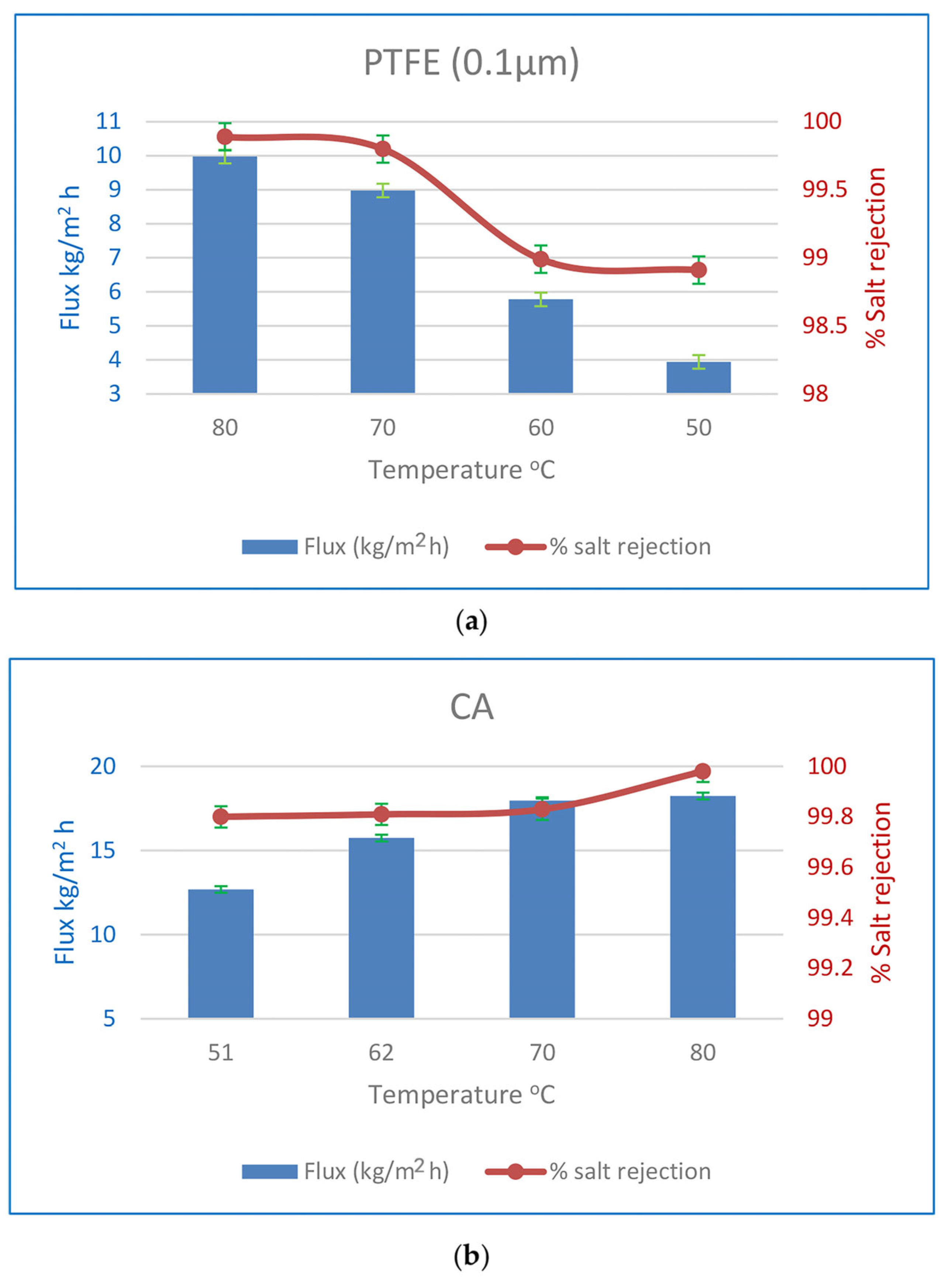
3.3.2. Flux and SR % Affects Due to Feed Temperature
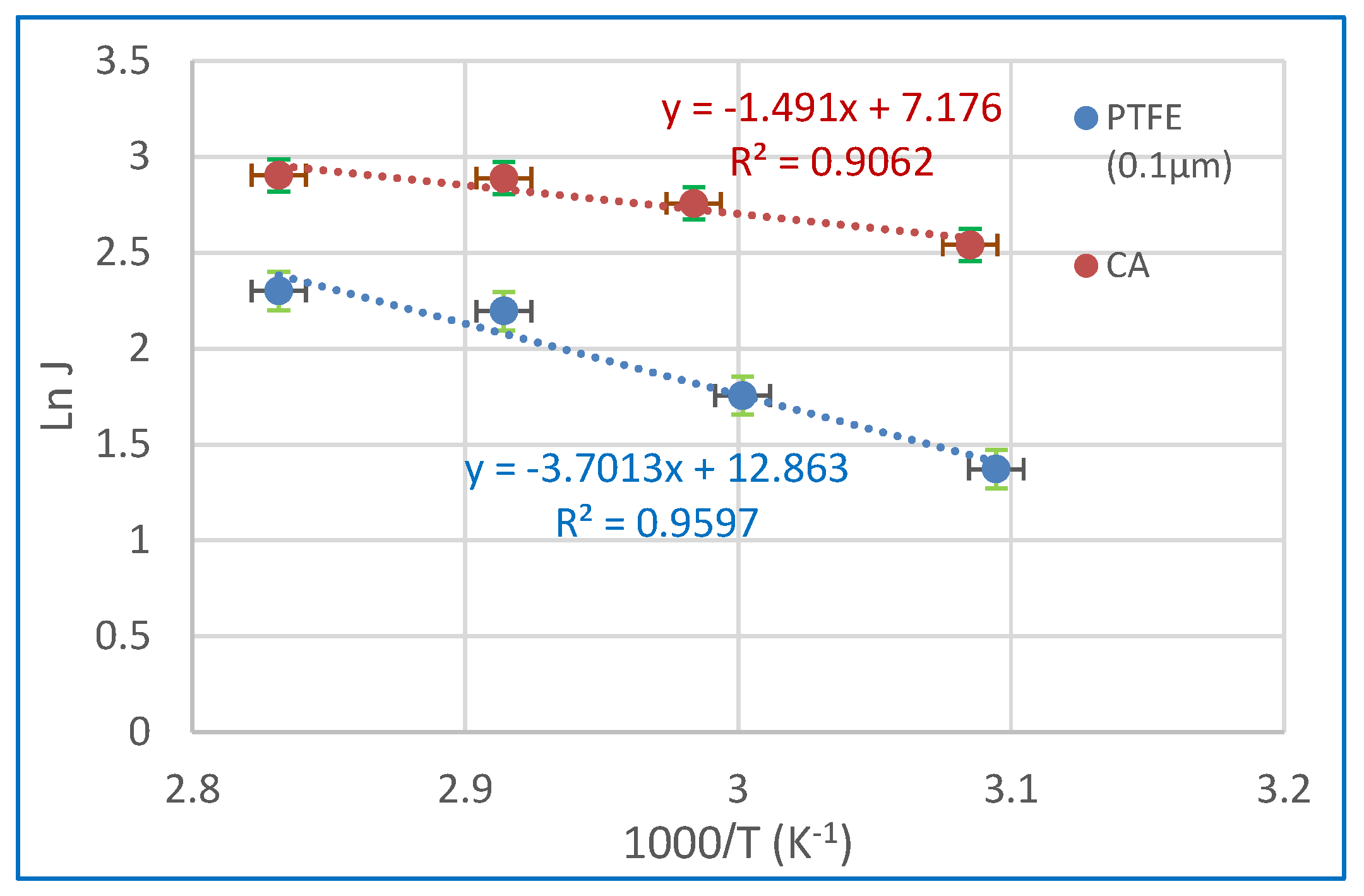
3.3.3. Compare Used Membranes with Others Cited
| Membrane Name | Thickness (nm) | Contact Angle (°) | Mem Config | PV Config | Feed NaCl (wt %) | Feed Temp (°C) | Activation Energy (kJ mol−1) | Flux (kg/m2 h) | SR (%) | Ref. |
|---|---|---|---|---|---|---|---|---|---|---|
| PVA | 730 ** | n/a | FS | VPV | 3.5 | 75 | n/a | 234.9 | 99.7 | [78] |
| PVA/PTFE | 2600 * | n/a | FS | VPV | 3.5 | 75 | n/a | 143.4 | 99.9 | [69] |
| MXene/PAN | ∼60 * | 49.5 | FS | VPV | 3.5 | 65 | 13.55 | 85.4 | 99.5 | [79] |
| MXene140 | ∼100 * | 52.5 | FS | VPV | 10 | 70 | 32.85 | 70 | 99.90 | [80] |
| HPAN-2 | 148,600 ** | 34 | FS | VPV | 10 | 65 | 20.1 | 58.7 | 99.91 | [81] |
| CTA/CNCs | 10,000 ** | 24.7 | FS | VPV | 10 | 70 | 37.8 | 58.5 | 99.8 | [25] |
| PA/mCNT | 160 * | 62 | FS | VPV | 10 | 70 | 36.97 | 40.8 | 99.99 | [82] |
| PVA-Lap2 MMM | 120,000–160,000 ** | 82 | FS | VPV | 10 | 70 | 12.24 | 39.9 | >99.9 | [83] |
| CS/GO-1MMM | 10,000–13,000 ** | 79 | FS | VPV | 5 | 81 | 31.28 | 30.0 | 99.99 | [84] |
| Laponite/PVA | 120,000–160,000 ** | n/a | FS | VPV | 10 | 40 | 5.73 | 21.1 | 99.99 | [85] |
| GO-PI | 1,000,000 ** | 59 | HF | VPV | 3.5 | 90 | 18.7 | 15.6 | >99.8 | [86] |
| GO/PAN | 100 * | n/a | FS | VPV | 10 | 30 | 22.19 | 11.23 | 99.8 | [87] |
| S-PVA/PAN | 4900 ** | 77.1 | FS | VPV | 10 | 70 | n/a | 11.2 | 99.8 | [88] |
| Polyimide/graphene oxide | 100,000 ** | 59 | FS | VPV | 10 | 75 | 15.7 | 10.7 | 99.9 | [89] |
| PVA/silica | 220 ** | n/a | HF | VPV | 3 | 60 | 14.44 | 10.4 | 99.9 | [21] |
| Cellulose diacetate on PTFE | 3500 ** | n/a | FS | VPV | 4 | 40 | n/a | 4.5–5.1 | 100 | [11] |
| Cellulose acetate | 20,000–25,000 * | 62 | FS | SGPV | 4–14 | 70 | 5.97–3.45 | 99.7 | [60] | |
| Alkali-treated cellulose acetate | 25,000 * | 24 | FS | SGPV | 3.5–16 | 70 | 12.4 | 17.96–11.08 | >99.8 | This study |
| Cellulose triacetate/Al2O3 | 13,000 ** | 52 | HF | VPV | 3–9 | 70 | 33.3–34.4 | 6.8–5 | 99.8 | [24] |
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Voutchkov, N. The Role of Desalination in an Increasingly Water-Scarce World; World Bank: Washington, DC, USA, 2019. [Google Scholar]
- Schunke, A.J.; Hernandez Herrera, G.A.; Padhye, L.; Berry, T.-A. Energy Recovery in SWRO Desalination: Current Status and New Possibilities. Front. Sustain. Cities 2020, 2, 9. [Google Scholar] [CrossRef]
- Galiano, F.; Castro-Muñoz, R.; Figoli, A. Pervaporation, Vapour Permeation and Membrane Distillation: From Membrane Fabrication to Application. Membranes 2021, 11, 162. [Google Scholar] [CrossRef] [PubMed]
- El Batouti, M.; Alharby, N.F.; Elewa, M.M. Review of New Approaches for Fouling Mitigation in Membrane Separation Processes in Water Treatment Applications. Separations 2021, 9, 1. [Google Scholar] [CrossRef]
- Li, Y.; Thomas, E.R.; Molina, M.H.; Mann, S.; Walker, W.S.; Lind, M.L.; Perreault, F. Desalination by Membrane Pervaporation: A Review. Desalination 2023, 547, 116223. [Google Scholar] [CrossRef]
- Mukherjee, M.; Roy, S.; Bhowmick, K.; Majumdar, S.; Prihatiningtyas, I.; Van der Bruggen, B.; Mondal, P. Development of High Performance Pervaporation Desalination Membranes: A Brief Review. Process Saf. Environ. Prot. 2022, 159, 1092–1104. [Google Scholar] [CrossRef]
- Ghazouani, N.; El-Bary, A.A.; Hassan, G.E.; Becheikh, N.; Bawadekji, A.; Elewa, M.M. Solar Desalination by Humidification–Dehumidification: A Review. Water 2022, 14, 3424. [Google Scholar] [CrossRef]
- Aburabie, J.; Mohammed, S.; Nassrullah, H.; Hashaikeh, R. Electrically-Conductive, Joule-Heated Pervaporation Membranes for Desalination: Investigating Energy-Saving, Self-Cleaning and Anti-Swelling Trifecta. Desalination 2023, 564, 116769. [Google Scholar] [CrossRef]
