Development of Novel PET-PAN Electrospun Nanocomposite Membrane Embedded with Layered Double Hydroxides Hybrid for Efficient Wastewater Treatment
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
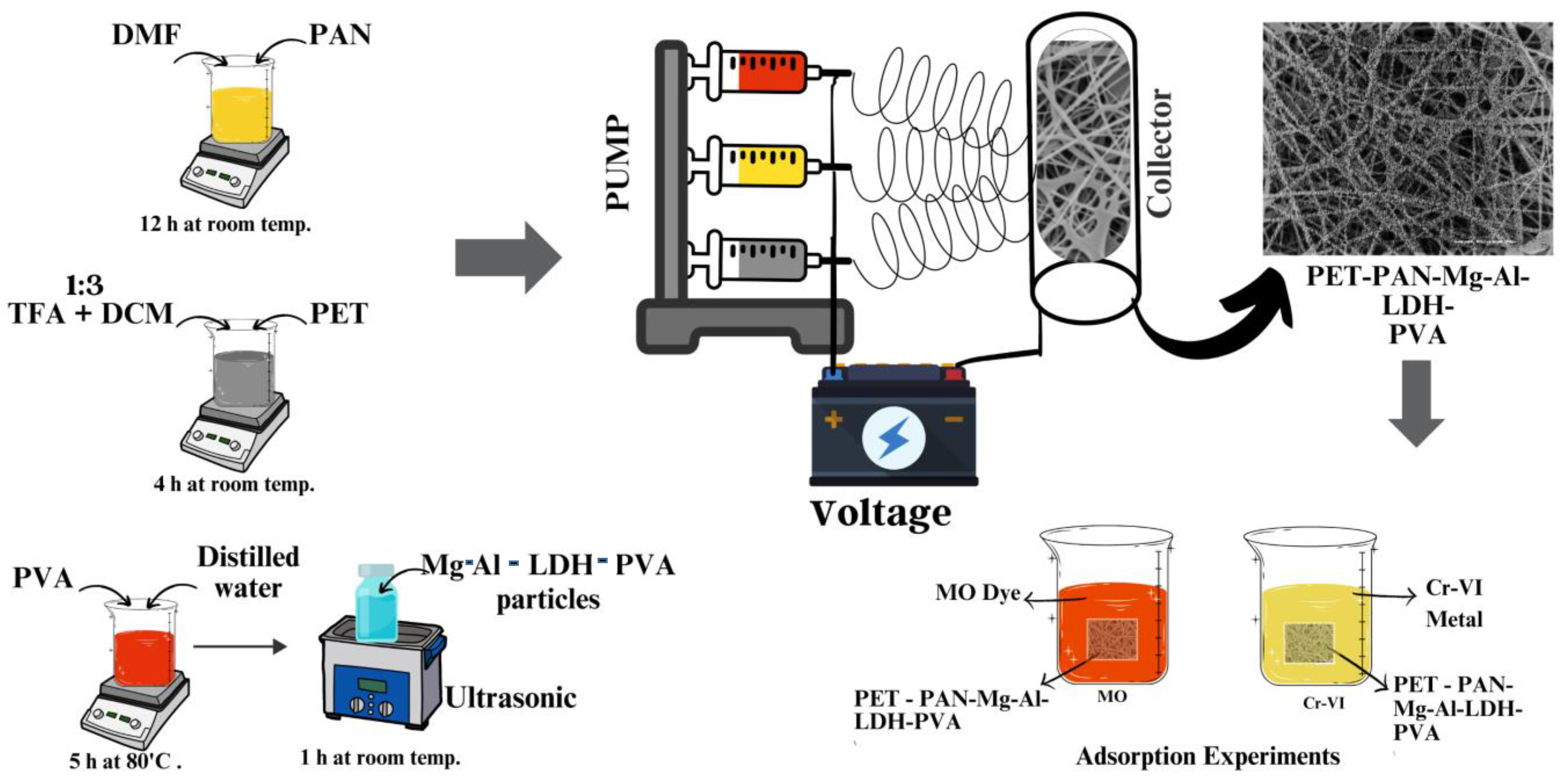
2.2. Synthesis of Mg-Al-LDH Nanoparticles
2.3. Preparation of Polymer Solutions for Electrospinning
2.4. Electrospinning of Membrane
2.5. Characterization of Electrospun Membranes
2.6. Batch Adsorption Studies
2.7. Reusability Experiment
2.8. Adsorption Isotherms
2.9. Adsorption Kinetics
3. Results and Discussion
3.1. Characterization
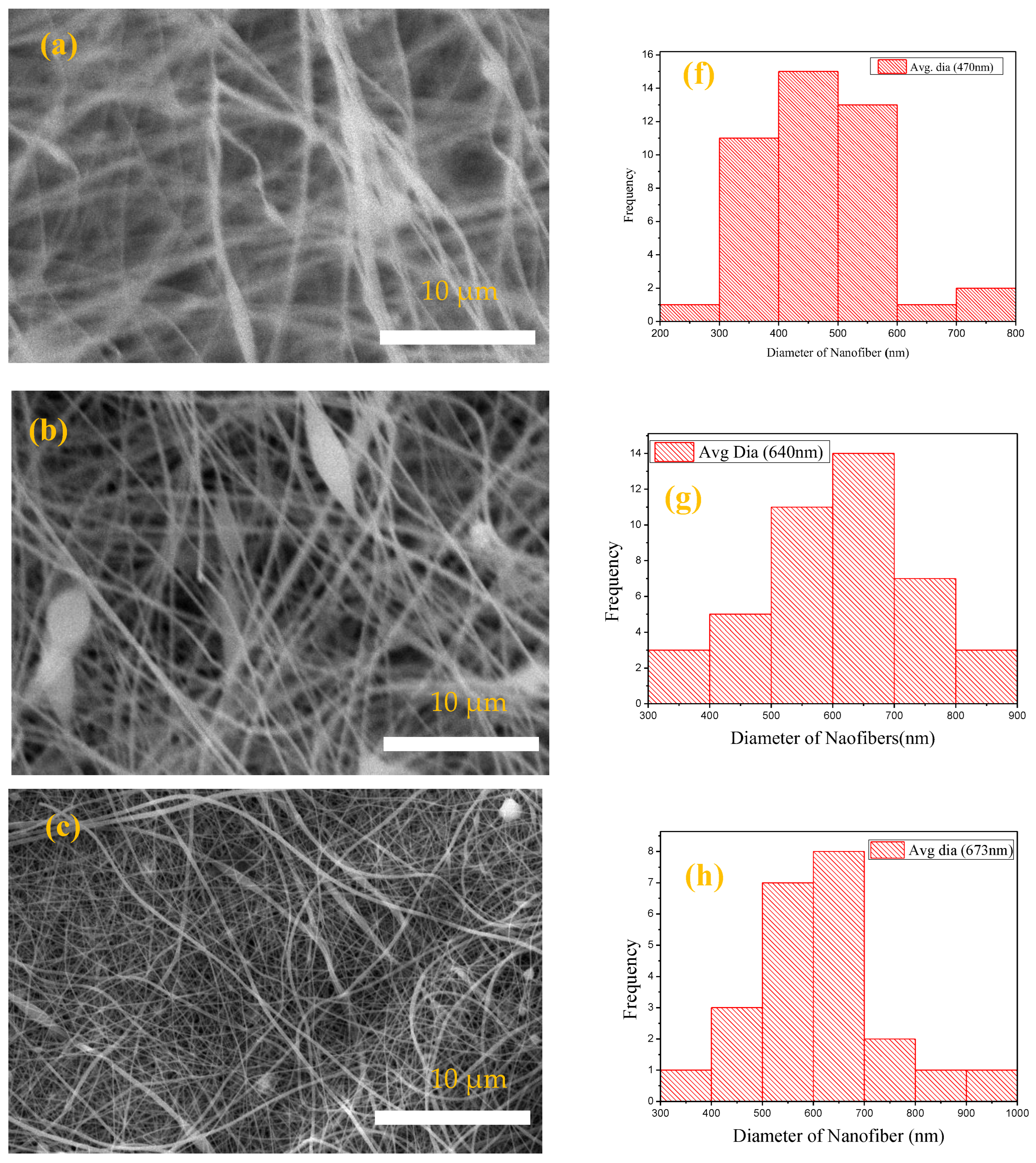
3.1.1. SEM Analysis
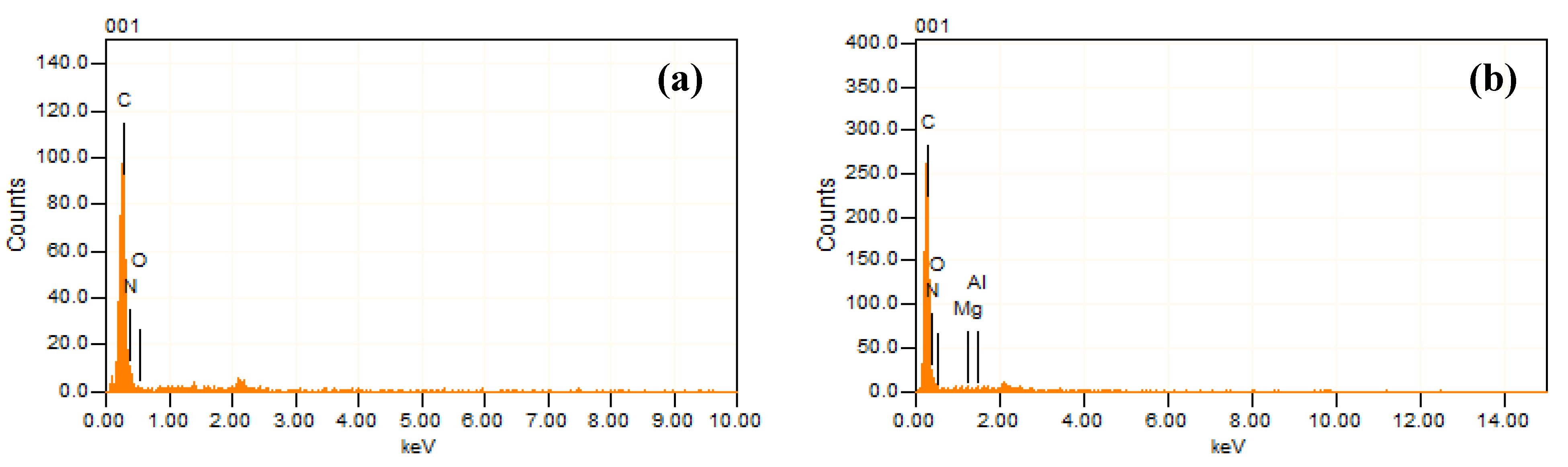
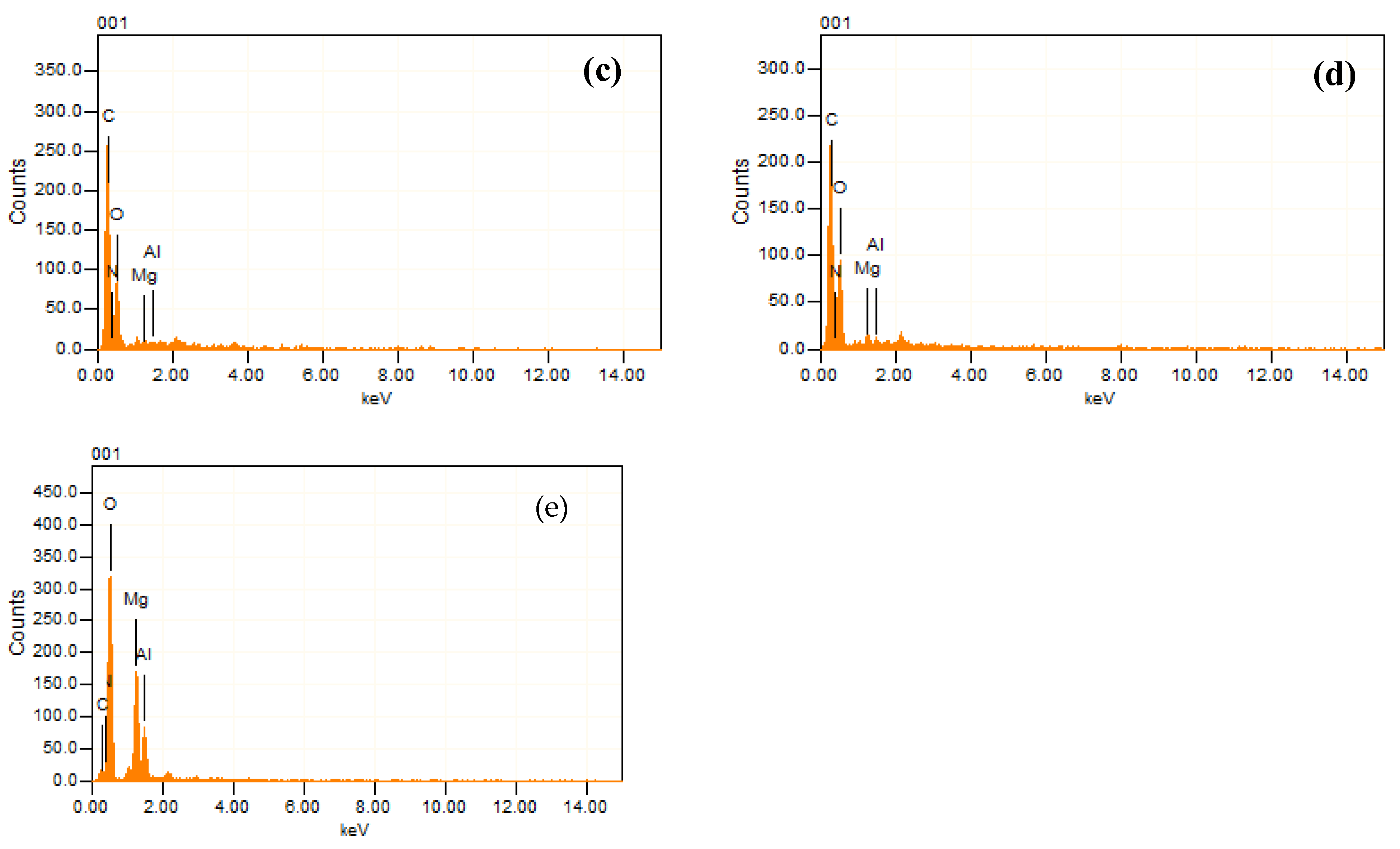
3.1.2. EDX Analysis
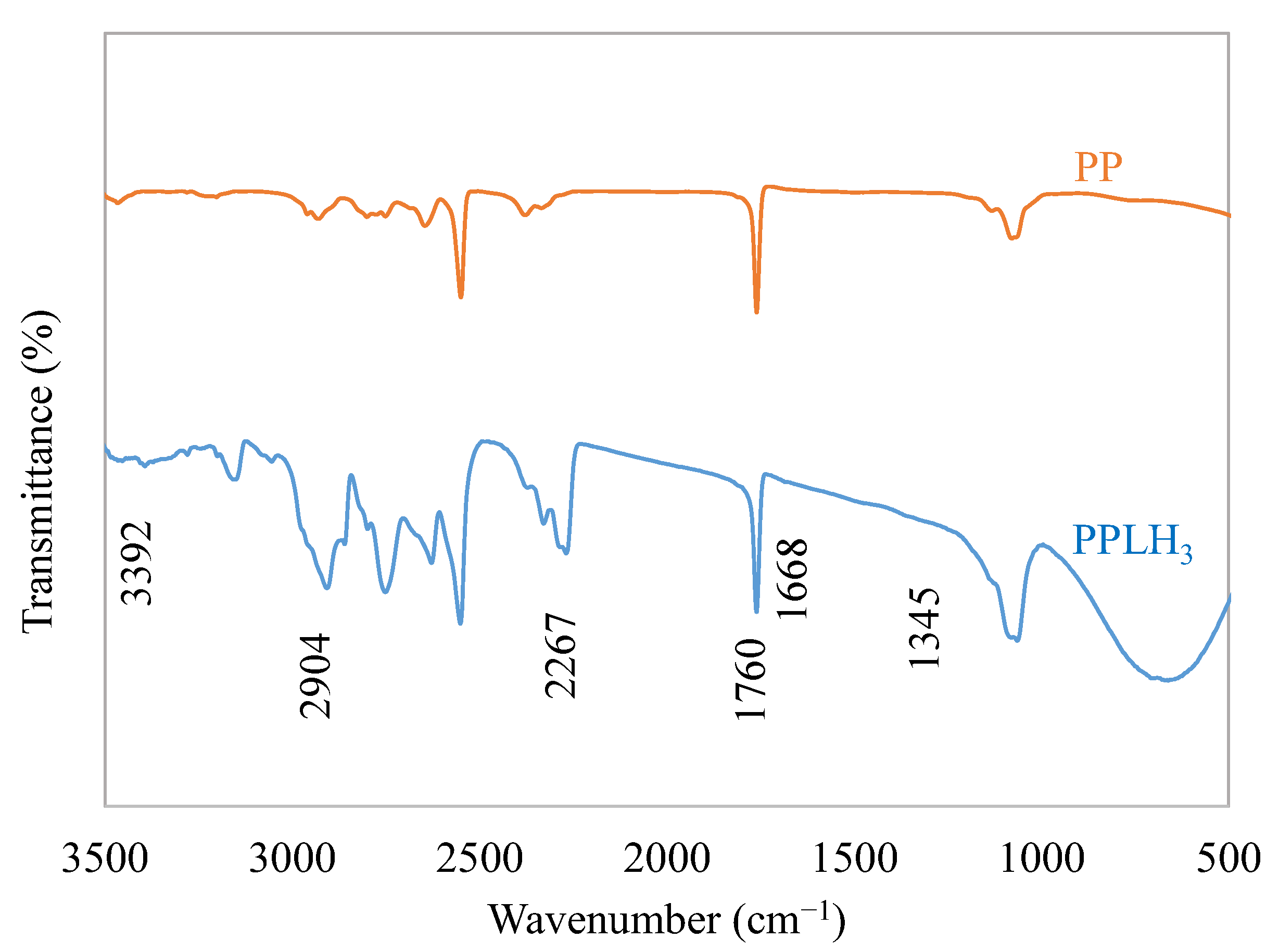
3.1.3. FTIR Analysis
3.1.4. AFM Analysis
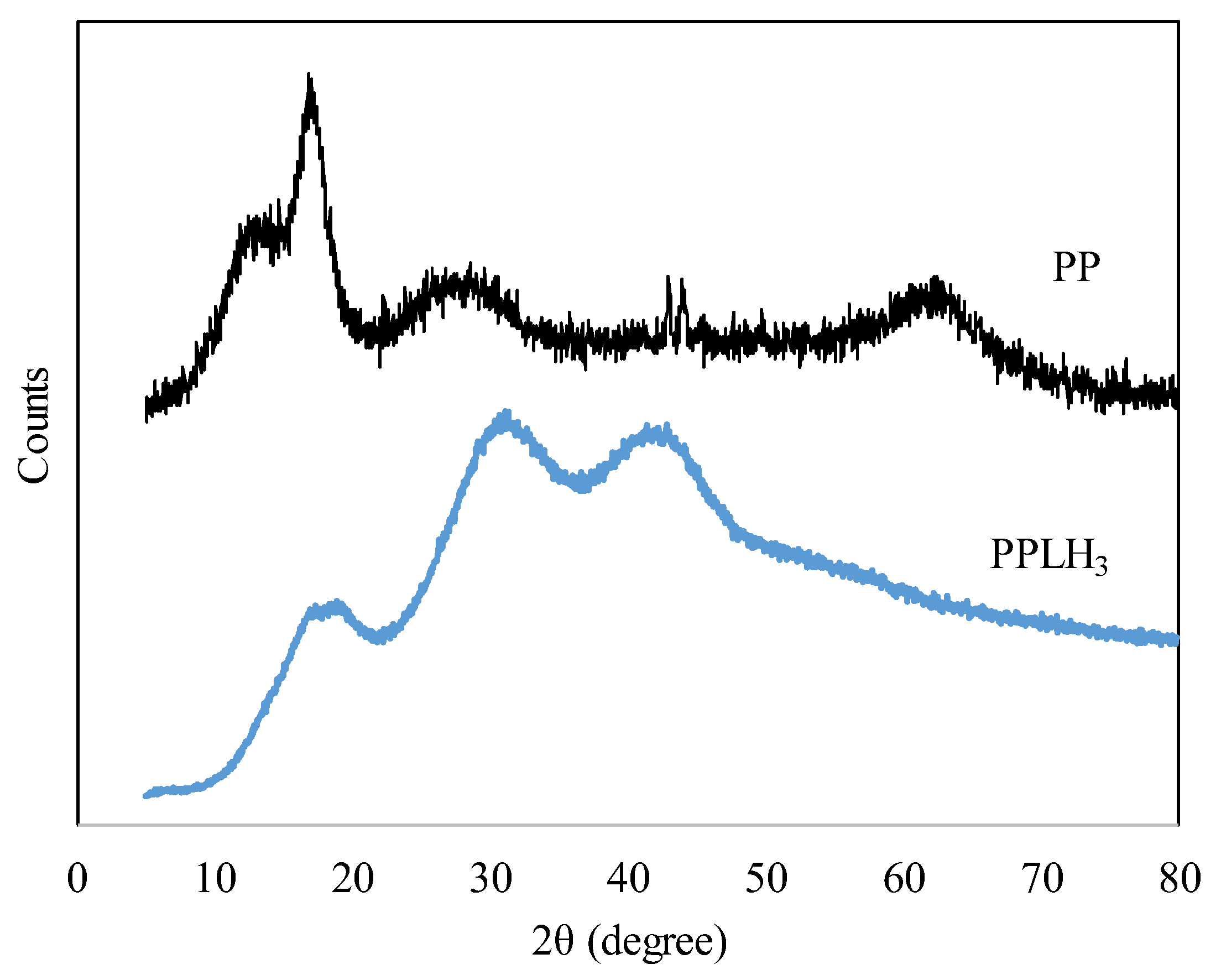
3.1.5. XRD Analysis
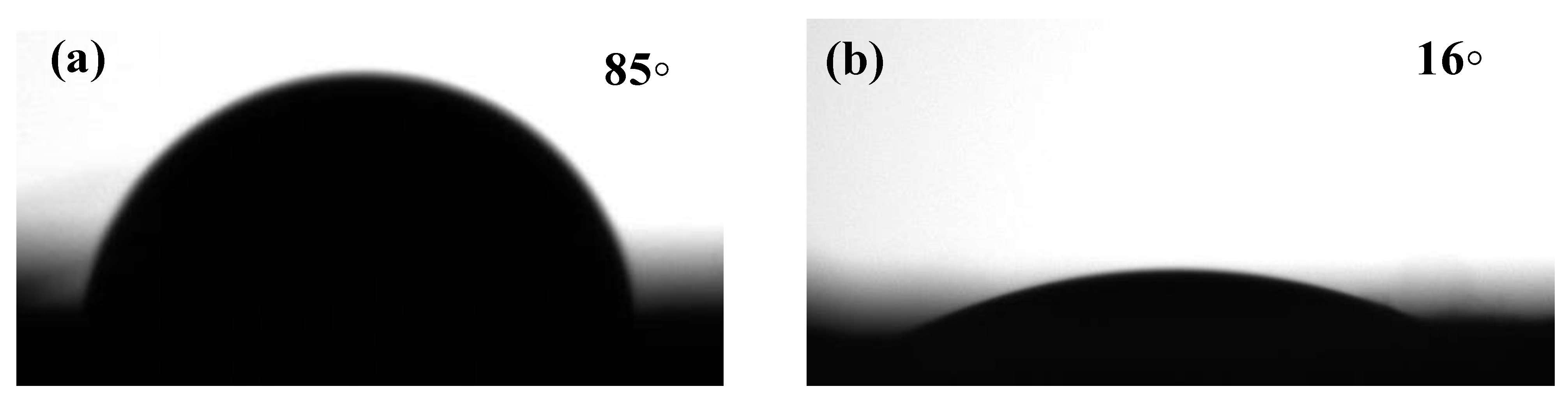
3.1.6. Water Contact Angle Measurement
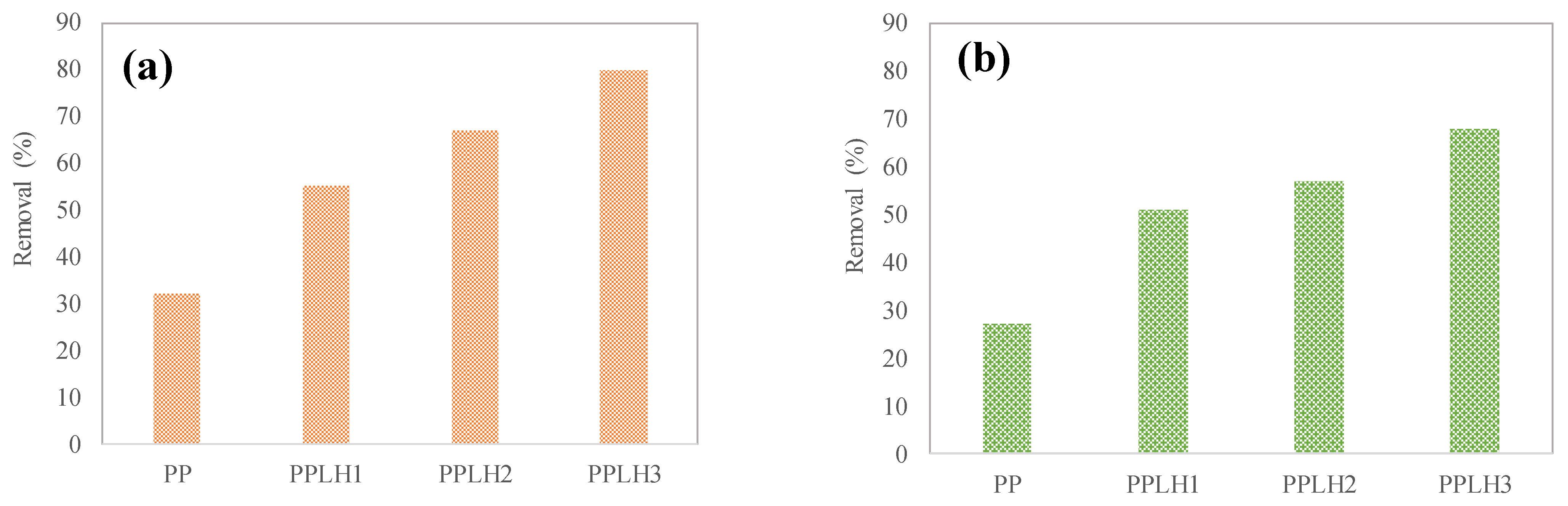
3.2. Basic Adsorption Experiment
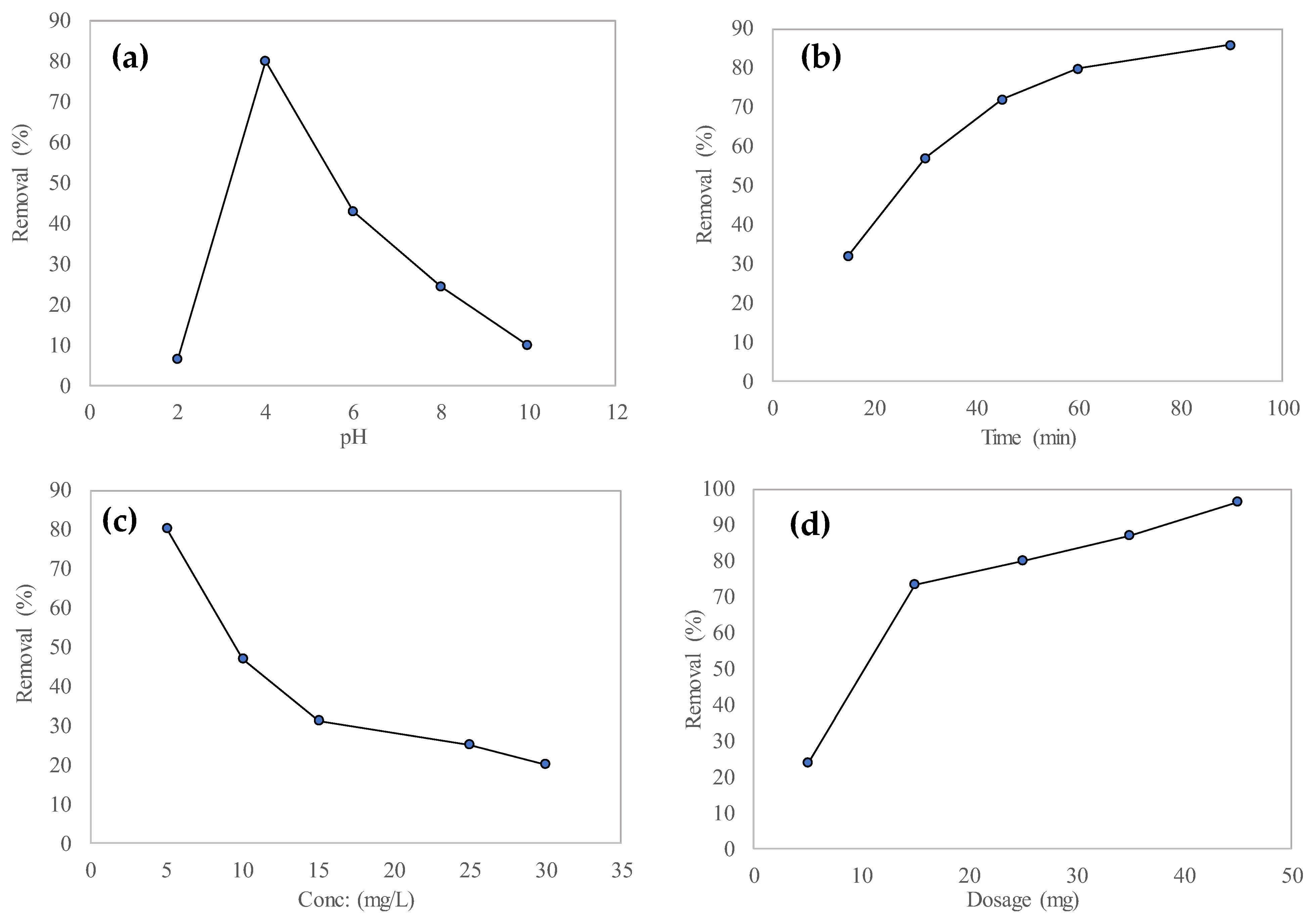
3.3. Optimization of Different Parameters for MO Adsorption
3.3.1. Effect of pH
3.3.2. Effect of Contact Time
3.3.3. Effect of Initial Dye Concentration
3.3.4. Effect of Adsorbent Dosage
3.4. Adsorption Isotherms
3.5. Adsorption Kinetics
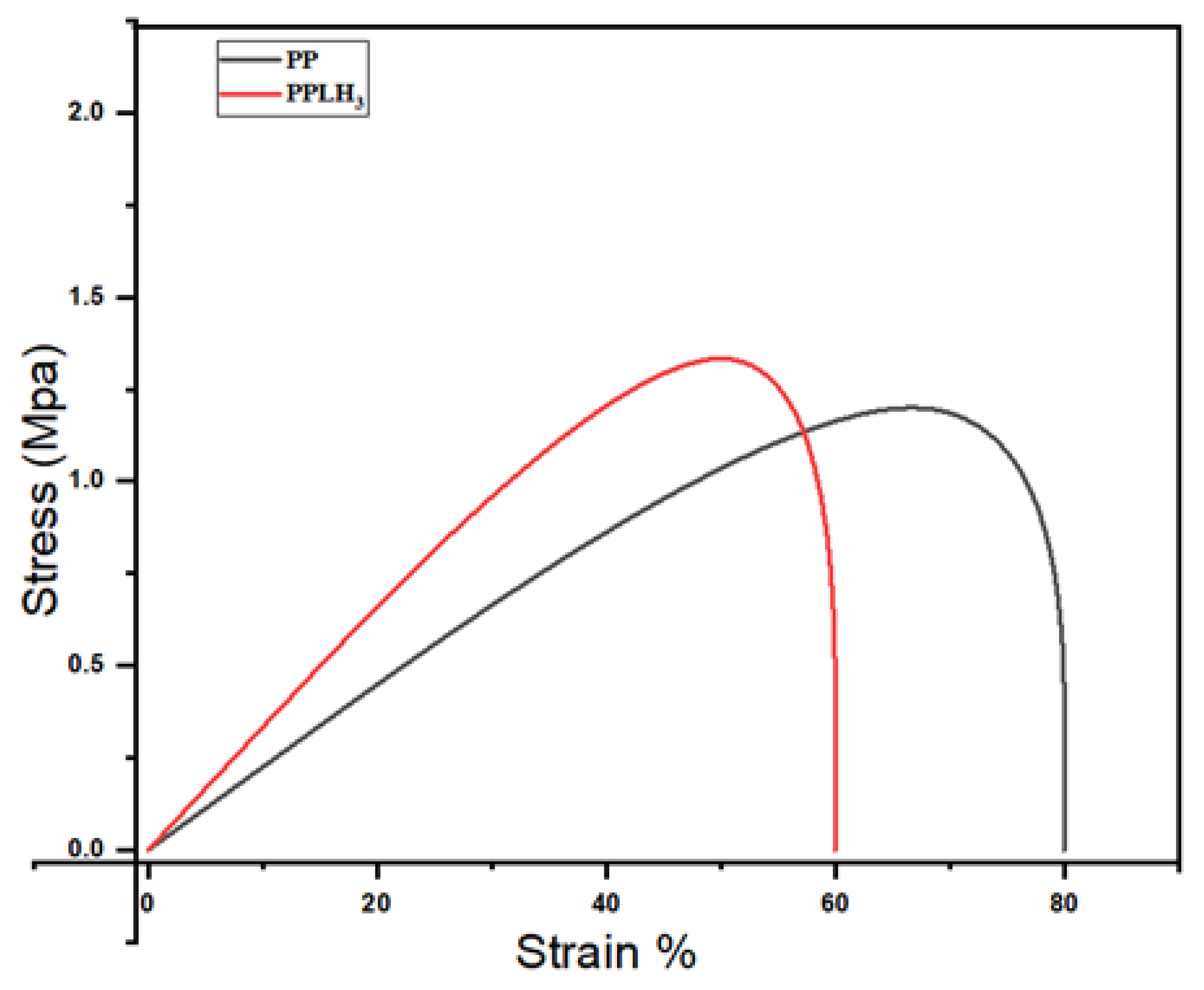
3.6. Mechanical Behavior
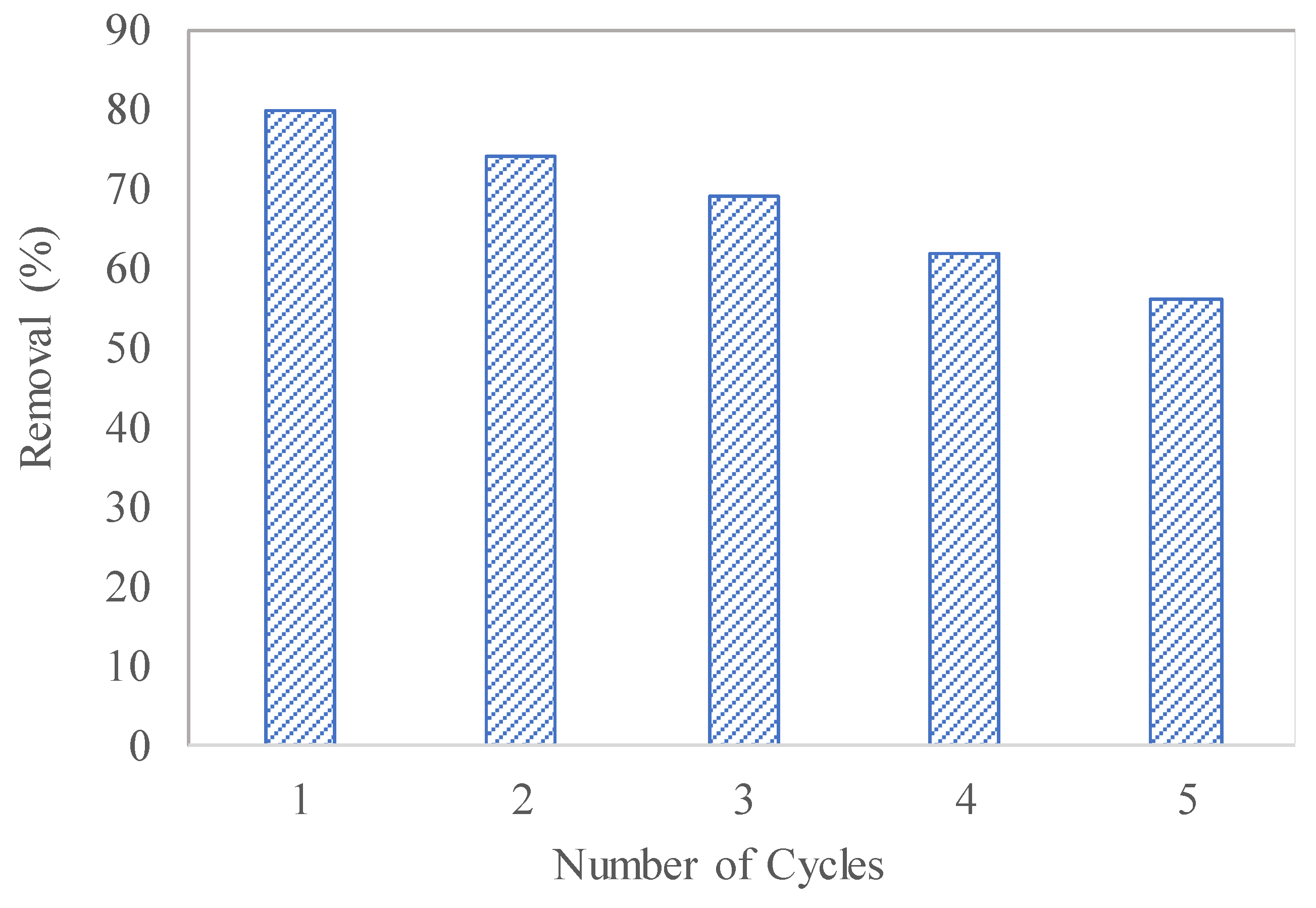
3.7. Recyclability Test
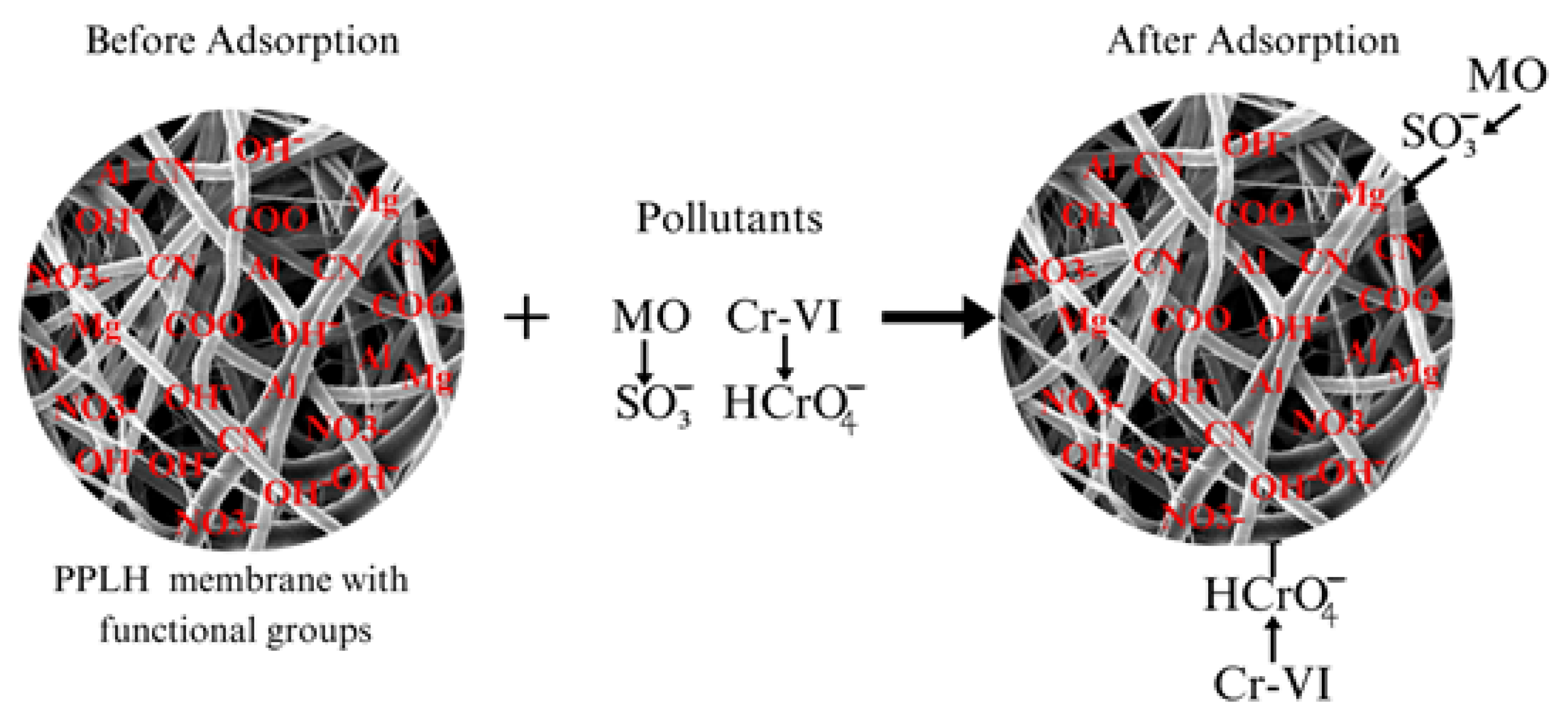
3.8. Proposed Adsorption Mechanism
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Tariq, M.; Ali Baig, S.; Shams, D.F.; Hussain, S.; Hussain, R.; Qadir, A.; Maryam, H.S.; Khan, Z.U.; Sattar, S.; Xu, X. Dye Wastewater Treatment Using Wheat Straw Biochar in Gadoon Industrial Areas of Swabi, Pakistan. Water Conserv. Sci. Eng. 2022, 7, 315–326. [Google Scholar]
- Baburaj, M.; Veeran, M.G.; Painuly, D.; Sreelekshmi, S.; Rajkumar, R.; Aprem, A.S. Fabrication and characterisation of polycaprolactone/gelatin/chitosan (PCL/GEL/CHI) electrospun nano-membranes for wastewater purification. Desalination 2023, 563, 116709. [Google Scholar]
- Wu, S.; Li, K.; Shi, W.; Cai, J. Preparation and performance evaluation of chitosan/polyvinylpyrrolidone/polyvinyl alcohol electrospun nanofiber membrane for heavy metal ions and organic pollutants removal. Int. J. Biol. Macromol. 2022, 210, 76–84. [Google Scholar] [PubMed]
- Li, L.; Guo, W.; Zhang, S.; Guo, R.; Zhang, L. Electrospun Nanofiber Membrane: An Efficient and Environmentally Friendly Material for the Removal of Metals and Dyes. Molecules 2023, 28, 3288. [Google Scholar] [PubMed]
- Alharbi, H.F.; Haddad, M.Y.; Aijaz, M.O.; Assaifan, A.K.; Karim, M.R. Electrospun bilayer PAN/chitosan nanofiber membranes incorporated with metal oxide nanoparticles for heavy metal ion adsorption. Coatings 2020, 10, 285. [Google Scholar] [CrossRef]
- Cheng, J.; Zhan, C.; Wu, J.; Cui, Z.; Si, J.; Wang, Q.; Peng, X.; Turng, L.-S. Highly efficient removal of methylene blue dye from an aqueous solution using cellulose acetate nanofibrous membranes modified by polydopamine. ACS Omega 2020, 5, 5389–5400. [Google Scholar] [CrossRef]
