Interfacial Water Stability between Modified Phosphogypsum Asphalt Mortar and Aggregate Based on Molecular Dynamics
Abstract
:1. Introduction
2. Materials and Methods
2.1. Construction of Asphalt Mortar
2.1.1. Matrix Asphalt Components
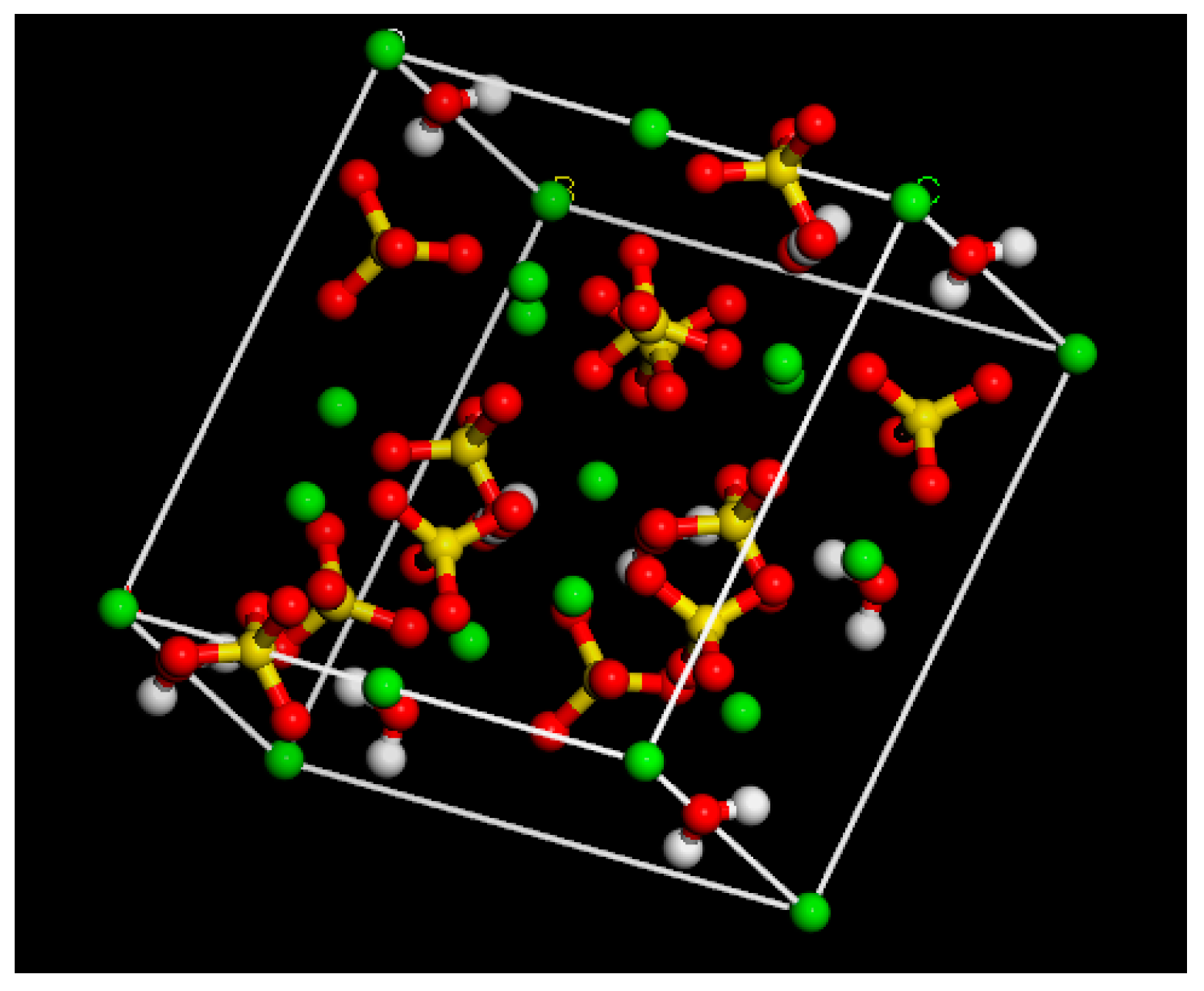
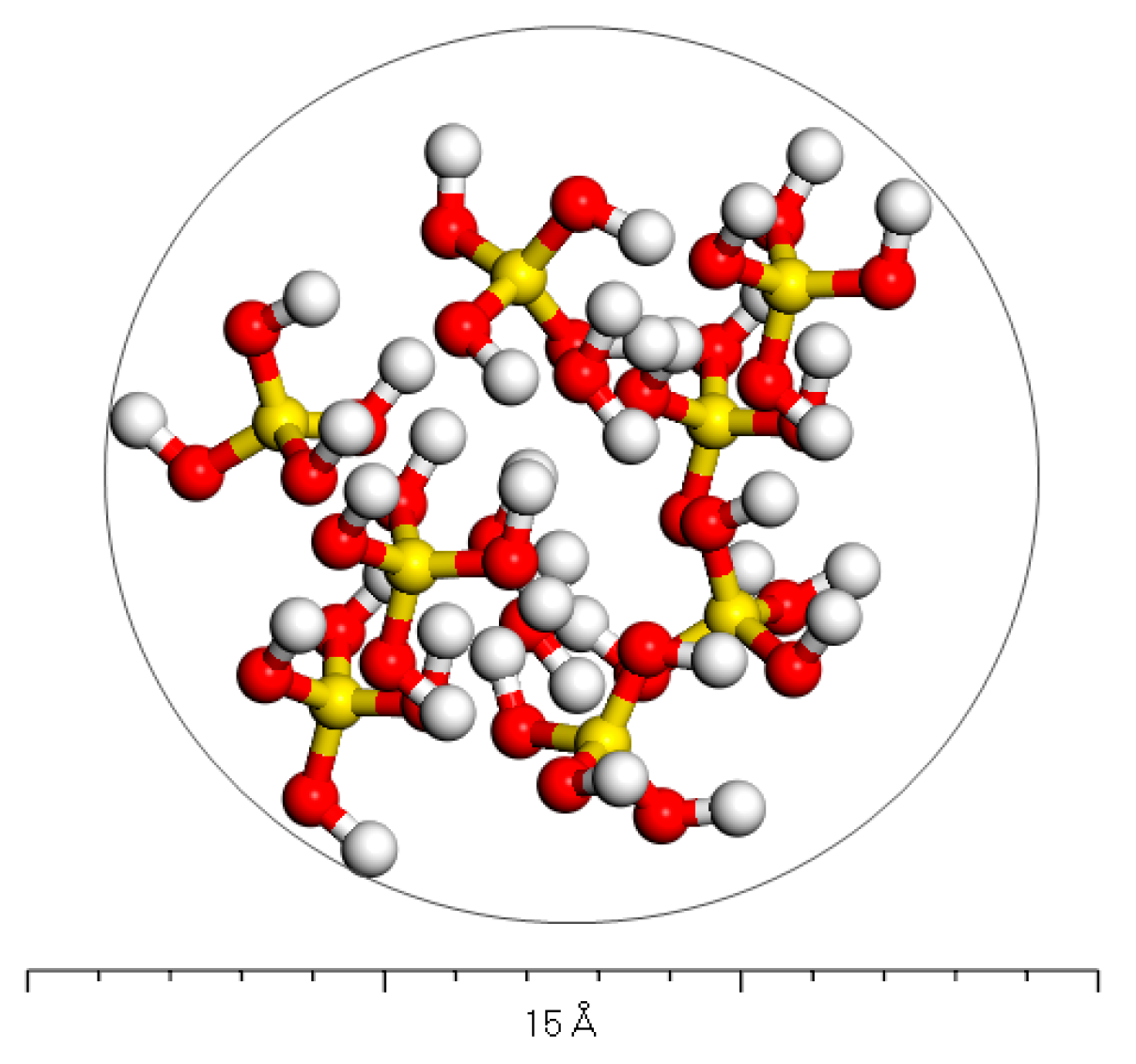
2.1.2. Phosphogypsum Molecular Model
