Morphological Reconstruction of a Critical-Sized Bone Defect in the Maxillofacial Region Using Modified Chitosan in Rats with Sub-Compensated Type I Diabetes Mellitus
Abstract
:1. Introduction
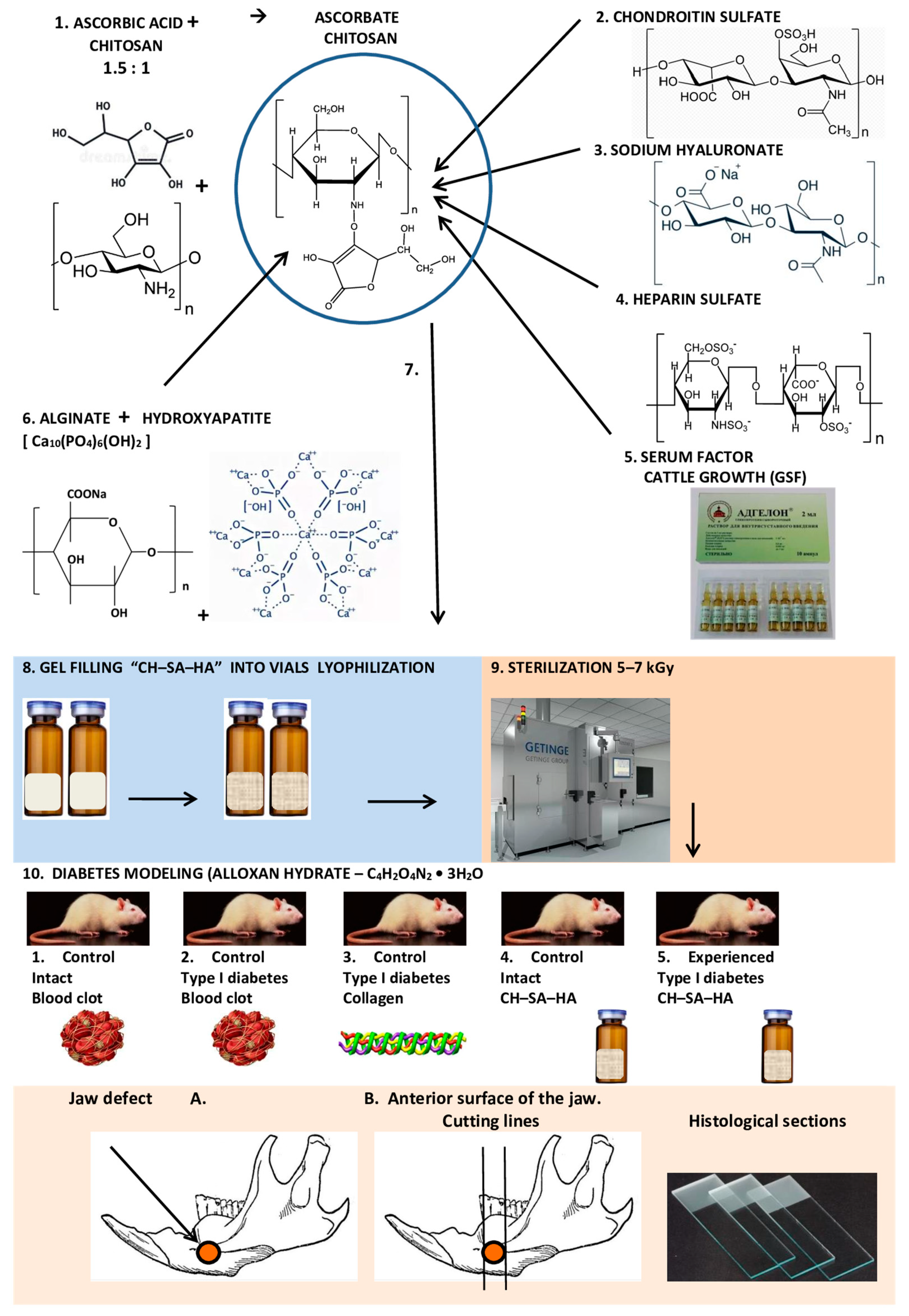
2. Materials and Methods
2.1. Composition and Production of Modified Chitosan
2.2. Animal Characterization and Modeling of Type I Diabetes Mellitus in Rats
2.3. The Conditions of Animal Detention
2.4. Modeling Defects of Critical Size in Rats
2.5. Postoperative Period
2.6. Morphological Analysis of Bone Tissue
- (BV)—Volumetric density of bone tissue, the percentage ratio of the volume occupied by bone structures to the total volume of the histological section;
- (BTT)—Thickness of bone trabeculae (mm). The criterion stipulates that the bone trabecula is a thin plate. Measurements were taken between the edges of the bone trabecula (5–8 measurements in relation to each trabecula with the calculation of the median);
- (ITS)—Intertrabecular spaces (mm), the distance between the edges of the cancellous bone trabeculae. The calculation is made in accordance with the so-called parallel plate model: BV minus BTT;
- (OBS)—Osteoblastic surface of bone trabeculae, the percentage ratio of the surface of bone trabeculae occupied by osteoblasts to the total bone surface;
- (OS)—Osteoid surface of bone trabeculae, the percentage ratio of the surface of bone trabeculae occupied by osteoid to the total bone surface, which was assessed by polarized light microscopy;
- (ES)—Eroded (osteoclastic) surface of bone trabeculae, the percentage ratio of the surface of bone trabeculae with the formation of gaps to the total bone surface, including the surface occupied by osteoclasts;
- (TBS)—Total bone surface;
- (FS)—Free surface of bone trabeculae, the percentage of the non-eroded surface of bone trabeculae and the surface not occupied by osteoblasts, osteoclasts to the total bone surface);
2.7. Statistical Analysis
3. Results
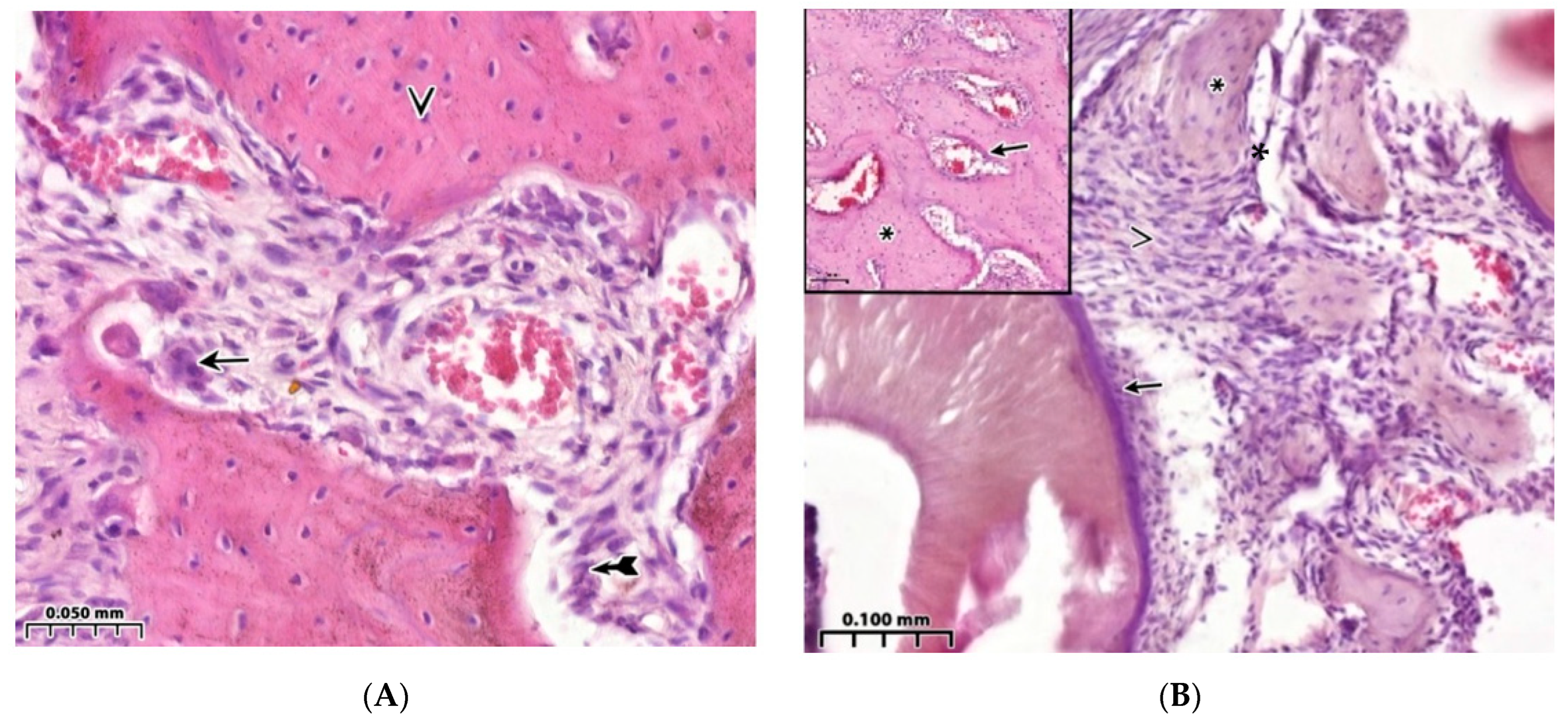
3.1. Morphological Analysis of the Bone Cavity Walls in Induced Type I Diabetes Mellitus Development
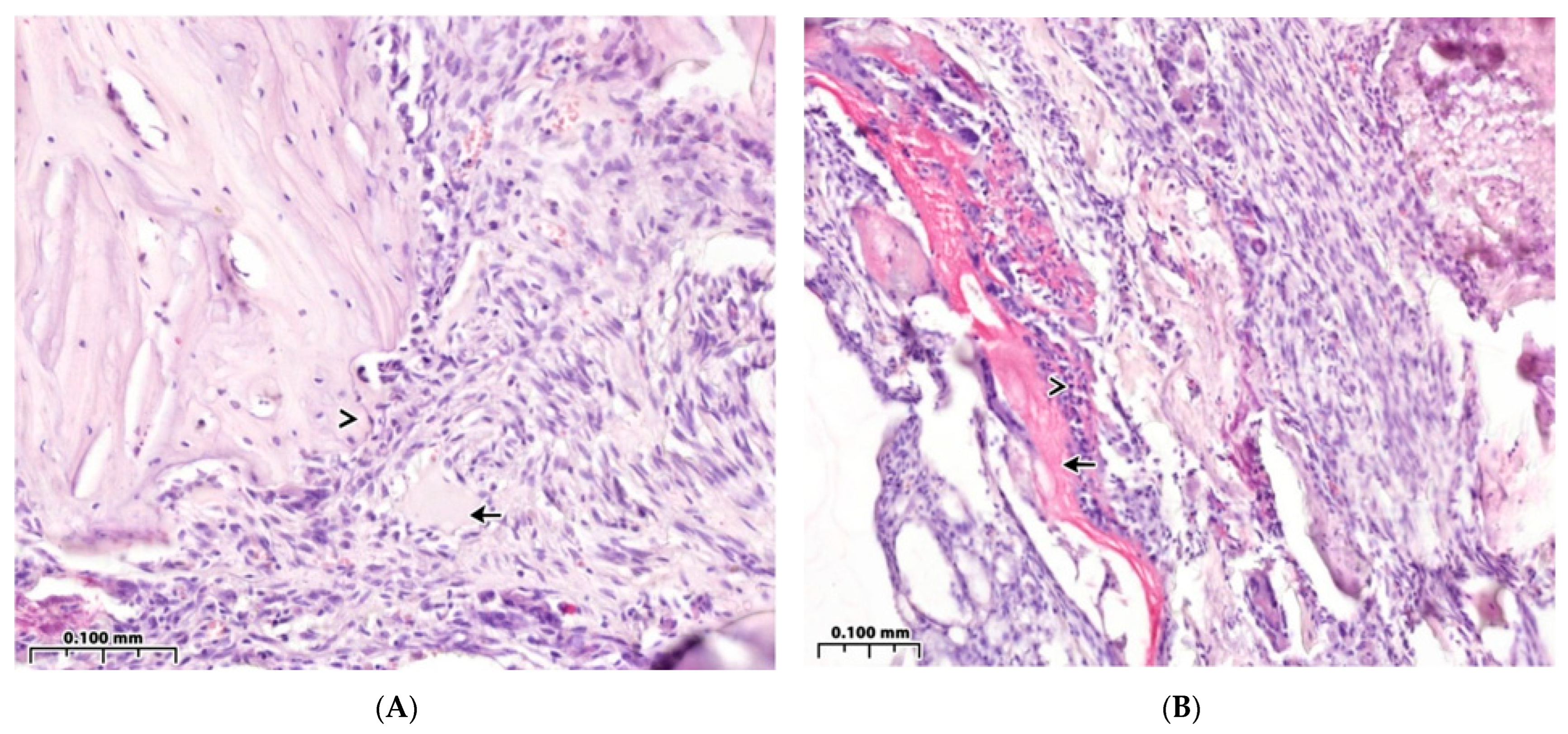
3.2. Regeneration of the Bone Cavity Walls Using a Collagen Sponge
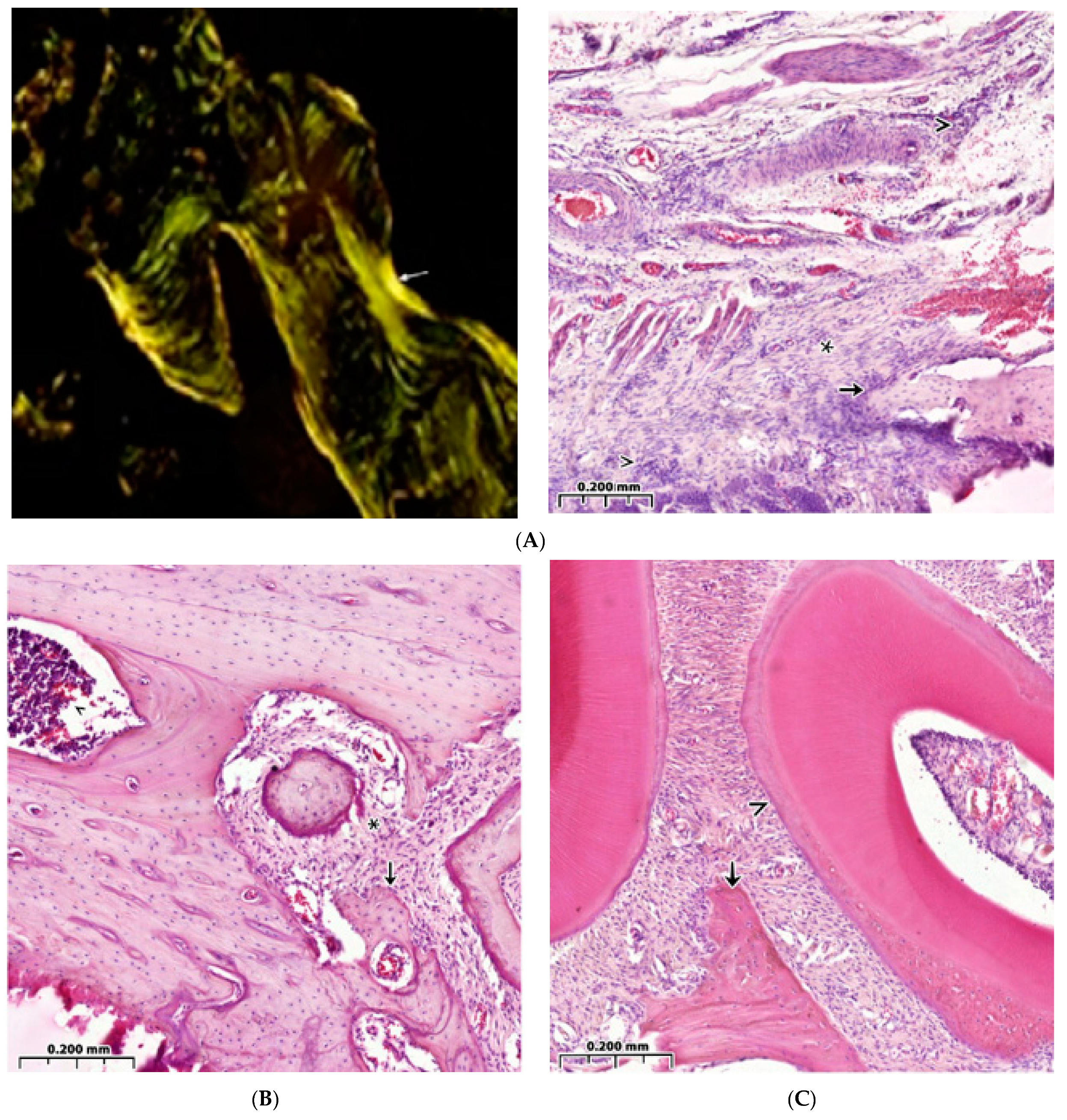
3.3. Regeneration of the Bone Cavity in Healthy Animals when Filling with CH–SA–HA
3.4. Bone Defect Regeneration in Animals with Sub-Compensated Diabetes Mellitus under CH–SA–HA Implantation
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Bolshakov, I.N.; Levenets, A.A.; Patlataya, N.N.; Nikolaenko, M.M.; Dmitrienko, A.E.; Ryaboshapko, E.I.; Matveeva, N.D.; Ibragimov, I.G.; Kotikov, A.R.; Furtsev, T.V. The Role of Modified Chitosan in Bone Engineering in Diabetes Mellitus: Analytical Review. Int. J. Dent. Oral. Health 2021, 7, 356. [Google Scholar]
- Napoli, N.; Chandran, M.; Pierroz, D.D.; Abrahamsen, B.; Schwartz, A.V.; Ferrari, S.L. Mechanisms of diabetes mellitus-induced bone fragility. Nat. Rev. Endocrinol. 2017, 13, 208–219. [Google Scholar] [CrossRef] [PubMed]