- Hu, Z.-P.; Gao, Z.-M.; Liu, X.; Yuan, Z.-Y. High-surface-area activated red mud for efficient removal of methylene blue from wastewater. Adsorpt. Sci. Technol. 2018, 36, 62–79. [Google Scholar] [CrossRef]
- Heide, A.; Wiebe, P.; Sabantina, L.; Ehrmann, A. Suitability of Mycelium-Reinforced Nanofiber Mats for Filtration of Different Dyes. Polymers 2023, 15, 3951. [Google Scholar] [CrossRef]
- Murillo, L.; Rivero, P.J.; Sandúa, X.; Pérez, G.; Palacio, J.F.; Rodríguez, R.J. Antifungal Activity of Chitosan/Poly (Ethylene Oxide) Blend Electrospun Polymeric Fiber Mat Doped with Metallic Silver Nanoparticles. Polymers 2023, 15, 3700. [Google Scholar]
- Tang, X.; Guo, X.; Duo, Y.; Qian, X. Preparation and Characterization of a One-Step Electrospun Poly (Lactic Acid)/Wormwood Oil Antibacterial Nanofiber Membrane. Polymers 2023, 15, 3585. [Google Scholar]
- Albiladi, A.; Gzara, L.; Organji, H.; Alkayal, N.S.; Figoli, A. Electrospun Poly (Vinylidene Fluoride-Co-Hexafluoropropylene) Nanofiber Membranes for Brine Treatment via Membrane Distillation. Polymers 2023, 15, 2706. [Google Scholar] [CrossRef] [PubMed]
- Ji, K.; Liu, C.; He, H.; Mao, X.; Wei, L.; Wang, H.; Zhang, M.; Shen, Y.; Sun, R.; Zhou, F. Research Progress of Water Treatment Technology Based on Nanofiber Membranes. Polymers 2023, 15, 741. [Google Scholar] [CrossRef] [PubMed]
- Avila, M.; Burks, T.; Akhtar, F.; Göthelid, M.; Lansåker, P.C.; Toprak, M.S.; Muhammed, M.; Uheida, A. Surface functionalized nanofibers for the removal of chromium (VI) from aqueous solutions. Chem. Eng. J. 2014, 245, 201–209. [Google Scholar] [CrossRef]
- Dai, S.; Wu, X.; Zhang, J.; Fu, Y.; Li, W. Coenzyme A-regulated Pd nanocatalysts for formic acid-mediated reduction of hexavalent chromium. Chem. Eng. J. 2018, 351, 959–966. [Google Scholar] [CrossRef]
- Farooqi, Z.H.; Akram, M.W.; Begum, R.; Wu, W.; Irfan, A. Inorganic nanoparticles for reduction of hexavalent chromium: Physicochemical aspects. J. Hazard. Mater. 2021, 402, 123535. [Google Scholar] [CrossRef]
- Zhao, R.; Li, X.; Li, Y.; Li, Y.; Sun, B.; Zhang, N.; Chao, S.; Wang, C. Functionalized magnetic iron oxide/polyacrylonitrile composite electrospun fibers as effective chromium (VI) adsorbents for water purification. J. Colloid Interface Sci. 2017, 505, 1018–1030. [Google Scholar] [CrossRef]
- Zhu, F.; Zheng, Y.-M.; Zhang, B.-G.; Dai, Y.-R. A critical review on the electrospun nanofibrous membranes for the adsorption of heavy metals in water treatment. J. Hazard. Mater. 2021, 401, 123608. [Google Scholar] [CrossRef]
- Li, C.; Wang, H.; Zhao, X.; Yang, K.; Meng, Q.; Zhang, L. Fabrication of Unidirectional Water Permeable PS/PET Composite Nanofibers Modified with Silver Nanoparticles via Electrospinning. Membranes 2023, 13, 257. [Google Scholar] [CrossRef]
- Pervez, M.N.; Talukder, M.E.; Mishu, M.R.; Buonerba, A.; Del Gaudio, P.; Stylios, G.K.; Hasan, S.W.; Zhao, Y.; Cai, Y.; Figoli, A. One-step fabrication of novel polyethersulfone-based composite electrospun nanofiber membranes for food industry wastewater treatment. Membranes 2022, 12, 413. [Google Scholar] [CrossRef]