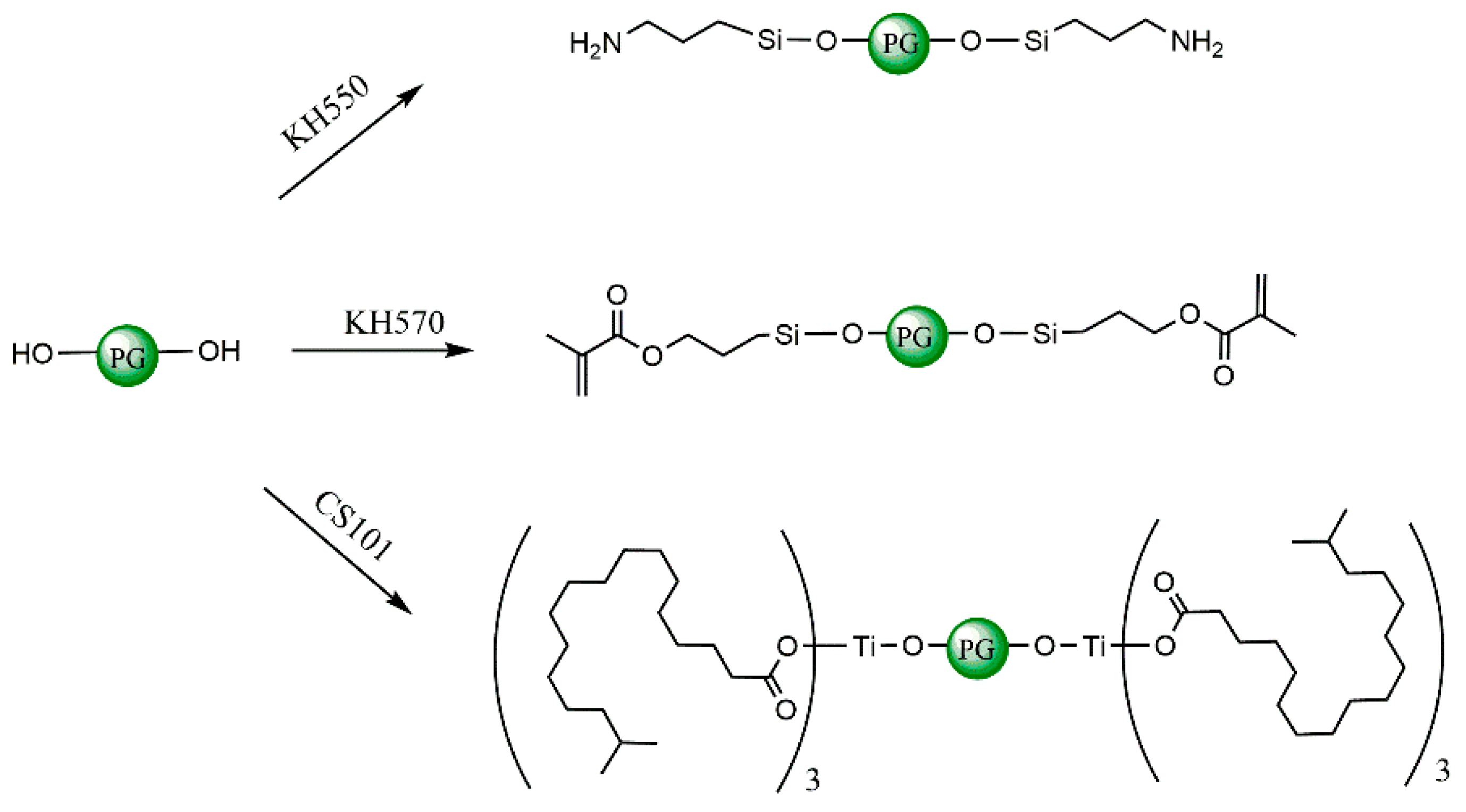
2.1.3. Construction of Coupling Agent Model
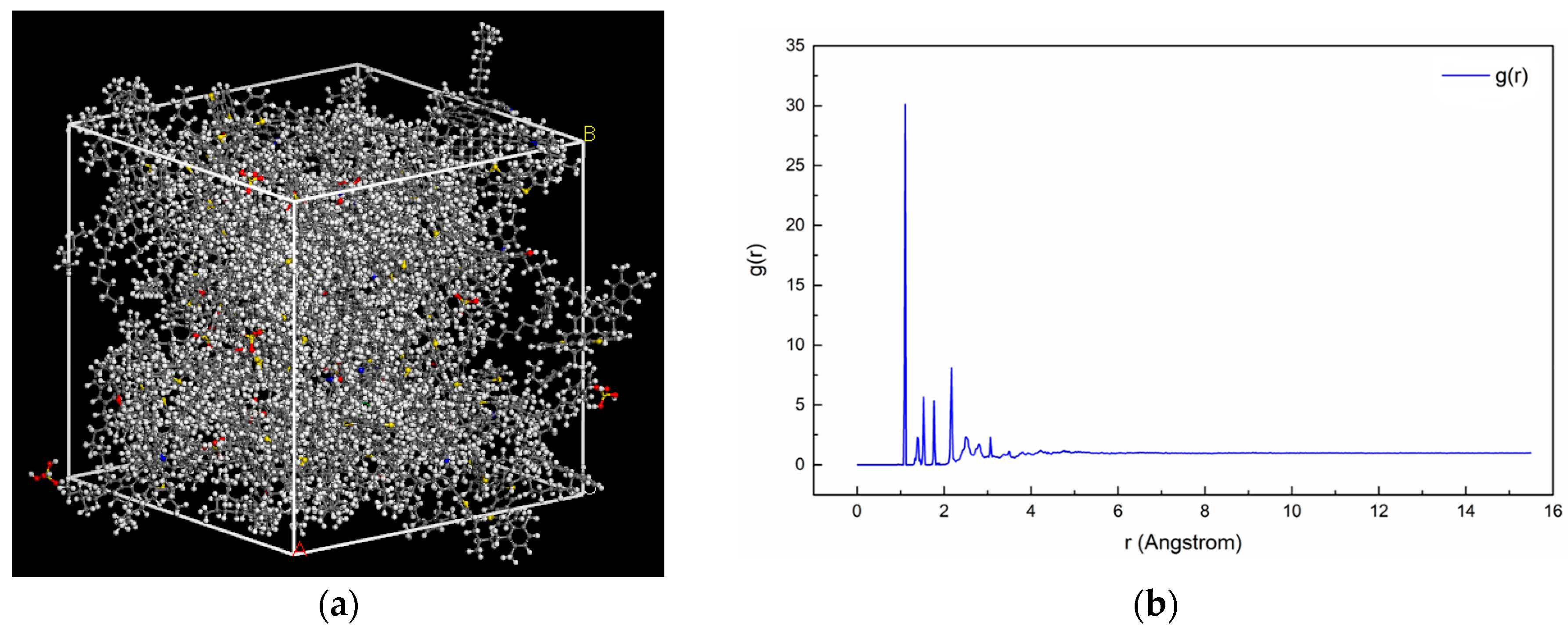
2.1.4. Assembly of Asphalt Mortar
2.2. Construction of Interfacial Model between PAM Mortar and Aggregate Mineral
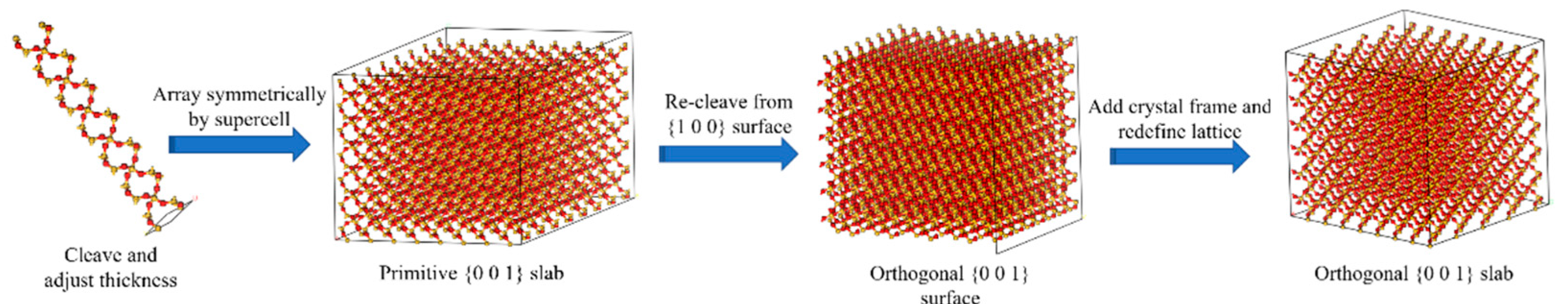
2.2.1. Aggregate Mineral Molecular Model
- The appropriate thickness of α-Quartz primary cells in the resource library of the MS software system is determined by cleaving them with the Clear function within the range of 6.3 ± 0.6 Å. The determination of the suitable threshold is founded on the premise that the molecular configuration of the rear of the “Cleave” lacks any atoms that are isolated or linked by a lone chemical bond.