- Napoli, N.; Strollo, R.; Paladini, A.; Briganti, S.I.; Pozzilli, P.; Epstein, S. The alliance of mesenchymal stem cells, bone, and diabetes. Int. J. Endocrinol. 2014, 2014, 690783. [Google Scholar] [CrossRef] [PubMed]
- Landis, W.J. The strength of a calcified tissue depends in part on the molecular structure and organization of its constituent mineral crystals in their organic matrix. Bone 1995, 16, 533–544. [Google Scholar] [CrossRef]
- Lee, N.K.; Choi, Y.G.; Baik, J.Y.; Han, S.Y.; Jeong, D.-W.; Bae, Y.S.; Kim, N.; Lee, S.Y. A crucial role for reactive oxygen species in RANKL-induced osteoclast differentiation. Blood 2005, 106, 852–859. [Google Scholar] [CrossRef]
- Tanaka, S.; Nakamura, K.; Takahasi, N.; Suda, T. Role of RANKL in physiological and pathological bone resorption and therapeutics targeting the RANKL-RANK signaling system. Immunol. Rev. 2005, 208, 30–49. [Google Scholar] [CrossRef]
- Ha, H.; Kwak, H.B.; Lee, S.W.; Jin, H.M.; Kim, H.-M.; Kim, H.-H.; Lee, Z.H. Reactive oxygen species mediate RANK signaling in osteoclasts. Exp. Cell Res. 2004, 301, 119–127. [Google Scholar] [CrossRef]
- Liu, R.; Bal, H.S.; Desta, T.; Krothapalli, N.; Alyassi, M.; Luan, Q.; Graves, D.T. Diabetes enhances periodontal bone loss through enhanced resorption and diminished bone formation. J. Dent. Res. 2006, 85, 510–514. [Google Scholar] [CrossRef]
- Andriankaja, O.M.; Galicia, J.; Dong, G.; Xiao, W.; Alawi, F.; Graves, D.T. Gene expression dynamics during diabetic periodontitis. J. Dent. Res. 2012, 91, 1160–1165. [Google Scholar] [CrossRef]
- Pacios, S.; Andriankaja, O.; Kang, J.; Alnammary, M.; Bae, J.; Bezerra, B.d.B.; Schreiner, H.; Fine, D.H.; Graves, D.T. Bacterial infection increases periodontal bone loss in diabetic rats through enhanced apoptosis. Am. J. Pathol. 2013, 183, 1928–1935. [Google Scholar] [CrossRef]
- Alblowi, J.; Tian, C.; Siqueira, F.M.; Kayal, R.A.; McKenzie, E.; Behl, Y.; Gerstenfeld, L.; Einhorn, T.A.; Graves, D.T. Chemokine expression is upregulated in chondrocytes in diabetic fracture healing. Bone 2013, 53, 294–300. [Google Scholar] [CrossRef] [PubMed]
- Katayama, Y.; Akatsu, T.; Yamamoto, M.; Kugai, N.; Nagata, N. Role of nonenzymatic glycosylation of type I collagen in diabetic osteopenia. J. Bone Miner. Res. 1996, 11, 931–937. [Google Scholar] [CrossRef] [PubMed]
- Stolzing, A.; Sellers, D.; Llewelyn, O.; Scutt, A. Diabetes induced changes in rat mesenchymal stem cells. Cells Tissues Organs 2010, 191, 453–465. [Google Scholar] [CrossRef]
- Bartell, S.M.; Kim, H.-N.; Ambrogini, E.; Han, L.; Iyer, S.; Ucer, S.S.; Rabinovitch, P.; Jilka, R.L.; Weinstein, R.S.; Zhao, H.; et al. FoxO proteins restrain osteoclastogenesis and bone resorption by attenuating H2O2 accumulation. Nat. Commun. 2014, 5, 3773. [Google Scholar] [CrossRef]
- Wang, Y.; Dong, G.; Jeon, H.H.; Elazizi, M.; La, L.B.; Hameedaldeen, A.; Xiao, E.; Tian, C.; Alsadun, S.; Choi, Y.; et al. FOXO1 mediates RANKL-induced osteoclast formation and activity. J. Immunol. 2015, 194, 2878–2887. [Google Scholar] [CrossRef]
