Development of Poly(acrylamide)-Based Hydrogel Composites with Powdered Activated Carbon for Controlled Sorption of PFOA and PFOS in Aqueous Systems
Abstract
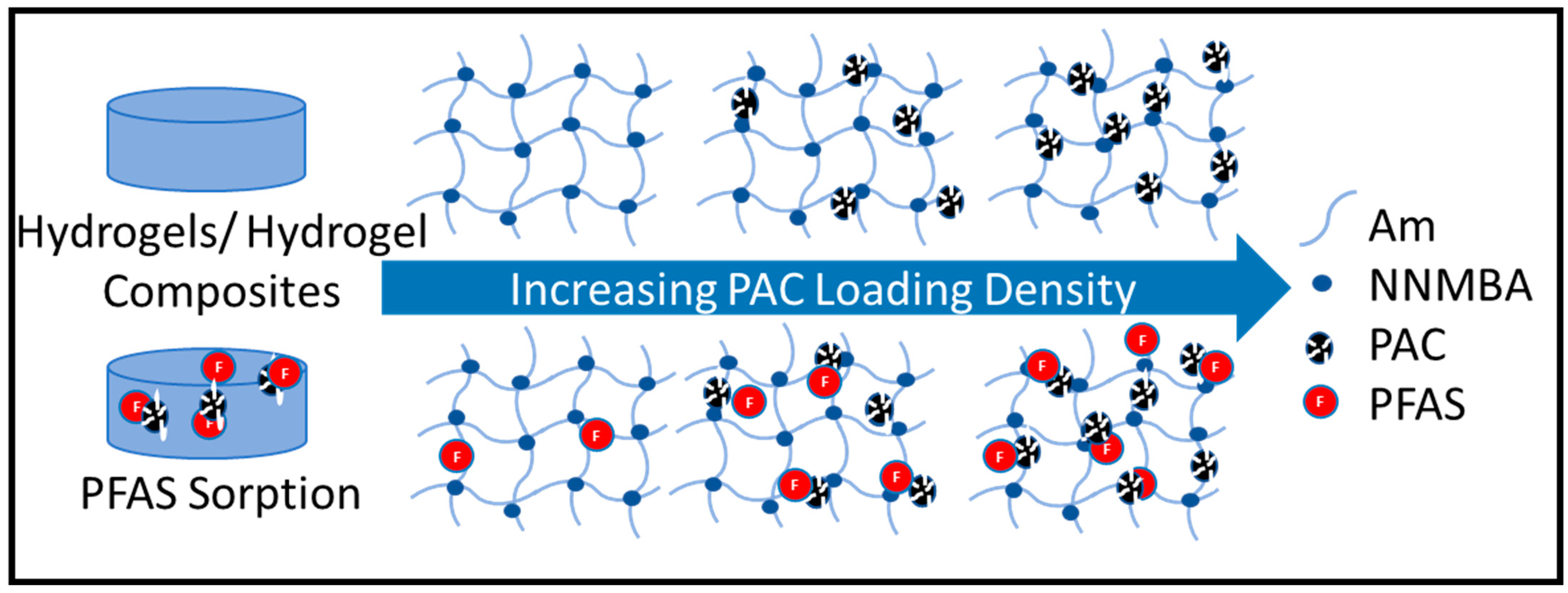
:1. Introduction
2. Materials and Methods
2.1. Materials
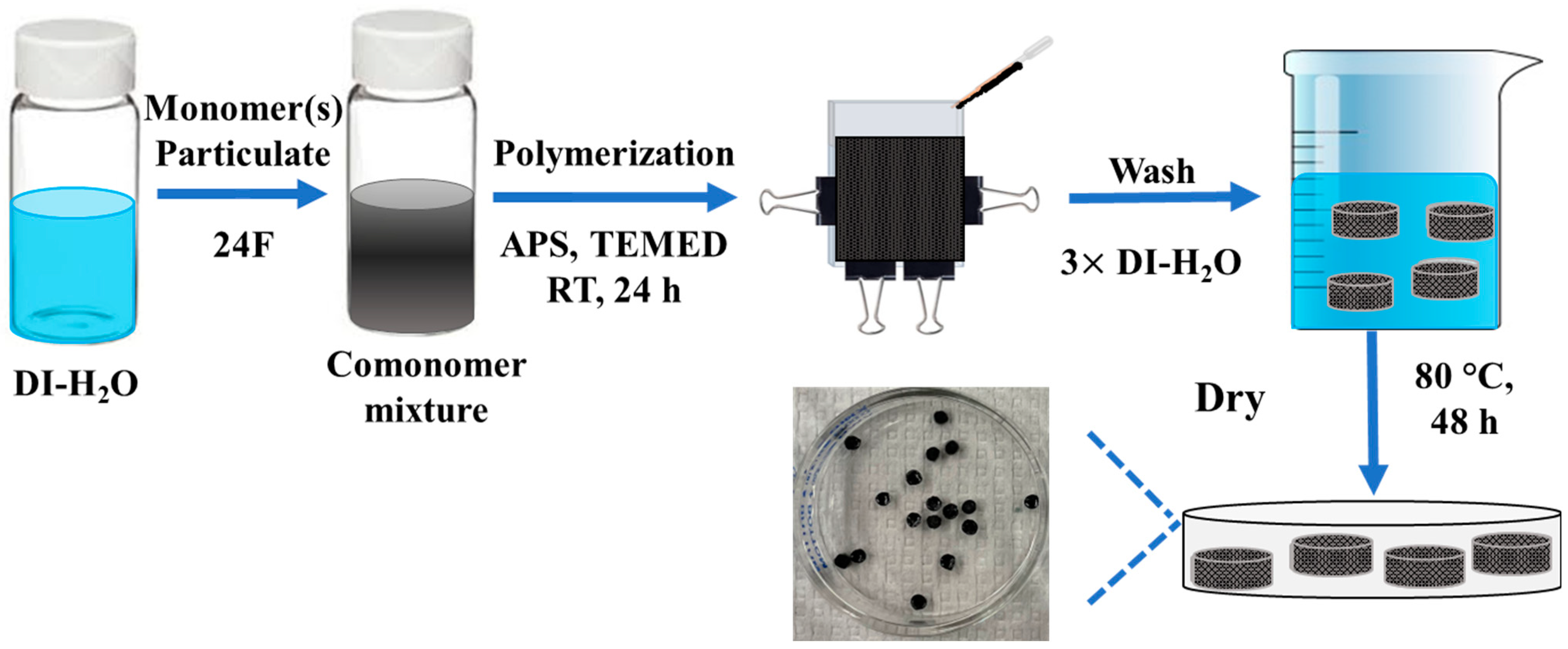
2.2. Preparation and Physicochemical Characterization of Hydrogels and Hydrogel Composites
2.3. Mass Yield of Monomer and Crosslinker to Polymer Conversion
2.4. Surface Characterization of Sorbent Materials
2.5. Aqueous Swelling Experiments of Hydrogels and Hydrogel Composites
2.6. PFAS Sorption Studies
3. Results and Discussion
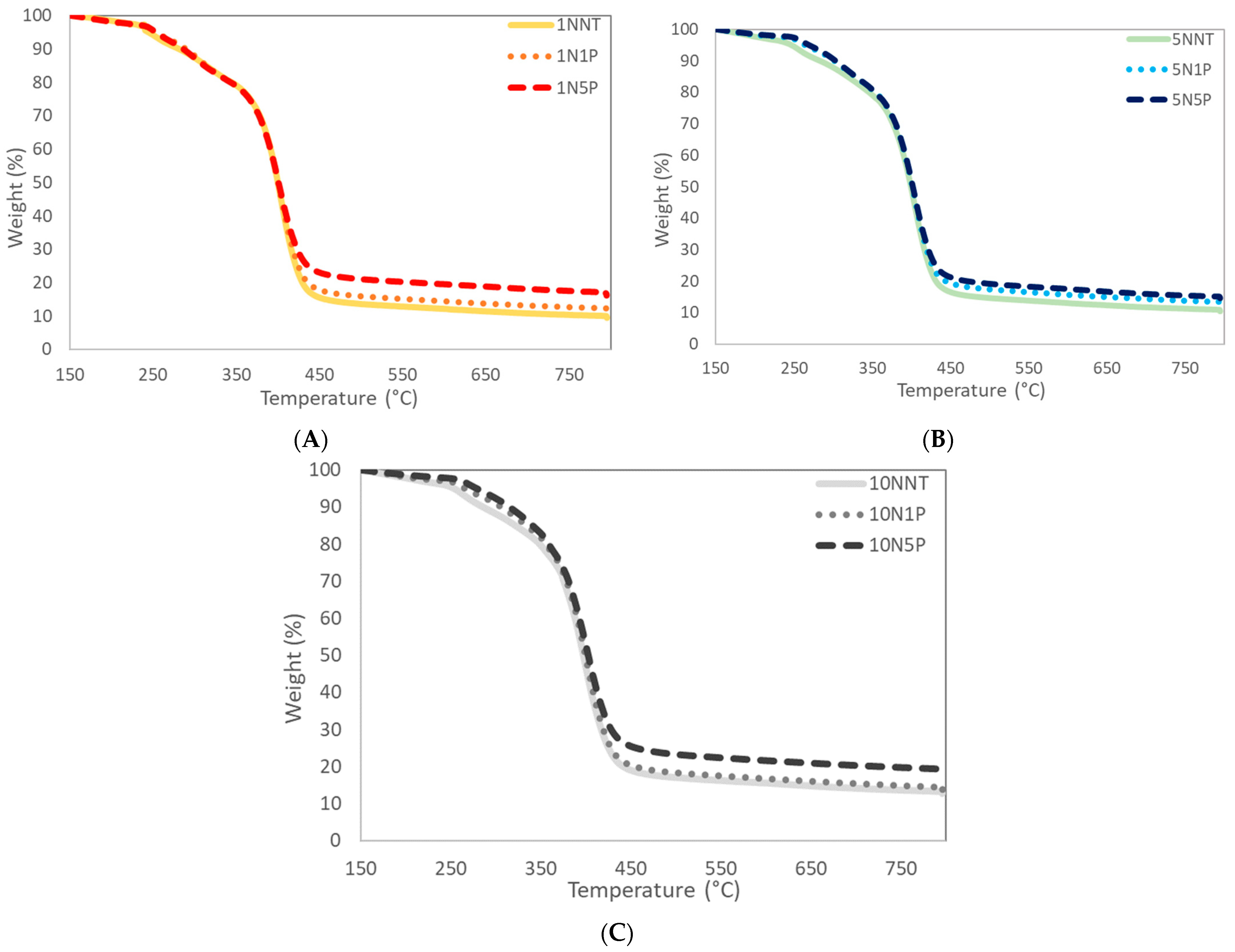