- The supercell function is utilized to perform array replication on the surface of the initial cleavage, resulting in the construction of a supercell based on the supercell parameters setting of u = 9 and v = 9, respectively.
- The non-orthogonal mineral composition model undergoes secondary sectioning through the “Reclave” operation. The selection of the profile here is based on [27]. After completion of the “Reclave” procedure, a 10 Å vacuum layer is added to the layered model to achieve the orthogonal aggregate model. Subsequently, the Miller surface of the construction target should be adjusted to face upward through the setting of crystal cell parameters, as depicted in Figure 8 (where the colour representation of atoms is the same as above).
2.2.2. Water Molecule Model
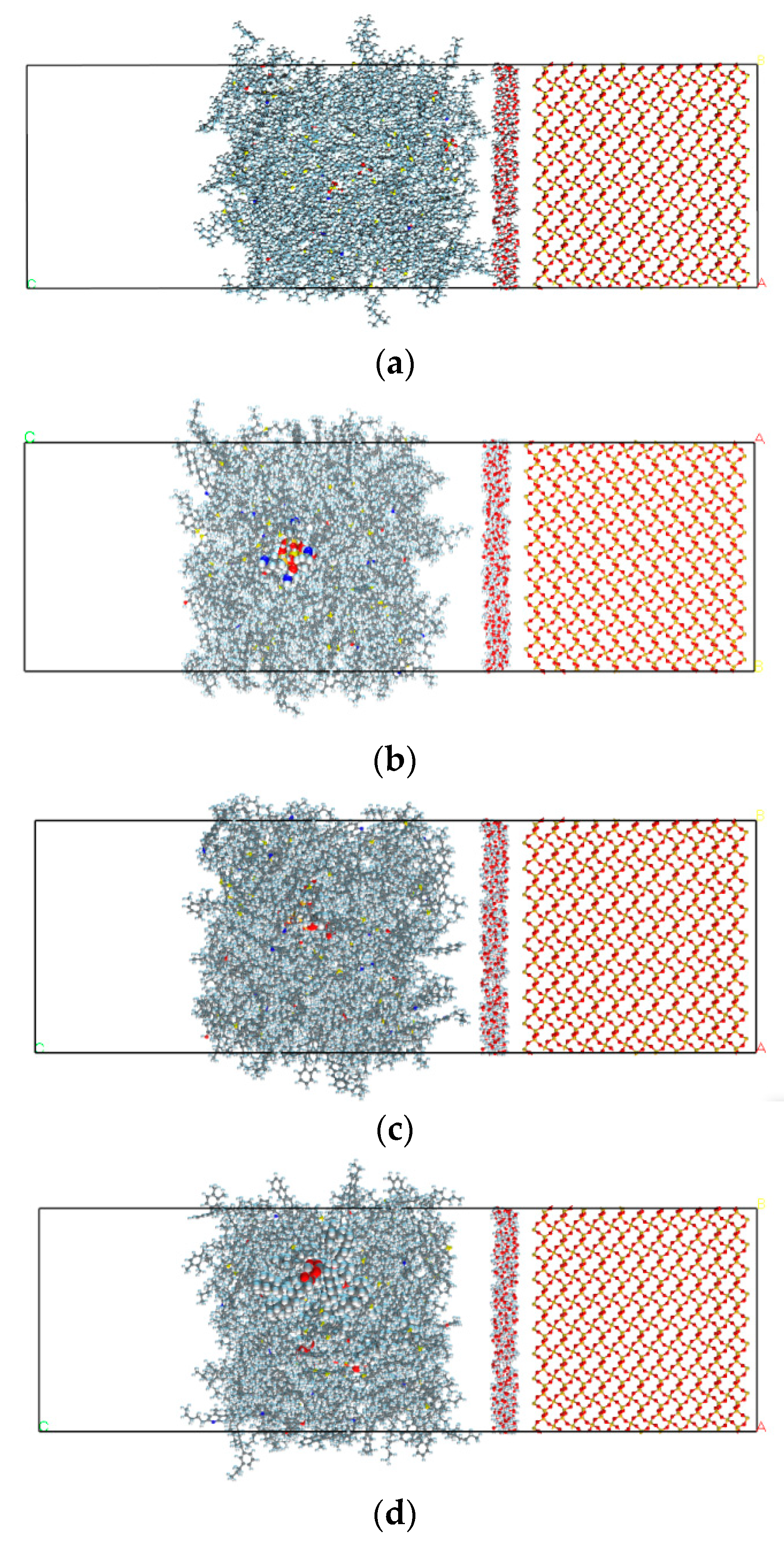
2.2.3. Interface Model between PAM Mortar and Aggregate Mineral Model
3. Result Analysis
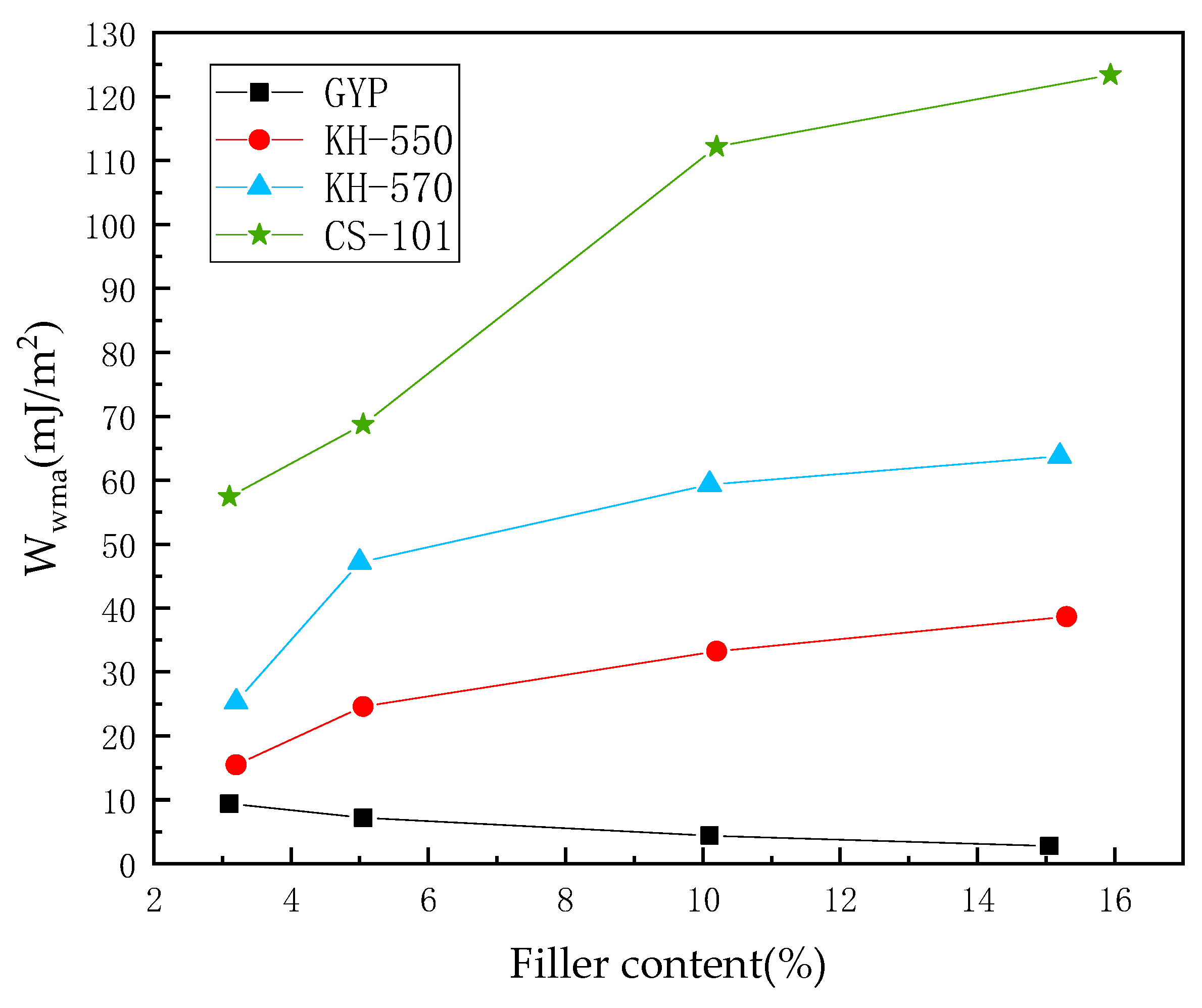
3.1. Adhesion Work of PAM–Water–Aggregate Interface
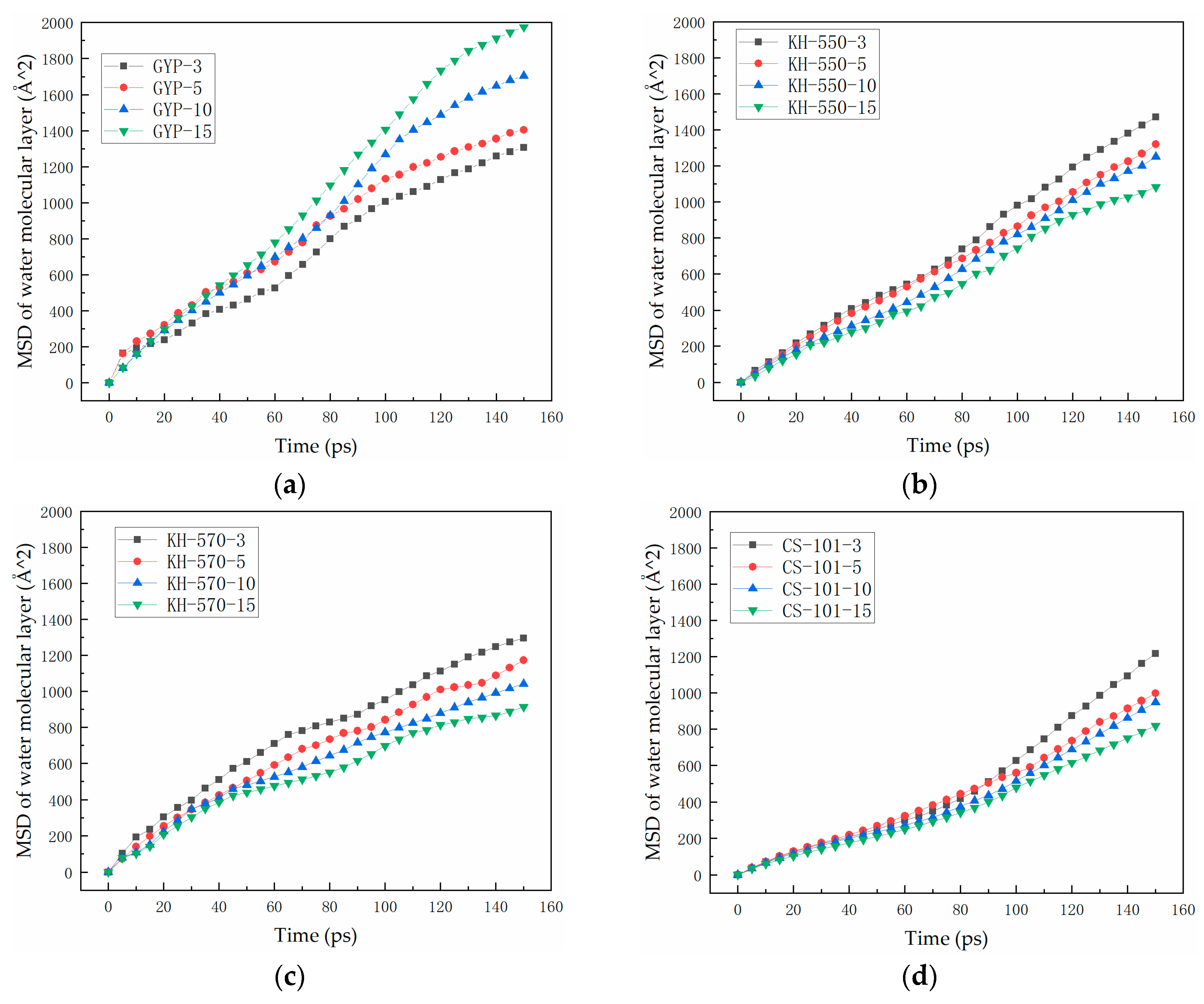
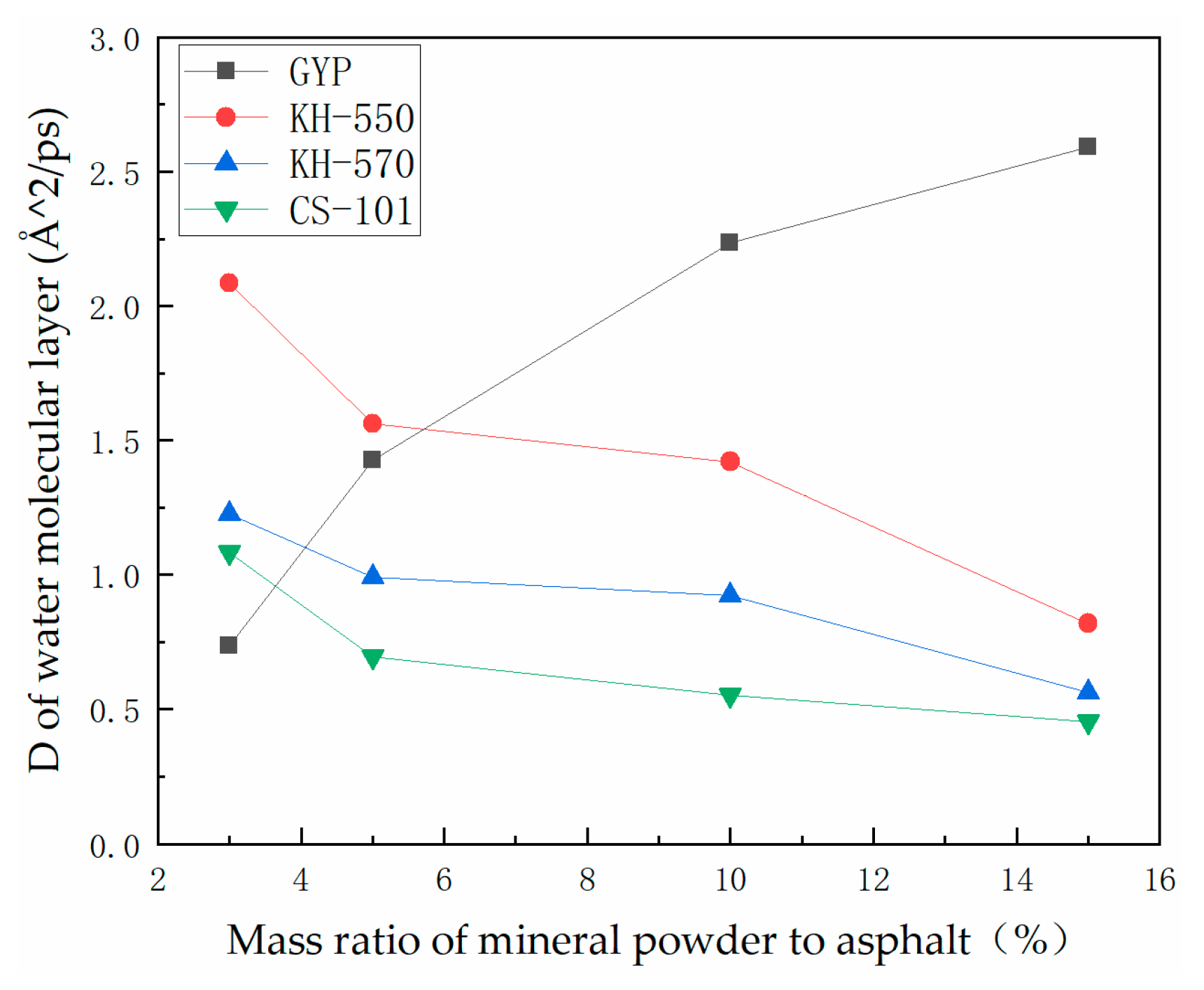
3.2. Analysis of the Degree of Diffusion of the Water Molecule Layer
4. Discussion
- The hydroxylated sulfate ions in phosphogypsum show significant hydrophilicity, and the diffusion rate of its small molecules in asphalt is faster, which leads to the existence of a water film and thus reduces the adhesion between asphalt and aggregate. After coupling agent modification, the hydrophilic group of sulfates is transformed into the long carbon chain of the coupling agent, which leads to the enhancement of the molecular polarity characteristics. This process may produce the phenomenon of the asphaltene molecules entangled in the aggregation, thus promoting the adsorption of asphalt and aggregate mineral components.