- Phan, D.-N.; Khan, M.Q.; Nguyen, N.-T.; Phan, T.-T.; Ullah, A.; Khatri, M.; Kien, N.N.; Kim, I.-S. A review on the fabrication of several carbohydrate polymers into nanofibrous structures using electrospinning for removal of metal ions and dyes. Carbohydr. Polym. 2021, 252, 117175. [Google Scholar] [CrossRef] [PubMed]
- Ozturk, E.A.; Ege, Z.R.; Murat, S.; Erdemir, G.; Kuruca, S.; Erkmen, Z.E.; Duygulu, O.; Gunduz, O.; Caykara, T.; Eroglu, M.S. Poly (L-lactic acid)/poly (ethylene oxide) based composite electrospun fibers loaded with magnesium-aluminum layered double hydroxide nanoparticles. Int. J. Biol. Macromol. 2022, 217, 562–571. [Google Scholar] [CrossRef]
- Xue, L.; Ren, J.; Wang, S.; Qu, D.; Wei, Z.; Yang, Q.; Li, Y. Preparation of nanofiber aerogels by electrospinning and studying of its adsorption properties for heavy-metal and dyes. J. Porous Mater. 2020, 27, 1589–1599. [Google Scholar] [CrossRef]
- Makarov, I.S.; Vinogradov, M.I.; Golova, L.K.; Arkharova, N.A.; Shambilova, G.K.; Makhatova, V.E.; Naukenov, M.Z. Design and Fabrication of Membranes Based on PAN Copolymer Obtained from Solutions in N-methylmorpholine-N-oxide. Polymers 2022, 14, 2861. [Google Scholar] [CrossRef]
- Feng, B.; Shen, W.; Shi, L.; Qu, S. Adsorption of hexavalent chromium by polyacrylonitrile-based porous carbon from aqueous solution. R. Soc. Open Sci. 2018, 5, 171662. [Google Scholar] [CrossRef] [PubMed]
- Kenawy, E.-R.; Tenhu, H.; Khattab, S.A.; Eldeeb, A.A.; Azaam, M.M. Highly efficient adsorbent material for removal of methylene blue dye based on functionalized polyacrylonitrile. Eur. Polym. J. 2022, 169, 111138. [Google Scholar] [CrossRef]
- Bonfim, D.P.; Cruz, F.G.; Bretas, R.E.; Guerra, V.G.; Aguiar, M.L. A sustainable recycling alternative: Electrospun PET-membranes for air nanofiltration. Polymers 2021, 13, 1166. [Google Scholar] [CrossRef] [PubMed]
- Incarnato, L.; Scarfato, P.; Di Maio, L.; Acierno, D. Structure and rheology of recycled PET modified by reactive extrusion. Polymer 2000, 41, 6825–6831. [Google Scholar] [CrossRef]
- Khorram, M.; Mousavi, A.; Mehranbod, N. Chromium removal using adsorptive membranes composed of electrospun plasma-treated functionalized polyethylene terephthalate (PET) with chitosan. J. Environ. Chem. Eng. 2017, 5, 2366–2377. [Google Scholar] [CrossRef]
- Zhang, S.; Shi, Q.; Korfiatis, G.; Christodoulatos, C.; Wang, H.; Meng, X. Chromate removal by electrospun PVA/PEI nanofibers: Adsorption, reduction, and effects of co-existing ions. Chem. Eng. J. 2020, 387, 124179. [Google Scholar] [CrossRef]
- Xu, P.; Wang, Y.; Wang, S.; Dai, W.; Chen, N.; Li, Q. Preparation of polyethyleneimine-modified porous polyacrylonitrile electrospun nanofibers for efficient removal of methyl orange. J. Macromol. Sci. Part A 2022, 59, 504–512. [Google Scholar] [CrossRef]
- Khalili, R.; Sabzehmeidani, M.M.; Parvinnia, M.; Ghaedi, M. Removal of hexavalent chromium ions and mixture dyes by electrospun PAN/graphene oxide nanofiber decorated with bimetallic nickel–iron LDH. Environ. Nanotechnol. Monit. Manag. 2022, 18, 100750. [Google Scholar] [CrossRef]