3.1. Synthesis Confirmation and Physicochemical Characterizations
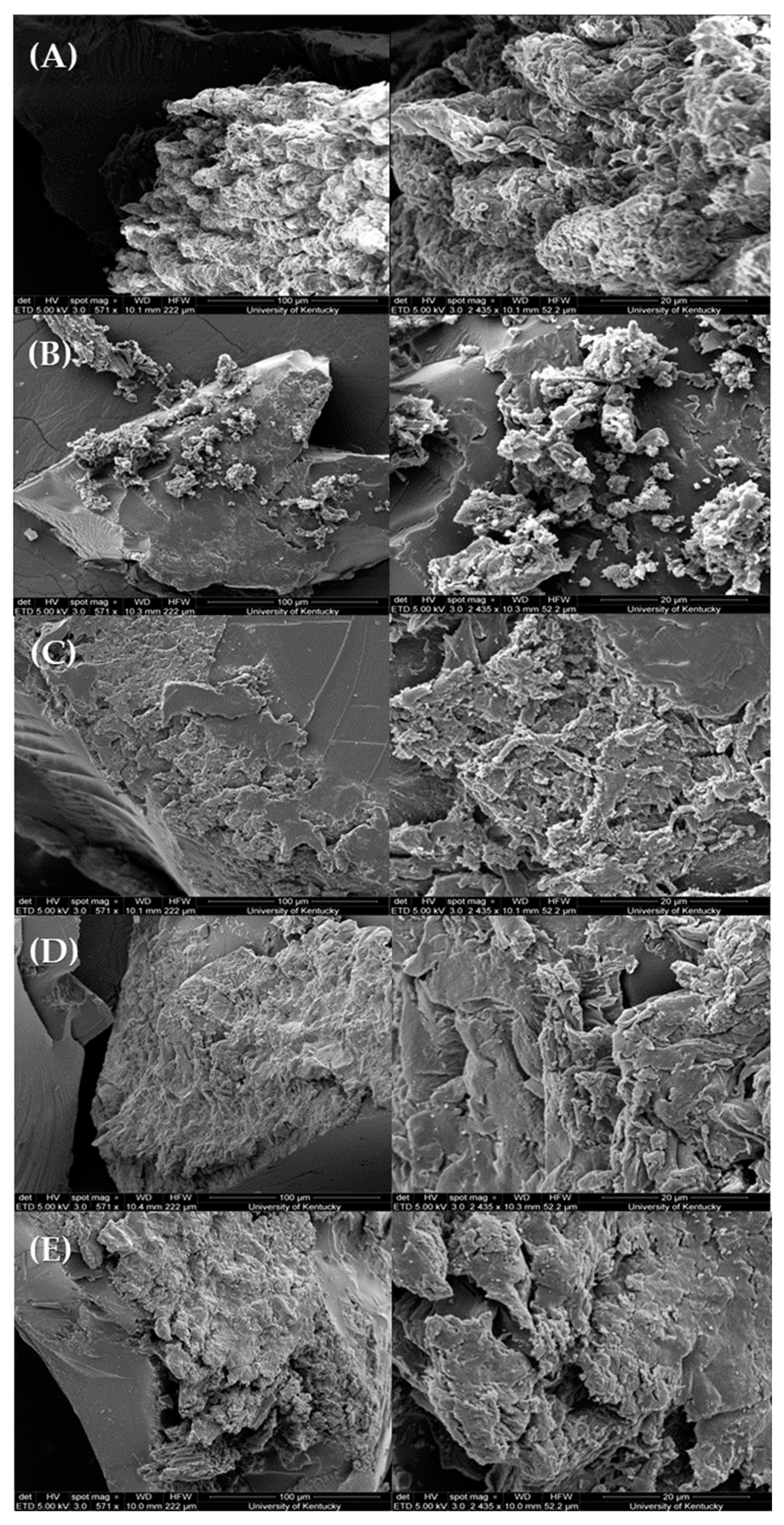
3.2. Surface Characterization
3.3. Aqueous Swelling Studies
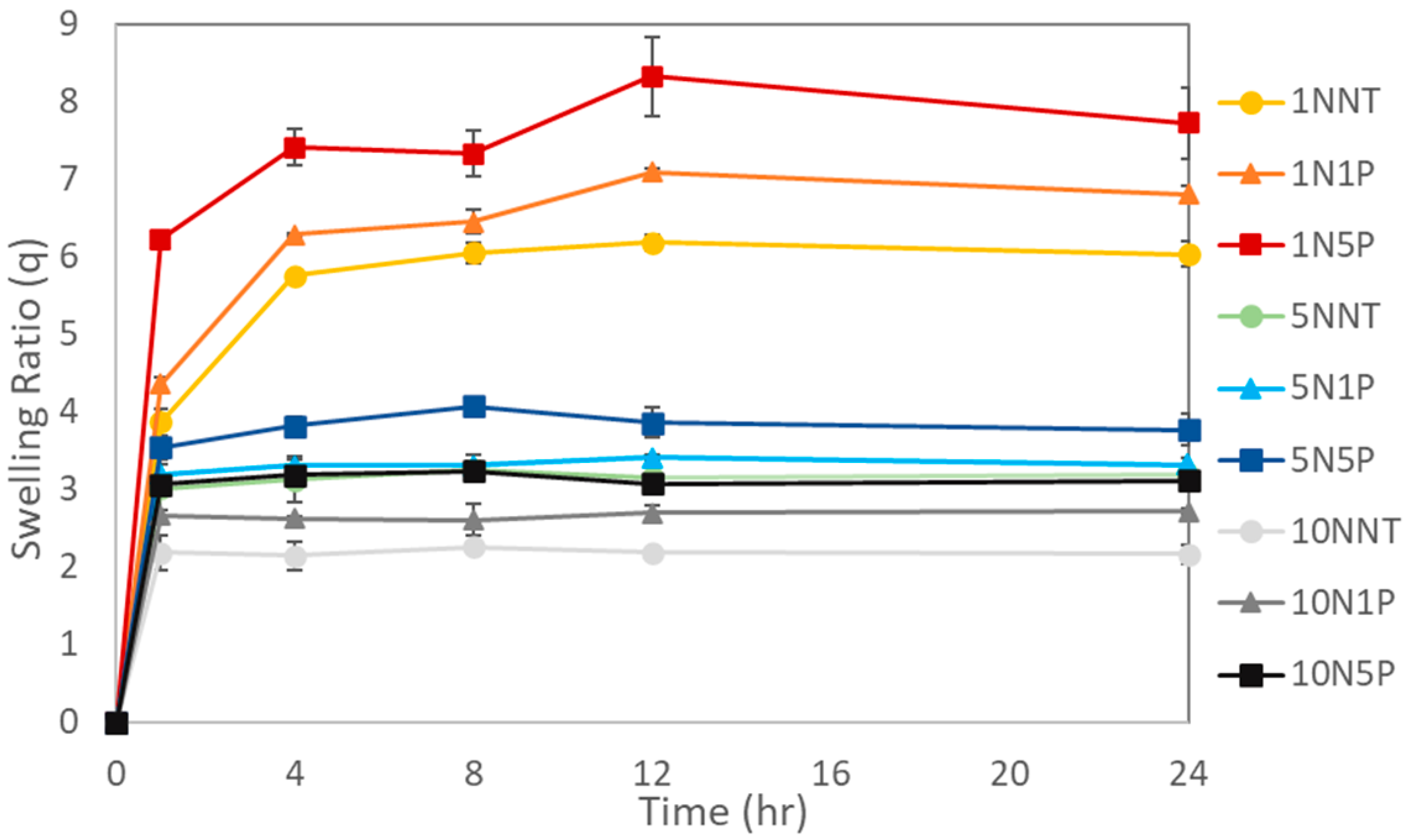
3.3.1. Kinetic Swelling Studies
3.3.2. Equilibrium Swelling Studies
3.4. Equilibrium Sorption Studies
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Dickman, R.A.; Aga, D.S. A Review of Recent Studies on Toxicity, Sequestration, and Degradation of per- and Polyfluoroalkyl Substances (PFAS). J. Hazard. Mater. 2022, 436, 129120. [Google Scholar] [CrossRef]
- Evich, M.G.; Davis, M.J.B.; McCord, J.P.; Acrey, B.; Awkerman, J.A.; Knappe, D.R.U.; Lindstrom, A.B.; Speth, T.F.; Tebes-Stevens, C.; Strynar, M.J.; et