- In the absence of a coupling agent, the addition of phosphogypsum causes diffusion of the water molecule layer in the asphalt mortar model. Because of phosphogypsum, the water sensitivity of asphalt increases, resulting in greater water molecule intervention on the asphalt–aggregate interface. Increasing the length of the carbon chain of the coupling agent can indirectly enhance the modification effect of the coupling agent on water stability. The limiting effect of KH-570 on interfacial water is stronger than that of KH-550.
- The diffusion coefficient of the water molecular layer of asphalt mastic modified with CS-101 coupling agent in phosphogypsum shows a significant decrease with the increase of CS-101-modified phosphogypsum (more than 5% mass ratio to asphalt). Compared with the KH coupling agent, the inhibition effect of CS-101 coupling agent on water diffusion is more significant. However, when these coupling agents are adopted in phosphogypsum-modified asphalt (with lower mass ratio to asphalt), it is preferable to use a KH type coupling agent when considering water stability.
- From the results of this study, it can be concluded that when the coupling agent-modified phosphogypsum is used as mineral powder filler (with higher mass ratio to asphalt), the coupling agent modification can significantly restrict the diffusion of water molecules and improve the adhesion performance of PAM, in which CS-101 coupling agent has the best effect, followed by KH-570 and KH-550.
- The modelling and findings in this paper provide a basis for further investigation of other properties of PAM such as mechanical properties, rheological properties, low temperature properties and self-healing properties. In addition, the research in this paper is mainly based on molecular simulation techniques, and experimental studies for the adhesion of PAM and aggregates and other influencing factors of adhesion are also not fully covered in the existing studies. Further studies will focus on the above-mentioned ideas.
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Conflicts of Interest
References
- Weiksnar, K.D.; Clavier, K.A.; Laux, S.J.; Townsend, T.G. Influence of trace chemical constituents in phosphogypsum for road base applications: A review. Resour. Conserv. Recycl. 2023, 199, 107237. [Google Scholar] [CrossRef]
- Bilal, E.; Bellefqih, H.; Bourgier, V.; Mazouz, H.; Dumitraş, D.-G.; Bard, F.; Laborde, M.; Caspar, J.P.; Guilhot, B.; Iatan, E.-L.; et al. Phosphogypsum circular economy considerations: A critical review from more than 65 storage sites worldwide. J. Clean. Prod. 2023, 414, 137561. [Google Scholar] [CrossRef]
- Haneklaus, N.; Barbossa, S.; Basallote, M.D.; Bertau, M.; Bilal, E.; Chajduk, E.; Chernysh, Y.; Chubur, V.; Cruz, J.; Dziarczykowski, K.; et al. Closing the upcoming EU gypsum gap with phosphogypsum. Resour. Conserv. Recycl. 2022, 182, 106328. [Google Scholar] [CrossRef]
- Wang, W.; Li, T.; Jia, Y.; Wei, J.; Cao, Z. The State-of-the-Art Review on Road Harmless Utilization Technology of Phosphogypsum. In Proceedings of the 7th International Technical Conference on Frontiers of Hydraulic and Civil Engineering Technology, HCET 2022, Online, 25–27 September 2022; pp. 390–395. [Google Scholar]
- Ou, L.; Zhu, H.Z.; Xu, Y.L.; Chen, R.P.; Yang, X.S. Gray correlation entropy analysis of zero shear viscosity and high-temperature rheological parameters of phosphogypsum-modified asphalt. Case Stud. Constr. Mater. 2022, 17, e01448. [Google Scholar] [CrossRef]
- Diao, M. Study on Performances of Phosphogypsum Whiskers Modified Asphalt. Master’s Thesis, Guizhou University, Guiyang, China, 2016. [Google Scholar]
- Qiu, H.; Tan, X.; Shi, S.; Zhang, H. Influence of filler-bitumen ratio on performance of modified asphalt mortar by additive. J. Mod. Transp. 2013, 21, 40–46. [Google Scholar] [CrossRef]
- Cuadri, A.A.; Navarro, F.J.; García-Morales, M.; Bolívar, J.P. Valorization of phosphogypsum waste as asphaltic bitumen modifier. J. Hazard. Mater. 2014, 279, 11–16. [Google Scholar] [CrossRef] [PubMed]
- Cuadri, A.A.; Perez-Moreno, S.; Altamar, C.L.; Navarro, F.J.; Bolivar, J.P. Phosphogypsum as additive for foamed bitumen manufacturing used in asphalt paving. J. Clean. Prod. 2021, 283, 124661. [Google Scholar] [CrossRef]
- Kim, Y.M.; Lee, S.Y. Experiments and numerical analysis of cold-recycled asphalt mixture modified with desulfurization gypsum additive. Constr. Build. Mater. 2022, 326, 126803. [Google Scholar] [CrossRef]
- Li, G. Research on Temperature Sensitivity and Viscosity of Calcium Sulfate Whisker Modified Asphalt. New Chem. Mater. 2017, 45, 235–237. [Google Scholar]
- Li, K. Study on the Effect of Phosphogypsum-Based Filler on the Properties of Asphalt Mortar and Its Mixture. Ph.D. Thesis, Wuhan Institute of Technology, Wuhan, China, 2022. [Google Scholar]