- Amirilargani, M.; Sadatnia, B. Poly(Vinyl Alcohol)/Zeolitic Imidazolate Frameworks (ZIF-8) Mixed Matrix Membranes for Pervaporation Dehydration of Isopropanol. J. Membr. Sci. 2014, 469, 1–10. [Google Scholar] [CrossRef]
- Wang, Z.; Deshmukh, A.; Du, Y.; Elimelech, M. Minimal and Zero Liquid Discharge with Reverse Osmosis Using Low-Salt-Rejection Membranes. Water Res. 2020, 170, 115317. [Google Scholar] [CrossRef]
- Kuznetsov, Y.P.; Kruchinina, E.V.; Baklagina, Y.G.; Khripunov, A.K.; Tulupova, O.A. Deep Desalination of Water by Evaporation through Polymeric Membranes. Russ. J. Appl. Chem. 2007, 80, 790–798. [Google Scholar] [CrossRef]
- Hamouda, S.B.; Boubakri, A.; Nguyen, Q.T.; Amor, M.B. PEBAX Membranes for Water Desalination by Pervaporation Process. High Perform. Polym. 2011, 23, 170–173. [Google Scholar] [CrossRef]
- Swenson, P.; Tanchuk, B.; Gupta, A.; An, W.; Kuznicki, S.M. Pervaporative Desalination of Water Using Natural Zeolite Membranes. Desalination 2012, 285, 68–72. [Google Scholar] [CrossRef]
- Cho, C.H.; Oh, K.Y.; Kim, S.K.; Yeo, J.G.; Sharma, P. Pervaporative Seawater Desalination Using NaA Zeolite Membrane: Mechanisms of High Water Flux and High Salt Rejection. J. Membr. Sci. 2011, 371, 226–238. [Google Scholar] [CrossRef]
- Wang, Q.; Li, N.; Bolto, B.; Hoang, M.; Xie, Z. Desalination by Pervaporation: A Review. Desalination 2016, 387, 46–60. [Google Scholar] [CrossRef]
- Sun, J.; Qian, X.; Wang, Z.; Zeng, F.; Bai, H.; Li, N. Tailoring the Microstructure of Poly(Vinyl Alcohol)-Intercalated Graphene Oxide Membranes for Enhanced Desalination Performance of High-Salinity Water by Pervaporation. J. Membr. Sci. 2020, 599, 117838. [Google Scholar] [CrossRef]
- Liang, B.; Li, Q.; Cao, B.; Li, P. Water Permeance, Permeability and Desalination Properties of the Sulfonic Acid Functionalized Composite Pervaporation Membranes. Desalination 2018, 433, 132–140. [Google Scholar] [CrossRef]
- Li, L.; Hou, J.; Ye, Y.; Mansour, J.; Zhang, Y.; Chen, V. Suppressing Salt Transport through Composite Pervaporation Membranes for Brine Desalination. Appl. Sci. 2017, 7, 856. [Google Scholar] [CrossRef]
- Wang, Z.; Sun, J.; Li, N.; Qin, Y.; Qian, X.; Xie, Z. Tuning Interlayer Structure to Construct Steady Dual-Crosslinked Graphene Oxide Membranes for Desalination of Hypersaline Brine via Pervaporation. Sep. Purif. Technol. 2022, 286, 120459. [Google Scholar] [CrossRef]
- Halakoo, E.; Feng, X. Layer-by-Layer Assembly of Polyethyleneimine/Graphene Oxide Membranes for Desalination of High-Salinity Water via Pervaporation. Sep. Purif. Technol. 2020, 234, 116077. [Google Scholar] [CrossRef]
- Qian, X.; Li, N.; Wang, Q.; Ji, S. Chitosan/Graphene Oxide Mixed Matrix Membrane with Enhanced Water Permeability for High-Salinity Water Desalination by Pervaporation. Desalination 2018, 438, 83–96. [Google Scholar] [CrossRef]
- Singh, P.S.; Chaudhri, S.G.; Kansara, A.M.; Schwieger, W.; Selvam, T.; Reuss, S.; Aswal, V.K. Cetyltrimethylammonium Bromide-Silica Membrane for Seawater Desalination through Pervaporation. Bull. Mater. Sci. 2015, 38, 565–572. [Google Scholar] [CrossRef]
- Prihatiningtyas, I.; Li, Y.; Hartanto, Y.; Vananroye, A.; Coenen, N.; Van der Bruggen, B. Effect of Solvent on the Morphology and Performance of Cellulose Triacetate Membrane/Cellulose Nanocrystal Nanocomposite Pervaporation Desalination Membranes. Chem. Eng. J. 2020, 388, 124216. [Google Scholar] [CrossRef]
- Prihatiningtyas, I.; Gebreslase, G.A.; Van der Bruggen, B. Incorporation of Al2O3 into Cellulose Triacetate Membranes to Enhance the Performance of Pervaporation for Desalination of Hypersaline Solutions. Desalination 2020, 474, 114198. [Google Scholar] [CrossRef]
- Prihatiningtyas, I.; Hartanto, Y.; Van der Bruggen, B. Ultra-High Flux Alkali-Treated Cellulose Triacetate/Cellulose Nanocrystal Nanocomposite Membrane for Pervaporation Desalination. Chem. Eng. Sci 2021, 231, 116276. [Google Scholar] [CrossRef]
