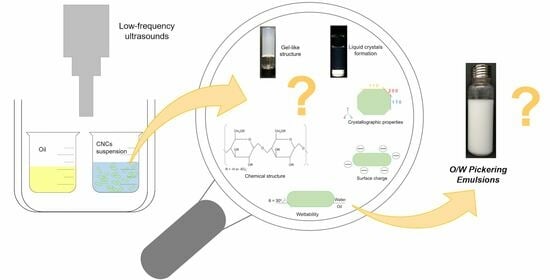
Low-Frequency Ultrasound Effects on Cellulose Nanocrystals for Potential Application in Stabilizing Pickering Emulsions
Abstract
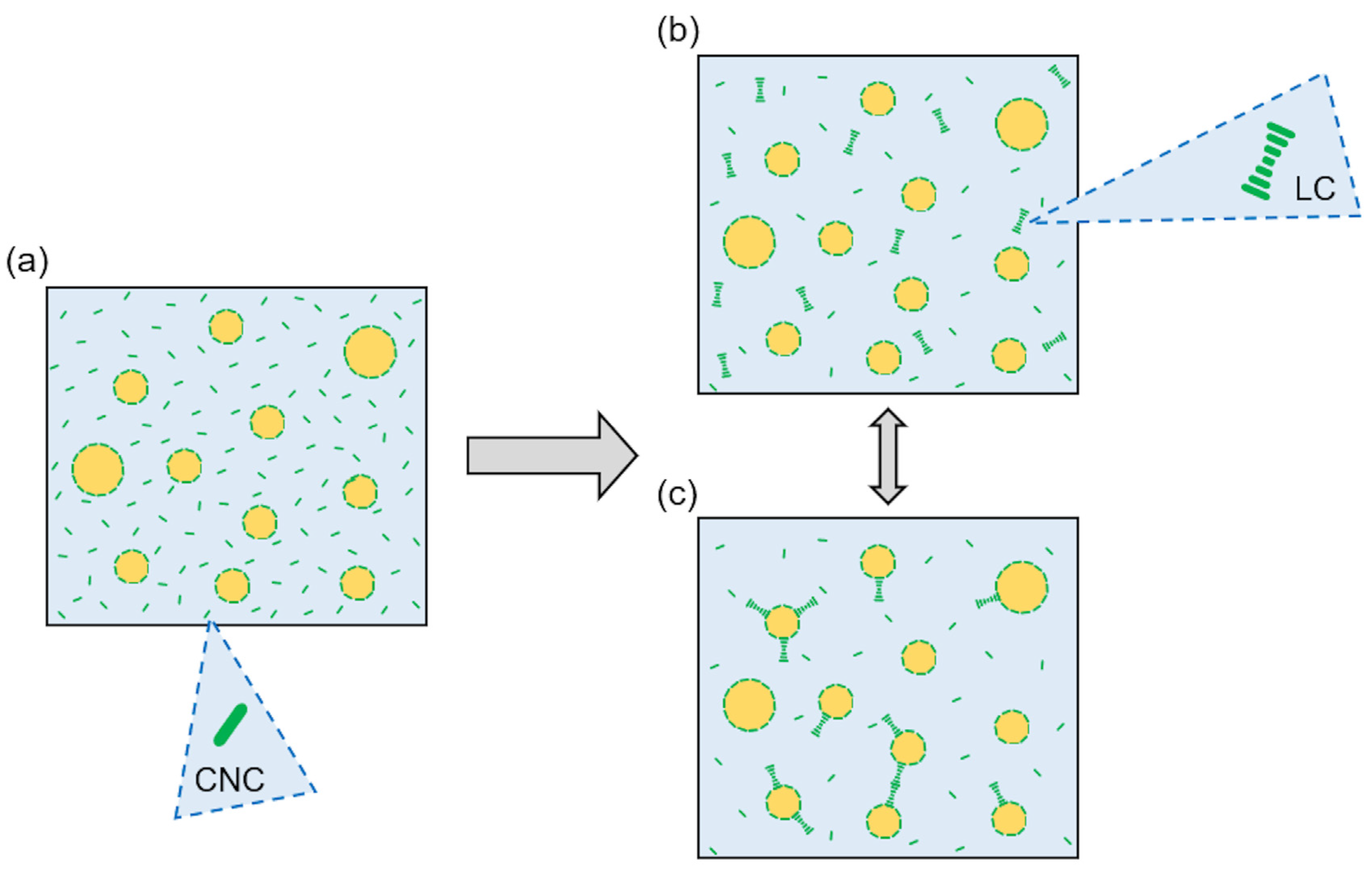
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. CNC Suspension Preparation and Low-Frequency Ultrasound Treatment
2.3. Size and ζ-Potential Measurements
2.4. Chemical Structure Analysis
2.5. X-ray Diffraction Measurements
2.6. Contact Angle Measurements
2.7. Rheological Measurements
2.8. Observation under Cross-Polarized Light
2.9. Suspension Stability Study
2.10. PE Preparation and Characterization
2.11. Statistical Analysis
3. Results and Discussion
3.1. LFU Effect on CNC Size
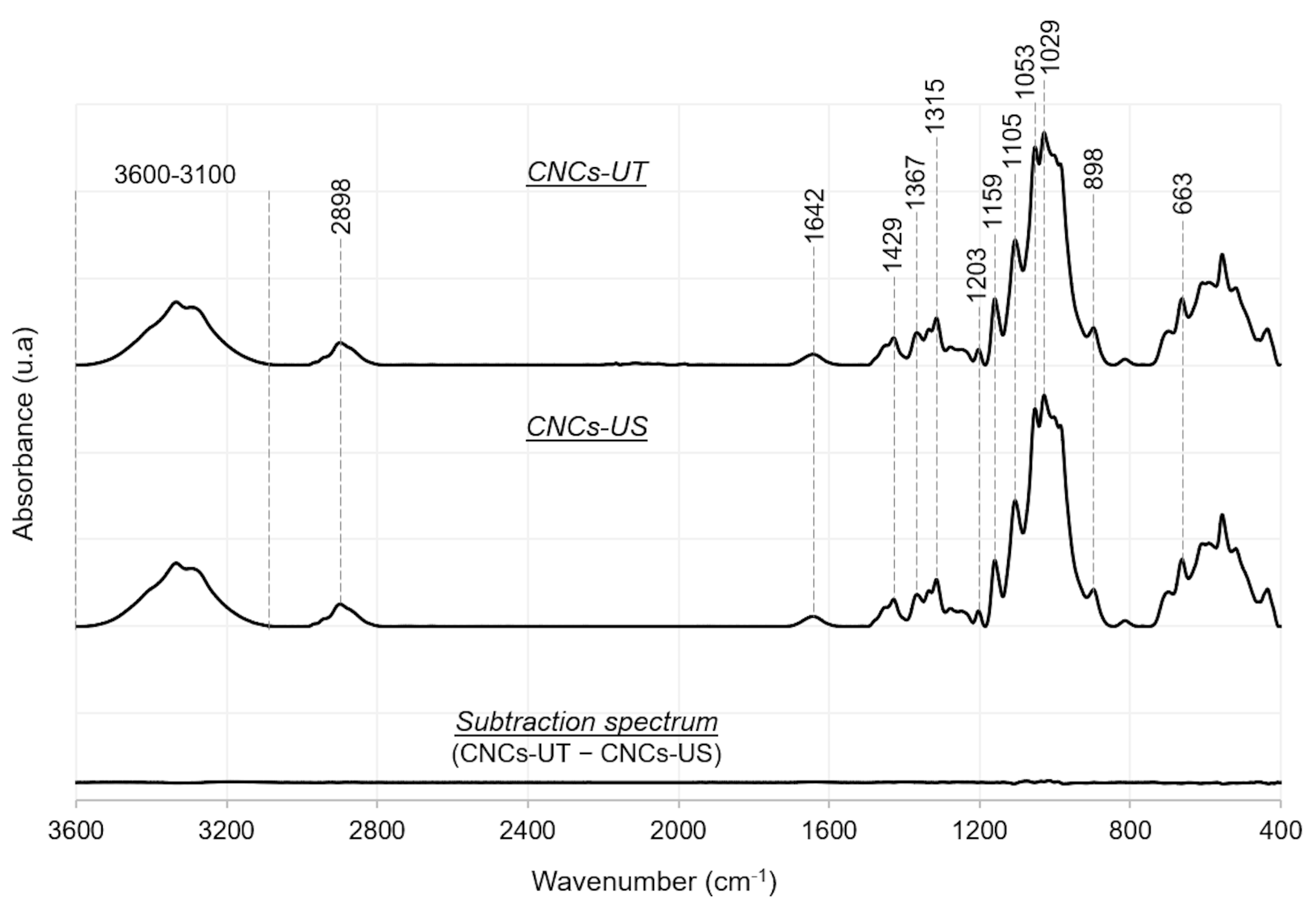
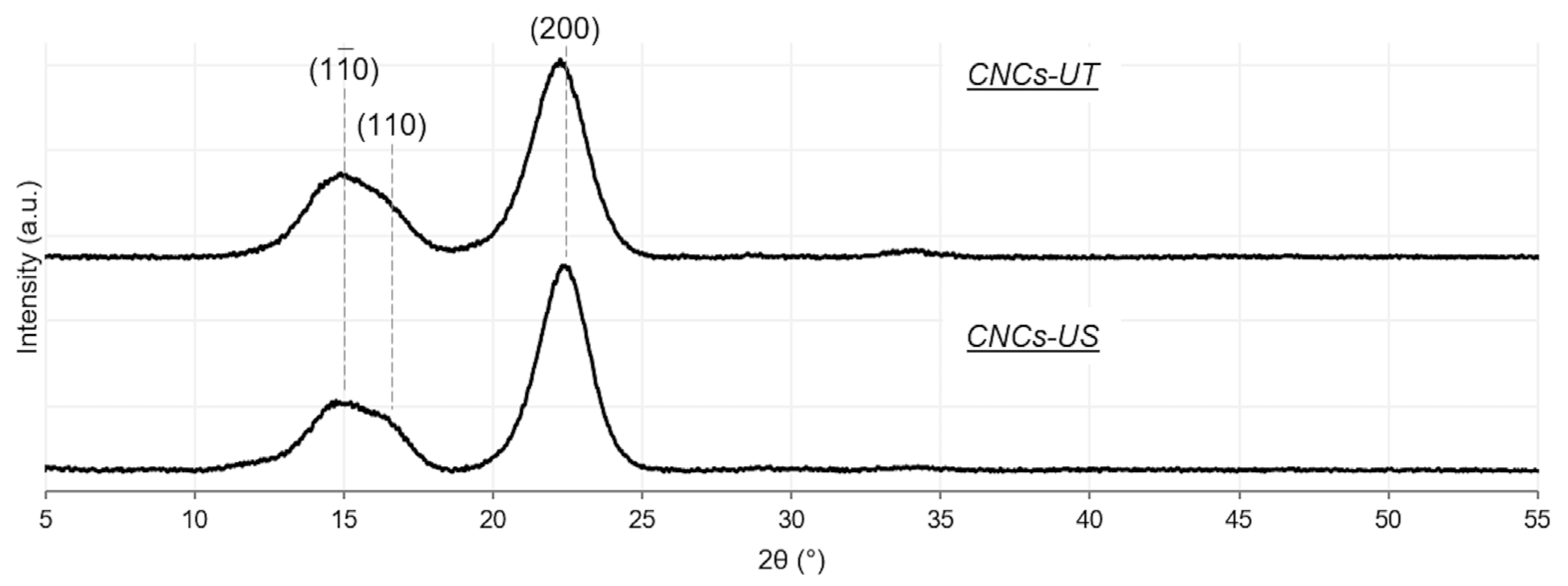
3.2. LFU Effect on the Chemical and Crystallographic Properties of CNCs
3.3. LFU Effect on CNC Charge
3.4. LFU Effect on CNC Hydrophilicity
3.5. LFU Effect on Rheological Properties
3.6. LFU Effect on Liquid Crystal Formation
3.7. LFU Effect on CNC Suspension Stability
3.8. LFU Effect on CNCs and Impact on PE Properties
3.8.1. Effect of CNC Particle Size and Shape on PEs
3.8.2. Effect of the CNC Particles’ Amphiphilic Properties and Charge on PEs
3.8.3. LC Effect on PE Stability
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Trache, D.; Hazwan Hussin, M.; Mohamad Haafiz, M.K.; Kumar Thakur, V. Recent Progress in Cellulose Nanocrystals: Sources and Production. Nanoscale 2017, 9, 1763–1786. [Google Scholar] [CrossRef] [PubMed]
- Nechyporchuk, O.; Belgacem, M.N.; Bras, J. Production of Cellulose Nanofibrils: A Review of Recent Advances. Ind. Crops Prod. 2016, 93, 2–25. [Google Scholar] [CrossRef]
- Rana, A.K.; Frollini, E.; Thakur, V.K. Cellulose Nanocrystals: Pretreatments, Preparation Strategies, and Surface Functionalization. Int. J. Biol. Macromol. 2021, 182, 1554–1581. [Google Scholar] [CrossRef]
- Nagarajan, K.J.; Ramanujam, N.R.; Sanjay, M.R.; Siengchin, S.; Surya Rajan, B.; Sathick Basha, K.; Madhu, P.; Raghav, G.R. A Comprehensive Review on Cellulose Nanocrystals and Cellulose Nanofibers: Pretreatment, Preparation, and Characterization. Polym. Compos. 2021, 42, 1588–1630. [Google Scholar] [CrossRef]
- Trache, D.; Tarchoun, A.F.; Derradji, M.; Hamidon, T.S.; Masruchin, N.; Brosse, N.; Hussin, M.H. Nanocellulose: From Fundamentals to Advanced Applications. Front. Chem. 2020, 8, 392. [Google Scholar] [CrossRef] [PubMed]