- Aldawsari, A.M.; Alsohaimi, I.; Hassan, H.M.; Abdalla, Z.E.; Hassan, I.; Berber, M.R. Tailoring an efficient nanocomposite of activated carbon-layered double hydroxide for elimination of water-soluble dyes. J. Alloys Compd. 2021, 857, 157551. [Google Scholar] [CrossRef]
- Zheng, G.; Peng, H.; Jiang, J.; Kang, G.; Liu, J.; Zheng, J.; Liu, Y. Surface functionalization of PEO nanofibers using a TiO2 suspension as sheath fluid in a modified coaxial electrospinning process. Chem. Res. Chin. Univ. 2021, 37, 571–577. [Google Scholar] [CrossRef]
- Alnaqbi, M.A.; Samson, J.A.; Greish, Y.E. Electrospun polystyrene/LDH fibrous membranes for the removal of Cd2+ ions. J. Nanomater. 2020, 2020, 5045637. [Google Scholar] [CrossRef]
- Qin, Q.; Liu, Y.; Chen, S.C.; Zhai, F.Y.; Jing, X.K.; Wang, Y.Z. Electrospinning fabrication and characterization of poly (vinyl alcohol)/layered double hydroxides composite fibers. J. Appl. Polym. Sci. 2012, 126, 1556–1563. [Google Scholar] [CrossRef]
- Yasin, S.A.; Sharaf Zeebaree, S.Y.; Sharaf Zeebaree, A.Y.; Haji Zebari, O.I.; Saeed, I.A. The efficient removal of methylene blue dye using CuO/PET nanocomposite in aqueous solutions. Catalysts 2021, 11, 241. [Google Scholar] [CrossRef]
- Dong, R.; Zhang, J.; Zhou, B.; Zhou, J. Application of a novel quaternized ammonium poly (vinyl alcohol)-based hybrid anion exchange membrane for the removal of Cr (VI) from wastewater. Water Sci. Technol. 2014, 70, 1602–1609. [Google Scholar] [CrossRef]
- Jin, X.; Wang, H.; Jin, X.; Wang, H.; Chen, L.; Wang, W.; Lin, T.; Zhu, Z. Preparation of keratin/PET nanofiber membrane and its high adsorption performance of Cr (VI). Sci. Total Environ. 2020, 710, 135546. [Google Scholar] [CrossRef]
- da Silva, R.J.; Mojica-Sánchez, L.C.; Gorza, F.D.; Pedro, G.C.; Maciel, B.G.; Ratkovski, G.P.; da Rocha, H.D.; do Nascimento, K.T.; Medina-Llamas, J.C.; Chávez-Guajardo, A.E. Kinetics and thermodynamic studies of Methyl Orange removal by polyvinylidene fluoride-PEDOT mats. J. Environ. Sci. 2021, 100, 62–73. [Google Scholar] [CrossRef]
- Mansor, E.S.; Ali, H.; Abdel-Karim, A. Efficient and reusable polyethylene oxide/polyaniline composite membrane for dye adsorption and filtration. Colloid Interface Sci. Commun. 2020, 39, 100314. [Google Scholar] [CrossRef]
- Habiba, U.; Lee, J.J.L.; Joo, T.C.; Ang, B.C.; Afifi, A.M. Degradation of methyl orange and congo red by using chitosan/polyvinyl alcohol/TiO2 electrospun nanofibrous membrane. Int. J. Biol. Macromol. 2019, 131, 821–827. [Google Scholar] [CrossRef]
- Pathirana, M.A.; Dissanayake, N.S.; Wanasekara, N.D.; Mahltig, B.; Nandasiri, G.K. Chitosan-graphene oxide dip-coated polyacrylonitrile-ethylenediamine electrospun nanofiber membrane for removal of the dye stuffs methylene blue and congo red. Nanomaterials 2023, 13, 498. [Google Scholar] [CrossRef] [PubMed]
- He, Y.; Wu, P.; Xiao, W.; Li, G.; Yi, J.; He, Y.; Chen, C.; Ding, P.; Duan, Y. Efficient removal of Pb (II) from aqueous solution by a novel ion imprinted magnetic biosorbent: Adsorption kinetics and mechanisms. PLoS ONE 2019, 14, e0213377. [Google Scholar] [CrossRef] [PubMed]