- Prihatiningtyas, I.; Hartanto, Y.; Ballesteros, M.S.R.; Van der Bruggen, B. Cellulose Triacetate/LUDOX-SiO2 Nanocomposite for Synthesis of Pervaporation Desalination Membranes. J. Appl. Polym. Sci. 2021, 138, 50000. [Google Scholar] [CrossRef]
- Yan, M.; Lu, Y.; Li, N.; Zeng, F.; Wang, Q.; Bai, H.; Xie, Z. Hyperbranch-Crosslinked S-SEBS Block Copolymer Membranes for Desalination by Pervaporation. Membranes 2020, 10, 277. [Google Scholar] [CrossRef]
- Yan, M.; Zeng, F.; Li, N.; Bian, W.; Shen, W.; Xie, Z. Benzene Ring Crosslinking of a Sulfonated Polystyrene-Grafted SEBS (S-SEBS-g-PSt) Membrane by the Friedel–Crafts Reaction for Superior Desalination Performance by Pervaporation. J. Mater. Chem. A Mater. 2022, 10, 11990–12004. [Google Scholar] [CrossRef]
- Zeng, H.; Liu, S.; Wang, J.; Li, Y.; Zhu, L.; Xu, M.; Wang, C. Hydrophilic SPEEK/PES Composite Membrane for Pervaporation Desalination. Sep. Purif. Technol. 2020, 250, 117265. [Google Scholar] [CrossRef]
- Xie, Z.; Ng, D.; Hoang, M.; Duong, T.; Gray, S. Separation of Aqueous Salt Solution by Pervaporation through Hybrid Organic-Inorganic Membrane: Effect of Operating Conditions. Desalination 2011, 273, 220–225. [Google Scholar] [CrossRef]
- Alwatban, A.M.; Alshwairekh, A.M.; Alqsair, U.F.; Alghafis, A.A.; Oztekin, A. Performance Improvements by Embedded Spacer in Direct Contact Membrane Distillation—Computational Study. Desalination 2019, 470, 114103. [Google Scholar] [CrossRef]
- Park, D.J.; Norouzi, E.; Park, C. Experimentally-Validated Computational Simulation of Direct Contact Membrane Distillation Performance. Int. J. Heat Mass Transf. 2019, 129, 1031–1042. [Google Scholar] [CrossRef]
- Alkhudhiri, A.; Darwish, N.; Hilal, N. Produced Water Treatment: Application of Air Gap Membrane Distillation. Desalination 2013, 309, 46–51. [Google Scholar] [CrossRef]
- Alsaadi, A.S.; Ghaffour, N.; Li, J.-D.; Gray, S.; Francis, L.; Maab, H.; Amy, G.L. Modeling of Air-Gap Membrane Distillation Process: A Theoretical and Experimental Study. J. Membr. Sci. 2013, 445, 53–65. [Google Scholar] [CrossRef]
- Zhang, Y.; Peng, Y.; Ji, S.; Wang, S. Numerical Simulation of 3D Hollow-Fiber Vacuum Membrane Distillation by Computational Fluid Dynamics. Chem. Eng. Sci. 2016, 152, 172–185. [Google Scholar] [CrossRef]
- Liu, J.; Liu, M.; Guo, H.; Zhang, W.; Xu, K.; Li, B. Mass Transfer in Hollow Fiber Vacuum Membrane Distillation Process Based on Membrane Structure. J. Membr. Sci. 2017, 532, 115–123. [Google Scholar] [CrossRef]
- Elsheniti, M.B.; Ibrahim, A.; Elsamni, O.; Elewa, M. Experimental and Economic Investigation of Sweeping Gas Membrane Distillation/Pervaporation Modules Using Novel Pilot Scale Device. Sep. Purif. Technol. 2023, 310, 123165. [Google Scholar] [CrossRef]
- Elsheniti, M.B.; Elbessomy, M.O.; Wagdy, K.; Elsamni, O.A.; Elewa, M.M. Augmenting the Distillate Water Flux of Sweeping Gas Membrane Distillation Using Turbulators: A Numerical Investigation. Case Stud. Therm. Eng. 2021, 26, 101180. [Google Scholar] [CrossRef]
- El Batouti, M.; Al-Harby, N.F.; Elewa, M.M. A Review on Promising Membrane Technology Approaches for Heavy Metal Removal from Water and Wastewater to Solve Water Crisis. Water 2021, 13, 3241. [Google Scholar] [CrossRef]
- Aijaz, M.O.; Karim, M.R.; Othman, M.H.D.; Samad, U.A. Anti-Fouling/Wetting Electrospun Nanofibrous Membranes for Membrane Distillation Desalination: A Comprehensive Review. Desalination 2023, 553, 116475. [Google Scholar] [CrossRef]
- González, D.; Amigo, J.; Suárez, F. Membrane Distillation: Perspectives for Sustainable and Improved Desalination. Renew. Sustain. Energy Rev. 2017, 80, 238–259. [Google Scholar] [CrossRef]