- Vanderfleet, O.M.; Cranston, E.D. Production Routes to Tailor the Performance of Cellulose Nanocrystals. Nat. Rev. Mater. 2021, 6, 124–144. [Google Scholar] [CrossRef]
- Grishkewich, N.; Mohammed, N.; Tang, J.; Tam, K.C. Recent Advances in the Application of Cellulose Nanocrystals. Curr. Opin. Colloid Interface Sci. 2017, 29, 32–45. [Google Scholar] [CrossRef]
- Seddiqi, H.; Oliaei, E.; Honarkar, H.; Jin, J.; Geonzon, L.C.; Bacabac, R.G.; Klein-Nulend, J. Cellulose and Its Derivatives: Towards Biomedical Applications. Cellulose 2021, 28, 1893–1931. [Google Scholar] [CrossRef]
- Meirelles, A.A.D.; Costa, A.L.R.; Cunha, R.L. Cellulose Nanocrystals from Ultrasound Process Stabilizing O/W Pickering Emulsion. Int. J. Biol. Macromol. 2020, 158, 75–84. [Google Scholar] [CrossRef]
- Chevalier, Y.; Bolzinger, M.-A. Emulsions Stabilized with Solid Nanoparticles: Pickering Emulsions. Colloids Surf. A Physicochem. Eng. Asp. 2013, 439, 23–34. [Google Scholar] [CrossRef]
- Cui, F.; Zhao, S.; Guan, X.; McClements, D.J.; Liu, X.; Liu, F.; Ngai, T. Polysaccharide-Based Pickering Emulsions: Formation, Stabilization and Applications. Food Hydrocoll. 2021, 119, 106812. [Google Scholar] [CrossRef]
- Pickering, S.U. CXCVI.—Emulsions. J. Chem. Soc. Trans. 1907, 91, 2001–2021. [Google Scholar] [CrossRef]
- Ramsden, W.; Gotch, F. Separation of Solids in the Surface-Layers of Solutions and ‘Suspensions’ (Observations on Surface-Membranes, Bubbles, Emulsions, and Mechanical Coagulation).—Preliminary Account. Proc. R. Soc. Lond. 1904, 72, 156–164. [Google Scholar] [CrossRef]
- Berton-Carabin, C.C.; Schroën, K. Pickering Emulsions for Food Applications: Background, Trends, and Challenges. Annu. Rev. Food Sci. Technol. 2015, 6, 263–297. [Google Scholar] [CrossRef] [PubMed]
- Gonzalez Ortiz, D.; Pochat-Bohatier, C.; Cambedouzou, J.; Bechelany, M.; Miele, P. Current Trends in Pickering Emulsions: Particle Morphology and Applications. Engineering 2020, 6, 468–482. [Google Scholar] [CrossRef]
- Xiao, J.; Li, Y.; Huang, Q. Recent Advances on Food-Grade Particles Stabilized Pickering Emulsions: Fabrication, Characterization and Research Trends. Trends Food Sci. Technol. 2016, 55, 48–60. [Google Scholar] [CrossRef]