- Kang, J.; de Bezerra, B.B.; Pacios, S.; Andriankaja, O.; Tsiagbe, V.; Li, Y.; Schreiner, H.; Fine, D.H.; Graves, D.T. Aggregatibacter actinomycetem comitans infection enhances apoptosis in vivo through a caspase-3-dependent mechanism in experimental periodontitis. Infect. Immun. 2012, 80, 2247–2256. [Google Scholar] [CrossRef] [PubMed]
- Saito, M.; Marumo, K. Collagen cross-links as a determinant of bone quality: A possible explanation for bone fragility in aging, osteoporosis, and diabetes mellitus. Osteoporos. Int. 2010, 21, 195–214. [Google Scholar] [CrossRef]
- Mahamed, D.A.; Marleau, A.; Alnaeeli, M.; Singh, B.; Zhang, X.; Penninger, J.M.; Teng, T.-Y.A. G(−) anaerobes-reactive CD4+ T-cells trigger RANKL-mediated enhanced alveolar bone loss in diabetic NOD mice. Diabetes 2005, 54, 1477–1486. [Google Scholar] [CrossRef]
- Shetty, S.; Kapoor, N.; Bondu, J.D.; Thomas, N.; Paul, T.V. Bone turnover markers: Emerging tool in the management of osteoporosis. Indian J. Endocrinol. Metab. 2016, 20, 846–852. [Google Scholar]
- Maggio, A.B.R.; Ferrari, S.; Kraenzlin, M.; Marchand, L.M.; Schwitzgebel, V.; Beghetti, M.; Rizzoli, R.; Farpour-Lambert, N.J. Decreased bone turnover in children and adolescents with well controlled type 1 diabetes. J. Pediatr. Endocrinol. Metab. 2010, 23, 697–707. [Google Scholar] [CrossRef]
- Kayal, R.A.; Tsatsas, D.; Bauer, M.A.; Allen, B.; Al-Sebaei, M.O.; Kakar, S.; Leone, C.W.; Morgan, E.F.; Gerstenfeld, L.C.; Einhorn, T.A.; et al. Diminished Bone Formation During Diabetic Fracture Healing is Related to the Premature Resorption of Cartilage Associated With Increased Osteoclast Activity. J. Bone Miner. Res. 2007, 22, 560–568. [Google Scholar] [CrossRef] [PubMed]
- Kayal, R.A.; Siqueira, M.; Alblowi, J.; McLean, J.; Krothapalli, N.; Faibish, D.; Einhorn, T.A.; Gerstenfeld, L.C.; Graves, D.T. TNF-alpha mediates diabetes-enhanced chondrocyte apoptosis during fracture healing and stimulates chondrocyte apoptosis through FOXO1. J. Bone Miner. Res. 2010, 25, 1604–1615. [Google Scholar] [CrossRef] [PubMed]
- Claes, L.; Recknagel, S.; Ignatius, A. Fracture healing under healthy and inflammatory conditions. Nat. Rev. Rheumatol. 2012, 8, 133–143. [Google Scholar] [CrossRef]
- Kozusko, S.D.; Riccio, C.; Goulart, M.; Bumgardner, J.; Jing, X.L.; Konofaos, P. Chitosan as a bone scaffold biomaterial. J. Craniofac. Surg. 2018, 29, 1788–1793. [Google Scholar] [CrossRef] [PubMed]
- Kumbhar, S. Self-functionalized, oppositely charged chitosan-alginate scaffolds for biomedical applications. BioTechnol. Indian J. 2017, 3, 1–15. [Google Scholar]
- Rodríguez-Vázquez, M.; Vega-Ruiz, B.; Ramos-Zúñiga, R.; Saldaña-Koppel, D.A.; Quiñones-Olvera, L.F. Chitosan and its potential use as a scaffold for tissue engineering in regenerative medicine. BioMed. Res. Int. 2015, 2015, 821279. [Google Scholar] [CrossRef]
- Jiang, T.; Kumbar, S.G.; Nair, L.S.; Laurencin, C.T. Biologically active chitosan systems for tissue engineering and regenerative medicine. Curr. Top. Med. Chem. 2008, 8, 354–364. [Google Scholar] [PubMed]
- Costa-Pinto, A.R.; Reis, R.L.; Neves, N.M. Scaffolds based bone tissue engineering: The role of chitosan. Rev. Tissue Eng. Part B Rev. 2011, 17, 331–347. [Google Scholar] [CrossRef]