- Khayet, M. Membranes and Theoretical Modeling of Membrane Distillation: A Review. Adv. Colloid Interface Sci. 2011, 164, 56–88. [Google Scholar] [CrossRef] [PubMed]
- Charfi, K.; Khayet, M.; Safi, M.J. Numerical Simulation and Experimental Studies on Heat and Mass Transfer Using Sweeping Gas Membrane Distillation. Desalination 2010, 259, 84–96. [Google Scholar] [CrossRef]
- Xu, D.; Zhu, Z.; Tan, G.; Xue, X.; Li, J. Mechanism Insight into Gypsum Scaling of Differently Wettable Membrane Surfaces with Antiscalants in Membrane Distillation. J. Membr. Sci. 2022, 652, 120499. [Google Scholar] [CrossRef]
- Yeszhanov, A.B.; Korolkov, I.V.; Dosmagambetova, S.S.; Zdorovets, M.V.; Güven, O. Recent Progress in the Membrane Distillation and Impact of Track-Etched Membranes. Polymers 2021, 13, 2520. [Google Scholar] [CrossRef] [PubMed]
- Wei, J.; Liao, M.; Ma, A.; Chen, Y.; Duan, Z.; Hou, X.; Li, M.; Jiang, N.; Yu, J. Enhanced Thermal Conductivity of Polydimethylsiloxane Composites with Carbon Fiber. Compos. Commun. 2020, 17, 141–146. [Google Scholar] [CrossRef]
- Pangarkar, B.L.; Sane, M.G.; Guddad, M. Reverse Osmosis and Membrane Distillation for Desalination of Groundwater: A Review. ISRN Mater. Sci. 2011, 2011, 523124. [Google Scholar] [CrossRef]
- Onsekizoglu, P. Membrane Distillation: Principle, Advances, Limitations and Future Prospects in Food Industry. In Distillation—Advances from Modeling to Applications; InTech: Houston, TX, USA, 2012. [Google Scholar]
- Kalla, S.; Piash, K.S.; Sanyal, O. Anti-Fouling and Anti-Wetting Membranes for Membrane Distillation. J. Water Process. Eng. 2022, 46, 102634. [Google Scholar] [CrossRef]
- Liu, J.; Albdoor, A.K.; Lin, W.; Hai, F.I.; Ma, Z. Membrane Fouling in Direct Contact Membrane Distillation for Liquid Desiccant Regeneration: Effects of Feed Temperature and Flow Velocity. J. Membr. Sci. 2022, 642, 119936. [Google Scholar] [CrossRef]
- Liu, L.; Charlton, L.; Song, Y.; Li, T.; Li, X.; Yin, H.; He, T. Scaling Resistance by Fluoro-Treatments: The Importance of Wetting States. J. Mater. Chem. A Mater. 2022, 10, 3058–3068. [Google Scholar] [CrossRef]
- Tan, G.; Xu, D.; Zhu, Z.; Zhang, X.; Li, J. Tailoring Pore Size and Interface of Superhydrophobic Nanofibrous Membrane for Robust Scaling Resistance and Flux Enhancement in Membrane Distillation. J. Membr. Sci. 2022, 658, 120751. [Google Scholar] [CrossRef]
- Ye, Z.-Y.; Broströmer, E.; Nan, J.; Cao, X.-F.; Liu, X.; Qiao, L.; Liu, Z.-J.; Su, X.-D. Protein Crystallization Benefits from the Rough Well Surface of a 48-Well Polystyrene Microplate. J. Cryst. Growth 2020, 532, 125425. [Google Scholar] [CrossRef]
- Liu, J.; Li, Z.; Wang, Y.; Liu, X.; Tu, G.; Li, W. Analyzing Scaling Behavior of Calcium Sulfate in Membrane Distillation via Optical Coherence Tomography. Water Res. 2021, 191, 116809. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Horseman, T.; Wang, Z.; Arafat, H.A.; Yin, H.; Lin, S.; He, T. Negative Pressure Membrane Distillation for Excellent Gypsum Scaling Resistance and Flux Enhancement. Environ. Sci. Technol. 2022, 56, 1405–1412. [Google Scholar] [CrossRef] [PubMed]
- Xie, S.; Li, Z.; Wong, N.H.; Sunarso, J.; Jin, D.; Yin, L.; Peng, Y. Gypsum Scaling Mechanisms on Hydrophobic Membranes and Its Mitigation Strategies in Membrane Distillation. J. Membr. Sci. 2022, 648, 120297. [Google Scholar] [CrossRef]
- Warsinger, D.M.; Swaminathan, J.; Guillen-Burrieza, E.; Arafat, H.A.; Lienhard, V.J.H. Scaling and Fouling in Membrane Distillation for Desalination Applications: A Review. Desalination 2015, 356, 294–313. [Google Scholar] [CrossRef]
- Zheng, Y.; Shen, J.; Yuan, J.; Khan, N.A.; You, X.; Yang, C.; Zhang, S.; El-Gendi, A.; Wu, H.; Zhang, R.; et al. 2D Nanosheets Seeding Layer Modulated Covalent Organic Framework Membranes for Efficient Desalination. Desalination 2022, 532, 115753. [Google Scholar] [CrossRef]