- Ribeiro, E.F.; Morell, P.; Nicoletti, V.R.; Quiles, A.; Hernando, I. Protein- and Polysaccharide-Based Particles Used for Pickering Emulsion Stabilisation. Food Hydrocoll. 2021, 119, 106839. [Google Scholar] [CrossRef]
- Dai, H.; Wu, J.; Zhang, H.; Chen, Y.; Ma, L.; Huang, H.; Huang, Y.; Zhang, Y. Recent Advances on Cellulose Nanocrystals for Pickering Emulsions: Development and Challenge. Trends Food Sci. Technol. 2020, 102, 16–29. [Google Scholar] [CrossRef]
- Kalashnikova, I.; Bizot, H.; Cathala, B.; Capron, I. Modulation of Cellulose Nanocrystals Amphiphilic Properties to Stabilize Oil/Water Interface. Biomacromolecules 2012, 13, 267–275. [Google Scholar] [CrossRef]
- Aw, Y.Z.; Lim, H.P.; Low, L.E.; Surjit Singh, C.K.; Chan, E.S.; Tey, B.T. Cellulose Nanocrystal (CNC)-Stabilized Pickering Emulsion for Improved Curcumin Storage Stability. LWT 2022, 159, 113249. [Google Scholar] [CrossRef]
- Ni, Y.; Li, J.; Fan, L. Production of Nanocellulose with Different Length from Ginkgo Seed Shells and Applications for Oil in Water Pickering Emulsions. Int. J. Biol. Macromol. 2020, 149, 617–626. [Google Scholar] [CrossRef] [PubMed]
- Gauthier, G.; Capron, I. Pickering Nanoemulsions: An Overview of Manufacturing Processes, Formulations, and Applications. JCIS Open 2021, 4, 100036. [Google Scholar] [CrossRef]
- Beuguel, Q.; Tavares, J.R.; Carreau, P.J.; Heuzey, M.-C. Ultrasonication of Spray-and Freeze-Dried Cellulose Nanocrystals in Water. J. Colloid Interface Sci. 2018, 516, 23–33. [Google Scholar] [CrossRef] [PubMed]
- Gicquel, E.; Bras, J.; Rey, C.; Putaux, J.-L.; Pignon, F.; Jean, B.; Martin, C. Impact of Sonication on the Rheological and Colloidal Properties of Highly Concentrated Cellulose Nanocrystal Suspensions. Cellulose 2019, 26, 7619–7634. [Google Scholar] [CrossRef]
- Metzger, C.; Drexel, R.; Meier, F.; Briesen, H. Effect of Ultrasonication on the Size Distribution and Stability of Cellulose Nanocrystals in Suspension: An Asymmetrical Flow Field-Flow Fractionation Study. Cellulose 2021, 28, 10221–10238. [Google Scholar] [CrossRef]
- Xu, H.-N.; Tang, Y.-Y.; Ouyang, X.-K. Shear-Induced Breakup of Cellulose Nanocrystal Aggregates. Langmuir 2017, 33, 235–242. [Google Scholar] [CrossRef]
- Ni, Y.; Li, J.; Fan, L. Effects of Ultrasonic Conditions on the Interfacial Property and Emulsifying Property of Cellulose Nanoparticles from Ginkgo Seed Shells. Ultrason. Sonochem. 2021, 70, 105335. [Google Scholar] [CrossRef]
