Revealing the Structure Formation on Polyglycerol Citrate Polymers—An Environmentally Friendly Polyester as a Seed-Coating Material
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
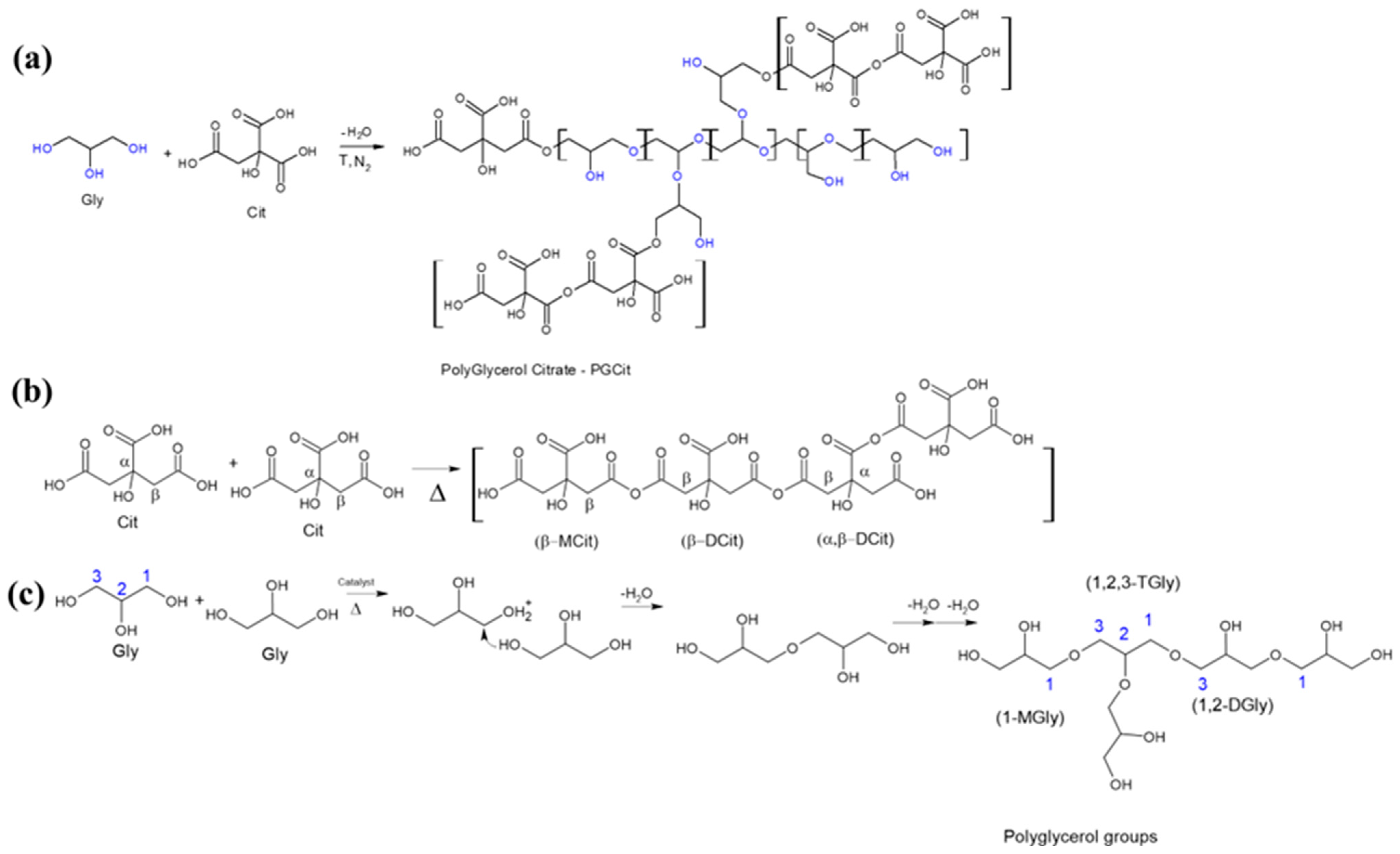
2.2. Materials Synthesis
2.3. Polymer Characterization
2.4. Preparation of PGCit-Coated Seeds
2.5. Germination Tests of Coated versus Non-Coated Soybean Seeds
3. Results and Discussion
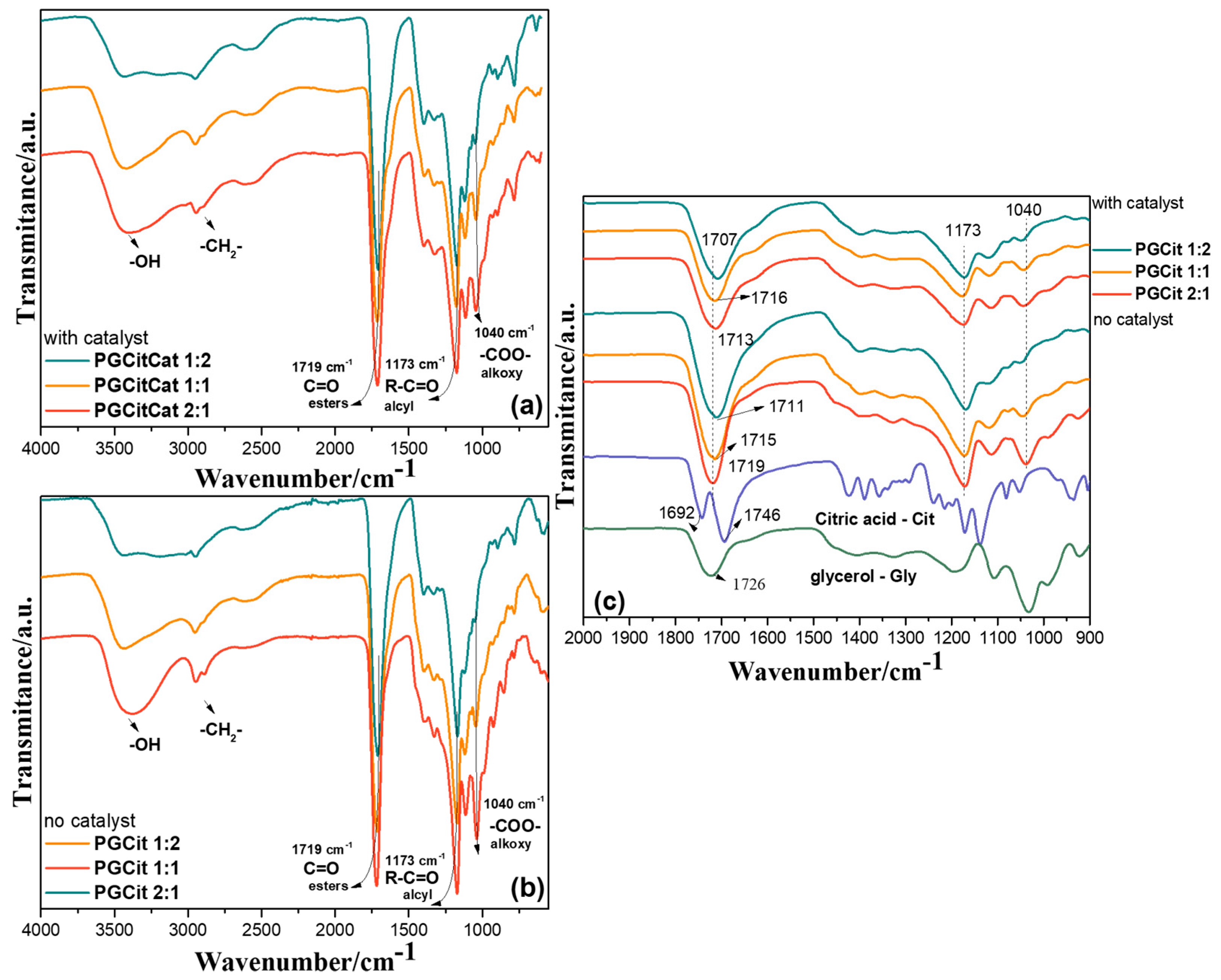
3.1. FTIR Analysis
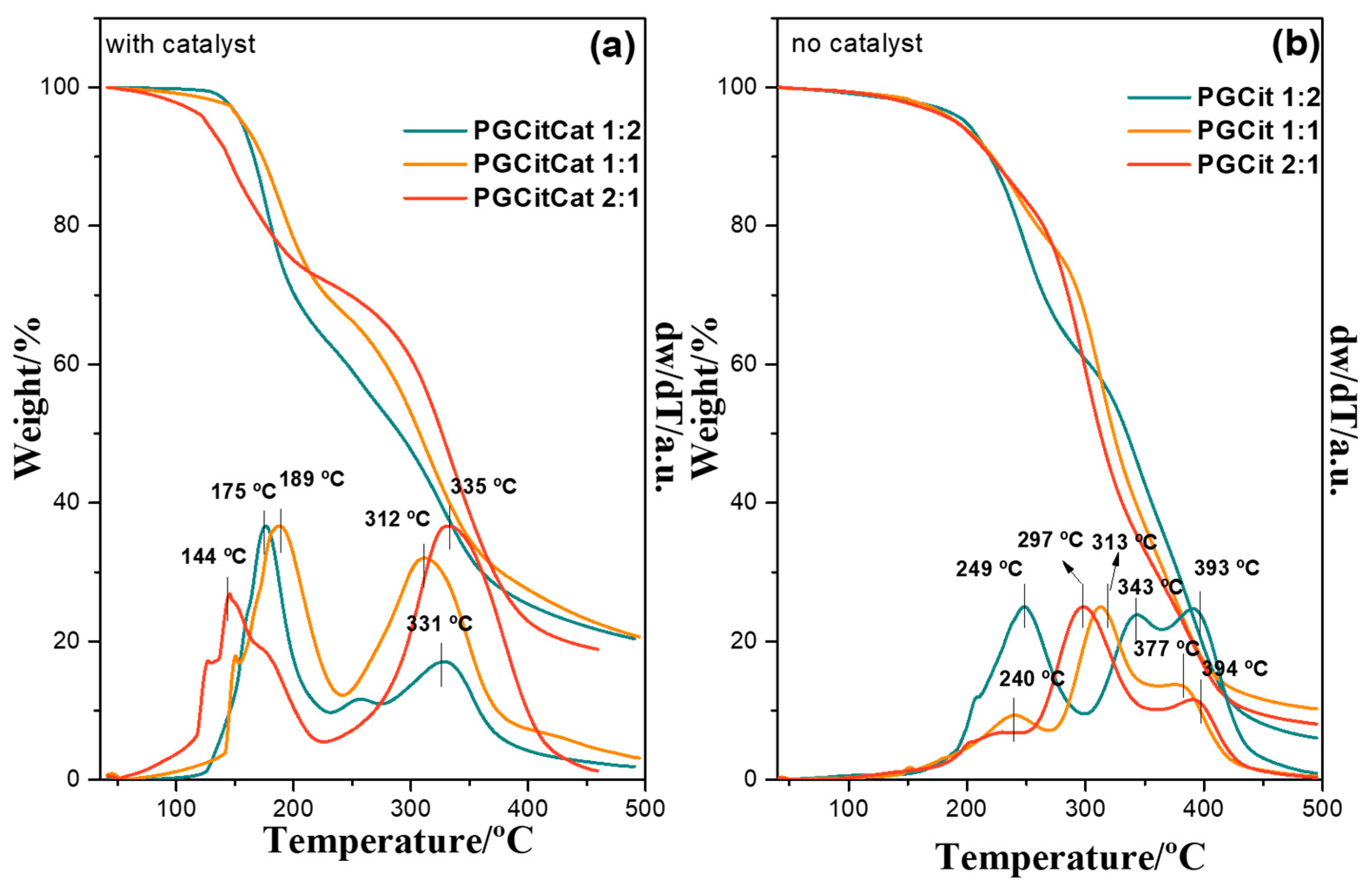
3.2. Thermic Analysis
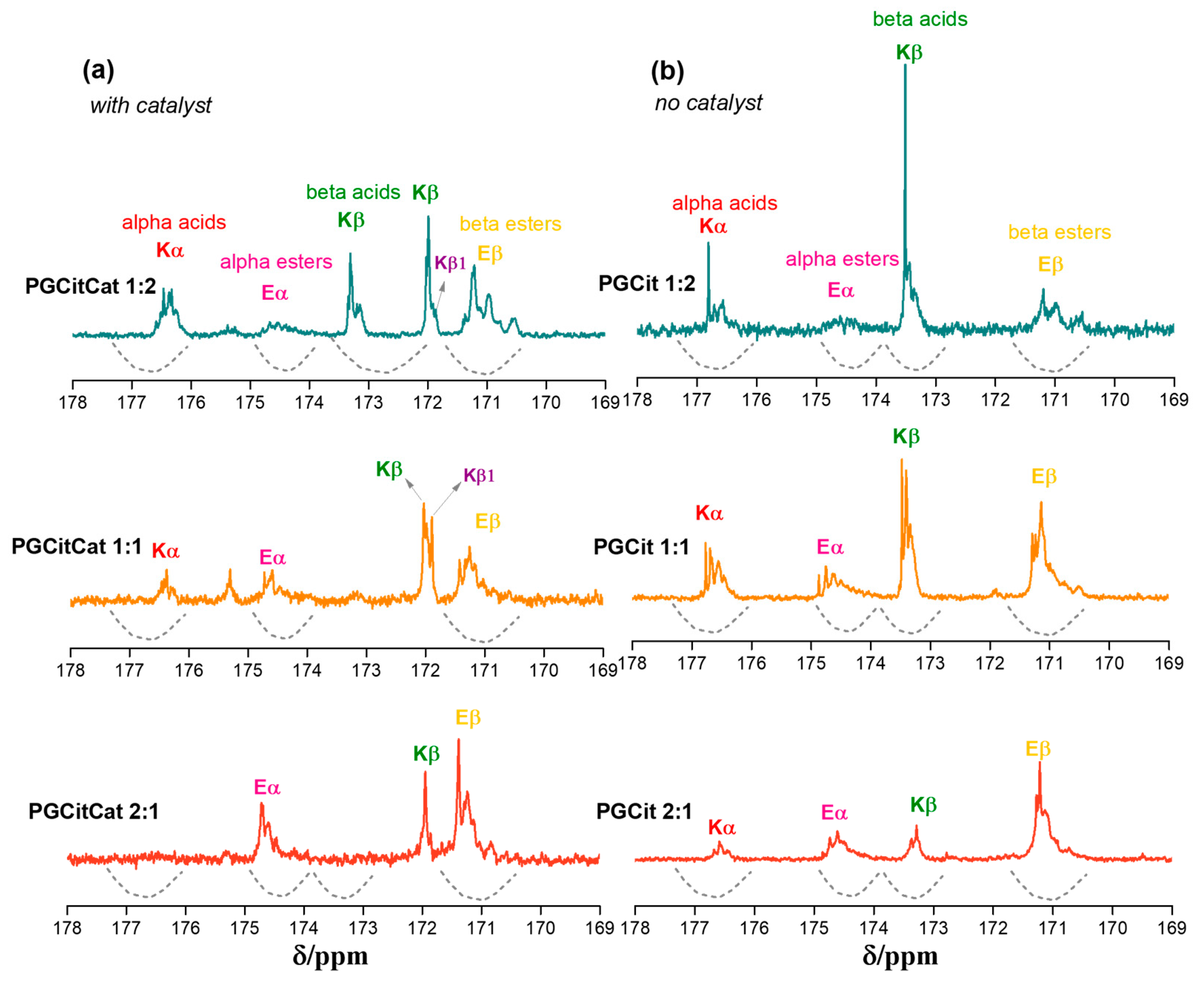
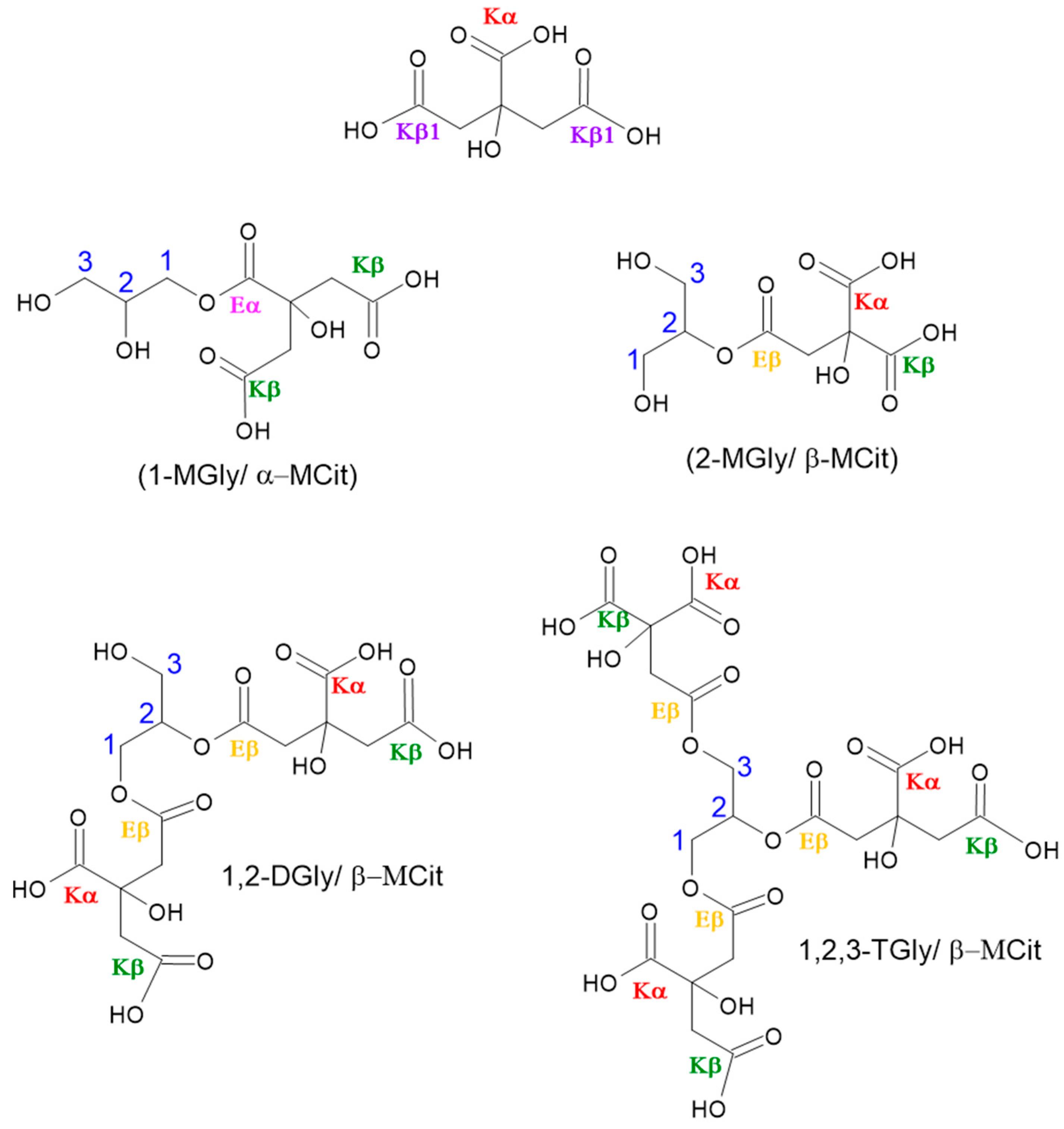
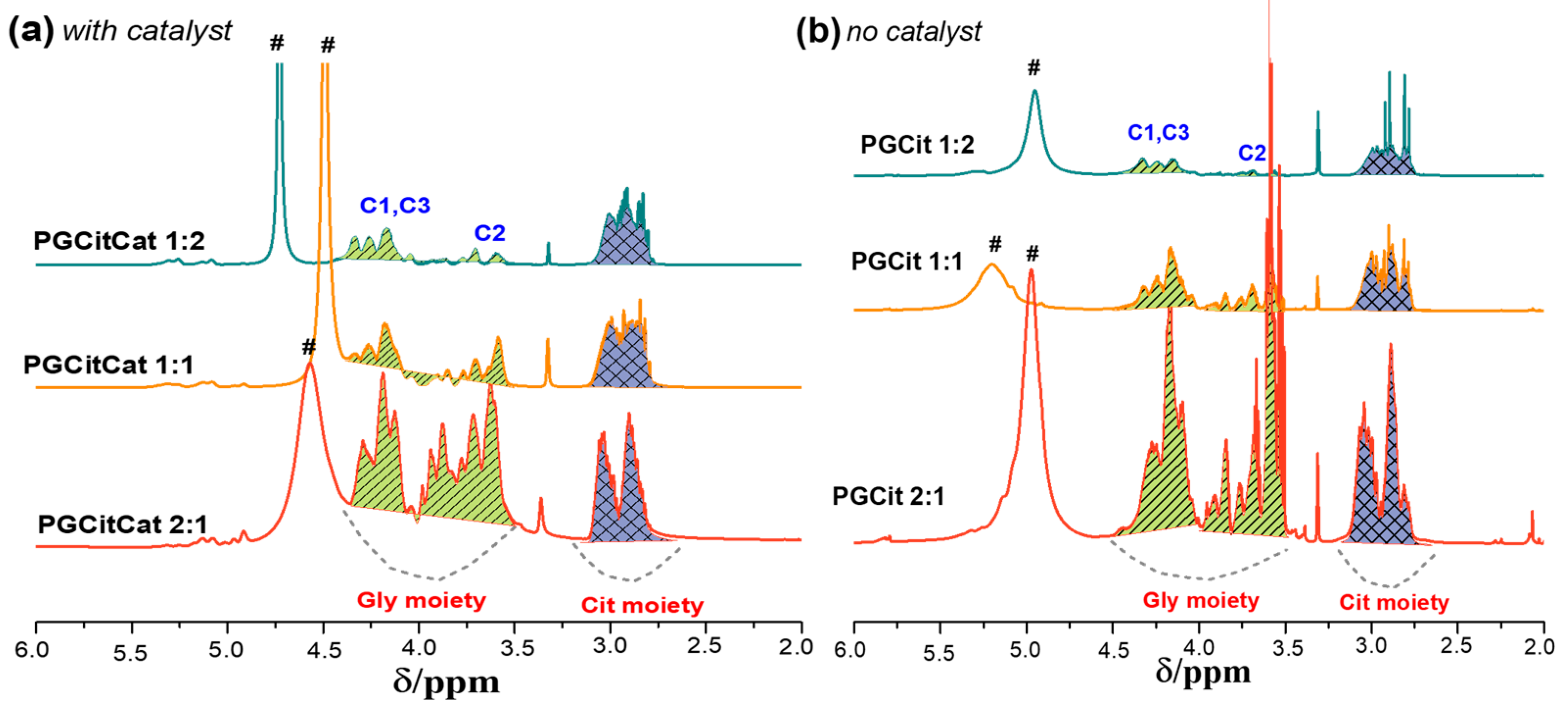
3.3. Polyester Structures by NMR Analysis
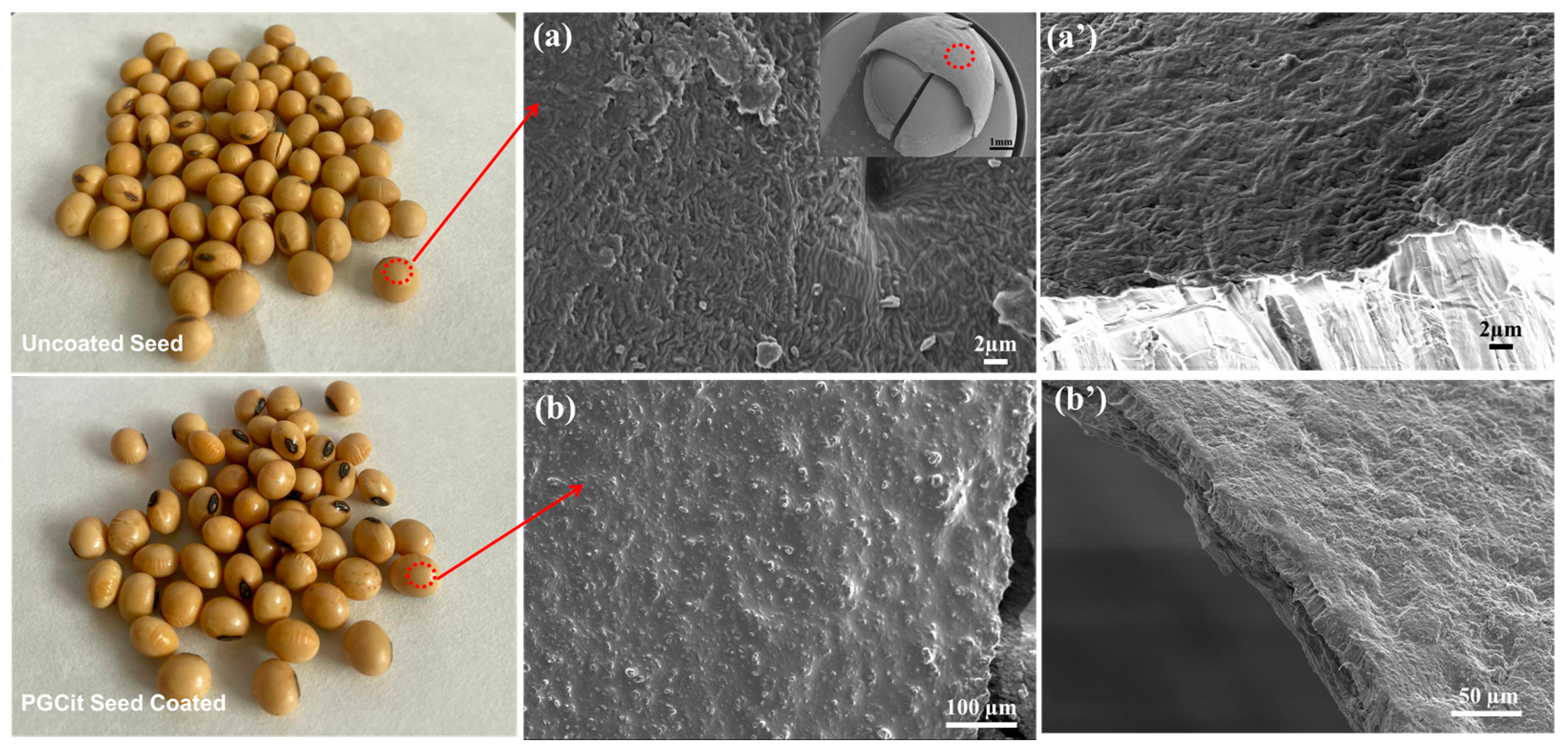