al. Per- and Polyfluoroalkyl Substances in the Environment. Science 2022, 375, eabg9065. [Google Scholar] [CrossRef] [PubMed]
- Sunderland, E.M.; Hu, X.C.; Dassuncao, C.; Tokranov, A.K.; Wagner, C.C.; Allen, J.G. A Review of the Pathways of Human Exposure to Poly- and Perfluoroalkyl Substances (PFASs) and Present Understanding of Health Effects. J. Expo. Sci. Environ. Epidemiol. 2019, 29, 131–147. [Google Scholar] [CrossRef]
- Committee on the Guidance on PFAS Testing and Health Outcomes; Board on Environmental Studies and Toxicology; Board on Population Health and Public Health Practice; Division on Earth and Life Studies; Health and Medicine Division; National Academies of Sciences, Engineering, and Medicine. Guidance on PFAS Exposure, Testing, and Clinical Follow-Up; National Academies Press: Washington, DC, USA, 2022; p. 26156. [Google Scholar] [CrossRef]
- Abunada, Z.; Alazaiza, M.Y.D.; Bashir, M.J.K. An Overview of Per- and Polyfluoroalkyl Substances (PFAS) in the Environment: Source, Fate, Risk and Regulations. Water 2020, 12, 3590. [Google Scholar] [CrossRef]
- Pérez, F.; Nadal, M.; Navarro-Ortega, A.; Fàbrega, F.; Domingo, J.L.; Barceló, D.; Farré, M. Accumulation of Perfluoroalkyl Substances in Human Tissues. Environ. Int. 2013, 59, 354–362. [Google Scholar] [CrossRef] [PubMed]