- Radoor, S.; Karayil, J.; Jayakumar, A.; Parameswaranpillai, J.; Siengchin, S. Efficient removal of methyl orange from aqueous solution using mesoporous ZSM-5 zeolite: Synthesis, kinetics and isotherm studies. Colloids Surf. A Physicochem. Eng. Asp. 2021, 611, 125852. [Google Scholar] [CrossRef]
- Novillo, C.; Guaya, D.; Avendaño, A.A.-P.; Armijos, C.; Cortina, J.; Cota, I. Evaluation of phosphate removal capacity of Mg/Al layered double hydroxides from aqueous solutions. Fuel 2014, 138, 72–79. [Google Scholar] [CrossRef]
- Arifeen, W.U.; Abideen, Z.U.; Parakash, N.G.; Xiaolong, L.; Ko, T.J. Effects of a high-performance, solution-cast composite electrolyte on the host electrospun polymer membrane for solid-state lithium metal batteries. Mater. Today Energy 2023, 33, 101270. [Google Scholar] [CrossRef]
- Habiba, U.; Afifi, A.M.; Salleh, A.; Ang, B.C. Chitosan/(polyvinyl alcohol)/zeolite electrospun composite nanofibrous membrane for adsorption of Cr6+, Fe3+ and Ni2+. J. Hazard. Mater. 2017, 322, 182–194. [Google Scholar] [CrossRef] [PubMed]
- Bakhsh, N.; Ahmed, Z.; Mahar, R.B.; Khatri, Z. Development and application of electrospun modified polyvinylidene fluoride (PVDF) nanofibers membrane for biofouling control in membrane bioreactor. Desalin. Water Treat 2021, 217, 74–82. [Google Scholar] [CrossRef]
- Wang, Q.; Geng, Y.; Lu, X.; Zhang, S. First-row transition metal-containing ionic liquids as highly active catalysts for the glycolysis of poly (ethylene terephthalate)(PET). ACS Sustain. Chem. Eng. 2015, 3, 340–348. [Google Scholar] [CrossRef]
- Ullah, S.; Hashmi, M.; Kharaghani, D.; Khan, M.Q.; Saito, Y.; Yamamoto, T.; Lee, J.; Kim, I.S. Antibacterial properties of in situ and surface functionalized impregnation of silver sulfadiazine in polyacrylonitrile nanofiber mats. Int. J. Nanomed. 2019, 14, 2693–2703. [Google Scholar] [CrossRef]
- Chiu, H.; Lin, J.; Cheng, T.; Chou, S. Fabrication of electrospun polyacrylonitrile ion-exchange membranes for application in lysozym. Express Polym. Lett. 2011, 5, 308–317. [Google Scholar] [CrossRef]
- Swain, S.K.; Barik, S.; Pradhan, G.C.; Behera, L. Delamination of Mg-Al layered double hydroxide on starch: Change in structural and thermal properties. Polym.-Plast. Technol. Eng. 2018, 57, 1585–1591. [Google Scholar] [CrossRef]
- Xuefeng, G. Water-repellent legs of water strider. Nature 2004, 432, 36. [Google Scholar]
- Li, X.; Ding, B.; Lin, J.; Yu, J.; Sun, G. Enhanced mechanical properties of superhydrophobic microfibrous polystyrene mats via polyamide 6 nanofibers. J. Phys. Chem. C 2009, 113, 20452–20457. [Google Scholar] [CrossRef]
- Skornyakov, I.; Komar, V. IR spectra and the structure of plasticized cellulose acetate films. J. Appl. Spectrosc. 1998, 65, 911–918. [Google Scholar] [CrossRef]
- Wang, N.; Zhao, Y.; Jiang, L. Low-cost, thermoresponsive wettability of surfaces: Poly (N-isopropylacrylamide)/Polystyrene composite films prepared by electrospinning. Macromol. Rapid Commun. 2008, 29, 485–489. [Google Scholar] [CrossRef]