3.4. Germination Tests of Coated vs. Non-Coated Soybean Seeds
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Javed, T.; Afzal, I.; Mauro, R.P. Seed Coating in Direct Seeded Rice: An Innovative and Sustainable Approach to Enhance Grain Yield and Weed Management under Submerged Conditions. Sustainability 2021, 13, 2190. [Google Scholar] [CrossRef]
- Baroni, D.F.; Vieira, H.D. Coating Seeds with Fertilizer: A Promising Technique for Forage Crop Seeds. Cienc. E Agrotecnologia 2020, 44, 1–11. [Google Scholar] [CrossRef]
- Accinelli, C.; Abbas, H.K.; Little, N.S.; Kotowicz, J.K.; Shier, W.T. Biological Control of Aflatoxin Production in Corn Using Non-Aflatoxigenic Aspergillus Flavus Administered as a Bioplastic-Based Seed Coating. Crop Prot. 2018, 107, 87–92. [Google Scholar] [CrossRef]
- Alexandre Gonçalves Avelar, S.; Valéria de Sousa, F.; Fiss, G.; Baudet, L.; Teichert Peske, S. The Use of Film Coating on the Performance of Treated Corn Seed 1. Rev. Bras. Sementes 2012, 34, 186–192. [Google Scholar] [CrossRef]
- Nehra, A.; Biswas, D.; Siracusa, V.; Roy, S. Natural Gum-Based Functional Bioactive Films and Coatings: A Review. Int. J. Mol. Sci. 2022, 24, 485. [Google Scholar] [CrossRef] [PubMed]
- Gohari, N.R.; Modiri, S.; Yari, H.; Saffari, M.; Baghizadeh, A. The Application of Hydrophilic Polyvinyl Alcohol Coatings Filled with Different Loadings of Zinc Oxide Nanoparticles to Mitigate Salinity Stress of the Wheat Seeds. J. Appl. Polym. Sci. 2023, 140, e53742. [Google Scholar] [CrossRef]