- Khayet, M.; Cojocaru, C. Artificial Neural Network Model for Desalination by Sweeping Gas Membrane Distillation. Desalination 2013, 308, 102–110. [Google Scholar] [CrossRef]
- Naim, M.; Elewa, M.; El-Shafei, A.; Moneer, A. Desalination of Simulated Seawater by Purge-Air Pervaporation Using an Innovative Fabricated Membrane. Water Sci. Technol. 2015, 72, 785–793. [Google Scholar] [CrossRef]
- Malucelli, L.C.; Lomonaco, D.; Filho, M.A.S.C.; Magalhães, W.L.E. Cellulose Triacetate from Different Sources: Modification Assessment through Thermal and Chemical Characterization. Holzforschung 2020, 74, 505–512. [Google Scholar] [CrossRef]
- Bali, G.; Meng, X.; Deneff, J.I.; Sun, Q.; Ragauskas, A.J. The Effect of Alkaline Pretreatment Methods on Cellulose Structure and Accessibility. ChemSusChem 2015, 8, 275–279. [Google Scholar] [CrossRef]
- Xu, E.; Wang, D.; Lin, L. Chemical Structure and Mechanical Properties of Wood Cell Walls Treated with Acid and Alkali Solution. Forests 2020, 11, 87. [Google Scholar] [CrossRef]
- Chieng, B.W.; Lee, S.H.; Ibrahim, N.A.; Then, Y.Y.; Loo, Y.Y. Isolation and Characterization of Cellulose Nanocrystals from Oil Palm Mesocarp Fiber. Polymers 2017, 9, 355. [Google Scholar] [CrossRef] [PubMed]
- Santos, E.B.C.; Moreno, C.G.; Barros, J.J.P.; de Moura, D.A.; Fim, F.d.C.; Ries, A.; Wellen, R.M.R.; da Silva, L.B. Effect of Alkaline and Hot Water Treatments on the Structure and Morphology of Piassava Fibers. Mater. Res. 2018, 21, 51845613. [Google Scholar] [CrossRef]
- Prihatiningtyas, I.; Volodin, A.; Van der Bruggen, B. 110th Anniversary: Cellulose Nanocrystals as Organic Nanofillers for Cellulose Triacetate Membranes Used for Desalination by Pervaporation. Ind. Eng. Chem. Res. 2019, 58, 14340–14349. [Google Scholar] [CrossRef]
- Wittmar, A.; Thierfeld, H.; Köcher, S.; Ulbricht, M. Routes towards Catalytically Active TiO2 Doped Porous Cellulose. RSC Adv. 2015, 5, 35866–35873. [Google Scholar] [CrossRef]
- Wu, D.; Gao, A.; Zhao, H.; Feng, X. Pervaporative Desalination of High-Salinity Water. Chem. Eng. Res. Des. 2018, 136, 154–164. [Google Scholar] [CrossRef]
- Meng, J.; Lau, C.H.; Xue, Y.; Zhang, R.; Cao, B.; Li, P. Compatibilizing Hydrophilic and Hydrophobic Polymersviaspray Coating for Desalination. J. Mater. Chem. A Mater. 2020, 8, 8462–8468. [Google Scholar] [CrossRef]
- Peng, F.; Lu, L.; Sun, H.; Jiang, Z. Analysis of Annealing Effect on Pervaporation Properties of PVA-GPTMS Hybrid Membranes through PALS. J. Membr. Sci. 2006, 281, 600–608. [Google Scholar] [CrossRef]
- Jiraratananon, R.; Chanachai, A.; Huang, R.Y.M.; Uttapap, D. Pervaporation Dehydration of Ethanol–Water Mixtures with Chitosan/Hydroxyethylcellulose (CS/HEC) Composite Membranes: I. Effect of Operating Conditions. J. Membr. Sci. 2002, 195, 143–151. [Google Scholar] [CrossRef]
- Yeom, C.K.; Lee, K.H. Vapor Permeation of Ethanol-Water Mixtures Using Sodium Alginate Membranes with Crosslinking Gradient Structure. J. Membr. Sci. 1997, 135, 225–235. [Google Scholar] [CrossRef]
- Kittur, A.A.; Kariduraganavar, M.Y.; Toti, U.S.; Ramesh, K.; Aminabhavi, T.M. Pervaporation Separation of Water-Isopropanol Mixtures Using ZSM-5 Zeolite Incorporated Poly(Vinyl Alcohol) Membranes. J. Appl. Polym. Sci. 2003, 90, 2441–2448. [Google Scholar] [CrossRef]
- Zhan, X.; Gao, Z.; Ge, R.; Lu, J.; Li, J.; Wan, X. Rigid POSS Intercalated Graphene Oxide Membranes with Hydrophilic/Hydrophobic Heterostructure for Efficient Pervaporation Desalination. Desalination 2022, 543, 116106. [Google Scholar] [CrossRef]
- Castro-Muñoz, R. Breakthroughs on Tailoring Pervaporation Membranes for Water Desalination: A Review. Water. Res. 2020, 187, 116428. [Google Scholar] [CrossRef] [PubMed]