- Ignatyev, A.; Gotovtsev, V.; Razgovorov, P. Effects of structural formation in the implementation of the technology for obtaining asphalt concrete mixtures with phosphogypsum and other additives. IOP Conf. Ser. Mater. Sci. Eng. 2021, 1100, 012016. [Google Scholar] [CrossRef]
- Chen, M.J.; Li, L. Waterproof Performance of Gypsum Modified by Silane Coupling Agent and Polyvinyl Alcohol. Bull. Chin. Ceram. Soc. 2014, 33, 1743–1747. [Google Scholar] [CrossRef]
- Zhang, A.; Wang, Y.; Sun, S. Improving Water Stability of Granite-Asphalt Interface by Adding Titanate Ester Coupling Agent. In Proceedings of the International Conference on Transportation Engineering, Chengdu, China, 25–27 July 2009. [Google Scholar]
- Peng, C.; Chen, P.X.; You, Z.P.; Lv, S.T.; Zhang, R.; Xu, F.; Zhang, H.; Chen, H.L. Effect of silane coupling agent on improving the adhesive properties between asphalt binder and aggregates. Constr. Build. Mater. 2018, 169, 591–600. [Google Scholar] [CrossRef]
- Cao, L.P.; Zhang, X.K.; Yang, C.; Luan, H.; Tian, Z.W.; Zhang, B.T.; Dong, Z.J. Modification mechanism of iron tailings asphalt mixture by silane coupling agents based on molecular dynamics. J. Cent. South Univ. Sci. Technol. 2021, 52, 2276–2286. [Google Scholar] [CrossRef]
- Li, D.D.; Greenfield, M.L. Chemical compositions of improved model asphalt systems for molecular simulations. Fuel 2014, 115, 347–356. [Google Scholar] [CrossRef]
- Tang, Y. Preparation Technology and Mechanism of High Performance Gypsum Cementitious Material. Ph.D. Thesis, Southeast University, Dhaka, Bangladesh, 2022. [Google Scholar]
- Liu, H. Study on In-Situ Polymerization of PMMA/Phosphogypsum Composite Microspheres and Applications. Ph.D. Thesis, Guizhou University, Guiyang, China, 2022. [Google Scholar]
- Xu, J.Y.; Ma, B.; Mao, W.J.; Si, W.; Wang, X.Q. Review of interfacial adhesion between asphalt and aggregate based on molecular dynamics. Constr. Build. Mater. 2023, 362, 129642. [Google Scholar] [CrossRef]
- Wu, H.M.; Liu, X.Y.; Fu, H.; Gong, W.; Chen, Z.; Yin, X.G. Phosphogypsum Modified by Titanate Coupling Agent and Its Application in PC/ABS. New Chem. Mater. 2020, 48, 254–258. [Google Scholar] [CrossRef]
- Chen, L.X.; Fu, H.; Gong, W.; Chen, Z.; Yin, X.G. Modification of Phosphogypsum by Silane Coupling Agent and Its Effect on Mechanical Properties of Polycarbonate/Acylonitrile Butadiene Styrene. Chem. World 2020, 61, 833–840. [Google Scholar] [CrossRef]
- Xu, S. Research on Crack Resistance of Asphalt Mastic at Medium Temperature Based on Filler Propertieses. Ph.D. Thesis, Chang’an University, Xi’an, China, 2021. [Google Scholar]
- Gao, Y.; Zhang, Y.; Gu, F.; Xu, T.; Wang, H. Impact of minerals and water on bitumen-mineral adhesion and debonding behaviours using molecular dynamics simulations. Constr. Build. Mater. 2018, 171, 214–222. [Google Scholar] [CrossRef]
- Guo, M.; Tan, Y.Q.; Wei, J.M. Using Molecular Dynamics Simulation to Study Concentration Distribution of Asphalt Binder on Aggregate Surface. J. Mater. Civ. Eng. 2018, 30, 04018075. [Google Scholar] [CrossRef]
- Chu, L.; Luo, L.; Fwa, T.F. Effects of aggregate mineral surface anisotropy on asphalt-aggregate interfacial bonding using molecular dynamics (MD) simulation. Constr. Build. Mater. 2019, 225, 1–12. [Google Scholar] [CrossRef]
- The NIST Reference on Constants, Units, and Uncertainty. Available online: https://physics.nist.gov/cuu/Constants/energy.html (accessed on 26 September 2023).



| Component | Numbers | Mass Ratio | ||
|---|---|---|---|---|
| Types | Name | Formula | ||
| Asphaltenes | Asphaltene-phenol | C42H54O | 3 | 5.3 |
| Asphaltene-pyrrole | C66H81N | 2 | 5.5 | |
| Asphaltene-thiophene | C51H62S | 3 | 6.6 | |
| Resins | Quinolinohopane | C40H59N | 4 | 5.8 |
| Thioisorenieratane | C40H60S | 4 | 7.1 | |
| Benzobisbenzothiophene | C18H10S2 | 15 | 13.5 | |
| Pyridinohopane | C36H57N | 4 | 6.2 | |
| Trimethybenzeneoxane | C29H50O | 5 | 6.4 | |
| Saturates | Squalane | C30H62 | 4 | 5.2 |
| Hopane | C35H62 | 4 | 6.0 | |
| Polycyclic aromatics | PHPN | C35H44 | 11 | 15.8 |
| DOCHN | C30H46 | 13 | 16.3 | |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Liang, C.; Li, Y.; Feng, P.; Li, Y. Interfacial Water Stability between Modified Phosphogypsum Asphalt Mortar and Aggregate Based on Molecular Dynamics. Polymers 2023, 15, 4412. https://doi.org/10.3390/polym15224412
Liang C, Li Y, Feng P, Li Y. Interfacial Water Stability between Modified Phosphogypsum Asphalt Mortar and Aggregate Based on Molecular Dynamics. Polymers. 2023; 15(22):4412. https://doi.org/10.3390/polym15224412
Chicago/Turabian StyleLiang, Cancan, Yilang Li, Ponan Feng, and Yuanle Li. 2023. "Interfacial Water Stability between Modified Phosphogypsum Asphalt Mortar and Aggregate Based on Molecular Dynamics" Polymers 15, no. 22: 4412. https://doi.org/10.3390/polym15224412