- Spin-Neto, R.; Coletti, F.L.; de Freitas, R.M.; Pavone, C.; Campana-Filholo, S.P.; Marcantonio, R.A.C. Chitosan-based biomaterials used in critical-size bone defects: Radiographic study in rat’s calvaria. Rev. Odontol. UNESP 2012, 41, 312–317. [Google Scholar] [CrossRef]
- Dhandayuthapani, B.; Yoshida, Y.; Maekawa, T.; Kumar, D.S. Polymeric scaffolds in tissue engineering application: A review. Int. J. Polym. Sci. 2011, 2011, 290602. [Google Scholar] [CrossRef]
- Bolshakov, I.N.; Levenetz, A.A.; Furtsev, T.V.; Kotikov, A.R.; Patlataya, N.N.; Ryaboshapko, E.I.; Dmitrienko, A.E.; Nikolaenko, M.M.; Matveeva, N.D.; Ibragimov, I.G. Experimental Reconstruction of Critical Size Defect of Bone Tissue in the Maxillofacial Region When Using Modified Chitosan. Biomed. Transl. Sci. 2022, 2, 1–8. [Google Scholar]
- Kirichenko, A.K.; Shulmin, A.V.; Sharkova, A.F.; Patlataya, N.N.; Bolshakov, I.N. Morphological Reconstruction of Main Arteries by Perivascular Implantation of Sulfated Chitosan in Experimental Atherosclerosis. Mod. Technol. Med. 2017, 9, 115–122. [Google Scholar] [CrossRef]
- Chatzipetros, E.; Christopoulos, P.; Donta, C.; Tosios, K.-I.; Tsiambas, E.; Tsiourvas, D.; Kalogirou, E.-M.; Tsiklakis, K. Application of nano-hydroxyapatite/chitosan scaffolds on rat calvarial critical-sized defects: A pilot study. Med. Oral Patol. Oral Cir. Bucal. 2018, 23, e625–e632. [Google Scholar] [CrossRef] [PubMed]
- Hu, J.X.; Ran, J.B.; Chen, S.; Jiang, P.; Shen, X.Y.; Tong, H. Carboxylated agarose (CA)-silk fibroin (SF) dual confluent matrices containing oriented hydroxyapatite (HA) crystals: Biomimetic organic/inorganic composites for tibia repair. Biomacromolecules 2016, 17, 2437–2447. [Google Scholar] [CrossRef] [PubMed]
- Luo, Y.; Lode, A.; Wu, C.; Chang, J.; Gelinsky, M. Alginate/nanohydroxyapatite scaffolds with designed core/shell structures fabricated by 3D plotting and in situ mineralization for bone tissue engineering. ACS Appl. Mater. Interfaces 2015, 7, 6541–6549. [Google Scholar] [CrossRef] [PubMed]
- Koshihara, Y.; Kawamura, M.; Oda, H.; Higaki, S. In vitro calcification in human osteoblastic cell line derived from periosteum. Biochem. Biophys. Res. Commun. 1987, 145, 651–657. [Google Scholar] [CrossRef]
- Chung, T.-W.; Liu, D.-Z.; Wang, S.-Y.; Wang, S.-S. Enhancement of the growth of human endothelial cells by surface roughness at nanometer scale. Biomaterials 2003, 24, 4655–4661. [Google Scholar] [CrossRef]
- Vissarionov, S.V.; Asadulaev, M.S.; Shabunin, A.S.; Yudin, V.E. Experimental evaluation of the efficiency of chitosan matrixes under conditions of modeling of a bone defect in vivo (preliminary report). Pediatr. Traumatol. Orthop. Reconstr. Surg. 2020, 8, 53–62. [Google Scholar] [CrossRef]
- Bolshakov, I.N.; Gorbunov, N.S.; Shamova, E.S.; Eremeev, A.V.; Sizykh, A.G.; Surkov, E.V.; Nasibov, S.M.; Maly, V.P.; Setkov, N.A. Wound Coating Based on Collagen-Chitosan Complex. Patent RF 2254145, 20 June 2005. [Google Scholar]