- Meirelles, A.A.D.; Costa, A.L.R.; Cunha, R.L. The Stabilizing Effect of Cellulose Crystals in O/W Emulsions Obtained by Ultrasound Process. Food Res. Int. 2020, 128, 108746. [Google Scholar] [CrossRef]
- Ming, Y.; Xia, Y.; Ma, G. Aggregating Particles on the O/W Interface: Tuning Pickering Emulsion for the Enhanced Drug Delivery Systems. Aggregate 2022, 3, e162. [Google Scholar] [CrossRef]
- Ni, Y.; Wu, J.; Jiang, Y.; Li, J.; Fan, L.; Huang, S. High-Internal-Phase Pickering Emulsions Stabilized by Ultrasound-Induced Nanocellulose Hydrogels. Food Hydrocoll. 2022, 125, 107395. [Google Scholar] [CrossRef]
- Perrin, L.; Desobry-Banon, S.; Gillet, G.; Desobry, S. Phase Diagram of Pickering Emulsions Stabilized by Cellulose Nanocrystals. Polymers 2023, 15, 2783. [Google Scholar] [CrossRef] [PubMed]
- Foster, E.J.; Moon, R.J.; Agarwal, U.P.; Bortner, M.J.; Bras, J.; Camarero-Espinosa, S.; Chan, K.J.; Clift, M.J.; Cranston, E.D.; Eichhorn, S.J. Current Characterization Methods for Cellulose Nanomaterials. Chem. Soc. Rev. 2018, 47, 2609–2679. [Google Scholar] [CrossRef] [PubMed]
- Zakani, B.; Entezami, S.; Grecov, D.; Salem, H.; Sedaghat, A. Effect of Ultrasonication on Lubrication Performance of Cellulose Nano-Crystalline (CNC) Suspensions as Green Lubricants. Carbohydr. Polym. 2022, 282, 119084. [Google Scholar] [CrossRef] [PubMed]
- Browne, C.; Raghuwanshi, V.S.; Lin, M.; Garnier, G.; Batchelor, W. Characterisation of Cellulose Nanocrystals by Rheology and Small Angle X-Ray Scattering (SAXS). Colloids Surf. A Physicochem. Eng. Asp. 2022, 651, 129532. [Google Scholar] [CrossRef]
- Chen, Y.W.; Lee, H.V.; Juan, J.C.; Phang, S.-M. Production of New Cellulose Nanomaterial from Red Algae Marine Biomass Gelidium Elegans. Carbohydr. Polym. 2016, 151, 1210–1219. [Google Scholar] [CrossRef] [PubMed]
- El Achaby, M.; El Miri, N.; Hannache, H.; Gmouh, S.; Aboulkas, A. Production of Cellulose Nanocrystals from Vine Shoots and Their Use for the Development of Nanocomposite Materials. Int. J. Biol. Macromol. 2018, 117, 592–600. [Google Scholar] [CrossRef]
- Jonoobi, M.; Oladi, R.; Davoudpour, Y.; Oksman, K.; Dufresne, A.; Hamzeh, Y.; Davoodi, R. Different Preparation Methods and Properties of Nanostructured Cellulose from Various Natural Resources and Residues: A Review. Cellulose 2015, 22, 935–969. [Google Scholar] [CrossRef]