- Clemente, M.; Rocha, R.J.; Iha, K.; Rocco, J.A.F.F. Development of Prepolymer Technology in the Synthesis of a Polyurethane Binder Used in Solid Rocket Fuels. Quim. Nova 2014, 37, 982–988. [Google Scholar] [CrossRef]
- Zafar, F.; Ghosal, A.; Sharmin, E.; Chaturvedi, R.; Nishat, N. A Review on Cleaner Production of Polymeric and Nanocomposite Coatings Based on Waterborne Polyurethane Dispersions from Seed Oils. Prog. Org. Coat. 2019, 131, 259–275. [Google Scholar] [CrossRef]
- Paravar, A.; Piri, R.; Balouchi, H.; Ma, Y. Microbial Seed Coating: An Attractive Tool for Sustainable Agriculture. Biotechnol. Rep. 2023, 37, e00781. [Google Scholar] [CrossRef]
- Ramakrishnan, R.; Kulandhaivelu, S.V.; Roy, S. Alginate/Carboxymethyl Cellulose/Starch-Based Active Coating with Grapefruit Seed Extract to Extend the Shelf Life of Green Chilli. Ind. Crops Prod. 2023, 199, 116752. [Google Scholar] [CrossRef]
- Bortoletto-Santos, R.; Guimarães, G.G.F.; Junior, V.R.; Da Cruz, D.F.; Polito, W.L.; Ribeiro, C. Biodegradable Oil-Based Polymeric Coatings on Urea Fertilizer: N Release Kinetic Transformations of Urea in Soil. Sci. Agric. 2020, 77, e20180033. [Google Scholar] [CrossRef]
- Kaikade, D.S.; Sabnis, A.S. Recent Advances in Polyurethane Coatings and Adhesives Derived from Vegetable Oil-Based Polyols. J. Polym. Environ. 2023, 31, 4583–4605. [Google Scholar] [CrossRef]
- International Energy Agency. Renewable Energy Market Update-Outlook for 2021 and 2022; International Energy Agency: Paris, France, 2021. [Google Scholar]
- Azerêdo, M.S.; Nunes, M.A.B.S.; Figueiredo, L.R.F.; Oliveira, J.E.; Tonoli, G.D.; de Barros, S.; Medeiros, E.S. Environmentally Friendly Adhesives Derived from Glycerol-Based Polymers. J. Adhes. Sci. Technol. 2022, 36, 98–108. [Google Scholar] [CrossRef]
- Mendonça, F.G.; Menezes, I.R.S.; Silva, I.F.; Lago, R.M. Multifunctional Glycerol/Citric Acid Crosslinked Polymer Hydrophilic Gel with Absorptive and Reducing Properties. New J. Chem. 2021, 45, 2410–2416. [Google Scholar] [CrossRef]
- Markets, R. Global Citric Acid Markets Report, 2011–2018 & 2019–2024. Cision. 2019. Available online: https://www.prnewswire.com/ (accessed on 20 October 2023).