- Patel, Z.S.; Young, S.; Tabata, Y.; Jansen, J.A.; Wong, M.E.; Mikos, A.G. Dual delivery of an angiogenic and an osteogenic growth factor for bone regeneration in a critical size defect model. Bone 2008, 43, 931–940. [Google Scholar] [CrossRef]
- Tumshevits, O.N.; Bolshakov, I.N.; Belousova, Y.B.; Zykova, L.D.; Tumshevits, V.O. Method for Treating Periodontitis in Insulin Dependent Diabetes with the Drug “CAH–-bol”. Patent RF 2309748, 10 November 2007. [Google Scholar]
- Dempster, D.W.; Compston, J.E.; Drezner, M.K.; Glorieux, F.H.; Kanis, J.A.; Malluche, H.; Meunier, P.J.; Ott, S.M.; Recker, R.R.; Parfitt, A.M. Standardized nomenclature, symbols, and units for bone histomorphometry: A 2012 update of the report of the ASBMR Histomorphometry Nomenclature Committee. J. Bone Miner. Res. 2013, 28, 2–17. [Google Scholar] [CrossRef] [PubMed]
- Morgan, C.J. Use of proper statistical techniques for research studies with small samples. Am. J. Physiol. Lung Cell Mol. Physiol. 2017, 313, L873–L877. [Google Scholar] [CrossRef] [PubMed]
- Lovett, M.; Lee, K.; Edwards, A.; Kaplan, D.L. Vascularization strategies for tissue engineering. Tissue Eng. Part B Rev. 2009, 15, 353–370. [Google Scholar] [CrossRef] [PubMed]
- Hasegawa, T.; Yamamoto, T.; Tsuchiya, E.; Hongo, H.; Tsuboi, K.; Kudo, A.; Abe, M.; Yoshida, T.; Nagai, T.; Khadiza, N.; et al. Ultrastructural and biochemical aspects of matrix vesiclemediated mineralization. Jpn. Dent. Sci. Rev. 2017, 53, 34–45. [Google Scholar] [CrossRef]
- Amaral, I.F.; Neiva, I.; da Silva, F.F.; Sousa, S.R.; Piloto, A.M.; Lopes, C.D.F.; Barbosa, M.A.; Kirkpatrick, C.J.; Pego, A.P. Endothelialization of chitosan porous conduits via immobilization of a recombinant fibronectin fragment (rhFNIII(7-10)). Acta Biomater. 2013, 9, 5643–5652. [Google Scholar] [CrossRef]
- Sivaraj, K.K.; Adams, R.H. Blood vessel formation and function in bone. Development 2016, 143, 2706–2715. [Google Scholar] [CrossRef]
- Gorustovich, A.A.; Roether, J.A.; Boccaccini, A.R. Effect of bioactive glasses on angiogenesis: A Review of in vitro and in vivo evidences. Tissue Eng. Part B Rev. 2010, 16, 199–207. [Google Scholar] [CrossRef] [PubMed]
- Thomas, A.M.; Gomez, A.J.; Palma, J.L.; Yap, W.T.; Shea, L.D. Heparin-chitosan nanoparticle functionalization of porous poly(ethylene glycol) hydrogels for localized lentivirus delivery of angiogenic factors. Biomaterials 2014, 35, 8687–8693. [Google Scholar] [CrossRef] [PubMed]
- Stegen, S.; van Gastel, N.; Carmeliet, G. Bringing new life to damaged bone: The importance of angiogenesis in bone repair and regeneration. Bone 2015, 70, 19–27. [Google Scholar] [CrossRef]
- Kuttappan, S.; Mathew, D.; Jo, J.-I.; Tanaka, R.; Menon, D.; Ishimoto, T.; Nakano, T.; Nair, S.V.; Nair, M.B.; Tabata, Y. Dual release of growth factor from nanocomposite fibrous scaffold promotes vascularisation and bone regeneration in rat critical sized calvarial defect. Acta Biomater. 2018, 78, 36–47. [Google Scholar] [CrossRef]
- Nguyen, L.H.; Annabi, N.; Nikkhah, M.; Bae, H.; Binan, L.; Park, S.; Kang, Y.; Yang, Y.; Khademhosseini, A. Vascularized bone tissue engineering: Approaches for potential improvement. Tissue Eng. Part B Rev. 2012, 18, 363–382. [Google Scholar] [CrossRef]