- Hedjazi, S.; Razavi, S.H. A Comparison of Canthaxanthine Pickering Emulsions, Stabilized with Cellulose Nanocrystals of Different Origins. Int. J. Biol. Macromol. 2018, 106, 489–497. [Google Scholar] [CrossRef]
- Kumar, A.; Negi, Y.S.; Choudhary, V.; Bhardwaj, N.K. Characterization of Cellulose Nanocrystals Produced by Acid-Hydrolysis from Sugarcane Bagasse as Agro-Waste. J. Mater. Phys. Chem. 2014, 2, 1–8. [Google Scholar] [CrossRef]
- Ilyas, R.A.; Sapuan, S.M.; Ishak, M.R. Isolation and Characterization of Nanocrystalline Cellulose from Sugar Palm Fibres (Arenga Pinnata). Carbohydr. Polym. 2018, 181, 1038–1051. [Google Scholar] [CrossRef]
- Beck-Candanedo, S.; Roman, M.; Gray, D.G. Effect of Reaction Conditions on the Properties and Behavior of Wood Cellulose Nanocrystal Suspensions. Biomacromolecules 2005, 6, 1048–1054. [Google Scholar] [CrossRef] [PubMed]
- Yu, L.; Lin, J.; Tian, F.; Li, X.; Bian, F.; Wang, J. Cellulose Nanofibrils Generated from Jute Fibers with Tunable Polymorphs and Crystallinity. J. Mater. Chem. A 2014, 2, 6402–6411. [Google Scholar] [CrossRef]
- Gong, J.; Li, J.; Xu, J.; Xiang, Z.; Mo, L. Research on Cellulose Nanocrystals Produced from Cellulose Sources with Various Polymorphs. RSC Adv. 2017, 7, 33486–33493. [Google Scholar] [CrossRef]
- Chen, Q.; Liu, P.; Nan, F.; Zhou, L.; Zhang, J. Tuning the Iridescence of Chiral Nematic Cellulose Nanocrystal Films with a Vacuum-Assisted Self-Assembly Technique. Biomacromolecules 2014, 15, 4343–4350. [Google Scholar] [CrossRef] [PubMed]
- Csiszar, E.; Kalic, P.; Kobol, A.; Ferreira, E.d.P. The Effect of Low Frequency Ultrasound on the Production and Properties of Nanocrystalline Cellulose Suspensions and Films. Ultrason. Sonochem. 2016, 31, 473–480. [Google Scholar] [CrossRef] [PubMed]
- Bao, Y.; Liu, K.; Zheng, Q.; Yao, L.; Xu, Y. A Review of Preparation and Tribological Applications of Pickering Emulsion. J. Tribol. 2022, 144, 011902. [Google Scholar] [CrossRef]
- Girard, M.; Bertrand, F.; Tavares, J.R.; Heuzey, M.-C. Rheological Insights on the Evolution of Sonicated Cellulose Nanocrystal Dispersions. Ultrason. Sonochem. 2021, 78, 105747. [Google Scholar] [CrossRef]