- Jiang, L.; Zhao, Y.; Zhai, J. A lotus-leaf-like superhydrophobic surface: A porous microsphere/nanofiber composite film prepared by electrohydrodynamics. Angew. Chem. Int. Ed. 2004, 43, 4338–4341. [Google Scholar] [CrossRef] [PubMed]
- Li, G.; Zhao, Y.; Lv, M.; Shi, Y.; Cao, D. Super hydrophilic poly (ethylene terephthalate)(PET)/poly (vinyl alcohol)(PVA) composite fibrous mats with improved mechanical properties prepared via electrospinning process. Colloids Surf. A Physicochem. Eng. Asp. 2013, 436, 417–424. [Google Scholar] [CrossRef]
- Jia, S.; Liang, Y.; Yang, N. High performance of polyacrylonitrile/[MgAl]-layered double hydroxide composite nanofiber separators for safe lithium-ion batteries. Solid State Ion. 2021, 370, 115735. [Google Scholar] [CrossRef]
- Karamipour, A.; Parsi, P.K.; Zahedi, P.; Moosavian, S.M.A. Using Fe3O4-coated nanofibers based on cellulose acetate/chitosan for adsorption of Cr (VI), Ni (II) and phenol from aqueous solutions. Int. J. Biol. Macromol. 2020, 154, 1132–1139. [Google Scholar] [CrossRef]
- Kummer, G.; Schonhart, C.; Fernandes, M.; Dotto, G.; Missio, A.; Bertuol, D.; Tanabe, E. Development of nanofibers composed of chitosan/nylon 6 and tannin/nylon 6 for effective adsorption of Cr (VI). J. Polym. Environ. 2018, 26, 4073–4084. [Google Scholar] [CrossRef]
- Anah, L.; Astrini, N. Influence of pH on Cr (VI) ions removal from aqueous solutions using carboxymethyl cellulose-based hydrogel as adsorbent. In Proceedings of the IOP Conference Series: Earth and Environmental Science—1st International Symposium on Green Technology for Value Chains, Tangerang, Indonesia, 3–5 October 2016; Volume 60, p. 012010. [Google Scholar]
- Chaukura, N.; Murimba, E.C.; Gwenzi, W. Synthesis, characterisation and methyl orange adsorption capacity of ferric oxide–biochar nano-composites derived from pulp and paper sludge. Appl. Water Sci. 2017, 7, 2175–2186. [Google Scholar] [CrossRef]
- Wu, Y.; Luo, H.; Wang, H.; Wang, C.; Zhang, J.; Zhang, Z. Adsorption of hexavalent chromium from aqueous solutions by graphene modified with cetyltrimethylammonium bromide. J. Colloid Interface Sci. 2013, 394, 183–191. [Google Scholar] [CrossRef]
- Ansari, S.; Ahmed, N.; Mahar, R.B.; Khatri, Z.; Khatri, M. Fabrication and characterization of electrospun zein/nylon-6 (ZN6) nanofiber membrane for hexavalent chromium removal. Environ. Sci. Pollut. Res. 2022, 29, 653–662. [Google Scholar] [CrossRef]
- Khan, M.I.; Shanableh, A.; Fernandez, J.; Lashari, M.H.; Shahida, S.; Manzoor, S.; Zafar, S.; ur Rehman, A.; Elboughdiri, N. Synthesis of dmea-grafted anion exchange membrane for adsorptive discharge of methyl orange from wastewaters. Membranes 2021, 11, 166. [Google Scholar] [CrossRef]
- Bensalah, H.; Younssi, S.A.; Ouammou, M.; Gurlo, A.; Bekheet, M.F. Azo dye adsorption on an industrial waste-transformed hydroxyapatite adsorbent: Kinetics, isotherms, mechanism and regeneration studies. J. Environ. Chem. Eng. 2020, 8, 103807. [Google Scholar] [CrossRef]
- Eltaweil, A.; Mohamed, H.A.; Abd El-Monaem, E.M.; El-Subruiti, G. Mesoporous magnetic biochar composite for enhanced adsorption of malachite green dye: Characterization, adsorption kinetics, thermodynamics and isotherms. Adv. Powder Technol. 2020, 31, 1253–1263. [Google Scholar] [CrossRef]