- Crone, B.C.; Speth, T.F.; Wahman, D.G.; Smith, S.J.; Abulikemu, G.; Kleiner, E.J.; Pressman, J.G. Occurrence of Per- and Polyfluoroalkyl Substances (PFAS) in Source Water and Their Treatment in Drinking Water. Crit. Rev. Environ. Sci. Technol. 2019, 49, 2359–2396. [Google Scholar] [CrossRef] [PubMed]
- Shahsavari, E.; Rouch, D.; Khudur, L.S.; Thomas, D.; Aburto-Medina, A.; Ball, A.S. Challenges and Current Status of the Biological Treatment of PFAS-Contaminated Soils. Front. Bioeng. Biotechnol. 2021, 8, 602040. [Google Scholar] [CrossRef]
- Gagliano, E.; Sgroi, M.; Falciglia, P.P.; Vagliasindi, F.G.A.; Roccaro, P. Removal of Poly- and Perfluoroalkyl Substances (PFAS) from Water by Adsorption: Role of PFAS Chain Length, Effect of Organic Matter and Challenges in Adsorbent Regeneration. Water Res. 2020, 171, 115381. [Google Scholar] [CrossRef]
- Kucharzyk, K.; Darlington, R.; Benotti, M.; Deeb, R.; Hawley, E. Novel Treatment Technologies for PFAS Compounds: A Critical Review. J. Environ. Manag. 2017, 204, 757–764. [Google Scholar] [CrossRef]
- Interstate Technology and Regulatory Council. Treatment Technologies and Methods for Per- and Polyfluoroalkyl Substances (PFAS). Available online: https://pfas-1.itrcweb.org/wp-content/uploads/2020/10/treatment_tech_508_Aug-2020-Final.pdf (accessed on 11 November 2020).