- Beck, S.; Bouchard, J. Ionic Strength Control of Sulfated Cellulose Nanocrystal Suspension Viscosity. TAPPI J. 2016, 15, 363–372. [Google Scholar] [CrossRef]
- Shafiei-Sabet, S.; Hamad, W.Y.; Hatzikiriakos, S.G. Rheology of Nanocrystalline Cellulose Aqueous Suspensions. Langmuir 2012, 28, 17124–17133. [Google Scholar] [CrossRef]
- Kádár, R.; Spirk, S.; Nypelö, T. Cellulose Nanocrystal Liquid Crystal Phases: Progress and Challenges in Characterization Using Rheology Coupled to Optics, Scattering, and Spectroscopy. ACS Nano 2021, 15, 7931–7945. [Google Scholar] [CrossRef]
- Schutz, C.; Agthe, M.; Fall, A.B.; Gordeyeva, K.; Guccini, V.; Salajková, M.; Plivelic, T.S.; Lagerwall, J.P.; Salazar-Alvarez, G.; Bergstrom, L. Rod Packing in Chiral Nematic Cellulose Nanocrystal Dispersions Studied by Small-Angle X-Ray Scattering and Laser Diffraction. Langmuir 2015, 31, 6507–6513. [Google Scholar] [CrossRef] [PubMed]
- Miller, D.S.; Carlton, R.J.; Mushenheim, P.C.; Abbott, N.L. Introduction to Optical Methods for Characterizing Liquid Crystals at Interfaces. Langmuir 2013, 29, 3154–3169. [Google Scholar] [CrossRef] [PubMed]
- Casado, U.; Mucci, V.L.; Aranguren, M.I. Cellulose Nanocrystals Suspensions: Liquid Crystal Anisotropy, Rheology and Films Iridescence. Carbohydr. Polym. 2021, 261, 117848. [Google Scholar] [CrossRef] [PubMed]
- Honorato-Rios, C.; Kuhnhold, A.; Bruckner, J.R.; Dannert, R.; Schilling, T.; Lagerwall, J.P.F. Equilibrium Liquid Crystal Phase Diagrams and Detection of Kinetic Arrest in Cellulose Nanocrystal Suspensions. Front. Mater. 2016, 3, 21. [Google Scholar] [CrossRef]
- Wu, J.; Ma, G.-H. Recent Studies of Pickering Emulsions: Particles Make the Difference. Small 2016, 12, 4633–4648. [Google Scholar] [CrossRef]
- Binks, B.P.; Lumsdon, S.O. Influence of Particle Wettability on the Type and Stability of Surfactant-Free Emulsions. Langmuir 2000, 16, 8622–8631. [Google Scholar] [CrossRef]
- Sarkar, A.; Dickinson, E. Sustainable Food-Grade Pickering Emulsions Stabilized by Plant-Based Particles. Curr. Opin. Colloid Interface Sci. 2020, 49, 69–81. [Google Scholar] [CrossRef]
- Jakubek, Z.J.; Chen, M.; Couillard, M.; Leng, T.; Liu, L.; Zou, S.; Baxa, U.; Clogston, J.D.; Hamad, W.Y.; Johnston, L.J. Characterization Challenges for a Cellulose Nanocrystal Reference Material: Dispersion and Particle Size Distributions. J. Nanoparticle Res. 2018, 20, 98. [Google Scholar] [CrossRef]