- Al-Sou’od, K. Adsorption isotherm studies of chromium (VI) from aqueous solutions using Jordanian pottery materials. Apcbee Procedia 2012, 1, 116–125. [Google Scholar] [CrossRef]
- Li, L.; Luo, C.; Li, X.; Duan, H.; Wang, X. Preparation of magnetic ionic liquid/chitosan/graphene oxide composite and application for water treatment. Int. J. Biol. Macromol. 2014, 66, 172–178. [Google Scholar] [CrossRef]
- Konicki, W.; Aleksandrzak, M.; Moszyński, D.; Mijowska, E. Adsorption of anionic azo-dyes from aqueous solutions onto graphene oxide: Equilibrium, kinetic and thermodynamic studies. J. Colloid Interface Sci. 2017, 496, 188–200. [Google Scholar] [CrossRef]
- Cha, D.I.; Kim, K.W.; Chu, G.H.; Kim, H.Y.; Lee, K.H.; Bhattarai, N. Mechanical behaviors and characterization of electrospun polysulfone/polyurethane blend nonwovens. Macromol. Res. 2006, 14, 331–337. [Google Scholar] [CrossRef]
- Veleirinho, B.; Rei, M.F.; Lopes-DA-Silva, J. Solvent and concentration effects on the properties of electrospun poly (ethylene terephthalate) nanofiber mats. J. Polym. Sci. Part B Polym. Phys. 2008, 46, 460–471. [Google Scholar] [CrossRef]
- Perrotti, T.C.; Freitas, N.S.; Alzamora, M.; Sanchez, D.R.; Carvalho, N.M. Green iron nanoparticles supported on amino-functionalized silica for removal of the dye methyl orange. J. Environ. Chem. Eng. 2019, 7, 103237. [Google Scholar] [CrossRef]
- Mallakpour, S.; Hatami, M. An effective, low-cost and recyclable bio-adsorbent having amino acid intercalated LDH@ Fe3O4/PVA magnetic nanocomposites for removal of methyl orange from aqueous solution. Appl. Clay Sci. 2019, 174, 127–137. [Google Scholar] [CrossRef]
- Yagub, M.T.; Sen, T.K.; Afroze, S.; Ang, H.M. Dye and its removal from aqueous solution by adsorption: A review. Adv. Colloid Interface Sci. 2014, 209, 172–184. [Google Scholar] [CrossRef]
- Dąbrowski, A. Adsorption—From theory to practice. Adv. Colloid Interface Sci. 2001, 93, 135–224. [Google Scholar] [CrossRef] [PubMed]
- Jin, Z.; Wang, X.; Sun, Y.; Ai, Y.; Wang, X. Adsorption of 4-n-nonylphenol and bisphenol-A on magnetic reduced graphene oxides: A combined experimental and theoretical studies. Environ. Sci. Technol. 2015, 49, 9168–9175. [Google Scholar] [CrossRef]
- Sheng, G.; Hu, J.; Li, H.; Li, J.; Huang, Y. Enhanced sequestration of Cr (VI) by nanoscale zero-valent iron supported on layered double hydroxide by batch and XAFS study. Chemosphere 2016, 148, 227–232. [Google Scholar] [CrossRef]
- Sheng, G.; Tang, Y.; Linghu, W.; Wang, L.; Li, J.; Li, H.; Wang, X.; Huang, Y. Enhanced immobilization of ReO4− by nanoscale zerovalent iron supported on layered double hydroxide via an advanced XAFS approach: Implications for TcO4− sequestration. Appl. Catal. B Environ. 2016, 192, 268–276. [Google Scholar] [CrossRef]
- Daud, M.; Hai, A.; Banat, F.; Wazir, M.B.; Habib, M.; Bharath, G.; Al-Harthi, M.A. A review on the recent advances, challenges and future aspect of layered double hydroxides (LDH)–Containing hybrids as promising adsorbents for dyes removal. J. Mol. Liq. 2019, 288, 110989. [Google Scholar] [CrossRef]