- Vojtová, L.; Pavliňáková, V.; Muchová, J.; Kacvinská, K.; Brtníková, J.; Knoz, M.; Lipový, B.; Faldyna, M.; Göpfert, E.; Holoubek, J.; et al. Healing and angiogenic properties of collagen/chitosan scaffolds enriched with hyperstable FGF2-STAB® protein: In vitro, ex novo and in vivo comprehensive evaluation. Biomedicines 2021, 9, 590. [Google Scholar] [CrossRef] [PubMed]
- Habraken, W.; Habibovic, P.; Epple, M.; Bohner, M. Calcium phosphates in biomedical applications: Materials for the future? Mater. Today 2015, 19, 69–87. [Google Scholar] [CrossRef]
- Kokubo, T.; Kim, H.M.; Kawashita, M. Novel bioactive materials with different mechanical properties. Biomaterials 2003, 24, 2161–2175. [Google Scholar] [CrossRef]
- Kamakura, S.; Nakajo, S.; Suzuki, O.; Sasano, Y. New scaffold for recombinant human bonemorphogenetic protein-2. J. Biomed. Mater. Res. Part A 2004, 71, 299–307. [Google Scholar] [CrossRef]
- Fernández-Cervantes, I.; Morales, M.; Agustín-Serrano, R.; Cardenas-García, M.; Pérez-Luna, P.; Arroyo-Reyes, B.; Maldonado-García, A. Polylactic acid/sodium alginate/hydroxyapatite composite scaffolds with trabecular tissue morphology designed by a bone remodeling model using 3D printing. J. Mater. Sci. 2019, 54, 9478–9496. [Google Scholar] [CrossRef]
- Hu, Y.; Ma, S.; Yang, Z.; Zhou, W.; Du, Z.; Huang, J.; Yi, H.; Wang, C. Facile fabrication of poly (L-lactic acid) microsphere-incorporated calcium alginate/hydroxyapatite porous scaffolds based on Pickering emulsion templates. Colloids Surf. B. 2016, 140, 382–391. [Google Scholar] [CrossRef]
- Hokmabad, V.R.; Davaran, S.; Aghazadeh, M.; Rahbarghazi, R.; Salehi, R.; Ramazani, A. Fabrication and characterization of novel ethyl cellulose-grafted-poly (ε-caprolactone)/alginate nanofibrous/macroporous scaffolds incorporated with nano-hydroxyapatite for bone tissue engineering. J. Biomater. Appl. 2019, 33, 1128–1144. [Google Scholar] [CrossRef] [PubMed]
- Shen, H.; Hu, X.; Yang, F.; Bei, J.; Wang, S. Cell affinity for bFGF immobilized heparin-containing poly(lactide-co-glycolide) scaffolds. Biomaterials 2011, 32, 3404–3412. [Google Scholar] [CrossRef]
- Hu, X.X.; Shen, H.; Yang, F.; Bei, J.Z.; Wang, S.G. Preparation and cell affinity of microtubular orientation-structured PLGA(70/30) blood vessel scaffold. Biomaterials 2008, 29, 3128–3136. [Google Scholar] [CrossRef]
- Yeo, Y.J.; Jeon, D.W.; Kim, C.S.; Choi, S.H.; Cho, K.S.; Lee, Y.K.; Kim, C.-K. Effects of chitosan nonwoven membrane on periodontal healing of surgically created one-wall intrabony defects in beagle dogs. J. Biomed. Mater. Res. B Appl. Biomater. 2005, 72, 86–93. [Google Scholar] [CrossRef]
- Darnell, M.; Sun, J.; Mehta, M.; Johnson, C.; Arany, P.R.; Suo, Z.; Mooneyl, D.J. Performance and biocompatibility of extremely tough alginate/polyacrylamide hydrogels. Biomaterials 2013, 34, 8042–8048. [Google Scholar] [CrossRef] [PubMed]
- Tiǧli, R.S.; Gumüşderelioǧlu, M. Evaluation of alginate-chitosan semi IPNs as cartilage scaffolds. J. Mater. Sci. Mater. Med. 2009, 20, 699–709. [Google Scholar] [CrossRef]