- Castro-Muñoz, R.; Ahmad, M.Z.; Fíla, V. Tuning of Nano-Based Materials for Embedding Into Low-Permeability Polyimides for a Featured Gas Separation. Front. Chem. 2020, 7, 897. [Google Scholar] [CrossRef] [PubMed]
- Galiano, F.; Briceño, K.; Marino, T.; Molino, A.; Christensen, K.V.; Figoli, A. Advances in Biopolymer-Based Membrane Preparation and Applications. J. Membr. Sci. 2018, 564, 562–586. [Google Scholar] [CrossRef]
- Xue, Y.L.; Huang, J.; Lau, C.H.; Cao, B.; Li, P. Tailoring the Molecular Structure of Crosslinked Polymers for Pervaporation Desalination. Nat. Commun. 2020, 11, 1461. [Google Scholar] [CrossRef]
- Selim, A.; Toth, A.J.; Fozer, D.; Haaz, E.; Mizsey, P. Pervaporative Desalination of Concentrated Brine Solution Employing Crosslinked PVA/Silicate Nanoclay Membranes. Chem. Eng. Res. Des. 2020, 155, 229–238. [Google Scholar] [CrossRef]
- Chaudhri, S.G.; Chaudhari, J.C.; Singh, P.S. Fabrication of Efficient Pervaporation Desalination Membrane by Reinforcement of Poly(Vinyl Alcohol)-Silica Film on Porous Polysulfone Hollow Fiber. J. Appl. Polym. Sci. 2018, 135, 45718. [Google Scholar] [CrossRef]
- Huang, A.; Feng, B. Synthesis of Novel Graphene Oxide-Polyimide Hollow Fiber Membranes for Seawater Desalination. J. Membr. Sci. 2018, 548, 59–65. [Google Scholar] [CrossRef]
- Liang, B.; Zhan, W.; Qi, G.; Lin, S.; Nan, Q.; Liu, Y.; Cao, B.; Pan, K. High Performance Graphene Oxide/Polyacrylonitrile Composite Pervaporation Membranes for Desalination Applications. J. Mater. Chem. A Mater. 2015, 3, 5140–5147. [Google Scholar] [CrossRef]
- Zhang, R.; Liang, B.; Qu, T.; Cao, B.; Li, P. High-Performance Sulfosuccinic Acid Cross-Linked PVA Composite Pervaporation Membrane for Desalination. Environ. Technol. 2019, 40, 312–320. [Google Scholar] [CrossRef] [PubMed]
- Feng, B.; Xu, K.; Huang, A. Synthesis of Graphene Oxide/Polyimide Mixed Matrix Membranes for Desalination. RSC Adv. 2017, 7, 2211–2217. [Google Scholar] [CrossRef]
- Liu, G.; Shen, J.; Liu, Q.; Liu, G.; Xiong, J.; Yang, J.; Jin, W. Ultrathin Two-Dimensional MXene Membrane for Pervaporation Desalination. J. Membr. Sci. 2018, 548, 548–558. [Google Scholar] [CrossRef]
- Fareed, H.; Jang, K.; Lee, W.; Kim, I.S.; Han, S. Dehydroxylation-Assisted Self-Crosslinking of MXene-Based Pervaporation Membranes for Treating High-Salinity Water. J. Ind. Eng. Chem. 2023, 119, 506–515. [Google Scholar] [CrossRef]
- Fareed, H.; Qasim, G.H.; Jang, J.; Lee, W.; Han, S.; Kim, I.S. Brine Desalination via Pervaporation Using Kaolin-Intercalated Hydrolyzed Polyacrylonitrile Membranes. Sep. Purif. Technol. 2022, 281, 119874. [Google Scholar] [CrossRef]
- Liu, Y.; Tong, Z.; Zhu, H.; Zhao, X.; Du, J.; Zhang, B. Polyamide Composite Membranes Sandwiched with Modified Carbon Nanotubes for High Throughput Pervaporation Desalination of Hypersaline Solutions. J. Membr. Sci. 2022, 641, 119889. [Google Scholar] [CrossRef]
- Selim, A.; Toth, A.J.; Haaz, E.; Fozer, D.; Szanyi, A.; Hegyesi, N.; Mizsey, P. Preparation and Characterization of PVA/GA/Laponite Membranes to Enhance Pervaporation Desalination Performance. Sep. Purif. Technol. 2019, 221, 201–210. [Google Scholar] [CrossRef]
- Goessi, M.; Tervoort, T.; Smith, P. Melt-Spun Poly(Tetrafluoroethylene) Fibers. J. Mater. Sci. 2007, 42, 7983–7990. [Google Scholar] [CrossRef]
- Ochoa, I.; Hatzikiriakos, S.G. Paste Extrusion of Polytetrafluoroethylene (PTFE): Surface Tension and Viscosity Effects. Powder Technol. 2005, 153, 108–118. [Google Scholar] [CrossRef]
- Feng, S.; Zhong, Z.; Wang, Y.; Xing, W.; Drioli, E. Progress and Perspectives in PTFE Membrane: Preparation, Modification, and Applications. J. Membr. Sci. 2018, 549, 332–349. [Google Scholar] [CrossRef]
- Liu, G.; Gao, C.; Li, X.; Guo, C.; Chen, Y.; Lv, J. Preparation and Properties of Porous Polytetrafluoroethylene Hollow Fiber Membrane through Mechanical Operations. J. Appl. Polym. Sci. 2015, 132, 43. [Google Scholar] [CrossRef]