- Niroula, A.; Gamot, T.D.; Ooi, C.W.; Dhital, S. Biomolecule-Based Pickering Food Emulsions: Intrinsic Components of Food Matrix, Recent Trends and Prospects. Food Hydrocoll. 2021, 112, 106303. [Google Scholar] [CrossRef]
- Yang, C.; Li, J.; Zhang, Y.; Wu, C.; Li, D. A Pesticide Sustained-Release Microcapsule from Cellulose Nanocrystal Stabilized Pickering Emulsion Template. J. Appl. Polym. Sci. 2023, 140, e53716. [Google Scholar] [CrossRef]
- Ng, S.-W.; Chong, W.-T.; Soo, Y.-T.; Tang, T.-K.; Karim, N.A.A.; Phuah, E.-T.; Lee, Y.-Y. Pickering Emulsion Stabilized by Palm-Pressed Fiber Cellulose Nanocrystal Extracted by Acid Hydrolysis-Assisted High Pressure Homogenization. PLoS ONE 2022, 17, e0271512. [Google Scholar] [CrossRef] [PubMed]
- Ataeian, P.; Aroyan, L.; Parwez, W.; Tam, K.C. Emulsions Undergoing Phase Transition: Effect of Emulsifier Type and Concentration. J. Colloid Interface Sci. 2022, 617, 214–223. [Google Scholar] [CrossRef] [PubMed]
- Kinra, S.; Pal, R. Rheology of Pickering Emulsions Stabilized and Thickened by Cellulose Nanocrystals over Broad Ranges of Oil and Nanocrystal Concentrations. Colloids Interfaces 2023, 7, 36. [Google Scholar] [CrossRef]
- Miao, C.; Mirvakili, M.-N.; Hamad, W.Y. A Rheological Investigation of Oil-in-Water Pickering Emulsions Stabilized by Cellulose Nanocrystals. J. Colloid Interface Sci. 2022, 608, 2820–2829. [Google Scholar] [CrossRef] [PubMed]
- Miao, C.; Tayebi, M.; Hamad, W.Y. Investigation of the Formation Mechanisms in High Internal Phase Pickering Emulsions Stabilized by Cellulose Nanocrystals. Philos. Trans. R. Soc. A Math. Phys. Eng. Sci. 2018, 376, 20170039. [Google Scholar] [CrossRef]
- Zhang, Z.; Cheng, M.; San Gabriel, M.; Neto, Â.A.T.; da Silva Bernardes, J.; Berry, R.; Tam, K.C. Polymeric Hollow Microcapsules (PHM) via Cellulose Nanocrystal Stabilized Pickering Emulsion Polymerization. J. Colloid Interface Sci. 2019, 555, 489–497. [Google Scholar] [CrossRef] [PubMed]
- Martins, D.; Estevinho, B.; Rocha, F.; Dourado, F.; Gama, M. A Dry and Fully Dispersible Bacterial Cellulose Formulation as a Stabilizer for Oil-in-Water Emulsions. Carbohydr. Polym. 2020, 230, 115657. [Google Scholar] [CrossRef]