- Ateia, M.; Arifuzzaman, M.; Pellizzeri, S.; Attia, M.F.; Tharayil, N.; Anker, J.N.; Karanfil, T. Cationic Polymer for Selective Removal of GenX and Short-Chain PFAS from Surface Waters and Wastewaters at Ng/L Levels. Water Res. 2019, 163, 114874. [Google Scholar] [CrossRef]
- Frazar, E.M.; Shah, R.A.; Dziubla, T.D.; Hilt, J.Z. Multifunctional Temperature-responsive Polymers as Advanced Biomaterials and Beyond. J. Appl. Polym. Sci. 2020, 137, 48770. [Google Scholar] [CrossRef] [PubMed]
- Tang, S.; Bhandari, R.; Delaney, S.P.; Munson, E.J.; Dziubla, T.D.; Hilt, J.Z. Synthesis and Characterization of Thermally Responsive N-Isopropylacrylamide Hydrogels Copolymerized with Novel Hydrophobic Polyphenolic Crosslinkers. Mater. Today Commun. 2017, 10, 46–53. [Google Scholar] [CrossRef] [PubMed]
- Gutierrez, A.M.; Frazar, E.M.; X. Klaus, M.V.; Paul, P.; Hilt, J.Z. Hydrogels and Hydrogel Nanocomposites: Enhancing Healthcare through Human and Environmental Treatment. Adv. Healthc. Mater. 2022, 11, 2101820. [Google Scholar] [CrossRef]
- Uspenskaya, M.V.; Sitnikova, V.E.; Dovbeta, M.A.; Olekhnovich, R.O.; Denisyuk, I.Y. Sorption Properties of Clay and Pectin-Containing Hydrogels. In Recent Research in Polymerization; Cankaya, N., Ed.; InTech: St. Petersburg, Russia, 2018. [Google Scholar] [CrossRef]
- Ateia, M.; Alsbaiee, A.; Karanfil, T.; Dichtel, W. Efficient PFAS Removal by Amine-Functionalized Sorbents: Critical Review of the Current Literature. Environ. Sci. Technol. Lett. 2019, 6, 688–695. [Google Scholar] [CrossRef]
- Kumarasamy, E.; Manning, I.M.; Collins, L.B.; Coronell, O.; Leibfarth, F.A. Ionic Fluorogels for Remediation of Per- and Polyfluorinated Alkyl Substances from Water. ACS Cent. Sci. 2020, 6, 487–492. [Google Scholar] [CrossRef]
- Zhang, Q.; Deng, S.; Yu, G.; Huang, J. Removal of Perfluorooctane Sulfonate from Aqueous Solution by Crosslinked Chitosan Beads: Sorption Kinetics and Uptake Mechanism. Bioresour. Technol. 2011, 102, 2265–2271. [Google Scholar] [CrossRef]
- Yu, Q.; Deng, S.; Yu, G. Selective Removal of Perfluorooctane Sulfonate from Aqueous Solution Using Chitosan-Based Molecularly Imprinted Polymer Adsorbents. Water Res. 2008, 42, 3089–3097. [Google Scholar] [CrossRef] [PubMed]
- Garg, S.; Wang, J.; Kumar, P.; Mishra, V.; Arafat, H.; Sharma, R.S.; Dumée, L.F. Remediation of Water from Per-/Poly-Fluoroalkyl Substances (PFAS)—Challenges and Perspectives. J. Environ. Chem. Eng. 2021, 9, 105784. [Google Scholar] [CrossRef]
- Son, H.; Kim, T.; Yoom, H.-S.; Zhao, D.; An, B. The Adsorption Selectivity of Short and Long Per- and Polyfluoroalkyl Substances (PFASs) from Surface Water Using Powder-Activated Carbon. Water 2020, 12, 3287. [Google Scholar] [CrossRef]
- Liu, C.; Hatton, J.; Arnold, W.A.; Simcik, M.F.; Pennell, K.D. In Situ Sequestration of Perfluoroalkyl Substances Using Polymer-Stabilized Powdered Activated Carbon. Environ. Sci. Technol. 2020, 54, 6929–6936. [Google Scholar] [CrossRef]
- Asgharzadehahmadi, S.A.; Muhamad, I.I.; Zaidel, D.N.A.; Supriyanto, E. Synthesis and Characterization of Polyacrylamide Based Hydrogel Containing Magnesium Oxide Nanoparticles for Antibacterial Applications. 6. Available online: https://www.semanticscholar.org/paper/Synthesis-and-characterization-of-polyacrylamide-Asgharzadehahmadi/c84a283b93362aae45e3d65795627009bf16e0fe (accessed on 1 January 2021).