- Matricardi, P.; Di Meo, C.; Coviello, T.; Hennink, W.E.; Alhaique, F. Interpenetrating polymer networks polysaccharide hydrogels for drug delivery and tissue engineering. Adv. Drug Deliv. Rev. 2013, 65, 1172–1187. [Google Scholar] [CrossRef] [PubMed]
- Venkatesan, J.; Bhatnagar, I.; Manivasagan, P.; Kang, K.-H.; Kim, S.-K. Alginate composites for bone tissue engineering: A review. Int. J. Biol. Macromol. 2015, 72, 269–281. [Google Scholar] [CrossRef]
- Muchová, J.; Hearnden, V.; Michlovská, L.; Vištejnová, L.; Zavad’áková, A.; Šmerková, K.; Kočiová, S.; Adam, V.; Kopel, P.; Vojtová, L. Mutual influence of selenium nanoparticles and FGF2-STAB® on biocompatible properties of collagen/chitosan 3D scaffolds: In vitro and ex novo evaluation. J. Nanobiotechnol. 2021, 19, 103. [Google Scholar] [CrossRef] [PubMed]
- Shchipunov, Y.A.; Postnova, I. Formation of calcium alginate-based macroporous materials comprising chitosan and hydroxyapatite. Colloid J. 2011, 73, 565–574. [Google Scholar] [CrossRef]
- Sharma, C.; Dinda, A.K.; Potdar, P.D.; Chou, C.-F.; Mishra, N.C. Fabrication and characterization of novel nano-biocomposite scaffold of chitosan–gelatin–alginate–hydroxyapatite for bone tissue engineering. Mater. Sci. Eng. C Mater. Biol. Appl. 2016, 64, 416–427. [Google Scholar] [CrossRef]
- Jin, H.-H.; Lee, C.-H.; Lee, W.-K.; Lee, J.-K.; Park, H.-C.; Yoon, S.-Y. In-situ formation of the hydroxyapatite/chitosan-alginate composite scaffolds. Mater. Lett. 2008, 62, 1630–1633. [Google Scholar] [CrossRef]
- Liu, D.; Liu, Z.; Zou, J.; Li, L.; Sui, X.; Wang, B.; Yang, N.; Wang, B. Synthesis and characterization of a hydroxyapatite-sodium alginate-chitosan scaffold for bone regeneration. Front. Mater. 2021, 8, 69. [Google Scholar] [CrossRef]
- Park, J.S.; Choi, S.H.; Moon, I.S.; Cho, K.S.; Chai, J.K.; Kim, C.K. Eight-week histological analysis on the effect of chitosan on surgically created one-wall intrabony defects in beagle dogs. J. Clin. Periodontol. 2003, 30, 443–453. [Google Scholar] [CrossRef] [PubMed]
- Belousova, Y.B. Use of the Medical Device “CAH–bol” for Secondary Prevention of Chronic Generalized Periodontitis in Patients with Type I Diabetes Mellitus. Master’s Thesis, Dissertation Research of Candidate of Medical Sciences. Specialty 01/14/14—Dentistry, Krasnoyarsk, Russia, 2010; 113p. [Google Scholar]
- Patlataya, N.N. Replacement of Bone Defects of the Jaws Using the Bone-Plastic Material “Bol-Chital”. Master’s Thesis, Dissertation Research of Candidate of Medical Sciences. Specialty 01/14/14—Dentistry, Krasnoyarsk, Russia, 2012; 126p. [Google Scholar]
- Levenets, A.A.; Bolshakov, I.N.; Chuchunov, A.A.; Barachtenko, N.N. Method for restoration of jaw bone tissue after cystectomy surgery. Patent RF No 2311181, 27 November 2007. [Google Scholar]
- Levenets, A.A.; Patlataya, N.N.; Bolshakov, I.N. Bol-chital is a new material for optimizing osteogenesis in dentistry. Sib. Med. Rev. 2009, 5, 84–86. [Google Scholar]