- Essalhi, M.; Khayet, M.; Ismail, N.; Sundman, O.; Tavajohi, N. Improvement of Nanostructured Electrospun Membranes for Desalination by Membrane Distillation Technology. Desalination 2021, 510, 115086. [Google Scholar] [CrossRef]
- Khayet, M.; García-Payo, M.C.; García-Fernández, L.; Contreras-Martínez, J. Dual-Layered Electrospun Nanofibrous Membranes for Membrane Distillation. Desalination 2018, 426, 174–184. [Google Scholar] [CrossRef]
- Ke, H.; Feldman, E.; Guzman, P.; Cole, J.; Wei, Q.; Chu, B.; Alkhudhiri, A.; Alrasheed, R.; Hsiao, B.S. Electrospun Polystyrene Nanofibrous Membranes for Direct Contact Membrane Distillation. J. Membr. Sci. 2016, 515, 86–97. [Google Scholar] [CrossRef]
- Su, C.; Li, Y.; Cao, H.; Lu, C.; Li, Y.; Chang, J.; Duan, F. Novel PTFE Hollow Fiber Membrane Fabricated by Emulsion Electrospinning and Sintering for Membrane Distillation. J. Membr. Sci. 2019, 583, 200–208. [Google Scholar] [CrossRef]
- Essalhi, M.; Khayet, M. Self-Sustained Webs of Polyvinylidene Fluoride Electrospun Nanofibers at Different Electrospinning Times: 1. Desalination by Direct Contact Membrane Distillation. J. Membr. Sci. 2013, 433, 167–179. [Google Scholar] [CrossRef]
- Wu, R.; Tan, Y.; Meng, F.; Zhang, Y.; Huang, Y.-X. PVDF/MAF-4 Composite Membrane for High Flux and Scaling-Resistant Membrane Distillation. Desalination 2022, 540, 116013. [Google Scholar] [CrossRef]
- Li, S.; Li, L.; Zhong, J.; Ma, R.; Xu, X.; Wu, H.; Yu, Y. Engineering Beads-on-String Structural Electrospun Nanofiber Janus Membrane with Multi-Level Roughness for Membrane Distillation. Desalination 2022, 539, 115950. [Google Scholar] [CrossRef]
- Moradi, R.; Karimi-Sabet, J.; Shariaty-Niassar, M.; Amini, Y. Air Gap Membrane Distillation for Enrichment of H218O Isotopomers in Natural Water Using Poly(Vinylidene Fluoride) Nanofibrous Membrane. Chem. Eng. Process. Process Intensif. 2016, 100, 26–36. [Google Scholar] [CrossRef]
- Makanjuola, O.; Anis, S.F.; Hashaikeh, R. Enhancing DCMD Vapor Flux of PVDF-HFP Membrane with Hydrophilic Silica Fibers. Sep. Purif. Technol. 2021, 263, 118361. [Google Scholar] [CrossRef]
- Rasekh, A.; Raisi, A. Electrospun Nanofibrous Polyether-Block-Amide Membrane Containing Silica Nanoparticles for Water Desalination by Vacuum Membrane Distillation. Sep. Purif. Technol. 2021, 275, 119149. [Google Scholar] [CrossRef]
- Huang, M.; Song, J.; Deng, Q.; Mu, T.; Li, J. Novel Electrospun ZIF/PcH Nanofibrous Membranes for Enhanced Performance of Membrane Distillation for Salty and Dyeing Wastewater Treatment. Desalination 2022, 527, 115563. [Google Scholar] [CrossRef]
- Woo, Y.C.; Tijing, L.D.; Park, M.J.; Yao, M.; Choi, J.-S.; Lee, S.; Kim, S.-H.; An, K.-J.; Shon, H.K. Electrospun Dual-Layer Nonwoven Membrane for Desalination by Air Gap Membrane Distillation. Desalination 2017, 403, 187–198. [Google Scholar] [CrossRef]
- Hu, X.; Chen, X.; Giagnorio, M.; Wu, C.; Luo, Y.; Hélix-Nielsen, C.; Yu, P.; Zhang, W. Beaded Electrospun Polyvinylidene Fluoride (PVDF) Membranes for Membrane Distillation (MD). J. Membr. Sci. 2022, 661, 120850. [Google Scholar] [CrossRef]
- Duong, H.C.; Chuai, D.; Woo, Y.C.; Shon, H.K.; Nghiem, L.D.; Sencadas, V. A Novel Electrospun, Hydrophobic, and Elastomeric Styrene-Butadiene-Styrene Membrane for Membrane Distillation Applications. J. Membr. Sci. 2018, 549, 420–427. [Google Scholar] [CrossRef]



| Solvents (g) | ||||||
|---|---|---|---|---|---|---|
| Acetone | DMF | MA | G | DMP | D | |
| CB | 13 | 100 | — | 3 | ||
| CAB | 10.5 | 74.5 | 10 | — | ||
| CA | 40 | 21.5 | 8 | 5 | 12 | 62 |
| Polymer | PTFE (0.1 µm) | PTFE (0.45 µm) |
|---|---|---|
| Support material | Laminated, PP non-woven | Laminated, PP netting |
| pH | 1–14 | 1–14 |
| Thickness | 152–254 µm | 64–127 µm |
| Water entry pressure | >4.1 bar (60 psi) | >0.76 bar (11 psi) |