- Gadomska-Gajadhur, A.; Bandzerewicz, A.; Wrzecionek, M.; Ruśkowski, P. Biobased Poly(Glycerol Citrate) Synthesis Optimization via Design of Experiments. Polym. Adv. Technol. 2021, 32, 3982–3994. [Google Scholar] [CrossRef]
- Wrzecionek, M.; Matyszczak, G.; Bandzerewicz, A.; Ruśkowski, P.; Gadomska-Gajadhur, A. Kinetics of Polycondensation of Citric Acid with Glycerol Based on a Genetic Algorithm. Org. Process Res. Dev. 2021, 25, 271–281. [Google Scholar] [CrossRef]
- Wrzecionek, M.; Howis, J.; Ruskowski, P.; Gadomska-Gajadhur, A. Optimizing the Conditions of Pgsu Synthesis with Simplex Method. Chem. Process Eng.-Inz. Chem. I Proces. 2020, 41, 119–128. [Google Scholar] [CrossRef]
- Chandra Kumari, M.; Jaisankar, V. Synthesis and Characterisation of Poly (Glycerol-Co-Citrate)/n-HAp Composite for Biomedical Applications. Mater. Today Proc. 2018, 5, 8824–8831. [Google Scholar] [CrossRef]
- Adeli, M.; Rasoulian, B.; Saadatmehr, F.; Zabihi, F. Hyperbranched Poly(Citric Acid) and Its Application as Anticancer Drug Delivery System. J. Appl. Polym. Sci. 2013, 129, 3665–3671. [Google Scholar] [CrossRef]
- Tisserat, B.; O’Kuru, R.H.; Hwang, H.; Mohamed, A.A.; Holser, R. Glycerol Citrate Polyesters Produced through Heating without Catalysis. J. Appl. Polym. Sci. 2012, 125, 3429–3437. [Google Scholar] [CrossRef]
- Anastas, P.; Eghbali, N. Green Chemistry: Principles and Practice. Chem. Soc. Rev. 2010, 39, 301–312. [Google Scholar] [CrossRef] [PubMed]
- Sengupta, S.; Singh, A.; Dutta, K.; Sahu, R.P.; Kumar, S.; Goswami, C.; Chawla, S.; Goswami, L.; Bandyopadhyay, A. Branched/Hyperbranched Copolyesters from Poly(Vinyl Alcohol) and Citric Acid as Delivery Agents and Tissue Regeneration Scaffolds. Macromol. Chem. Phys. 2021, 222, 2100134. [Google Scholar] [CrossRef]
- Bodaghi, A.; Adeli, M.; Dadkhahtehrani, A.; Tu, Z. Synthesis of Polyglycerol-Citric Acid Nanoparticles as Biocompatible Vectors for Biomedical Applications. J. Mol. Liq. 2017, 242, 53–58. [Google Scholar] [CrossRef]
- Maria, R.M.; Moraes, T.B.; Magon, C.J.; Venâncio, T.; Altei, W.F.; Andricopulo, A.D.; Colnago, L.A. Processing of High Resolution Magic Angle Spinning Spectra of Breast Cancer Cells by the Filter Diagonalization Method. Analyst 2012, 137, 4546–4551. [Google Scholar] [CrossRef] [PubMed]
- Meneguzzo, M.R.R.; Meneghello, G.E.; Nadal, A.P.; Xavier, F.D.M.; Dellagostin, S.M.; Carvalho, I.R.; Gonçalves, V.P.; Lautenchleger, F.; Lângaro, N.C. Seedling Length and Soybean Seed Vigor. Cienc. Rural. 2021, 51. [Google Scholar] [CrossRef]
- Pramanick, D.; Ray, T.T. Synthesis and Biodegradation of Copolyesters from Citric Acid and Glycerol. Polym. Bull. 1988, 19, 365–370. [Google Scholar] [CrossRef]
- Zahlan, H.; Saeed, W.S.; Alrasheed, R.; Alandes, N.M.; Aouak, T. Synthesis of Poly (Citric Acid-Co-Glycerol) and Its Application as an Inhibitor of CaCO3 Deposition. Materials 2019, 12, 3800. [Google Scholar] [CrossRef]
- Berube, M.A.; Schorr, D.; Ball, R.J.; Landry, V.; Blanchet, P. Determination of In Situ Esterification Parameters of Citric Acid-Glycerol Based Polymers for Wood Impregnation. J. Polym. Environ. 2018, 26, 970–979. [Google Scholar] [CrossRef]
- Valerio, O.; Misra, M.; Mohanty, A.K. Poly(Glycerol-Co-Diacids) Polyesters: From Glycerol Biorefinery to Sustainable Engineering Applications, A Review. ACS Sustain. Chem. Eng. 2018, 6, 5681–5693. [Google Scholar] [CrossRef]
- Giroto, A.S.; do Valle, S.F.; Ribeiro, T.; Ribeiro, C.; Mattoso, L.H.C. Towards Urea and Glycerol Utilization as “Building Blocks” for Polyurethane Production: A Detailed Study about Reactivity and Structure for Environmentally Friendly Polymer Synthesis. React. Funct. Polym. 2020, 153, 104629. [Google Scholar] [CrossRef]
- Halpern, J.M.; Urbanski, R.; Weinstock, A.K.; Iwig, D.F.; Mathers, R.T.; Von Recum, H.A. A Biodegradable Thermoset Polymer Made by Esterification of Citric Acid and Glycerol. J. Biomed. Mater. Res. A 2014, 102, 1467–1477. [Google Scholar] [CrossRef] [PubMed]