- Shatat, R.S.; Niazi, S.K.; Ariffin, A. Synthesis and Characterization of Different Molecular Weights Polyacrylamide. IOSR J. Appl. Chem. 2017, 10, 67–73. [Google Scholar] [CrossRef]
- Abdelkader, R.; Mohammed, B. Green Synthesis of Cationic Polyacrylamide Composite Catalyzed by An Ecologically Catalyst Clay Called Maghnite-H+ (Algerian MMT) Under Microwave Irradiation. Bull. Chem. React. Eng. Catal. 2016, 11, 170. [Google Scholar] [CrossRef]
- Leung, W.M.; Axelson, D.E.; Van Dyke, J.D. Thermal Degradation of Polyacrylamide and Poly(Acrylamide-Co-Acrylate). J. Polym. Sci. Part Polym. Chem. 1987, 25, 1825–1846. [Google Scholar] [CrossRef]
- Wang, X.; Jing, S.; Qiu, X.; Zhao, S.; Liu, Y.; Tan, Y. Novel Bile Acid Sequestrant: A Biodegradable Hydrogel Based on Amphiphilic Allylamine Copolymer. Chem. Eng. J. 2016, 304, 493–502. [Google Scholar] [CrossRef]
- Lebkiri, I.; Abbou, B.; Kadiri, L.; Ouass, A.; Elamri, A.; Ouaddari, H.; Elkhattabi, O.; Lebkiri, A.; Rifi, E.H. Swelling Properties and Basic Dye Adsorption Studies of Polyacrylamide Hydrogel. Desalination Water Treat. 2021, 233, 361–376. [Google Scholar] [CrossRef]
- Sato, N.; Aoyama, Y.; Yamanaka, J.; Toyotama, A.; Okuzono, T. Particle Adsorption on Hydrogel Surfaces in Aqueous Media Due to van Der Waals Attraction. Sci. Rep. 2017, 7, 6099. [Google Scholar] [CrossRef]
- Zhao, L.; Bian, J.; Zhang, Y.; Zhu, L.; Liu, Z. Comparison of the Sorption Behaviors and Mechanisms of Perfluorosulfonates and Perfluorocarboxylic Acids on Three Kinds of Clay Minerals. Chemosphere 2014, 114, 51–58. [Google Scholar] [CrossRef]



| Hydrogel/ Hydrogel Composite Samples | Crosslinking Density Am:NNMBA (mol%) | PAC Loading Percentage (mass %) | Mass Yield % | PAC Final Loading % |
|---|---|---|---|---|
| 1NNT | 99:1 | - | 91 | - |
| 5NNT | 95:5 | - | 98 | - |
| 10NNT | 90:10 | - | 99 | - |
| 1N1P | 99:1 | 1 | 90 | 2.44 |
| 5N1P | 95:5 | 1 | 95 | 1.49 |
| 10N1P | 90:10 | 1 | 96 | 1.00 |
| 1N5P | 99:1 | 5 | 78 | 7.50 |
| 5N5P | 95:5 | 5 | 69 | 3.87 |
| 10N5P | 90:10 | 5 | 79 | 5.89 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Klaus, M.V.X.; Gutierrez, A.M.; Hilt, J.Z. Development of Poly(acrylamide)-Based Hydrogel Composites with Powdered Activated Carbon for Controlled Sorption of PFOA and PFOS in Aqueous Systems. Polymers 2023, 15, 4384. https://doi.org/10.3390/polym15224384
Klaus MVX, Gutierrez AM, Hilt JZ. Development of Poly(acrylamide)-Based Hydrogel Composites with Powdered Activated Carbon for Controlled Sorption of PFOA and PFOS in Aqueous Systems. Polymers. 2023; 15(22):4384. https://doi.org/10.3390/polym15224384
Chicago/Turabian StyleKlaus, Maria Victoria X., Angela M. Gutierrez, and J. Zach Hilt. 2023. "Development of Poly(acrylamide)-Based Hydrogel Composites with Powdered Activated Carbon for Controlled Sorption of PFOA and PFOS in Aqueous Systems" Polymers 15, no. 22: 4384. https://doi.org/10.3390/polym15224384
APA StyleKlaus, M. V. X., Gutierrez, A. M., & Hilt, J. Z. (2023). Development of Poly(acrylamide)-Based Hydrogel Composites with Powdered Activated Carbon for Controlled Sorption of PFOA and PFOS in Aqueous Systems. Polymers, 15(22), 4384. https://doi.org/10.3390/polym15224384