- Bolshakov, I.N.; Levenets, A.A.; Patlataya, N.N. Experience of using the new osteoplastic material “Bol-Chital” in patients with tumor-like diseases of the jaw bones. Sib. Med. Rev. 2010, 1, 83–84. [Google Scholar]

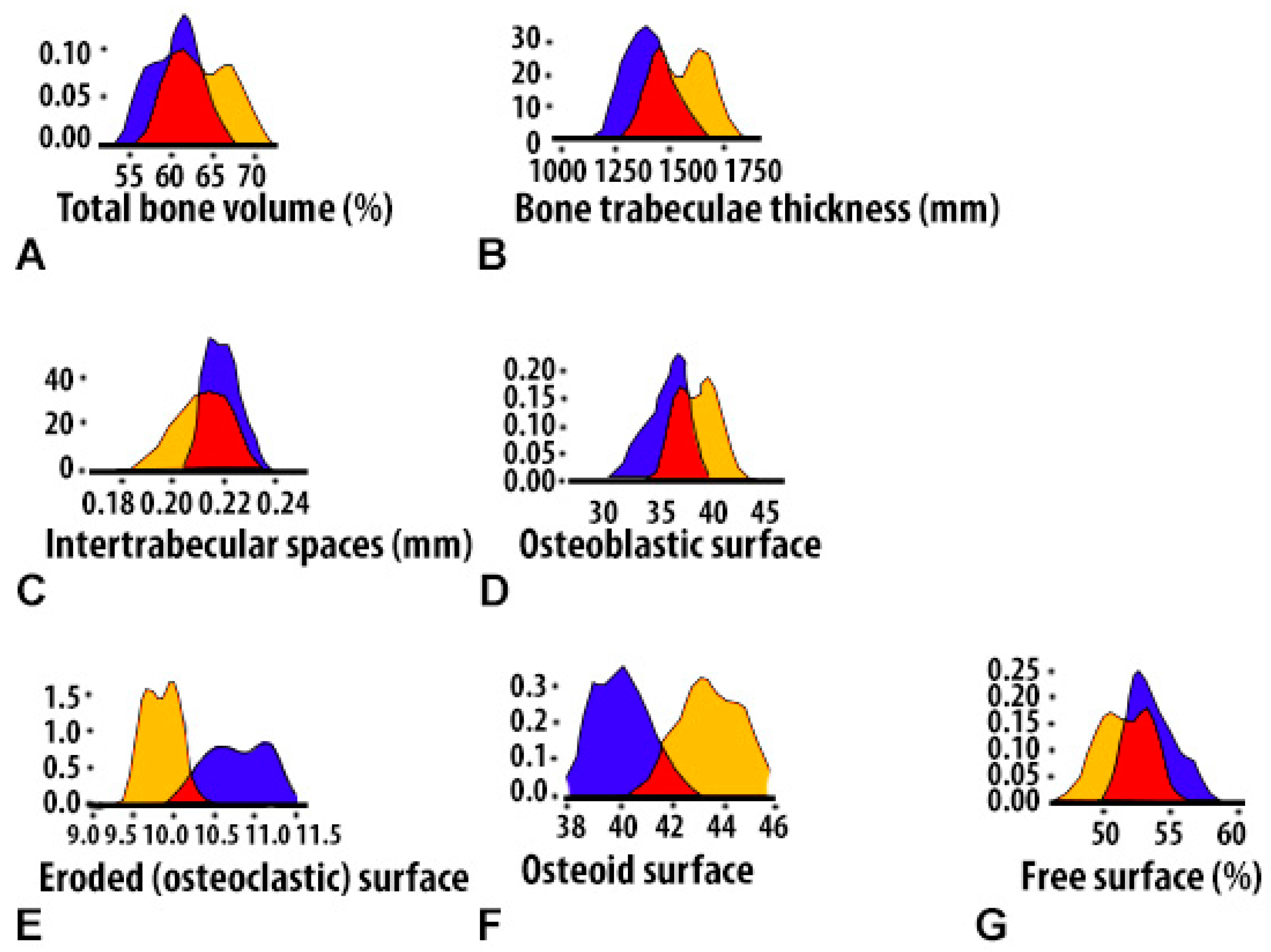
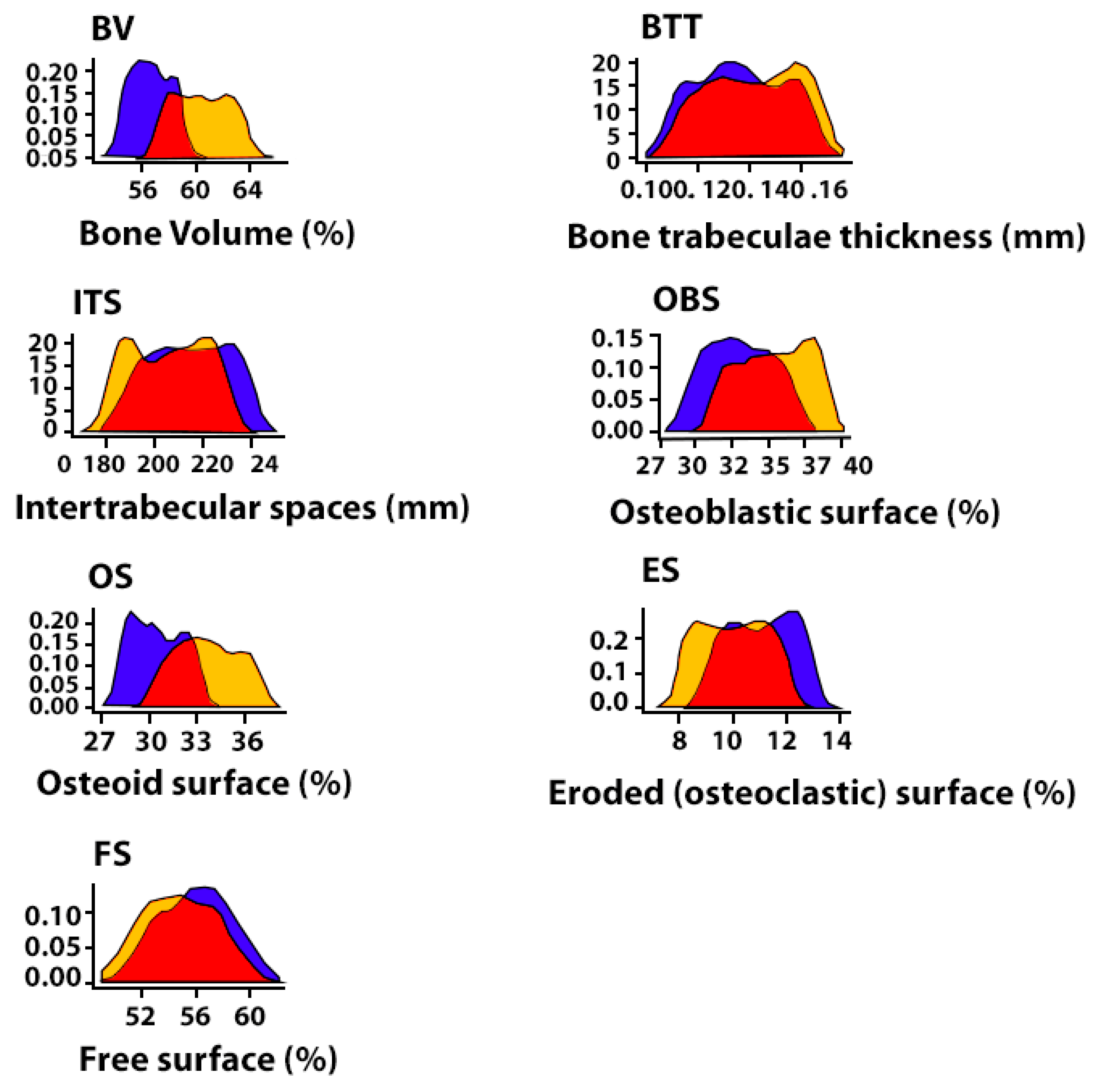


| Histomorpho Metric Criterion | 4-Week-Old Mandibular Defect Area | Peripheral Zone of Bone Control 4 Healthy (CH–SA–HA) | Peripheral Zone of Bone (Diabetes Mellitus) Collagen + CH–SA–HA | ||||
|---|---|---|---|---|---|---|---|
| Control 1 Healthy (under the Blood Clot) | Control 2 (Diabetes Mellitus) (under the Blood Clot) | Control 3 (Diabetes Mellitus) (Collagen) | Control 4 Healthy (CH–SA–HA) | Experienced (Diabetes Mellitus) (CH–SA–HA) | |||
| 1 | 2 | 3 | 4 | 5 | 6 | 7 | 8 |
| BV 1 (%) | 60.9[58.0;62.0] ** | 50.4[49.5;51.3] + | 56.4[55.3;57.7] *** | 62.9[60.7;66.8] | 60.3[58.5;62.1] † | 66.5[64.3;68.1] •• | 61.2[58.0;64.3] ++ |
| BTT 1 (mm) | 0.14[0.13;0.15] * | 0.12[0.10;0.13] + | 0.13[0.12;0.15] *** | 0.16[0.15;0.17] | 0.14[0.12;0.16] † | 0.16[0.15;0.17] •• | 0.14[0.13;0.16] ++ |
| ITS 1 (mm) | 0.22[0.21;0.22] ** | 0.20[0.19;0.21] | 0.21[0.20;0.22] *** | 0.21[0.21;0.22] | 0.20[0.19;0.21] † | 0.21[0.20;0.22] | 0.18[0.17;0.20] ++ |
| OBS 1 (%) | 36.0[34.7;37.0] ** | 29.1[28.5;29.8] + | 32.9[31.1;34.8] *** | 38.9[37.1;40.1] | 35.5[33.4;37.5] † | 7.2[6.3;7.8] •• | 4.5[3.9;5.3] ++ |
| OS 1 (%) | 40.0[39.1;40.7] ** | 27.1[26.0;28.2] + | 30.2[28.9;31.8] *** | 43.3[42.8;44.3] | 33.5[32.0;35.3] † | 10.3[9.6;11.0] •• | 6.9[5.9;8.0] ++ |
| ES 1 (%) | 10.7[10.5;11.1] ** | 15.4[14.2;16.6] + | 11.2[10.0;12.1] *** | 9.9[9.7;10.0] | 10.0[8.9;11.0] † | 1.3[1.2;1.4] •• | 1.3[1.1;1.4] ++ |
| FS 1 (%) | 53.1[52.5;54.7] ** | 62.0[60.3;63.7] + | 56.1[54.0;57.8] *** | 51.5[50.1;53.2] | 54.6[52.7;56.9] † | 91.4[90.9;92.6] •• | 94.1[93.4;94.8] ++ |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Patlataya, N.N.; Bolshakov, I.N.; Khorzhevskii, V.A.; Levenets, A.A.; Medvedeva, N.N.; Cherkashina, M.A.; Nikolaenko, M.M.; Ryaboshapko, E.I.; Dmitrienko, A.E. Morphological Reconstruction of a Critical-Sized Bone Defect in the Maxillofacial Region Using Modified Chitosan in Rats with Sub-Compensated Type I Diabetes Mellitus. Polymers 2023, 15, 4337. https://doi.org/10.3390/polym15214337
Patlataya NN, Bolshakov IN, Khorzhevskii VA, Levenets AA, Medvedeva NN, Cherkashina MA, Nikolaenko MM, Ryaboshapko EI, Dmitrienko AE. Morphological Reconstruction of a Critical-Sized Bone Defect in the Maxillofacial Region Using Modified Chitosan in Rats with Sub-Compensated Type I Diabetes Mellitus. Polymers. 2023; 15(21):4337. https://doi.org/10.3390/polym15214337
Chicago/Turabian StylePatlataya, Nadezhda N., Igor N. Bolshakov, Vladimir A. Khorzhevskii, Anatoli A. Levenets, Nadezhda N. Medvedeva, Mariya A. Cherkashina, Matvey M. Nikolaenko, Ekaterina I. Ryaboshapko, and Anna E. Dmitrienko. 2023. "Morphological Reconstruction of a Critical-Sized Bone Defect in the Maxillofacial Region Using Modified Chitosan in Rats with Sub-Compensated Type I Diabetes Mellitus" Polymers 15, no. 21: 4337. https://doi.org/10.3390/polym15214337