| Application temperature range | 82 °C | 82 °C |
| Alcohol bubble point (MPa) | 0.2–0.24 | 0.07–0.1 |
| Alcohol flow rate (25 °C, Δp = −0.07 MPa) (mL/min/cm2) | 5–10 | 25–40 |
| Water contact angle | 134 ± 3 | 127 ± 3 |
| Membrane Type | NaCl Ci (g/L) | Thot (°C) | Tcold (°C) | Operation Time (h) | Flux (kg/m2 h) | Salt Rejection (%) |
|---|---|---|---|---|---|---|
| CB | 35 | 70 | 13 | 3.46 | 14.3 | 43.3 |
| CAB | 35 | 70 | 13 | 4.35 | 3.35 | 87.6 |
| PTFE (0.1 µm) | 35 | 80 | 13 | 08:25 | 9.98 | 99.89 |
| 35 | 70 | 13 | 08:25 | 8.98 | 99.91 | |
| 35 | 60 | 13 | 08:25 | 5.78 | 98.99 | |
| 35 | 50 | 12 | 08:10 | 3.94 | 98.91 | |
| PTFE (0.45 µm) | 35 | 70 | 12 | 07:50 | 13.35 | 99.86 |
| 35 | 60 | 12 | 08:30 | 12.18 | 99.78 | |
| 96 | 60 | 13 | 06:40 | 8.88 | 99.83 | |
| 101.5 | 60 | 11 | 06:30 | 7.99 | 99.73 | |
| 138.8 | 60 | 12 | 06:43 | 6.23 | 99.68 | |
| 157 | 60 | 12 | 06:20 | 5.43 | 99.86 | |
| 157 | 60 | 10 | 07:30 | 5.62 | 99.89 | |
| CA | 35 | 51 | 12 | 07:05 | 12.68 | 99.8 |
| 35 | 62 | 12 | 07:10 | 15.74 | 99.81 | |
| 35 | 70 | 12 | 07:15 | 17.96 | 99.83 | |
| 35 | 80 | 12 | 07:12 | 18.24 | 99.98 | |
| 98 | 60 | 11 | 06:05 | 13.74 | 99.83 | |
| 135 | 60 | 12 | 05:55 | 12.87 | 99.83 | |
| 160 | 60 | 12 | 06:10 | 10.62 | 99.83 | |
| 160 | 60 | 9.5 | 07:10 | 11.08 | 99.88 |
| Polymer | MD Module (Flat Sheet) | Thickness (nm) | Contact Angle (°) | LEP (kPa) | MD Configuration | Flux (kg/m2·h) | SR (%) | Time (h) | Ref. |
|---|---|---|---|---|---|---|---|---|---|
| PVDF | Single layer | ~350 | 145.8 | ~52 | DCMD | ~58 | 99.9 | 65 | [94] |
| PVDF/PSf | Dual layer | ~710/800 | 140 | ~79 | DCMD | ~48 | 99.9 | NA | [95] |
| PS | Single layer | ~200 | 114 | ~150 | DCMD | ~31 | 99.9 | 10 | [96] |
| PTFE | Hollow fibre | ~210 | 158 | ~249 | DCMD | ~30 | NA | 8 | [97] |
| PVDF | Single layer | ~1150 | 139 | ~110 | DCMD | ~28 | 99.4 | 25 | [98] |
| PVDF/MOFs | Single layer | ~501 | 147.5 | ~41 | DCMD | ~27 | NA | 6 | [99] |
| PAN/PVDF-HFP-PS/PDMS | Multi-layer | ~266/531 | 148.5 | ~41 | DCMD | ~27 | 100 | 36 | [100] |
| PVDF | Single layer | ~350 | 124 | ~260 | AGMD | ~26 | NA | 30 | [101] |
| PVDF-HFP/SiF | Single layer | ~160 | >90 | ~250 | DCMD | ~26 | 99.99 | 11 | [102] |
| PEBAX/SiNPs | Single layer | ~267 | 116.5 | NA | VMD | ~23 | NA | 14 | [103] |
| PVDF-HFP/MOFs | Single layer | ~293 | 134 | ~90 | DCMD | ~20 | 99.99 | 3 | [104] |
| PVDF-HFP/N6 | Dual layer | ~240/125 | 126.3 | ~184 | AGMD | ~15 | 99 | 20 | [105] |
| PVDF | Single layer | ~378 | 139.6 | ~58 | DCMD | ~12 | NA | 20 | [106] |
| SBS | Single layer | NA | 132 | NA | DCMD | ~11 | 99.97 | 120 | [107] |
| PTFE (0.45 µm) | Single layer | 64,000–127,000 | 126 | 75 | SGMD | 13.35 | 99.86 | 8 | This study |
| PTFE (0.1 µm) | Single layer | 152,000–254,000 | 126 | 413 | SGMD | 8.98 | 99.91 | 8 | This study |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Al-Harby, N.F.; El Batouti, M.; Elewa, M.M. A Comparative Analysis of Pervaporation and Membrane Distillation Techniques for Desalination Utilising the Sweeping Air Methodology with Novel and Economical Pervaporation Membranes. Polymers 2023, 15, 4237. https://doi.org/10.3390/polym15214237
Al-Harby NF, El Batouti M, Elewa MM. A Comparative Analysis of Pervaporation and Membrane Distillation Techniques for Desalination Utilising the Sweeping Air Methodology with Novel and Economical Pervaporation Membranes. Polymers. 2023; 15(21):4237. https://doi.org/10.3390/polym15214237
Chicago/Turabian StyleAl-Harby, Nouf F., Mervette El Batouti, and Mahmoud M. Elewa. 2023. "A Comparative Analysis of Pervaporation and Membrane Distillation Techniques for Desalination Utilising the Sweeping Air Methodology with Novel and Economical Pervaporation Membranes" Polymers 15, no. 21: 4237. https://doi.org/10.3390/polym15214237