- Deng, L.; Shi, Z.; Peng, X. Adsorption of Cr (VI) onto a magnetic CoFe2O4/MgAl-LDH composite and mechanism study. RSC Adv. 2015, 5, 49791–49801. [Google Scholar] [CrossRef]




| Sr # | Membrane Type | PET (%) | PAN (%) | LDH (g) | PVA (%) |
|---|---|---|---|---|---|
| 1 | PP | 5 | 8 | _ | _ |
| 2 | PPLH1 | 5 | 8 | 0.08 | 8 |
| 3 | PPLH2 | 5 | 8 | 0.12 | 8 |
| 4 | PPLH3 | 5 | 8 | 0.16 | 8 |
| Sample | Al | Mg | C | O | N |
|---|---|---|---|---|---|
| LDH | 5.39 | 16.63 | 11.37 | 66.61 | - |
| PP | - | - | 86.61 | 6.95 | 6.43 |
| PPLH1 | 0.17 | 0.20 | 72.71 | 23.11 | 3.81 |
| PPLH2 | 0.22 | 0.42 | 58.17 | 36.10 | 5.09 |
| PPLH3 | 0.40 | 0.94 | 57.29 | 37.34 | 4.03 |
| Membrane Type | Pollutant Type | Solution pH | Adsorption Time (min) | Concentration (mg/L) | Dosage (mg) | Solution Volume (mL) |
|---|---|---|---|---|---|---|
| PP | Cr(VI) | 2 | 90 | 5 | 25 | 20 |
| PPLH1 | ||||||
| PPLH2 | ||||||
| PPLH3 | ||||||
| PP | MO | 4 | 60 | 5 | 25 | 20 |
| PPLH1 | ||||||
| PPLH2 | ||||||
| PPLH3 |
| Langmuir Isotherm | Freundlich Isotherm | ||||
|---|---|---|---|---|---|
| qmax (mg g–1) | KL (L mg–1) | R2 | KF (mg·g−1)(L·mg−1)1/n | 1/n | R2 |
| 5.2 | 0.558 | 0.9788 | 3.1 | 0.1 | 0.8146 |
| Pseudo-1st Order | Pseudo-2nd Order | ||||
|---|---|---|---|---|---|
| qe (mg g–1) | k1 (h–1) | R2 | qe (mg g–1) | k2 (g mg–1 h–1) | R2 |
| 2.90 | 0.018 | 0.966 | 4.39 | 0.009 | 0.994 |
| S.No | Adsorbent | pH | Pollutant | Equilibrium Time (min) | qmax (mg/g) | Reference |
|---|---|---|---|---|---|---|
| 1 | PET-PAN-Mg-AI-LDH-PVA | 4 | MO | 60 | 5.2 | This study |
| 2 | NiFeLDH/PAN/GO | 6 | Cr(VI) | - | 6.19 | [31] |
| 3 | Fe NPs/SiO2–NH2/glycerol | 3 | MO | 180 | 3.02 | [74] |
| 4 | LDH@Fe3O4/PVA | 6 | MO | 420 | 19.5 | [75] |
| 5 | Zein/nylon-6 nanofibrous membrane | 2 | Cr(VI) | 60 | 4.73 | [65] |
| 6 | Chitosan/nylon 6 | 3 | Cr(VI) | 240 | 23.9 | [61] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Pirzada, A.M.; Ali, I.; Mallah, N.B.; Maitlo, G. Development of Novel PET-PAN Electrospun Nanocomposite Membrane Embedded with Layered Double Hydroxides Hybrid for Efficient Wastewater Treatment. Polymers 2023, 15, 4388. https://doi.org/10.3390/polym15224388
Pirzada AM, Ali I, Mallah NB, Maitlo G. Development of Novel PET-PAN Electrospun Nanocomposite Membrane Embedded with Layered Double Hydroxides Hybrid for Efficient Wastewater Treatment. Polymers. 2023; 15(22):4388. https://doi.org/10.3390/polym15224388
Chicago/Turabian StylePirzada, Abdul Majeed, Imran Ali, Nabi Bakhsh Mallah, and Ghulamullah Maitlo. 2023. "Development of Novel PET-PAN Electrospun Nanocomposite Membrane Embedded with Layered Double Hydroxides Hybrid for Efficient Wastewater Treatment" Polymers 15, no. 22: 4388. https://doi.org/10.3390/polym15224388
APA StylePirzada, A. M., Ali, I., Mallah, N. B., & Maitlo, G. (2023). Development of Novel PET-PAN Electrospun Nanocomposite Membrane Embedded with Layered Double Hydroxides Hybrid for Efficient Wastewater Treatment. Polymers, 15(22), 4388. https://doi.org/10.3390/polym15224388