- Chu, G.; Vasilyev, G.; Vilensky, R.; Boaz, M.; Zhang, R.; Martin, P.; Dahan, N.; Deng, S.; Zussman, E. Controlled Assembly of Nanocellulose-Stabilized Emulsions with Periodic Liquid Crystal-in-Liquid Crystal Organization. Langmuir 2018, 34, 13263–13273. [Google Scholar] [CrossRef]
- Wan, W.; Zhao, Z.; Hughes, T.C.; Qian, B.; Peng, S.; Hao, X.; Qiu, J. Graphene Oxide Liquid Crystal Pickering Emulsions and Their Assemblies. Carbon 2015, 85, 16–23. [Google Scholar] [CrossRef]
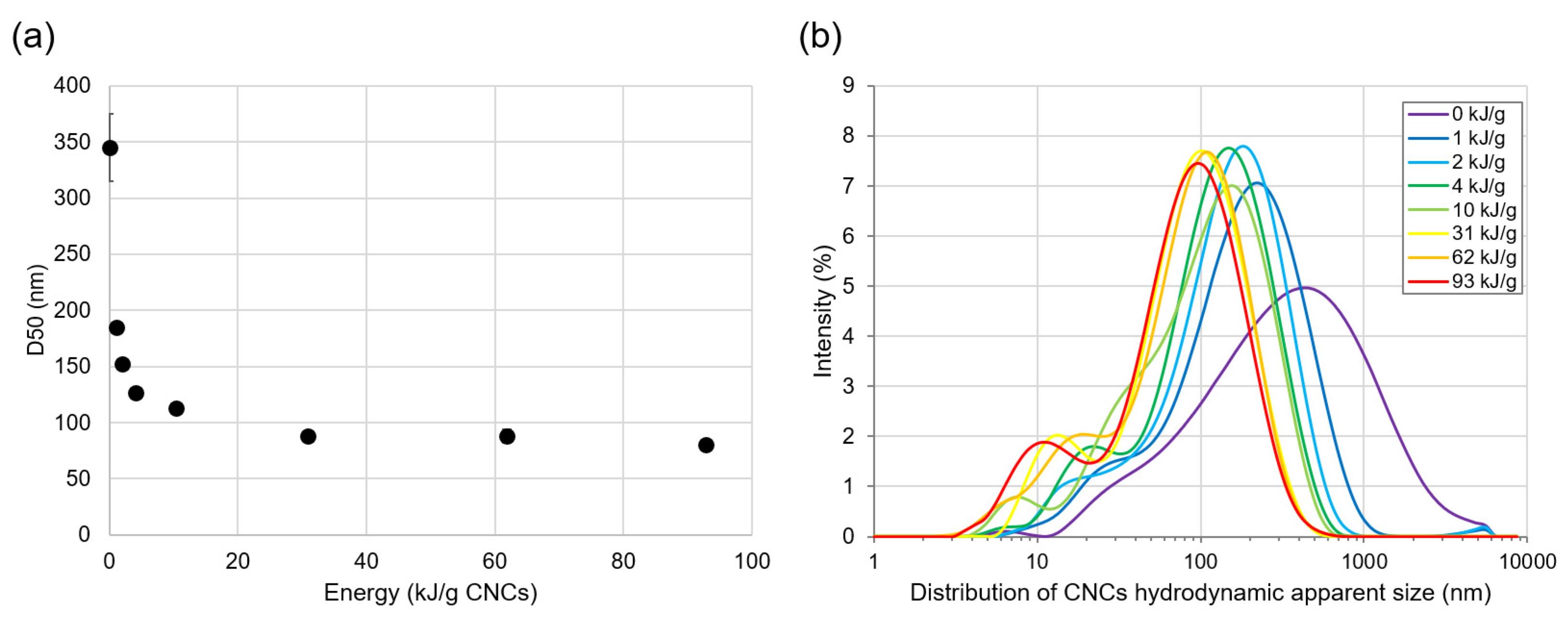




| CNC Suspension Concentration (%—w/w) | Energy (kJ) | Energy (kJ/g CNCs) | D10 (nm) | D50 (nm) | D90 (nm) |
|---|---|---|---|---|---|
| 0.5 | 0 | 0 | 59 ± 9.3 a | 345 ± 30.0 a | 1350 ± 155 a |
| 58 | 93 | 13 ± 1.2 b | 80 ± 2.1 b | 203 ± 22.7 b | |
| 5 | 0 | 0 | 57 ± 2.6 a | 366 ± 7.6 a | 1370 ± 260 a |
| 58 | 9.3 | 12 ± 1.7 b | 100 ± 5.6 c | 269 ± 19.8 c |
| Wavenumber (cm−1) | Assignments | References |
|---|---|---|
| Between 3100 and 3600 | O-H stretching of primary and secondary hydroxyl groups | [32,36,38] |
| 2898 | C-H stretching | [32,35] |
| 1642 | O-H bending of adsorbed water | [32,35,36] |
| 1429 | In-plane bending vibration of H-C-H and O-C-H bonds | [38] |
| 1367 | C-H asymmetric stretching and C-O symmetric stretching | [35] |
| 1315 | C-H2 wagging | [39] |
| 1203 | S=O stretching | [36] |
| 1159 | C-O-C asymmetric stretching | [32,36] |
| 1105 | C-O-C wagging and twisting | [35] |
| 1053 | C-O stretching | [38] |
| 1029 | C-O-C deformation | [36] |
| 898 | C-H rocking and O-H bending | [36,40] |
| 663 | Out-plane hydrogen bonded O–H groups twisting | [32] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Perrin, L.; Desobry, S.; Gillet, G.; Desobry-Banon, S. Low-Frequency Ultrasound Effects on Cellulose Nanocrystals for Potential Application in Stabilizing Pickering Emulsions. Polymers 2023, 15, 4371. https://doi.org/10.3390/polym15224371
Perrin L, Desobry S, Gillet G, Desobry-Banon S. Low-Frequency Ultrasound Effects on Cellulose Nanocrystals for Potential Application in Stabilizing Pickering Emulsions. Polymers. 2023; 15(22):4371. https://doi.org/10.3390/polym15224371
Chicago/Turabian StylePerrin, Louise, Stephane Desobry, Guillaume Gillet, and Sylvie Desobry-Banon. 2023. "Low-Frequency Ultrasound Effects on Cellulose Nanocrystals for Potential Application in Stabilizing Pickering Emulsions" Polymers 15, no. 22: 4371. https://doi.org/10.3390/polym15224371