- Noordover, B.A.J.; Duchateau, R.; van Benthem, R.A.T.M.; Ming, W.; Koning, C.E. Enhancing the Functionality of Biobased Polyester Coating Resins through Modification with Citric Acid. Biomacromolecules 2007, 8, 3860–3870. [Google Scholar] [CrossRef] [PubMed]
- Castellane, T.C.L.; Campanharo, J.C.; Colnago, L.A.; Coutinho, I.D.; Lopes, É.M.; Lemos, M.V.F.; de Macedo Lemos, E.G. Characterization of New Exopolysaccharide Production by Rhizobium Tropici during Growth on Hydrocarbon Substrate. Int. J. Biol. Macromol. 2017, 96, 361–369. [Google Scholar] [CrossRef] [PubMed]
- Duncan, T.M. 13C Chemical Shieldings in Solids. J. Phys. Chem. Ref. Data 1987, 16, 125–151. [Google Scholar] [CrossRef]
- Castro, J.M.; Montalbán, M.G.; Martínez-Pérez, N.; Domene-López, D.; Pérez, J.M.; Arrabal-Campos, F.M.; Fernández, I.; Martín-Gullón, I.; García-Quesada, J.C. Thermoplastic Starch/Polyvinyl Alcohol Blends Modification by Citric Acid–Glycerol Polyesters. Int. J. Biol. Macromol. 2023, 244, 125478. [Google Scholar] [CrossRef]
- Nair, L.S.; Laurencin, C.T. Biodegradable Polymers as Biomaterials. Prog. Polym. Sci. 2007, 32, 762–798. [Google Scholar] [CrossRef]
- Shon, K.-J.; Kim, Y.; Colnago, L.A.; Opella, S.J. NMR Studies of the Structure and Dynamics of Membrane-Bound Bacteriophage Pf1 Coat Protein. Science 1991, 252, 1303–1305. [Google Scholar] [CrossRef]
- Leo, G.C.; Colnago, L.A.; Valentine, K.G.; Opella, S.J. Dynamics of Fd Coat Protein in Lipid Bilayers. Biochemistry 1987, 26, 854–862. [Google Scholar] [CrossRef]
- Bundy, J.G.; Osborn, D.; Weeks, J.M.; Lindon, J.C.; Nicholson, J.K. An NMR-Based Metabonomic Approach to the Investigation of Coelomic Fluid Biochemistry in Earthworms under Toxic Stress. FEBS Lett. 2001, 500, 31–35. [Google Scholar] [CrossRef]
- Ma, Y.; Mou, Q.; Wang, D.; Zhu, X.; Yan, D. Dendritic Polymers for Theranostics. Theranostics 2016, 6, 930–947. [Google Scholar] [CrossRef]
- Scarsi, M.; Brandelero, R.P.H.; Deuner, C. Desempenho Germinativo de Sementes de Soja Revestidas Com Polímeros Hidrofílicos. Colloq. Agrar. 2020, 16, 48–59. [Google Scholar] [CrossRef]
- Hungria, M.; Nogueira, M.A.; Araujo, R.S. Soybean Seed Co-Inoculation with Bradyrhizobium spp. and Azospirillum Brasilense: A New Biotechnological Tool to Improve Yield and Sustainability. Am. J. Plant. Sci. 2015, 6, 811–817. [Google Scholar] [CrossRef]




| Nomenclature | Molar Ratio Gly:Cit | Glycerol | Citric Acid | Catalyst (H2SO4) | ||
|---|---|---|---|---|---|---|
| (g) | (mol) | (g) | (mol) | (mL) | ||
| PGCitCat 1:2 | 1:2 | 9.59 | 0.104 | 40 | 0.208 | - |
| PGCitCat 1:1 | 1:1 | 9.59 | 0.104 | 20 | 0.104 | |
| PGCitCat 2:1 | 2:1 | 19.18 | 0.208 | 20 | 0.104 | |
| PGCit 1:2 | 1:2 | 9.59 | 0.104 | 40 | 0.208 | 0.24 |
| PGCit 1:1 | 1:1 | 9.59 | 0.104 | 20 | 0.104 | 0.12 |
| PGCit 2:1 | 2:1 | 19.18 | 0.208 | 20 | 0.104 | 0.12 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Giroto, A.S.; Valle, S.F.; Borges, R.; Colnago, L.A.; Ribeiro, T.S.; Jablonowski, N.D.; Ribeiro, C.; Mattoso, L.H.C. Revealing the Structure Formation on Polyglycerol Citrate Polymers—An Environmentally Friendly Polyester as a Seed-Coating Material. Polymers 2023, 15, 4303. https://doi.org/10.3390/polym15214303
Giroto AS, Valle SF, Borges R, Colnago LA, Ribeiro TS, Jablonowski ND, Ribeiro C, Mattoso LHC. Revealing the Structure Formation on Polyglycerol Citrate Polymers—An Environmentally Friendly Polyester as a Seed-Coating Material. Polymers. 2023; 15(21):4303. https://doi.org/10.3390/polym15214303
Chicago/Turabian StyleGiroto, Amanda S., Stella F. Valle, Roger Borges, Luiz A. Colnago, Tatiana S. Ribeiro, Nicolai D. Jablonowski, Caue Ribeiro, and Luiz H. C. Mattoso. 2023. "Revealing the Structure Formation on Polyglycerol Citrate Polymers—An Environmentally Friendly Polyester as a Seed-Coating Material" Polymers 15, no. 21: 4303. https://doi.org/10.3390/polym15214303
APA StyleGiroto, A. S., Valle, S. F., Borges, R., Colnago, L. A., Ribeiro, T. S., Jablonowski, N. D., Ribeiro, C., & Mattoso, L. H. C. (2023). Revealing the Structure Formation on Polyglycerol Citrate Polymers—An Environmentally Friendly Polyester as a Seed-Coating Material. Polymers, 15(21), 4303. https://doi.org/10.3390/polym15214303










